Abstract
We have found that the hyperthermophilic archaeon Pyrobaculum calidifontis VA1 produced a thermostable esterase. We isolated and sequenced the esterase gene (estPc) from strain VA1. estPc consisted of 939 bp, corresponding to 313 amino acid residues with a molecular mass of 34,354 Da. As estPc showed significant identity (30%) to mammalian hormone-sensitive lipases (HSLs), esterase of P. calidifontis (Est) could be regarded as a new member of the HSL family. Activity levels of the enzyme were comparable or higher than those of previously reported enzymes not only at high temperature (6,410 U/mg at 90°C), but also at ambient temperature (1,050 U/mg at 30°C). The enzyme displayed extremely high thermostability and was also stable after incubation with various water-miscible organic solvents at a concentration of 80%. The enzyme also exhibited activity in the presence of organic solvents. Est of P. calidifontis showed higher hydrolytic activity towards esters with short to medium chains, with p-nitrophenyl caproate (C6) the best substrate among the p-nitrophenyl esters examined. As for the alcoholic moiety, the enzyme displayed esterase activity towards esters with both straight- and branched-chain alcohols. Most surprisingly, we found that this Est enzyme hydrolyzed the tertiary alcohol ester tert-butyl acetate, a feature very rare among previously reported lipolytic enzymes. The extreme stability against heat and organic solvents, along with its activity towards a tertiary alcohol ester, indicates a high potential for the Est of P. calidifontis in future applications.
Carboxylesterases (EC 3.1.1.1) and lipases (EC 3.1.1.3) are enzymes that hydrolyze carboxylate esters and are widespread in various organisms including animals, plants, and microorganisms. Interfacial activation and/or the presence of a surface loop covering the active site (lid) have been previously used as criteria for the distinction of the two enzymes (17). An alternative criterion has recently been proposed in which carboxylesterases are defined as enzymes that catalyze the hydrolysis of acylglycerols with short chains (<10 carbon atoms), while lipases are defined as enzymes that catalyze the hydrolysis of acylglycerols with long chains (≥10 carbon atoms) (16). The standard substrate for carboxylesterase activity is tributyrine, and that for lipase activity is triolein. As many of these enzymes exhibit activity in organic solvents, they have become two of the most widely used enzymes in organic synthesis and industrial processes (5, 6, 18, 30).
Carboxylesterases and lipases share a similar active site consisting of three residues: a nucleophilic serine residue in a GXSXG motif, an acidic residue (aspartic acid or glutamic acid), and a histidine. These residues act cooperatively in the catalytic mechanism of ester hydrolysis. The enzymes also display a common α/β hydrolase fold (27) which is also found in other hydrolases, such as haloalkane dehalogenase (11), acetylcholinesterase (37), dienelactone hydrolase (29), and serine carboxypeptidase (22).
At present, lipolytic enzymes from prokaryotes are classified into eight families (I to VIII) according to their amino acid sequences (3). Enzymes belonging to Family I are called true lipases and are further classified into six subfamilies. Some of the best-studied lipases, such as Pseudomonas lipases, Bacillus lipases, and Staphylococcus lipases, are members of Family I.
Family IV is also referred to as the HSL family, as enzymes belonging to this family show remarkable similarity to mammalian hormone-sensitive lipases (HSLs). HSL is a lipase which catalyzes the hydrolysis of triacylglycerols in adipose tissue and is a rate-limiting enzyme in the mobilization of fatty acids from stored lipids. Human HSL consists of an N-terminal domain with unknown function and a catalytic domain into which a regulatory module is inserted (13, 28). Bacterial enzymes belonging to Family IV display similarity to the catalytic domain of HSL, implying that mammalian HSLs have evolved from these bacterial Family IV enzymes.
Family IV includes enzymes from Alicyclobacillus acidocaldarius (23), Pseudomonas sp. B11-1 (7), Archaeoglobus fulgidus (24), Alcaligenes eutrophus, Moraxella sp. (10), and Escherichia coli (19). These members commonly harbor a consensus pentapeptide GDSAG corresponding to the GXSXG motif. Another HGGG motif with unknown function is also conserved in this family. The three-dimensional structures of the carboxylesterase from A. acidocaldarius (8) and A. fulgidus (9) and the brefeldin A esterase from Bacillus subtilis (39) have been determined. The analyses of these structures revealed that these enzymes harbor multiple α-helices at their N terminals that comprise cap structures located above their active sites.
As for archaea, a lipase has yet to be identified, while a few esterases have so far been characterized. The primary structures of these esterases have been elucidated in only three cases. Besides the esterase from A. fulgidus mentioned above, another Family IV esterase, from Sulfolobus solfataricus, has recently been characterized (26). In addition, an esterase from Sulfolobus acidocaldarius has been found to belong to Family V (4). Besides these three enzymes, three other esterases have been purified and studied. One is an esterase from S. acidocaldarius, distinct from the Family V enzyme mentioned above (35, 36). A DNA fragment that includes an esterase gene has been isolated from Pyrococcus furiosus (15). The third is the esterase from Sulfolobus shibatae, an extracellular enzyme that is induced when Tween compounds are used as sole carbon source (14). Although the number of archaeal lipolytic enzymes is still low, genome analyses have revealed that some other archaea strains harbor putative esterase or lipase genes.
We have recently isolated a facultatively aerobic, hyperthermophilic archaeon, Pyrobaculum calidifontis VA1 (1). Through a halo-forming plate assay using tributyrine or olive oil as substrates, we detected an esterase activity in the cell extracts of P. calidifontis VA1. Here we report the cloning of the esterase gene from P. calidifontis VA1, along with purification and detailed characterization of the gene product.
MATERIALS AND METHODS
Organisms and culture conditions.
