Abstract
Objective To evaluate whether routine administration of sublingual misoprostol 600 μg after delivery reduces postpartum haemorrhage.
Design Randomised double blind placebo controlled trial.
Setting Primary health centre in Bissau, Guinea-Bissau, West Africa.
Participants 661 women undergoing vaginal delivery.
Intervention Misoprostol 600 μg or placebo administered sublingually immediately after delivery.
Main outcome measures Postpartum haemorrhage, defined as a loss of ≥ 500 ml and decrease in haemoglobin concentration after delivery.
Results The incidence of postpartum haemorrhage was not significantly different between the two groups, the relative risk being 0.89 (95% confidence interval 0.76 to 1.04) in the misoprostol group compared with the placebo group. Mean blood loss was 10.5% (-0.5% to 20.4%) lower in the misoprostol group than in the control group. Severe postpartum haemorrhage of ≥ 1000 ml or ≥ 1500 ml occurred in 17% (56) and 8% (25) in the placebo group and 11% (37) and 2% (7) in the misoprostol group. Significantly fewer women in the misoprostol group experienced a loss of ≥ 1000 ml (0.66, 0.45 to 0.98) or ≥ 1500 ml (0.28, 0.12 to 0.64). The decrease in haemoglobin concentration tended to be less in the misoprostol group, the mean difference between the two groups being 0.16 mmol/l (-0.01 mmol/l to 0.32 mmol/l).
Conclusion Sublingual misoprostol reduces the frequency of severe postpartum haemorrhage.
Introduction
In low income countries many pregnant women are anaemic.1 Maternal mortality is four times higher in severely anaemic women than in those who are not anaemic.2 In Guinea-Bissau, West Africa, 31% of pregnant women are anaemic3 and the maternal mortality is more than 8 per 1000 live births, postpartum haemorrhage being the most common cause of death.4 The primary cause of postpartum haemorrhage is uterine atony, which accounts for 70% of cases.5
Several drugs reduce postpartum haemorrhage by causing the uterus to contract. Ergot derivatives have been used for decades, although oxytocin is the drug of choice in some centres. Several prostaglandins are used as second or third line agents. These drugs, however, must be refrigerated to remain effective. Moreover, most uterotonics must be administered by injection, which requires sterile equipment and training in safe administration, prerequisites unavailable for most women delivering in low income countries.
Misoprostol, a prostaglandin E1 analogue, is heat stable and can be administered orally, rectally, or sublingually. A multicentre study sponsored by the World Health Organization found that misoprostol was less effective for prophylaxis than intravenous or intramuscular injections of oxytocin but did not investigate the possible benefit of misoprostol to the large number of women who give birth outside a health facility with electricity.6 Community based distribution of misoprostol in Indonesia reduced the perceived frequency of excessive bleeding and the need for emergency referral for postpartum haemorrhage compared with data from a control area where misoprostol was not available.7
To date all randomised studies of prophylactic misoprostol have used oral and rectal administration, though a recent pharmacokinetic study showed that sublingual administration secures the highest peak concentration and the best bioavailability.8 A recent pilot study found that sublingual misoprostol and intravenous syntometrine have comparable effects on blood loss in the third stage of labour.9 None of the above clinical studies, however, was sufficiently blinded. We tested whether routine administration of sublingual misoprostol could reduce the incidence of postpartum haemorrhage in a resource poor, primary healthcare setting in Guinea-Bissau, West Africa, when assessed in an adequately blinded manner.
Methods
Setting and participants
In Bissau, the capital of Guinea-Bissau, most women give birth at the national hospital—the Hospital National de Simão Mendes (HNSM). A growing number, however, give birth at local health centres such as the Centro de Saúde de Bandim, where four midwives attend about 500 deliveries a year. Midwives may seek support from a general physician, not trained in obstetrics. If complications occur, women are transferred immediately to the national hospital, though this is often delayed because of lack of transport. Public electricity is not usually available and the generator at the health centre works less than half of the nights because of lack of diesel or spare parts. There is no running water at the centre. The local health centre is supplied with essential drugs, but they are often out of stock and relatives are often sent to the nearest pharmacy to buy drugs as necessary.
