Abstract
Background
Sepsis is a major contributor to high morbidity and mortality, often leading to coagulation disorders (CD) in affected individuals. Baicalein, a natural compound with well-established anti-inflammatory properties, shows promise as a potential treatment for sepsis. However, its molecular mechanisms in sepsis-associated CD remain poorly understood. This study investigated the therapeutic effects of baicalein in sepsis and identified candidate genes involved in its mechanism of action.
Methods
Transcriptomic data, baicalein-related targets from public databases, and CD-related genes from the literature were analyzed to identify potential candidate genes. Machine learning algorithms and expression validation techniques were employed to screen initial candidate genes from the candidates. A nomogram was then constructed based on these candidate genes. Functional enrichment and immune infiltration analyses were conducted to explore the underlying mechanisms, while molecular docking was used to assess interactions between baicalein and the candidate genes. Gene expression was further validated by reverse transcription-quantitative PCR (RT-qPCR).
Results
Seven initial candidate genes were identified. Machine learning and expression validation confirmed MMP9, ARG1, and FYN as the final candidate genes involved in sepsis. A highly accurate nomogram, constructed using these candidate genes, demonstrated strong predictive value for sepsis diagnosis. Functional enrichment analysis revealed their pivotal roles in sepsis pathogenesis, while immune infiltration analysis indicated immune dysregulation in sepsis. Additionally, molecular docking revealed strong binding interactions between baicalein and proteins encoded by these candidate genes, supporting further investigation of its therapeutic potential in sepsis. However, these in silico findings are preliminary and require validation through in vitro and in vivo experiments to confirm biological activity. RT-qPCR further validated differential expression of these genes in patients with sepsis compared to healthy controls, confirming the results.
Conclusion
This study identified MMP9, ARG1, and FYN as candidate genes in sepsis involved in immune regulation. Additionally, molecular docking revealed strong binding interactions between baicalein and the proteins encoded by these candidate genes, supporting further investigation of its therapeutic potential in sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40246-025-00818-6.
Keywords: Sepsis, Coagulation disorders, Machine learning, Baicalein, Immune regulation
Introduction
Sepsis is a life-threatening condition characterized by organ dysfunction resulting from a dysregulated host response to infection that stems from an abnormal host reaction to an infection. This abnormal response results in a condition with significantly high mortality [1]. The global mortality rate for sepsis is approximately 26%, with an incidence of 270 cases per 100,000 population annually [2]. Over the past two decades, the incidence of sepsis has increased by 8.7% per year [3], resulting in up to 2.8 million deaths annually in high-income countries [4]. Sepsis is now the leading cause of death worldwide [5]. Its pathogenesis is multifaceted, involving endothelial dysfunction, coagulation abnormalities, altered cellular function, and disrupted cardiovascular responses [6]. Among these, coagulation disorders (CD) are prevalent and severe complications of sepsis, significantly contributing to mortality [2]. During sepsis, pathogens and their byproducts trigger an inflammatory response, releasing a cascade of mediators that damage endothelial cells. This process impairs anticoagulant and fibrinolytic functions while promoting procoagulation [7]. Additionally, inflammatory mediators activate platelets, enhancing their adhesion, aggregation, and the risk of thrombosis [8]. Simultaneously, the activities of anticoagulants like protein C and antithrombin III are substantially reduced, further diminishing the body’s ability to control coagulation [8]. Persistent CD, if uncorrected, can progress to disseminated intravascular coagulation (DIC), which significantly heightens sepsis-related mortality [9]. Currently, there are no specific therapies targeting sepsis-related CD, and existing treatments primarily focus on anticoagulation and replacement therapies. However, the appropriate dosing and timing of anticoagulants remain challenging, with potential complications such as bleeding. Alternative therapies, such as plasma transfusions and platelet infusions, are limited by resources and may provoke immune responses [10].
Baicalein, a prominent natural flavonoid [11], has demonstrated beneficial effects in CD research [12]. With its anti-inflammatory and antioxidant properties, baicalein effectively inhibits the release of inflammatory mediators, scavenges free radicals, and mitigates disease-induced bodily damage [12, 13]. Baicalein modulates the expression and activity of coagulation factors, reducing platelet aggregation, which in turn improves blood hypercoagulability and prevents thrombosis formation [14, 15]. Additionally, it may influence the fibrinolytic system by promoting fibrin breakdown and preventing complications arising from coagulation abnormalities [13]. Baicalein also regulates excessive immune responses during sepsis, suppressing the release of inflammatory mediators, minimizing tissue damage, and enhancing immune cell function to restore immune balance [16].
Despite its promising therapeutic potential for sepsis-related coagulation dysfunction, the precise molecular mechanisms through which baicalein exerts its effects remain unclear. This study employed various bioinformatics approaches to analyze the CD-related genes (CDRGs) associated with baicalein treatment in sepsis, aiming to elucidate the biological roles of candidate genes in sepsis pathogenesis. This study aims to identify potential candidate genes and mechanisms by which baicalein may address coagulation dysfunction in patients with sepsis, ultimately contributing to a reduction in sepsis-related mortality.
Materials and methods
The collection of data and identification of coagulation disorders (CD)-related genes (CDRGs)
Gene expression profiles for the training set were sourced from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) by downloading the GSE65682 dataset (GPL13667) [17]. This dataset includes blood samples from both patients with sepsis and control subjects, comprising 760 samples in total, with survival data available for 479 patients with sepsis. The validation dataset, GSE95233 (GPL570), contained 51 sepsis and 22 control samples. For external validation of the nomogram model, an independent validation dataset GSE54514 (GPL6947) was obtained from the GEO database, which contained 163 samples including 127 sepsis patients and 36 control samples from blood specimens. A total of 904 CDRGs were identified based on their association with CD and thrombosis, as referenced in prior studies [5, 18] (Supplementary Table 1).
Identification of Baicalein-related targets (BRGs)
The structure of baicalein was retrieved from the Public Chemical Database (PubChem) (https://pubchem.ncbi.nlm.nih.gov/), and its effective targets were identified through multiple databases (Supplementary Table 2). The target genes were then standardized using the Universal Protein Resource (UniProt) database (https://www.uniprot.org/), and were subsequently referred to as baicalein-related genes (BRGs). The interactions among these BRGs were visualized using Cytoscape (v 3.7.1) [19].
Differential expression analysis and candidate gene screening
To identify differentially expressed genes (DEGs) between patients with sepsis and healthy controls, the “limma” R package (v 3.54.1) [20] was utilized with criteria of p < 0.05 and |log2FoldChange (FC)| >1.0. A volcano plot was created with the “ggplot2” R package (v 3.4.1) [21], highlighting the top five upregulated and five downregulated genes based on Log2FC. A heatmap of the DEGs was generated using the “ComplexHeatmap” R package (v 2.14.0) [22]. Initial candidate genes were identified through intersecting analysis of DEGs, BRGs, and CDRGs, visualized with the “ggvenn” R package (v 0.1.9) [23].
Identification of feature genes via machine learning
To further refine the initial candidate genes, multiple feature selection methods were applied. First, least absolute shrinkage and selection operator (LASSO) regression was performed using the “glmnet” R package (v 4.1.4) [24], selecting feature genes 1 based on the lambda value that minimized model error. Support vector machine-recursive feature elimination (SVM-RFE) was also conducted using the “e1071” R package (v 1.7.13) [25], where genes corresponding to the highest accuracy were identified as feature genes 2.
Additionally, random forest (RF) classification was performed using the “randomForest” R package (v 4.7.1.1) [26]. The optimal ntree value was selected within the range of 10 to 100 with a step size of 2 to minimize the out-of-bag error. The top three most important genes were designated as feature genes 3.
Finally, genes common to feature genes from LASSO (feature genes 1), SVM-RFE (feature genes 2), and RF (feature genes 3) were identified through intersecting analysis using the “ggvenn” R package (v 0.1.9), resulting in a set of feature genes.
Identification of candidate genes from feature genes using machine learning
The expression levels of feature genes between patients with sepsis and healthy controls were assessed in both the training and validation datasets using the Wilcoxon rank-sum test (p < 0.05). Box plots, generated with the “ggpubr” R package (v 0.6.0) (https://CRAN.R-project.org/package=ggpubr), were used to visualize the expression differences between patients with sepsis and healthy controls in both datasets. Feature genes that exhibited significant expression differences and consistent trends across the datasets were identified as candidate genes. These candidate genes were subsequently analyzed using receiver operating characteristic (ROC) analysis with the “pROC” R package (v 1.18.0) [27]. The area under the curve (AUC) for each candidate gene was calculated, with genes having an AUC greater than 0.7 considered as candidate gene.
Construction and evaluation of nomogram
A nomogram was then constructed based on the expression of candidate gene in the training set using the “rms” R package (v 6.5.0) [28]. Calibration curves were plotted with the “ResourceSelection” R package (v 0.3.5) [29] to evaluate the accuracy of the nomogram. The Hosmer-Lemeshow (HL) test assessed the model fit (p > 0.05), and a mean absolute error (MAE) < 0.1 indicated high model accuracy. A calibration curve slope closer to 1 signified better predictive capability of the nomogram. Decision curve analysis (DCA) and ROC analysis were conducted using the “rmda” package (v 1.6) [30] and the “pROC” R package (v 1.18.0), respectively, with an AUC value > 0.7 in ROC analysis indicating favorable performance. Additionally, a clinical impact curve (CIC) was generated using the “rmda” R package (v 1.6).