P. calidifontis VA1 is a facultatively aerobic hyperthermophilic archaeon that was isolated from a hot spring in the Philippines (1). E. coli DH5α was used as a host of the genomic DNA library and a host for cloning various DNA fragments. E. coli BL21-CodonPlus(DE3)-RIL was used as a host for overexpression of the esterase gene from strain VA1 (estPc gene) with the plasmid pET-21a-d(+). P. calidifontis VA1 was grown at 90°C in a medium containing 1.0% tryptone, 0.1% yeast extracts, and 0.1% Na2S2O3 · 5H2O. E. coli strains were cultivated at 37°C in Luria-Bertani (LB) medium. Ampicillin was used at a final concentration of 100 μg/ml.
Cloning of the esterase gene.
P. calidifontis genomic DNA was partially digested with Sau3AI, and the resultant DNA fragments were ligated into pUC118/BamHI BAP. E. coli DH5α was transformed with the library and plated onto LB agar plates containing 100 μg of ampicillin/ml. The transformants were subsequently replicated onto LB agar plates containing 100 μg of ampicillin/ml, 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and 1% tributyrin. After incubation at 37°C until colonies were observed, these plates were further incubated at 60°C. Transformants with clear halos around the colonies were chosen as candidate clones, and their plasmids were isolated and sequenced.
DNA manipulation and sequencing.
Genomic DNA was purified by standard methods (32). Purification of plasmid DNA was performed with plasmid Mini- and Midi-kits (Qiagen, Hilden, Germany). Restriction enzymes and other DNA-modifying enzymes were used as recommended by the manufacturers (Takara Shuzo, Kyoto, Japan; Toyobo, Osaka, Japan; New England Biolabs, Beverly, Mass.). DNA sequencing was performed with an ABI PRISM kit and a model 310 capillary DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). Sequence data were analyzed and compared with other sequences using DNASIS software (Hitachi Software Engineering, Yokohama, Japan). Sequence alignment and phylogenetic analyses were performed with the ClustalW program (38), available at the DNA Data Bank of Japan website (http://www.ddbj.nig.ac.jp/E-mail/clustalw-e.html).
Gene expression.
The two primers est-5′ (5′-TTTTTTCATATGCCTCTTAGCCCTATACTAAGG-3′) and est-3′ (5′-TTTTTTGGATCCTTACGCCACAGCCATCGACTTTATTGAGGC-3′), which included restriction enzyme sites (underlined) for NdeI and BamHI, respectively, were used to amplify the estPc gene. PCR was performed with these primers, with P. calidifontis genomic DNA as the template and KOD Plus (Toyobo) as a DNA polymerase. The PCR product digested with NdeI and BamHI was inserted into pET-21a-d(+) and introduced into E. coli BL21-CodonPlus(DE3)-RIL. The transformants were cultivated, and 0.1 mM IPTG was added to induce gene expression when the optical density at 660 nm reached 0.4. After induction for 8 h, cells were harvested and disrupted.
Protein purification.
Cells were suspended in 20 mM Tris-HCl buffer (pH 8.0). Cell extracts were obtained by sonication and centrifugation (10,000 × g for 20 min at 4°C). The supernatant was incubated at 80°C for 20 min and then centrifuged (16,000 × g for 20 min at 4°C) to discard the denatured proteins. The supernatant was then applied to a 5-ml HiTrap Q anion exchange column (Amersham Pharmacia Biotech, Uppsala, Sweden) equilibrated with 20 mM Tris-HCl buffer (pH 8.0) and eluted with a linear 0-to-0.5 M NaCl gradient in 20 mM Tris-HCl (pH 8.0) using an ÄKTA Explorer 10S (Amersham Pharmacia Biotech). The fractions containing esterase activity were collected and concentrated using an Ultrafree-4 Centrifugal Filter Unit Biomax-10 apparatus (Millipore, Bedford, Mass.). The sample was then applied to a Superdex 200HR 10/30 gel filtration column (Amersham Pharmacia Biotech) in 20 mM Tris-HCl (pH 8.0) containing 0.1 M NaCl. The fractions containing esterase activity were collected and used as purified enzyme. The N-terminal amino acid sequence of the purified enzyme was determined using a protein sequencer (model 270; Perkin-Elmer Applied Biosystems). The protein concentration was determined with the Bio-Rad protein assay system (Bio-Rad, Hercules, Calif.) with bovine serum albumin as a standard.
Electrophoretic methods.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with standard methods (32). The protein standards used were phosphorylase b (Mr, 97,000), albumin (Mr, 66,000), ovalbumin (Mr, 45,000), carbonic anhydrase (Mr, 30,000), trypsin inhibitor (Mr, 20,100), and α-lactalbumin (Mr, 14,400). Gels were stained with Coomassie brilliant blue R-250.
Molecular mass determination by gel filtration.
Molecular mass determinations were performed by gel filtration chromatography. Pure enzyme or protein standards were applied to a Superdex 200HR 10/30 gel filtration column (Amersham Pharmacia Biotech) in 20 mM Tris-HCl (pH 8.0) containing 0.1 M NaCl and 1 mM dithiothreitol (DTT). Ferritin (440,000), catalase (232,000), aldolase (158,000), albumin (67,000), ovalbumin (43,000), chymotrypsinogen A (25,000), and RNase A (13,700) were used as protein standards, and Bluedextran (2,000,000) was used for determination of void volume. The molecular mass of the esterase was calculated by interpolation on a plot of (Ve−Vo)/(Vt−Vo) against log molecular mass, where Ve is the elution volume of the protein, Vo is the void volume of the column, and Vt is the geometric bed volume.
Enzyme assays.
