Abstract
Hepatitis A virus (HAV) and Norwalk-like virus (NLV) were detected by reverse transcription-PCR in clams imported into the United States from China. An epidemiological investigation showed that these clams were associated with five cases of Norwalk-like gastroenteritis in New York State in August 2000 (Food and Drug Administration Import Alert 16-50). They were labeled “cooked” but appeared raw. Viral RNA extraction was performed by using dissected digestive tissues rather than whole shellfish meats; this was followed by glycine buffer elution, polyethylene glycol precipitation, Tri-Reagent treatment, and purification of poly(A) RNA with magnetic beads coupled to poly(dT) oligonucleotides. We identified HAV and NLV as genotype I and genogroup II strains, respectively. Both viruses have high levels of homology to Asian strains. An analysis of fecal coliforms revealed a most-probable number of 93,000/100 g of clam meat, which is approximately 300-fold higher than the hygienic standard for shellfish meats.
Shellfish are filter feeders that can readily bioconcentrate human pathogens found within fecally contaminated growing waters. Viral pathogens, such as hepatitis A virus (HAV) and Norwalk-like viruses (NLVs), are potential causes of viral illness associated with raw shellfish consumption. NLVs are a leading cause of food-borne illness in the United States (18), and most adults are seropositive for this virus, indicating that exposure to NLVs is quite common (4). Approximately 80,000 illnesses due to HAV occur in the United States per annum (18); however, the potential for a widespread shellfish-associated hepatitis A outbreak is high. For example, approximately 300,000 people in Shanghai, China, or 5% of the city's population, developed hepatitis A after the consumption of contaminated clams in 1988 (9).
In August of 2000, five cases of gastroenteritis consistent with symptoms associated with Norwalk-like illness were reported after the consumption of raw clams in a restaurant in Cortland Manor, N.Y. These clams were imported from China and, although packaged and labeled as “cooked,” had the physical appearance and texture of raw clams when thawed. With the cooperation of the importing firm, stocks of these clams were embargoed by the New Jersey State Health Department at the request of the U.S. Food and Drug Administration (USFDA; Import Alert 16-50). Our laboratory received frozen clams on the half shell directly from the USFDA.
To access viral contamination of these clams, we employed a recently developed rapid RNA extraction strategy, termed the GPTT procedure, for the detection of viral RNA by reverse transcription (RT)-PCR (15). This procedure uses a high-pH glycine buffer to elute the virus, polyethylene glycol precipitation to concentrate the virus, Tri-Reagent to extract the RNA, and oligo(dT)-labeled magnetic beads to purify viral RNA in less than 8 h. A modified version of this procedure, combining meats from 12 whole clams for RT-PCR screening, successfully amplified a 275-bp HAV nucleotide sequence (15). Identification of HAV by RT-PCR using RNA extracted from these clams was also reported by the USFDA (8). However, previous attempts by our laboratory to identify NLV, the suspect agent for which these clams were embargoed, were unsuccessful.
In this publication, we describe a modification of the GPTT procedure that resulted in the successful detection of Norwalk virus (NV) within these clams by RT-PCR. Also, for strain identification, a larger amplicon of HAV was generated and sequenced.
MATERIALS AND METHODS
Viruses and shellfish.
NV strain 8FIIa (14) was obtained from human stool produced during a volunteer study (25). A genogroup II NLV-positive stool sample was obtained from Lillian Stark at the Florida State Health Department, Tampa. The NV and NLV stocks were produced by diluting the stool 10-fold in Dulbecco's minimum essential medium (Gibco-BRL, Gaithersburg, Md.), centrifuging it at 16,200 × g for 20 min, and serially filtering it through 10% serum-treated (in Dulbecco's minimum essential medium) Millex 0.45-μm (HV) and 0.1-μm (VV) low protein binding filters (Millipore Corp., Bedford, Mass.). One-milliliter aliquots were frozen at −80°C. HAV stock was obtained from the American Type Culture Collection as VR-1402, a cell culture-adapted, cytopathic clone of strain HM-175 that was originally designated HM-175/18f (17). The HAV was propagated in fetal rhesus monkey kidney (FRhK-4) cells obtained from Stanley Lemon, University of Texas Medical Center, Galveston.
