Abstract
The initial analysis of the recently sequenced genome of Acanthamoeba polyphaga Mimivirus, the largest known double-stranded DNA virus, predicted a proteome of size and complexity more akin to small parasitic bacteria than to other nucleocytoplasmic large DNA viruses and identified numerous functions never before described in a virus. It has been proposed that the Mimivirus lineage could have emerged before the individualization of cellular organisms from the three domains of life. An exhaustive in silico analysis of the noncoding moiety of all known viral genomes now uncovers the unprecedented perfect conservation of an AAAATTGA motif in close to 50% of the Mimivirus genes. This motif preferentially occurs in genes transcribed from the predicted leading strand and is associated with functions required early in the viral infectious cycle, such as transcription and protein translation. A comparison with the known promoter of unicellular eukaryotes, amoebal protists in particular, strongly suggests that the AAAATTGA motif is the structural equivalent of the TATA box core promoter element. This element is specific to the Mimivirus lineage and may correspond to an ancestral promoter structure predating the radiation of the eukaryotic kingdoms. This unprecedented conservation of core promoter regions is another exceptional feature of Mimivirus that again raises the question of its evolutionary origin.
Keywords: nucleocytoplasmic large DNA viruses, viral evolution, viral promoter
The recent discovery and genome analysis of Acanthamoeba polyphaga Mimivirus, the largest known double-stranded DNA virus, challenged much of the accepted dogma regarding viruses (1, 2). Its particle size (>400 nm), genome length (1.2 million bp), and huge gene repertoire (911 protein-coding genes) all contribute to blur the established boundaries between viruses and the smallest parasitic cellular organisms. Phylogenetic analysis placed the evolutionary origin of Mimivirus before the emergence of the extant eukaryotic kingdoms, raising the possibility that large DNA viruses might define a domain distinct from the three other domains of life, Eucarya, Archaea, and Bacteria (2). The exceptionally large gene content of the Mimivirus genome, which includes key protein translation genes as well as a very diverse set of enzymes belonging to different metabolic pathways, is consistent with the hypothesis that Mimivirus (and other large DNA viruses) evolved from a free living organism through a genome-reduction process akin to the one experienced by parasitic intracellular bacteria (3–5). However, less radical scenarios have been proposed, such as the recruitment of numerous host-acquired genes complementing a set of core genes common to all nucleocytoplasmic large DNA viruses (NCLDVs) (6). In the latter case, the acquired ORFs would have to be transferred with their own promoter or be put under the control of a suitable viral promoter to function properly. These questions about the origin of the Mimivirus genes prompted us to systematically analyze the DNA sequences immediately 5′ upstream of each of the Mimivirus ORFs in search for transcriptional motifs. Unexpectedly, this analysis revealed the presence of the perfectly conserved octamer AAAATTGA in the putative core promoter regions of nearly half of all Mimivirus genes. The same analysis, applied to the genomes of other large DNA viruses, confirmed that such a homogeneity of the core promoter sequences is a unique characteristic of Mimivirus.
Materials and Methods
The Mimivirus genome sequence and the corresponding gene annotations used here are identical to those deposited in GenBank under accession no. AY653733. The annotation of the Mimivirus genome sequence defines 911 predicted protein-coding genes plus an additional set of 347 less convincing unidentified reading frames (URFs). Intergenic regions have an average size of 157 nt, with a standard deviation of 113 nt (excluding the two-tailed 5% most extreme data points). Starting from the predicted gene map, we searched for conserved motifs within the 150-nt regions upstream of each of the predicted translation start codons (ATG) of the 911 genes. These subsequences were identified (i) as statistically overrepresented short oligomers (by using in-house perl scripts that are available from the authors on request) or (ii) by using the Gibbs sampler algorithm implemented in meme (7).
The genome data for Invertebrate iridescent virus 6 (NC_003038), Fowlpox (NC_002188), and Amsacta moorei entomopoxvirus (NC_002520) were downloaded from RefSeq and analyzed by using an identical approach.
To search for a potential occurrence of related motifs in the Acanthamoeba castellanii genome, we used the available data from a recent genomic survey sequencing project (≈19 mega-bases of sequence data are available at www.ncbi.nlm.nih.gov).