Treatment protocol and consent
In the first month of the trial, an expatriate midwife with experience in research was posted to the health centre. All four local (auxiliary) midwives and the physician were informed about the project, and all staff members were trained during a two week period. The midwives were taught how to apply controlled cord traction and how to cut and clamp the cord at delivery. Though we attempted to improve all routines at the maternity centre, the only intervention studied was the impact of misoprostol on postpartum haemorrhage.
All women who gave birth at the local centre from March 2003 to August 2004 were asked to take part in the study. When the women arrived at the health centre, the midwife in charge explained the purpose of the study to the women. As most women of child bearing age are illiterate we obtained verbal consent. All the women were given the same clinical care. Before the delivery the midwife let the woman choose an envelope containing three tablets of either misoprostol or placebo, and these were administered sublingually as soon as the baby had been delivered. Most women received the tablets within two minutes of delivery.
In cases of severe postpartum haemorrhage, local guidelines for treatment were followed; oxytocin was freely available to the midwives during the trial.
Randomisation and masking
Misoprostol and placebo tablets of identical form, size, colour, and packing were produced by U-liang Pharmaceutical, Taipei, Taiwan. Seven hundred opaque envelopes were consecutively numbered and filled with either three tablets of placebo or three tablets of misoprostol 200 μg, distributed randomly by using a list of random numbers.
Measurement of blood loss
After delivery of the baby and drainage of the amniotic fluid, a clean plastic lined absorbent drape was placed under the woman's buttocks to collect all the blood lost. The drape was changed as many times as needed. The woman stayed on the drape or was asked to wear a pad over the next 60 minutes. In the case of severe haemorrhage, midwives were instructed to follow the usual guidelines for management of postpartum haemorrhage, and the supplemental treatment was registered. All drapes and pads were weighed on an electronic scale and the known dry weight of the linen was subtracted. As 1 ml of blood weighs close to 1 g, the balance in grams was assumed to be the total blood loss in ml.
Haemoglobin concentration was measured by finger prick before and 24 hours after delivery. If the woman left the local centre within 20 hours of delivery, she was visited at home to have her haemoglobin concentration measured.
Outcome measures
The primary outcome measure was postpartum haemorrhage, defined as a blood loss of ≥ 500 ml. We analysed the blood loss, the incidence of different thresholds of blood loss, change in haemoglobin concentration between admission and discharge, and the incidence of a 10% decrease in haemoglobin concentration between admission and discharge.
The midwives documented potential side effects by interviewing the women about nausea, diarrhoea, and vomiting one hour after delivery. The midwife in charge measured rectal temperature and recorded any shivering.
Power calculation and analysis
The average blood loss in the third stage of labour is 250-350 ml, and 12% of women will loose > 500 ml.10 Use of uterotonics should reduce this proportion to 5%.10 We calculated that we needed 300 women in each group to have a power of 80% (1 - β) with a risk of type 2 error (α) of 5%.
