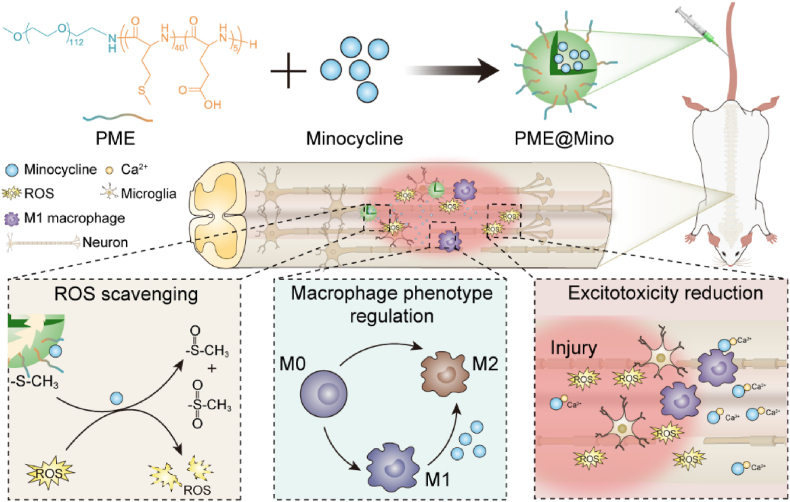
Abstract
The microenvironments play a crucial role in secondary injury following spinal cord injury (SCI). Deterioration of the microenvironments, including oxidative stress, inflammation, and excitotoxicity, exacerbates SCI. However, due to the complexity of these microenvironments, synergistic modulation of multiple factors remains challenging. In this study, we developed a reactive oxygen species (ROS) responsive nanoformulation system based on a methoxy poly(ethylene glycol)-block-poly(L-methionine-co-L-glutamic acid) copolymer (PME) and loaded it with minocycline (PME@Mino) to promote SCI repair. This nanoformulation was administered intravenously, accumulating at the lesion site where PME@Mino was exposed to ROS, triggering the release of minocycline. Through the synergistic modulation of multiple microenvironment factors, including reduction of oxidative stress, regulation of pro-inflammatory M1 macrophages polarization to anti-inflammatory M2 macrophages, and reduction of calcium ion influx, PME@Mino achieved neuronal and myelin protection. This study highlights advanced approaches for modulating microenvironments using nanoscale treatments for SCI.
Keywords: poly(amino acid) nanoformulation, Modulation of microenvironments, Neuroprotection, Anti-Inflammation, Spinal cord injury therapy
Graphical abstract
1. Introduction
Spinal cord injury (SCI) usually results from trauma, such as accidental falls, vehicular accidents, or violence, and is associated with sensory, motor, and autonomic dysfunctions [1]. SCI affects approximately 3 million people worldwide, with about 18,000 new cases annually [2]. Current clinical treatment strategies for SCI include early surgical decompression and stabilization, along with high-dose steroid pulse therapy. However, the outcomes of these strategies remain unsatisfactory. Few evidences show that early surgical treatment has significant clinical benefits in improving the long-term function of SCI [3]. Additionally, high-dose steroids can cause complications, such as systemic bleeding, tissue edema, increased susceptibility to infections, and hyperglycemia [4]. Therefore, there is an urgent need to develop new treatment strategies.
The SCI microenvironments refer to the metabolic and function-active microenvironments of spinal cord tissue and cells after injury [5]. These microenvironments undergo dramatic changes shortly after SCI. SCI triggers the production of large amounts of reactive oxygen species (ROS) [6]. The increased concentration of ROS in the microenvironment damages mitochondria, affects tissue metabolism, and triggers cell apoptosis [7,8]. Inflammation is another critical factor influencing secondary injury. Macrophages can differentiate into two phenotypes, M1, which promotes inflammation, and M2, which promotes tissue repair [9]. After SCI, macrophages are recruited to the lesion site and tend to polarize as M1. While M1 macrophages are beneficial in the early stages of inflammation by removing tissue fragments, their prolonged presence leads to chronic inflammation, scarring, and suppression of tissue regeneration [[10], [11], [12]]. Due to vascular destruction and cell death, large amounts of glutamate are released into the microenvironments, leading to excitotoxicity [13]. Excessive glutamates bind to ionophilic and metabolic receptors, causing calcium influx, eventually leading to mitochondrial dysfunction and cell damage [14]. These changes in the microenvironments, including inflammation, oxidative stress, and excitotoxicity, exacerbate secondary injury after SCI. This suggests that modulation of the microenvironments can be targeted to promote the outcomes of SCI treatment. However, given the complexity of the SCI microenvironments, regulating a single factor may be limited in SCI therapy. Multi-level modulation of the microenvironments may be a suitable approach for treating SCI.
In this study, we designed a ROS responsive platform based on methoxy poly(ethylene glycol)-block-poly(L-methionine-co-L-glutamic acid) copolymer (PME) and loaded it with minocycline (Mino) to obtain a poly(amino acid) nanoformulation (PME@Mino) (see Scheme 1). In vitro, PME@Mino reduced oxidative stress in cells, inhibited excitotoxicity, and decreased inflammation by inducing M2 polarization of macrophages. In vivo, PME@Mino significantly promoted functional recovery in rats, reduced spinal cord cavities, inhibited demyelination, and achieved neuroprotection.
Scheme 1.
Mechanisms of PME@Mino for SCI therapy.
2. Materials and methods
2.1. Materials
L-Methionine and minocycline hydrochloride were purchased from Aladdin Scientific Corp. (Shanghai, P. R. China). H-Glu(OBzl)-OH were purchased from GL Biochem, Ltd. (Shanghai, P. R.China). mPEG-OH were purchased from Sigma-Aldrich (St. Louis, MO, USA). Triphosgene was obtained from Shanghai Duodian Chemical Co., Ltd. (Shanghai, P. R. China). N, N-dimethylformamide (DMF), tetrahydrofuran (THF), toluene, and other required chemicals were purchased from Beijing Chemical Industry Group Co., Ltd. (P. R. China).
2.2. Synthesis and characterization of copolymer
First, mPEG-NH2 (Mn = 5000) was synthesized by terminal amination of mPEG-OH (Mn = 5000) as described in a previous study [15]. L-methionine or L-glutamyl benzyl ester was reacted with excess triphosgene in anhydrous THF under continuous nitrogen flow to prepare L-methionine N-carboxylicanhydride (Met-NCA) and benzyl L-glutamic acid N-carboxylicanhydride (BLG-NCA). Subsequently, mPEG-NH2 was dehydrated and dissolved in anhydrous DMF; Met-NCA and BLG-NCA were added subsequently. The mixture was stirred for 3 days at room temperature (23 °C ± 2 °C), then precipitated, dialyzed, lyophilized and deprotected under the acidic microenvironments of hydrogen bromide and trifluoroacetic acid to obtain PME. The copolymer results were characterized using 1H nuclear magnetic resonance spectroscopy (NMR) profiles.
2.3. Preparation and characterization of nanoformulation
Forty mg of PME and 40 mg of Mino were separately dissolved in 2 mL of DMF. The solutions were mixed in a 1:1 ratio with the drug solution and then added at a rate of 0.2 mL/min by drops to a 40 % PBS solution. After stirring for 3 h, the mixture was dialyzed in deionized water for 8 h to obtain PME@Mino. For PME nanoparticle, the synthesis method was the same as above with 10 mg/mL of PME in DMF solution. The particle size of the resulting nanoparticle was examined using dynamic light scattering (DLS), and their ultramorphology was examined using transmission electron microscopy (TEM).