Results and Discussion
A Highly Conserved Motif in the 5′ Upstream Regions of Mimivirus Genes. In contrast to the common difficulty of extracting well conserved signals from eukaryotic promoters (8), our results were unexpectedly clear cut: We found that 403 of the 911 (45%) predicted Mimivirus genes exhibited a strictly conserved AAAATTGA motif within their 150-nt upstream region. The statistical significance of this finding in the context of the exact nucleotide composition of the Mimivirus genome was assessed as follows. We cut the Mimivirus genome into 15,752 consecutive 150-nt-long segments (using both strands) and counted the occurrences of the above motif in each of them. Only 661 (4%) of these segments were found to contain the AAAATTGA motif at least once. Such a strong preferential occurrence of this motif in the 5′ upstream region of Mimivirus genes is indeed highly significant (Fisher exact test, P < 10-194). Overall, 60% of the occurrences of this motif are located in front of a gene. Moreover, the distribution of the motif within these 5′ upstream regions is nonrandom, with most of the motifs being located in a narrow range, between 50 and 110 nt from the translation start (Fig. 1). A sequence logo (9) including the flanking regions of these AAAATTGA motifs shows that they are preceded by two to three AT-rich positions and followed by eight to nine AT-rich positions (Fig. 2).
Fig. 1.
The distribution of the position of the AAAATTGA motif with respect to the predicted gene start shows the location of the conserved element at ≈50–110 nt.
Fig. 2.
The sequence logo shows the conserved AT-rich neighborhood of the exactly conserved AAAATTGA octamer. The logo was based on 400 genes with a strictly conserved AAAATTGA motif; 3 genes with a motif that is <20 nt from the predicted translation start were not included in the computation of the logo. (See Fig. 5 for the corresponding logo of the meme motif.)
The same set of 150-nt upstream regions was also analyzed by using the more sophisticated Gibbs sampler approach, as implemented in meme (7). A position-weight matrix (PWM) that corresponds to a motif very similar to the previous AAAATTGA was identified (meme motif; Fig. 5, which is published as supporting information on the PNAS web site). Using a score cutoff of 1,000 (based on the bimodal distribution of the PWM scores; Fig. 6, which is published as supporting information on the PNAS web site), 446 genes (49%) with a motif were detected. For comparison, only 464 (3%) of the above 15,752 consecutive genomic segments exhibit the meme motif (score ≥ 1,000). Most of them are thus found upstream of a gene-coding region. (Fisher exact test, P < 10-280). We note that no other predominant motif was found in the upstream regions of genes not exhibiting the AAAATTGA motif. Finally, we verified that the prevalence of the AAAATTGA motif in the 150-nt segment upstream of the ORFs was not a mere statistical consequence of their high A + T content. When these sequences were randomized, the AAAATTGA octamer was found only 22.7 times (compared with 403), and the meme motif was found 7.2 times (compared with 446) on average over 100 repetitions of the randomization protocol.
The Motif Is Less Frequent in the More Hypothetical URFs. The same analysis was performed with the 347 unlikely genes, annotated URFs, in the Mimivirus genome. Only 20 (6%) of them exhibited the AAAATTGA motif in their 150-nt upstream region. Moreover, many of them did not fall within the [-110, -50] range or were not flanked by AT-rich positions. Consistently, only 11 (3%) of the URFs exhibited the previously defined meme motif (score > 1,000). This motif distribution is not significantly different from the one observed in the 15,752 consecutive 150-nt-long segments covering the whole genome.
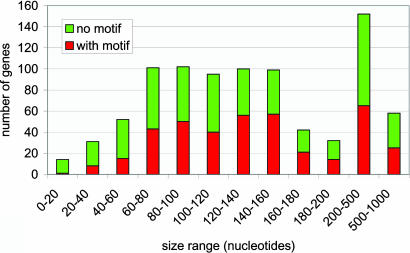
The Presence/Absence of the Motif Does Not Correlate with the Intergenic Distance. We verified that the occurrences of the AAAATTGA motif were not trivially linked to the distance between successive ORFs. Fig. 3 shows that the proportion of genes exhibiting the motif is fairly constant across the whole range of intergenic distances, except for the smallest ones (<60 nt). It is worth noting that a fraction of these short distances might be artifactual, corresponding to cases where the proximal ATG does not coincide with the actual translation start. The net result of this artifact is to slightly minimize the number of genes exhibiting the AAAATTGA motif.
