Abstract
Bioassays are little used to detect individual toxins in the environment because, compared to analytical methods, these assays are still limited by several problems, such as the sensitivity and specificity of detection. We tentatively solved these two drawbacks for detection of anatoxin-a(s) by engineering an acetylcholinesterase to increase its sensitivity and by using a combination of mutants to obtain increased analyte specificity. Anatoxin-a(s), a neurotoxin produced by some freshwater cyanobacteria, was detected by measuring the inhibition of acetylcholinesterase activity. By using mutated enzyme, the sensitivity of detection was brought to below the nanomole-per-liter level. However, anatoxin-a(s) is an organophosphorous compound, as are several synthetic molecules which are widely used as insecticides. The mode of action of these compounds is via inhibition of acetylcholinesterase, which makes the biotest nonspecific. The use of a four-mutant set of acetylcholinesterase variants, two mutants that are sensitive to anatoxin-a(s) and two mutants that are sensitive to the insecticides, allows specific detection of the cyanobacterial neurotoxin.
Toxic substances which are hazardous to human, domestic animal, and wild animal health may contaminate water resources and drinking water supplies. Some of these substances, the cyanotoxins, are produced by cyanobacteria which occur naturally in freshwaters and proliferate with increasing eutrophication (8, 19). The most widely reported cyanotoxins are the cyclic peptides microcystins, which are potent hepatotoxins and tumor promoters. In addition to these toxins, cyanobacteria may produce neurotoxins that have been responsible for lethal poisonings of mammals and birds, including anatoxin-a(s) (Fig. 1). This toxin is a natural organophosphate (16) which irreversibly inhibits acetylcholinesterase (AChE), similar to organophosphorous and carbamate insecticides and some chemical warfare agents. When AChE is inhibited, the neurotransmitter acetylcholine is no longer hydrolyzed in the synapse, the postsynaptic membrane cannot be repolarized, and nerve influx is blocked. Anatoxin-a(s) is highly toxic for mammals when the toxigenic cyanobacteria produce mass populations in drinking water (4, 13, 15, 18).
FIG. 1.
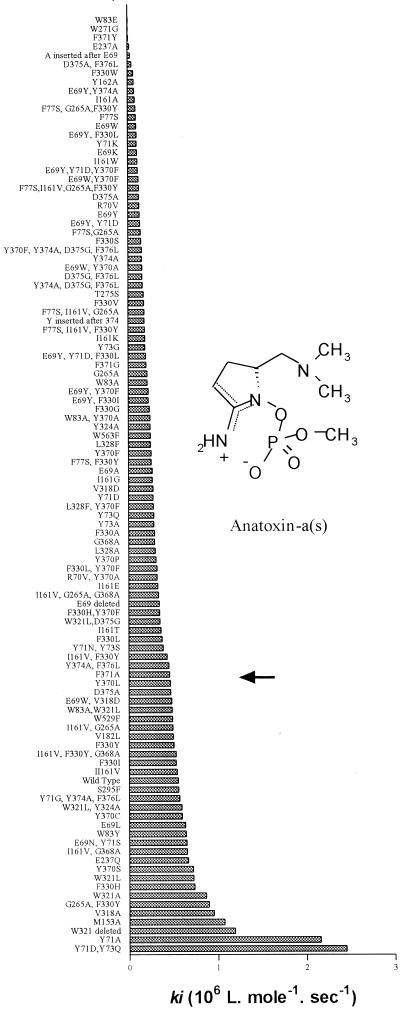
Inhibition rates of Drosophila AchE mutants. The highest ki values indicate the mutants most sensitive to inhibition by purified anatoxin-a(s). The arrow indicates the position of wild-type AChE.
Detection of anatoxin-a(s) in freshwater presents several problems. The toxicity of the cyanobacteria is strain specific, and morphological observations alone cannot predict the hazard level. As anatoxin-a(s) lacks a chromophore, the only analytical method that may be considered for detection is high-performance liquid chromatography plus mass spectroscopy. An alternative is to use the strong inhibition of AChE by the toxin as a sensor (14). However, AChE is inhibited by all known organophosphate and carbamate insecticides used in crop protection and animal husbandry. We have already used a biosensor bearing several enzymes with different sensitivities to detect and identify insecticides (3). Here we adapted this technology for detection of anatoxin-a(s). First we investigated an AChE which is sensitive to anatoxin-a(s) to obtain high sensitivity, and then we investigated a set of mutant enzymes which allowed unambiguous identification of the cyanobacterial toxin.
MATERIALS AND METHODS
Reagents.
