Abstract
Background
Localized prostate cancer (PCa) is a largely heterogeneous disease regarding its clinical behavior. Current risk stratification relies on clinicopathological parameters and distinguishing between indolent and aggressive cases remains challenging. To improve risk stratification, we aimed to identify new prognostic markers for PCa.
Methods
We performed an in silico analysis on publicly available PCa transcriptome datasets. The top 20 prognostic genes were assessed in PCa tissue samples of our institutional cohort (n = 92) using the NanoString nCounter technology. The three most promising candidates were further assessed by immunohistochemistry (IHC) in an institutional (n = 121) and an independent validation cohort from the EMPACT consortium (n = 199). Cancer-specific survival (CSS) and progression-free survival (PFS) were used as endpoints.
Results
Our in silico analysis identified 113 prognostic genes. The prognostic values of seven of the top 20 genes were confirmed in our institutional radical prostatectomy (RPE) cohort. Low CENPO, P2RX5, ABCC5 as well as high ASF1B, NCAPH, UBE2C, and ZWINT gene expressions were associated with shorter CSS. IHC analysis confirmed the significant associations between NCAPH and UBE2C staining and worse CSS. In the external validation cohort, higher NCAPH and ZWINT protein expressions were associated with shorter PFS. The combination of the newly identified tissue protein markers improved standard risk stratification models, such as D’Amico, CAPRA, and Cambridge prognostic groups.
Conclusions
We identified and validated high tissue levels of NCAPH, UBE2C, and ZWINT as novel prognostic risk factors in clinically localized PCa patients. The use of these markers can improve routinely used risk estimation models.
Subject terms: Prognostic markers, Cancer, Prostate cancer
Introduction
Prostate cancer (PCa) is the most common cancer in males, with over 1.2 million new cases yearly worldwide, ranking fifth in cancer-related deaths [1]. The use of serum prostate-specific antigen (PSA) for early detection of PCa has resulted in a shift toward earlier diagnosis. However, it led to the detection of a high number of indolent PCa that do not necessitate active treatment and may remain asymptomatic throughout patient’s life [2]. Approximately 30–45% of PCa patients detected through screening are overdiagnosed, leading to unnecessary treatments like radical prostatectomy (RPE) or radiation therapy (RT) [3]. To avoid the risk of overtreatment, current guidelines do not recommend wide-spread population-based PSA screening, though debate persists [4]. Improved risk stratification is crucial for accurately distinguishing between low- and high-risk PCa cases, guiding treatment decisions towards active surveillance or treatment [5].
The D’Amico risk stratification - based on PSA level, clinical stage, and biopsy Gleason score - stratifies patients into low-, intermediate-, and high-risk groups. The original, retrospective study revealed that patients with intermediate- and high-risk PCa benefit more from RPE or RT regarding 5-year biochemical recurrence (BCR)-free survival compared to implant with or without neoadjuvant androgen deprivation therapy. On the other hand, patients with low-risk PCa showed no significant differences in response to various therapies [6]. The prognostic relevance of D’Amico classification for the prediction of BCR, progression, overall-, and cancer-specific survival was subsequently confirmed in patients who underwent RPE [7]. More recently, the Cambridge Prognostic Groups (CPG), a five-strata model utilizing PSA at diagnosis, clinical stage, and biopsy Gleason score, demonstrated superior predictive capability for PCa-related death compared to the previously described three-strata model [8]. Additional relevant risk estimation model, the Cancer of the Prostate Risk Assessment (CAPRA) scoring system, has been developed with the purpose to predict recurrence after RPE [9]. Each described model provides accuracies ranging from 75 to 90% for the prediction of adverse outcomes, however, did not take into account the tumor’s biology. In addition, gene panel-based classifiers were developed and validated earlier, however the selection of the included genes was performed using single mRNA datasets and thus may be limited. Additionally, the assay platform requirements hinder the widespread, decentralized application of these gene panels, while simple immunohistochemical analyses could be easily integrated into daily clinical routine.
In the last years, large, transcriptome datasets with detailed clinical annotation and the necessary bioinformatics tools have become accessible for PCa, enabling systematic search for prognostic biomarkers [10]. In the present work, we analyzed PCa transcriptome datasets [11, 12] to identify novel genes for the prediction of outcomes of clinically localized PCa after RPE. The top 20 prognostic genes were further assessed at the gene expression (GE) level in our institutional PCa cohort. Subsequently, the three most promising novel prognostic genes were further assessed at the protein level in two independent PCa cohorts.
Materials and methods
Overview of the analysis pipeline and patient cohorts
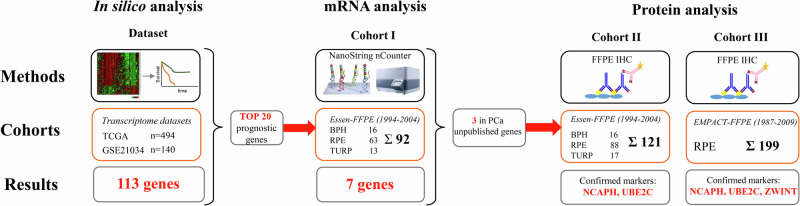
Figure 1 provides an overview of the analysis steps, methods applied, and patient cohorts. Details of the in silico data analysis and description of the two institutional patient cohorts and external validation cohort are described in the supplementary materials.
Fig. 1.
Overview of the performed analyses, patient cohorts and key findings of the present study.