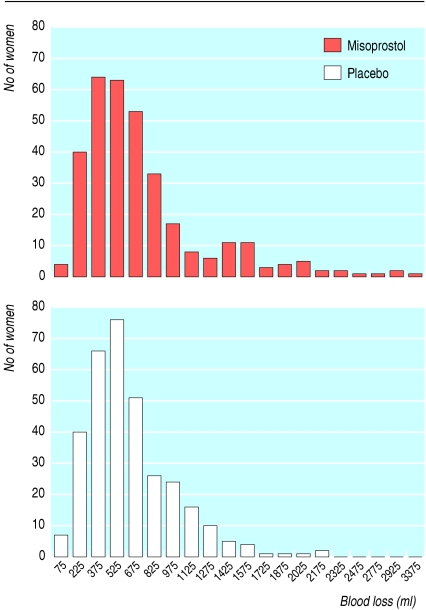
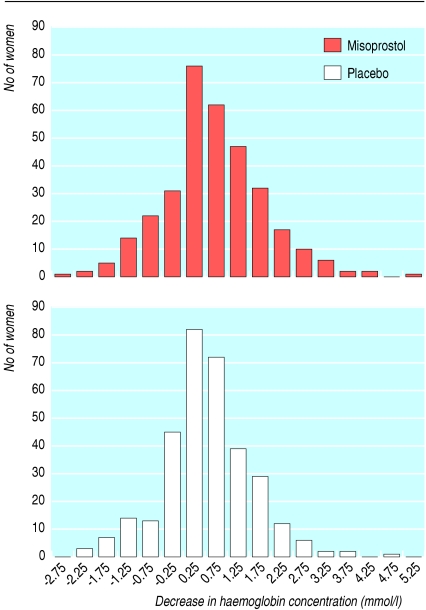
We entered data onto dBase V and used Stata version 9.0 for Windows for statistical analysis. Transformed blood loss (fig 1) and decrease in haemoglobin concentration approximated normal distributions (fig 2), so we analysed log (blood loss) and decrease in haemoglobin using linear regression. We used Pearson's χ2 test to compare categorical variables and risks ratios to express relative risks.
Fig 1.
Distribution of postpartum blood loss in women according to treatment
Fig 2.
Distribution of decrease in haemoglobin concentration after delivery in women according to treatment
Results
Randomisation and progress through trial
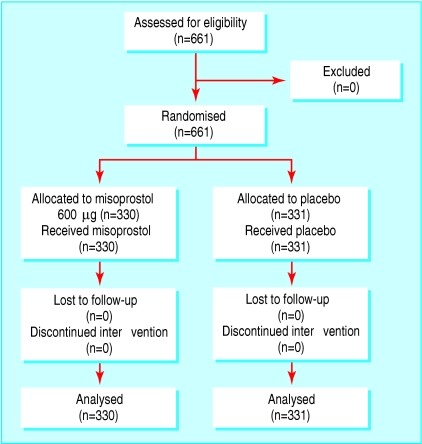
A total of 661 women were enrolled in the study, 330 in the misoprostol group and 331 in the placebo group. All eligible women were included and received the randomised drug; there was no loss to follow-up and no one discontinued the intervention. Hence, all enrolled women were included in the analysis (fig 3).
Fig 3.
Flow of participants in placebo controlled trial of misoprostol for prevention of postpartum haemorrhage
Baseline characteristics
Few women in Bissau possess a reliable birth certificate; the median reported age of enrolled women was 23 years (interquartile range 19-27 years). The median weight was 61 kg (interquartile range 57-69 kg) (table 1). There were no significant differences between the two groups with respect to duration of the third stage of labour, weight, or Apgar score (table 2).
Table 1.
Baseline characteristics of 661 women giving birth at a primary health centre, Guinea-Bissau, 2003-4. Figures are median (interquartile range) unless stated otherwise
| Characteristic | Misoprostol (n=330) | Control (n=331) |
|---|---|---|
| Age (years) | 23 (19-26) | 24 (20-28) |
| Parity | 1 (0-3) | 1 (0-3) |
| Weight (kg) | 61 (57-69) | 62 (57-69) |
| Initial Hb concentration (mmol/l) | 6.6 (5.8-7.3) | 6.7 (5.9-7.5) |
| No (%) with fever at admission (≥38.0°C) | 7 (2.1) | 5 (1.5) |
| No (%) with history of PPH | 20 (6.1) | 32 (9.7) |
| No (%) with history of caesarean section | 6 (1.8) | 4 (1.2) |
Hb=haemoglobin; PPH=postpartum haemorrhage.
