Abstract
The amygdala is centrally involved in formation of emotional memory and response to fear or risk. We have demonstrated that the lateral and basolateral amygdala nuclei fail to form in neuroD2 null mice and neuroD2 heterozygotes have fewer neurons in this region. NeuroD2 heterozygous mice show profound deficits in emotional learning as assessed by fear conditioning. Unconditioned fear was also diminished in neuroD2 heterozygotes compared to wild-type controls. Several key molecular regulators of emotional learning were diminished in the brains of neuroD2 heterozygotes including Ulip1, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, and GABAA receptor. Thus, neuroD2 is essential for amygdala development and genes involved in amygdala function are altered in neuroD2-deficient mice.
Keywords: basic helix-loop-helix, haploinsufficiency
The amygdala is an almond-shaped brain structure that integrates emotional learning and other emotional responses such as fear perception. The role of the amygdala in fear perception and response was established through lesioning studies in animals and confirmed through positron emission tomography and functional magnetic resonance imaging in normal human subjects and in schizophrenic patients with deficits in fear processing (1-9).
We previously reported that neuroD2-null mice on a mixed genetic background are indistinguishable from littermates at birth, but fail to thrive and die within weeks of birth. Before death, nullizygous mice develop ataxia, seizures, cerebellar degeneration, and growth retardation. NeuroD2 heterozygotes appear normal as adults, but have reduced seizure threshold and mild ataxia. We observed aggressive behavior between neuroD2 heterozygotes, which prompted us to evaluate the function and structure of the amygdala and related limbic system brain regions that control aggression, fear, and other emotions. Unconditioned fear (also referred to as anxiety or risk perception) is the response to environmental cues that signal danger. The basolateral amygdala and ventral subiculum of hippocampus are involved in unconditioned fear responses; however, the neuronal circuitry and molecular mechanisms are poorly understood (10).
In contrast to unconditioned fear, the neurochemical and molecular basis of emotional learning related to conditioned fear are being elucidated through studies that evaluate fear conditioning in the context of pharmacologic or genetic manipulation of neurotransmitter receptors and second messengers. Fear conditioning involves coupling a neutral conditioned stimulus (CS) such as an audible tone with a noxious unconditioned stimulus (US) such as foot shock and observing that after training and rest periods, that the CS alone is sufficient to elicit behavioral (e.g., freezing) and autonomic responses (11, 12). During the training and rest period, the lateral amygdala acquires and consolidates short- and long-term memory that associates the conditioned and unconditioned stimuli through a process known as emotional learning.
The acquisition phase of emotional memory involves activation of calcium-dependent second messenger systems following calcium influx through the NMDA receptors and voltage-gated calcium channels. In particular, Ca2+/calmodulin-dependent protein kinase II is autophosphorylated and translocated to the NR2B subunit of the NMDA receptor, where it remains in an active state even after intracellular calcium levels return to baseline. Glutamate activation of the metabotropic glutamate receptor, mGluR5, may enhance NMDA channel conductance by phosphorylating the NR2B subunit of the NMDA receptor via stimulation of protein kinase C. These processes have been implicated in synaptic strengthening, a key component to emotional learning. In contrast to glutamatergic activation of amygdala neurons, GABAergic interneurons maintain the inhibitory tone of the lateral amygdala (13, 14).
Synaptic strengthening or remodeling continues during a period referred to as the consolidation phase. During consolidation, the cAMP response element-binding protein is activated by kinases such as protein kinase A and mitogen-associated protein kinase resulting in transcriptional activation of genes that contribute to synaptic remodeling. Cytoskeletal proteins, such as Ulip family members, have been implicated in structural changes associated with synaptic strengthening (15-17). During the association of the conditioned and unconditioned stimuli, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor is synthesized and inserted into active synapses, a possible mechanism for strengthening glutamatergic signaling in the postsynaptic neuron.
The neuronal circuits and molecular mechanisms underlying emotional memory have been partly elucidated. However, little is known about the factors that might generate individual variability in emotional learning and memory. Here, we demonstrate that neuroD2, a basic helix-loop-helix (bHLH) transcription factor, is necessary for development of the amygdala. We further show that the dose of neuroD2 gene is critical for regulation of molecules that underlie emotional learning and that neuroD2 heterozygous mice fail to elicit appropriate responses in fear conditioning and unconditioned fear analyses. The haploinsufficiency phenotype at the cellular and behavioral level indicates that both copies of the neuroD2 gene are necessary for amygdala development and function. To our knowledge, neuroD2 is the first among the bHLH transcription factor family to show a haploinsufficiency phenotype in brain development and mammalian behavior.
Materials and Methods
Mouse Husbandry. Mice were bred and genotyped as described in accordance with institutional approved procedures. Congenic strains were generated by crossing neuroD2 heterozygotes with wild-type pure 129SV or C57BL6 mice for at least eight generations.
Histochemistry and Immunostaining. β-Galactosidase staining was performed as described (18). Cryo-protected brains were OCT embedded, and 12-μm frozen sections were cut for Nissl staining (every 10 sections). Mice were anesthetized with avertin and perfused with PBS and then 4% paraformaldehyde. The 50-μm sections were immunostained with antibodies against AMPA receptor (Chemicon, dilution 1:500), GABAA receptors γ subunit (Chemicon, dilution 1:1,000), GluR5/6/7 (Chemicon, dilution 1:1,000), NeuN (Chemicon, dilution 1:1,000) Ulip1/TUC4 (Chemicon, dilution 1:250), and NMDA receptor 1 (Chemicon, dilution 1:1,000). Alexa Fluor 488-conjugated secondary antibody (Molecular Probes) was used for color detection under Ziess LSM 510 META confocal microscope. DAPI was used for counterstain to verify the amygdala region for imaging and counting. Counting was performed on six wild-type and six heterozygous littermates. For E14, the 12-μm sagital frozen sections were incubated with Ulip/TUC4 antibody, followed by secondary biotinylated-rabbit IgG (vector,1:200 dilution), ABC elite, and DAB substrate kit (Vector Laboratories) were used for signal detection.