For external validation of the nomogram model, the nomogram model constructed from the training set was applied to this external dataset to evaluate its generalizability and predictive performance. Calibration curves were plotted to assess the accuracy of the nomogram using the “ResourceSelection” R package (v 0.3.5) [31]. The HL test was used to assess model fit (p > 0.05) and MAE < 0.1 indicated high accuracy. DCA and ROC analysis were performed using the “rmda” package (v 1.6) and “pROC” R package (v 1.18.0), respectively, to evaluate clinical utility and predictive performance. Clinical impact curves (CIC) were also constructed to assess the model’s clinical applicability. Internal cross-validation was performed using the bootstrap method with the bootcov function from the “rms” R package (v 6.8-1) to assess the stability and robustness of the nomogram coefficients. The bootstrap validation was conducted with 1000 bootstrap samples to evaluate model performance metrics including C-index, Brier score, and calibration.
Diagnostic value and clinical expression differences of candidate genes
Survival analysis was performed on the 479 sepsis individuals with available survival information from the training set. The patients were divided into high-expression and low-expression groups based on the median expression level of each candidate gene, using the “survminer” R package (v 0.4.9) [32]. Kaplan-Meier (KM) survival curves for patients with sepsis were constructed with the “survival” R package (v 3.5.3) (https://CRAN.R-project.org/package=survival), and differences in overall survival were evaluated using the log-rank test, with statistical significance set at p < 0.05. Additionally, expression differences among various clinical subgroups were analyzed using the Wilcoxon rank-sum test (p < 0.05).
Gene set analysis of candidategene
Gene set enrichment analysis (GSEA) was conducted to explore the biological functions and pathways of candidate gene in sepsis. Spearman correlation analysis was performed using the “psych” R package (v 2.2.9) [33] to calculate the correlation coefficients between each candidate gene and all other genes. The genes in the sepsis samples were then ranked from high to low based on their correlation coefficients. GSEA was carried out using the “clusterProfiler” R package (v 4.2.2) [34], based on the Molecular Signatures Database (MSigDB) (https://www.gsea-msigdb.org/gsea/msigdb). Enrichment was considered significant if the normalized enrichment score (NES) was greater than 1, with a false discovery rate (FDR) < 0.25 and a p-value < 0.05.
To further investigate biological pathway alterations during the pathogenesis of sepsis, gene set variation analysis (GSVA) was applied using the “GSVA” R package (v 1.46.0) [35], with the background gene set “Hallmark.all.v2022.1.Hs.symbols” from MSigDB. GSVA scores were calculated for patients with sepsis and healthy controls to assess pathway activity in each sample. Differential analysis of the GSVA scores was conducted using the “limma” R package (v 3.54.1) to identify pathways with significant changes between sepsis and control samples. Subsequently, differential analysis of the GSVA scores was conducted via the “limma” R package (v 3.54.1), which incorporates FDR correction, to detect pathways that showed significant alterations between sepsis and control samples (adjusted p < 0.05). The correlation coefficients between candidate genes and pathways were calculated using Spearman analysis with the “psych” R package (v 2.2.9).
Immune infiltration analysis
To explore immune infiltration in sepsis, the relative proportions of 28 immune cell types [36] were evaluated across all samples in the training set using the “GSVA” R package (v 1.46.0). Differential immune cell infiltration between patients with sepsis and healthy controls was analyzed using the Wilcoxon rank-sum test (p < 0.05). Additionally, Spearman’s correlation coefficients were calculated with the “psych” R package (v 2.2.9) to examine associations between differentially infiltrated immune cells and candidate genes (|correlation (cor)| >0.3, p < 0.05).
Establishment of regulatory network and tissue expression examination
To investigate the upstream regulatory factors of candidate genes, a microRNA (miRNA)-mRNA-transcription factor (TF) network was constructed. Potential miRNAs targeting candidate genes were identified using the DIANA-microT (http://www.microrna.gr/microT) and TargetScan (http://www.targetscan.org) databases. The intersection of miRNAs predicted by both databases was considered the key miRNAs for each critical gene. TFs associated with the candidate genes were retrieved from the TRRUST database (http://www.grnpedia.org/trrust/). The resulting miRNA-mRNA-TF network was visualized using Cytoscape (v 3.7.1). Finally, to examine the expression levels of candidate genes across various tissues, gene expression data from the GTEx database (https://ngdc.cncb.ac.cn/databasecommons/) were analyzed (p < 0.05).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) experiment
In this study, five pairs of whole blood samples were collected from The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, including five healthy controls (samples 1–5) and five patients with sepsis (samples 6–10). The study was approved by Guizhou University of Traditional Chinese Medicine (approval number: 20230014), and all participants provided informed consent. The expression levels of candidate genes were validated using reverse transcription quantitative PCR (RT-qPCR). Total RNA from the 10 samples was extracted using TRIzol (Ambion, Austin, USA) following the manufacturer’s protocol. Reverse transcription to cDNA was carried out using the SureScript First-Strand cDNA Synthesis Kit (Servicebio, Wuhan, China) in accordance with the protocol guidelines. RT-qPCR was performed using the 2× Universal Blue SYBR Green qPCR Master Mix (Servicebio, Wuhan, China), with forward and reverse primers listed in Supplementary Table 3. GAPDH served as the internal reference gene. The expression levels of the candidate genes were calculated using the 2−ΔΔCt method [37]. GraphPad Prism 10 [38] was used for data visualization, and statistical significance was evaluated using the Student’s t-test (p < 0.05).
Molecular Docking
The 3D structures of the proteins encoded by the candidate genes were retrieved from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) (http://www.rcsb.org). The 3D structure of baicalein’s active compound was obtained from PubChem. To investigate the molecular docking potential between baicalein and the candidate genes, molecular docking was performed using AutoDock (v.1.2.5) [39], with a binding energy threshold set at < -5.0 kcal/mol.
Statistical analysis
Bioinformatics analyses were conducted using R programming language (v 4.2.2). For multiple hypothesis testing correction, Benjamini-Hochberg FDR correction was applied for pathway-level analyses (GSVA) with adjusted p < 0.05 as the significance threshold. For gene set enrichment analysis (GSEA), FDR < 0.25 and |normalized enrichment score (NES)| >1 were used as significance thresholds. The Wilcoxon rank-sum test was employed for gene expression validation, with p < 0.05 considered statistically significant.
Results
Initial candidate genes identification for Baicalein in sepsis
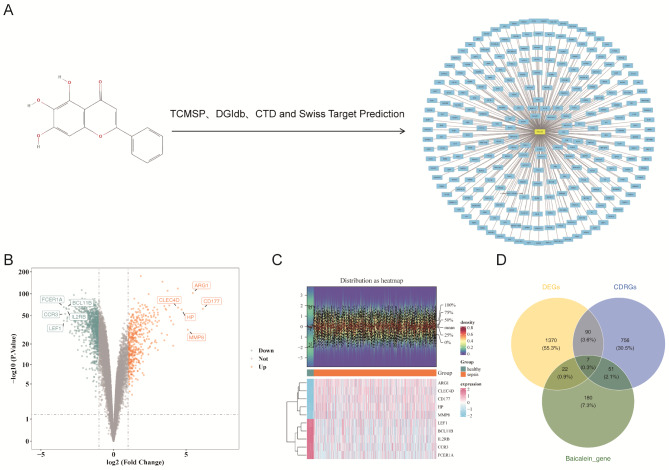
A total of 260 unique effective genes were identified as BRGs from multiple databases (Fig. 1A and Supplementary Table 4). These genes are potentially crucial in identifying key regulatory elements involved in sepsis.
Fig. 1.
Candidate genes identification for baicalein in sepsis. (A) Network diagram of the chemical structure and effective targets of baicalein. (B) Volcano plot of differentially expressed genes (DEGs). (C) Heatmap of DEGs. (D) Screening of candidate genes
Subsequently, transcriptomic analysis revealed 1,489 DEGs between sepsis and control samples (p < 0.05, |Log2FC| >1), with 489 upregulated and 1000 downregulated genes (Fig. 1B). The significant changes in gene expression indicate widespread molecular disruptions in sepsis, which likely contribute to its pathophysiology. Heatmaps also highlighted distinct expression patterns between sepsis and control samples (Fig. 1C).
Further analysis identified seven initial candidate genes by intersecting DEGs, BRGs, and CDRGs (Fig. 1D). These initial candidate genes may represent critical regulatory nodes that baicalein could target to modulate sepsis, providing valuable insights into potential therapeutic strategies.