2.4. Determination of drug loading and encapsulation efficiency of nanoparticle
The drug loading (DL) and encapsulation efficiency (EE) of PME@Mino were determined by high-performance liquid chromatography (HPLC). The mobile phase consisted of 0.2 mol/L ammonium acetate, DMF, and tetrahydrofuran in a ratio of 600:398:2, with 0.1 mol/L ethylenediaminetetraacetic acid disodium solution. The flow rate was 1 mL/min, and detection was performed at 280 nm.
The DL and EE of the PME@Mino were determined using organic solvent extraction. The nanoformulation was dissolved in DMF and then vortexed for 5 min to extract Mino. The total Mino content was determined using HPLC. The DL and EE were then calculated using the following formulas:
where M encapsulated is the mass of Mino encapsulated in nanoparticles, M nanoformulation is the mass of nanoformulation, and M added is the mass of Mino added.
2.5. Responsive release of PME drug-loaded nanoparticle
Rhodamine B was used as a model drug for evaluating the responsive release from PME nanoparticles. Rhodamine B-loaded PME was prepared as described above. The responsive release was then studied by dialyzing the rhodamine B-loaded PME (4 mg/mL, 5 mL) in 15 mL PBS/PBS with H2O2 (300 μM) at 37 °C with horizontal shaking. At 1, 3, 6, 12, 24, and 48 h, samples from the outside of the dialysis bags were collected, and the release medium was replaced with fresh solution. Fluorescence intensity in the samples was measured using a UV–Vis spectrometer (microplate reader) with excitation at 535 nm.
2.6. Cell culture
PC12 cells were purchased from the BeNa Culture Collection, and BV2 cells were obtained from Wuhan Pricella Biotechnology Co., Ltd. Both cell types were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 U/mL streptomycin. Cells were maintained in a humidified incubator at 37 °C with 5 % CO2. Trypsin-EDTA was used to subculture the cells.
2.7. Cellular uptake
Cy5.5-labeled PME was prepared as described. Briefly, PME and Cy-5.5-NHS were mixed in DMF and stirred for 12 h. After dialysis, Cy5.5-labeled PME was obtained. An LPS solution with a concentration of 100 ng/mL was then added to stimulate BV2 cells. The media was replaced by DMEM with Cy5.5-PME (20 μg/mL). According to the time point of 1 h, 3 h, 6 h, and12 h, PBS was added to wash away unabsorbed Cy5.5-PME, and the cells were collected for Flow cytometry at 633 nm.
2.8. Assay of antioxidant effects in vitro
PC12 cells were seeded in a 24-well plate at 40000 cells/well and cultured for 24 h, the culture media was then replaced with DMEM containing saline, PME (20 μg/mL), Mino (5 μg/mL), or PME@Mino (25 μg/mL) and incubated for 30 min. A solution of H2O2 (H2O2 concentration: 300 μM) was then added and cells were cultured for 24 h. Calcein-AM/PI working solution configuration was performed according to the manufacturer's instructions. Subsequently, 250 μL of the working solution was added to each well and incubated in the dark at 37 °C for 30 min.
Furthermore, a CCK-8 assay was conducted to assess cell viability in different groups. After 24 h of oxidative stress (300 μM H2O2), the culture medium was replaced with CCK-8 working solution, and cells were incubated in the dark for 1 h. The absorbance was then measured at 450 nm using a microplate reader. Cell viability was calculated using the following formula:
where Asample and Asham are the absorbance values of the sample and sham wells, respectively.
To assess ROS levels, after being cultured in a microenvironment of oxidative stress (300 μM of H2O2), the culture media was replaced with DCFH-DA working solution. After incubation in the dark for 20 min, cells were analyzed using confocal laser scanning microscopy (CLSM) with an excitation wavelength of 488 nm. The mean intracellular fluorescence intensity was quantified using ImageJ software (National Institutes of Health, USA).
2.9. Reduction excitotoxicity in vitro
PC12 cells were seeded in confocal dishes at 50000 cells/well and cultured for 24 h, before applying the treatment described above. Cells were stained with Fluo-4 AM working solution (1 μM) and incubated in the dark for 10 min. Fluorescence images were captured using CLSM at 488 nm, and the mean intracellular fluorescence intensity was calculated using ImageJ software.
2.10. Fluorescent staining of nitric oxide synthase (iNOS)/arginase 1 (Arg1)
BV2 cells were seeded on slides in 6-well plates at 50,000 cells/well and cultured for 24 h. The cells were then induced with 100 ng/mL of LPS for 10 h. After replacing the media with DMEM containing saline, PME (20 μg/mL), Mino (5 μg/mL), or PME@Mino (25 μg/mL), cells were cultured for an additional 24 h. Cells were fixed using 4 % paraformaldehyde, permeabilized with Triton-X100, and blocked with 5 % FBS. The primary antibody solution was added and incubated overnight, followed by a 2 h incubation with secondary antibodies at 37 °C with shaking. Samples were analyzed by CLSM after DAPI staining at an excitation wavelength of 488 nm. Arg1 staining was performed identically to iNOS staining. The mean fluorescence intensity for both iNOS and Arg1 was quantified using ImageJ software.
2.11. Polarization of macrophage phenotypes in vitro
After exposure to LPS and treatment with different groups, BV2 cells were collected and incubated with CD11b (FITC) and CD86 (BV605) at 4 °C for 30 min. Cells were fixed, permeabilized and incubated with CD206 (PE-CY7) in the dark for 30 min. Flow cytometry was then performed for quantitative analysis of macrophage polarization.
2.12. Inflammation cytokine secretion
After exposing BV cells to LPS and treating them with different groups, the culture media was collected. The levels of TNF-α, IL-6, and TGF-β were measured using ELISA kits according to the manufacturer's instructions. Absorbance was recorded at 450 nm using a microplate reader.
2.13. Establishment of animal models
Adult female Sprague–Dawley rats (SD rats) (age, 6–8 weeks; weighing 200–250 g) were were obtained from Spefu (Beijing) Biotechnology Co., LTD. In this study, a stable and reproducible SCI model was established using the Allen method. Rats were anesthetized using an intraperitoneal injection of 2 % sodium pentobarbital. T10 was exposed as the center, and a 40-g metal rod (2.5 mm in diameter) was used to smash the exposed spinal cord segment from a height of 50 mm in free fall. After contusion, the muscle, fascia, and skin were closed in layers.
The rats were randomly divided into 5 groups before establishing SCI model. The group without contusion injury was the control group. The SCI rat models were intravenously injected with saline (the saline group), PME (the PME group), Mino (the Mino group), and PME@Mino (the PME@Mino group) (Mino dose: 10 mg/kg, PME dose: 40 mg/kg) for 3 days. All rat subjects were injected with cefazolin (25 mg/kg) twice daily for 5 days to prevent infection. The urinary bladder of each SCI rat was massaged twice daily until bladder function recovered.
2.14. Bio-distribution analysis
SCI rats were intravenously injected with Cy5.5-labeled PME (20 mg/kg). At 1, 6, 12, and 24 h post-injection, the rats were sacrificed, and various organs (heart, liver, spleen, lungs, kidneys, and spinal cord) were collected. Fluorescence in the collected tissue was imaged and analyzed using the IVIS® Spectrum system.
2.15. Histological staining and detection
Eight weeks post-SCI, rats were anesthetized with sodium pentobarbital, and the heart was fully exposed with a blunt needle inserted along the lower edge of the left ventricle, and the needle was fixed at the exit of the aorta. The right atrial appendage was cut open and perfused with 150 mL of normal saline; when the effluent became clear and transparent, it was replaced with 4 % paraformaldehyde solution. After perfusion, spinal cord tissue was exposed, and a 2 cm segment of the injured spinal cord was collected and fixed in 4 % paraformaldehyde for further analysis.