Apoptosis Detection. TUNEL assay was used to stain for apoptotic cells. E17 and E18 embryos were perfused/fixed and the brains were processed into 12-μm coronal or sagital sections. Every fifth section was analyzed by TUNEL staining according the to manufacturer's directions (Roche Diagnostics, Cell Death Detection kit). Thirteen wild-type and 12 neuroD2-null mouse embryos were analyzed. The percentage of TUNEL-positive cells/DAPI was used for quantitation. Histochemical studies on neuroD2-null mouse brain were conducted on F1 hybrid offspring of C57Bl6 and 129 heterozygous breeding pairs because these mice live longer than purebred neuroD2-null mice.
Behavioral Studies. The stainless steel elevated plus-maze contained two open arms (30 × 5 cm) and two arms (30 × 5 cm) enclosed by 30-cm-high walls. The platform was 40 cm from the floor and lit by dim light. Mice were placed in the central square at the beginning of 2-min trials. The numbers of entries into open and wall-enclosed arms and the time spent in open and closed arms were recorded. An arm entry or exit was defined as all four paws passing the arm entry plane. The light/dark box test was based on the initial model described by Crawley and Goodwin (21).
Contextual and Cued Fear Conditioning Method. Contextual and cued fear conditioning paradigms were performed as described with some modifications (11).
Chromatin Immunoprecipitation (ChIP) for Ulip. Primary neuronal culture from ≈P3-P5 mouse brains were cultured and pulled down by neuroD2 antibody to identify the regulatory region of Ulip promoter. Briefly, brains were dissected, trypsinized, and cultured in DMEM/F12 medium with 2% BSA and N2 supplement. Cells were fixed with 2% formaldehyde before sonication; subsequently, lysate was precleared with rabbit serum before pulled down by neuroD2 antibody and protein A-Sepharose. Pull-down mixture was decrosslinked and DNA was purified by QiaQuick spin kit (Qiagen). PCR was performed with primers surrounding potential E box (CAGATG) about -400 and -300 from ATG on Ulip promoter. Input DNA before pull-down was used as positive control. The same amount of DNA was analyzed by PCR with pancreatic amylase primer set for nonspecific ChIP control (19). ChIP by β-tubulin pull-down and PCR was used as negative control.
Western Blot. Protein extract from forebrain of wild-type and neuroD2+/- mice was equally loaded into SDS/10% PAGE for electrophoresis, then transferred to PROTRAN nitrocellulose membrane (Schleicher & Schuell). After blotting with primary Ulip/TUC4 (1:1,000) and secondary HRP conjugate antibody, Lumi-Light Western blotting substrate kit (Roche Diagnostics) was used for signal detection according to the manufacturer's instructions. γ-Tubulin was used as internal control for normalization.
Results
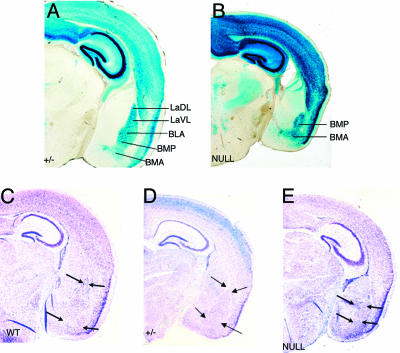
NeuroD2 Null Mice Lack Nuclei of the Lateral and Basolateral Amygdala. The coding region of neuroD2 is replaced by the LacZ gene in neuroD2+/- and -/- mice, allowing detection of neuroD2-positive cells in β-galactosidase stained sections (20). Analysis of serial coronal sections through amygdala of neuroD2+/- mice revealed that neuroD2 is expressed in the dorsal and ventral parts of lateral amygdala (LaDL and LaVL; together, LA), basolateral amygdala (BLA), and basomedial amgdala posterior (BMP) (Fig. 1A). In neuroD2-null mice, β-galactosidase staining was similar to the expression pattern in heterozygous mice in most brain regions, but was absent in LaDL, LaVL, and BLA (Fig. 1B). Histopathologic evaluation by Nissl staining, which stains nuclei of neurons and glia, confirmed the absence of the LA and BLA nuclei in neuroD2-null mice and the presence of these nuclei in heterozygous mice (Fig. 1 C-E). Analysis of Nissl-stained serial sections from Bregma approximately -4.8 to -1.7 mm revealed that no other nuclei or identifiable neuronal populations were absent in amygdala, hippocampus, lateral cortex, or subiculum. The absence of neuroD2 locus expression in specific regions of amygdala suggested that the formation of these nuclei depends on neuroD2 activity.
Fig. 1.
Histochemical analysis. (A and B) X-gal staining on 50-μm coronal sections through amygdala region of neuroD2-heterozygote and -null mouse brain; images were taken from brain regions covering amygdala with representative phenotype. Nullizygous mouse brains stain darker because they express two copies of the β-galactosidase gene compared to one in heterozygotes. (C and D) Nissl-stained 12-μm coronal sections confirm the absence of LA and BLA. This finding was consistent in all serial sections from Bregma ≈4.8-1.7 mm (representative section shown). Arrows indicates the amygdala region. BLA, basolateral amygdala anterior; BMA, basomedial amygdala anterior; BMP, basomedial amygdala posterior; LaDL, dorsal lateral amygdala; LaVL, ventral lateral amygdala.