Identification of key feature genes associated with sepsis via machine learning algorithms
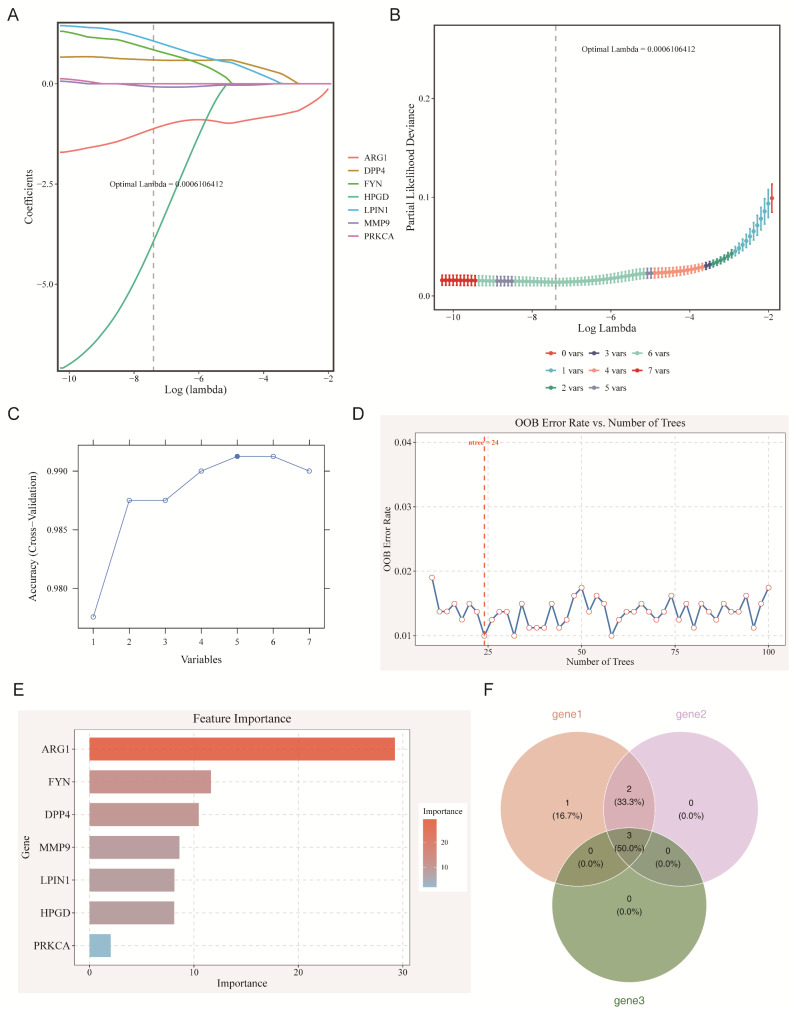
To refine the feature genes, three feature selection algorithms—LASSO, SVM-RFE, and RF—were applied to the training set of sepsis data. LASSO regression identified six genes (ARG1, MMP9, DPP4, HPGD, LPIN1, and FYN) as feature genes 1, with an optimal lambda of 0.0006106412 (Fig. 2A-B). SVM-RFE, using ten-fold cross-validation, selected five genes (ARG1, DPP4, FYN, MMP9, and LPIN1) as feature genes 2, with the highest accuracy when the feature set was reduced to five genes (Fig. 2C). RF analysis, with an ntree value of 24, identified MMP9, ARG1, and FYN as feature genes 3 (candidate genes) based on their importance (Fig. 2D-E).
Fig. 2.
Identification of feature genes associated with sepsis via machine learning algorithms. (A-B) LASSO logic coefficient penalty chart (A) and LASSO cross-validation curve (B). (C) SVM-RFE algorithm screening results. (D-E) Relationship between out-of-bag (OOB) error rate and ntree in random forest model (D) and candidate gene importance ranking (E). (F) Venn diagram of feature genes identified by three algorithms
The intersection of these three methods led to the final selection of three candidate genes: MMP9, ARG1, and FYN (Fig. 2F). These genes emerged as potential therapeutic targets, warranting further validation of their roles in sepsis pathology.
Expression analysis of candidate genes in sepsis
The expression levels of the three identified candidate genes were compared between sepsis and control samples from both the training and validation datasets. All three genes exhibited consistent and significant differential expression across datasets (Fig. 3A-B) (p < 0.0001). MMP9 and ARG1 were upregulated in sepsis samples, whereas FYN was downregulated. Based on these results, MMP9, ARG1, and FYN were selected as candidate genes.
Fig. 3.
Expression analysis of candidate genes in sepsis. (A-B) Gene expression levels of candidate genes in patients with sepsis and control samples from the training set (A) and verification of gene expression levels in patients with sepsis and control samples (B). (C-E) ROC analysis of core genes across all samples from the training set
ROC analysis demonstrated that all three genes had AUC values greater than 0.7, indicating satisfactory predictive performance for sepsis (Fig. 3C-E). These findings supported the inclusion of MMP9, ARG1, and FYN as candidate genes for sepsis and provided a foundation for evaluating their diagnostic potential in future studies.
Evaluation of candidate genes-based nomogram for sepsis prediction
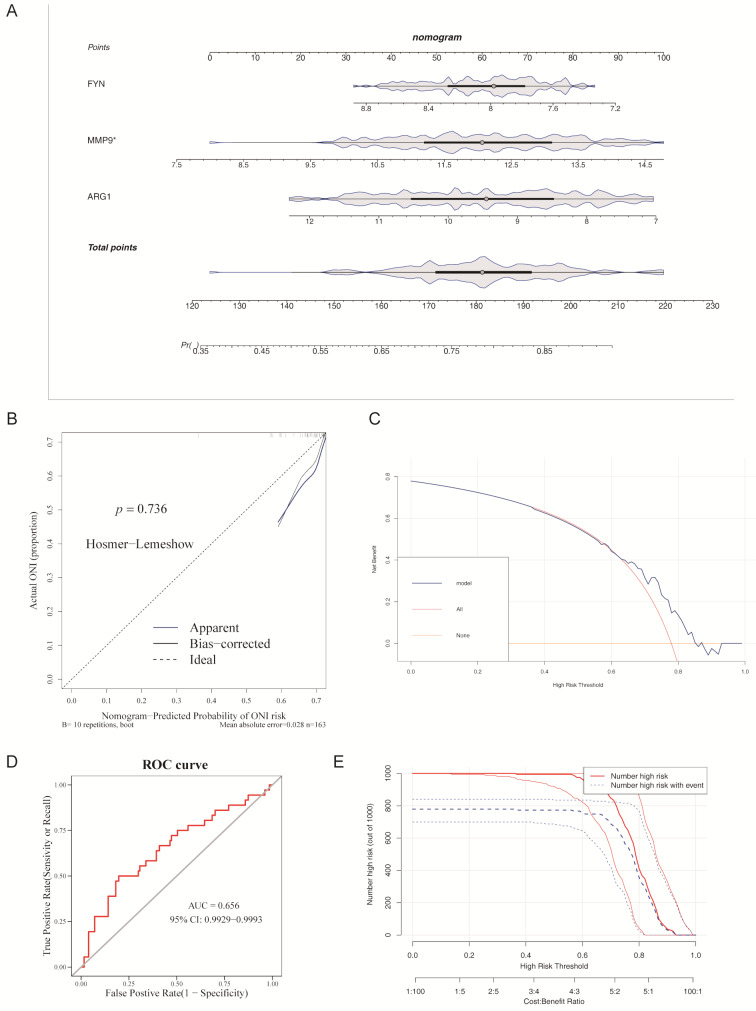
To evaluate the predictive capability of candidate genes for sepsis, a nomogram was developed based on gene expression data from the training set. Notably, ARG1 and FYN contributed significantly to the predictive value of the nomogram, while changes in MMP9 expression had minimal impact on the predictive results. The nomogram aggregated gene expression scores, with higher scores indicating an increased likelihood of sepsis (Fig. 4A).
Fig. 4.
Evaluation of candidate gene-based nomogram for sepsis prediction. (A) Column chart model of candidate genes. (B) Calibration curve of the nomogram model. (C) Decision curve of the nomogram model. (D) ROC curve of the nomogram model. (E) Clinical impact curve of the nomogram model
Calibration curves demonstrated that the slope was very close to 1, indicating high predictive value for the nomogram. Additionally, the HL test confirmed good agreement between predicted and observed values (p = 0.541). The MAE was 0.003, indicating excellent model accuracy (Fig. 4B).
DCA further supported the net benefit provided by the nomogram (Fig. 4C), and the ROC curve yielded an AUC of 0.996, demonstrating outstanding predictive performance (Fig. 4D). The CIC further validated the model’s accuracy, confirming its strong predictive power at higher risk thresholds (Fig. 4E).
These results collectively suggest that the nomogram, based on candidate genes, can serve as a reliable tool for sepsis prediction in clinical practice.
External validation and internal cross-validation of nomogram
To address concerns about model generalizability, we performed both external validation and internal cross-validation using bootstrap methods. For external validation, the calibration curve demonstrated good agreement between predicted and observed values (Hosmer-Lemeshow test p > 0.05, MAE < 0.1) (Fig. 5A-B). Decision curve analysis showed positive net benefit across the risk threshold range, confirming clinical utility (Fig. 5C). ROC analysis and clinical impact curves further validated the model’s predictive performance in this external cohort (Fig. 5D-E).
Fig. 5.
External and internal validation of the nomogram model for sepsis prediction. (A) Nomogram based on candidate genes (ARG1, FYN, and MMP9) used to predict the risk of sepsis. (B) Calibration curve of the nomogram using the independent GSE54514 dataset (n = 163), demonstrating good agreement between predicted and observed outcomes (Hosmer–Lemeshow test, p > 0.05; MAE < 0.1). (C) Decision curve analysis (DCA) showing positive net clinical benefit across a wide range of threshold probabilities (0–1), confirming clinical utility of the nomogram. (D) Receiver Operating Characteristic (ROC) curve showing excellent predictive performance in the external validation cohort (AUC = 0.996). (E) Clinical impact curve (CIC) demonstrating the predicted number of high-risk patients (blue) and the actual number of patients with events (red) across different risk thresholds, further supporting model reliability and clinical applicability
For internal cross-validation, the validation demonstrated excellent model stability with completely unchanged regression coefficients after validation. The intercept remained stable at 13.6099, while the coefficients for MMP9, ARG1, and FYN were − 0.0142, 1.4277, and − 2.6452, respectively, showing no change after validation. The model achieved a C-index of 0.996 and Brier score of 0.011, indicating excellent discrimination and calibration. The likelihood ratio test statistic was 271.34 with 3 degrees of freedom (p < 0.001), confirming overall statistical significance. Both Dxy and Gamma values reached 0.992, further confirming excellent discriminative ability. R² values ranged from 0.852 to 0.897, showing the model explains over 85% of outcome variance, with a maximum derivative of 6.3e-08 indicating stable calibration (Supplementary Table 5).
