Abstract
Glycohaemoglobins were first used in routine clinical laboratories for diabetes monitoring around 1977 and at the time all methods had either no calibrators, or used material with assayed values derived from individual manufacturers’ assays. Over the next five to fifteen years, lyophilised and whole blood sample exchanges were shown to improve inter-laboratory variability markedly. The use of a precise HPLC method as the “standard method” in the Diabetes Control and Complications Trial (DCCT) led to significant further improvement.
National standardisation schemes in the mid to late 1990s in the USA, Japan and Sweden further improved the quality and accuracy of HbA1c assays in clinical use.
The work of the IFCC Working Group on Standardisation of HbA1c in establishing true International Reference Methods for HbA1c and the successful preparation of pure HbA1c calibration material should lead to further improvements in inter-method and inter-laboratory variability, essential to the long-term monitoring of patients with diabetes.
Introduction – A Historical Perspective
HbA1c, glycated haemoglobin or glycohaemoglobin (GHb) is formed by the attachment of glucose to various amino groups, N-terminal valines and side chain lysine groups of both the two α and two β chains of normal adult haemoglobin A (HbA). HbA is the major component of adult Hb, being approximately 97%, along with 2.5% HbA2 and 0.5% HbF (Foetal haemoglobin).
60% of the glucose is bound to the N-terminal valines of the β chains. A small amount of glucose binding occurs at the α chain N-terminal valines. The remainder occurs at the forty- four lysine side chains, eleven on each of the two α and two β chains. HbA1c is therefore a heterogenous molecular structure. Between 1958 and 1961, Allen, Schroeder and colleagues chromatographically separated several fast moving minor Hb components in red cell haemolysates from healthy adults.1–3 These moved down a cation exchange (CE) column faster than HbA. They were described as HbA1a, HbA1b, HbA1c, in the order they were eluted, and accounted for about 7% of HbA with HbA1c being approximately 70–80% of these components.
In 1962 Huisman and Dozy noted that these “fast” moving haemoglobins were elevated in the red cells of diabetic individuals, but did not investigate the “fast” moving haemoglobin further because they attributed it to tolbutamide treatment in their patients.4
In 1968 Rahbar of the University of Tehran (Iran) reported a fast moving Hb fraction on starch and agar gel electrophoresis which was found in two out of 1200 patients.5 The two patients had diabetes mellitus. Later in 1969 he and colleagues determined that the component related to diabetes, was also present in the blood of people without diabetes, but there was about twice as much of this component in the blood of patients with diabetes.6
In 1971 the first suggestion of a relationship between mean blood glucose, long-term diabetic complications and fast haemoglobins was made by Trivelli.7
Koenig et al in 1975 using genetically modified diabetic mice, reported that the HbA1c fraction was present in diabetes after the onset of diabetes and appeared to be post-translational8 and in 1976 found a highly significant relationship between HbA1c, fasting blood glucose and oral glucose tolerance tests.9
Bunn et al in 1975 using in vivo studies, observed that the glycation process included the formation of a Schiff base (aldimine) and that much of the aldimine undergoes conversion to a stable ketoamine. Using Fe59 as a tracer, they demonstrated that the HbA1c levels increased over the 120 day life span of a red blood cell, and that this was probably a non-enzymatic reaction because of the slow rate of HbA1c formation.10
The usefulness of HbA1c in monitoring diabetic control was demonstrated in diabetic outpatients and was shown to correlate with urine and blood glucose over extended periods by Gabbay et al in 1977.11 The best correlation between HbA1c and 24 hour urine glucose occurred 43–70 days prior to the HbA1c sample. Physicians’ clinical ratings of metabolic control were shown to be correlated with HbA1c levels in patients with diabetes.12
HbA1c Methodology and Manufacturer Standardisation
Methods for glycated haemoglobins fall into three basic chemical principles.
Methods based on charge differences
Methods based on structural differences
Methods based on chemical reactivity
1. Methods Based on Charge Differences
These methods depend on the extra negative charge that is gained when glucose becomes attached to the N-terminal valine of the β chain of HbA. This extra charge allows the fast HbA1 fractions to migrate more rapidly in an electric field and to elute more rapidly from a negatively charged CE resin.
