Evidence of biogenic activity on Mars has profound scientific implications for our understanding of the origin of life on Earth and the presence and diversity of life within the Cosmos. Analysis of the Martian meteorite Allan Hills 84001 (ALH84001) revealed several lines of evidence that has led some investigators to suggest that microbial life existed on Mars approximately 4 billion years ago (45). One of the strongest lines of evidence is the presence of tens-of-nanometer-size magnetite (Fe3O4) crystals found within carbonate globules and their associated rims in the meteorite (57, 58). Approximately one-quarter of these magnetites have remarkable morphological and chemical similarities to magnetite particles produced by magnetotactic bacteria, which occur in aquatic habitats on Earth. Moreover, these types of magnetite particles are not known or expected to be produced by abiotic means either through geological processes or synthetically in the laboratory. We have therefore argued that these Martian magnetite crystals are in fact magnetofossils (57, 58). If this is true, such magnetofossils would constitute evidence of the oldest life forms known. In this respect, we note there is now considerable uncertainty concerning when the earliest terrestrial life forms existed. Until recently, results from the ∼3.5-billion-year-old Apex cherts of the Warrawoona group in western Australia held this record (52), although this work is now in question (12).
The remaining three-quarters of the ALH84001 magnetites are likely products of processes including, but not limited to, precipitation from a hydrothermal fluid, thermal decomposition of the carbonate matrix in which they are embedded, and/or extracellular formation by dissimilatory Fe-reducing bacteria. No single process, either biogenic or inorganic, can explain the full distribution of magnetite crystal morphologies observed in the ALH84001 carbonates. Unlike most meteorites which experienced only one brief period of aqueous and thermal activity during their formation in the early Solar System, Martian meteorite ALH84001 represents part of a dynamic and evolving planetary surface. From its formation approximately 4.5 billion years ago (49; L. E. Nyquist, B. M. Bansal, H. Wiesmann, and C. Y. Shih, Abstr. Pap. Submit. Lunar Planet. Sci. Conf. 26:1065-1066, 1995) to its landing in Antarctica ∼13,000 years ago (35), it experienced multiple shock events (59) and at least one, if not more, episodes of aqueous alteration (51, 61). We propose that the magnetite crystals in ALH84001 can be best explained as the products of biogenic and inorganic processes that operated on early Mars.
The report of McKay et al. (45) and subsequent papers on ALH84001 magnetite (57, 58) have not been without controversy. The identification of a population of ALH84001 magnetite identical to magnetite produced by terrestrial magnetotactic bacteria is not in contention. Rather, the debate and surrounding controversy is over whether this “biogenic” magnetite is an exclusive product of biology. To date, the most credible alternative inorganic hypothesis is centered on whether partial thermal decomposition of the ALH84001 carbonates could produce a population of biogenic-like magnetite (27, 28; D. C. Golden, D. W. Ming, H. V. Lauer, Jr., C. S. Schwandt, R. A. Morris, G. E. Lofgren, and G. A. McKay, Lunar Planet. Sci. Conf. 33, CD-ROM no. 1839, 2002). However, there are serious inconsistencies with this hypothesis. First, a preliminary report suggesting the inorganic formation of biogenic-like magnetite (D. C. Golden et al., Lunar Planet. Sci. Conf. 33, CD-ROM no. 1839, 2002) has been invalidated. Second, decomposition of a solid solution [(Fex,Mg1-x)CO3] carbonate, as observed in ALH84001, forms a mixed Fe-Mg spinel (2, 15, 23) and not pure magnetite, as observed for the biogenic fraction of the ALH84001 crystals (57, 58). Therefore, although controversial, the biogenic interpretation for one-quarter of the ALH84001 magnetites remains the most plausible hypothesis yet advanced. Additionally, such a biogenic interpretation is consistent with the broader scenario of how the carbonates themselves formed (45, 51, 60, 61).
BRIEF HISTORY OF MARTIAN METEORITE ALH84001
On 27 December 1984, during a snowmobile ride on the far western Allan Hills icefield, located at the eastern terminus of the Transantarctic mountain range (76°54′S, 157°01′E), a National Science Foundation team discovered the ALH84001meteorite (http://curator.jsc.nasa.gov/curator/antmet/mmc/mmc.htm). The field notes documenting the find describe it as a “highly-shocked, grayish-green, achondrite, 90% covered with fusion crust (with the additional comment ‘Yowza-Yowza’)” (http://curator.jsc.nasa.gov/curator/antmet/mmc/mmc.htm). Since this meteorite was visually the most unusual rock collected during the 1984-1985 field season, it was the first to be processed and was classified (49, 54) incorrectly as a rare achondritic meteorite known as a diogenite. It took another 9 years before it was correctly identified as a Martian meteorite (47, 53) based on oxygen isotope analysis (18).
ALH84001 is the oldest of the 18 age-dated samples (49) in our current inventory of 26 identified Martian meteorites (http://curator.jsc.nasa.gov/curator/antmet/mmc/mmc.htm). It has a radiogenic Rb-Sr crystallization age of ∼4.5 billion years (49; Nyquist et al., Abstr. Pap. Submit. Lunar Planet. Sci. Conf. 26:1065-1066, 1995), indicating that it formed shortly after the planet Mars itself was formed. Note that the crust of Earth, unlike Mars, is continually being modified, either being created or destroyed. As a result, there are no rocks on Earth that have an age comparable to the age of ALH84001. The oldest known terrestrial samples are zirconium grains with U-Pb ages of ∼4.4 billion years (62). The oldest known terrestrial rocks are in the Acasta Gneiss Complex in North America, dated at ∼4.03 billion years old (56), and the Isua Supercrustal rocks in west Greenland, dated at ∼3.7 to 3.8 billion years old (1). ALH84001 was subsequently ejected into interplanetary space from the Martian surface ∼16 million years ago (30), presumably as a consequence of a collision of an asteroid or comet with Mars (46). About 13,000 years ago it was captured by the Earth's gravity field and fell as a meteorite in Antarctica (35).