Diagnostic value of candidate genes in sepsis
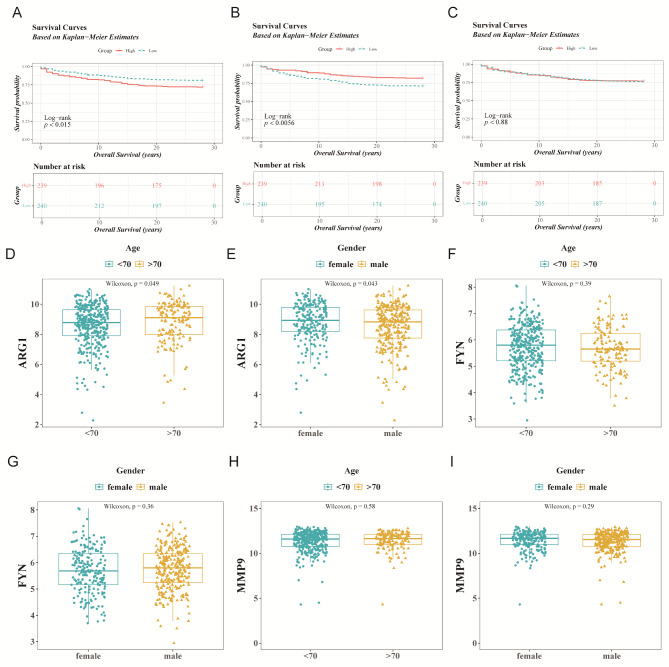
Additionally, the diagnostic significance of MMP9, ARG1, and FYN was evaluated using overall survival data from 479 sepsis individuals with available survival information in the training set. Based on the median expression values of the candidate genes (MMP9: 11.60321, ARG1: 8.873225, FYN: 5.737927), patients with sepsis were classified into two groups. KM survival analysis revealed that high expression levels of ARG1 and FYN were associated with shorter overall survival (p < 0.05), while MMP9 showed no significant difference in survival (Fig. 6A-C). Further subgroup analyses indicated significant expression differences in ARG1 based on age and gender (p < 0.05) (Fig. 6D-I), suggesting that ARG1 may be influenced by clinical characteristics and highlighting its potential diagnostic value in sepsis.
Fig. 6.
Diagnostic value of three candidate genes in sepsis. (A-C) Kaplan-Meier (KM) curves for patients with sepsis in high and low expression groups of candidate genes from the training set (ARG1, FYN, MMP9, from left to right). (D-I) Differential expression of candidate genes among different clinical subgroups of patients with sepsis in the training set (ARG1, FYN, MMP9, from left to right)
Biological pathways involved in candidate genes of sepsis
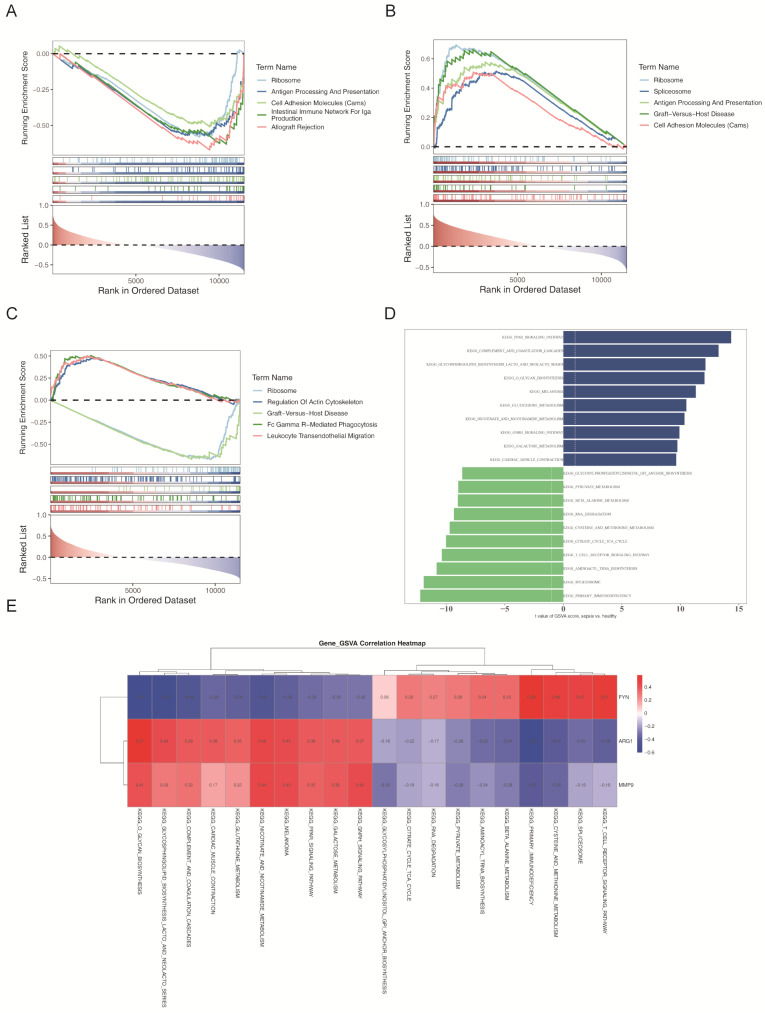
To explore the biological functions and pathways associated with the candidate genes, Spearman correlation analysis was conducted between the candidate genes and all other genes in the training set. The results revealed that ARG1 was linked to 42 pathways, FYN to 34 pathways, and MMP9 to 64 pathways (Fig. 7A-C). Notably, GSEA demonstrated that ARG1, FYN, and MMP9 were significantly enriched in several key biological pathways.
Fig. 7.
Biological pathways involved in candidate genes of sepsis. (A-C) GSEA of candidate genes. (D) Differential analysis of Hallmark pathways enriched in GSVA between patients with sepsis and control samples. (E) Correlation analysis between candidate genes and the top 10 Hallmark enrichment pathways (A gradient from blue to red indicates the extent of correlation from negative to positive; the deeper the color, the stronger the correlation. The horizontal axis represents the top 10 pathways, and the vertical axis represents candidate genes)
GSVA identified 148 differentially regulated pathways between patients with sepsis and healthy controls (Fig. 7D). Among the upregulated pathways were “cardiac muscle contraction,” “galactose metabolism,” and “complement and coagulation cascades.” In contrast, downregulated pathways included “primary immunodeficiency,” “T cell receptor signaling,” and “citric acid cycle.”
Subsequent correlation analysis between the three candidate genes and the top 10 upregulated and 10 downregulated pathways identified by GSVA revealed distinct patterns. FYN showed positive associations with the downregulated pathways and negative associations with the upregulated pathways, while ARG1 and MMP9 exhibited the opposite pattern (Fig. 7E). These results suggest that ARG1, FYN, and MMP9 play pivotal roles in the pathogenesis of sepsis.
Immune infiltration analysis in sepsis
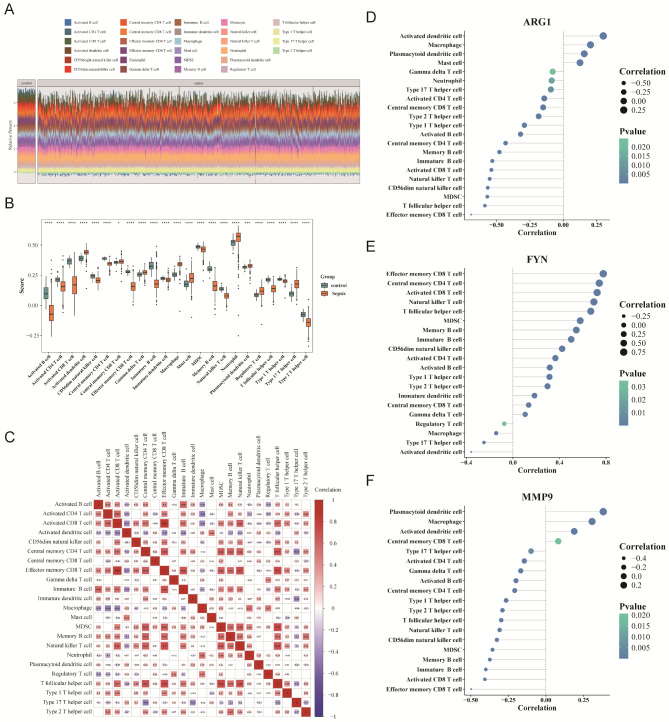
To further investigate immune cell infiltration in sepsis, single-sample GSEA (ssGSEA) was performed on 28 immune cell types, revealing notable disparities in immune cell infiltration between sepsis and control samples (Fig. 8A). A total of 23 immune cell types showed significant variations in infiltration levels (p < 0.05) (Fig. 8B), indicating immune dysregulation in sepsis.
Fig. 8.