Cohort I for GE analyses included 92 PCa patients who underwent either RPE (n = 63) or palliative transurethral resection (pTURP, n = 13) at the Department of Urology, University Hospital Essen as well as 16 men with benign prostatic hyperplasia (BPH). Cohort II for immunohistochemistry (IHC) analyses had a large overlap with cohort I and consisted of 121 men (RPE n = 88, pTURP n = 17, BPH n = 16). Cohort III included 199 intermediate and high-risk PCa patients who underwent RPE in eight European centers [13].
To gain a broader understanding of the expression patterns of selected candidate markers in various disease stages, we included not only clinically localized (RPE-treated) PCa patients but also samples from nonmalignant cases (BPH) and advanced PCa cases (pTURP). The results of these subgroups (BPH, pTURP) were only considered for comparison with clinically localized PCa but were not used for survival analyses. All analyses were performed blinded to the clinical data to minimize bias.
GE analysis
Total RNA was extracted from FFPE sections with >40% tumor cell content as described in the supplementary materials.
Immunohistochemistry
Based on the results of GE analyses, three protein markers (NCAPH Cat.Nr.: HPA003008, Sigma-Aldrich, St. Louis, Missouri, USA; UBE2C Cat.Nr.: H00011065-M01, Abnova, Taipei City, Taiwan; and ZWINT Cat.Nr.: ab84367, Abcam, Cambridge, Great-Britain) were selected for validation. The detailed description of IHC staining and evaluation is provided in the supplementary material.
Statistical analyses
Ordinal clinicopathological characteristics were compared using nonparametric, two-tailed Wilcoxon rank-sum test. The relation between gene and protein expressions and ordinal variables with more than two categories were evaluated using Kruskal–Wallis test. Metric or continuous associations between protein/gene expressions and clinicopathological parameters were analyzed using Pearson chi-squared test and Spearman’s rank correlation coefficient. Cancer-specific and progression-free survival (CSS and PFS) analyses were performed using Cox proportional hazards analysis and visualized using the Kaplan–Meier curves with log-rank test. Parameters with significant values in the univariate analyses were further assessed in multivariate Cox models. In all analyses, p-value ≤ 0.05 was considered statistically significant. For evaluations of institutional cohorts, the SPSS software packages (IBM SPSS Statistics for Windows, version 25, IBM Corp., Armonk, NY, USA) and R Studio were used.
Results
Patients’ characteristics
The median follow-up for the RPE treated patients in cohort I, II, III were 179, 182 and 77 months, respectively. The median preoperative PSA values in the cohort I, II and III were 11.5, 10.8 and 36.8 ng/ml, respectively, which reflects the composition of cohorts; cohort I and II included low-, intermediate- and high-risk patients, while cohort III included only intermediate- and high-risk patients. Accordingly, both the clinical and pathological stage as well as lymph node (LN) positivity rate were less favorable in cohort III. The 5-year CSS rate was 87%, 90% and 94% for cohort I, II and III, respectively (Table 1).
Table 1.
Patients’ characteristics for cohort I (for GE analysis) and cohorts II and III (for protein expression analysis).
| Cohorts | Institutional GE cohort (I) | Institutional IHC cohort (II) | Multicentre IHC cohort (III) | |||||
|---|---|---|---|---|---|---|---|---|
| Whole cohort | pTURP | RPE | Whole cohort | pTURP | RPE | RPE | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Patients | 76 | 13 | 63 | 105 | 17 | 88 | 199 | |
| Age median [range] | 64 [51–87] | 67 [60–87] | 62 [51–74] | 63 [50–87] | 69 [57–87] | 62 [50–74] | 67 [43–81] | |
| PSA median [range] | 13 [0–341] | 39 [4–341] | 11.5 [0–86] | 11.3 [0–341] | 62.6 [3–6–341] | 10.8 [0–86] | 36.8 [20–597] | |
| cT stage | cT1 | 3 (4) | 1 (8) | 2 (3) | 6 (6) | 1 (6) | 5 (6) | 18 (9) |
| cT2 | 46 (60) | 5 (38) | 41 (65) | 61 (58) | 4 (24) | 57 (65) | 79 (40) | |
| cT3 | 21 (28) | 4 (31) | 17 (27) | 25 (24) | 4 (24) | 21 (24) | 99 (50) | |