2.16. Behavioral analysis
Rats were randomly divided into four groups (n = 8). After SCI model establishment, the rats were intravenously injected with saline, PME, Mino, or PME@Mino (10 mg/kg of Mino and 40 mg/kg of blank PME). The Basso, Beattie, Bresnahan (BBB) locomotor rating score was used to evaluate the recovery of hind limb motor function of rats at a predetermined time point (1 day or 1–8 weeks after injury). Two independent researchers performed the assessments, and the score was finalized when both investigators agreed. Additionally, the body weight and the recovery time of urination function after SCI were recorded.
2.17. Preparation of paraffin tissue sections, hematoxylin & eosin (H&E) staining, and Luxol fast blue (LFB) staining
Eight weeks post-injury, rats were sacrificed using overdose anesthetics and perfused with PBS and 4 % paraformaldehyde. Afterward, T9–T11 spinal cord segments were collected, fixed with 4 % paraformaldehyde, embedded in paraffin, cut into 4-μm thick sections, and finally stained with H&E or 0.1 % LFB. The stained sections were then studied using an optical microscope.
2.18. Immunofluorescence staining
Sections were blocked in PBS containing 3 % bovine serum protein for 30min and incubated with primary antibodies overnight at 4 °C. In this study, antibodies against Iba1 (for activated macrophages/microglia), CD68 (for activated macrophages/microglia), CD163 (for M2 macrophage), 3-nitrotyrosine (to indicate oxidative stress), neurofilament-200 kD (NF200, for axons), and glial fibrillary acidic protein (GFAP, for astrocytes) were used. After incubation with appropriate secondary antibodies, the nuclei were stained with DAPI. Samples were analyzed using confocal laser scanning microscopy (CLSM).
2.19. Spinal ultrastructure observation
Eight weeks post-SCI, three rats from each group were randomly selected and sacrificed using an anesthetic overdose. Cardiac perfusion was performed using a mixture of 4 % paraformaldehyde and 2.5 % glutaraldehyde, followed by the removal of approximately 2 cm of the spinal cord and fixation overnight in 2.5 % glutaraldehyde solution. After gradient dehydration, the sections were stained using uranyl acetate and lead citrate and subsequently subjected to transmission electron microscopy (TEM). The G-ratio (ratio of axon diameter to myelin diameter) was measured using ImageJ software.
2.20. Statistical analysis
All experiments were repeated in triplicate, and data are expressed as the mean ± standard deviation (SD). Comparisons between groups were evaluated using the one-way analysis of variance (ANOVA). Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA), Statistically significant difference was determined when p < 0.05.
3. Results and discussion
3.1. Preparation and characterization of PME@Mino
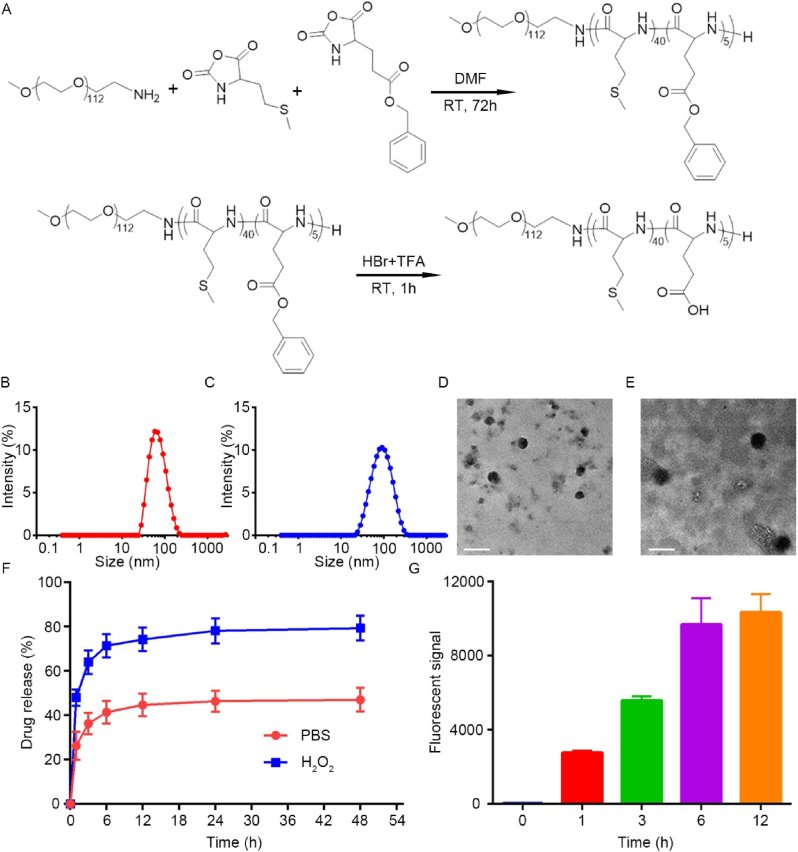
The ring-opening polymerization of Met N-carboxyanhydride (NCA) and Glu NCA, initiated by mPEG-NH2 (Mn = 5000), was employed to synthesize the PME polymer, as shown in Fig. 1A. The chemical structures of the monomer were characterized using 1H NMR (Fig. S1 and S2, Supplementary Information), with all peaks precisely assigned. The molecular weight of the PME polymer was determined by comparing the integration of the methionine unit methionine peak in the main chain (−COCHNH−) and the methylene peak of the benzyl ester in the glutamic acid benzyl ester (−COOCH2C6H6) with the methylene peak of PEG (−CH2CH2O−). The copolymer of the PMet block and the PGlu block had polymerization degrees of 40 and 5, respectively. The benzyl ester was subsequently removed by deprotection to yield PME, which was characterized by 1H NMR (Fig. S3, Supplementary Information).
Fig. 1.
Synthesis and characterization of nanoparticles. (A) The synthetic route of PME nanoparticle. (B, C) DLS measurement of PME and PME@Mino size distribution. (D, E) TEM images present the morphology of PME and PME@Mino (scale bar = 100 nm). (F) Drug release versus time of nanoparticles in PBS and PBS with H2O2 (300 μM). (G) Effect of BV2 on endocytosis of PME-Cy5.5 at 1 h, 6 h, 12 h, and 24 h post-SCI in vitro. Data are represented as mean ± SD (n = 3).
The amphiphilic nature of PME enables it to self-assemble into nanoparticles. DLS analysis showed that the hydrodynamic average diameter of PME was 66.76 nm, with a polydispersity index (PDI) of 0.22 (Fig. 1B). After Mino encapsulation, the hydrodynamic average diameter of PME@Mino increased to 78.84 nm, with a PDI of 0.20 (Fig. 1C). PME utilizes electrostatic interactions to encapsulate positively charged drugs, such as Mino, forming the PME@Mino nanoparticles. The size increase after drug loading was consistent with the DLS data. TEM revealed that PME nanoparticle had a uniform spherical shape with an average diameter of about 52.9 nm, slightly smaller than the DLS measurement (Fig. 1D). After Mino encapsulation, the average diameter of PME@Mino increased to 67.8 nm (Fig. 1E), confirming the successful drug loading. The zeta potential also increased significantly after drug incorporation (Fig. S4), further validating the successful encapsulation of Mino. The encapsulation efficiency of Mino by the nanoparticle was also measured, with a DL of 19.5 wt% and an EE of 72.6 %. This efficient drug loading is likely due to the electrostatic interactions between Mino and PME.