Clams implicated in an outbreak of viral gastroenteritis were provided by Jerrold Mulnick and Richard Manney, USFDA, Jamaica, N.Y. (USFDA Import Alert 16-50). These clams were imported from China, were packaged frozen on the half shell, and were believed to be Ruditapes philipparium (Manila clams). It is not known where these clams were harvested.
Viral RNA extraction.
Stomachs and digestive diverticula with some surrounding tissue were dissected from 59 thawed clams. These digestive tissues were pooled (total weight, approximately 10 g) and extracted by the GPTT procedure (15). Briefly, this procedure involves blending tissue with glycine buffer (0.1 M glycine, 0.3 M NaCl, pH 9.5), precipitation of viral particles with 8% polyethylene glycol 8000, total-RNA extraction with Tri-reagent, and poly(A) RNA purification with magnetic beads containing poly(dT) oligonucleotides (Dynal, Oslo, Norway).
Primers and RT-PCR.
RT-PCR was performed with 10 μl of extracted RNA, gene-specific primers, the one-step RT-PCR kit (using procedures recommended by the manufacturer [Qiagen, Valencia, Calif.]), and 10 U of cloned RNase inhibitor (Gibco-BRL) per 50-μl reaction mixture.
For NLVs, positive-strand primers p36 (5′ ATAAAAGTTGGCATGAACA 3′; nucleotides 4487 to 4501), p69 (5′ GGCCTGCCATCTGGATTGCC 3′; nucleotides 4733 to 4752), and NI (5′ GAATTCCATCGCCCACTGGCT 3′; nucleotides 4768 to 4788) were paired with degenerate negative-strand primer NVp110 (5′ AC[A/T/G]AT[C/T]TCATCATCACCATA 3′; nucleotides 4865 to 4884). Primer sequences were from Le Guyader et al. (16). Primer locations are according to GenBank nucleotide sequence accession no. M87661.
Positive-strand primer 2870 (5′ GACAGATTCTACATTTGGATTGGT 3′) and negative-strand primer 3381 (5′ CCATTTCAAGAGTCCACACACT 3′), which were used for detection of HAV, were from Hutin et al. (11). The designations of primer locations are according to the HM-175 strain (5). For RT-PCR of HAV, RT was done at 50°C for 30 min and Taq activation was done for 15 min at 95°C, followed by 40 cycles of PCR (annealing at 60°C for 1 min, extension at 72°C for 1 min, and denaturation at 95°C for 30 s). The final cycle was 2 min of annealing at 60°C and a 10-min extension at 72°C. For NV, the same conditions were used except that the PCR annealing temperature was 55°C for primers p36 and NVp110 and 50°C for primers NI and NVp110 and primers p69 and NVp110.
RT-PCR negative controls were prepared by using (i) eluate extracted from uncontaminated oysters (uncontaminated Manila clams were not available) and (ii) RT-PCR cocktails with RNase-free H2O in place of eluate. RT-PCR positive controls were prepared by using 1 μl of cultured HAV stock, 10 U of cloned RNase inhibitor, 8 μl of RNase-free H2O, or 9 μl of Norwalk disease stool filtrate with 1 μl of RNase inhibitor, followed by heating to 99°C for 5 min to release the viral RNA from its capsid. All of the primers used were synthesized by Midland Certified Reagent Co. (Midland, Tex.). After the RT-PCRs, amplified nucleic acids were visualized by polyacrylamide gel electrophoresis (PAGE) using 4 to 20% gradient gels (Bio-Rad, Hercules, Calif.) and ethidium bromide or SYBR green staining (Molecular Probes Inc., Eugene, Oreg.).