Fig. 3.
No relationship between the presence/absence of the AAAATTGA motif and the distance between two genes can be identified in the size distribution of Mimivirus intergenic regions. The exception is the virtual absence of the motif in intergenic regions that are too short to host it.
The Motif Significantly Correlates with Genes Transcribed from the Leading Strand. We then examined other possible correlations between the presence of the AAAATTGA motifs and the positions of the corresponding genes within the genome. Overall, the distribution of Mimivirus protein-coding genes does not exhibit any significant strand bias: 450 genes are found on the positive strand (R genes), and 461 are found on the negative strand (L genes; Fisher exact test, P = 0.8). The distribution of the AAAATTGA motif is similarly unbiased, with 196 occurrences in front of the R genes and 207 in front of the L genes. The meme motif exhibits a similar distribution, with 217 in front of R genes and 229 in front of L genes (score > 1,000). There is thus no significant global strand preference for the occurrence of the upstream motif (Fisher exact test, P = 0.8).
However, a previous analysis (2) identified a putative origin of replication (OR) of the chromosome near position 380,000 (between genes L294 and L295). On the basis of this prediction, one can distinguish a “leading” strand, with 578 genes transcribed away from the OR, and a “lagging” strand, from which 333 genes are transcribed. Interestingly, the AAAATTGA motif occurs significantly more frequently (Fisher exact test, P = 0.027) in front of the genes transcribed from the leading strand (281/578 = 48.6%) than in front of the genes transcribed from the lagging strand (122/333 = 36.6%). A similar asymmetry is found for the meme motif (313/578 = 54.2% and 133/313 = 39.9%; Fisher exact test, P = 0.015).
We also examined whether the presence of the AAAATTGA motif might correlate with certain categories of gene functions (Table 1). One hundred fifty-seven Mimivirus genes can be associated to one of the Clusters of Orthologous Groups of proteins (COG) (10) functional classes. We found that most translation- and transcription-related genes exhibited the AAAATTGA motif, which was also true of genes related to nucleotide transport and metabolism. In contrast, the motif was absent from the upstream region of most genes related to DNA replication, recombination, and repair, as well as from genes classified in the cell envelope biogenesis/outer membrane COGs. Overall, the upstream motif does not occur more frequently in front of genes associated with functional annotations (88/232 = 38%) compared with anonymous ones (315/679 = 46%) (Table 2, which is published as supporting information on the PNAS web site).
Table 1. Number of “COGed” Mimivirus genes with or without a conserved AAAATTGA motif.
| With motif | Without motif | Total | COG function |
|---|---|---|---|
| 7 (9) | 23 (21) | 30 | DNA replication, recombination, and repair |
| 0 (0) | 9 (9) | 9 | Cell envelope biogenesis, outer membrane |
| 3 (3) | 5 (5) | 8 | Amino acid transport and metabolism |
| 5 (5) | 11 (11) | 16 | Posttranslational modification, protein turnover, chaperones |
| 1 (1) | 2 (2) | 3 | Lipid metabolism |
| 34 (38) | 26 (22) | 60 | Function unknown or general function prediction only |
| 2 (2) | 1 (1) | 3 | Secondary metabolites biosynthesis, transport, and catabolism |
| 5 (5) | 0 (0) | 5 | Nucleotide transport and metabolism |
| 7 (7) | 2 (2) | 9 | Transcription |
| 6 (5) | 1 (2) | 7 | Translation, ribosomal structure, and biogenesis |
Only COG classes that contain at least three Mimivirus genes are shown. Numbers corresponding to genes with or without a meme motif are given in parentheses.