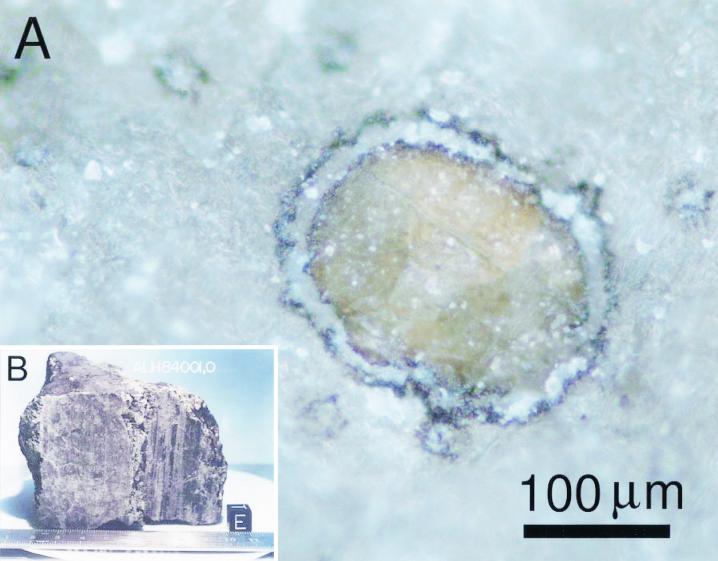
With a mass of 1.94 kg, ALH84001 is primarily a volcanic, silicate-rich rock composed mainly of orthopyroxene [(Mg, Fe)SiO3] with minor phases of chromite (FeCr2O4), olivine [(Mg, Fe)SiO4], pyrite (FeS2), apatite [Ca3(PO4)2], and Si-rich glass (47). Secondary carbonate mineral assemblages, formed at temperatures compatible with terrestrial microbes (51, 60, 61), range in size from ∼1 to 500 μm and are found within small cracks and fissures in the meteorite (45, 47). They comprise ∼1% (vol/vol) of the rock (47) and have radiogenic Rb-Sr ages of ∼3.90 ± 0.04 billion years (11), indicating that they formed while the rock was still on Mars. The δ13C isotopic composition (∼40‰ relative to Pee Dee Belemnite) for the carbonates further substantiates the Martian origin of these secondary minerals (51). The carbonate globules consist of an optically golden core concentrically zoned in Ca, Mn, Fe, and Mg carbonate (45, 51, 60, 61) in which nanometer-size magnetites (Fe3O4) are evenly distributed as a minor phase (45, 57, 58). Surrounding the core is an inner rim and an outer rim composed mainly of tens-of-nanometer-size magnetite crystals embedded in a Mg-rich Fe carbonate matrix (45, 57, 58), which are separated by a band of nearly pure Mg carbonate (45). Optically, this appears as a layered black-white-black rim (Fig. 1). Since the carbonate globules are Martian, then by association the magnetites are also of Martian origin. In support of this we note that even though the Antarctic environment is oxidizing, little or no conversion of the magnetites to maghemite and hematite has occurred. Magnetite is a mixture of Fe and O, with one-third of the Fe atoms occurring as Fe2+ and two-thirds of the atoms occurring as Fe3+. The near-surface environment on the ice fields of Antarctica is oxidizing, and nearly all Antarctic meteorites show evidence of rust on their outer surfaces (29). Nanometer-size magnetite crystals exposed to atmospheric levels of oxygen rapidly oxidize to maghemite (γ-Fe2O3;Fe3+) or hematite (α-Fe2O3;Fe3+) (48). The fact that little or no conversion of the magnetites to maghemite and hematite has occurred in ALH84001 suggests that during the meteorite's residence time in Antarctica, there was little terrestrial alteration of the carbonate assemblages.
FIG. 1.
(A) Optical image of a freshly fractured surface of a chip from the ALH84001 meteorite, showing the gray Si-rich orthopyroxene matrix with embedded carbonate globules. The large carbonate globule in the center has an ellipsoidal shape (∼150 μm along the major axis) with a golden central core of Fe-Ca-Mg-Mn carbonate surrounded by black (magnetite-rich), white (Mg carbonate), and black (magnetite-rich) rims. (B) View of Martian meteorite ALH84001 after curatorial processing at NASA's Johnson Space Center, showing a freshly cut interior surface. Note the vertical crushed zone running through the center of the image containing numerous cracks and fissures in which carbonate globules are found.
MAGNETITE AS A UNIVERSAL BIOSIGNATURE: THE MAGNETITE ASSAY FOR BIOGENICITY
Magnetite is a common mineral on Earth and is formed by a range of inorganic processes, including hydrothermal or volcanic precipitation from an Fe-rich fluid (57 and references therein). With the discovery by Blakemore in 1975 (10) that single-domain magnetite crystals are utilized by procaryotic organisms called magnetotactic bacteria came the realization that magnetite might be used as a potential biosignature. Here we define a biosignature to be a physical and/or chemical marker of life that does not occur through random, stochastic interactions or through directed human intervention.
Magnetotactic bacteria are now known to be ubiquitous in terrestrial aquatic environments, and they appear to utilize the magnetic properties of magnetite (5, 6, 24) in conjunction with the Earth's global geomagnetic field as an orientation mechanism (10, 22). This is a process known as magnetotaxis, and when it is coupled with flagellar motility and aerotaxis, it allows an organism to locate and maintain an optimal position in vertical chemical gradients within aquatic environments by reducing a three-dimensional search problem to a one-dimensional search problem (25, 37).
Now we consider a specific strain of a magnetotactic bacterium, designated strain MV-1. Cells of this marine strain are gram negative, have vibrioid to helicoid cell morphology, and generally produce a single intracellular chain of ∼12 well-ordered magnetite crystals, each encapsulated within a coating or membrane (4). The chain of magnetite crystals acts much like a compass needle (5), allowing passive alignment of the bacterium along the Earth's geomagnetic field lines. Magnetotaxis is the term used to describe the passive alignment and active motility of a bacterial cell along magnetic field lines. All magnetotactic bacteria, including strain MV-1, produce only one morphological type of magnetite crystals, all of which fall in a narrow size distribution range from ∼30 to 120 nm long (5, 6). Particles in this size range are single magnetic domains (5). The cell appears to exert strict physical and chemical control over the biomineralization process(es) involved in magnetite synthesis (5, 6).
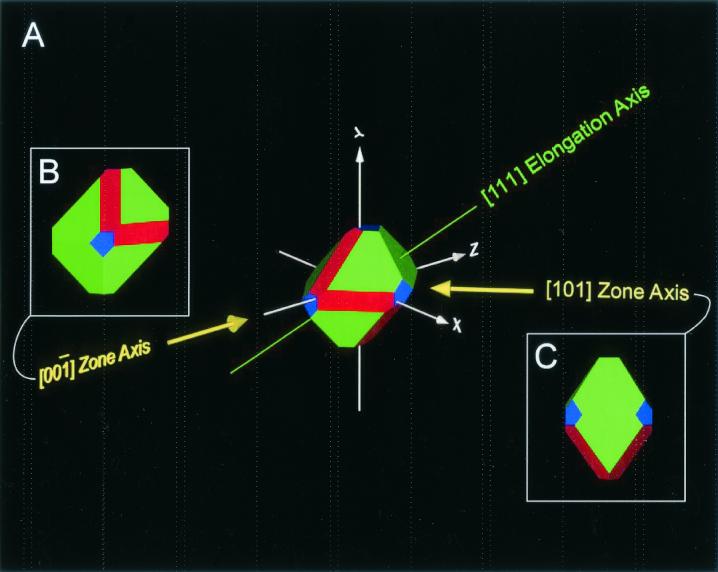
The intracellular magnetite crystals produced by strain MV-1 display six distinctive properties that allow them to be distinguished from any known population of inorganically produced magnetites (for a complete discussion of these properties see reference 57). These six properties are: (i) narrow size range (a non-log-normal size distribution centered in the single-magnetic-domain size range); (ii) restricted width-to-length ratios (optimized for the largest overlap in the single-domain region of a Butler-Banerjee diagram); (iii) chemical purity (essentially stoichiometrically pure Fe3O4); (iv) few crystallographic defects (defect free with the exception of occasional twinning perpendicular to the [111] axis of elongation; (v) crystal morphology (Fig. 2) with unusual truncated hexa-octahedral geometry consisting of a combination of the three crystallographic forms for the 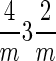 point group, the cube {100}, the octrahedron {111}, and the rhombic dodecahedron {110}, with only 6 of the 12 possible {110} faces being expressed, namely, those that satisfy the relationship {110} · [111]☰0, with elongation defined to be along the [111]↔[
point group, the cube {100}, the octrahedron {111}, and the rhombic dodecahedron {110}, with only 6 of the 12 possible {110} faces being expressed, namely, those that satisfy the relationship {110} · [111]☰0, with elongation defined to be along the [111]↔[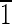
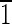
 ] axis [i.e., (1
] axis [i.e., (1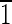 0),(
0),(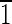 10),(10
10),(10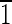 ),(
),(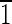 01),(01
01),(01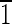 ),(0
),(0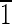 1)] (note that the term truncated hexa-octahedron should not be confused with hexoctahedron, which is another crystallographic form in the isometric system with 48 equivalent faces in the
1)] (note that the term truncated hexa-octahedron should not be confused with hexoctahedron, which is another crystallographic form in the isometric system with 48 equivalent faces in the 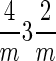 point group denoted by the symbol {321}); and (vi) elongation along only one of the possible four threefold rotation axes of a regular octahedron.