Amplicons were excised from 2% agarose gels and purified for cloning with the Zymoclean Gel DNA Recovery Kit (Zymo Research, Orange, Calif.) in accordance with the instructions of the manufacturer. Amplicons were cloned into the pGem Vector (Promega Corp., Madison, Wis.) and sequenced by automated dideoxy sequencing (Nucleic Acids Facility, U.S. Department of Agriculture Eastern Regional Research Center, Wyndmoor, Pa.). Homology searches were performed with the National Center for Biotechnology Information Blast program, version 2.2.1 (1). The NLV dendrogram was constructed with the Megalign Lasergene software for Windows (DNAstar Inc., Madison, Wis.).
Fecal coliform analysis.
The most-probable-number (MPN) method for the detection of fecal coliforms was used on meat pooled from 13 of the thawed contaminated clams imported from China by using the three-tube procedure described in reference 3. The meat was diluted 1:10 in sterile phosphate-buffered saline (pH 7.2) and blended for 1 min.
Nucleotide sequence accession number.
The HAV and NLV nucleotide sequences reported here have been submitted to the GenBank database and assigned accession nos. AF399912 and AF399913, respectively. Also, a third unknown RNA-dependent RNA polymerase-like sequence, which was isolated from these claims, was submitted to GenBank under accession no. AF401047.
RESULTS
To assess the sanitary quality of the imported clams, an MPN fecal coliform test was performed on 13 frozen clams. A fecal coliform MPN value of 93,000/100 g of shellfish tissue was obtained. This value is 300-fold higher than the MPN hygienic market standard of 230/100 g for shellfish meats (3a).
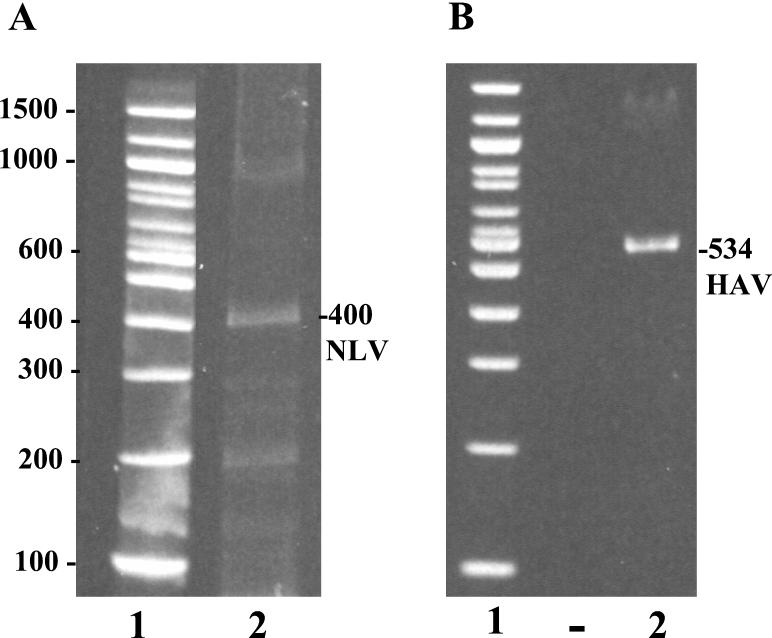
For detection of NLV by RT-PCR, the GPTT procedure was modified. Instead of using whole shellfish, we dissected the digestive tissues from 59 individual clams and used the GPTT extraction procedure to extract viral RNA from a pooled sample of these tissues. Attempts to amplify viral RNA sequences with genogroup I primers p69 and p110 and genogroup II primers N1 and p110 were unsuccessful. Subsequently, RT-PCR was performed with primers p36 and p110. This RT-PCR amplicon was then resolved by agarose gel electrophoresis and purified by gel extraction. A 400-bp amplicon was obtained (Fig. 1A). The fragment was cloned, sequenced, and submitted to the GenBank database (accession no. AF399913). Of the three NLV strains used within the laboratory, primers p36 and p110 successfully amplified only genogroup I strain 8FIIa, precluding the possibility of cross-contamination by the other two strains. The entire sequence of 8FIIa is known, and it clearly differs from the sequence of the strain identified in the imported clams.