The amygdala nuclei are generated during mouse E16 and E17. To distinguish between the possibilities that the nuclei fail to form versus that they form but undergo apoptosis, we evaluated TUNEL staining in serial brain sections from E17 or E18 pups. There was no significant difference in TUNEL staining in the amygdala region of neuroD2-null mice compared to wild-type controls (E17: 15 ± 6% for wild type, 11 ± 12% for null; E18: 5 ± 3% for wild type, 4 ± 3% for null, n ≥ 6 per condition, data not shown). The absence of elevated apoptosis in the amygdala at E17 and E18 and the absence of the LA and BLA at later time points suggest that formation of the LA and BLA depends on neuroD2 activity.
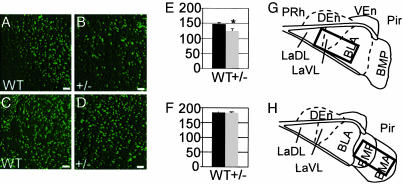
NeuroD2 Heterozygous Mice Show Incomplete Amygdala Development. The profound neuroanatomic phenotype in the amygdala of neuroD2-null mice prompted us to evaluate the neuroD2 heterozygous mice to determine whether the dose of neuroD2 was important for amygdala development or function. RT-PCR and Western analysis demonstrated that the level of neuroD2 mRNA and protein in heterozygotes is 16 ± 6% and 31 ± 14% of wild type, respectively (not shown, n = 3). To quantitatively determine whether neuroD2 heterozygotes exhibit neuronal loss, we stained for mature neurons with NeuN antibody on serial coronal sections of mouse brain (Bregma approximately -3.8 to -1.2 mm). In the regions of lateral and basolateral amygdala, the number of NeuN-positive cells was 147 ± 6 in wild type and 124 ± 8 in neuroD2+/-. In the regions of anterior and posterior basomedial amygdala, the number of NeuN positive cells was 198 ± 8 in wild type and 191 ± 4 in neuroD2+/-. The neuronal cell loss was significant in lateral and basolateral amygdala (Fig. 2 A, B, and E), but no significant difference was found in basomedial amygdala (Fig. 2 C, D, and F).
Fig. 2.
Counting of mature neurons with NeuN immunostaining. Confocal images of NeuN immunostaining for wild-type (A) and neuroD2+/- (B) are shown. Sections covering lateral and basolateral amygdala were stained and counted (Bregma approximately -1.4 to -1.7 mm). Confocal images of NeuN immunostaining for wild-type (C) and neuroD2+/- (D) are shown. Sections covering anterior and posterior of basomedial amygdala were stained and counted (Bregma approximately -1.4 to -1.7 mm). (E) Graph represents the cell count results for NeuN immunostaining of wild-type and heterozygotes in lateral and basolateral amygdala. Data shown as mean ± SEM with significance determined by t test (*, P < 0.05). (F) Graph represents the cell count results for NeuN immunostaining of wild-type and heterozygotes in anterior and posterior of basomedial amygdala. Data are shown as mean ± SEM, no significant difference was observed. (Scale bar, 50 μm.) Analysis was conducted in three sets of littermates. The boxed regions in G and H represent pictures shown in A-D and cell count shown in E and F.
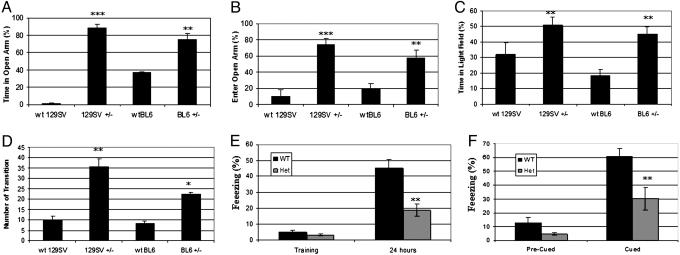
NeuroD2 Heterozygous Mice Respond Inappropriately to Risk. The amygdala and subiculum are involved in responding to environmental cues of risk that have not been experimentally conditioned. The standard behavioral tests for unconditioned anxiety are the elevated plus-maze and the light-dark box test (21-24), which are based on avoidance of unprotected (open) arms and unprotected (light) box, respectively. In a 2-min trial of the elevated plus-maze, neuroD2+/- mice on a pure 129SV background spent an average of 89 ± 4% of time in the open arms as opposed to wild-type controls that spent 1.1 ± 0.9% of time in the open arms (Fig. 3A). Because mouse strain can affect behavioral studies, the experiment was repeated on a nearly pure C57BL6 background and showed similar results (Fig. 3A). NeuroD2+/- mice entered the open arms 74 ± 7% of the time as opposed to wild-type controls that made 10 ± 3% of transitions into the open arms (Fig. 3B). NeuroD2-null mice were not suited for behavioral testing because they die within 48 h of birth on pure C57BL6 or 129SV backgrounds. During the trial, five mice fell from the open arm and two had seizures. These mice were excluded from statistical analyses.
Fig. 3.