point group denoted by the symbol {321}); and (vi) elongation along only one of the possible four threefold rotation axes of a regular octahedron.
FIG. 2.
(A) Idealized schematic representation of a magnetite crystal displaying a truncated hexa-octahedral geometry with eight {111} octahedral (green) faces, six {110} dodecahedral (red) faces, and six {100} cubic (blue) faces. This is the most commonly observed geometry of magnetite crystals isolated from magnetotactic bacterial strain MV-1. One-quarter of the magnetite crystals in Martian meteorite ALH84001 carbonates also have this geometry. The principal Cartesian axes (x, y, and z) are indicated in crystallographic nomenclature which corresponds to the [100],[010],[001] zone axes. The crystal is elongated along the [111]↔[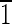
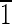
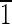 ] axis, as shown by the green vector transecting the crystal. Note that because of the symmetry of the truncated hexa-octahedral geometry, all expressed {110} faces, shown in red, are equivalent [i.e., (1
] axis, as shown by the green vector transecting the crystal. Note that because of the symmetry of the truncated hexa-octahedral geometry, all expressed {110} faces, shown in red, are equivalent [i.e., (1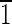 0),(
0),(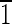 10),(10
10),(10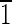 ),(
),(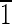 01),(01
01),(01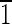 ),(0
),(0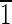 1)]. (B) Crystal viewed in the [00
1)]. (B) Crystal viewed in the [00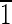 ] direction. Note that the truncated hexa-octahedral geometry displays six equivalent {100} faces, shown in blue [(100),(010),(001),(
] direction. Note that the truncated hexa-octahedral geometry displays six equivalent {100} faces, shown in blue [(100),(010),(001),(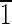 00),(0
00),(0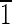 0),(00
0),(00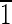 )]. (C) Crystal viewed down the [101] axis. The truncated hexa-octahedron representation has a characteristic diamond-shaped outline. This orientation displays the width of two parallel {100} and two parallel {110} faces.
)]. (C) Crystal viewed down the [101] axis. The truncated hexa-octahedron representation has a characteristic diamond-shaped outline. This orientation displays the width of two parallel {100} and two parallel {110} faces.
These six properties are referred to below as the magnetite assay for biogenicity (MAB). A seventh property, the presence of magnetite crystals aligned in chains, could have been included in the MAB criteria. However, it is rare for magnetite chains to remain intact after the death of the host organism. This is because a magnetite chain exists in a state of high magnetostatic potential energy and tends to spontaneously collapse into a clump, releasing the energy unless it is actively maintained by the host organism. In some instances, fortuitous fossilization can preserve a small fraction of magnetite chains, and such chains in ALH84001 carbonate have been reported by Friedmann et al. (26). Properties i, ii, v, and vi are related to the external physical morphology of the magnetite crystal, while property iv refers to the internal crystal structure and property iii is related to the chemical composition. Although these properties are interrelated, each can be expressed independent of the others. Perhaps the most unusual property and the most difficult to ascertain is the truncated hexa-octahedral morphology illustrated in Fig. 2. This morphology is remarkable in that only one-half of the faces comprising the {110} dodechedral crystallographic form are exhibited. While in general many crystals may not display all faces in a given crystallographic form, it is very unlikely that through a random process exactly one-half of a form would be missing and that all of the faces that are exhibited would be the same size.
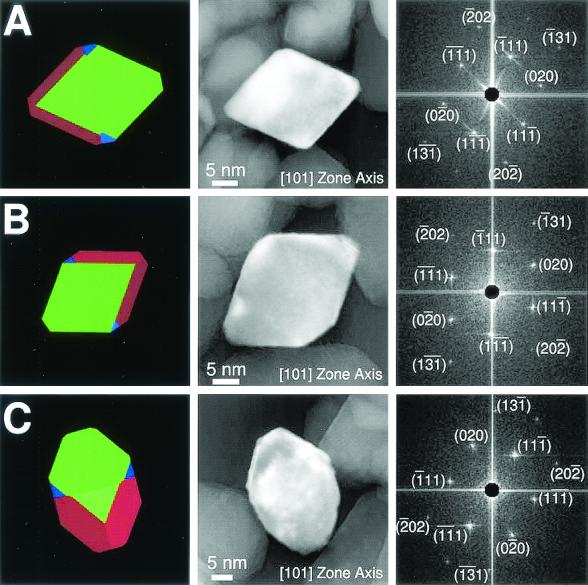
Classical transmission electron microscopy imaging of strain MV-1 magnetite yielded the two-dimensional projection of the crystal parallel to the imaging plane (Fig. 3). In order to determine whether a given crystal has a truncated hexa-octahedral morphology, it is necessary to view the crystal along one of the principal crystallographic axes of magnetite to establish unambiguously the spatial orientation of the crystal relative to the imaging plane. The two-dimensional projection can then be compared to the projection which would be expected for a truncated hexa-octahedral geometry viewed in an identical orientation. Clearly, no single two-dimensional projection is uniquely characteristic of a three-dimensional morphology, and so multiple projections of the same crystal under rotation are necessary for unambiguous identification of the underlying three-dimensional morphology (20). However, some projections are more diagnostic of a given geometry than others; for a truncated hexa-octahedron such projections occur when the crystal is viewed down the [101], [100], and [111] zone axes. In Fig. 3, three MV-1 magnetites are all shown along the [101] zone axis and have projections consistent with an appropriately oriented truncated hexa-octahedron, as indicated. To date, no inorganic magnetite population produced inorganically, either naturally or synthetically, has simultaneously displayed all six MAB properties.
FIG. 3.