| cT4 | 4 (5) | 2 (15) | 2 (3) | 5 (5) | 2 (12) | 3 (3) | 3 (2) | |
| na | 2 | 1 | 1 (2) | 8 | 6 | 2 (2) | 0 | |
| T1 | 2 (3) | 0 | 2 (3) | 3 (3) | 1 (6) | 2 (2) | 0 (0) | |
| pT2 | 36 (47) | 5 (38) | 31 (49) | 49 (47) | 3 (18) | 46 (52) | 33 (17) | |
| pT3 | 35 (46) | 6 (46) | 29 (46) | 42 (40) | 6 (35) | 36 (41) | 130 (65) | |
| pT4 | 3 (4) | 2 (15) | 1 (2) | 6 (6) | 3 (18) | 3 (3) | 36 (18) | |
| na | 0 | 0 | 0 | 5 | 4 | 1 | 0 | |
| PostOP ISUP/WHO grade group | 1 | 31 (41) | 0 | 31 (49) | 48 (46) | 0 | 48 (55) | na |
| 2 | 16 (21) | 0 | 16 (25) | 22 (21) | 0 | 22 (25) | na | |
| 3 | 5 (7) | 0 | 5 (8) | 5 (5) | 0 | 5 (6) | na | |
| 4 | 11 (14) | 3 (23) | 8 (13) | 9 (9) | 2 (12) | 7 (8) | na | |
| 5 | 12 (16) | 9 (69) | 3 (5) | 13 (13) | 9 (53) | 4 (4) | na | |
| na | 1 | 1 | 0 | 8 | 6 | 2 | 199 | |
| LN metastasis (>2 cm) | 8 (10) | 3 (23) | 5 (8) | 10 (9) | 3 (18) | 7 (8) | 77 (39) | |
| D’Amico risk | low | 5 (7) | – | 5 (8) | 10 (9) | – | 10 (11) | 0 |
| interm. | 19 (26) | – | 19 (30) | 26 (24) | – | 26 (30) | 120 (60) | |
| high | 51 (67) | 12 (92) | 39 (62) | 63 (60) | 13 (76) | 50 (57) | 79 (40) | |
| na | 1 | 1 | 0 | 6 | 4 | 2 | 0 | |
| Capra risk | low | 9 (12) | – | 9 (14) | 21 (20) | – | 21 (24) | na |
| interm. | 33 (43) | – | 33 (52) | 37 (35) | – | 37 (42) | na | |
| high | 31 (55) | 11 (85) | 20 (32) | 37 (35) | 11 (65) | 26 (30) | na | |
| na | 3 | 2 | 1 | 10 | 6 | 4 | 199 | |
| Death | 59 (78) | 12 (92) | 47 (75) | 83 (79) | 15 (88) | 68 (77) | 30 (15) | |
| Cancer-spec. death | 29 (38) | 11 (85) | 18 (29) | 37 (35) | 14 (82) | 23 (26) | 13 (7) | |
| Median follow-up in months | [range] | 151 [8–340] | 43 [1–151] | 179 [1–340] | 156 [1–340] | 18 [1–99] | 182 [1–340] | 77 [1–154] |
| Progression | na | na | na | na | na | na | 57 (29) | |
| PSA-progression | na | na | na | na | na | na | 56 (28) | |
LN lymph node metastasis, interm intermediate, na no available data, RPE radical prostatectomy, pTURP palliative transurethral resection, GE gene expression.
GE analysis of 20 genes in the institutional GE cohort (cohort I)
Our in silico analysis identified 113 prognostic genes in TCGA and GSE21034 datasets (Supplementary Table 1). The top 20 of 113 genes with the highest average hazard ratio values and with the lowest p-values were selected. Then, we measured the GE levels of these genes in our institutional GE cohort (cohort I). GE as continuous variables were correlated with clinicopathological data (Supplementary Table 2).
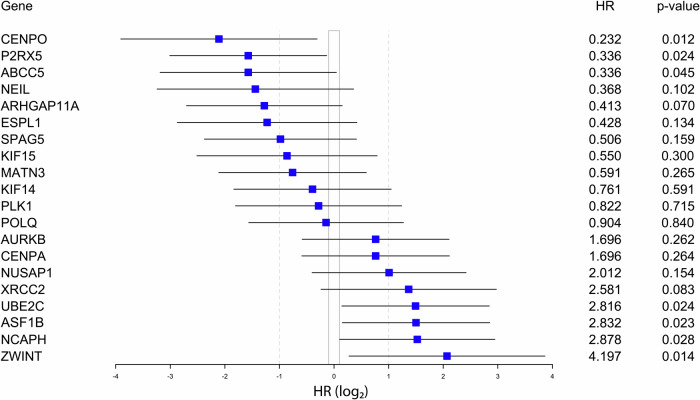
Seven of 20 genes showed significant prognostic value: high expression of UBE2C, NCAPH, ASF1B, and ZWINT (risk genes), and low expression of CENPO, P2RX5, and ABCC5 (protective genes) were associated with shorter CSS after RPE (Fig. 2). For further investigation, we focused on those three risk markers which have formerly not been assessed in PCa (UBE2C, NCAPH, ZWINT).
Fig. 2. Forest plot presentation of Cox univariate CSS analyses for the 20 genes with dichotomized GE levels in the institutional GE cohort (cohort I).
HR hazard ratio.
These genes were significantly overexpressed in advanced PCa cases (pTURP) compared to both BPH and RPE groups. In addition, UBE2C showed significantly lower expression levels in BPH samples compared to RPEs (Fig. 3A).
Fig. 3. Gene expression (GE) values of selected risk genes in BPH, RPE and pTURP samples.
GE levels of NCAPH, UBE2C and ZWINT were significantly higher in pTURP compared to BPH samples (A). Cancer-specific survival (CSS) analyses stratified by GE levels in the institutional GE cohort (cohort I) (B). CSS analyses stratified by protein expressions in the institutional IHC cohort (cohort II) (C). Progression-free survival (PFS) analyses of protein expressions in the multicentre validation cohort (cohort III) (D). BPH benign prostate hyperplasia, RPE radical prostatectomy, pTURP palliative transurethral resection of prostate.
NCAPH and ZWINT GE showed no association with clinicopathological parameters, while higher UBE2C GE levels were associated with positive LNs (p = 0.022), higher ISUP/WHO grade (p = 0.013), and higher CAPRA score (p = 0.036) (Table 2).
Table 2.