Esterase activity against p-nitrophenyl esters was determined by measuring the amount of p-nitrophenol released by esterase-catalyzed hydrolysis. The production of p-nitrophenol was monitored at 405 nm continuously by a UV-1600PC spectrophotometer with a thermal control unit (Shimadzu, Kyoto, Japan). Unless otherwise mentioned, in the standard assay, esterase activity was measured with 1 mM p-nitrophenyl caproate as a substrate in 50 mM HEPES (pH 7.1) containing 1% acetonitrile at 70°C. After preincubation for 3 min, the reaction was initiated by adding enzyme into the reaction mixture. One unit of esterase activity is defined as the amount of activity which causes the release of 1 μmol of p-nitrophenol/min from p-nitrophenyl caproate. In every measurement, the effect of nonenzymatic hydrolysis of substrates was taken into consideration and subtracted from the value measured when the enzyme was added. Measurements were carried out at least three times, and the extinction coefficients of p-nitrophenol were determined prior to the measurements under every condition. Activity was described as the initial rate for hydrolysis of p-nitrophenyl ester.
Substrate specificity towards p-nitrophenyl esters was determined using p-nitrophenyl propionate (0.02 to 5 mM), p-nitrophenyl butyrate (0.02 to 2 mM), p-nitrophenyl valerate (0.01 to 1 mM), p-nitrophenyl caproate (0.008 to 1 mM), and p-nitrophenyl caprylate (0.03 to 0.4 mM) as substrates in 50 mM HEPES (pH 7.1) with 1% acetonitrile at 70°C. An additional 0.04% Triton X-100 was included in the reaction mixture in the case of p-nitrophenyl caprylate. With p-nitrophenyl palmitate as a substrate, 4% 2-propanol was included in the reaction mixture in order to solubilize the substrate. Kinetic analyses by curve fitting were calculated with SigmaPlot (SPSS Science, Chicago, Ill.).
We examined the activity towards acetate esters by using methyl acetate, ethyl acetate, propyl acetate, isopropyl acetate, butyl acetate, isobutyl acetate, sec-butyl acetate, tert-butyl acetate, hexyl acetate, and octyl acetate as substrates in a batch reaction. The assay mixture contained a 50 mM concentration of substrate, 90 mM sodium phosphate (pH 7.0), and 10% acetonitrile. The reaction was initiated by adding enzyme into the mixture after a 3-min preincubation. After reaction for 20 s at 40°C, the reaction was terminated by cooling the samples in ice water. The reaction mixture was then applied to an RSpak DE-413 column (Shodex, Tokyo, Japan) in an elution buffer consisting of 50 mM sodium phosphate (pH 7.0) and 50% acetonitrile and analyzed by high-performance liquid chromatography (HPLC; Shimadzu). Effluents were detected by absorbance at 205 nm. The amount of acetic acid released in this batch reaction was calculated from the peak area corresponding to acetic acid which eluted from the HPLC column.
The effect of pH on esterase activity was studied in a pH range of 3.0 to 9.0. The buffers used were 50 mM sodium citrate (pH 3.0 to 4.0), 50 mM sodium acetate (pH 4.0 to 5.5), 50 mM morpholineethanesulfonic acid (MES; pH 5.5 to 7.0), 50 mM HEPES (pH 7.0 to 8.0), and 50 mM Bicine (pH 8.0 to 9.0). Production of p-nitrophenoxide and p-nitrophenol was monitored at 348 nm (the pH-independent isosbestic wavelength of p-nitrophenoxide and p-nitrophenol). The effect of temperature on esterase activity was studied in the range of 30 to 95°C. p-Nitrophenyl caproate was dissolved in 50 mM HEPES (pH 7.1) and incubated at each temperature for 3 min prior to measurements. The pH values of buffers were adjusted at each temperature.
The inhibitory effect of the modifying reagent for Ser and His was examined using phenylmethylsulfonyl fluoride (PMSF) and diethyl pyrocarbonate (DEPC), respectively. The enzyme (20.5 μg/ml) was incubated with various concentrations of PMSF in 50 mM MES (pH 7.0) or DEPC in 50 mM MES (pH 6.0) at 37°C for 10 min. The reaction was stopped by cooling samples in ice water. Residual activities were subsequently measured by the standard assays described above.
Thermostability was measured by incubating the enzyme (0.205 mg/ml) in 20 mM Tris-HCl (pH 8.0) at 100 or 110°C for various intervals of time. Stability against organic solvents was measured by incubating the enzyme (100 μg/ml) in 20 mM Tris-HCl (pH 8.0) containing 0, 50, and 80% organic solvent (vol/vol) at 30°C for 60 min. In order to measure the residual activities, aliquots were taken from these mixtures and added as the enzyme sample in the standard assay. The final concentration of the solvent in the assay solution is therefore 0.01 or 0.016% and does not affect the extinction coefficient. Activity measurements in the presence of 50% solvent were carried out at 70°C. In this case, the respective extinction coefficients were 14,000 M−1 cm−1 (no solvent), 5,530 M−1 cm−1 (methanol), 4,500 M−1 cm−1 (ethanol), 3,910 M−1 cm−1 (2-propanol), 4,660 M−1 cm−1 (acetonitrile), 6,920 M−1 cm−1 (dimethyl sulfoxide), and 5,390 M−1 cm−1 (dimethylformamide).
Nucleotide sequence accession number.
The nucleotide sequence data reported here are available in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. AB078331.
RESULTS
Esterase activity in cell extracts of P. calidifontis VA1.
In order to examine the lipolytic activity in cell extracts of P. calidifontis VA1, a plate assay was performed using tributyrine and olive oil (mainly composed of triolein) as substrates. A clear halo was detected on the tributyrine plates, while no signal was observed on those with olive oil. According to the definition of carboxylesterases and/or lipases, it was suggested that P. calidifontis VA1 harbored a carboxylesterase activity.
Cloning and sequence analysis of estPc.