Truncated hexa-octahedron representation, transmission electron micrograph, and fast Fourier transform for each of three magnetite crystals (A to C) extracted from magnetotactic bacterial strain MV-1. All of the crystals are viewed down the [101] zone axis and have a diamond-shaped outline. Variations in appearance are due to differences in the sizes of the displayed {110} (red) and {100} (blue) faces. The widths of the {110} faces range from ∼3 to 10 nm, and the widths of the {100} range from ∼3 to 8 nm. The {100} faces correspond to the {200} crystallographic lattice planes, and the {110} faces correspond to the {220} crystallographic lattice planes, as shown in the micrographs. (A) Magnetite that is ∼30 nm long. The (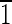 01),(10
01),(10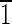 ),(0
),(0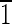 0),(010) faces are ∼3 nm wide. (B) Magnetite that is ∼35 nm long. This crystal is slightly asymmetric, having an upper (
0),(010) faces are ∼3 nm wide. (B) Magnetite that is ∼35 nm long. This crystal is slightly asymmetric, having an upper (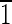 01) face that is ∼5 nm wide and a lower (10
01) face that is ∼5 nm wide and a lower (10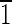 ) face that is ∼8 nm wide. The (0
) face that is ∼8 nm wide. The (0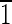 0) and (010) faces are ∼5 nm wide. (C) Magnetite that is ∼30 nm long with (
0) and (010) faces are ∼5 nm wide. (C) Magnetite that is ∼30 nm long with (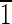 01) and (10
01) and (10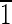 ) faces that are ∼10 nm wide and (010) and (0
) faces that are ∼10 nm wide and (010) and (0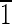 0) faces that are ∼6 nm wide.
0) faces that are ∼6 nm wide.
The influence of the Earth's magnetic field on the outcome of a chemical reaction is infinitesimal (i.e., μB · BEarth ≪ k · T)]; the magnetic field interaction [μB · BEarth = ∼5 × 10−28 J] is considerably less than the thermal energy [k · T = ∼4 × 10−21 J] at the standard temperature). Thus, it is impossible for a magnetic field to influence the outcome of a chemical reaction. Furthermore, statistically, simultaneous observation of all six properties as a result of random-chance inorganic chemical reactions asymptotically approaches zero. Since “the utility of any biomarker is inversely proportional to the ease of making it inorganically, the harder it is to make inorganically, the better the bio[signature]” (A. Knoll, personal communication). Hence, the MAB criteria constitute a robust biosignature. The term robust is used here to imply the exclusivity of the MAB, in that it even discounts many demonstrably intracellularly produced biogenic magnetites. For example, on average, ∼30% of magnetites from strain MV-1 fail to meet the MAB criteria and so would not be considered biogenic. The advantage of such an exclusive biosignature is that any magnetite population meeting the MAB criteria is almost certainly a biogenic precipitate. To reiteriate, no inorganic magnetite population has met the MAB criteria. Although considerable effort has been expended unsuccessfully to find or produce such a population of inorganic magnetites (27, 28; Golden et al., Lunar Planet. Sci. Conf. 33, CD-ROM no. 1839, 2002), the validity of the MAB criteria remains intact. Since planetary magnetic fields and magnetite crystals are not purely the provenance of Earth, the MAB biosignature is as applicable to extraterrestrial samples as it is to terrestrial samples.
MAGNETITE CRYSTALS IN ALH84001 CARBONATES
Nanometer-size magnetite crystals present in ALH84001 carbonates have been extensively studied, both in situ and in extracts, by high-resolution transmission electron microscopy (57, 58). Chemically and physically diverse magnetites have been observed; these magnetites range in size from ∼10 to 500 nm, have aspect (width/length) ratios of ∼0.1 to 1.0, exhibit poorly defined to well-faceted geometries, and vary from chemically impure to pure with and without defects.
Since MAB criteria have been established, they can be applied to the ALH84001 magnetite population. Approximately 75% of the ALH84001 magnetites can be immediately discounted as biogenic precipitates because they either (i) are irregular shapes having two-dimensional projections that can be described as semicircular, convex irregular, concave irregular, or teardrop shaped; (ii) have a whisker-platelet shape; or (iii) have a regular geometry consistent with inorganic magnetite, such as octahedral or cubo-octahedral. In general, these particles are not consistently chemically pure, and some contain minor amounts of Al and/or Cr (57). While the origin of these particles has been ascribed to processes such as in situ thermal or shock decomposition of Fe carbonate (27, 28; Golden et al., Lunar Planet. Sci. Conf. 33, CD-ROM no. 1839, 2002), it is not clear that these processes occurred within the carbonates or could be responsible for the origin of the majority of the ALH84001 magnetites. The heat necessary to decompose pure Fe carbonate and form magnetite is greater than the experimentally measured blocking temperature of the carbonate and so would require homogenization of all magnetic dipoles. However, considerable magnetic inhomogeneity is still present within the ALH84001 carbonates (38). Oxygen isotope fractionation of the ALH84001 carbonates does not support the thermal history required for carbonate decomposition (61). Overall, decomposition of carbonate to magnetite is an exothermic process, so that once decomposition begins, the reaction is self-sustaining and it would be difficult to achieve only partial conversion. In ALH84001 carbonate, magnetite is distributed throughout the carbonate with widely different abundances and with a fine spatial heterogeneity inconsistent with thermal decomposition (57). A considerable decrease in volume occurs during the conversion of Fe-bearing carbonate (ρ = 3.96 g/cm3) to magnetite (ρ = 5.15 g/cm3), and while some magnetites appear to be associated with void space, the majority are not. Impure ALH84001 magnetites containing minor to trace amounts of Al and/or Cr are dispersed among chemically pure magnetites (57). Since neither Al nor Cr can be incorporated into the carbonate structure, these magnetites must predate the surrounding carbonate. Finally, chemically pure magnetites are found embedded in a Fe-Mg carbonate, yet decomposition of such a carbonate produces only Mg-Fe spinels, not pure magnetite (2, 15, 16, 23). This is an unavoidable consequence of combinatorial entropy (31).
A more tenable hypothesis for the origin of the particles involves the allochthonous accumulation of magnetites during the growth of the carbonate from hydrothermal fluids. Another hypothesis is that the magnetites were formed by dissimilatory Fe-reducing organisms which facilitate mineral formation by creating external chemical environments suitable for extracellular magnetite precipitation (41). This is consistent with the emerging perception that extensive reservoirs of liquid water existed on early Mars (13, 14, 42, 43) and that active volcanism has persisted on the planet to at least the last few hundred million years (50).