Association between patients’ clinicopathological parameters and NCAPH, UBE2C, and ZWINT gene and protein expressions in the institutional GE and IHC cohorts (I and II).
| Institutional GE cohort (cohort I) | Institutional IHC cohort (cohort II) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | NCAPH | UBE2C | ZWINT | NCAPH IHC score | UBE2C IHC score | ZWINT IHC score | ||||||||||||||||||||||
| n | Med | Range | P | Med | Range | P | Med | Range | P | n | 0 n | % | ≥1 n | % | P | n | ≤1 n | % | >1 n | % | P | n | 0 n | % | ≥1 n | % | P | |
| Age | ||||||||||||||||||||||||||||
| ≤65 years | 38 | 6 | 1–92 | 0.784 | 36 | 3–226 | 0.452 | 29 | 5–349 | 0.415 | 52 | 47 | 90% | 5 | 10% | 0.566 | 55 | 26 | 47% | 29 | 53% | 0.058 | 55 | 51 | 93% | 4 | 7% | 0.461 |
| >65 years | 25 | 10 | 1–61 | 34 | 5–246 | 33 | 10–75 | 29 | 25 | 86% | 4 | 14% | 29 | 20 | 69% | 9 | 31% | 30 | 29 | 97% | 1 | 3% | ||||||
| Pathological stage | ||||||||||||||||||||||||||||
| T1-pT2 | 33 | 6 | 1–61 | 0.291 | 37 | 5–137 | 0.880 | 32 | 9–214 | 0.262 | 44 | 41 | 93% | 3 | 7% | 0.180 | 46 | 26 | 57% | 20 | 43% | 0.721 | 46 | 43 | 93% | 3 | 7% | 0.786 |
| pT3-pT4 | 30 | 8.5 | 1–92 | 35 | 3–226 | 28.5 | 5–349 | 37 | 31 | 84% | 6 | 16% | 38 | 20 | 53% | 18 | 47% | 39 | 37 | 95% | 2 | 5% | ||||||
| Lymph node status | ||||||||||||||||||||||||||||
| LN0 | 58 | 7 | 1–92 | 0.079 | 34 | 3–246 | 0.022 | 29 | 5–349 | 0.461 | 74 | 66 | 89% | 8 | 11% | 0.780 | 77 | 45 | 58% | 32 | 42% | 0.025 | 78 | 73 | 94% | 5 | 6% | 0.490 |
| LN+ | 5 | 17 | 3–29 | 73 | 49–229 | 43 | 17–59 | 7 | 6 | 86% | 1 | 14% | 7 | 1 | 14% | 6 | 86% | 7 | 7 | 100% | 0 | 0% | ||||||
| PSA | ||||||||||||||||||||||||||||
| <10 ng/ml | 24 | 7 | 2–59 | 0.496 | 28 | 5–134 | 0.065 | 29 | 10–100 | 0.800 | 36 | 36 | 100% | 0 | 0% | 0.004 | 40 | 29 | 73% | 11 | 28% | 0.003 | 39 | 36 | 92% | 3 | 8% | 0.530 |
| ≥10 ng/ml | 38 | 8.5 | 1–92 | 38 | 3–246 | 32.5 | 5–349 | 44 | 35 | 80% | 9 | 20% | 43 | 17 | 40% | 26 | 60% | 45 | 43 | 96% | 2 | 4% | ||||||
| PSA as continuous (KW) | 63 | 8 | 1–92 | 0.405 | 36 | 3–246 | 0.424 | 30 | 5–349 | 0.522 | 80 | – | 83 | – | 84 | – | ||||||||||||
| ISUP/WHO grade group | ||||||||||||||||||||||||||||
| 1 | 31 | 6 | 2–61 | 0.461 | 26 | 5–246 | 0.013 | 33 | 8–214 | 0.885 | 46 | 44 | 96% | 2 | 4% | 0.023 | 46 | 30 | 65% | 16 | 35% | 0.025 | 46 | 43 | 93% | 3 | 7% | 0.808 |
| 2–5 | 32 | 9 | 1–92 | 47 | 3–226 | 28.5 | 5–349 | 34 | 27 | 79% | 7 | 21% | 37 | 15 | 41% | 22 | 59% | 38 | 36 | 95% | 2 | 5% | ||||||
| ISUP continuous (KW) | 63 | 8 | 1–92 | 0.365 | 36 | 3–246 | 0.073 | 30 | 5–349 | 0.403 | 80 | – | 83 | – | 84 | |||||||||||||
| D'Amico | ||||||||||||||||||||||||||||
| Risk group 1-2 | 24 | 6 | 1–61 | 0.542 | 32 | 3–137 | 0.232 | 32 | 5–214 | 0.944 | 35 | 31 | 89% | 4 | 11% | 0.964 | 34 | 20 | 59% | 14 | 41% | 0.483 | 34 | 30 | 88% | 4 | 12% | 0.063 |
| Risk group 3 | 39 | 8 | 2–92 | 36 | 8–246 | 28.5 | 8–349 | 45 | 40 | 89% | 5 | 11% | 49 | 25 | 51% | 24 | 49% | 50 | 49 | 98% | 1 | 2% | ||||||
| D'Amico continuous (KW) | 63 | 8.5 | 1–92 | 0.392 | 36 | 3–246 | 0.458 | 30 | 5–349 | 0.613 | 80 | – | 83 | – | 84 | – | ||||||||||||
| CAPRA | ||||||||||||||||||||||||||||
| Risk group 1-2 | 42 | 7 | 1–92 | 0.402 | 31.5 | 3–246 | 0.036 | 32.5 | 5–349 | 0.470 | 56 | 51 | 91% | 5 | 9% | 0.282 | 55 | 34 | 62% | 21 | 38% | 0.049 | 57 | 53 | 93% | 4 | 7% | 0.573 |
| Risk group 3 | 20 | 8.5 | 2–30 | 49.5 | 14–226 | 26.5 | 13–78 | 23 | 19 | 83% | 4 | 17% | 26 | 10 | 38% | 16 | 62% | 26 | 25 | 96% | 1 | 4% | ||||||
| CAPRA continuous (KW) | 62 | 8 | 1–92 | 0.394 | 36 | 3–246 | 0.066 | 30 | 5–349 | 0.430 | 79 | – | 81 | – | 84 | – | ||||||||||||
Bold printed p-values were significant (≤0.05).