In order to isolate the gene(s) responsible for the carboxylesterase activity detected above, genomic DNA was digested with Sau3AI and inserted into pUC118. E. coli DH5α transformants were plated onto LB-ampicillin-IPTG-tributyrin plates. Among 4,000 transformants, three clones formed clear halos around the colonies. The three plasmids were isolated and sequenced and were found to contain identical inserts. Further characterization indicated the presence of an open reading frame of 939 bp (estPc) which showed similarity to previously reported esterase and lipase genes. The deduced amino acid sequence consisting of 313 residues displayed approximately 50% identity with the esterase of A. fulgidus and a putative lipase gene product (lipP-2) from S. solfataricus. Although the similarities were relatively lower than those towards the enzymes from archaea, the Est of P. calidifontis also showed similarity to mammalian HSLs (≈30% identity), lipase 2 from Moraxella sp. TA144 (27% identity), and esterase from E. coli (26% identity), indicating that Est of P. calidifontis is a member of the Family IV lipolytic enzymes. The pentapeptide GDSAG including catalytic serine was conserved in the enzyme at positions 155 to 159, suggesting that Ser157 is the catalytic residue in the enzyme. Sequence alignment among Family IV enzymes indicated that the other catalytic components (Asp254 and His284) and the HGGG motif were also conserved. A secondary structure prediction of Est from P. calidifontis (http://www.embl-heidelberg.de/predictprotein/predictprotein.html) suggested the presence of two α-helices at the N-terminal region of the protein, a feature shared in the Family IV enzymes from B. subtilis (39) and A. acidocaldarius (8) whose three-dimensional structures have been determined.
Overexpression and purification of recombinant Est from P. calidifontis.
We produced recombinant Est of P. calidifontis in E. coli. Cell extracts were heat treated, and most of the proteins from the host cells were removed. The enzyme remained in the soluble fraction after heat treatment and was further purified by anion exchange chromatography and gel filtration chromatography. Through this purification procedure, the recombinant Est was purified 6.5-fold with a yield of 66% (Table 1). The homogeneity of the protein was confirmed by SDS-PAGE (Fig. 1). The molecular mass of this protein was 34 kDa, in good agreement with the molecular mass deduced from the nucleotide sequence (34,354 Da). The N-terminal amino acid sequence of the protein, Phe-Leu-Ser-Phe-Ile-Leu-Arg-Gln-Ile-Leu, was identical to the sequence deduced from the nucleotide sequence, confirming that the purified enzyme was the protein product of estPc. In order to examine the subunit composition of the recombinant enzyme, gel filtration chromatography was performed at room temperature in the presence of 1 mM DTT. The protein was found to be 98 kDa, suggesting that the enzyme was a trimer under these conditions.
TABLE 1.
Purification table for the recombinant Est
| Step | Total protein (mg) | Total activity (U) | Sp act (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 129 | 7,740 | 599 | 100 | 1 |
| Heat treatment | 40.3 | 7,350 | 1,820 | 95 | 3.1 |
| HiTrap Q | 18.6 | 7,020 | 3,780 | 91 | 6.3 |
| Superdex 200 | 13.1 | 5,130 | 3,910 | 66 | 6.5 |
FIG. 1.
SDS-PAGE of the purified Est. Lane 1, molecular mass standards; lane 2, purified Est of P. calidifontis (5 μg).
Search for a stabilizing reagent.
Esterase activity was first measured spectrophotometrically using p-nitrophenyl esters as substrates. During these measurements, we observed that the Est was unstable at low concentrations (10 to 100 nM). The half-life of the enzyme at a concentration of 60 nM was 5.2 h in 20 mM Tris-HCl (pH 8.0) at 4°C. This instability was not observed at micromolar concentrations. In order to stabilize the enzyme at low concentrations, we examined the effects of adding various compounds, such as Triton X-100 (0.1 to 1%), Tween 20 (0.1 to 1%), 1 mM DTT, 1 mM 2-mercaptoethanol, glycerol (20 to 50%), 10% sucrose, EDTA (1 to 10 mM), 10% acetone, and 10% ethanol. Among these, addition of 0.1% Triton X-100 exhibited a significant stabilizing effect without leading to a change in activity levels. Therefore, 0.1% Triton X-100 was added in the enzyme stock solution when performing further experiments unless otherwise stated.
Effect of pH and temperature.
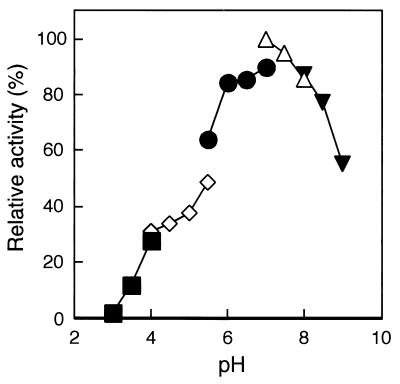
The effect of pH towards Est activity was investigated using p-nitrophenyl caproate as a substrate (Fig. 2). The absorption of p-nitrophenol varies when pH is altered because of changes in equilibrium between p-nitrophenol and p-nitrophenoxide. Therefore, the release of p-nitrophenol was monitored at 348 nm, the isosbestic point of p-nitrophenol and p-nitrophenoxide. The activity was measured in a pH range of 3.0 to 9.0. Est displayed high activity at neutral pH, with an optimal pH of approximately 7.0.
FIG. 2.
pH profile of Est from P. calidifontis. The activity of the enzyme at different pH levels was measured at 70°C. Buffers used were sodium citrate (closed boxes) (pH 3.0 to 4.0), sodium acetate (open diamonds) (pH 4.0 to 5.5), MES (closed circles) (pH 5.5 to 7.0), HEPES (open triangles) (pH 7.0 to 8.0), and Bicine (closed triangles) (pH 8.0 to 9.0).