Unconditioned and conditioned fear test. (A) Time in open arm of elevated plus maze. (B) Frequency of entry into open arm of elevated plus maze. (C) Time in open field of light dark box. (D) Number of transitions between open and dark field. Data shown as mean ± SEM for 10-12 mice per condition. (E) Contextual long-term memory was observed by freezing response during the training period and 24 h later. Data are presented as mean ± SEM for 10-13 mice per condition. (F) Cued-contextual long-term memory was assessed by freezing percentage during the precued and cued periods after 24-h training. Data are presented as mean ±SEM for 10-13 mice per condition. Significance was determined by Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
In the light-dark box test, wild-type 129SV mice spent the majority of the observation period in the dark box, whereas neuroD2+/- mice spent approximately equal time in the dark and light box (Fig. 3C). Similar results were obtained on the C57BL6 background (Fig. 3C). NeuroD2+/- mice transitioned between light and dark fields ≈3 times more frequently than wild-type controls (Fig. 3D). The results of the plus maze and light-dark box test indicate that neuroD2+/- mice fail to avoid unprotected areas in unconditioned anxiety paradigms. The behavioral phenotype of neuroD2 heterozygous mice demonstrates that neuroD2 is essential for risk perception and response.
Emotional Learning Is Impaired in neuroD2 Heterozygous Mice. Albeit modest, the reduction in neuron number in the basolateral and lateral amygdala prompted evaluation of emotional memory generation in neuroD2+/- mice. Fear conditioning, a powerful procedure for studying emotional learning, was used because sensory inputs to the amygdala terminate in the lateral nucleus (LA) and damage to LA interferes with fear conditioning (25). The initial training of mice involved a pure tone CS and a mild foot shock US and the primary endpoint was freezing response. All mice obtained in this test was responded to tone stimulus indicating they have no hearing problem. Contextual freezing at 24 h was 45 ± 5 times for wild type and 19 ± 4 in neuroD2+/- mice. Precued freezing occurred 13 ± 4 times in wild type and 5 ± 1 times in neuroD2+/- mice, whereas postcued freezing occurred 61 ± 6 times in wild type and 30 ± 8 in heterozygous mice. Thus, neuroD2+/- mice had impaired contextual and cued-contextual long-term memory (Fig. 3 E and F).
Receptors Involved in Emotional Memory Consolidation Are Reduced in neuroD2 Heterozygotes. Acquisition of fear conditioning involves the convergence of neuronal inputs from the CS and US pathway to lateral amygdala during training. CS inputs specifically lead to the release of glutamate, which binds to glutamate receptors. These glutamate receptors, including AMPA receptors, NMDA receptors, and metabotropic glutamate receptors (mGluRs), play a key role in fear conditioning in the lateral amygdala (14). The AMPA receptor differs from the others in that it is synthesized and inserted into LA synapses during fear conditioning (26). To quantitatively determine whether a neuronal subtype of glutamate receptors is affected in neuroD2 heterozygotes, we immunostained for AMPA receptors (Fig. 4 A and B), mGluRs (Fig. 4 D and E), and NMDA receptors (Fig. 4 G and H). In the region of lateral and basolateral amygdala, the number of AMPA receptor-positive cells was 76 ± 3 in wild type and 11 ± 2 in neuroD2+/- (Fig. 4C). The number of mGluR5-positive cells was 52 ± 3 in wild type and 57 ± 4 in neuroD2+/- (Fig. 4F). The number of NMDA receptor-positive cells was 53 ± 9 in wild type and 57 ± 9 in neuroD2+/- (Fig. 4I). These results demonstrated that AMPA receptor is reduced in lateral and basolateral amygdala in neuroD2 heterozygotes. However, there is no significant difference of AMPA receptor-positive cells in hippocampus and neocortex between wild type and neuroD2+/- (data not shown).
Fig. 4.
Reduction of molecular determinants of fear conditioning. (A, D, G, and J) Confocal images of AMPA receptor, GluR5 receptor, NMDA receptor, and GABAA receptor γ immunostaining in wild type, respectively. Sections used for this analysis were registered with adjacent sections stained for NeuN to ensure that the entire population of neurons in the lateral and basolateral amygdala were counted (Bregma approximately -1.4 to -1.7 mm). (B, E, H, and K) Confocal images of AMPA receptor, GluR5 receptor, NMDA receptor, and GABAA receptor γ immunostaining in neuroD2+/-, respectively. Sections covering lateral and basolateral amygdala were stained and counted (Bregma approximately -1.4 to -1.7 mm). (C, F, I, and L) Graph represents the cell count results for AMPA receptor, GluR5 receptor, NMDA receptor, and GABAA receptor γ immunostaining of wild type and heterozygotes. Data shown as mean ± SEM with significance determined by t test (P < 0.001 for AMPA receptor). Representative images from basolateral amygdala region are shown. (Scale bar, 50 μm.) Analysis was conducted in three sets of littermates. Sections used for this analysis were registered with adjacent sections stained for NeuN to ensure that the same population of neurons in the lateral and basolateral amygdala were counted. Regions counted were identical to that shown in Fig. 3G.
GABAergic neurons maintain inhibitory tone in the LA. The GABAA receptor γ subunit is enriched in the basolateral amygdala of both humans and rodents (27-31). We analyzed GABAA receptor γ expression in the amygdala of wild-type and neuroD2+/- mice by immunostaining (Fig. 4 J and K). In the region of lateral and basolateral amygdala, the number of GABAA receptor γ-positive cells was 84 ± 2 in wild type and 51 ± 3 in neuroD2+/- (Fig. 4L). These results demonstrated significant reduction in GABAA receptor γ staining in neuroD2 heterozygotes.