From the late 1970s to the mid 1980s the major assays were either electrophoretic or mini-column assays for HbA1 and later the more specific HbA1c. The mini-column assays were rapid “short column” variations of the original 1971 Trivelli macro-column Amberlite IRC-50 CE resin procedure7 which took several days to perform. Fractions were eluted in the order HbA1a, HbA1b, HbF, HbA1c, HbAO and HbA2 with buffers of varying ionic strength.
Kynoch and Lehman developed the first rapid (2½ hour) mini-column assay13 which was to become the forerunner of several commercial applications such as BioRad (Richmond, California), Helena (Beaumont, Texas) and Isolab (Akron, Ohio). Similar early developments used centrifuge assisted CE columns and resin slurry adaptations.
Many interferences were present in these methods, e.g. aldimine HbA1c, HbF, carbamylated haemoglobin and Hb variants. Assay temperature was shown to be a critical factor in the use of mini-columns based on CE utilizing BioRex 70 resin (BioRad Laboratories).
Rosenthal showed that using a stable preparation with a macro-column value of 8.6% HbA1, when assayed on commercial mini-columns, values from 3.7% HbA1 at 16°C, to 24% HbA1 at 35°C could be obtained. Only between 22–24°C were the mini-column values close to the macro-column value.14 Significant variations occurred even over this narrow temperature range. Temperature nomograms were initially used to correct assay temperatures to “true” macro-column HbA1 values.15
It was recognised that to get consistent results, mini-columns had to be temperature thermostatted. Kaplan et al showed that with assays performed in a temperature controlled system at 23°C, excellent precision with between day CVs <2% was achieved.16
The use of multilevel lyophilised bloods with values assigned by manufacturers using their own kit methods to determine the HbA1c values became common. Correction of achieved standard values to stated standard values was achieved by graphing the standards.17 The use of temperature control was shown to achieve better precision than the use of calibrators for correcting temperature variation.18 Later developments and current HbA1c HPLC assays based on CE, incorporate both thermostatted temperature control and the use of calibrators.
Other assays which are based on charge differences are agarose gel electrophoresis, isoelectric focusing (IEF), and capillary electrophoresis. These methods are no longer used for routine clinical use.
2. Methods Based on Structural Differences
Mallia et al. in 1981 described a method which separated glycated haemoglobins based on the binding of the cis-diol groups on glucose bound to haemoglobin as HbA1c to affinity chromatography (AC) resins.19 The resin was a cross-linked agarose, activated with carbonyl diimidazole bound to m-amino phenylboronic acid. Glycated haemoglobins bind to the affinity resin, whilst non-glycated haemoglobin does not bind. The glycated haemoglobins are then eluted with a counter polyalcohol ligand, sorbitol. Quantitation is by spectrophotometry at 415nm of the glycated and non-glycated fractions.
AC is much less temperature sensitive than CE and does not suffer from interfering factors such as HbF and carbamylated haemoglobin, and has long been recognised as the “reference” method for use in patients with haemoglobin variants.20
CE and AC give similar values in non-diabetic patients, but affinity methods are up to 40–50% higher than CE assays in patients with poorly controlled diabetes when the affinity value is reported directly from the column as % total glycated haemoglobin.
Non-diabetic AC reference ranges varied widely between manufacturers e.g. Clinovative (Pierce Chemical Co, Rochford, Illinois) 6.2 – 8.0% GHb; Isolab (Akron, Ohio) 5.5 – 8.5% GHb; and Endocrine Sciences (Tarzana, California) 4.0 – 6.8% GHb.
The non-specificity of CE methods for measuring glycated haemoglobin was originally determined using AC comparisons with both chemical techniques and CE chromatography.