NCLDV Core Genes and the AAAATTGA Motif. Iyer et al. (6) identified a set of homologues genes that have been identified in all or most members of the four main NCLDV groups: Iridoviridae, Asfarviridae, Phycodnaviriae, and Poxviridae. Some of these “core” genes are also found in baculoviridae and phages. These core genes are divided into four classes, from the most conserved to the least conserved. Remarkably, we found that none of the nine class I core genes found in Mimivirus has the exact octamer motif in its 5′ upstream region, and only two exhibit the meme motif (Table 3, which is published as supporting information on the PNAS web site). In contrast, all but one of the six class II core genes have the octamer motif and the meme motif. The motif distribution for the class III (7/11) and class IV (10/16) core genes is more balanced. However, none of the above distributions significantly differs from the 446/911 ratio observed for all Mimivirus genes (Fisher exact test, P > 0.1).
The Presence of Such a Highly Conserved 5′ Upstream Motif Is Unique to Mimivirus. For many years, the 5′ upstream region of eukaryotic genes has been under intense scrutiny in many different organisms from different kingdoms (e.g., fungi, plant, and metazoan) in an attempt to decipher the sequence-based signal involved in the initiation and regulation of the transcription process (11, 12). The sole common result that emerged from these numerous studies is that sequence conservation is the exception rather than the rule in the 5′ upstream regions of eukaryotic genes (13, 14). Other recent studies have confirmed this lack of conservation in several lineages of parasitic protists (15). However, large eukaryotic DNA viruses have been much less studied than their cellular counterparts. The analysis as applied to the Mimivirus genome was thus performed on the genomes of NCLDVs of the Iridiviridae, Phycodnaviridae, and Poxviridae families to establish the pattern of sequence conservation in the 5′ upstream regions of their genes. Our results show that none of these other NCLDVs exhibits a pattern of conservation comparable to the one observed for Mimivirus (Table 4, which is published as supporting information on the PNAS web site). For instance, only 30 of 178 (17%) genes of Invertebrate iridescent virus 6 (CIV, Iridoviridae) have a conserved AAAATTGA motif within their 150-nt upstream region. Fourteen of 231 (6%) Fowlpox (FOP, Poxviridae) genes and 10 of 218 (4.5%) Amsacta moorei entomopoxvirus (AME, Poxviridae) genes also exhibit this motif. In all other NCLDVs, fewer or no occurrences of this motif were detected. Note, however, that 47 of the 218 (22%) AME genes display a conserved TTTTGAAA motif (Table 4). Finally, an analysis by Gibbs sampling of all viral genomes containing >100 annotated genes showed that the Mimivirus pattern of 5′ upstream motif conservation is truly unique among large DNA viruses. Such a conservation is also absent from archaea viruses such as AFV1 (16). In the absence of experimental data, we cannot formally exclude that the intergenic AAAATTGA motif may act on both upstream and downstream adjacent genes. However, a symmetrical analysis of the 150-nt-long intergenic regions 3′ downstream of each gene identified only half (203 of 403) of the previously identified motifs and exhibited no preferential location for these motifs with respect to the preceding stop codon (Fig. 7, which is published as supporting information on the PNAS web site). These results are thus in favor of a 5′ polarity of function.
The Conserved AAAATTGA Motif Is Likely to Be a Main Core Promoter Element. Analyses of the structure and expression of a number of genes from amoebal protists have shown that they are expressed in single transcription units and that few of them have introns (15). Because the mechanisms of gene expression used by a virus and its host must be compatible, it is reasonable to propose that the short genome regions (157-nt average) separating two consecutive Mimivirus ORFs contain most of the promoter sequence information. The eukaryotic core promoter includes DNA elements that can extend 35 bp upstream and/or downstream of the transcription initiation site. Most core promoter elements appear to interact directly with components of the basal transcription machinery. In metazoan, the most conserved and recognizable core promoter element is the TATA box, located 25–30 bp from the transcription start site and present in approximately one-third of human genes (17). The average position (-60 from the predicted initiator ATG) of the conserved octamer found in Mimivirus is consistent with it playing a role similar to the TATA box for the expression of the viral genes, provided the 3′ UTRs (5′ UTRs) of viral mRNA are ≈30 nt long on average. Such a compact promoter/3′ UTR structure has been observed in amitochondriate protists such as Giardia intestinalis or Entamoeba histolytica (15). The sequences of the TATA box-like element of these protozoa are also different from the 5′-TATATAAG-3′ consensus identified in the other eukaryotic kingdoms. Accordingly, we propose that the AAAATTGA motif found in 50% of Mimivirus intergenic regions is the virus TATA box-like motif.