Immune infiltration analysis in sepsis. (A) Distribution ratio of immune cells between patients with sepsis and normal control samples (different colors represent different immune cells, and the vertical axis shows the percentage of each cell in the sample). (B) Screening of differential immune cells. (C) Correlation analysis between differential immune cells. (D-F) Correlation analysis between differential immune cells (different colors represent different levels of p-value, with darker colors indicating more significant differences. The size of the circle represents different levels of correlation, with smaller shapes indicating stronger negative correlation and larger shapes indicating stronger positive correlation)
Additionally, correlation analysis was conducted to examine the relationships between immune cells with differential infiltration and the candidate genes. Activated CD4+ T cells exhibited strong positive correlations with other immune cells, such as activated CD8+ T cells, while regulatory T cells showed negative correlations with several immune cell types (Fig. 8C). Moreover, correlation analysis indicated that ARG1 and MMP9 were negatively correlated with effector memory CD8+ T cells (cor = -0.69 and cor = -0.49, respectively), while FYN was positively correlated with effector memory CD8+ T cells (cor = 0.78, p < 0.05) (Fig. 8D-F). These correlations suggest that ARG1, MMP9, and FYN may influence immune cell infiltration and immune responses in sepsis, particularly in regulating the function of effector memory CD8+ T cells.
Construction of miRNA-mRNA-TF networks and expression analysis of candidate genes
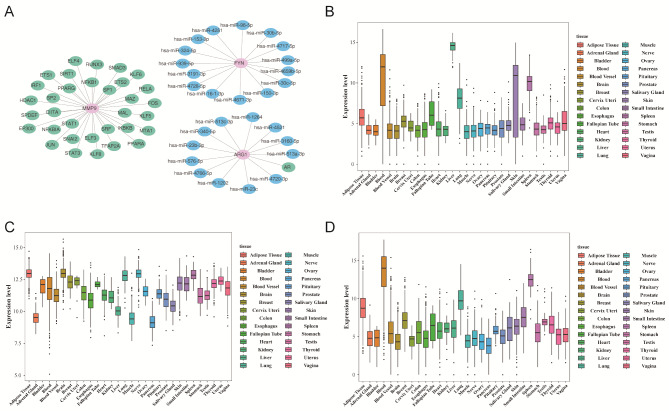
To explore upstream regulatory mechanisms, a miRNA-mRNA-TF network was constructed. miRNA predictions for ARG1 and FYN were derived from DIANA-microT and TargetScan, identifying 12 and 15 key miRNAs, respectively (Fig. 9A). For instance, hsa-miR-30b-5p was predicted to regulate FYN expression. These miRNAs typically bind to the 3’ untranslated region (3’ UTR) of the mRNA, leading to mRNA degradation or inhibition of translation. Additionally, TFs for MMP9 and ARG1 were retrieved from TRRUST, identifying 33 TFs for MMP9 and 1 TF for ARG1. For example, MAL was found to target MMP9.
Fig. 9.
Construction of miRNA-mRNA-TF networks and expression analysis of candidate genes. (A) miRNA-mRNA-TF regulatory network (pink represents candidate genes, green represents transcription factors, and blue represents miRNA). (B-D) The expression profile of candidate genes in human body tissues and organs: ARG1 (B), FYN (C), and MMP9 (D)
Expression patterns of ARG1, FYN, and MMP9 across human tissues were analyzed using GTEx data. ARG1 exhibited high expression in blood, liver, lung, skin, and spleen. FYN was ubiquitously expressed, while MMP9 was predominantly expressed in adipose tissue, blood, lung, and spleen (Fig. 9B-D).
Validation of critical gene expression levels by RT-qPCR
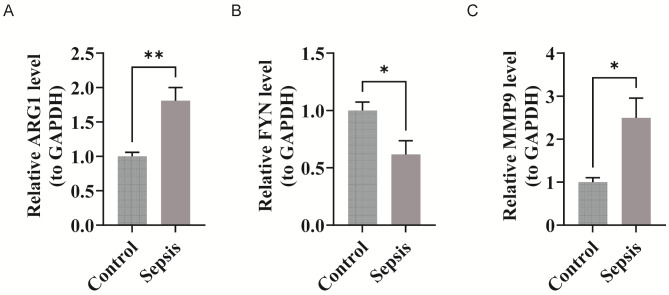
To validate the bioinformatics findings, the expression levels of ARG1, FYN, and MMP9 were measured in both healthy controls and patients with sepsis. Significant differences in expression levels were observed between the two groups (p < 0.05). MMP9 and ARG1 were upregulated in sepsis samples compared to controls, while FYN was downregulated in sepsis (Fig. 10A-C). These results confirmed the consistency between the PCR experiments and bioinformatics predictions regarding critical gene expression levels.
Fig. 10.
Validation of critical gene expression levels by RT-qPCR. (A-C) The expression levels of ARG1, FYN, and MMP9 in healthy controls and septic patients (from left to right, they are ARG1, FYN, and MMP9)
Molecular Docking of Baicalein with candidate genes
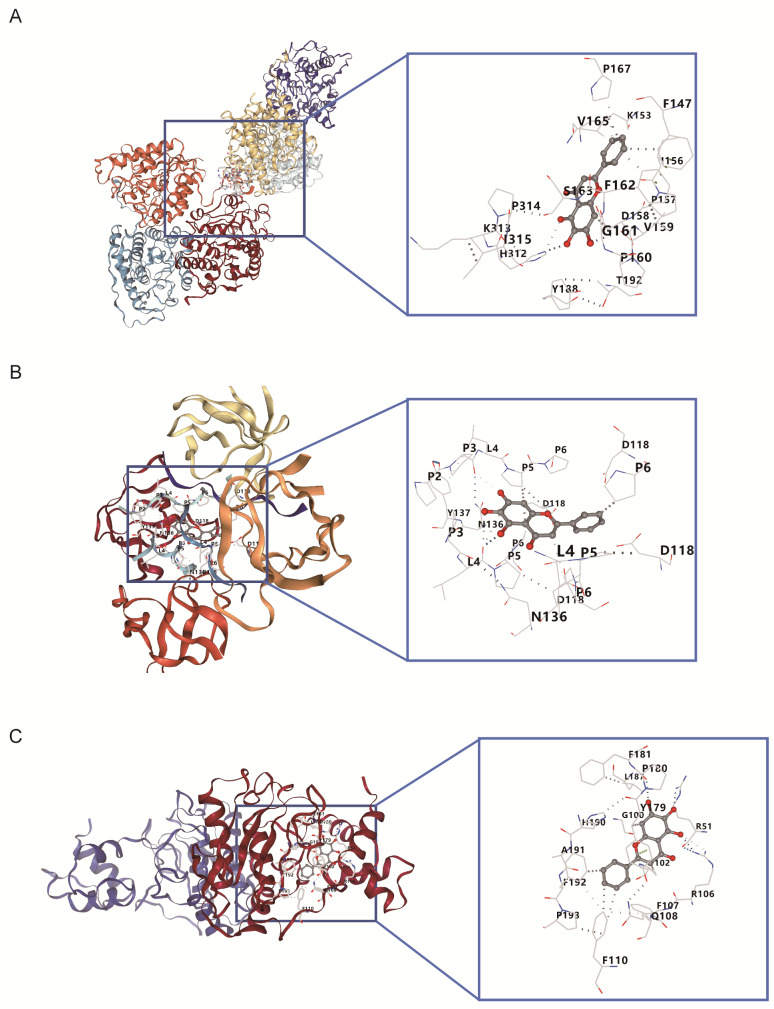
Molecular docking simulations were conducted to assess baicalein’s binding affinity with ARG1, FYN, and MMP9. The results revealed strong binding affinities between baicalein and the proteins encoded by these candidate genes (binding energy < -5.0 kcal/mol). The binding energies for baicalein with ARG1, FYN, and MMP9 were − 8.4, -7.4, and − 9.7 kcal/mol, respectively (Table 1 and Fig. 11A-C). These results suggest that baicalein may exert therapeutic effects through its interaction with these critical gene-encoded proteins.
Table 1.
Molecular docking of baicalein with candidate genes
| Molecular docking binding energy (kcal/mol) | ||||
|---|---|---|---|---|
| MOL ID | Molecular name | gene name | PDB ID | score |
| 5281605 | Baicalein | ARG1 | 8E5M | -8.4 |
| FYN | 4ZNX | -7.4 | ||
| MMP9 | 8K5Y | -9.7 | ||
Fig. 11.
Molecular docking sites of baicalein with candidate genes. (A) ARG1-baicalein. (B) FYN-baicalein. (C) MMP9-baicalein. The gray part is baicalein, where the light gray part represents the amino acid binding site, and the gray dashed lines connect the two interacting parts
Discussion
Sepsis is a prevalent and fatal condition globally, responsible for 30% of in-hospital deaths [40]. Its pathogenesis is highly complex, involving the interplay of multiple systems, including immunity, inflammation, coagulation, and metabolic disorders [41]. This study employed a range of bioinformatics approaches to identify candidate genes associated with coagulation dysfunction in sepsis: MMP9, ARG1, and FYN. These genes are implicated in sepsis-related coagulation abnormalities and represent potential therapeutic targets for baicalein. The robustness of these biomarkers was validated through various analytical methods, including RF algorithms, support vector machines (SVM), and ROC curve analysis. Additionally, GSEA, molecular regulatory network construction, and immune infiltration analyses were performed to explore the mechanisms of these candidate genes, providing a theoretical foundation for sepsis treatment.