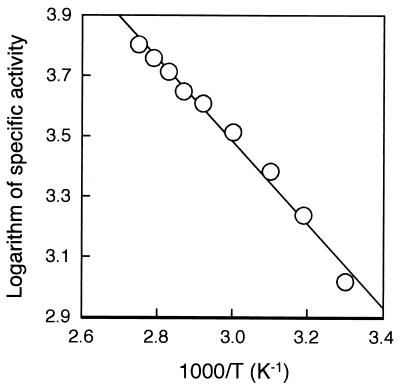
The effect of temperature on esterase activity was investigated using p-nitrophenyl caproate as a substrate. The activity was measured in the range of 30 to 95°C. The enzyme showed higher activity at higher temperature, with an optimal temperature of 90°C. Est activity at 30°C was 16% of the maximum activity. The activation energy was calculated from the Arrhenius plot to be 26.4 kJ/mol (Fig. 3).
FIG. 3.
Temperature profile of Est of P. calidifontis at pH 7.1. The logarithm of specific activity was plotted against 1,000/T (Arrhenius plot).
Stability.
The thermostability of the Est of P. calidifontis was investigated (Fig. 4). The enzyme was incubated at 100 or 110°C in a sealed cuvette at a concentration of 0.205 mg/ml with no addition of Triton X-100. Activities prior to and after incubation were compared at 70°C. The Est protein was found to be remarkably thermostable. No decrease in activity could be observed after 2 h at 100°C. The half-life (± standard deviation) for the enzyme at 110°C was 56 ± 4 min.
FIG. 4.
Thermostability of Est of P. calidifontis. The enzyme was incubated at 100°C (open boxes) or 110°C (closed circles) in a sealed cuvette. Residual activity was measured by standard assay. Activity prior to incubation was defined as 100%.
The stability of the esterase against water-miscible organic solvents was also investigated (Table 2). Methanol, ethanol, 2-propanol, acetonitrile, dimethyl sulfoxide, and dimethylformamide were added to final concentrations of 50 and 80% and incubated at 30°C for 60 min. Also in these experiments, the enzyme displayed extreme stability, with no drastic decreases in residual activity. These results indicate that the Est of P. calidifontis is a remarkably stable enzyme against both heat and organic solvents.
TABLE 2.
Stability against and activity in water-miscible organic solvents
| Organic solvent | Residual activity after 1 h incubationa (%)
|
Activity in 50% solventb (U/mg) | |
|---|---|---|---|
| 50% concn | 80% concn | ||
| None | 100 | 100 | 4,053 ± 241 |
| Methanol | 109 ± 2 | 89 ± 4 | 556 ± 9 |
| Ethanol | 105 ± 5 | 103 ± 1 | 101 ± 41 |
| 2-Propanol | 110 ± 2 | 94 ± 1 | 42 ± 14 |
| Acetonitrile | 117 ± 9 | 116 ± 5 | 119 ± 53 |
| Dimethyl sulfoxide | 101 ± 2 | 90 ± 2 | 2,148 ± 131 |
| Dimethylformamide | 124 ± 3 | 96 ± 1 | 296 ± 28 |
Incubation was carried out at 30°C, and activity measurements were at 70°C.
Activity measurements were carried out at 70°C. Extinction coefficients for each solvent (50%) are indicated in Materials and Methods.
Activity in solutions with 50% water-miscible organic solvents.
We measured the enzyme activity of Est in reaction mixtures including 50% of the organic solvents mentioned above (Table 2). Activity was observed in all cases, and a particularly high level of activity was found in 50% dimethyl sulfoxide.
Inhibition.
The effects of various inhibitors were investigated. From the deduced amino acid sequence, it was suggested that this Est harbored a catalytic triad consisting of Ser, His, and Asp. In order to confirm this point experimentally, we examined the effect of two chemical reagents on the activity of the enzyme. PMSF, a typical modification reagent for Ser, drastically inhibited the reaction. Addition of 25 μM PMSF inhibited the reaction by approximately 94%. DEPC, a modification reagent for His, also inhibited the reaction, but higher concentrations of DEPC than PMSF were necessary. Addition of 2.0 mM DEPC inhibited the reaction by about 98%.
Substrate specificity.
As the Est of P. calidifontis was shown to be a highly stable esterase with the potential for further application, we examined the substrate specificity of the enzyme. Specificity of the enzyme towards the length of the acyl chains was investigated using p-nitrophenyl esters (propionate, C3; butyrate, C4; valerate, C5; caproate, C6; and caprylate, C8) as substrates (Table 3). The kcat value had a tendency to increase with the increase in acyl chain length of substrates until the C6 substrate caproate. The kcat value towards p-nitrophenyl caprylate (C8) displayed a sharp decrease from that of p-nitrophenyl caproate (61%). Likewise, the Km value decreased with the increase in acyl chain length until C6. The Km value for C8 was larger than that for C6. Therefore, the kcat/Km ratios indicated that p-nitrophenyl caproate (C6) was the best substrate among the p-nitrophenyl esters examined. Est exhibited very low levels of activity against the long-chain substrate p-nitrophenyl palmitate (C16). Compared to the activity against p-nitrophenyl caproate at 70°C (4,050 U/mg), the p-nitrophenyl palmitate hydrolyzing activity was 127 U/mg at this temperature.
TABLE 3.