When the HbA1c peak isolated from BioRex 70 resin was chromatographed on an affinity resin, only 65% to 75% of the peak was glycated, whilst 4.7% to 5.7% of the non-glycated peak was glycated.21
Manufacturers’ standardisation of manual AC assays was rarely performed. Most published assays had no standards and simply calculated ratios of absorbances of the glycated and non-glycated fractions directly from the AC resins used in individual methods and manufacturers’ kits.
Later AC column assays used lyophilised blood as standards and graphed calculated assay standard values on linear graph paper against stated levels to obtain patient values. Because of the large differences between CE HbA1c values and AC total GHb values, linear regressions were performed against CE methods to report AC values as the same numerical values as CE HbA1c values.
When the glycated fraction bound to affinity resin was assayed by colorimetric techniques, it was shown to contain 9.5% HbA1a + b, 52.3% HbA1c and 38.1% HbAO. 29% of HbA1a + b, 67% of HbA1c and 7% of HbAO appeared in the glycated fraction.22
Despite the considerable differences in specificity for glycated and non-glycated haemoglobins between CE and AC assays, linear regression analysis between the two methods, using patient samples, produces almost identical values, across the entire spectrum of HbA1c values in non-diabetic and diabetic patients, when the % total GHb affinity result is expressed as % HbA1c.21
3. Methods Based on Chemical Reactivity
(a) Thiobarbituric acid colorimetry
This assay was the first HbA1 assay suitable to large scale routine patient testing and was the first assay used by the author commencing in 1977. Lysed red cells react with oxalic acid to form 5-(hydroxymethyl)-furfural (5HMF). Thiobarbituric acid was added and a chromogen measured at 443nm. The assay was standardised against HbA1 values obtained from CE at 443nm (0.029 OD units = 1% HbA1c).23 In retrospect this assay was the first glycated haemoglobin assay to be used with a form of pure standards, albeit not pure HbA1c, using either HMF standards or fructose.24 This assay was never developed commercially and is no longer used in routine laboratories for blood analysis, but is still used in the measurement of advanced glycosylation end products.
(b) Immunoassays
In 1978 Javid et al described a radioimmunoassay for HbA1c using antiserum produced from sheep.25 The antiserum was relatively specific for HbA1c with minor cross reactivity to HbA1a and HbA1b and did not react with HbAO. The assay was never developed commercially.
In 1991 Dako Diagnostics Ltd (Ely, UK) marketed the first commercial immunoassay, Novoclone HbA1c, which used enzyme linked micro-titre plates with an antibody specific to the N-terminal 8 amino acids of ketoamine HbA1c. This antibody did not react with any abnormal heterozygous or homozygous haemoglobin variants and therefore gave much lower HbA1c results in patients with these variants. The assay was discontinued within a few years.
In 1992 Bayer Diagnostics (Elkhart, Indiana) introduced the DCA 2000 Point of Care HbA1c analyser. This manual analyser was originally marketed with an 8 minute assay programme, and subsequently converted to a 6 minute assay. It is now very widely used in hospital diabetes clinics, general practitioner medical clinics, remote aboriginal health centres and small laboratories.
Immunological based assays for HbA1c became more widely used in the mid 1990s with the advent of automated immunoanalysers. Many commercial assays are now available and are targeted against the β N-terminal glycated tetrapeptide or hexapeptide group.
Assay design is variable from immunoturbidimetry (Tinaquant - Boehringer Mannheim, Mannheim, Germany); latex agglutination inhibition method using monoclonal antibodies (DCA 2000 - Bayer, Elkhart, Indiana); to latex enhanced competitive immunoturbidimetry (Roche Diagnostics, Penzberg, Germany). These assays have stored lot specific standard curves, or utilize HbA1c standards calibrated against CE values and total haemoglobin standards.
Overall Manufacturer Standardisation
In the early to mid 1980s HbA1c methods produced inconsistent results which varied between assay types and between manufacturers. This was mainly due to the fact that glycohaemoglobins are heterogeneous and that numerous different methods measured different glycated species with different reliability, including lot to lot variability, and often without any form of standardisation.