Molecular docking analysis revealed that baicalein exhibited high binding affinities with the proteins encoded by the candidate genes MMP9, ARG1, and FYN, with binding energies of − 9.7, − 8.4, and − 7.4 kcal/mol, respectively, providing computational evidence for potential direct molecular interactions. These findings are consistent with previous studies supporting the therapeutic potential of baicalein in the treatment of sepsis. As a major plant-derived flavonoid, baicalein has been shown to modulate various biological networks and effectively alleviate the inflammatory response associated with sepsis [16, 42]. Bayram et al. reported that baicalein exerted protective effects in septic animals by inhibiting the production of pro-inflammatory cytokines [43]. Previous research has also demonstrated that baicalein can exert anti-inflammatory, antioxidant, and immunomodulatory effects by regulating key signaling pathways, including NF-κB, MAPK, and JNK, which are closely linked to inflammation and coagulation dysfunction in sepsis. Our docking-based predictions of molecular interactions provide mechanistic insight into how baicalein might exert therapeutic effects in sepsis-associated coagulopathy.
MMP9 (Matrix metalloproteinase-9) is a matrix metalloproteinase [44], which plays an important role in extracellular matrix remodeling and inflammatory response [45]. As a key gene for sepsis [45–47], its high expression is associated with increased severity of sepsis and enhanced vascular permeability [51]. Studies have shown that MMP9 can inhibit platelet aggregation [48], interfere with coagulation and inflammatory responses, suppress platelet-fibrin interactions and the formation of platelet-monocyte aggregates [49]. The expression of MMP9 is regulated by the NF-κB signaling pathway and the MAPK pathway. During tissue damage, the JNK signaling cascade reaction can activate MMP9 and participate in the cell’s response to stress. Kim’s [50] research shows that the MMP9 promoter contains the binding site of NF-κB. Under inflammatory conditions, NF-κB can regulate the activity of the MMP9 promoter, enhance the expression of MMP9, and thereby promote the release of inflammatory mediators [51]. The MAPK signaling pathway enhances the transcription of MMP9 through its downstream transcription factors [52]. Under oxidative stress and inflammatory conditions, the JNK pathway increases the expression of MMP9 [53], and inhibiting the JNK signal can have an indirect regulatory effect on MMP9 [54]. Baicalein can inhibit the transcriptional activity of NF-κB by reducing the phosphorylation of p65 [55, 56], regulate the level of phosphorylated JNK by targeting the phosphorylation of JNK [53], and directly inhibit the phosphorylation of MAPK Thereby inhibiting the production of MMP9 [54], reducing the accumulation of ROS and the synthesis of inflammatory factors [57, 58], down-regulating the expression of TNF-α and IL-6 [59, 60], and ultimately alleviating inflammatory damage [61].
ARG1 (Arginase-1) is capable of being discharged from human granulocytes and sustaining extremely high activity within the extracellular environment during the course of inflammatory reactions [62], exerting a potent suppressive influence on the immune system [63]. The high expression of ARG1 may lead to vasodilatory dysfunction at different stages of sepsis [63]. ARG1 is involved in nitrogen metabolism and ROS regulation during oxidative stress in sepsis [64], and its high expression may exacerbate immune dysregulation [65] and vascular dysfunction [56]. As a key factor in inflammatory immune regulation [66], overexpression of ARG1 can promote inflammatory polarization [67]. The expression of ARG1 is regulated by NF-κB signaling and MAPK signaling. The NF-κB signaling indirectly regulates the expression of ARG1 through the interferon γ pathway [57]. MAPK signaling affects the regulation of ARG1 under oxidative stress conditions, and its downstream effector molecules regulate the transcription of ARG1 [47]. In immune cells, MAPK activation promotes the generation of ARG1 and participates in oxidative stress responses [47, 50, 60]. Baicalein regulates the function of immune cells and alleviates oxidative damage by inhibiting the activation of NF-κB and the phosphorylation of MAPK, and down-regulating the expression of ARG1 [50, 68–70].
FYN (Tyrosine-protein kinase Fyn), belonging to the Src family of kinases, is a key player in sepsis [71]. It has been shown to be involved in signal transduction pathways during the development and activation of T lymphocytes and NK cells [72], and it is associated with inflammatory responses and macrophage function [73], its downregulation may lead to immune dysfunction [70]. Studies have shown that Fyn inhibitors from plant extracts inhibit the activation of NF-κB [74]. For instance, discophorol F and manassantin B inhibit the phosphorylation of Fyn and the signal transduction process of the IKK/IκB pathway, thereby suppressing the nuclear translocation of NF-κB p65 and the expression of cytokines [74–76]. Overexpression of Fyn in skeletal muscle in vivo stimulates mTORC1, leading to the activation of JNK, promoting cell invasion [77] and increasing cell death [78]. Knockdown of FYN inhibits JNK/CHOP signaling [79]. Additionally, Fyn is considered an upstream activator of p38 MAPK, suggesting that p38 MAPK may play a role in the expression of pro-inflammatory genes [73]. Baicalein reduces ROS generation by inhibiting FYN kinase activity, intervenes in the FYN-TIM3 interaction, weakens STAT3 phosphorylation, improves mitochondrial function and reduces oxidative damage [59]. It also inhibits the production of inflammatory factors such as TNF-α and IL-6 by blocking the Fyn-STAT3 signal [60].
GSEA enrichment analysis revealed that these candidate genes participate in critical pathways, including the “complement and coagulation cascade,” “antigen processing and presentation,” and “cell adhesion molecules (CAMs).” Notably, the upregulation of the complement and coagulation cascades may contribute to the excessive thromboinflammatory response observed in sepsis [80]. Both the complement and coagulation cascades are pivotal in the progression of sepsis, and modulating these pathways could potentially reduce organ damage and mortality associated with the condition [81]. Previous studies have highlighted the crucial, interconnected roles of these cascades in the host’s innate defense against invading pathogens [82]. CAMs are complex transmembrane proteins that mediate adhesion between cells and the extracellular matrix, initiating mechanical adhesion reactions [83]. These molecules are involved in inflammatory responses, microthrombosis, and endothelial damage by facilitating interactions between leukocytes and endothelial cells [83]. This process significantly impacts coagulation dysfunction. Additionally, antigen processing and presentation are fundamental to adaptive immunity [84]. By modulating Th1 cell differentiation, antigen presentation can reduce inflammation [85]. It is hypothesized that these pathways contribute to sepsis by promoting platelet activation, forming microthrombosis, and releasing large amounts of pro-inflammatory factors, thereby establishing a “vicious cycle of inflammation-coagulation.”
Immune infiltration analysis further suggested that ARG1, MMP9, and FYN may influence immune cell infiltration and immune responses in sepsis, particularly in regulating the function of effector memory CD8+ T cells. Effector memory CD8+ T cells, a subset of memory T cells [86], respond more rapidly and robustly to secondary infections and maintain immune memory [87–89]. Clinical research has shown that sepsis affects the number of memory CD8+ T cells, thereby impairing the immune response to secondary infections [90]. A reduction in CD8+ T cells contributes to immune suppression [65]. The immunosuppressive state induced by high ARG1 expression can interfere with the activation and proliferation of effector memory CD8+ T cells [91]. Additionally, MMP9 may impair the migration and aggregation of these cells to infection sites by altering the extracellular matrix environment. Conversely, the downregulation of FYN weakens its positive regulatory effect on effector memory CD8+ T cells, further compromising immune function [46]. These candidate genes may influence sepsis by modulating effector memory CD8+ T cells, enhancing anti-infection responses, and contributing to the immunopathology of sepsis.
Based on these findings, we hypothesize that baicalein may exert its therapeutic effects in sepsis-induced coagulopathy through a dual mechanism: directly via molecular interactions with MMP9, ARG1, and FYN proteins, as predicted by our docking analysis; and indirectly by modulating the NF-κB, MAPK, and JNK signaling pathways. This combined regulatory mechanism may contribute to the attenuation of inflammation and coagulation dysfunction in sepsis. However, these computational predictions and mechanistic hypotheses require further experimental validation, including protein-binding assays, pathway analysis, and functional studies, to confirm the biological relevance and therapeutic potential of baicalein in the context of sepsis.
RT-qPCR experiments confirmed the differential expression of MMP9, ARG1, and FYN in patients with sepsis. However, gene expression data alone do not fully capture the functional roles of the proteins encoded by these genes in sepsis [92, 93]. Therefore, follow-up studies should expand the sample size and include protein-level verification to further validate the involvement of these genes in sepsis-related coagulation dysfunction.
Additionally, several limitations exist in this study. First, the sample size for RT-qPCR validation was relatively small (n = 5 per group), primarily due to practical constraints in patient recruitment and sample collection, which may affect the statistical power of the results. Second, the study was primarily based on transcriptomic analysis and lacked validation at the protein level, as well as functional assays in cell models and in vivo experiments. Therefore, it remains unclear whether the observed transcriptional changes translate into corresponding alterations in functional protein expression. Moreover, the molecular docking results were derived from computational simulations and do not necessarily reflect the actual pharmacological activity or in vivo efficacy of baicalein in biological systems. Another limitation lies in the lack of detailed clinical phenotypic information in the public databases used, which prevented correlation analyses between candidate gene expression and clinical severity indicators such as disseminated intravascular coagulation (DIC) status or organ failure scores.