Anatoxin-a(s) was obtained from freeze-dried Anabaena lemmermanii strain PH-160 isolated from Lake Knud S/o in Denmark (13). The lyophilized powder was solubilized in water (10 mg ml−1), ultrasonicated, and centrifuged (10,000 × g). The supernatant was used as a source of toxin (18). It was free of irreversible AChE inhibitors of nonbiological origin since it was cultivated aseptically in laboratory conditions and since other Anabaena sp. strains did not show any inhibition. To test for the occurrence of toxin in aquatic environments, blooms or scums dominated by Anabaena spp. were sampled from several European freshwater lakes and centrifuged for 10 min at 10,000 × g, and the pellets were lyophilized. Ten milligrams (dry weight) of each pellet was subsequently rehydrated in 1 ml of water, ultrasonicated, and then centrifuged for 10 min at 10,000 × g. The supernatants were used as potential sources of toxin.
Drosophila AchEs and mutated derivatives were produced in baculovirus and purified by affinity chromatography as previously described (10). The residue numbering and structural data were obtained from reference 12.
Enzyme kinetics.
Incubation of the enzyme with the toxin leads to progressive phosphorylation of the enzyme, which is then devoid of any activity. The mechanism of irreversible inhibition follows scheme 1 (1):
 |
where E is AChE, PX is anatoxin-a(s), EP is the phosphorylated enzyme, and X is the residual group (dephosphorylated toxin residue). The inhibitor phosphorylates the AChE active site serine, and the inhibition can be considered irreversible in the initial hours. This scheme can be simplified with the bimolecular rate constant ki = k2/Kd, as follows:
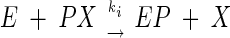 |
To monitor inhibition, the enzyme was incubated for various times with inhibitor (toxin or insecticide) at 25°C in 25 mM phosphate buffer (pH 7). The variation in the remaining free enzyme ([E]/[E0]) versus time was estimated by sampling aliquots at various times and recording the remaining activity with 1 mM acetylthiocholine (9). Disappearance of free enzyme (E) follows second-order kinetics:
 |
where t is the time of incubation, [PX0] and [E0] are the initial concentrations of inhibitor and enzyme, respectively, and [E] is the concentration of enzyme at different times. In this equation, [PX0], and ki are unknown. These two parameters were estimated by performing multiple nonlinear regression on inhibition with different concentrations of anatoxin-a(s).
Preparation of the electrochemical sensor and measurement procedure.
The sensor system consisted of two electrodes, an Ag/AgCl reference electrode and a graphite working electrode, both made by using screen-printing technology. The fabrication technique was based on consecutive depositions of several layers on a polyvinyl chloride sheet, including a silver conducting layer, a carbon pad, a reference electrode (Ag/AgCl), and an insulating layer (2). After each deposition the electrodes were dried at 60°C for 30 min.
AChE immobilization was carried out by entrapment in a photo-cross-linkable PVA-SbQ polymer (Toyo Gosei Kogyo Co., Tokyo, Japan). A 3-μl homogeneous mixture of enzyme solution in 0.025 M sodium phosphate buffer (pH 7) and PVA-SbQ polymer (50%, vol/vol) was deposited on the working electrode. The amount of enzyme immobilized on each electrode was calculated to be 0.02 pmol. The electrodes were then placed for 3 h under a 16-W neon lamp to obtain polymerization. The electrodes were stored at 4°C in sealed bags.
The amperometric measurements were carried out with a PRG-DEL potentiostat (Tacussel, Villeurbanne, France), and the working potential was poised at 100 mV versus the Ag/AgCl reference electrode. The output current was recorded with a personal computer by using PICO ADC software (release 3.07; Picolog Software, Cambridge, United Kingdom). The biosensor was inserted vertically into the body of an analytical cell containing 5 ml of 0.025 M phosphate buffer (pH 7) with constant magnetic stirring. Fifty microliters of a substrate solution (0.1 M thiocholine) was then added, and the signal initiated by the enzymatic reaction was recorded (dI0). After inhibition by immersion of the biosensor in aqueous solutions of different samples for 10 min, a new lower current intensity (dI1) was recorded in the same mode, after which relative inhibition (RI) could be calculated with the equation: RI = [(dI0 − dI1)/dI0)] × 100. The limit of detection corresponded to 10% inhibition of the enzyme immobilized on the electrode.
RESULTS AND DISCUSSION
Engineering an AChE for increased sensitivity to anatoxin-a(s).