FIG. 1.
HAV and NLV in imported Chinese clams. Viral RNA was extracted from dissected stomachs and digestive diverticula from clams by the GPTT procedure; this was followed by one-step RT-PCR and PAGE analysis of 10% of the RNA extracted. The NLV and HAV amplicons were visualized by SYBR green and ethidium bromide staining, respectively. (A) Lanes: 1, 100-bp molecular size ladder; 2, 400-bp RT-PCR amplicon obtained from RNA extracted from clams with NLV primers p36 (positive) and p110 (negative). (B) Lanes: 1, 100-bp molecular size ladder; 2, a 534-bp RT-PCR amplicon obtained from RNA extracted from clams with HAV positive-strand primer 2870 and negative-strand primer 3381. The values on the left are molecular sizes in base pairs.
Comparison with NLV sequences from the GenBank database with the National Center for Biotechnology Information Blast program, version 2.1.1 (1), shows strong sequence similarity to strains SWRL-1998-JP (accession no. AB019276), 970249F (accession no. AB026482), 9915-02F (accession no. AB044370), SRSV-OTH-25/89/JP (accession no. L23830), and 97-320084F (accession no. AB034828), as well as calicivirus strain Toronto TV24 (accession no. U02030). The SWRL-1998-JP strain is a genogroup II NLV (20). A dendrogram comparing the nucleotide sequences of the corresponding RNA-dependent RNA polymerase regions of different NLV strains (Norwalk [accession no. M87661], Southampton [accession no. L07418], Hawaii [accession no. U07611], Lordsdale [accession no. X86557], Melksham [accession no. X81879], SWRL-1998-JP [accession no. AB019276], and Toronto TV24 [accession no. U02030]) is shown in Fig. 2. An amino acid alignment with calicivirus strain Toronto TV24 is shown in Fig. 3.
FIG. 2.
Dendrogram comparison of nucleotide sequence homologies among selected NLVs and the NLV isolated from imported clams. The 357-bp nucleotide sequence amplified from imported clams with primers p36 and p110 (primer sequences excluded) is compared to the corresponding region within the RNA-dependent RNA polymerase gene of selected genogroup I and genogroup II NLV strains (Norwalk [accession no. M87661], Southampton [accession no. L07418], Hawaii [accession no. U07611], Lordsdale [accession no. X86557], Melksham [accession no. X81879], SWRL-1998-JP [accession no. AB019276], and Toronto TV24 [accession no. U02030]). Primer sequences were excluded from the comparison. Percent nucleotide identities with the amplified sequence from imported clams are shown to the right of the dendrogram.
FIG. 3.
Alignment with the NLV sequence identified in imported clams. The predicted peptide sequence underlined was obtained from an RT-PCR amplicon (accession no. AF399913) with RNA-dependent RNA polymerase primers p36 and p110; primer sequences were excluded. An alignment with the NLV strain TV24 (accession no. AAA18929) peptide sequence is shown. One hundred sixteen of the 118 amino acids are identical.
Since HAV strains are highly homologous, the amplicon originally obtained from whole clams (15) was not large enough for strain characterization or genotype identification. For strain identification, HAV-specific primers 2870 and 3375 were used to amplify a 534-bp HAV amplicon (Fig. 1B), which was cloned and sequenced (GenBank accession no. AF399912). Comparison of the HAV amplicon with other HAV sequences shows this strain to be most closely related to strain AH-1 (accession no. AB020564). Comparison with different strains of HAV indicates this to be a genotype I virus with 99 and 98% nucleotide sequence homology to the Japanese AH-1 (accession no. AB020564) and Chinese C-81 (accession no. L07712) strains, respectively. The nucleotide sequence identity between reference strain HM-175 (accession no. M16632) and the imported clam sequence is 91% (Fig. 4).
FIG. 4.