To address these limitations, future studies will focus on several key areas. In terms of experimental validation, we plan to expand the RT-qPCR sample size, utilize proteomic techniques to verify the consistency between transcriptomic and proteomic levels, and perform cell-based assays to explore the functional roles of the candidate genes in sepsis. In vivo validation using animal models will also be conducted. Regarding mechanistic exploration, protein-binding assays and cellular functional experiments will be employed to verify the molecular docking results and investigate the interactions between candidate genes and key signaling pathways. From a clinical application perspective, future studies will involve sepsis cohorts with well-annotated clinical phenotype data to explore associations between candidate gene expression and disease severity, conduct subgroup analyses, and develop stratification models for sepsis based on gene expression profiles. These validation efforts will provide more comprehensive evidence to support the clinical translation of our findings.
Conclusions
By employing various bioinformatics approaches, this study identified candidate genes of baicalein for treating sepsis, focusing on pathways involved in coagulation function. This study identified candidate genes associated with sepsis-induced coagulopathy and provided computational evidence for the potential of baicalein to interact with these genes, laying a theoretical foundation for further experimental exploration of its role in treating sepsis-related coagulopathy.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1: Specific screening of genes
Supplementary Table 2: Baicalein effective targets identified through multiple databases
Supplementary Table 3: Sequences of forward and reverse primers for PCR
Supplementary Table 4: Candidate genes identification for baicalein in sepsis
Supplementary Table 5: Consistency of regression coefficients before and after validation
Acknowledgements
None.
Author contributions
All authors had full access to all data in the study and take responsibility for the integrity and accuracy of the data analysis. MW and ZFL contributed to the study concept and design. YXZ and TTY were responsible for data collection. DSZ handled the statistical analysis. LFM was responsible for drafting the manuscript, and LZ provided critical reading and approval of the final version.
Funding
This study was funded by the National Natural Science Foundation of China (82360903), the Guizhou Science and Technology Plan Project (Guizhou Science and Technology Foundation -ZK[2024] General 396), the PhD Start-up Fund (GYZYYFYBS-2023[09]), and the Guizhou Thousand Level Innovative Talent Project.
Data availability
The datasets analyzed in the current study are available in the Gene Expression Omnibus (GEO) database (GPL13667, GPL570) repository, accessible at https://www.ncbi.nlm.nih.gov/geo/.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Guizhou University of Traditional Chinese Medicine (20230014).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lifang Mu and Yuxue Zhang contributed equally to this work.
References
- 1.Liu D, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raeven P, Zipperle J, Drechsler S. Extracellular vesicles as markers and mediators in sepsis. Theranostics. 2018;8(12):3348–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol. 2021;34(2):71–6. [DOI] [PubMed] [Google Scholar]
- 4.Giustozzi M, et al. Coagulopathy and sepsis: pathophysiology, clinical manifestations and treatment. Blood Rev. 2021;50:100864. [DOI] [PubMed] [Google Scholar]
- 5.Desposito L, Bascara C. Review: sepsis guidelines and core measure bundles. Postgrad Med. 2024;136(7):702–11. [DOI] [PubMed] [Google Scholar]
- 6.Evans T. Diagnosis and management of sepsis. Clin Med (Lond). 2018;18(2):146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, et al. [Advance in sepsis-related coagulation disorders and immunity response]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(12):1519–23. [DOI] [PubMed] [Google Scholar]
- 8.Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology. 2020;132(5):1238–45. [DOI] [PubMed] [Google Scholar]
- 9.Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192(5):803–18. [DOI] [PubMed] [Google Scholar]
- 10.Maneta E, et al. Endothelial dysfunction and immunothrombosis in sepsis. Front Immunol. 2023;14:1144229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, et al. Baicalin and Baicalein in modulating tumor microenvironment for cancer treatment: A comprehensive review with future perspectives. Pharmacol Res. 2024;199:107032. [DOI] [PubMed] [Google Scholar]
- 12.Wan Y, et al. Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free Radic Biol Med. 2023;196:108–20. [DOI] [PubMed] [Google Scholar]
- 13.Lai JQ, et al. Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis. Acta Pharmacol Sin. 2024;45(8):1715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, et al. Baicalein mediates protection against Staphylococcus aureus-induced pneumonia by inhibiting the coagulase activity of vWbp. Biochem Pharmacol. 2020;178:114024. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Discovery of anti-stroke active substances in Guhong injection based on multi-phenotypic screening of zebrafish. Biomed Pharmacother. 2022;155:113744. [DOI] [PubMed] [Google Scholar]
- 16.Chen HY, et al. Baicalein attenuates severe polymicrobial sepsis via alleviating immune dysfunction of T lymphocytes and inflammation. Chin J Integr Med. 2022;28(8):711–8. [DOI] [PubMed] [Google Scholar]
- 17.Jin T, et al. Identification and validation of a novel 17 coagulation-related genes signature for predicting prognostic risk in colorectal cancer. Heliyon. 2024;10(12):e32687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, et al. Clinical correlation between coagulation disorders and sepsis in patients with liver failure. Clin Hemorheol Microcirc. 2022;80(3):219–31. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, et al. Potential molecular mechanisms of plantain in the treatment of gout and hyperuricemia based on network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:3023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie ME, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva RAM, Chen ZJ. ggplot2: elegant graphics for data analysis. Volume 17, 2nd ed. Measurement: Interdisciplinary Research and Perspectives; 2019. pp. 160–7. 3. [Google Scholar]
- 22.Gu Z. Complex heatmap visualization. Imeta. 2022;1(3):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, et al. Ferroptosis and Autophagy-Related genes in the pathogenesis of ischemic cardiomyopathy. Front Cardiovasc Med. 2022;9:906753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Cinelli M, et al. Feature selection using a one dimensional Naïve bayes’ classifier increases the accuracy of support vector machine classification of CDR3 repertoires. Bioinformatics. 2017;33(7):951–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alderden J, et al. Predicting pressure injury in critical care patients: A Machine-Learning model. Am J Crit Care. 2018;27(6):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robin X, et al. pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachs MC. PlotROC: A tool for plotting ROC curves. J Stat Softw, 2017. 79. [DOI] [PMC free article] [PubMed]
- 29.Lele SR, Keim JL. Weighted distributions and Estimation of resource selection probability functions. Ecology. 2006;87(12):3021–8. [DOI] [PubMed] [Google Scholar]
- 30.Duo L, et al. Machine learning model to estimate probability of remission in patients with idiopathic membranous nephropathy. Int Immunopharmacol. 2023;125Pt A:p111126. [DOI] [PubMed] [Google Scholar]
- 31.McDonald TL. The point process use-availability or presence-only likelihood and comments on analysis. J Anim Ecol. 2013;82(6):1174–82. [DOI] [PubMed] [Google Scholar]
- 32.Ramsay IS, et al. Model selection and prediction of outcomes in recent onset schizophrenia patients who undergo cognitive training. Schizophr Res Cogn. 2018;11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orifjon S et al. Translation and Adaptation of the Adult Developmental Coordination Disorder/Dyspraxia Checklist (ADC) into Asian Uzbekistan. Sports (Basel). 2023;11(7). [DOI] [PMC free article] [PubMed]
- 34.Wu T, et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov (Camb). 2021;2(3):100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, et al. Novel diagnostic biomarkers related to oxidative stress and macrophage ferroptosis in atherosclerosis. Oxid Med Cell Longev. 2022;2022:p8917947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 38.Mitteer DR, Greer BD. Using GraphPad prism’s heat maps for efficient, Fine-Grained analyses of Single-Case data. Behav Anal Pract. 2022;15(2):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trott O, Olson AJ. AutoDock vina: improving the speed and accuracy of Docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee C, et al. Prevalence, underlying causes, and preventability of Sepsis-Associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2):e187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Liu CF. Sepsis heterogeneity. World J Pediatr. 2023;19(10):919–27. [DOI] [PubMed] [Google Scholar]
- 42.Dogra A. Baicalein: unveiling the multifaceted Marvel of hepatoprotection and beyond. J Asian Nat Prod Res, 2025: pp. 1–13. [DOI] [PubMed]
- 43.Bayram P, et al. Two flavonoids, Baicalein and naringin, are effective as anti-inflammatory and anti-oxidant agents in a rat model of polymicrobial sepsis. Immunopharmacol Immunotoxicol. 2023;45(5):597–606. [DOI] [PubMed] [Google Scholar]
- 44.Brummel NE, et al. Inflammation and coagulation during critical illness and Long-Term cognitive impairment and disability. Am J Respir Crit Care Med. 2021;203(6):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W. Critical roles of S100A12, MMP9, and PRTN3 in sepsis diagnosis: insights from multiple microarray data analyses. Comput Biol Med. 2024;171:108222. [DOI] [PubMed] [Google Scholar]