Mimivirus TATA Box-Like Motif Is Not Prevalent in Amoebal Organisms. Interestingly, the Mimivirus TATA box-like motif does not bear a particular resemblance (if any) with the different TATA box-like consensus sequences (if any) that have been identified in various protozoan. For instance, the E. histolytica TATA box-like consensus is TATTTAAA (15). Using the available data from a genomic survey sequencing of A. castellanii, we verified that the AAAATTGA motif is not particularly prevalent in the genome of the closest (partially sequenced) relative of the Mimivirus host A. polyphaga. In addition, no significant difference in codon usage between the sets of genes harboring or not harboring the AAAATTGA motif could be detected. Symmetrically, of the 29 Mimivirus genes most likely to have been acquired by lateral transfer (18), 15 exhibit the AAAATTGA motif, and 14 do not have it. These results argue against the hypothesis that this promoter element, together with the large proportion of associated Mimivirus genes, was simply acquired from its host. Interestingly, clusters of paralogous genes that have been produced by multiple rounds of duplications from an ancestral Mimivirus gene (19) conserved the AAAATTGA motifs amidst the divergence of their respective promoter regions. The example of the large gene cluster L175–L185 is shown in Fig. 4.
Fig. 4.
The alignment of the intergenic region of the paralogous gene cluster L175–L185 shows the perfect conservation of the AAAATTGA motif within intergenic regions that have otherwise more extensively diverged. Note that the AAAATTGA motif is part of the C terminus of gene L185 (indicated by white Xs).
The Two Types of Mimivirus Promoters Might Correspond to Early Versus Late Functions. Homologues of the three main proteins involved in the formation of the transcription preinitiation complex have been identified in the Mimivirus genome: the two RNA polymerase II subunits (Rbp1 and Rpb2) and the TFIID (TATA box-binding) initiation factor. The corresponding amino acid sequences are extremely distant from their closest matches: Candida albicans Rpb1 (34% identical), Dictyostelium discoideum Rpb2 (36% identical), and Plasmodium falciparum TFIID (24% identical). We propose that Mimivirus's unique TATA box-like sequence AAAATTGA might have coevolved and might be recognized by a preinitiation complex including these divergent Mimivirus proteins. A second type of promoter that is highly degenerate might then be recognized by the host preinitiation complex or involve a combination of Mimivirus and host-encoded transcription factors. This hypothesis is consistent with the preferential occurrence of the AAAATTGA (type I promoter) in front of Mimivirus genes encoding functions required for the early (or late-early) phase of viral infection (transcription, nucleotide transport, and protein translation) (Table 1). According to this scenario, the corresponding genes could be transcribed in the host cytoplasm. Conversely, the AAAATTGA motif is preferentially absent from the promoter of Mimivirus genes encoding “late” functions such as DNA replication and particle biogenesis and assembly (Table 1). These genes could be transcribed in the host nucleus.
Conclusion
Our bioinformatics and comparative genomics study revealed a unique feature of Mimivirus among the eukaryotic domain: the presence of a highly conserved AAAATTGA motif in the immediate 5′ upstream region of 50% of its protein-encoding genes. By analogy with the known promoter structures of unicellular eukaryotes, amoebal organisms in particular, we propose that this motif corresponds to a TATA box-like core promoter element. This element, and its conservation, appears to be specific of the Mimivirus lineage and might correspond to an ancestral promoter structure predating the radiation of the eukaryotic kingdoms (2). Mimivirus genes exhibiting this type of promoter might be ancestral as well. Interestingly, this observation is true in the case of all translation apparatus-related genes initially identified in a virus (four aminoacyl tRNA synthetases, mRNA cap binding protein, translation factor eF-Tu, and tRNA methyltransferase) as well as in Mimivirus Topoisomerase 1A (bacterial type). However, it is also possible, but less likely, that horizontally acquired ORFs could have been inserted downstream of a preexisting AAAATTGA motif.