Comparison of the HAV nucleotide sequences spanning the VP1-2A junction (nucleotides 2870 to 3375, excluding primer sequences). Nucleotide numbers are according to the wild-type strain HM-175 sequence reported by Cohen et al. (5). The sequence obtained from the imported clams (ccHAV) is compared to Japanese strain AH-1 (accession no. AB020564) and the nucleotide 3024 to 3190 fragment identified from Shanghai, China, in 1981 (C-81; accession no. L07712). Nucleotides identical to those of the imported-clam HAV are shown as dots, and variable nucleotides are shown as letters.
DISCUSSION
Comparison of the 400-bp portion of the sequence encoding RNA-dependent RNA polymerase of NLV from the imported clams indicates a genogroup II virus with a high level of homology to several Japanese isolates (20). A high level of homology to the Toronto NLV strain, a proposed cluster prototype strain (2), indicates that this virus is most probably a member of the Toronto clade. Although both genogroup I and genogroup II NLVs are common worldwide, genogroup II is more commonly associated with outbreaks of viral gastroenteritis (12, 19). Seropositivity rates of 100 and 98% for genogroup I and II antigens, respectively, for children in Beijing, China, by 9 years of age (13) confirm that NLVs are common in China.
Different primer sets have been used to detect genogroup I and II NLVs with various degrees of efficiency. The NI and p110 primers detected 36 (84%) of 43 genogroup II strains, and p36 and p110 detected 16 (37%) of 43 strains from NLV-positive stools (10). Le Guyader et al. (16) reported that p36 and p110 successfully amplified 12 of 15 NLV sequences from stool samples known to be positive. Why primers p36 and p110 did amplify NLV sequences and genogroup II-specific primers NI and p110 did not successfully amplify the NLV RNA isolated from these clams is unclear. However, investigation of the putative NI primer site, located within the p36-p110 amplicon, indicates three nucleotide mismatches, including one at the second-to-last base from the 3′ end.
The HAV sequence comparisons indicate that the identified HAV strain is highly homologous to the Japanese AH-1 and Chinese C81 isolates. Similarities between genotype I viruses and the imported clam sequence for the 168-nucleotide regions encoding the VP1-2A junction indicate that this is a genotype I virus. This is not surprising, since approximately 80% of the human HAV strains isolated are type I, with most of the remaining human strains being type III (22). Clear delineation and assignment of this strain as type Ia or type Ib are somewhat problematic since the levels of nucleotide sequence identity between type Ia or Ib strains and the imported clam sequence are approximately equal. However, the C-81 strain of HAV is a Ia subgenotype (6), indicating that the imported-clam HAV is most probably a subgenotype Ia virus. The high overall level of nucleotide sequence identity between the Japanese AH-1 and Chinese C-81 strains is consistent with the previous observation that the sequences of HAV isolates often correlate with different geographic regions (22). To date, there are no reports of hepatitis associated with the consumption of the imported clams. The availability of sequence information may permit monitoring of recent isolates to determine whether this strain has been, or is, circulating in North America.
Because of the high fecal coliform counts, it is evident that these clams were harvested from an area with fecal pollution or held in an area that had high levels of fecal pollution and/or processed under grossly unsanitary conditions. The clams were marketed in plastic bags containing approximately 60 clams, each with one shell removed. Bacterial contamination during shucking may have contaminated the external portions of the product; however, the presence of virus within the digestive tract strongly suggests contamination of live clams in their native setting. Low fecal coliform levels in shellfish do not always indicate that shellfish are free of viral contamination, since virus may persist within shellfish for relatively long periods after bacterial levels have been reduced in surrounding waters (7). However, the fecal coliform standard for shellfish does prevent the majority of contaminated shellfish from reaching the marketplace. It is important to note that had these clams been tested by standard fecal coliform testing methods, they probably would have been deemed unfit for human consumption. Also, the high fecal coliform counts corroborate the raw appearance of these clams, since cooked and subsequently frozen clams should not have high coliform counts.