- 46.Liang J, et al. Analysis of sepsis markers and pathogenesis based on gene differential expression and protein interaction network. J Healthc Eng. 2022;2022:p6878495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng M, et al. Exploring the anti-inflammatory and immune regulatory effects of Taohe Chengqi Decoction in sepsis-induced lung injury. J Ethnopharmacol. 2024;333:118404. [DOI] [PubMed] [Google Scholar]
- 48.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol. 2005;78(1):279–88. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Q, et al. Microenvironment-responsive coating for vascular stents to regulate coagulation-inflammation interaction and promote vascular recovery. Bioact Mater. 2025;48:443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HS, et al. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp Mol Med. 2007;39(1):106–13. [DOI] [PubMed] [Google Scholar]
- 51.Mao Y, et al. PI3K/AKT/mTORC1 signalling pathway regulates MMP9 gene activation via transcription factor NF-κB in mammary epithelial cells of dairy cows. Anim Biotechnol. 2024;35(1):2314100. [DOI] [PubMed] [Google Scholar]
- 52.Li W, et al. Tumor necrosis factor stimulates matrix metalloproteinase 9 secretion from cultured human chorionic trophoblast cells through TNF receptor 1 signaling to IKBKB-NFKB and MAPK1/3 pathway. Biol Reprod. 2010;83(3):481–7. [DOI] [PubMed] [Google Scholar]
- 53.Bai X, et al. Baicalin suppresses interleukin-1β-induced apoptosis, inflammatory response, oxidative stress, and extracellular matrix degradation in human nucleus pulposus cells. Immunopharmacol Immunotoxicol. 2023;45(4):433–42. [DOI] [PubMed] [Google Scholar]
- 54.Zong B et al. Baicalin weakens the Porcine ExPEC-Induced inflammatory response in 3D4/21 cells by inhibiting the expression of NF-κB/MAPK signaling pathways and reducing NLRP3 inflammasome activation. Microorganisms, 2023. 11(8). [DOI] [PMC free article] [PubMed]
- 55.Liu Y, et al. Investigation of the mechanism of Baicalein in the treatment of periodontitis based on network pharmacology, molecular Docking and experimental validation. BMC Oral Health. 2024;24(1):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Y, et al. Exploring the therapeutic potential and in vitro validation of Baicalin for the treatment of triple-negative breast cancer. Front Pharmacol. 2025;16:1530056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chi F, et al. Baicalein disrupts the KEAP1-NRF2 interaction to alleviate oxidative stress injury by inhibiting M1 macrophage polarization. Free Radic Biol Med. 2025;227:557–69. [DOI] [PubMed] [Google Scholar]
- 58.Kuwar OK, Kalia N. Anti-inflammatory and antioxidant effects of baicalein: targeting Nrf2, and nfĸb in neurodegenerative disease. Inflammopharmacology. 2025;33(3):1303–10. [DOI] [PubMed] [Google Scholar]
- 59.Feng J, et al. Preliminary investigation on the mechanism of anti-periodontitis effect of scutellariae radix based on bioinformatics analysis and in vitro verification. Heliyon. 2024;10(16):e35744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, et al. Targeting inflammation-associated AMPK//Mfn-2/MAPKs signaling pathways by Baicalein exerts anti-atherosclerotic action. Phytother Res. 2021;35(8):4442–55. [DOI] [PubMed] [Google Scholar]
- 61.Ren M, et al. Baicalein inhibits inflammatory response and promotes osteogenic activity in periodontal ligament cells challenged with lipopolysaccharides. BMC Complement Med Ther. 2021;21(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang JX, et al. ARG1 as a promising biomarker for sepsis diagnosis and prognosis: evidence from WGCNA and PPI network. Hereditas. 2022;159(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munder M, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108(5):1627–34. [DOI] [PubMed] [Google Scholar]
- 64.Zhu R, et al. Wogonoside alleviates microglia-mediated neuroinflammation via TLR4/MyD88/NF-κB signaling axis after spinal cord injury. Eur J Pharmacol. 2024;973:176566. [DOI] [PubMed] [Google Scholar]
- 65.Shigematsu Y, et al. Decreased ARG1 expression as an adverse prognostic phenotype in non-alcoholic non-virus-related hepatocellular carcinoma. Virchows Arch. 2022;481(2):253–63. [DOI] [PubMed] [Google Scholar]
- 66.Hu S, et al. Disrupted eNOS activity and expression account for vasodilator dysfunction in different stage of sepsis. Life Sci. 2021;264:118606. [DOI] [PubMed] [Google Scholar]
- 67.Shao X, et al. Low-dose decitabine promotes M2 macrophage polarization in patients with primary immune thrombocytopenia via enhancing KLF4 binding to PPARγ promoter. Clin Transl Med. 2023;13(7):e1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gan YL, et al. FKBP51 is involved in LPS-induced microglial activation via NF-κB signaling to mediate neuroinflammation. Life Sci. 2024;351:122867. [DOI] [PubMed] [Google Scholar]
- 69.Li D, et al. Panax Notoginseng saponins regulate the polarization of microglia by inhibiting the hematopoietic progenitor kinase 1 signaling pathway. NeuroReport. 2025;36(13):737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganguly R, Gupta A, Pandey AK. Role of Baicalin as a potential therapeutic agent in hepatobiliary and Gastrointestinal disorders: A review. World J Gastroenterol. 2022;28(26):3047–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge J, et al. Identification of key biomarkers and therapeutic targets in sepsis through coagulation-related gene expression and immune pathway analysis. Front Immunol. 2024;15:1470842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. [DOI] [PubMed] [Google Scholar]
- 73.Mok HL, et al. Modified Zhenwu Decoction suppresses chronic colitis via targeting macrophage CCR2/Fyn/p38 MAPK signaling axis. Phytomedicine. 2024;129:155694. [DOI] [PubMed] [Google Scholar]
- 74.Jeong H et al. Caffeoyl-Prolyl-Histidine Amide Inhibits Fyn and Alleviates Atopic Dermatitis-Like Phenotypes via Suppression of NF-κB Activation. Int J Mol Sci. 2020;21(19). [DOI] [PMC free article] [PubMed]
- 75.Lu Y, et al. Saucerneol F inhibits tumor necrosis factor-α and IL-6 production by suppressing Fyn-mediated pathways in FcεRI-mediated mast cells. Food Chem Toxicol. 2013;59:696–702. [DOI] [PubMed] [Google Scholar]
- 76.Lu Y, et al. Manassantin B isolated from Saururus chinensis inhibits cyclooxygenase-2-dependent prostaglandin D2 generation by blocking Fyn-mediated nuclear factor-kappaB and mitogen activated protein kinase pathways in bone marrow derived-mast cells. Biol Pharm Bull. 2013;36(8):1370–4. [DOI] [PubMed] [Google Scholar]
- 77.Liu M, et al. Highly expressed FYN promotes the progression of placenta accreta by activating STAT3, p38, and JNK signaling pathways. Acta Histochem. 2023;125(1):151991. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, et al. Fyn activation of mTORC1 stimulates the IRE1α-JNK pathway, leading to cell death. J Biol Chem. 2015;290(41):24772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dorotea D, et al. Pan-Src kinase inhibitor treatment attenuates diabetic kidney injury via Inhibition of Fyn kinase-mediated Endoplasmic reticulum stress. Exp Mol Med. 2022;54(8):1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei X, et al. Unraveling the intricate web: complement activation shapes the pathogenesis of Sepsis-Induced coagulopathy. J Innate Immun. 2024;16(1):337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang H et al. The dysfunction of complement and coagulation in diseases: the implications for the therapeutic interventions. MedComm (2020). 2024;5(11):e785. [DOI] [PMC free article] [PubMed]
- 82.Shimizu J, et al. Extracellular CIRP promotes Kupffer cell inflammatory polarization in sepsis. Front Immunol. 2024;15:1411930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stevens AJ, et al. Programming multicellular assembly with synthetic cell adhesion molecules. Nature. 2023;614(7946):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22(12):751–64. [DOI] [PubMed] [Google Scholar]
- 85.Ming S, et al. Immunoglobulin-Like transcript 5 inhibits Macrophage-Mediated bacterial killing and antigen presentation during sepsis. J Infect Dis. 2019;220(10):1688–99. [DOI] [PubMed] [Google Scholar]
- 86.Han J, et al. Memory CD8(+) T cell responses to cancer. Semin Immunol. 2020;49:101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turner SJ, Bennett TJ, La Gruta NL. CD8(+) T-Cell memory: the why, the when, and the how. Cold Spring Harb Perspect Biol, 2021. 13(5). [DOI] [PMC free article] [PubMed]
- 88.Zhang H, et al. Dysfunction of S100A4(+) effector memory CD8(+) T cells aggravates asthma. Eur J Immunol. 2022;52(6):978–93. [DOI] [PubMed] [Google Scholar]
- 89.Wong YC, et al. Sustained viremia suppression by SHIVSF162P3CN-recalled effector-memory CD8 + T cells after PD1-based vaccination. PLoS Pathog. 2021;17(6):e1009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danahy DB, et al. Clinical and experimental sepsis impairs CD8 T-Cell-Mediated immunity. Crit Rev Immunol. 2016;36(1):57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huangfu L, et al. Fraxetin inhibits IKKβ, blocks NF-κB pathway and NLRP3 inflammasome activation, and alleviates spleen injury in sepsis. Chem Biol Interact. 2025;408:111406. [DOI] [PubMed] [Google Scholar]
- 92.Amirshahrokhi K, Imani M. Edaravone reduces brain injury in hepatic encephalopathy by upregulation of Nrf2/HO-1 and Inhibition of NF-κB, iNOS/NO and inflammatory cytokines. Mol Biol Rep. 2025;52(1):222. [DOI] [PubMed] [Google Scholar]
- 93.Jiang T, et al. Dual role of Baimao-Longdan-Congrong-Fang in inhibiting Staphylococcus aureus virulence factors and regulating TNF-α/TNFR1/NF-κB/MMP9 axis. Phytomedicine. 2025;139:156477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Specific screening of genes
Supplementary Table 2: Baicalein effective targets identified through multiple databases
Supplementary Table 3: Sequences of forward and reverse primers for PCR
Supplementary Table 4: Candidate genes identification for baicalein in sepsis
Supplementary Table 5: Consistency of regression coefficients before and after validation
Data Availability Statement
The datasets analyzed in the current study are available in the Gene Expression Omnibus (GEO) database (GPL13667, GPL570) repository, accessible at https://www.ncbi.nlm.nih.gov/geo/.