KW Kruskal–Wallis test was performed for the continuous variables.
In the univariate analysis, high PSA (≥10 ng/ml, p = 0.009), continuous CAPRA score (p = 0.001), and high NCAPH (p = 0.034), UBE2C (p = 0.028) and ZWINT (p = 0.023) GE levels were associated with shorter CSS. In the multivariate analysis, only PSA remained independent significant risk-factor for CSS (p = 0.018) (Supplementary Table 3A, B).
IHC results of UBE2C, NCAPH, ZWINT in the institutional IHC cohort (cohort II)
Positive NCAPH staining showed a significant association with higher PSA levels (p = 0.004) and ISUP/WHO grade (p = 0.023). Consistent with the observations at the GE level, higher UBE2C protein expression significantly associated with LN positivity (p = 0.025), and higher PSA level (p = 0.003), ISUP/WHO grade (p = 0.025), and CAPRA risk score (p = 0.049). Finally, neither ZWINT GE nor protein expression showed an association with any of the clinicopathological parameters (Table 2).
We compared GE and protein levels of the three genes in overlapping samples and observed a significant correlation between gene and protein expression levels for UBE2C (p = 0.015), but not for NCAPH and ZWINT (Supplementary Fig. 2).
Similar to the results found at the GE level, NCAPH and UBE2C protein expressions were significantly associated with CSS (p < 0.001, and p = 0.026, respectively). However, high ZWINT protein expression showed no association with CSS, possibly due to the fact that only 5 of 86 (6%) PCa cases exhibited positive ZWINT staining (Fig. 3B, C).
In addition to the markers, high PSA (p = 0.002), LN positivity (p = 0.029), high ISUP/WHO grade (p = 0.026), continuous CAPRA score (p = 0.017), and high D’Amico scores (p = 0.049) proved to be significantly associated with poor CSS (Supplementary Table 3A). In the multivariate analysis, only high PSA and NCAPH expression remained independent significant risk-factors for CSS (p = 0.023, p = 0.04) (Supplementary Table 3B).
IHC results of UBE2C, NCAPH, ZWINT in the independent multicentre IHC cohort (cohort III)
Protein expressions of NCAPH, UBE2C and ZWINT were investigated in an independent multicentre validation cohort (cohort III) consisting of intermediate and high-risk PCa patients treated with RPE (n = 199). Patients with high NCAPH and ZWINT protein expressions showed significantly shorter PFS (p = 0.024, p = 0.012) (Fig. 3D). In cohort III, a substantially higher proportion of patients were tested positive for ZWINT (26%), compared to to 6% in cohort II, which may be explained by the higher rate of high-risk patients. In cohort III, PFS was used as an endpoint, since only 13 PCa-related deaths (6%) were registered largely limiting the statistical evaluability of CSS as an endpoint. Therefore, results for CSS are only detailed in the supplementary materials.
We found no relationship between the clinicopathological parameters and CSS or PFS, only high ZWINT for CSS (p = 0.041), and high NCAPH and ZWINT expressions showed a significant association with higher risk of progression after RPE (p = 0.04, p = 0.010). Both markers proved to be prognostic in the multivariate analysis (p = 0.002, p = 0.010) (Supplementary Table 3A, B).
Evaluation of clinical risk groups with and without NCAPH, UBE2C or ZWINT expressions
According to current guidelines, patients are classified into low-, intermediate-, and high-risk groups based on clinicopathological variables. In our cohorts, the D’Amico and CAPRA risk scores adequately identified patients with low-risk PCa (100% 5- and 10-year CSS). In contrast, intermediate and high-risk groups had similar CSS or PFS rates showing a limited prognostic value for the above risk models. Most importantly, high-risk patients (according to both D’Amico and CAPRA) could be further stratified by NCAPH, UBE2C, and ZWINT gene and protein expressions into subgroups with significantly different prognosis. The one-by-one addition of NCAPH, UBE2C and ZWINT expressions to the established prognostic models are shown in Supplementary Figs. 3 and 4.
Evaluation of clinical risk groups with and without the three protein IHC score
The combined protein expressions of NCAPH, UBE2C and ZWINT proved to be strong risk factors for patients’ outcome in both, the institutional and validation cohorts (cohort II and III) (Supplementary Table 4A, C). High expression of one of the three markers were associated with decreased CSS in cohort II and decreased PFS in cohort III (p = 0.006, p = 0.023).