Ulip1 Is Reduced in neuroD2-Deficient Mice and Is a Direct neuroD2 Target. Ulip1 (TUC4/CRMP/UNC-33/Dpysl3) is a neuroD2-regulated gene identified in unpublished microarray studies in our laboratory. Ulip1 protein is involved in synapse structural remodeling by promoting microtubule assembly and in neurite outgrowth during embryogenesis (15-17). Synaptic remodeling also occurs during consolidation of emotional memory in lateral amygdala (17); therefore, we asked whether Ulip1 expression was altered in neuroD2 mutant mice.
We analyzed Ulip1 expression in neuroD2-null mouse embryos at E14 by immunostaining. Ulip1 expression is reduced in neopallial cortex and adjacent brain regions including developing amygdala, but is not significantly decreased in the midbrain or diencephalon (data not shown). Immunostaining of the amygdala region of postnatal day 7 brain sections also revealed lower expression of Ulip1 in neuroD2 heterozygous mice (Fig. 5 A and B), suggesting that neuroD2 plays a role in regulation of neurite outgrowth through Ulip1 during the peak period of neuronal differentiation. Consistent with these results, four sets of littermates were analyzed by Western blot and showed reduced Ulip1 levels in neuroD2+/- mice compared to controls (Fig. 5 C and D).
Fig. 5.
Ulip1 is the direct target of neuroD2. Immunostaining of Ulip1 on postnatal day 7 brain sections from wild type (A) and neuroD2 (B) heterozygotes. Image shows the coronal view of the amygdala. (C) Western blot analysis of Ulip1 expression in forebrain of wild type and neuroD2+/-; internal loading control γ-tubulin is shown in D. (E) ChIP for primary neuronal progenitor cells was performed with neuroD2 antibody and PCR with primer set surrounding a neuroD2-preferred E box sequence on Ulip promoter. Input titration and nonspecific ChIP (NS) was performed at the same PCR condition. (F) The same amount of DNA was amplified by PCR with pancreatic amylase primers as nonspecific ChIP control.
We previously showed that P19 embryonal carcinoma cells underwent neuronal differentiation in response to transfected neuroD2 and a neuroD2 dimerization partner, E12. To determine whether Ulip1 is a direct target of neuroD2, we assessed Ulip1 mRNA levels by RT-PCR in P19 cells 48 h after transfection with neuroD2 and E12. Ulip1 mRNA was induced 5.4 ± 0.1 fold by neuroD2/E12 in P19 cells (data not shown). We next applied ChIP assays to determine whether Ulip1 is a direct target of neuroD2. The promoter of Ulip1 contains an E box sequence that binds neuroD2/E12 heterodimers in vitro. ChIP assays using a neuroD2 antibody in three sets of primary neuronal cell cultures from ≈P3-P5 mouse brains, showed enrichment of Ulip1 promoter (Fig. 5E). Under the same conditions, the amylase promoter was not enriched (Fig. 5F), indicating specificity of neuroD2 for the Ulip1 promoter. Based on the induction of Ulip1 mRNA in P19 cells by neuroD2, direct association of neuroD2 with the Ulip1 promoter, the presence of a neuroD2-preferred E box in the Ulip1 promoter, and the reduction of Ulip1 in neuroD2 null mice, we conclude that Ulip1 is a direct target of neuroD2.
Discussion
Although the function and neuronal circuitry of the amygdala are well described, little is known about the genetic and molecular basis of amygdala development. In this study, we demonstrate that both copies of the neuroD2 bHLH transcription factor gene are necessary for amygdala development and function. Immune system bHLH proteins have been shown to be haplo-sensitive (32, 33). In this study, neuroD2 showed a haploinsufficiency phenotype and regulated amygdala development and function.
The absence of the BLA and LA in neuroD2-null mice is reminiscent of the absence of specific neuronal populations in mice that lack neurogenin, neurogenin2, math1, mash1, and neuroD (34-43). Little is known about the reasons that certain populations of neurons are formed but others are absent in mice that are nullizygous for certain neurogenic bHLH factors.
The Potential Mechanism of neuroD2 in Neuronal Outgrowth and Differentiation. Nervous system development requires neurite outgrowth and guidance to establish accurate synaptic contacts. During corticogenesis at ≈E14-E17, a homogenous population of proliferative precursor cells develop into a diverse array of terminally differentiated neurons. Attracting and repelling signaling molecules such as the netrins and collapsins, members of the semaphorin protein family, direct neuronal outgrowth and axonal guidance. Ulip1, an intracellular phosphoprotein that is regulated by Rho kinase, has been implicated as a regulator of semaphorin signaling. Studies in the nematode demonstrate that Unc33, the Ulip1 homolog, is necessary for both axon guidance and outgrowth (44). In Xenopus, a closely related family member, Ulip2 (CRMP-62, CRMP-2) regulates semaphorin mediated growth cone collapse (45). In a manner analogous to axon genesis and guidance, synaptic remodeling in memory formation requires reorganization of cytoskeletal proteins (14, 17).
Ulip1 is up-regulated during generation of postmitotic neurons and is expressed in newly born neurons in the developing nervous system and in cells that undergo neuronal differentiation in response to retinoic acid (46-48). Ulip1 reaches its highest expression level in differentiating neurons during peak periods of axonal growth and is down-regulated afterward in most brain regions, but remains expressed in LA and BLA, where emotional memory is formed (49, 50). During embryogenesis, neuroD2 is expressed in advance of Ulip1. We have shown that Ulip is decreased in LA and BLA of neuroD2 heterozygotes and that neuroD2 directly regulates Ulip1 expression. Thus, neuroD2 appears to transcriptionally regulate a key component of the signal amplification system involved in axon outgrowth, guidance, and synaptic remodeling.