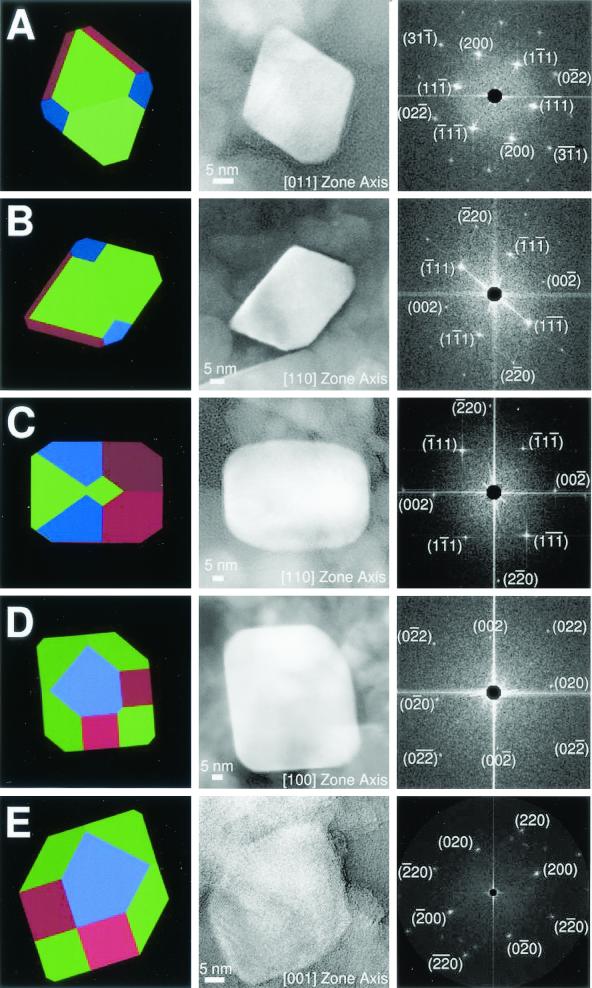
The remaining ∼25% of the ALH84001 magnetites appear to meet all the MAB requirements described above. Figure 4 shows five chemically pure, defect-free ALH84001 magnetite crystals in the single-domain size range with elongation along the [111] axis having projections consistent with an appropriately oriented truncated hexa-octahedron. In a terrestrial context, the presence of this population of magnetite crystals would be interpreted as a biosignature indicative of the presence of past metabolic activity by magnetotactic bacteria. In ALH84001 carbonates, we consider these magnetites to be biosignatures of Martian magnetotactic bacteria. The only alternative explanation is that they are the products of an unknown inorganic process for which no analog exists on the Earth and which has yet to be recreated in the laboratory. The best inorganic magnetites meet only three of the criteria, despite the fact that the entire ferrite industry (which is an approximately $35-billion-per-year business) has worked to perfect magnetic recording technology for 50 years. The fact that only one-quarter of the ALH84001 magnetites meet the MAB requirements for a biosignature is not surprising for the following reasons: (i) terrestrial magnetofossils are typically found in environments where inorganic magnetites are abundant, resulting in an intimate mixture of biogenic and inorganic magnetites (see reference 57 and references therein); (ii) the vast majority of information on ALH84001 magnetites has come from extracts obtained by acid dissolution of the carbonate, which destroys the distribution and spatial relationship between magnetites, resulting in a homogenized sample; (iii) other strains of magnetotactic bacteria or dissimilatory Fe-reducing organisms on the Earth do not necessarily produce magnetite crystals like MV-1 that are easily distinguished from inorganically produced magnetite; and (iv) even in strain MV-1 only ∼70% of the magnetites fulfill the MAB criteria. In fact, perhaps the most surprising observation about the ALH84001 magnetites is that as many as 25% meet the MAB criteria of biogenicity.
FIG. 4.
Truncated hexa-octahedron representation, transmission electron micrograph, and fast Fourier transform for each of five magnetite crystals (A to E) extracted from ∼3.90-billion-year-old ALH84001 carbonate. (A to C) Crystals viewed down the [011] and [110] zone axes. The views in panels A, B, and C are directly comparable to those in Fig. 3 for biogenic MV-1 magnetites. Note the variation in size of the {110} and {100} faces, whose sizes increase relative to the sizes of the {111} faces from panel A to panel C. (A) Magnetite that is ∼35 nm long. The (01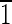 ) and (0
) and (0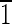 1) faces are ∼3 nm wide. The (100) face is ∼5 nm wide, and the (
1) faces are ∼3 nm wide. The (100) face is ∼5 nm wide, and the (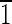 00) face is ∼7 nm wide. (B) Magnetite that is ∼40 nm long and is asymmetric for the displayed {100} and {110} faces. The (
00) face is ∼7 nm wide. (B) Magnetite that is ∼40 nm long and is asymmetric for the displayed {100} and {110} faces. The (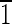
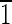 0) face is ∼3 nm wide, and the (1
0) face is ∼3 nm wide, and the (1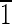 0) face is ∼7 nm wide. The (001) face is ∼3 nmwide, and the (00
0) face is ∼7 nm wide. The (001) face is ∼3 nmwide, and the (00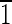 ) face is ∼7 nm wide. (C) Magnetite that is ∼55 nm long and is symmetrical for the displayed {100} and {110} faces. The (001),(00
) face is ∼7 nm wide. (C) Magnetite that is ∼55 nm long and is symmetrical for the displayed {100} and {110} faces. The (001),(00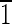 ),(
),(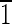 10),(1
10),(1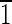 0) faces are ∼20 nm wide. (D and E) Crystals viewed down the [100] and [001] zone axes. Again note the variation in the face sizes between the displayed {110} and {100} surfaces. (D) Magnetite that is ∼60 nm in the longest direction, with (0
0) faces are ∼20 nm wide. (D and E) Crystals viewed down the [100] and [001] zone axes. Again note the variation in the face sizes between the displayed {110} and {100} surfaces. (D) Magnetite that is ∼60 nm in the longest direction, with (0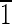 1),(01
1),(01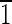 ) faces that are ∼8 nm long. The (001),(00
) faces that are ∼8 nm long. The (001),(00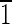 ),(0
),(0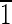 0),(010) faces are ∼30 nm wide. (E) Magnetite that is ∼35 nm in the longest direction, with (110) and (
0),(010) faces are ∼30 nm wide. (E) Magnetite that is ∼35 nm in the longest direction, with (110) and (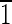
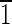 0) faces that are ∼5 nm long. The (010),(0
0) faces that are ∼5 nm long. The (010),(0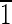 0),(100),(
0),(100),(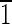 00) faces are all ∼15 nm wide.
00) faces are all ∼15 nm wide.
ARE THE MAGNETOTACTIC BACTERIA AN ANCIENT GROUP THAT COULD HAVE EXISTED ON EARLY MARS?
The findings discussed above ultimately raise the question of whether magnetotactic bacteria can be considered an ancient group of procaryotes and whether they exist or could ever have existed on Mars. Although evidence from Martian meteorites may never unequivocally answer this, we address the first question by utilizing the known phylogenetic relationships of magnetotactic bacteria. The second question we address by using the known metabolic properties of the magnetotactic bacteria, particularly those of strain MV-1, and how they might be suitable for a bacterium's survival and growth on Mars.
Observations from ancient rocks suggest that biogenic magnetite is present in some terrestrial rocks as old as ∼2 billion years (17). We currently do not know how long magnetotactic bacteria have been on the Earth or even how widely this phenotype is currently distributed among contemporary prokaryotes. Phylogenetic analysis, based on the sequences of 16S rRNA genes of many cultured and uncultured magnetotactic bacteria, show that most of the Fe3O4-producing strains are associated with the α subgroup of the Proteobacteria (6, 55). Exceptions include the cultured, Fe3O4-producing, SO42−-reducing organism Desulfovibrio magneticus strain RS-1 (36), which is a member of the δ subgroup of the Proteobacteria, and the uncultured organism Magnetobacterium bavaricum, which is phylogenetically associated with the newly designated Nitrospira group in the domain Bacteria. Only one greigite (Fe3S4)-producing magnetotactic bacterium has been analyzed to date, and this organism has been found to be associated with the SO42−-reducing bacteria in δ subgroup of the Proteobacteria (22). Neither the Proteobacteria nor the Nitrospira group is a deeply branching lineage in the domain Bacteria, and thus these groups are not generally considered to be ancient groups of procaryotes (63). However, dating the evolution of most bacterial groups is currently impossible or at best incredibly difficult; in addition, phylogenetic analyses suggest that magnetotaxis may have evolved several times in the past (22, 55).