We first tested the sensitivity of AChEs from three animals, an insect (Drosophila), a fish (electric eel), and a mammal (ox), by using equation 1, which allowed to us estimate the concentration of anatoxin-a(s) and the rate constants of inhibition (kis). The kis obtained for the three AChEs were 0.55, 0.12, and 0.35 106 liters · mol−1 · s−1, respectively. These data show that the insect enzyme was slightly more sensitive than the two vertebrate AChEs. They also indicate that anatoxin-a(s) is among the most neurotoxic organophosphorous compounds since the inhibition rates were higher than those obtained for most insecticides. For example, the rate of inhibition of Drosophila AChE by organophosphates varies over 4 orders of magnitude, from 7.0 × 102 liters · mol−1 · s−1 for omethoate to 1.4 × 107 liters · mol−1 · s−1 for chlorpyriphos-oxon (6).
To further increase the sensitivity of the Drosophila enzyme, we mutated amino acids in the region of the active site by using various strategies, including residue replacement, deletion, insertion, and combination of mutations (5). The sensitivity of each mutant to anatoxin-a(s) was estimated (Fig. 1). Some mutations appeared to provide higher sensitivity to the toxin, while others resulted in resistance. Mutant Y71A exhibited 3.6-fold-increased sensitivity compared to wild-type Drosophila AChE, and the double mutant Y71D Y73Q exhibited 4.1-fold-increased sensitivity. It should be noted that the Y71D or Y73Q mutation alone did not increase sensitivity, but a combination of these mutations clearly did. This phenomenon has been observed previously for sensitivity to insecticides (5); it originates from the proximity of the two mutations and the high level of allostery between the different residues lining the active site of AchE. The localization of mutations affecting the sensitivity to anatoxin-a(s) was determined by using the recently determined structure of Drosophila AChE (12). The active site is located at the bottom of a 20-Å-deep narrow gorge. Mutations that enhance the sensitivity to the toxin are located at the entrance of this gorge (Fig. 2), showing that the increase in sensitivity was obtained by making entrance of the toxin into the active site easier.
FIG. 2.
Twenty-angstrom slab of Drosophila AChE, showing the positions of mutations that provided the best sensitivities to anatoxin-a(s) (Tyr71 and Tyr73) in white at the entrance of the active site gorge. Amino acids important for catalysis (Ser238 and Trp83) at the bottom of the active site are shown in yellow.
Quantification of anatoxin-a(s).
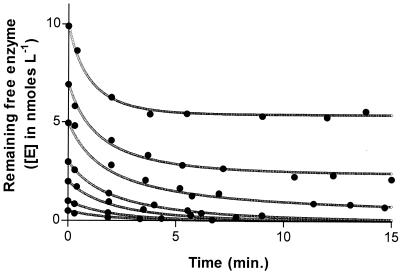
In addition to detection of anatoxin-a(s), inhibition of AChE may be used to quantify the cyanobacterial toxin. As the inhibition is irreversible and stoichiometric, the reaction takes place until the entire available toxin present in a sample has reacted with the enzyme. Thus, recording the remaining active free enzyme after anatoxin-a(s) is added shows that the concentration of free enzyme reaches a plateau. An example is shown in Fig. 3, where inhibition of an enzyme solution at 10 nmol liter−1 shows a plateau at 5.4 nmol liter−1, equivalent to a toxin concentration of 4.6 nmol liter−1. To confirm and refine the toxin concentration, we used several concentrations of enzyme and fitted data by a multiple nonlinear fit with equation 1. In this example, the concentration of anatoxin-a(s) was estimated to be 4.6 ± 0.14 pmol per ml.
FIG. 3.
Determination of the amount of anatoxin-a(s) by progressive inhibition. In this experiment, 10, 7, 5, 3, 2, 1, and 0.5 pmol of AChE were mixed with aliquots of an anatoxin-a(s)-containing sample. Inhibition was monitored over time. The plateaus correspond to the free enzyme remaining when all of the neurotoxin had reacted with the AChE.
Selectivity of detection.
A prerequisite of a bioassay is selectivity. This does not occur with inhibition of AChE because man-made toxins, such as organophosphates and carbamates used as insecticides, may also inhibit the enzyme (11). To make the assay selective, we first searched for mutants sensitive to anatoxin-a(s) and resistant to most insecticides. This requirement was met by Y71D-Y73Q and Y71A, the two most anatoxin-a(s)-sensitive mutants. Then we did the reverse and obtained two mutants resistant to anatoxin-a(s) and sensitive to the majority of the insecticides: E69W and E69Y. To specifically detect the presence of anatoxin-a(s), inhibition rates (ki) were estimated for the four mutants. The ki ratio for the two mutants belonging to each group allowed us to unambiguously discriminate between anatoxin-a(s) and the insecticides tested (Fig. 4).