Table 2.
Characteristics of labour in women according to treatment group. Figures are median (interquartile range) unless stated otherwise
| Misoprostol (n=330) | Control (n=331) | |
|---|---|---|
| Birth weight (g) | 3100 (2800-3420) | 3100 (2720-3400) |
| Apgar score | 10 (10-10) | 10 (9-10) |
| Length of third stage of labour (mins) | 12 (10-17) | 12 (10-17) |
| No (%) with retained placenta | 11 (3.3) | 11 (3.3) |
| No (%) transferred to HNSM | 3 (0.9) | 3 (0.9) |
HNSM=Hospital National de Simão Mendes.
Outcome measures
Of the 661 women, 150 (45%) in the misoprostol group and 170 (51%) in the control group had postpartum haemorrhage of ≥500 ml, the relative risk being 0.89 (0.76 to 1.04). The mean blood loss was 10.5% (-0.5% to 20.4%) lower in the misoprostol group than in the placebo group (table 3). Significantly fewer women in the misoprostol group (11%) suffered from severe postpartum haemorrhage, with a blood loss of ≥ 1000 ml (11% v 17%; 0.66, 0.45 to 0.98). For a blood loss of ≥ 1500 ml, the relative risk between the misoprostol group (2%) and the control group (8%) was 0.28 (0.12 to 0.64).
Table 3.
Blood loss and change in haemoglobin concentration in women according to treatment group. Means are shown with 95% confidence intervals in brackets
| Misoprostol (n=330) | Control (n=331) | Relative risk or mean difference (95% CI) | |
|---|---|---|---|
| No (%) with blood loss ≥500 ml | 150 (45) | 170 (51) | 0.89 (0.76 to 1.04) |
| Mean measured blood loss (ml)* | 443 (408 to 481) | 496 (456 to 538) | 10.5 (−0.5 to 20.4)† |
| No (%) with blood loss ≥1000 ml | 37 (11) | 56 (17) | 0.66 (0.45 to 0.98) |
| No (%) with blood loss ≥1500 ml | 7 (2) | 25 (8) | 0.28 (0.12 to 0.64) |
| Mean decrease in Hb concentration (mmol/l) | 0.29 (0.17 to 0.41) | 0.45 (0.33 to 0.57) | 0.16 (−0.01 to 0.32)† |
| No (%) with 10% fall in Hb concentration | 105 (32) | 115 (35) | 0.92 (0.74 to 1.14) |
HB=haemoglobin.
Log transformed.
Mean difference.
Differences in haemoglobin concentrations at admission and one day postpartum varied considerably (table 3); the haemoglobin concentration increased in 33% (218/661) of the women. The mean decrease in haemoglobin concentration was 0.16 mmol/l (-0.01 mmol/l to 0.32 mmol/l) lower in the misoprostol group than in the placebo group (table 3). The proportion of women with a reduction of more than 10% of the admission value did not differ between the two groups.
Side effects
Significantly more women in the misoprostol group experienced shivering and pyrexia (table 4). There were few complaints about nausea, and few in either group had vomiting or diarrhoea. One woman in the misoprostol group died. She was a 19 year old primigravida who gave birth to a 2630 g boy and had postpartum haemorrhage of 1417 ml. Apparently this was under control, but she suddenly died 90 minutes after delivery. The verbal autopsy audit reached no definitive conclusion as to the cause of death.
Table 4.