To verify the ROS responsiveness of PME, 300 μM of H2O2 was used to simulate the oxidative stress microenvironments after SCI. DLS results indicated that the PME nanoparticles maintained stable in PBS at room temperature within 24 h (Fig. S5A, Supplementary Information), but when exposed to H2O2 solution, the hydrodynamic average diameter increased, and the particle distribution became disordered with uneven sizes (Fig. S5B, Supplementary Information). This was due to the oxidation of the PME side chains, which formed sulfoxides and sulfones, thereby enhancing the hydrophilicity and causing the change in particle size. This suggests that PME is responsive under ROS microenvironments. To further study ROS responsiveness of PME, rhodamine B was used as a model drug to track the release behavior under oxidative conditions. As shown in Fig. 1F, the release of rhodamine B in PBS was slow, reaching 41.34 % of the total amount within 6 h and 47.02 % within 48 h. However, in the presence of H2O2, the release rate of rhodamine B was significantly increased, reaching 71.39 % of the total amount within 6 h, and 79.33 % within 48 h, which was 1.68 times the total release in PBS. This suggests that PME is ROS responsive. This responsiveness is conferred by the thioether bond of methionine in the PME.
To investigate cellular uptake, PME was labeled with Cy5.5 to form PME-Cy5.5, which was then co-cultured with activated BV2 cells. Flow cytometry was used to quantify the fluorescence intensity inside the cells at different time points to reflect the phagocytosis of PME-Cy5.5. As shown in Fig. 1G, the fluorescence intensity increased with prolonged co-culture time, indicating continuous nanoparticle uptake. Notably, PME-Cy5.5 was rapidly internalized by BV2 cells within 1 h, and although the uptake rate decreased afterward, the cells continued to slowly take up the nanoparticles, suggesting consistent and successful nanoparticle ingestion.
3.2. Neuroprotective activity of PME@Mino in vitro
Biocompatibility is essential for the application of biological materials. The biocompatibility of PME was evaluated at various concentrations. As shown in Fig. S6, Supplementary Information, PME showed good biocompatibility and slightly promoted cell proliferation as the concentration increased from 12.5 to 200 μM. PME@Mino also showed no cytotoxicity.
ROS in the microenvironments increases dramatically after SCI, originating from damaged mitochondria, inflammatory cells, and dead cells [6]. Excessive ROS oxidizes the unsaturated fatty acids in cell membrane, producing cytotoxic oxidation products such as 4-hydroxynonenal and 2-propenal [16]. Moreover, the function and integrity of the cell membranes are disrupted by oxidation, triggering ionic disorders and cell death [7].
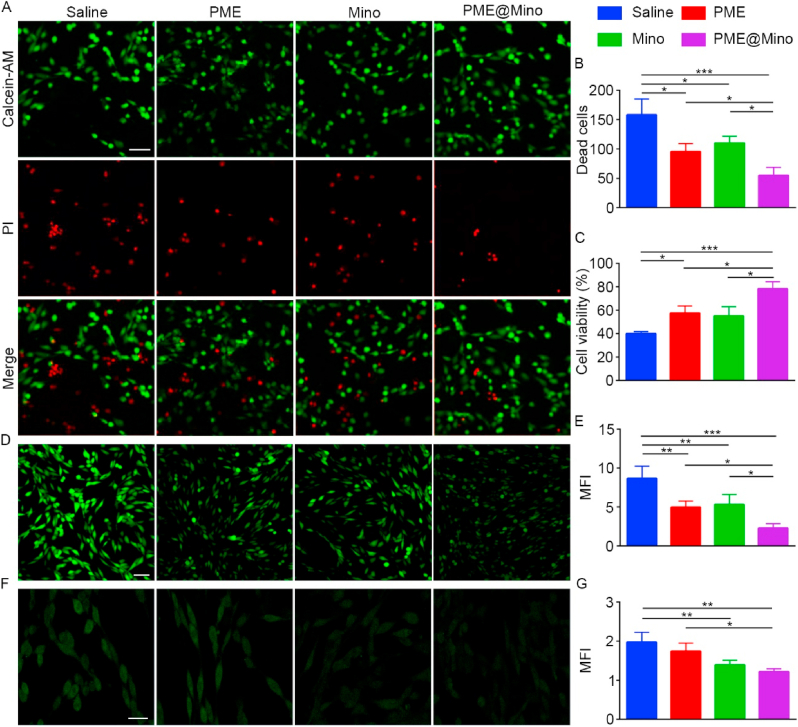
Scavenging ROS is critical for reducing secondary injury after SCI. In this study, the antioxidant effects of PME@Mino were studied in vitro. PC12 cells were co-cultured in medium with saline, PME, Mino, and PME@Mino for 30 min, while H2O2 was used to mimic the oxidative stress microenvironments after SCI. Live/Dead staining results (Fig. 2A) demonstrated significant cell death in the saline group following oxidative stress induction. In contrast, the treating groups showed a reducing number of dead cells. Quantitative analysis (Fig. 2B) revealed that the PME, Mino and PME@Mino group reduced dead cells by 39.6 %, 30.5 % and 65.3 %, respectively, compared to the saline group. Notably, the PME@Mino group exhibited the lowest number of dead cells, indicating the capacity to mitigate oxidative stress-induced cytotoxicity of PME@Mino nanoformulation. These findings were further confirmed by CCK-8 assay (Fig. 2C). Under oxidative stress, cell viability was significantly decreased, with an absorbance indicating that only 40 % of the cells were viable. However, after applying PME, Mino, and PME@Mino, the absorbance significantly increased. Compared with the saline group, the PME group showed a 42.5 % increase, while the PME@Mino group increased by 96 %, indicating a significantly better response than the PME group. Although the Mino group also showed improvement, it was not significantly different from that of the saline group. These results suggest that PME@Mino effectively protects cell viability in a microenvironment of oxidative stress.
Fig. 2.
Neuroprotective effects of nanoparticles in vitro. (A) Live/Dead staining images of PC12 cells in different treatment groups under oxidative stress microenvironment (scale bar = 25 μm) and (B) the quantitative analysis result of the number of dead cells (dead cells/0.025 mm2). (C) CCK-8 analysis of PC12 cell viability in different treatment groups under oxidative stress microenvironment. (D) The antioxidant effect of different groups on PC12 cells in oxidative stress microenvironment (scale bar = 200 μm), and (E) the quantitative analysis result. (F) Intracellular Ca2+ concentration in each treatment group under excitotoxicity (scale bar = 20 μm) and (G) the quantitative analysis result. Data are shown as the mean ± SD (n = 3; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001).
To assess intracellular ROS scavenging, DCFH-DA was used. PC12 cells were co-cultured in medium with saline, PME, Mino, and PME@Mino and subsequently exposed to oxidative stress mimicked using H2O2. CLSM showed minimal fluorescence in the control group, while fluorescence significantly increased in the saline group after being exposed to oxidative stress (Fig. 2D). The application of PME, Mino, and PME@Mino reduced intracellular fluorescence; with PME@Mino showing the most significant reduction. Semi-quantitative analysis by ImageJ software revealed a 42.7 % reduction in fluorescence in the PME group, a 38.5 % reduction in the Mino group, and a 61.1 % reduction in the PME@Mino group (Fig. 2E). These results were further supported by flow cytometry, where the mean fluorescence intensity (MFI) of the PME, Mino, and PME@Mino groups decreased by 41.8 %, 40.2 %, and 61.1 %, respectively (Fig. S7A, B, Supplementary Information). We also examined how nanoformulation protected PC12 cells undergoing neural differentiation against oxidative stress (Fig. S8A, B, Supplementary Information). Compared with other groups, cells in the PME@Mino group exhibited a more extended morphology, and the expression of microtubule-associated protein 2 (MAP2) was 1.52 times that of the saline group. These results indicating that PME@Mino has superior ROS scavenging compared to PME and Mino alone. The ROS scavenging effect of PME is attributed to the thioether bonds on its side chain, which are derived from methionine. Methionine can be oxidized to methionine sulfoxide by a variety of oxidants, including ROS, making the material more hydrophilic and promoting drug release [17,18]. Mino, a phenolic antioxidant, directly eliminates ROS in the SCI microenvironments through its phenol ring structure. In our study, Mino was shown to have antioxidant effects in the Mino and PME@Mino groups. Furthermore, the PME@Mino group benefited from the combined effect of PME and Mino and obtained the best antioxidant effect.