Cells of strain MV-1 are metabolically versatile and can grow either as chemoorganoheterotrophs (6) or as chemolithoautotrophs (6; D. A. Bazylinski, A. J. Dean, B. L. Dubbels, L. Kimble-Long, and S. L. Middleton, submitted for publication) under both microaerobic and anaerobic conditions with O2 or nitrous oxide (N2O) as the terminal electron acceptor (7). As sources of electrons, MV-1 cells oxidize certain reduced sulfur compounds and formate by using the Calvin-Benson-Bassham (reductive pentose phosphate) pathway for CO2 fixation and autotrophy (although the possibility that other autotrophic pathways also occur in strain MV-1 has not been eliminated). Fe2+ is not used as an electron source when it is supplied as amorphous iron sulfide (FeS) or siderite (FeCO3) in O2 gradient cultures, although other forms of Fe2+ have not yet been evaluated. In the Calvin-Benson-Bassham pathway the CO2-fixing enzyme is ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO). There are two forms of this enzyme, forms I and II, which differ in the types of subunits present and in the ability to discriminate between different substrates at given CO2/O2 ratios (33). Although both forms are capable of fixing CO2 or O2, the form II RuBisCO generally has a lower specificity than the form I RuBisCO and requires a higher CO2/O2 ratio to function as an efficient carboxylase (33). A form II RuBisCO gene has been cloned from strain MV-1 (Bazylinski et al., submitted). All known magnetotactic bacteria are strict microaerophiles and/or anaerobes (6); therefore, the optimal growth conditions for strain MV-1 should include a high CO2/O2 ratio. Thus, it appears reasonable that strain MV-1 and other autotrophic magnetotactic bacterial strains possess and rely on a form II RuBisCO enzyme for CO2 fixation. In this respect, it is noteworthy that ancient Mars probably had and present Mars has a microaerobic atmosphere with a very high CO2/O2 ratio (see below).
How life might have existed on Mars based on environmental conditions has been discussed in the past (39, 44, 57). Important considerations include the presence of liquid water, microaerobic atmospheric conditions, the presence of a possible carbon source (either organic matter or CO2), the presence of potential energy (electron) sources, and, particularly in the case of the magnetotactic bacteria, the presence of a magnetic field.
Mars has surface erosional features that strongly suggest there were significant quantities of standing water, such as oceans and large lakes, on its surface during its first 2 billion years (13, 14). More recent surface features (<1 million years) suggest that liquid water may have flowed out of surficial depths and may still be present relatively close to the Martian surface (42, 43). Mars has an atmospheric surface pressure that is 2 orders of magnitude less than that of Earth (3, 50). Its atmosphere consists of about 95.3% CO2, 0.13% O2, 2.7% N2, and 1.6% Ar; the remainder is smaller amounts of CO, H2O vapor, and trace gases (3, 40, 50). It is also likely that early Mars had higher levels of O2 than Mars has now due to the dissociation of atmospheric O2-bearing species (3). Organic carbon levels on Mars appear to be very low. No organic carbon was detected in four soil samples taken by Viking I and Viking II (9), but these findings may have been due to photocatalytic oxidation on the Martian surface (8) and/or the formation of benzenecarboxylic acid salts (8). It is not known whether organic compounds are present in soil below the surface. Indigenous organic compounds have been detected in some Martian meteorites (34, 64), including ALH84001 (19). Thus, Mars appears to have had and may still have relatively large reservoirs of liquid water in which microbes could have existed and may continue to exist. In addition, Mars has a microaerobic atmosphere with a very high concentration of CO2 (32) and a very high CO2/O2 ratio, which might be favorable for autotrophic growth of microaerophiles, such as the magnetotactic bacteria, using a form II RuBisCO enzyme. If autotrophic organisms existed or still exist on Mars, then what electron sources supported or support this lifestyle is another important question. Certainly there is generally enough light to support photoautotrophy. For chemolithoautotrophs, iron appears to be a tempting possibility based on its great abundance on Mars. However, much of the iron appears to be in the form of Fe3+, at least on the Martian surface, although Fe2+ is known to exist in silicates in Martian meteorites (45, 47).
The question of whether Mars has a magnetic field strong enough to support magnetotaxis in the way that we currently understand it (21) and the question of whether magnetotactic bacteria require a magnetic field to exist or to synthesize magnetite are more complex. Results from the Mars Global Surveyor indicate that Mars had a significant global magnetic field until approximately 4 billion years ago (21), and although there appears to be little of this field left today, remnant crustal magnetism appears to be widespread on the planet and to be sufficiently strong (>5 μT) to presumably support magnetotaxis (21, 38). However, laboratory experiments clearly show that most magnetotactic bacteria in pure culture do not require a magnetic field to synthesize magnetite (Bazylinski, unpublished data), and it is unclear why magnetotaxis would be needed under microaerobic conditions, which they prefer (6). It is possible, however, that other chemical gradients might occur in aquatic habitats on Mars, where magnetotaxis might confer an advantage. Much more must be known about Mars and the magnetotactic bacteria before any of these questions can be definitively answered.
CONCLUSIONS
Perhaps the most profound implication of this study is that approximately one-quarter of the magnetite crystals embedded in the carbonate assemblages in Martian meteorite ALH84001 require the intervention of biology to explain their presence. No single inorganic process or sequence of inorganic processes, however complex, is known that can explain the full distribution of magnetites observed in ALH84001 carbonates. Under these circumstances, our best working hypothesis is that early Mars supported the evolution of Martian biota that had several traits (e.g., truncated hexa-octahedral magnetite and magnetotaxis) consistent with the traits of contemporary magnetotactic bacteria on Earth.
Acknowledgments
We thank E. F. DeLong for valuable discussions and suggestions and the STI group at the NASA Johnson Space Center, S. R. Keprta, and K. White for technical support. The manuscript was improved by the insightful comments of the reviewers.
This work was funded and supported by NASA's Astrobiology Institute and Exobiology Program. D.A.B. was also supported by U.S. National Science Foundation grant CHE-9714101.
REFERENCES
- 1.Appel, P. W. U., C. M. Fedo, S. Moorbath, and J. S. Myers. 1998. Early Archean Isua supercrustal belt, West Greenland: pilot study of the Isua Interdisciplinary Research Project. Geol. Greenl. Surv. Bull. 180:94-99. [Google Scholar]
- 2.Bagin, V. I., T. S. Gendler, R. S. Rybak, and R. N. Kut'min. 1974. Transformation temperatures of the natural solid solution (Fe, Mg) CO3. Izv. Earth Phys. 6:73-84. [Google Scholar]
- 3.Barth, C. A., A. I. F. Stewart, S. W. Bougher, D. M. Hunten, S. J. Bauer, and A. F. Nagy. 1992. The aeronomy of the current Martian atmosphere, p. 1054-1089. In H. H. Keiffer, B. M. Jakosky, C. W. Snyder, and M. S. Matthews (ed.), Mars. The University of Arizona Press, Tucson.
- 4.Bazylinski, D. A. 1990. Anaerobic production of single-domain magnetite by the marine, magnetotactic bacterium, strain MV-1, p. 69-77. In R. B. Frankel and R. P. Blakemore (ed.), Iron biominerals. Plenum Press, New York, N.Y.