The high protein expression of any of the three markers (three IHC marker score) significantly improved D’Amico risk stratification by splitting the high-risk patients into a favorable vs. a very poor prognosis subgroup in cohort III for PFS and CSS (p = 0.007 and p = 0.065). In addition, patients in the D’Amico high-risk group but favorable IHC results (all three markers negative) had even longer CSS and PFS than D’Amico intermediate risk-group patients (Fig. 4, Supplementary Fig. 5).
Fig. 4. Progression-free survival (PFS) analysis by D’Amico and Cambridge risk scores (left plots), three IHC marker score (middle plots), and the combination of D’Amico or Cambridge with IHC marker scores (right plots) in the multicentre IHC validation cohort (cohort III).
In the D’Amico high-risk and Cambridge prognostic groups (CPG 4 and 5) patients were further stratified by the three maker IHC marker score (at least one marker high). P-values represent PFS differences for all groups (black), and between D’Amico high-risk patients with negative vs. positive marker groups (blue), and between Cambridge risk group 4 (CPG) patients with negative vs. positive marker groups (light blue) and between CPG 5 patients with negative vs. positive marker groups (dark blue). The three IHC marker score evaluation was not feasible in 36 samples due to missing IHC staining of any of the three markers. Neg.: negative marker expression, Pos.: positive expression of any of the three marker.
The five-tired Cambridge model (CPG 1–5) was superior to the three-tired D’Amico model regarding its prognostic value for both PFS and CSS in the external validation cohort (cohort III) (CSS: p = 0.247 vs. p < 0.001 (Supplementary Fig. 5) and PFS: p = 0.468 vs. p = 0.055 (Fig. 4)). The higher risk heterogeneity and lower number of patients in each risk group did not allow for the feasible application of the Cambridge model to the institutional cohort II. As the validation cohort (cohort III) included only intermediate and high-risk patients, only three patients were included in the CPG 3 according to the Cambridge model (not presented on the Figures), while 146 patients were classified into the CPG 4 and 50 in the CPG 5 groups. The CPG 5 subgroup showed significantly worse CSS (p < 0.001, Supplementary Fig. 5) and slightly shorter PFS (p = 0.055, Fig. 4) compared to CPG 4. We divided the two subgroups with the three IHC marker score (combination of NCAPH, UBE2C and ZWINT – at least one positive), and found no PCa-caused death in CPG high-risk [4, 5] but marker negative groups (Supplementary Fig. 5). When considering CSS endpoint, no PCa-caused death was observed in the marker negative subgroup (p = 0.023, Supplementary Fig. 5) and only 8 (18%) patients progressed after RPE (p = 0.019, Fig. 4).
Discussion
In the present work, we demonstrate, for the first time, that high gene and protein expressions of NCAPH, UBE2C, and ZWINT are associated with poor prognosis in clinically localized PCa. Furthermore, the addition of GE or protein expression of these markers to established clinical risk scores substantially increased their prognostic value, especially in the high-risk groups.
PCa exhibits diverse molecular and histological patterns, leading to a wide range of clinical presentations. While current prognostic classifications combine PSA level, clinical stage and biopsy Gleason score, they do not take into account the tumor’s molecular characteristics. Recent advancements in molecular techniques have facilitated a comprehensive exploration of the molecular underpinnings of PCa, which led to the description of distinct molecular subtypes on the DNA and mRNA levels. However, the clinical application of subtype classification remains experimental and awaits further validation [14].
Further efforts aimed to incorporate molecular GE signatures into routine prognostication. The 31-gene panel Prolaris was shown to predict BCR after RPE [15]. The 22-gene Decipher classifier was found to predict metastatic progression after RPE [16]. Although these tools are recommended by the current NCCN guidelines, they are not yet widely adopted in the routine [17, 18].
Since the elaboration of the above GE-tools, larger independent transcriptome datasets became available to identify novel, easy-to-use prognostic biomarkers. We performed a systematic in silico analysis and identified 113 prognostic genes. GE of the 20 most significant genes were determined in our institutional RPE cohort. Of note, our top 20 genes showed some overlapping with the previously published commercially available gene panels. For instance, the Prolaris panel included the NUSAP1, PLK1, and ASF1B [15], while the Decipher panel includes NUSAP1 and UBE2C [16]. In the first validation step, we confirmed the prognostic values of 7 of the top 20 genes; low GEs of CENPO, P2RX5 and ABCC5 and high GEs of ASF1B, NCAPH, UBE2C, and ZWINT showed significant associations with shorter CSS in our RPE cohort (cohort I). Consequently, we selected NCAPH, UBE2C, and ZWINT for further validation on the protein level in our institutional cohort and an independent multicentre cohort.
NCAPH regulates mitotic chromosome architecture and segregation, impacting cell growth, proliferation, and migration. Inhibition in non-small cell lung cancer cells arrested cell cycle at the G2/M phase, leading to apoptosis [19], and its high expression was associated with advanced stage and poor survival [20]. In cervical cancer, NCAPH is significantly upregulated, correlating with tumor size, invasion, and LN metastasis [21]. In the TCGA PCa dataset, NCAPH is significantly overexpressed in tumors, associated with advanced stage, LN involvement, and worse OS [22]. Silencing NCAPH expression inhibited proliferation and migration in PCa cell lines [23]. Accordingly, in our study, higher gene and protein expressions were associated with worse CSS and PFS in localized PCa. High NCAPH GE tended to associate with the presence of LN metastasis, while higher protein expression was significantly associated with higher PSA level, and ISUP/WHO grade.