The intracellular Ca2+ concentration reflects excitotoxicity. Fluo-4 AM was used to label the intracellular Ca2+ concentration of PC12 cells. PC12 cells were co-cultured in medium with saline, PME, Mino, and PME@Mino and subsequently induced with glutamate to simulate excitotoxicity after SCI. CLSM showed significant Ca2+ fluorescence in the saline group after the induction of excitotoxicity, while Ca2+ fluorescence decreased after treatment with PME, Mino, and PME@Mino (Fig. 2F). Although PME showed limited anti-excitotoxicity, the fluorescence intensity was significantly reduced in the Mino and PME@Mino groups, with no significant difference between them. Semi-quantitative analysis using ImageJ (Fig. 2G) showed that fluorescence intensity decreased by 29.5 % in the Mino group and by 38.3 % in the PME@Mino group. Flow cytometry further confirmed the inhibitory effect on calcium influx (Fig. S9A, B, Supplementary Information). The Mino group showed a 41.8 % reduction in average fluorescence intensity, while PME@Mino was more effective, with a 48.2 % reduction. Although the PME group showed a slight decrease in fluorescence, this was not statistically significant compared to the saline group.
The therapeutic effect of Mino and PME@Mino was significantly better than that of PME. Glutamate-induced excitotoxicity leads to excessive calcium influx, mitochondrial depolarization, ATP dysfunction, and eventual cell death [[19], [20], [21]]. Mino reduces Ca2+ concentration in the microenvironments by chelating divalent and trivalent metal ions [22]. Furthermore, through the selective partial inhibition of the electron transport chain complex I and IV and the modulation of voltage-dependent anion channels, Mino depolarizes the mitochondrial membrane and decreases mitochondrial Ca2+ uptake [22,23]. In agreement with previous studies, Mino has been shown to significantly reduce intracellular calcium concentration in excitotoxic conditions [24]. The inhibition of Ca2+ influx by PME can be attributed to membrane-sealing effect of PEG. PEG is a common membrane-sealing agent and has been proven to alleviate Ca2+ influx and promote recovery after SCI [24,25]. PEG removes the water of hydration at the damaged edge of membrane lipids, inducing the membrane to collapse, fuse, and seal, thus sealing the plasmalemma [26]. These sealed membranes protect cells from Ca2+ influx.
3.3. Anti-inflammation activity of PME@Mino in vitro
Anti-inflammatory therapy is crucial for modulation of the microenvironments after SCI. Immunofluorescence staining was used to evaluate the regulatory effects various treatments on macrophage polarization. As shown in Fig. 3A, the saline group showed high expression of iNOS after induction of inflammation, while the expression of iNOS significantly decreased in the PME, Mino, and PME@Mino groups. Compared with the saline group, iNOS expression decreased by 33.8 % in the PME group, 74.3 % in the Mino group, and 79.9 % in the PME@Mino group (Fig. 3B). Arg1, a marker of M2 macrophages, exhibited the lowest expression in the saline group (Fig. 3A). The PME@Mino group exhibited the strongest Arg1 fluorescence. In semi-quantitative analysis (Fig. 3C), Arg1 expression in the Mino and PME@Mino groups was 3.16 and 4.1 times higher, respectively, than that in the saline group. Although Arg1 expression was higher in the PME group, it was not significantly different from the saline group.
Fig. 3.
Polarization regulation of macrophages. (A) Representative confocal images of iNOS and Arg1 staining in BV2 cells (scale bar = 20 μm). (B) The quantitative analysis result of iNOS. (C) The quantitative analysis result of Arg1. (D) The polarization modulation effects of different treatment groups on BV2 cultured with H2O2. (E) Quantitative analysis result of the percentage of M1 macrophages. (F) Quantitative analysis result of the percentage of M2 macrophages. (G) Quantitative analysis result of M1/M2 ratio. (H–J) Effect of different treatment groups on the expression of TNF-α, IL-6 and TGF-β in BV2. H). TNF-α. I) IL-6. J) TGF-β. Data are shown as the mean ± SD (n = 3; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001).
To further validate the impact of PME@Mino on macrophage polarization, the proportions of M1 and M2 macrophages were measured through flow cytometry (Fig. 3D). Compared to the saline group, the M1 phenotype differentiation (Fig. 3E) in the PME, Mino, and PME@Mino groups decreased 17.7 %, 20.6 %, and 22.6 %, respectively. Meanwhile, the M2 phenotype increased by 15.6 %, 72.3 %, and 104 % in these groups (Fig. 3F), with the M1/M2 ratio decreasing by 27.3 %, 55.1 %, and 60.1 %, respectively (Fig. 3G). These results indicate that PME has some anti-inflammatory properties, likely due to its ROS scavenging effect. Mino exhibited a more pronounced anti-inflammatory effect, promoting M2 polarization. The PME@Mino group had more M2 macrophages than the Mino group, though the difference was not statistically significant, indicating that the anti-inflammatory effect of PME@Mino is primarily driven by Mino, with PME's antioxidant effect contributing to the slight enhancement observed.
Cytokine levels in the cell culture medium were also measured. Inflammatory cytokines (TNF-α and IL-6) decreased in all treatment groups compared to the saline group (Fig. 3H and I). The Mino group had a 52.5 % decrease in TNF-α and a 57.7 % decrease in IL-6 levels, while the PME@Mino group showed a 54.5 % decrease in TNF-α and a 64.7 % decrease in IL-6 levels. Although TNF-α and IL-6 levels decreased in the PME group by 8.1 % and 5.4 % compared to those in the saline group, the differences were not statistically significant. As shown in Fig. 3J, the expression of TGF-β, an anti-inflammatory cytokine, significantly increased in the Mino and PME@Mino groups, reaching 3.4 and 3.8 times that of the saline group. The anti-inflammatory effects were also similar between the Mino and PME@Mino groups, with no significant differences. These results indicate that the anti-inflammatory effect of PME@Mino was mainly due to the actions of Mino, while the antioxidant properties of PME endowed PME@Mino with a slight advantage.
After SCI, the pro-inflammatory polarization of astrocytes aggravates inflammation and promotes scar formation [27]. Compared with the saline group, C3 expression in the PME group, Mino group, and PME@Mino group decreased by 14.5 %, 35.8 %, and 44 % (Fig. S10A, B, Supplementary Informmation). Both the Mino group and the PME@Mino group showed significant anti-polarization efficacy versus saline, though no statistical difference existed between these two groups. This indicates that Mino primarily mediates the observed polarization inhibition, and inhibiting the pro-inflammatory polarization of astrocytes may be one of the anti-inflammatory mechanisms of the nanoformulation.
The anti-inflammatory effect of Mino may be attributed to the inhibition of the phosphorylation of p38 MAPK [22]. p38 MAPK, being a protein kinase, has a variety of downstream proinflammatory effects. Proinflammatory phosphorylation will result in the activation of transcription factors and nuclear transfer, including nuclear factor kappa b (NF-κB) predominance, lipopolysaccharide-induced tumor necrosis factor alpha (LITAF), and Nur77 [[28], [29], [30]]. These changes also alter the white blood cell profile, enhancing the synthesis of proinflammatory cytokines and chemokines such as TNF-α, IL-1β, and IL-6. The application of Mino reduces the expression of proinflammatory factors, inhibiting M1 polarization. Furthermore, the Mino group showed increased expression of TGF-β, which is consistent with the reports of previous research [31]. The TGF-β/Smad signaling pathways are associated with M2 polarization. Overexpressed TGF-β binds to type II receptors on macrophages leading to the phosphorylation of type I receptors. This activates the Smad2 and Smad3 signaling cascade, promoting M2 polarization, and suppressing M1 polarization [32,33].