Side effects after delivery in women who received misoprostol to treat postpartum haemorrhage. Figures are numbers (percentages) of women
| Misoprostol (n=330) | Control (n=331) | Relative risk (95% CI) | |
|---|---|---|---|
| Shivering | 189 (57) | 78 (24) | 2.43 (1.96 to 3.01) |
| Fever (≥38.0°C) | 78 (24) | 11 (3) | 7.09 (3.84 to 13.1) |
| Nausea | 2 (1) | 4 (1) | 0.50 (0.09 to 2.72) |
| Vomiting | 10 (3) | 4 (1) | 2.51 (0.79 to 7.91) |
| Diarrhoea | 10 (3) | 4 (1) | 2.50 (0.79 to 7.89) |
Discussion
Sublingual misoprostol is effective in reducing the incidence of severe postpartum haemorrhage. Though we expected 12% of the women in the control group to have a blood loss of > 500 ml,10 our measurement technique showed that as many as 51% of the women in the control group had a loss of ≥ 500 ml. We therefore redefined severe postpartum haemorrhage as a loss of ≥ 1000 ml or ≥ 1500 ml. These levels of bleeding affected 17% and 8% of the women in the control group—that is, proportions exceeding those estimated in our power calculations. Hence, the present randomised double blinded study showed that routine misoprostol had a clear protective effect on severe postpartum haemorrhage in excess of 1000 ml. Also, the reduction in haemoglobin concentrations after delivery was less in the women in the misoprostol group.
Comparison with other studies
As in other studies, we found a significant increase in shivering and pyrexia in the misoprostol group.6,11,12 According to the midwives, however, these side effects were transient and of little disadvantage to the women.
Four randomised controlled trials tested oral and rectal misoprostol against placebo and found no significant reduction in the number of women experiencing postpartum haemorrhage, if the threshold was 500 ml.11,13-15 The explanation for this finding might be found in the study done by Tang et al, which compared the pharmacokinetics of three routes of administration: sublingual, oral, and vaginal.8 The time to peak concentration was shortest in the sublingual route, at 26.0 minutes (SD 11.5 minutes). As bleeding in an uncomplicated third stage of labour normally ceases within 10 minutes, we would not expect to see any effect of misoprostol on “normal” bleeding. Continuous bleeding for more than 15-25 minutes, however, should be reduced by its uterotonic properties.
Strengths of study
Most studies on misoprostol as a uterotonic agent in postpartum haemorrhage have been insufficiently blinded because the company that owns the patent has been unwilling to cooperate in studies of obstetric use of misoprostol by manufacturing a placebo. However, the rights have now been sold and we were able to obtain the active and the placebo tablets manufactured and packed so that they were completely identical.
Estimation of blood loss is known to be difficult and inaccurate. In several of the previous studies, the birth attendant estimated blood loss, a method known to underestimate the quantity by about 30%.16 We tried to estimate blood loss more precisely, though we could have overestimated it because of the addition of amniotic fluid or underestimated it because of loss of blood outside the drapes. As these errors are likely to be distributed equally between the two study groups they are unlikely to have introduced any systematic bias that could have affected the significance of our results.
Conclusion
In rural Guinea-Bissau, 75% of women give birth at home,17 and worldwide only about 50% of women give birth in health facilities. Therefore, strategies must be identified to increase the safety of deliveries attended by unskilled birth attendants. According to the study from Indonesia,7 it might be safe and useful to give misoprostol to women during antenatal care and to teach midwives to use the drug safely. Our randomised trial suggests that misoprostol would play an important part in such a strategy to reduce complications of delivery and maternal mortality.
What is already known on this topic
Conventional uterotonics require refrigeration and equipment for injection and are therefore inappropriate for the control of postpartum haemorrhage in poor areas with little infrastructure
Though misoprostol tablets do not require refrigeration, oral misoprostol 600 μg is less effective than conventional uterotonics in reducing postpartum haemorrhage of ≥ 1000 ml
What this study adds
Sublingual misoprostol 600 μg reduces the incidence of severe haemorrhage (≥ 1000 ml)
Misoprostol tablets may reduce the morbidity and mortality associated with postpartum haemorrhage in the developing world
More studies on the use of misoprostol outside developed health facilities are warranted. If the drug is found to be consistently beneficial and safe, sublingual misoprostol should be offered to labouring women at the start of the third stage of labour if injectable uterotonics are not available. The mothers should be informed that shivering and mild fever can be expected.