Kinetic parameters for hydrolysis of various p-nitrophenyl esters
| p-Nitrophenyl substrate | Km (μM) | kcat (s−1) | kcat/Km (s−1 μM−1) |
|---|---|---|---|
| Propionate (C3) | 348 ± 80 | 1,480 ± 110 | 4.3 |
| Butyrate (C4) | 164 ± 7 | 1,690 ± 20 | 10 |
| Valerate (C5) | 71.0 ± 10.0 | 2,070 ± 80 | 29 |
| Caproate (C6) | 44.4 ± 5.9 | 2,620 ± 90 | 59 |
| Caprylate (C8) | 51.0 ± 25.6 | 1,600 ± 270 | 31 |
We further compared the activities towards acetate esters with various alcoholic moieties (methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, hexyl, and octyl) (Table 4). Among straight-chained alcohols, butanol ester was the most preferred substrate, with high activities also observed for the C3 and C6 substrates. Methyl acetate was found to be a poor substrate. Interestingly, activity with isobutyl acetate as a substrate led to the highest level of activity. Moreover, although activity levels were low, this Est enzyme could also catalyze the hydrolysis of the tert-butyl acetate substrate.
TABLE 4.
Specific activity towards various acetate esters
| Substrate | Sp act (U/mg) |
|---|---|
| Methyl acetate | 160 ± 24 |
| Ethyl acetate | 313 ± 42 |
| Propyl acetate | 805 ± 61 |
| Isopropyl acetate | 390 ± 40 |
| Butyl acetate | 1,160 ± 27 |
| Isobutyl acetate | 1,510 ± 6 |
| sec-Butyl acetate | 876 ± 17 |
| tert-Butyl acetate | 272 ± 16 |
| Hexyl acetate | 747 ± 80 |
| Octyl acetate | 559 ± 55 |
DISCUSSION
We have reported the characterization of a highly active, thermostable carboxylesterase from the hyperthermophilic archaeon P. calidifontis VA1. Using p-nitrophenyl caproate as a substrate, Est displayed an activity of 1,050 U/mg at 30°C, and the level of activity increased with an elevation in temperature (6,410 U/mg at 90°C). As the variety of assay methods for esterases and lipases has hampered an accurate comparison of enzyme activities, we compared the activity of Est with that of enzymes examined with similar assay methods. The catalytic efficiency of the enzyme was comparable or higher than those of previously reported enzymes over a broad temperature range (2, 7, 21, 23, 24, 31). The high catalytic efficiency of the enzyme was also indicated by comparing the activation energies of several enzymes. The activation energy of Est from P. calidifontis (26.4 kJ/mol) was comparable to that of A. fulgidus (26 kJ/mol) (24) and was lower than that of the carboxylesterase from A. acidocaldarius (31 kJ/mol) (23) and the lipase from Pseudomonas sp. B11-1 (47 kJ/mol for p-nitrophenyl butyrate) (7). The Arrhenius plot for Est from P. calidifontis was linear at temperatures ranging from 30 to 90°C, indicating that the conformation of this enzyme was not altered throughout this temperature range.
Est from P. calidifontis was found to be one of the most thermostable and thermophilic lipolytic enzymes to be reported, along with the enzyme from P. furiosus (15). High activity and stability at elevated temperatures should be favorable for dissolution of hydrophobic substrates in the reaction system. In addition, the high reaction temperature provides a means to utilize a variety of substrates with high melting temperatures that would otherwise have to be dissolved in an organic solvent at ambient temperatures. Furthermore, as mentioned above, this Est displayed relatively high activity at ambient temperatures, implying that the enzyme could also be utilized at lower temperatures for long periods of time as an extremely stable enzyme.
The Est enzyme was also stable against water-miscible organic solvents. The lipases from Pseudomonas sp. B11-1 (7) and Fusarium heterosporum (34) were drastically inactivated after incubation with acetonitrile. We found that the P. calidifontis enzyme, after 1 h of incubation in the presence of 80% organic solvents, retained its activity in the standard assays. This indicates that the enzyme did not denature, at least to an irreversible extent, during the incubation. The enzyme also exhibited activity in solutions including 50% organic solvents (Table 2). Stability against organic solvent is important when using enzymes for synthesis of esters. These features, along with the thermostability of the enzyme, make it a very attractive enzyme for future application in industry as well as in biochemistry.
Concerning substrate specificity, the Est of P. calidifontis was found to be active against esters with short to medium chains in the acyl moiety. As for alcoholic moiety, the Est protein displayed an interesting feature: the ability to hydrolyze tert-butyl acetate. At present, enzymes that can catalyze the hydrolysis and/or the synthesis of tertiary esters are very rare (12, 40), and this further raises the potential of this Est protein as an enzyme in organic synthesis.
The substrate specificity of carboxylesterase from A. acidocaldarius could be altered by site-directed and saturation mutagenesis experiments (25). The acyl binding pocket of this enzyme was engineered to increase affinity towards long acyl chains, and an improvement of enzymatic activity towards p-nitrophenyl esters with longer acyl chains was observed. At present, a lipase from a thermophilic organism has not been used in industry. However, future engineering of these thermostable enzymes, as mentioned above, may provide a new array of stable, lipolytic enzymes for use in industry.
In contrast to the enormous number of bacterial lipolytic enzymes that have been studied, only six enzymes have been characterized from archaea. With the growing number of complete archaeal genome sequences in the databases, we were tempted to examine the presence of putative esterase and/or lipase genes in these archaeal strains. In order to cover enzymes from all families, one or two typical enzymes were chosen from each family, and a BLAST search was performed against the archaeal genome databases (http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/genom_table_cgi). Interestingly, some putative genes were identified in BLAST searches with Family IV or Family V enzymes, while no candidates with notable similarity to lipolytic enzymes could be retrieved with enzymes from other families. With the data available at present, it seems that the archaeal enzymes are confined to Family IV and V. It is also interesting that these two families are comprised of enzymes from diverse organisms, including psychrophiles, mesophiles, and (hyper)thermophiles (3), a tendency not found in other families.