3.4. Biological distribution and safety in vivo
PME-Cy5.5 was administered to rats after SCI, and the distribution of the Cy5.5 signal was assessed at various time points. One-hour post-injection, Cy5.5 was detected in the heart, liver, spleen, lungs, kidneys, and spinal cord (Fig. 4A). The liver showed the strongest signal, and the fluorescence intensity in the organs gradually decreased over time. Notably, the signal at the injury site of the spinal cord gradually increased, peaking at 12 h post-administration (Fig. 4B). These results indicate that PME and PME@Mino successfully reached and accumulate at the lesion site, likely due to the disruption of the blood-spinal cord barrier (BSCB) and increased vascular permeability. The nanoformulations remained at the lesion site for at least 24 h, suggesting that they may have a persistent effect on modulation of the microenvironments [34].
Fig. 4.
Evaluation of the biodistribution of nanoformulation in vivo. (A) Fluorescent image of spinal cord and organs after administration with PME@Cy5.5. (B) Quantitative analysis of fluorescent intensity in spinal cord shown in Fig. 4A. Data are shown as the mean ± SD (n = 3).
To further evaluate the safety of PME and PME@Mino in vivo, histological analysis of major organs (heart, liver, spleen, lungs, and kidneys) was conducted at 8 weeks post-administration. H&E staining revealed no significant histopathological alterations in either treatment group compared to the sham group (Fig. S11, Supplementary Information). These findings demonstrate that both PME and PME@Mino maintain biocompatibility at therapeutic concentrations, with no observable organ toxicity.
3.5. Therapeutic effect of PME@Mino in vivo
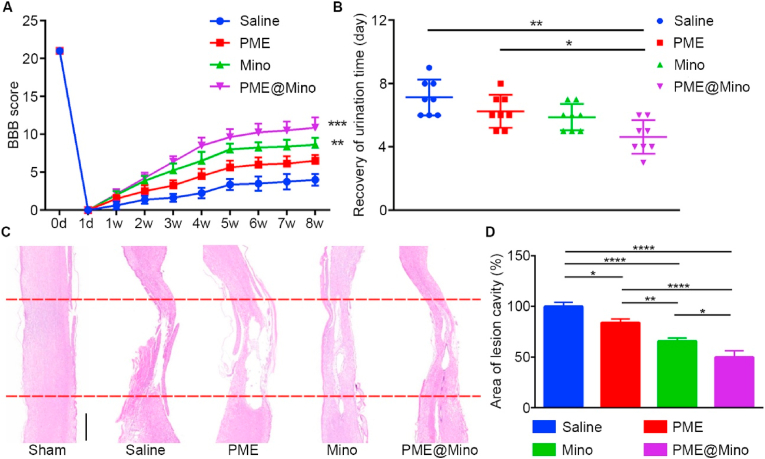
Based on the promising in vitro results, a rat contusion model was used to evaluate the therapeutic efficacy of PME@Mino. At 8 weeks post-injury, the motor function of the rats was assessed using the BBB behavior test. As shown in Fig. 5A, the BBB scores of the PME, Mino, and PME@Mino groups were all significantly higher than those of the saline group. The PME group showed a 62.5 % improvement, the Mino group a 115 % improvement, and the PME@Mino group exhibited the most significant recovery, with a 171 % improvement, significantly outperforming both the PME and Mino groups. SCI leads to a neurogenic bladder dysfunction, characterized by the spontaneous cessation of urination. Therefore, alleviation of the secondary injury is beneficial for the functional recovery of the bladder [35,36]. As shown in Fig. 5B, rats in the treated groups regained spontaneous urination faster than those in the saline group, with the PME@Mino group showing the fastest recovery, consistent with its superior BBB scores. These results indicate that PME@Mino treatment resulted in the best functional recovery.
Fig. 5.
Assessment of functional and histological repair after SCI. (A) BBB scores of SCI rats receiving various treatments. (B) Recovery of urination time. (C) H&E staining of spinal cord tissue, and the red lines indicated the injury site (scale bar = 1 mm). (D) Quantitative analysis of the relative area of lesion cavity in SCI rats with different treatments when compared with that of saline group. Data are shown as the mean ± SD (n = 8 for A, B, n = 3 for D; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Histological analysis was performed to confirm anatomical recovery after SCI. In the saline group, H&E staining of spinal cord sections from rats 8 weeks post-SCI revealed severe tissue disruption, large cavities, and almost complete loss of continuity (Fig. 5C). In contrast, the PME, Mino, and PME@Mino groups showed improved tissue integrity. Semi-quantitative analysis showed that the relative area of cavities in the PME, Mino, and PME@Mino groups decreased by 16.97 %, 34.19 %, and 50.08 %, respectively, compared to the saline group (Fig. 5D). The PME@Mino group exhibited the best tissue continuity, which is essential for functional recovery.
SCI often leads to demyelination and nerve function damage. In the saline group, large voids with negative LFB staining indicated severe demyelination (Fig. 6A). In the PME, Mino, and PME@Mino groups, demyelination alleviated significantly, with dense myelin sheaths observed. The LFB-positive area in the PME group increased by 62.3 %, by 130.9 % in the Mino group, and by 188 % in the PME@Mino group, compared to the saline group (Fig. 6B). The protective effect of the myelin sheath in the PME@Mino group was significantly better than that in other groups. These results are consistent with the ultrastructure of myelin sheath observed through TEM (Fig. 6C). G-ratio refers to the ratio of the inner diameter to the outer diameter of the myelin sheath, which reflects the condition of the myelin sheath; when the ratio is 1, it indicates demyelination. The myelin sheath in the saline group was severely damaged, leaving only a small amount of very thin myelin sheath with a G-ratio close to 1. The G-ratio was reduced by 8.7 % in the PME group, 13.4 % in the Mino group, and 25.8 % in the PME@Mino group (Fig. 6D). These results provide histological evidence for functional recovery after SCI. The results also indicate that PME@Mino had the most significant protective effect, as it does not only inhibit the formation of cavities but also inhibits demyelination.
Fig. 6.
Promotion of SCI repair in vivo. (A) LFB staining of spinal cord tissue and the red lines indicated the injury site (scale bar = 1 mm). (B) Quantitative analysis of LFB staining area in the lesion site in SCI rats with different treatments when compared with that of saline group. (C) The ultrastructure of the nerve myelin sheath of different treatment groups (scale bar = 1 μm) and (D) the G-ratio. (E) Immunofluorescence staining in the injured spinal cord, the astrocyte marker GFAP (red) and the neuronal marker NF200 (green). (F) The quantitative analysis result of NF200. Data are shown as the mean ± SD (n = 3 for B, D, F, n = 5 for H; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and∗∗∗∗p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Neurons are crucial for functional recovery after SCI, and immunohistochemical staining of GFAP (red) and NF200 (green) was performed on spinal cord from rats at 8 weeks post-SCI (Fig. 6E). The sham group exhibited dense green fluorescence, indicating intact neurons, while the saline group showed almost no NF200 expression. The PME, Mino, and PME@Mino groups showed increased NF200 expression, which was 2.68 times, 4.34 times, and 6.7 times higher, respectively, compared with the saline group (Fig. 6F). The PME@Mino group had the highest NF200 expression, which may be explained by the antioxidant and anti-inflammatory effects of PME@Mino. These results indicate that PME@Mino can successfully protect neurons and promote SCI repair.