UBE2C is involved in cell cycle regulation. Increased UBE2C expression is associated with larger tumor size, higher grade, lymphovascular invasion, LN metastasis, and poor survival in various tumors [24–27]. In PCa, in silico analyses revealed that high UBE2C GE is associated with higher Gleason scores and worse survival [28]. In a recent study, UBE2C was associated with higher PSA, Gleason score and pathological stage but no associations were found with outcomes [29]. In accordance, we observed that high gene and protein expressions are associated with higher PSA levels, ISUP/WHO grade, LN positivity and poor CSS.
ZWINT is a component of the kinetochore complex, critical for cell division. Little is known about the role and prognostic value ZWINT in various malignancies. In NSCLC, higher ZWINT GE was observed in tumors compared to non-cancerous tissues. High GE was associated with significantly worse OS and PFS in patients with pulmonary adenocarcinoma [30]. In the present study, ZWINT GE was significantly higher in advanced PCa cases (pTURP) and higher ZWINT levels were associated with shorter CSS and PFS.
When applying established clinicopathological risk scores like D’Amico and CAPRA to our cohorts, we found that they accurately identify low-risk patients with excellent prognosis. However, CSS and PFS between intermediate and high-risk patients were not significantly different with either risk classifications. Combining the three molecular markers with the D’Amico risk score revealed prognostic value specifically in high-risk patients, suggesting heterogeneity within this group. D’Amico high-risk patients with low NCAPH, UBE2C and ZWINT levels have significant better prognosis compared to those with high expression levels. Similarly, for the CAPRA model, our markers were able to split high-risk patients in two significantly different prognostic groups. We could reach an even better prognostic stratification when combining the three protein markers (at least one highly expressed). The five-tired Cambridge risk model was superior compared to the three-tired D’Amico model in terms of CSS and PFS. The combined use of IHC markers (all three markers negative) could identify patients with improved survival in the CPG 4-5 subgroups. More importantly, no cancer-specific death was detected in the three marker negative subgroup. Based on these, we concluded that our newly identified and validated markers can improve currently used risk models independent of using the markers on the GE or protein levels. Therefore, a straightforward IHC staining of three markers could identify patients with excellent prognosis after RPE.
Our study is limited by its inherent retrospective nature, which may introduce uncontrolled selection bias and is associated with missing data points (marked as ‘NA’ in separate rows of Table 1). Additionally, there is variability in the availability of some endpoints; for example, PFS data was available only for cohort III, while CSS data was available for cohorts I and II but limited by the shorter follow-up time in cohort III. Finally, the number of patients with available mRNA samples was limited.
In our study, using a systematic hypothesis-free approach, we identified and validated – for the first time - UBE2C, NCAPH, and ZWINT as novel prognostic tissue markers - for localized PCa. Combining these biomarkers with established prognostic scores, we were able to substantially improve risk stratification especially within the high-risk group.
Supplementary information
Enhancing Risk Stratification models in Localized Prostate Cancer by Novel Validated Tissue Biomarkers
Author contributions
CO: writing-original draft, data analysis, visualization. FM, MW and BG: methodology, data analysis. SJ, MS and MKJ resources, writing-review, and editing. BH: resources, supervision, writing-review, and editing. AS: writing-review, and editing. PN: writing-review, and editing. HR: conceptualization, methodology, data analysis, visualization, writing-review, and editing. TS: conceptualization, methodology, funding acquisition, data analysis, writing-original draft. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Funding
This project was supported by National Research, Development and Innovation Office of Hungary (PharmaLab, RRF-2.3.1-21-2022-00015 and TKP2021-NVA-15). Open access funding provided by Semmelweis University.
Data availability
Data sources and handling of the publicly available datasets used in this study are described in the Materials and Methods. Further details and other data that support the findings of this study are available from the corresponding authors upon request.
Competing interests
BH: Advisory boards for Janssen, Bayer, ABX, Lightpoint, Amgen, MSD, Pfizer, Novartis. Invited speaker for Accord, Astellas, Janssen R&D. Honoraria from Uromed. Research funding from AAA/Novartis, Bristol Myers Squibb, and German Research Foundation. Leadership roles for DKG AUO and DGU. The authors declare no potential conflicts of interest with the present project and publication.
Ethics
The study was performed in compliance with the Declaration of Helsinki and the institutional ethics committees approved the study protocol (21-9991-BO and KEK-BE128/2015).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Henning Reis, Tibor Szarvas.
Supplementary information
The online version contains supplementary material available at 10.1038/s41391-024-00918-9.