The function of carboxylesterases in archaea has not been revealed. It can be speculated that the esterases function to hydrolyze ester compounds, providing (short-chain) carboxylic acids and/or alcohols to be assimilated by the cells. Genome analyses revealed that A. fulgidus (20) and S. solfataricus (33) have putative genes encoding enzymes involved in β-oxidation as well as esterase genes. Interestingly, a putative enoyl-coenzyme A (CoA) hydratase gene was found adjacent to the estPc gene in P. calidifontis VA1. It will be necessary to confirm whether P. calidifontis can catabolize carboxylate esters and whether the other components of β-oxidation, namely acyl-CoA synthetase, acyl-CoA dehydrogenase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase, are active in P. calidifontis.
REFERENCES
- 1.Amo, T., M. Paje, A. Inagaki, S. Ezaki, H. Atomi, and T. Imanaka. Pyrobaculum calidifontis sp. nov., a novel hyperthermophilic archaeon that grows under atmospheric air. Archaea, in press. [Online.] [DOI] [PMC free article] [PubMed]
- 2.Anguita, J., L. B. Rodríguez Aparicio, and G. Naharro. 1993. Purification, gene cloning, amino acid sequence analysis, and expression of an extracellular lipase from an Aeromonas hydrophila human isolate. Appl. Environ. Microbiol. 59:2411-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arpigny, J. L., and K. E. Jaeger. 1999. Bacterial lipolytic enzymes: classification and properties. Biochem. J. 343:177-183. [PMC free article] [PubMed] [Google Scholar]
- 4.Arpigny, J. L., D. Jendrossek, and K. E. Jaeger. 1998. A novel heat-stable lipolytic enzyme from Sulfolobus acidocaldarius DSM 639 displaying similarity to polyhydroxyalkanoate depolymerases. FEMS Microbiol. Lett. 167:69-73. [DOI] [PubMed] [Google Scholar]
- 5.Bornscheuer, U. T. 2002. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol. Rev. 733:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Bornscheuer, U. T., and R. J. Kazlauskas. 1999. Hydrolases in organic synthesis — regio- and stereoselective biotransformations. Wiley-VCH, Weinheim, Germany.
- 7.Choo, D. W., T. Kurihara, T. Suzuki, K. Soda, and N. Esaki. 1998. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol. 64:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Simone, G., S. Galdiero, G. Manco, D. Lang, M. Rossi, and C. Pedone. 2000. A snapshot of a transition state analogue of a novel thermophilic esterase belonging to the subfamily of mammalian hormone-sensitive lipase. J. Mol. Biol. 303:761-771. [DOI] [PubMed] [Google Scholar]
- 9.De Simone, G., V. Menchise, G. Manco, L. Mandrich, N. Sorrentino, D. Lang, M. Rossi, and C. Pedone. 2001. The crystal structure of a hyper-thermophilic carboxylesterase from the archaeon Archaeoglobus fulgidus. J. Mol. Biol. 314:507-518. [DOI] [PubMed] [Google Scholar]
- 10.Feller, G., M. Thiry, J. L. Arpigny, and C. Gerday. 1991. Cloning and expression in Escherichia coli of three lipase-encoding genes from the psychrotrophic antarctic strain Moraxella TA144. Gene 102:111-115. [DOI] [PubMed] [Google Scholar]
- 11.Franken, S. M., H. J. Rozeboom, K. H. Kalk, and B. W. Dijkstra. 1991. Crystal structure of haloalkane dehalogenase: an enzyme to detoxify halogenated alkanes. EMBO J. 10:1297-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi, N. N., and K. D. Mukherjee. 2000. Specificity of papaya lipase in esterification with respect to the chemical structure of substrates. J. Agric. Food Chem. 48:566-570. [DOI] [PubMed] [Google Scholar]
- 13.Hemilä, H., T. T. Koivula, and I. Palva. 1994. Hormone-sensitive lipase is closely related to several bacterial proteins, and distantly related to acetylcholinesterase and lipoprotein lipase: identification of a superfamily of esterases and lipases. Biochim. Biophys. Acta 1210:249-253. [DOI] [PubMed] [Google Scholar]
- 14.Huddleston, S., C. A. Yallop, and B. M. Charalambous. 1995. The identification and partial characterisation of a novel inducible extracellular thermostable esterase from the archaeon Sulfolobus shibatae. Biochem. Biophys. Res. Commun. 216:495-500. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda, M., and D. S. Clark. 1998. Molecular cloning of extremely thermostable esterase gene from hyperthermophilic archaeon Pyrococcus furiosus in Escherichia coli. Biotechnol. Bioeng. 57:624-629. [DOI] [PubMed] [Google Scholar]
- 16.Jaeger, K. E., B. W. Dijkstra, and M. T. Reetz. 1999. Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu. Rev. Microbiol. 53:315-351. [DOI] [PubMed] [Google Scholar]
- 17.Jaeger, K. E., S. Ransac, B. W. Dijkstra, C. Colson, M. van Heuvel, and O. Misset. 1994. Bacterial lipases. FEMS Microbiol. Rev. 15:29-63. [DOI] [PubMed] [Google Scholar]
- 18.Jaeger, K. E., and M. T. Reetz. 1998. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 16:396-403. [DOI] [PubMed] [Google Scholar]