To assess mechanism of neuroprotective effects, 3-nitrotyrosine (3-NT) staining was performed 1-day post-injury. 3-NT is an oxidative stress biomarker that is formed due to nitration of tyrosine and free tyrosine residues of bound proteins by reactive peroxynitrite molecules [37]. The expression of 3-NT was positively correlated with the degree of oxidative stress. As shown in Fig. 7A, the fluorescence of 3-NT was very strong in the saline group, indicating significant oxidative stress following SCI. In the PME, Mino, and PME@Mino groups, the fluorescence intensity of 3-NT decreased, with the most significant reduction observed in the PME@Mino group. Semi-quantitative analysis (Fig. 7B) showed that the MFI of the PME group decreased by 45.1 % compared to the saline group. In contrast, the Mino group showed a modest reduction of 26.8 %, while the PME@Mino group exhibited a 64.6 % decrease, significantly lower than both the PME and Mino groups. These findings align with the in vitro antioxidant results.
Fig. 7.
The microenvironment modulation effect and mechanisms of PME@Mino in vivo. (A) The antioxidant effect of different treatment groups in vivo (scale bar = 50 μm), and (B) the quantitative analysis result. (C) Immunofluorescence images of macrophage/microglia distribution in the rostral, injury and caudal of lesion sites, Iba1 (red) and DAPI (blue), scale bar = 50 μm. (D-F) The number of Iba1+ cells in the D) rostral, E) injury and F) caudal of the lesion sites (Iba1+ cells/0.25 mm2). (G) Immunofluorescence images of M2 polarization in the lesion at 7 days after SCI, CD68 (red), CD163 (green), DAPI (blue), scale bar = 50 μm. (H, I) Quantitative analysis results of H) CD68 and I) CD163. Data are shown as the mean ± SD (n = 3 for B, n = 5 for D, E, F, H, I; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Anti-inflammatory effect of nanoformulation is important for modulation of SCI microenvironment. The distribution of activated macrophages/microglia indicates the severity of inflammation [38]. As shown in Fig. 7C, the saline group exhibited the highest red fluorescence intensity (Iba1+) at 8 weeks post-SCI, demonstrating significant accumulation of Iba1+ cells at the injury site, and within 2 mm of rostral and caudal sites, suggesting the presence of chronic inflammation. In contrast, the PME, Mino, and PME@Mino groups showed reduced Iba1 fluorescence intensity compared to the saline group, with PME@Mino demonstrating the lowest intensity. Quantitative analysis of Iba1+ cell numbers showed that, the PME group showed reductions of 4 %, 15.4 %, and 18.6 % in the rostral, injury, and caudal sites compared to the saline group. The Mino group exhibited decreases of 52.9 %, 50.1 %, and 48.2 %, while the PME@Mino group showed the most pronounced reductions of 76.2 %, 73.8 %, and 71.9 % (Fig. 7D–F). The PME@Mino group displayed the lowest number of Iba1+ cells across all examined regions and demonstrated statistically significant superiority over other treatment groups. These results indicated that PME@Mino significantly attenuates chronic inflammation that persists after SCI.
To further investigate the mechanism of anti-inflammation, the polarization of M2 macrophages were analyzed at the lesion site 7 days after SCI. As shown in Fig. 7G, the saline group exhibited the highest expression of CD68, indicating sever inflammation at the injury site, while demonstrating weak CD163 fluorescence, suggesting little M2 macrophage polarization. In contrast, the PME, Mino, and PME@Mino group showed reduced CD68 expression and enhanced CD163 expression. Notably, the PME@Mino group displayed the weakest CD68 fluorescence and strongest CD163 fluorescence among all groups, demonstrating superior anti-inflammatory effects and M2 polarization capacity. Semi-quantitative analysis of MFI revealed that compared to the saline group, the expression of CD68 in the PME, Mino, and PME@Mino groups decreased by 14.7 %, 34.3 %, and 57.6 % (Fig. 7H), while the expression of CD163 increased 1.2-fold, 1.9-fold, and 2.8-fold, respectively (Fig. 7I). The minimal CD68 expression indicated that PME@Mino had the strongest anti-inflammation ability, which was consistent with the results of immunofluorescence staining of Iba1. Moreover, the maximal CD163 expression indicated that PME@Mino showed the best modulation ability of M2 polarization, and promotion of M2 polarization might be one of the anti-inflammatory mechanisms of PME@Mino. Compared with the Mino group, the PME@Mino group showed significant improvement in anti-inflammation and M2 polarization. This may be explained from the antioxidant property provided by PME nanoparticles, as well as the protection to Mino from being rapidly cleared in vivo.
4. Conclusion
In this study, a bioactive ROS-responsive nanoformulation, PME@Mino, was designed to modulate the SCI microenvironments. Upon intravenous administration, PME@Mino accumulated at the lesion site and released Mino in response to ROS. The treatment alleviated oxidative stress, mitigated excitotoxicity and promoted M2 macrophage phenotype polarization. These findings demonstrate that PME@Mino provide multi-level modulation of the SCI microenvironment, reducing secondary injury and promoting both histological and functional recovery. This study offers an alternative treatment strategy for SCI.
CRediT authorship contribution statement
Yuehong Li: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Qingzheng Zhang: Writing – review & editing, Conceptualization. Zongtai Liu: Writing – review & editing, Methodology. Weiguo Xu: Writing – review & editing, Funding acquisition. Changfeng Fu: Supervision, Funding acquisition.
Ethics approval and consent to participate
In vivo studies were performed adhering to the Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Animal Care and Use Committee of the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences.
Consent for publication
All authors consent for publication.
Funding
This work was financially supported by the National Key R&D Program of China (Grant No. 2022YFB3808000) was granted by Ministry of Science and Technology of the People's Republic of China; the National Natural Science Foundation of China (Grant No. 82071391) was granted by National Natural Science Foundation of China; the Science and Technology Development Program of Jilin Province (Grant Nos. 20230204089YY and 20240602025RC) were granted by Jilin Provincial Science and Technology Department, China.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We greatly appreciate the Key Laboratory of Polymer Ecomaterials of Changchun Institute of Applied Chemistry, Chinese Academy of Sciences for guidance and support on material characterization.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2025.102227.