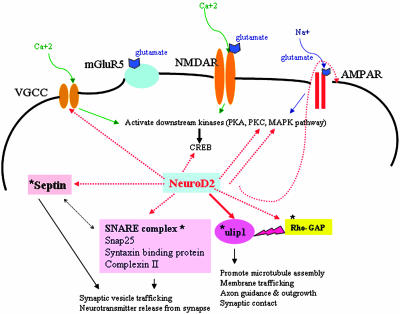
Molecules That Form Emotional Memory Are Decreased in neuroD2-Null Mice. In neuroD2-heterozygous mice, at least two other molecules besides Ulip1 that contribute to emotional memory formation are decreased. The AMPA receptor and the GABAA receptor γ subunit were diminished to 14% and 60% of control, respectively. This decease cannot be explained by cell loss, as other neurotransmitter receptor levels were normal and the number of NeuN-positive cells was decreased to 84% of controls. Fig. 6 shows the molecules that are known to be involved in emotional memory formation that are diminished in neuroD2 heterozygotes. We propose that the reduction of Ulip1, the AMPA receptor, and the GABAA receptor γ subunit coupled with the reduced number of LA and BLA neurons underlies the deficiencies in emotional learning in neuroD2 heterozygous mice (Fig. 6).
Fig. 6.
Potential molecular mechanism of neuroD2 influence on amygdala development and emotional learning. The reduction of AMPA receptor, GABAA receptor γ subunit, and Ulip1 underlies the deficiencies in emotional learning in neuroD2 heterozygous mice. Other neuroD2 targets are involved in synaptic vesicle trafficking, signal transduction pathways, or neuronal structural protein remodeling. Molecules marked with an asterisk are from unpublished microarray data from our laboratory. Dotted red arrows represent genes that are decreased in neuroD2 heterozygous or null mice, and the solid red arrow represents a direct target of neuroD2.
Neither the AMPA receptor nor the GABAA receptor γ subunit were increased at the RNA level by neuroD2 in P19 cells (data not shown). These genes are either context-specific targets of neuroD2 or they are altered in response to aberrant neurotransmission in nuclei affected by the dose of neuroD2. Alternatively, it is possible that neurotransmitter receptor genes are late direct or indirect targets of neuroD2, in a manner analogous to certain targets of the myogenic bHLH gene, MyoD (51).
Our data support a model in which neuroD2 is not only essential for amygdala development but also for regulation of molecules involved in emotional learning, although we cannot exclude the possibility that the changes in AMPA receptor levels or GABAA receptor γ subunit were secondary to secondary developmental changes in neuroD2-deficient mice. This finding is analogous to neuroD, which is essential for beta cell formation in pancreas and also for transcriptional regulation of insulin in the developed pancreas (52, 53).
Evolutionary Considerations for the Role of neuroD2 in Emotional Memory Formation. Previous studies have pointed out parallels between the role of bHLH proteins in the neurogenin, Mash, and Math families in developing mouse brain and their respective orthologs in Drosophila (39, 42, 54-57). For example, mouse atonal homolog 1 (Math1) is necessary for development of cerebellar granule cells, pontine ganglia, and multiple neuronal populations involved in proprioception (41-43), and the Drosophila homolog atonal has been sufficient to rescue the CNS and PNS phenotype in math1 null mice (56); this has led to the suggestion that certain neurogenic bHLH proteins are functionally conserved between Drosophila and mammals.
Unlike ngn, Mash, and Math family members, neuroD and neuroD2 lack Drosophila orthologs, although they seem most likely to have evolved from atonal homologs (54, 58). This study revealed a previously unrecognized role for neuroD2 in the generation and differentiation of neurons in the amygdala. The expression of Ulip1, AMPA receptor, and GABAA receptor γ is decreased in neuroD2 heterozygous mouse brain in LA and BLA. Therefore, the dosage of neuroD2 affects the molecules that govern emotional learning and fear response. This finding raises the question of whether neurogenic bHLH proteins that evolved beyond insects play a role in development of brain regions that have no known anatomic or functional equivalent in invertebrates. The fact that neuroD2 heterozygous mice have a single copy of the gene, yet still show profound differences from normal mice, raises the possibility that polymorphisms that affect neuroD2 expression or function during brain development might contribute to the manner in which mice, and perhaps humans, respond to one another and their environment.
Acknowledgments
We thank Thomas Russell for technical assistance. This work was supported by National Institutes of Health Grants NS36086 and AR045113 (to S.J.T.) and a Burroughs Wellcome Career Award (to J.M.O.).
Abbreviations: CS, conditioned stimulus; US, unconditioned stimulus; bHLH, basic helix-loop-helix; ChIP, chromatin immunoprecipitation; LA, lateral amygdala; BLA, basolateral amygdala; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; En, embryonic day n; Pn, postnatal day n.