FIG. 4.
Identification of organophosphorous compounds inhibiting AChE by determining the relative inhibition rates for two mutants. The inhibition rate constants of four enzymes (Y71D Y73Q, Y71A, E69W, and E69Y) were estimated for anatoxin-a(s) and for some insecticides. Four inhibition ratios were then tested to obtain a signature for inhibition by anatoxin-a(s) versus inhibition by an insecticide. Y71D Y73Q and Y71A were more rapidly inhibited than E69W and E69Y by anatoxin-a(s) but not by the insecticides tested.
Occurrence of anatoxin-a(s).
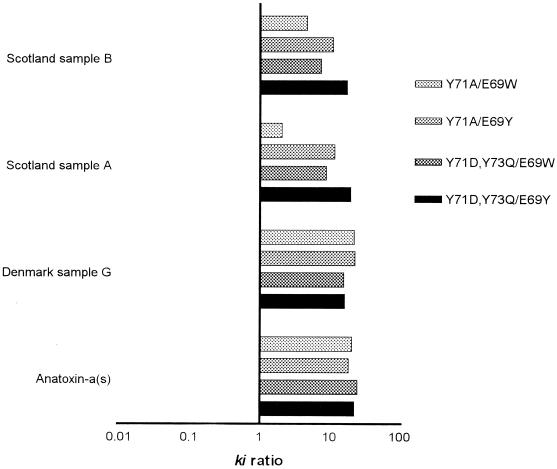
Anatoxin-a(s) has been found in North America (16), South America (17), and Europe (7, 13). However, the natural occurrence of anatoxin-a(s) remains poorly documented, at least in part because no specific detection method has been available. To test our four-mutant set with environmental material, we examined cyanobacterial bloom samples from Spain, Greece, France, Scotland, and Denmark. In three samples, two from Scotland and one from Denmark, we found irreversible inhibition of AChE. The inhibition patterns were consistent with those of anatoxin-a(s) (Fig. 5). While lower ki ratios were obtained with some mutants tested with the Scottish environmental cyanobacterial samples than with the Danish environmental cyanobacterial samples, all ratios were found to be due to anatoxin-a(s), consistent with ki ratios of >1. The estimated concentrations of the toxin in the Scotland B, Denmark, and Scotland A samples were 30, 10, and 1.5 nmol per g (dry weight), values which can be compared to the intraperitoneal 50% lethal dose of anatoxin-a(s) in mice, which is 121 nmol per kg (14).
FIG. 5.
Detection of anatoxin-a(s) in cyanobacterial bloom samples from three freshwater lakes and analysis of purified anatoxin-a(s) reference toxin by using four AChE mutants.
Development of a biosensor.
A sensitive screen-printed amperometric sensor suitable for determination of the concentration of anatoxin-a(s) was developed. All of the measurements were carried out after the enzyme electrode was immersed in an aqueous solution for 10 min. The two sensitive enzymes (mutants Y71D Y73Q and Y71A) allowed detection of 0.5 nmol of toxin per liter. Furthermore, the limits of detection with the two insensitive mutants (E69W and E69Y) were found to be 16- and 50-fold higher than the limits of detection for the two sensitive enzymes. Thus, the specificity observed in spectrophotometric assays was conserved in the immobilized enzymes.
Acknowledgments
We are grateful to Thomas Lanaras (Aristotle University of Thessaloniki, Thessaloniki, Greece), Antonio Quesada (Universidad Autonoma de Madrid, Madrid, Spain), and Helene Ducobu (CESAC-Toulouse, Toulouse, France) for kindly providing natural bloom samples and to Kenneth A. Beattie (University of Dundee) for technical assistance.
This research was supported by grants from INSERM (Programme Environnement et Santé) and CEE (grants Cyanotox IC18-CT980293, ACHEB QLK3-CT-2000-00650, and Safegard QLK3-CT-2000-000481) and by grant PRA BT-98-04.