Appendix A. Supplementary data
The following is the supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.David G., Mohammadi S., Martin A.R., Cohen-Adad J., Weiskopf N., Thompson A., Freund P. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat. Rev. Neurol. 2019;15(12):718. doi: 10.1038/s41582-019-0270-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee B.B., Cripps R.A., Fitzharris M., Wing P.C. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52(2):110. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 3.Hutson T.H., Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15(12):732. doi: 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- 4.Weissman D.E., Dufer D., Vogel V., Abeloff M.D. Corticosteroid toxicity in neuro-oncology patients. J. Neuro Oncol. 1987;5(2):125. doi: 10.1007/BF02571300. [DOI] [PubMed] [Google Scholar]
- 5.Fan B.Y., Wei Z.J., Yao X., Shi G.D., Cheng X., Zhou X.H., Zhou H.X., Ning G.Z., Kong X.H., Feng S.Q. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018;27(6):853. doi: 10.1177/0963689718755778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X., He X., Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. 2014;9(20):1787. doi: 10.4103/1673-5374.143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzzocrea S., Riley D.P., Caputi A.P., Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001;53(1):135. [PubMed] [Google Scholar]
- 8.Duchen M.R. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflügers Archiv. 2012;464(1):111. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David S., Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011;12(7):388. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 10.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29(43) doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi K., Gu Y., Tang J., Chen H., Xu Y., Wu L., Cai F., Deng L., Yang H., Shi Q., Cui W., Chen L. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat. Commun. 2020;11(1):4504. doi: 10.1038/s41467-020-18265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Z., Zang H., Zhu L., Huang K., Yi T., Zhang S., Cheng S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomedicine. 2019;14:721. doi: 10.2147/IJN.S187854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. 2011;71(2):281. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 14.Káradóttir R., Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145(4):1426. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J.X., Shi F.H., Xiao C.S., Lin L., Chen L., He C.L., Zhuang X.L., Chen X.S. One-step preparation of reduction-responsive poly(ethylene glycol)-poly (amino acid)s nanogels as efficient intracellular drug delivery platforms. Polym. Chem. 2011;2(12):2857. [Google Scholar]
- 16.Hall E.D. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8(2):152. doi: 10.1007/s13311-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine R.L., Moskovitz J., Stadtman E.R. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50(4–5):301. doi: 10.1080/713803735. [DOI] [PubMed] [Google Scholar]
- 18.Kim G., Weiss S.J., Levine R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta-Gen. Subj. 2014;1840(2):901. doi: 10.1016/j.bbagen.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park E., Velumian A.A., Fehlings M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J. Neurotrauma. 2004;21(6):754. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 20.Norenberg M.D., Rao K.V. The mitochondrial permeability transition in neurologic disease. Neurochem. Int. 2007;50(7–8):983. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasola A., Bernardi P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium. 2011;50(3):222. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Shultz R.B., Zhong Y.H. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regeneration Research. 2017;12(5):702. doi: 10.4103/1673-5374.206633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Martinez E.M., Sanz-Blasco S., Karachitos A., Bandez M.J., Fernandez-Gomez F.J., Perez-Alvarez S., de Mera R., Jordan M.J., Aguirre N., Galindo M.F., Villalobos C., Navarro A., Kmita H., Jordán J. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem. Pharmacol. 2010;79(2):239. doi: 10.1016/j.bcp.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Wang X.J., Shu G.F., Xu X.L., Peng C.H., Lu C.Y., Cheng X.Y., Luo X.C., Li J., Qi J., Kang X.Q., Jin F.Y., Chen M.J., Ying X.Y., You J., Du Y.Z., Ji J.S. Combinational protective therapy for spinal cord injury medicated by sialic acid-driven and polyethylene glycol based micelles. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119326. [DOI] [PubMed] [Google Scholar]
- 25.Krause T.L., Bittner G.D. Rapid morphological fusion of severed myelinated axons by polyethylene glycol. Proc. Natl. Acad. Sci. U. S. A. 1990;87(4):1471. doi: 10.1073/pnas.87.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittner G.D., Spaeth C.S., Poon A.D., Burgess Z.S., McGill C.H. Repair of traumatic plasmalemmal damage to neurons and other eukaryotic cells. Neural Regeneration Research. 2016;11(7):1033. doi: 10.4103/1673-5374.187019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escartin C., Galea E., Lakatos A., O'Callaghan J.P., Petzold G.C., Serrano-Pozo A., Steinhäuser C., Volterra A., Carmignoto G., Agarwal A., Allen N.J., Araque A., Barbeito L., Barzilai A., Bergles D.E., Bonvento G., Butt A.M., Chen W.T., Cohen-Salmon M., Cunningham C., Deneen B., De Strooper B., Díaz-Castro B., Farina C., Freeman M., Gallo V., Goldman J.E., Goldman S.A., Götz M., Gutiérrez A., Haydon P.G., Heiland D.H., Hol E.M., Holt M.G., Iino M., Kastanenka K.V., Kettenmann H., Khakh B.S., Koizumi S., Lee C.J., Liddelow S.A., MacVicar B.A., Magistretti P., Messing A., Mishra A., Molofsky A.V., Murai K.K., Norris C.M., Okada S., Oliet S.H.R., Oliveira J.F., Panatier A., Parpura V., Pekna M., Pekny M., Pellerin L., Perea G., Pérez-Nievas B.G., Pfrieger F.W., Poskanzer K.E., Quintana F.J., Ransohoff R.M., Riquelme-Perez M., Robel S., Rose C.R., Rothstein J.D., Rouach N., Rowitch D.H., Semyanov A., Sirko S., Sontheimer H., Swanson R.A., Vitorica J., Wanner I.B., Wood L.B., Wu J., Zheng B., Zimmer E.R., Zorec R., Sofroniew M.V., Verkhratsky A. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24(3):312. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson C.M., Hedrick M.N., Izadi H., Bates T.C., Olivera E.R., Anguita J. p38 mitogen-activated protein kinase controls NF-κB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect. Immun. 2007;75(1):270. doi: 10.1128/IAI.01412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceccarelli S., Panera N., Mina M., Gnani D., De Stefanis C., Crudele A., Rychlicki C., Petrini S., Bruscalupi G., Agostinelli L., Stronati L., Cucchiara S., Musso G., Furlanello C., Svegliati-Baroni G., Nobili V., Alisi A. LPS-induced TNF-α factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget. 2015;6(39) doi: 10.18632/oncotarget.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang T., Wang J., Benicky J., Saavedra J.M. Minocycline ameliorates LPS-induced inflammation in human monocytes by novel mechanisms including LOX-1, Nur77 and LITAF inhibition. Biochim. Biophys. Acta. 2012;1820(4):503. doi: 10.1016/j.bbagen.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H., Gao X.J., Li Y.J., Su J.B., E T.Z., Zhang X., Ni W., Gu Y.X. Minocycline reduces intracerebral hemorrhage-induced white matter injury in piglets, CNS Neurosci. Ther. 2019;25(10):1195. doi: 10.1111/cns.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis M.A., Sheppard D. In: Vol 32, Littman D.R., Yokoyama W.M., editors. vol. 32. Annual Reviews; Palo Alto: 2014. TGF-β activation and function in immunity; p. 51. (Annual Review of Immunology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie D., Shi O.Y. The role and mechanisms of macrophage polarization and hepatocyte pyroptosis in acute liver failure. Front. Immunol. 2023;14:15. doi: 10.3389/fimmu.2023.1279264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi Y., Duan W., Chen J., You T., Li S., Jiang W., Li M., Wang G., Pan X., Wu J., Liu D., Li J., Wang Y. Neutrophil decoys with anti-inflammatory and anti-oxidative properties reduce secondary spinal cord injury and improve neurological functional recovery. Adv. Funct. Mater. 2021;31(34):14. [Google Scholar]
- 35.Li L.M., Xiao B., Mu J.F., Zhang Y., Zhang C.Y., Cao H.C., Chen R.J., Patra H.K., Yang B., Feng S.Q., Tabata Y., Slater N.K.H., Tang J.B., Shen Y.Q., Gao J.Q. A MnO2 nanoparticle-dotted Hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano. 2019;13(12) doi: 10.1021/acsnano.9b07598. [DOI] [PubMed] [Google Scholar]
- 36.Luo W.Q., Wang Y.M., Lin F., Liu Y.X., Gu R., Liu W.G., Xiao C.S. Y selenium-doped carbon quantum dots efficiently ameliorate secondary spinal cord injury via scavenging reactive oxygen species. Int. J. Nanomedicine. 2020;15 doi: 10.2147/IJN.S282985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandookwala M., Sengupta P. 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020;130(10):1047. doi: 10.1080/00207454.2020.1713776. [DOI] [PubMed] [Google Scholar]
- 38.Lin F., Liu Y., Luo W., Liu S., Wang Y., Gu R., Liu W., Xiao C. Minocycline-Loaded Poly(α-Lipoic Acid)-Methylprednisolone prodrug nanoparticles for the combined anti-inflammatory treatment of spinal cord injury. Int. J. Nanomedicine. 2022;17:91. doi: 10.2147/IJN.S344491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.