References
- 1.Adolphs, R., Tranel, D., Damasio, H. & Damasio, A. (1994) Nature 372, 669-672. [DOI] [PubMed] [Google Scholar]
- 2.Pitkanen, A., Savander, V. & LeDoux, J. E. (1997) Trends Neurosci. 20, 517-523. [DOI] [PubMed] [Google Scholar]
- 3.Scott, S. K., Young, A. W., Calder, A. J., Hellawell, D. J., Aggleton, J. P. & Johnson, M. (1997) Nature 385, 254-257. [DOI] [PubMed] [Google Scholar]
- 4.Sprengelmeyer, R., Young, A. W., Schroeder, U., Grossenbacher, P. G., Federlein, J., Buttner, T. & Przuntek, H. (1999) Proc. R. Soc. London B 266, 2451-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adolphs, R., Tranel, D. & Damasio, H. (2001) Neuropsychology 15, 396-404. [DOI] [PubMed] [Google Scholar]
- 6.Krolak-Salmon, P., Henaff, M. A., Vighetto, A., Bertrand, O. & Mauguiere, F. (2004) Neuron 42, 665-676. [DOI] [PubMed] [Google Scholar]
- 7.Adolphs, R., Tranel, D. & Buchanan, T. W. (2005) Nat. Neurosci. 8, 512-518. [DOI] [PubMed] [Google Scholar]
- 8.Adolphs, R., Gosselin, F., Buchanan, T. W., Tranel, D., Schyns, P. & Damasio, A. R. (2005) Nature 433, 68-72. [DOI] [PubMed] [Google Scholar]
- 9.Gur, R. E., McGrath, C., Chan, R. M., Schroeder, L., Turner, T., Turetsky, B. I., Kohler, C., Alsop, D., Maldjian, J., Ragland, J. D. & Gur, R. C. (2002) Am. J. Psychiatry 159, 1992-1999. [DOI] [PubMed] [Google Scholar]
- 10.French, S. J., Hailstone, J. C. & Totterdell, S. (2003) Brain Res. 981, 160-167. [DOI] [PubMed] [Google Scholar]
- 11.Impey, S., Smith, D. M., Obrietan, K., Donahue, R., Wade, C. & Storm, D. R. (1998) Nat. Neurosci. 1, 595-601. [DOI] [PubMed] [Google Scholar]
- 12.LeDoux, J. (2003) Cell Mol. Neurobiol. 23, 727-738. [DOI] [PubMed] [Google Scholar]
- 13.Stutzmann, G. E. & LeDoux, J. E. (1999) J. Neurosci. 19, RC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues, S. M., Schafe, G. E. & LeDoux, J. E. (2004) Neuron 44, 75-91. [DOI] [PubMed] [Google Scholar]
- 15.Gu, Y. & Ihara, Y. (2000) J. Biol. Chem. 275, 17917-17920. [DOI] [PubMed] [Google Scholar]
- 16.Fukata, Y., Itoh, T. J., Kimura, T., Menager, C., Nishimura, T., Shiromizu, T., Watanabe, H., Inagaki, N., Iwamatsu, A., Hotani, H. & Kaibuchi, K. (2002) Nat. Cell Biol. 4, 583-591. [DOI] [PubMed] [Google Scholar]
- 17.Govek, E. E., Newey, S. E. & Van Aelst, L. (2005) Genes Dev. 19, 1-49. [DOI] [PubMed] [Google Scholar]
- 18.Lin, C. H., Stoeck, J., Ravanpay, A. C., Guillemot, F., Tapscott, S. J. & Olson, J. M. (2004) Dev. Biol. 265, 234-245. [DOI] [PubMed] [Google Scholar]
- 19.Penn, B. H., Bergstrom, D. A., Dilworth, F. J., Bengal, E. & Tapscott, S. J. (2004) Genes Dev. 18, 2348-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson, J. M., Asakura, A., Snider, L., Hawkes, R., Strand, A., Stoeck, J., Hallahan, A., Pritchard, J. & Tapscott, S. J. (2001) Dev. Biol. 234, 174-187. [DOI] [PubMed] [Google Scholar]
- 21.Crawley, J. & Goodwin, F. K. (1980) Pharmacol. Biochem. Behav. 13, 167-170. [DOI] [PubMed] [Google Scholar]
- 22.File, S. E. & Aranko, K. (1988) Neuropsychobiology 20, 82-86. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers, R. J., Haller, J., Holmes, A., Halasz, J., Walton, T. J. & Brain, P. F. (1999) Physiol. Behav. 68, 47-53. [DOI] [PubMed] [Google Scholar]
- 24.Bourin, M. & Hascoet, M. (2003) Eur. J. Pharmacol. 463, 55-65. [DOI] [PubMed] [Google Scholar]
- 25.LeDoux, J. E., Cicchetti, P., Xagoraris, A. & Romanski, L. M. (1990) J. Neurosci. 10, 1062-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rumpel, S., Ledoux, J., Zador, A. & Malinow, R. (2005) Science 308, 83-88. [DOI] [PubMed] [Google Scholar]
- 27.Crestani, F., Lorez, M., Baer, K., Essrich, C., Benke, D., Laurent, J. P., Belzung, C., Fritschy, J. M., Luscher, B. & Mohler, H. (1999) Nat. Neurosci. 2, 833-839. [DOI] [PubMed] [Google Scholar]
- 28.Crestani, F., Martin, J. R., Mohler, H. & Rudolph, U. (2000) Nat. Neurosci. 3, 1059. [DOI] [PubMed] [Google Scholar]
- 29.Benes, F. M. & Berretta, S. (2001) Neuropsychopharmacology 25, 1-27. [DOI] [PubMed] [Google Scholar]
- 30.McDonald, A. J. & Mascagni, F. (2004) J. Comp. Neurol. 473, 137-146. [DOI] [PubMed] [Google Scholar]