REFERENCES
- 1.Aldrige, W. N. 1950. Some properties of specific cholinesterase with particular reference to the mechanism of inhibition by diethyl p-nitrophenyl thiophosphate (E 605) and analogues. Biochem. J. 46:451-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, T. T., and R. D. Schmid. 1999. A disposable multielectrode biosensor for the discrimination of organophosphate and carbonate insecticides in the submicromolar range using native and recombinant variants of acetylcholinesterase. Anal. Chim. Acta 401:95-103. [Google Scholar]
- 3.Bachmann, T. T., B. Leca, F. Vilatte, J.-L. Marty, D. Fournier, and R. D. Schmid. 2000. Improved multianalyte detection of organophosphates and carbamates with disposable multielectrode biosensors using recombinant mutants of Drosophila acetylcholinesterase and artificial neural network. Biosens. Bioelect. 15:193-201. [DOI] [PubMed] [Google Scholar]
- 4.Beasley, V. R., R. W. Coppock, J. Simon, R. Ely, W. B. Buck, and R. A. Corley. 1983. Apparent blue-green algae poisoning in swine subsequent to ingestion of a bloom dominated by Anabaena spiroides. J. Am. Vet. Assoc. 182:413-414. [PubMed] [Google Scholar]
- 5.Boublik, Y., P. Saint-Aguet, A. Lougarre, M. Arnaud, F. Villatte, S. Estrada-Mondaca, and D. Fournier. 2002. Acetylcholinesterase engineering for detection of insecticide residues. Protein Eng. 15:43-50. [DOI] [PubMed] [Google Scholar]
- 6.Charpentier, A., P. Menozzi, V. Marcel, F. Villatte, and D. Fournier. 2000. A method to estimate acetylcholinesterase-active sites and turnover in insects. Anal. Biochem. 285:76-81. [DOI] [PubMed] [Google Scholar]
- 7.Codd, G. A. 1995. The toxicity of benthic blue-green algae in Scottish freshwaters. The Scottish Office, Foundation for Water Research, Marlow, Bucks., England.
- 8.Codd, G. A. 2000. Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol. Eng. 16:51-60. [Google Scholar]
- 9.Ellman, G. L., K. D. Courtney, K. D. Andres, and R. M. Featherstone. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88-95. [DOI] [PubMed] [Google Scholar]
- 10.Estrada-Mondaca, S., and D. Fournier. 1998. Stabilization of recombinant Drosophila acetylcholinesterase. Protein Express. Purif. 12:166-172. [DOI] [PubMed] [Google Scholar]
- 11.Harada, K.-I., F. Kondo, and L. Lawton. 1999. Laboratory analysis of cyanotoxins, p. 369-405. In I. Chorus and J. Bartram (ed.), Toxic cyanobacteria in water. E and FN Spon, London, United Kingdom.
- 12.Harel, M., G. Kryger, T. L. Rosenberry, W. D. Mallender, T. Lewis, R. J. Fletcher, J. M. Guss, I. Silman, and J. L. Sussman. 2000. Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Sci. 9:1063-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriksen, P., W. W. Carmichael, J. An, and O. Moestrup. 1997. Detection of an anatoxin-a(s)-like anticholinesterase in natural blooms and cultures of cyanobacteria/blue-green algae from Danish lakes and in stomach contents of poisoned birds. Toxicon 35:901-903. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood, N. A., and W. W. Carmichael. 1987. Anatoxin-a(S), an anticholinesterase from the cyanobacterium Anabaena flos-aquae NRC-525-17. Toxicon 25:1221-1227. [DOI] [PubMed] [Google Scholar]
- 15.Mahmood, N. A., W. W. Carmichael, and D. Pfahler. 1998. Anticholinesterase poisoning in dogs from cyanobacterial (blue green algae) bloom dominated by Anabaena flos-aquae. Am. J. Vet. Res. 49:500-503. [PubMed] [Google Scholar]
- 16.Matsunaga, S., R. E. Moore, W. P. Niemczura, and W. W. Carmichael. 1989. Anatoxin-a(s), a potent anticholinesterase from Anabaena flos-aquae. J. Am. Chem. Soc. 111:8021-8023. [Google Scholar]
- 17.Monserrat, J. M., J. S. Yunes, and A. Bianchini. 2001. Effects of Anabaena spiroides (Cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ. Toxicol. Chem. 20:1228-1235. [DOI] [PubMed] [Google Scholar]
- 18.Onodera, H., Y. Oshima, P. Henriksen, and T. Yasumoto. 1997. Confirmation of anatoxin-a(s) in the cyanobacterium Anabaena lemmermannii as the cause of bird kills in Danish lakes. Toxicon 35:1645-1648. [DOI] [PubMed] [Google Scholar]
- 19.Sivonen, K., and G. Jones. 1999. Cyanobacterial toxins, p. 41-111. In I. Chorus and J. Bartram (ed.), Toxic cyanobacteria in water. E and FN Spon, London, United Kingdom.