Isolation of both HAV and NLV in these clams validates extraction of digestive diverticula and GPTT extraction as a rapid and efficient method of isolation of viral RNA from contaminated shellfish that is suitable for RT-PCR amplification of limited quantities of virus. Although extraction of stomachs and digestive diverticula does require additional time, a single pooled sample can be readily extracted by the GPTT method and analyzed by RT-PCR and PAGE in 24 h. The greater success of this method over the use of whole shellfish may be due to concentration of the virus and a reduced amount of RT-PCR inhibitors (23, 24) within the digestive tissues of the clam. However, since digestive tracts were pooled, it is conceivable that positive HAV and NLV tests resulted from a small number of disparate or even single clams that may have been mixed with uncontaminated clams during processing and packaging.
Generally speaking, it can be argued that the detection of RNA from human viral pathogens by RT-PCR of shellfish extracts may not provide an indication of the health risk to consumers since inactivated virions may still contain intact RNA and therefore yield a positive RT-PCR result (21). However, the unmodified GPTT test detected as few as 22.4 RT-PCR 50% end point units of NV (15) but was unable to detect the presence of NLV within whole clams. Further, dissection and extraction of digestive tissues from an entire bag of clams were required before a positive test was obtained. Therefore, it is evident that the level of virus causing a potential health risk is very small and most probably below our current threshold of RT-PCR detectability.
Acknowledgments
We thank Richard Manny and Jerrold Mulnick of the USFDA for providing seized clams imported from China, Lillian Stark of the Florida State Health Department for providing two genogroup II NLV-positive stool samples, and Stanley Lemon of the University of Texas Medical Center for the FRhK-4 cell line. We also thank Kim Green of the National Institutes of Health and Theresa Cromeans of the Centers for Disease Control and Prevention for review of the manuscript and helpful discussions.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, T., J. S. Noel, and R. L. Fankhauser. 2000. Genetic classification of “Norwalk-like viruses.” J. Infect. Dis. 18:S336-S348. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1995. Multiple-tube fermentation technique for members of the coliform group, p. 944-950. In M. H. Franson (ed.), Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington D.C.
- 3a.Anonymous. 1991. Council directive 91/492/EEC. Laying down the health conditions for the production and placing on the market of live bivalve mollusks. Off. J. Euro. Comm. 268:1-14. [Google Scholar]
- 4.Blacklow, N. R., G. Cukor, M. K. Bedigian, P. Echeverria, H. B. Greenberg, D. S. Schreiber, and J. S. Trier. 1979. Immune response and prevalence of antibody to Norwalk enteritis virus as determined by radioimmunoassay. J. Clin. Microbiol. 10:903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. I., J. R. Ticehurst., R. H. Purcell., A. Buckler-White., and B. M. Baroudy. 1987. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J. Virol. 61:50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz, B. I., C. A. Sariol, A. Normann, L. Rodriguez, and B. Flehmig. 2001. Genetic relatedness of Cuban wild-type HAV isolates. J. Med. Virol. 64:96-103. [DOI] [PubMed] [Google Scholar]
- 7.Gerba, C. P., and S. M. Goyal. 1978. Detection and occurrence of enteric viruses in shellfish: a review. J. Food Prot. 41:743-754. [DOI] [PubMed] [Google Scholar]
- 8.Goswami, B. B., M. Kulka, D. Ngo, P. Istafanos, and T. A. Cebula. 2002. A PCR-based method for the detection of hepatitis A virus in produce and shellfish. J. Food Prot. 65:393-402. [DOI] [PubMed]