References
- 1.Bray F, Ferlay J, Soerjomataram I. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. A Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Chistiakov DA, Myasoedova VA, Grechko AV, Melnichenko AA, Orekhov AN. New biomarkers for diagnosis and prognosis of localized prostate cancer. Semin Cancer Biol. 2018;52:9–16. [DOI] [PubMed] [Google Scholar]
- 3.Jalloh M, Cooperberg MR. Implementation of PSA-based active surveillance in prostate cancer. Biomark Med. 2014;8:747–53. [DOI] [PubMed] [Google Scholar]
- 4.Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. ERSPC Investigators. Screening and Prostate-Cancer Mortality in a Randomized European Study. N Engl J Med. 2009;360:1320–8. [DOI] [PubMed] [Google Scholar]
- 5.Chys B, Devos G, Everaerts W, Albersen M, Moris L, Claessens F, et al. Preoperative Risk-Stratification of High-Risk Prostate Cancer: A Multicenter Analysis. Front Oncol. 2020;10:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Bruce Malkowicz S, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc. 1998;280:969–74. [DOI] [PubMed] [Google Scholar]
- 7.Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic Validation of the D’Amico Risk Group Classification for Predicting Survival Following Radical Prostatectomy. J Urol. 2008;179:1354–61. [DOI] [PubMed] [Google Scholar]
- 8.Gnanapragasam VJ, Bratt O, Muir K, Lee LS, Huang HH, Stattin P, et al. The Cambridge Prognostic Groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer: A validation study. BMC Med. 2018;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperberg M, Pasta D, Elkin E, Litwin M, Latini DM, Du Chane J, et al. The UCSF Cancer of the Prostate Risk Assessment (CAPRA) Score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy Á, Munkácsy G, Győrffy B. Pancancer survival analysis of cancer hallmark genes. Sci Rep. 2021;11:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer.The Cancer Genome Atlas Research Network. Cell. 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell. 2010;18:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castiglione F, Dell’Oglio P, Tosco L, Everaerts W, Albersen M, Hakim L, et al. European Multicenter Prostate Cancer Clinical and Translational Research Group (EMPaCT). Tumor Volume and Clinical Failure in High-Risk Prostate Cancer Patients Treated With Radical Prostatectomy. Prostate. 2017;77:3–9. [DOI] [PubMed] [Google Scholar]
- 14.Szarvas T, Csizmarik A, Szűcs M, Nyirády P. Molecular subtypes and perspectives of targeted therapies in prostate cancer. Orv Hetil. 2019;160:252–63. [DOI] [PubMed] [Google Scholar]
- 15.Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Transatlantic Prostate Group. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011;12:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, et al. Discovery and Validation of a Prostate Cancer Genomic Classifier that Predicts Early Metastasis Following Radical Prostatectomy. PLoS One. 2013;8:e66855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis. wt al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79:243–62. [DOI] [PubMed] [Google Scholar]
- 18.Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate cancer, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. JNCCN J Natl Compr Cancer Netw. 2019;17:479–505. [DOI] [PubMed] [Google Scholar]
- 19.Kim B, Kim SW, Lim J-Y, Park S-J. NCAPH Is Required for Proliferation, Migration and Invasion of Non-small-cell Lung Cancer Cells. Anticancer Res. 2020;40:3239–46. [DOI] [PubMed] [Google Scholar]
- 20.Xiong Q, Fan S, Duan L, Liu B, Jiang X, Chen X, et al. NCAPH is negatively associated with Mcl-1 in non-small cell lung cancer. Mol Med Rep. 2020;22:2916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Qiao X, Cooper T, Pan W, Liu L, Hayball J, et al. HPV E7-mediated NCAPH ectopic expression regulates the carcinogenesis of cervical carcinoma via PI3K/AKT/SGK pathway. Cell Death Dis. 2020;11:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui F, Hu J, Xu Z, Tan J, Tang H. Overexpression of ncaph is upregulated and predicts a poor prognosis in prostate cancer. Oncol Lett. 2019;17:5768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arai T, Kojima S, Yamada Y, Sugawara S, Kato M, Yamazaki K, et al. Micro-ribonucleic acid expression signature of metastatic castration-resistant prostate cancer: Regulation of NCAPH by antitumor miR-199a/b-3p. Int J Urol. 2019;26:506–20. [DOI] [PubMed] [Google Scholar]
- 24.Kariri Y, Toss MS, Alsaleem M, Elsharawy KA, Joseph C, Mongan NP, et al. Ubiquitin-conjugating enzyme 2C (UBE2C) is a poor prognostic biomarker in invasive breast cancer. Breast Cancer Res Treat. 2022;192:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H-Q, Zhao G, Ke B, Ma G, Liu G-L, Liang H, et al. Overexpression of UBE2C correlates with poor prognosis in gastric cancer patients. Eur Rev Med Pharmacol Sci. 2018;22:1665–71. [DOI] [PubMed] [Google Scholar]
- 26.Shen Z, Jiang X, Zeng C, Zheng S, Luo B, Zeng Y, et al. High expression of ubiquitin-conjugating enzyme 2C (UBE2C) correlates with nasopharyngeal carcinoma progression. BMC Cancer. 2013;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhao R, Chi S, Zhang W, Xiao C, Zhou X, et al. UBE2C is upregulated by estrogen and promotes epithelial-mesenchymal transition via p53 in endometrial cancer. Mol Cancer Res. 2020;18:204–15. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Wang J, Tang Q, Ren G. Identification of UBE2C as hub gene in driving prostate cancer by integrated bioinformatics analysis. PLoS One. 2021;16:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Lee KW, Yang HJ, Park JJ, Lee CH, Jeon YS, et al. The correlation between the expression of ubiquitin-conjugating enzyme 2C and prostate cancer prognosis. J Cancer Res Clin Oncol. 2023;149:6351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan W, Xie S, Wang M, Pan S, Huang X, Xiong M, et al. Bioinformatic analysis of prognostic value of ZW10 interacting protein in lung cancer. Onco Targets Ther. 2018;11:1683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enhancing Risk Stratification models in Localized Prostate Cancer by Novel Validated Tissue Biomarkers
Data Availability Statement
Data sources and handling of the publicly available datasets used in this study are described in the Materials and Methods. Further details and other data that support the findings of this study are available from the corresponding authors upon request.