- 5.Bazylinski, D. A., and B. M. Moskowitz. 1997. Microbial biomineralization of magnetic iron minerals: microbiology, magnetism, and environmental significance. Rev. Mineral. 35:181-223. [Google Scholar]
- 6.Bazylinski, D. A., and R. B. Frankel. 2000. Biologically controlled mineralization of magnetic iron minerals by magnetotactic bacteria, p. 109-144. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 7.Bazylinski, D. A., R. B. Frankel, and H. W. Jannasch. 1988. Anaerobic magnetite production by a marine magnetotactic bacterium. Nature (London) 334:518-519. [Google Scholar]
- 8.Benner, S. A., K. G. Devine, L. N. Matveeva, and D. H. Powell. 2000. The missing organic molecules on Mars. Proc. Natl. Acad. Sci. USA 97:2425-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biemann, K., J. Oro, P. Toulmin, L. E. Orgel, A. O. Nier, D. M. Anderson, P. G. Simmonds, D. Flory, A. V. Diaz, D. R. Rushneck, J. E. Biller, and A. L. LaFleur. 1977. The search for organic substances and inorganic volatile compounds in the surface of Mars. J. Geophys. Res. 82:4641-4658. [Google Scholar]
- 10.Blakemore, R. P. 1975. Magnetotactic bacteria. Science 190:377-379. [DOI] [PubMed] [Google Scholar]
- 11.Borg, L. E., J. N. Connelly, L. E. Nyquist, C.-Y. Shih, H. Wiesmann, and Y. Reese. 1999. The age of carbonates in Martian meteorite ALH84001. Science 286:90-94. [DOI] [PubMed] [Google Scholar]
- 12.Brasier, M. D., O. R. Green, A. P. Jephcoat, A. K. Kleppe, M. J. van Kranendonk, J. F. Lindsay, A. Steele, and N. V. Grassineau. 2002. Questioning the evidence for Earth's oldest fossils. Nature (London) 416:76-81. [DOI] [PubMed] [Google Scholar]
- 13.Carr, M. H. 1996. Water erosion on Mars and its biologic implications. Endeavor 20:56-60. [DOI] [PubMed] [Google Scholar]
- 14.Carr, M. H. 1996. Water on early Mars. Ciba Found. Symp. 202:249-265. [PubMed] [Google Scholar]
- 15.Chai, L., and A. Navrotsky. 1996. Synthesis, characterization, and enthalpy of mixing of the (Fe, Mg)CO3 solid solution. Geochim. Cosmochim. Acta 60:4377-4383. [Google Scholar]
- 16.Chang, L. L. Y., R. A. Howie, and J. Zussman. 1996. Siderite, vol. 5B. Longman Group Limited, Burnt Mill, United Kingdom.
- 17.Chang, S.-B. R., J. F. Stolz, J. L. Kirschvink, and S. M. Awaramik. 1989. Biogenic magnetite in stromatolites. II. Occurrence in ancient sedimentary environments. Precamb. Res. 43:305-315. [Google Scholar]
- 18.Clayton, R. N. 1993. Oxygen isotope analysis of ALH8400. Antarct. Meteorite Newsl. (JSC Curator's Office) 16:4. [Google Scholar]
- 19.Clemett, S. J., M. T. Dulay, J. S. Gillette, X. D. Chillier, T. B. Mahajan, and R. N. Zare. 1998. Evidence for the extraterrestrial origin of polycyclic aromatic hydrocarbons in the Martian meteorite ALH84001. Faraday Discuss. 109:417-436. [DOI] [PubMed] [Google Scholar]
- 20.Clemett, S. J., K. L. Thomas-Keprta, J. Shimmin, M. Morphew, J. R. McIntosh, D. A. Bazylinski, J. L. Kirschvink, S. J. Wentworth, D. S. McKay, H. Vali, E. K. Gibson, Jr., and C. S. Romanek. Crystal morphology of MV-1 magnetite: part I. Am. Mineral., in press.
- 21.Connerney, J. E. P., M. H. Acuna, P. J. Wasilewski, N. F. Ness, H. Rème, C. Mazelle, D. Vignes, R. P. Lin, D. L. K. Mitchell, and P. A. Cloutier. 1999. Magnetic lineations in the ancient crust of Mars. Science 284:794-798. [DOI] [PubMed] [Google Scholar]
- 22.DeLong, E. F., R. B. Frankel, and D. A. Bazylinski. 1993. Multiple evolutionary origins of magnetotaxis in bacteria. Science 259:803-806. [DOI] [PubMed] [Google Scholar]
- 23.Dubrawski, J. V. 1991. Thermal decomposition of some siderite-magnesite minerals using DSC. J. Therm. Anal. 37:1213-1221. [Google Scholar]
- 24.Frankel, R. B., R. P. Blakemore, and R. S. Wolfe. 1979. Magnetite in freshwater magnetotactic bacteria. Science 203:1355-1356. [DOI] [PubMed] [Google Scholar]
- 25.Frankel, R. B., D. A. Bazylinski, M. S. Johnson, and B. S. Taylor. 1997. Magneto-aerotaxis in marine coccoid bacteria. Biophys. J. 73:994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedmann, E. I., J. Wierzchos, C. Ascaso, and M. Winklhofer. 2001. Chains of magnetite crystals in the meteorite ALH84001: evidence of biological origin. Proc. Natl. Acad. Sci. USA 98:2176-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golden, D. C., D. W. Ming, C. S. Schwandt, R. A. Morris, and G. E. Lofgren. 2000. An experimental study on kinetically-driven precipitation of calcium-magnesium-iron carbonates from solution: implications for the low-temperature formation of carbonates in Martian meteorite Allan Hills 84001. Meteoritics 35:457-465. [Google Scholar]
- 28.Golden, D. C., D. W. Ming, C. S. Schwandt, H. V. Lauer, Jr., R. V. Socki, R. A. Morris, G. E. Lofgren, and G. A. McKay. 2001. A simple inorganic process for formation of carbonates, magnetite, and sulfides in Martian meteorite ALH84001. Am. Mineral. 86:370-375. [Google Scholar]
- 29.Gooding, J. L. 1992. Soil mineralogy and chemistry on Mars: possible clues from salts and clays in SNC meteorites. Icarus 99:28-41. [Google Scholar]
- 30.Goswami, J. N., N. Sinha, S. V. S. Murty, R. K. Mohapatra, and C. J. Clement. 1997. Nuclear tracks and light noble gases in ALH84001: preatmospheric size, fall characteristics, cosmic ray exposure duration and formation age. Meteoritics 32:91-96. [Google Scholar]
- 31.Graetzel, M., and P. Infelta. 2000. The bases of chemical thermodynamics, vol. 2. Universal Publishers, Parkland, Fla.
- 32.James, P. B., H. H. Kieffer, and D. A. Paige. 1992. The seasonal cycle of carbon dioxide on Mars, p. 934-968. In H. H. Keiffer, B. M. Jakosky, C. W. Snyder, and M. S. Matthews (ed.), Mars. The University of Arizona Press, Tucson.