- 19.Kanaya, S., T. Koyanagi, and E. Kanaya. 1998. An esterase from Escherichia coli with a sequence similarity to hormone-sensitive lipase. Biochem. J. 332:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klenk, H. P., R. A. Clayton, J. F. Tomb, O. White, K. E. Nelson, K. A. Ketchum, R. J. Dodson, M. Gwinn, E. K. Hickey, J. D. Peterson, D. L. Richardson, A. R. Kerlavage, D. E. Graham, N. C. Kyrpides, R. D. Fleischmann, J. Quackenbush, N. H. Lee, G. G. Sutton, S. Gill, E. F. Kirkness, B. A. Dougherty, K. McKenney, M. D. Adams, B. Loftus, S. Peterson, C. I. Reich, L. K. McNeil, J. H. Badger, A. Glodek, L. Zhou, R. Overbeek, J. D. Gocayne, J. F. Weidman, L. McDonald, T. Utterback, M. D. Cotton, T. Spriggs, P. Artiach, B. P. Kaine, S. M. Sykes, P. W. Sadow, K. P. D'Andrea, C. Bowman, C. Fujii, S. A. Garland, T. M. Mason, G. J. Olsen, C. M. Fraser, H. O. Smith, C. R. Woese, and J. C. Venter. 1997. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364-370. [DOI] [PubMed] [Google Scholar]
- 21.Lesuisse, E., K. Schanck, and C. Colson. 1993. Purification and preliminary characterization of the extracellular lipase of Bacillus subtilis 168, an extremely basic pH-tolerant enzyme. Eur. J. Biochem. 216:155-160. [DOI] [PubMed] [Google Scholar]
- 22.Liao, D. I., K. Breddam, R. M. Sweet, T. Bullock, and S. J. Remington. 1992. Refined atomic model of wheat serine carboxypeptidase II at 2.2-Å resolution. Biochemistry 31:9796-9812. [DOI] [PubMed] [Google Scholar]
- 23.Manco, G., E. Adinolfi, F. M. Pisani, G. Ottolina, G. Carrea, and M. Rossi. 1998. Overexpression and properties of a new thermophilic and thermostable esterase from Bacillus acidocaldarius with sequence similarity to hormone-sensitive lipase subfamily. Biochem. J. 332:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manco, G., E. Giosuè, S. D'Auria, P. Herman, G. Carrea, and M. Rossi. 2000. Cloning, overexpression, and properties of a new thermophilic and thermostable esterase with sequence similarity to hormone-sensitive lipase subfamily from the archaeon Archaeoglobus fulgidus. Arch. Biochem. Biophys. 373:182-192. [DOI] [PubMed] [Google Scholar]
- 25.Manco, G., L. Mandrich, and M. Rossi. 2001. Residues at the active site of the esterase 2 from Alicyclobacillus acidocaldarius involved in substrate specificity and catalytic activity at high temperature. J. Biol. Chem. 276:37482-37490. [DOI] [PubMed] [Google Scholar]
- 26.Morana, A., N. Di Prizito, V. Aurilia, M. Rossi, and R. Cannio. 2002. A carboxylesterase from the hyperthermophilic archaeon Sulfolobus solfataricus: cloning of the gene, characterization of the protein. Gene 283:107-115. [DOI] [PubMed] [Google Scholar]
- 27.Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, J. Schrag, J. L. Sussman, K. H. G. Verschueren, and A. Goldman. 1992. The α/β hydrolase fold. Protein Eng. 5:197-211. [DOI] [PubMed] [Google Scholar]
- 28.Østerlund, T., B. Danielsson, E. Degerman, J. A. Contreras, G. Edgren, R. C. Davis, M. C. Schotz, and C. Holm. 1996. Domain-structure analysis of recombinant rat hormone-sensitive lipase. Biochem. J. 319:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak, D., and D. Ollis. 1990. Refined structure of dienelactone hydrolase at 1.8 Å. J. Mol. Biol. 214:497-525. [DOI] [PubMed] [Google Scholar]
- 30.Phytian, S. J. 1998. Esterases, p. 193-241. In D. R. Kelly (ed.), Biotechnology, 2nd ed., vol. 8a. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 31.Rashid, N., Y. Shimada, S. Ezaki, H. Atomi, and T. Imanaka. 2001. Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A. Appl. Environ. Microbiol. 67:4064-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.She, Q., R. K. Singh, F. Confalonieri, Y. Zivanovic, G. Allard, M. J. Awayez, C. C.-Y. Chan-Weiher, I. G. Clausen, B. A. Curtis, A. De Moors, G. Erauso, C. Fletcher, P. M. K. Gordon, I. Heikamp-de Jong, A. C. Jeffries, C. J. Kozera, N. Medina, X. Peng, H. P. Thi-Ngoc, P. Redder, M. E. Schenk, C. Theriault, N. Tolstrup, R. L. Charlebois, W. F. Doolittle, M. Duguet, T. Gaasterland, R. A. Garrett, M. A. Ragan, C. W. Sensen, and J. Van der Oost. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 98:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada, Y., C. Koga, A. Sugihara, T. Nagao, N. Takada, S. Tsunasawa, and Y. Tominaga. 1993. Purification and characterization of a novel solvent-tolerant lipase from Fusarium heterosporum. J. Ferment. Bioeng. 75:349-352. [Google Scholar]
- 35.Sobek, H., and H. Görisch. 1989. Further kinetic and molecular characterization of an extremely heat-stable carboxylesterase from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochem. J. 261:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobek, H., and H. Görisch. 1988. Purification and characterization of a heat-stable esterase from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochem. J. 250:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sussman, J. L., M. Harel, F. Frolow, C. Oefner, A. Goldman, L. Toker, and I. Silman. 1991. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 253:872-879. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, Y., J. A. Contreras, P. Sheffield, T. Osterlund, U. Derewenda, R. E. Kneusel, U. Matern, C. Holm, and Z. S. Derewenda. 1999. Crystal structure of brefeldin A esterase, a bacterial homolog of the mammalian hormone-sensitive lipase. Nat. Struct. Biol. 6:340-345. [DOI] [PubMed] [Google Scholar]
- 40.Yeo, S. H., T. Nihira, and Y. Yamada. 1998. Purification and characterization of tert-butyl ester-hydrolyzing lipase from Burkholderia sp. YY62. Biosci. Biotechnol. Biochem. 62:2312-2317. [DOI] [PubMed] [Google Scholar]