More genomic data on amoebal species, other protozoa, and their associated viruses are now needed to better understand the evolutionary scenario through which the unique promoter structure of Mimivirus genes might have emerged. Our findings can now be used to guide the experimental characterization of Mimivirus transcription units and identify the transcription factors involved in the recognition of its uniquely conserved promoter element.
Supplementary Material
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique and a grant from the French National Genopole Network.
Abbreviations: NCLDV, nucleocytoplasmic large DNA virus; COG, Clusters of Orthologous Groups of proteins; URF, unidentified reading frame.
References
- 1.La Scola, B., Audic, S., Robert, C., Jungang, L., de Lamballerie, X., Drancourt, M., Birtles, R., Claverie, J.-M. & Raoult, D. (2003) Science 299, 2033. [DOI] [PubMed] [Google Scholar]
- 2.Raoult, D., Audic, S., Robert, C., Abergel, C., Renesto, P., Ogata, H., La Scola, B., Suzan, M. & Claverie, J.-M. (2004) Science 306, 1344-1350. [DOI] [PubMed] [Google Scholar]
- 3.Ogata, H., Audic, S., Renesto-Audiffren, P., Fournier, P. E., Barbe, V., Samson, D., Roux, V., Cossart, P., Weissenbach, J., Claverie, J.-M. & Raoult, D. (2001) Science 293, 2093-2098. [DOI] [PubMed] [Google Scholar]
- 4.van Ham, R. C., Kamerbeek, J., Palacios, C., Rausell, C., Abascal, F., Bastolla, U., Fernandez, J. M., Jimenez, L., Postigo, M., Silva, F. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters, E., Hohn, M. J., Ahel, I., Graham, D. E., Adams, M. D., Barnstead, M., Beeson, K. Y., Bibbs, L., Bolanos, R., Keller, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12984-12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer, L. M., Aravind, L. & Koonin, E. V. (2001) J. Virol. 75, 11720-11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey, T. L. & Gribskov, M. (1998) Bioinformatics 14, 48-54. [DOI] [PubMed] [Google Scholar]
- 8.Fickett, J. W. & Hatzigeorgiou, A. G. (1997) Genome Res. 7, 861-878. [DOI] [PubMed] [Google Scholar]
- 9.Schneider, T. D. & Stephens, R. M. (1990) Nucleic Acids Res. 18, 6097-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatusov, R. L., Fedorova, N. D., Jackson, J. D., Jacobs, A. R., Kiryutin, B., Koonin, E. V., Krylov, D. M., Mazumder, R., Mekhedov, S. L., Nikolskaya, A. N., et al. (2003) BMC Bioinformatics 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid, C. D., Praz, V., Delorenzi, M., Perier, R. & Bucher, P. (2004) Nucleic Acids Res. 32, D82-D85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao, F., Xuan, Z., Liu, L. & Zhang, M. Q. (2005) Nucleic Acids Res. 33, D103-D107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smale, S. T. & Kadonaga, J. T. (2003) Annu. Rev. Biochem. 72, 449-479. [DOI] [PubMed] [Google Scholar]
- 14.Davuluri, R. V., Grosse, I. & Zhang, M. Q. (2001) Nat. Genet. 29, 412-417. [DOI] [PubMed] [Google Scholar]
- 15.Vanacova, S., Liston, D. R., Tachezy, J. & Johnson, P. J. (2003) Int. J. Parasitol. 33, 235-255. [DOI] [PubMed] [Google Scholar]
- 16.Bettstetter, M., Peng, X., Garrett, R. A. & Prangishvili, D. (2003) Virology 315, 68-79. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki, Y., Tsunoda, T., Sese, J., Taira, H., Mizushima-Sugano, J., Hata, H., Ota, T., Isogai, T., Tanaka, T., Nakamura, Y., et al. (2001) Genome Res. 11, 677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogata, H., Abergel, C., Raoult, D. & Claverie, J.-M. (2005) Science 308, 1114b.15905382 [Google Scholar]
- 19.Suhre, K. (2005) J. Virol., in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.