- 31.Yilmazer-Hanke, D. M., Roskoden, T., Zilles, K. & Schwegler, H. (2003) Behav. Brain Res. 145, 145-159. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang, Y., Soriano, P. & Weintraub, H. (1994) Cell 79, 875-884. [DOI] [PubMed] [Google Scholar]
- 33.Quong, M. W., Martensson, A., Langerak, A. W., Rivera, R. R., Nemazee, D. & Murre, C. (2004) J. Exp. Med. 199, 1101-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parras, C. M., Schuurmans, C., Scardigli, R., Kim, J., Anderson, D. J. & Guillemot, F. (2002) Genes Dev. 16, 324-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cau, E., Gradwohl, G., Fode, C. & Guillemot, F. (1997) Development (Cambridge, U.K.) 124, 1611-1621. [DOI] [PubMed] [Google Scholar]
- 36.Fode, C., Ma, Q., Casarosa, S., Ang, S. L., Anderson, D. J. & Guillemot, F. (2000) Genes Dev. 14, 67-80. [PMC free article] [PubMed] [Google Scholar]
- 37.Pattyn, A., Simplicio, N., Van Doorninck, J. H., Goridis, C., Guillemot, F. & Brunet, J. F. (2004) Nat. Neurosci. 7, 589-595. [DOI] [PubMed] [Google Scholar]
- 38.Ma, Q., Kintner, C. & Anderson, D. J. (1996) Cell 87, 43-52. [DOI] [PubMed] [Google Scholar]
- 39.Ma, Q., Chen, Z., del Barco Barrantes, I., de la Pompa, J. L. & Anderson, D. J. (1998) Neuron 20, 469-482. [DOI] [PubMed] [Google Scholar]
- 40.Fode, C., Gradwohl, G., Morin, X., Dierich, A., LeMeur, M., Goridis, C. & Guillemot, F. (1998) Neuron 20, 483-494. [DOI] [PubMed] [Google Scholar]
- 41.Bermingham, N. A., Hassan, B. A., Price, S. D., Vollrath, M. A., Ben-Arie, N., Eatock, R. A., Bellen, H. J., Lysakowski, A. & Zoghbi, H. Y. (1999) Science 284, 1837-1841. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Arie, N., Hassan, B. A., Bermingham, N. A., Malicki, D. M., Armstrong, D., Matzuk, M., Bellen, H. J. & Zoghbi, H. Y. (2000) Development (Cambridge, U.K.) 127, 1039-1048. [DOI] [PubMed] [Google Scholar]
- 43.Bermingham, N. A., Hassan, B. A., Wang, V. Y., Fernandez, M., Banfi, S., Bellen, H. J., Fritzsch, B. & Zoghbi, H. Y. (2001) Neuron 30, 411-422. [DOI] [PubMed] [Google Scholar]
- 44.Hedgecock, E. M., Culotti, J. G., Thomson, J. N. & Perkins, L. A. (1985) Dev. Biol. 111, 158-170. [DOI] [PubMed] [Google Scholar]
- 45.Goshima, Y., Nakamura, F., Strittmatter, P. & Strittmatter, S. M. (1995) Nature 376, 509-514. [DOI] [PubMed] [Google Scholar]
- 46.Minturn, J. E., Fryer, H. J., Geschwind, D. H. & Hockfield, S. (1995) J. Neurosci. 15, 6757-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minturn, J. E., Geschwind, D. H., Fryer, H. J. & Hockfield, S. (1995) J. Comp. Neurol. 355, 369-379. [DOI] [PubMed] [Google Scholar]
- 48.Byk, T., Ozon, S. & Sobel, A. (1998) Eur. J. Biochem. 254, 14-24. [DOI] [PubMed] [Google Scholar]
- 49.Quinn, C. C., Gray, G. E. & Hockfield, S. (1999) J. Neurobiol. 41, 158-164. [PubMed] [Google Scholar]
- 50.Quinn, C. C., Chen, E., Kinjo, T. G., Kelly, G., Bell, A. W., Elliott, R. C., McPherson, P. S. & Hockfield, S. (2003) J. Neurosci. 23, 2815-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergstrom, D. A., Penn, B. H., Strand, A., Perry, R. L., Rudnicki, M. A. & Tapscott, S. J. (2002) Mol. Cell 9, 587-600. [DOI] [PubMed] [Google Scholar]
- 52.Naya, F. J., Huang, H. P., Qiu, Y., Mutoh, H., DeMayo, F. J., Leiter, A. B. & Tsai, M. J. (1997) Genes Dev. 11, 2323-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang, H. P., Chu, K., Nemoz-Gaillard, E., Elberg, D. & Tsai, M. J. (2002) Mol. Endocrinol. 16, 541-551. [DOI] [PubMed] [Google Scholar]
- 54.Ben-Arie, N., McCall, A. E., Berkman, S., Eichele, G., Bellen, H. J. & Zoghbi, H. Y. (1996) Hum. Mol. Genet. 5, 1207-1216. [DOI] [PubMed] [Google Scholar]
- 55.Hassan, B. A. & Bellen, H. J. (2000) Genes Dev. 14, 1852-1865. [PubMed] [Google Scholar]
- 56.Wang, V. Y., Hassan, B. A., Bellen, H. J. & Zoghbi, H. Y. (2002) Curr. Biol. 12, 1611-1616. [DOI] [PubMed] [Google Scholar]
- 57.Bertrand, N., Castro, D. S. & Guillemot, F. (2002) Nat. Rev. Neurosci. 3, 517-530. [DOI] [PubMed] [Google Scholar]
- 58.Ledent, V., Paquet, O. & Vervoort, M. (2002) Genome Biol. 3, RESEARCH0030. [DOI] [PMC free article] [PubMed] [Google Scholar]