- 33.Jordan, D. B., and W. L. Ogren. 1981. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature (London) 291:513-515. [Google Scholar]
- 34.Jull, A. J. T., J. W. Beck, and G. S. Burr. 2000. Isotopic evidence for extraterrestrial organic material in the Martian meteorite, Nakhla. Geochim. Cosmochim. Acta 64:3763-3772. [Google Scholar]
- 35.Jull, A. J. T., C. J. Eastoe, S. Xue, and G. F. Herzog. 1995. Isotopic composition of carbonates in the SNC meteorites Allan Hills 84001 and Nakhla. Meteoritics 30:311-318. [Google Scholar]
- 36.Kawaguchi, R., J. G. Burgess, T. Sakaguchi, H. Takeyama, R. H. Thornhill, and T. Matsunaga. 1995. Phylogenetic analysis of a novel sulfate-reducing magnetic bacterium, RS-1, demonstrates its membership of the δ-proteobacteria. FEMS Microbiol. Lett. 126:277-282. [DOI] [PubMed] [Google Scholar]
- 37.Kirschvink, J. L. 1980. South-seeking magnetic bacteria. J. Exp. Biol. 86:345-347. [Google Scholar]
- 38.Kirschvink, J. L., A. T. Maine, and H. Vali. 1997. Paleomagnetic evidence of a low-temperature origin of carbonate in the Martian meteorite ALH84001. Science 275:1629-1633. [DOI] [PubMed] [Google Scholar]
- 39.Klein, H. P., N. H. Horowitz, and K. Biemann. 1992. The search for extant life on Mars, p. 1221-1233. In H. H. Keiffer, B. M. Jakosky, C. W. Snyder, and M. S. Matthews (ed.), Mars. The University of Arizona Press, Tucson.
- 40.Krasnopolsky, V. A., and P. D. Feldman. 2001. Detection of molecular hydrogen in the atmosphere of Mars. Science 294:1914-1917. [DOI] [PubMed] [Google Scholar]
- 41.Lovley, D. R., J. F. Stolz, G. L. Nord, Jr., and J. P. Phillips. 1987. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature (London) 330:252-254. [Google Scholar]
- 42.Malin, M. C., and M. H. Carr. 1999. Groundwater formation of Martian valleys. Nature (London) 397:560-561. [DOI] [PubMed] [Google Scholar]
- 43.Malin, M. C., and K. S. Edgett. 2000. Evidence for recent groundwater seepage and surface runoff on Mars. Science 288:2330-2335. [DOI] [PubMed] [Google Scholar]
- 44.McKay, C. P., R. L. Mancinelli, C. R. Stoker, and R. A. Wharton, Jr. 1992. The possibility of life on Mars during a water-rich past, p. 1234-1245. In H. H. Keiffer, B. M. Jakosky, C. W. Snyder, and M. S. Matthews (ed.), Mars. The University of Arizona Press, Tucson.
- 45.McKay, D. S., E. K. Gibson, Jr., K. L. ThomasKeprta, H. Vali, C. S. Romanek, S. J. Clemett, X. D. F. Chillier, C. R. Maechling, and R. N. Zare. 1996. Search for past life on Mars: possible relic biogenic activity in Martian meteorite ALH84001. Science 273:924-930. [DOI] [PubMed] [Google Scholar]
- 46.Melosh, H. J. 1995. Cratering dynamics and the delivery of meteorites to the Earth. Meteoritics Planet. Sci. 30:545-546. [Google Scholar]
- 47.Mittlefehldt, D. W. 1994. ALH84001, a cumulate orthopyroxenite member of the Martian meteorite clan. Meteoritics Planet. Sci. 29:214-221. [Google Scholar]
- 48.Nicholls, D. 1987. Complexes and first-row transition elements. Macmillan Education Ltd., London, United Kingdom.
- 49.Nyquist, L. E., D. D. Bogard, C.-Y. Shih, A. Greshake, D. Stoffler, and O. Eugster. 2001. Ages and geologic histories of Martian meteorites. Space Sci. Rev. 96:105-164. [Google Scholar]
- 50.Owen, T. 1992. Composition and early history of the atmosphere of Mars, p. 818-834. In H. H. Keiffer, B. M. Jakosky, C. W. Snyder, and M. S. Matthews (ed.), Mars. The University of Arizona Press, Tucson.
- 51.Romanek, C. S., M. M. Grady, I. P. Wright, D. W. Mittlefehldt, R. A. Socki, C. T. Pillinger, and E. K. Gibson, Jr. 1994. Record of fluid-rock interactions on Mars from the meteorite ALH84001. Nature (London) 372:655-657. [DOI] [PubMed] [Google Scholar]
- 52.Schopf, J. W. 1993. Microfossils of the Early Archean Apex chert: new evidence of the antiquity of life. Science 260:640-646. [DOI] [PubMed] [Google Scholar]
- 53.Score, R. 1997. Finding ALH84001. Planet. Rep. XVII:5-7. [Google Scholar]
- 54.Score, R., and D. W. Mittlefehldt. 1993. Macroscopic and thin section description of ALH84001. Antarct. Meteorite Newsl. (JSC Curator's Office) 16:3. [Google Scholar]
- 55.Spring, S., and D. A. Bazylinski. 2000. Magnetotactic bacteria. In The prokaryotes. [Online.] Springer-Verlag New York, Inc., New York, N.Y. http://www.springer-ny.com/.
- 56.Stern, R. A., and W. Bleeker. 1998. Age of the world's oldest rocks refined using Canada's SHRIMP: the Acasta gneiss complex, Northwest Territories, Canada. Geosci. Can. 25:27-32. [Google Scholar]
- 57.Thomas-Keprta, K. L., D. A. Bazylinski, J. L. Kirchvink, S. J. Clemett, D. S. McKay, S. J. Wentworth, H. Vali, E. K. Gibson, Jr., and C. S. Romanek. 2000. Elongated prismatic magnetite crystals in ALH84001 carbonate globules: potential Martian magnetofossils. Geochim. Cosmochim. Acta 64:4049-4081. [DOI] [PubMed] [Google Scholar]
- 58.Thomas-Keprta, K. L., S. J. Clemett, D. A. Bazylinski, J. L. Kirschvink, D. S. McKay, S. J. Wentworth, H. Vali, E. K. Gibson, Jr., M. F. McKay, and C. S. Romanek. 2001. Truncated hexa-octahedral magnetite crystals in ALH84001: presumptive biosignatures. Proc. Natl. Acad. Sci. USA 98:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treiman, A. H. 1998. The history of ALH 84001 revised: multiple shock events. Meteoritics Planet. Sci. 33:753-764. [DOI] [PubMed] [Google Scholar]
- 60.Treiman, A. H., and C. S. Romanek. 1998. Bulk and stable isotopic composition of carbonate minerals in Martian meteorite ALH84001: no proof of high formation temperature. Meteoritics Planet. Sci. 33:737-742. [DOI] [PubMed] [Google Scholar]
- 61.Valley, J. W., J. M. Eiler, C. M. Graham, E. K. Gibson, C. S. Romanek, and E. M. Stolper. 1997. Low-temperature carbonate concretions in the Martian meteorite ALH84001: evidence from stable isotopes and mineralogy. Science 275:1633-1638. [DOI] [PubMed] [Google Scholar]
- 62.Wilde, S. A., J. W. Valley, W. H. Peck, and C. M. Graham. 2001. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature (London) 409:175-178. [DOI] [PubMed] [Google Scholar]
- 63.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright, I. P., M. M. Grady, and C. T. Pillinger. 1989. Organic materials in a Martian meteorite. Nature (London) 340:220-222. [Google Scholar]