Contributors: LH was the principal investigator, contributed to the study design, wrote the draft manuscript, and is guarantor. BBN, LH, and PA contributed to the study design and were involved in the data interpretation and writing of the manuscript. PC supervised the execution of the study, did the data entry, and contributed to the write up. Rosa Amelia Caceres Perez taught the midwives, who were Angelina da Silva, Ermelinda Tchuda, Suzana Rodrigues, and Felismina António Gomes. JN conducted the statistical analysis and contributed to the data interpretation.
Funding: The Danish Society of Obstetrics and Gynaecology, the Illum Foundation, and the Danish International Development Agency.
Competing interests: None declared.
Ethical approval: The scientific committee of the Ministry of Health in Guinea Bissau and the Danish central scientific ethical committee (No 624-02-0036).
References
- 1.Rush D. Nutrition and maternal mortality in the developing world. Am J Clin Nutr 2000;72: 212S-40S. [DOI] [PubMed] [Google Scholar]
- 2.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr 2001;131: 604-615S. [DOI] [PubMed] [Google Scholar]
- 3.Kaestel P. Micronutrient supplementation and other predictors of birth size and perinatal mortality in Guinea-Bissau [thesis]. Copenhagen, Denmark: Department of Epidemiology Research, Statens Serum Institute, 2004.
- 4.Hoj L, Stensballe J, Aaby P. Maternal mortality in Guinea-Bissau: the use of verbal autopsy in a multi-ethnic population. Int J Epidemiol 1999;28: 70-6. [DOI] [PubMed] [Google Scholar]
- 5.Kwast BE. Postpartum haemorrhage: its contribution to maternal mortality. Midwifery 1991;7: 64-70. [DOI] [PubMed] [Google Scholar]
- 6.Gulmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358: 689-95. [DOI] [PubMed] [Google Scholar]
- 7.Maternal and Neonatal Health Program. Preventing postpartum hemorrhage: a community-based approach proves effective in rural Indonesia. Baltimore USA: MNH Program, 2005.
- 8.Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod 2002;17: 332-6. [DOI] [PubMed] [Google Scholar]
- 9.Lam H, Tang OS, Lee CP, Ho PC. A pilot-randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstet Gynecol Scand 2004;83: 647-50. [DOI] [PubMed] [Google Scholar]
- 10.Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database Syst Rev 2000;(3): CD000007. [DOI] [PubMed]
- 11.Hofmeyr GJ, Nikodem VC, de Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour—a randomised placebo-controlled trial. S Afr Med J 2001;91: 432-5. [PubMed] [Google Scholar]
- 12.Lumbiganon P, Villar J, Piaggio G, Gulmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG 2002;109: 1222-6. [DOI] [PubMed] [Google Scholar]
- 13.Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. Am J Obstet Gynecol 1998;179: 1043-6. [DOI] [PubMed] [Google Scholar]
- 14.Hofmeyr GJ, Nikodem VC, de Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. Br J Obstet Gynaecol 1998;105: 971-5. [DOI] [PubMed] [Google Scholar]
- 15.Surbek DV, Fehr PM, Hosli I, Holzgreve W. Oral misoprostol for third stage of labor: a randomized placebo-controlled trial. Obstet Gynecol 1999;94: 255-8. [DOI] [PubMed] [Google Scholar]
- 16.Prasertcharoensuk W, Swadpanich U, Lumbiganon P. Accuracy of the blood loss estimation in the third stage of labor. Int J Gynaecol Obstet 2000;71: 69-70. [DOI] [PubMed] [Google Scholar]
- 17.Hoj L, da Silva D, Hedegaard K, Sandstrom A, Aaby P. Factors associated with maternal mortality in rural Guinea-Bissau. A longitudinal population-based study. BJOG 2002;109: 792-9. [PubMed] [Google Scholar]