- 9.Halliday, M. L., L. Y. Kang, T. K. Zhou, M. D. Hu, Q. C. Pan, T. Y. Fu, Y. S. Huang, and S. L. Hu. 1991. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J. Infect. Dis. 164:852-859. [DOI] [PubMed] [Google Scholar]
- 10.Honma, S., S. Nakata, K. Kinoshita-Numata, K. Kogawa, and S. Chiba. 2000. Evaluation of nine primer sets of PCR primers in the RNA dependent RNA polymerase region for detection and differentiation of members of the family Caliciviridae, Norwalk virus and Sapporo virus. Microbiol. Immunol. 44:411-419. [DOI] [PubMed] [Google Scholar]
- 11.Hutin, Y. J., V. Pool, E. H. Cramer, O. V. Nainan, J. Weth, I. T. Williams, S. T. Goldstein, K. F. Gensheimer, B. P. Bell, C. N. Shapiro, M. J. Alter, and H. S. Margolis. 1999. A multistate food-borne outbreak of hepatitis A. N. Engl. J. Med. 340:595-602. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, X., D. O. Matson, W. D. Cubitt, and M. K. Estes. 1996. Genetic and antigenic diversity of human caliciviruses (HuCVs) using RT-PCR and new EIAs. A review. Arch. Virol. Suppl. 12:251-262. [DOI] [PubMed] [Google Scholar]
- 13.Jing, Y., Y. Qian, Y. Huo, L.-P. Wang, and X. Jiang. 2000. Seroprevalence against Norwalk-like human calicivirus in Beijing, China. J. Med. Virol. 60:97-101. [DOI] [PubMed] [Google Scholar]
- 14.Kapikian, A. Z., R. G Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingsley, D. H., and G. P. Richards. 2001. A rapid and efficient extraction method for reverse transcription-PCR detection of hepatitis A and Norwalk-like viruses in shellfish. Appl. Environ. Microbiol. 67:4152-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Guyader, F., M. K. Estes, M. E. Hardy, F. H. Neill, J. Green, D. W. Brown, and R. L. Atmar. 1996. Evaluation of a degenerate primer for the PCR detection of human caliciviruses. Arch. Virol. 141:2225-2235. [DOI] [PubMed] [Google Scholar]
- 17.Lemon, S. M., P. C. Murphy, P. A. Shields, L. H. Ping, S. M. Feinstone, T. Cromeans, and R. W. Jansen. 1991. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: evidence for genetic recombination. J. Virol. 65:2056-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mead, P. S., L. Slutsker., V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noel, J. S., T. Ando, J. P. Leite, K. Y. Green., K. E. Dingle, M. K. Estes, Y. Seto, S. S. Monroe, and R. I. Glass. 1997. Correlation of patient immune response with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990-1995. J. Med. Virol. 53:372-383. [DOI] [PubMed] [Google Scholar]
- 20.Ohyama, T., S. Yoshizumi, H. Sawada, Y. Uchiyama, Y. Katoh, N. Hamaoka, and E. Utagawa. 1999. Detection and nucleotide sequence analysis of human caliciviruses (HuCVs) from samples in non-bacterial gastroenteritis outbreaks in Hokkaido, Japan. Microbiol. Immunol. 43:543-550. [DOI] [PubMed] [Google Scholar]
- 21.Richards, G. P. 1999. Limitations of molecular biological techniques for assessing the virological safety of foods. J. Food Prot. 62:691-697. [DOI] [PubMed] [Google Scholar]
- 22.Robertson, B. H., R. W. Jansen, B. Khanna, A. Totsuka, O. V. Nainan, G. Siegl, A. Widell, H. S. Margolis, S. Isomura, K. Ito, T. Ishizu, Y. Mortisugu, and S. M. Lemon. 1992. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J. Gen. Virol. 73:1365-1377. [DOI] [PubMed] [Google Scholar]
- 23.Romalde, J. L., M. K. Estes, G. Szücs, R. L. Atmar, C. M. Woodley, and T. G. Metcalf. 1994. In situ detection of hepatitis A virus in cell cultures and shellfish tissues. Appl. Environ. Microbiol. 60:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab, K. J., F. H. Neill, M. K. Estes, T. G. Metcalf, and R. L. Atmar. 1998. Distribution of Norwalk virus within shellfish following bioaccumulation and subsequent depuration by detection using RT-PCR. J. Food Prot. 61:1674-1680. [DOI] [PubMed] [Google Scholar]
- 25.Xi, J. N., D. Y. Graham, K. N. Wang, and M. K. Estes. 1990. Norwalk virus genome cloning and characterization. Science 250:1580-1583. [DOI] [PubMed] [Google Scholar]