Slightly later methodologies used lyophilised standards with calibrator values obtained by correlation from other methods in which the calibrator values had been simply obtained by calculating the % elution of non-specific glycated peaks to non-specific non-glycated peaks obtained from the average of multiple determinations.
Variability of glycated haemoglobin values also resulted from many different units being used: fast haemoglobin, HbA1, HbA1c and total glycohaemoglobins.
Inter-laboratory Harmonisation
The increasing use of HbA1c in the routine monitoring of diabetic control and the recognition of significant inter-laboratory variability prompted laboratories and diabetologists especially in the USA, UK and the Netherlands to investigate the feasibility of inter-laboratory comparability and harmonisation.
In the UK, Boucher et al performed a collaborative study, using blood samples from five diabetic and five non-diabetic patients, packed in ice and distributed to hospitals in England, Scotland and Wales using a variety of methods, minicolumn CE, thiobarbituric acid colorimetry, IEF and electroendosmosis. 26
Problems were experienced with aldimine HbA1c adducts which were at that stage not being routinely eliminated in HbA1c assays. They noted significant differences between methods and stated that “no useful inter-laboratory standards were clearly identified for any of the assay systems”.
Peterson et al in the USA (1984) similarly exchanged blood samples stored at 4°C and studied HPLC, thiobarbituric acid colorimetry, AC, and mini-column CE assays for factors such as the ability to discriminate between samples taken from diabetic and normal subjects, inter-method correlation, and reproducibility between laboratories. They noted that for HPLC CE and AC assays the blood samples were only stable for 4 days at 4°C, and that transport and transit times were significant aspects in the variability and that “in view of the increasingly widespread clinical and investigative use of glycosylated haemoglobin assays, international agreement regarding these issues is overdue”.27
Important developments towards reducing inter-laboratory variability occurred from 1985 to 1995, mainly from two centres, one in the USA and the second in the Netherlands.
The Department of Child Health in Columbia, Missouri under the direction of David Goldstein and Randie Little had pioneered the BioRex 70 HPLC assay, which was used as the reference anchor for the DCCT from 1983 onwards.
They used blood samples and frozen haemolysates and compared the DCCT assay with a manual mini-column CE assay and an AC manual assay. “Standards” derived as whole blood samples analysed at least 12 times with the DCCT assay were designated as the “standard values”. Using such standards, both manual column assays gave nearly identical values to the reference HPLC in both healthy individuals and patients with diabetes. Using crude haemolysate standards stored under proper long-term storage conditions (−70°C), even with assays measuring different GHb components they noted “harmonised values in these different types of kits were remarkably close to the actual measured HPLC values”.28
The same laboratory in 1992 studied seven different assays using a common frozen haemolysate calibrator. These assays comprised the HPLC reference assay, and assays using the following method principles, CE-HPLC, AC-HPLC, automated AC, electrophoresis, immunoassay and manual AC.29
They noted not only significant improvements in inter-laboratory variability due to the harmonised assays, e.g. a range of 0.7% HbA1c versus a range of 4.2% HbA1c using the non-harmonised assays, but also that long-term imprecision was improved substantially by common calibration e.g. manual affinity methods were lowered from CV of 10.1% for non calibrated assays to CV of 4.3% using calibrators. They chose crude frozen assayed haemolysates as the calibrator material because of the lack of availability of pure material.
A different approach using lyophilised haemolysate calibrators was chosen by the Dutch laboratory under the direction of Cas Weykamp and Theo Penders to study HbA1c variability and harmonisation. In 1993 they prepared stable lyophilised EDTA blood haemolysates in a Dutch external quality assurance programme SKZL. These were distributed to 101 laboratories using 12 different analytical methods.30
The HbA1c methods covered electrophoresis, ion-exchange chromatography, AC and enzyme immunoassay.
The mean intra-laboratory CV was 5.2% (range 0.2% – 28.7%). 47% of laboratories did not meet the criteria of <5% CV, whereas 68% did not meet the clinically more desirable 3.3 – 3.6% CV. Their conclusion was that “the mean inter-laboratory CV of 10% established for one method performed in many laboratories, and the mean inter-laboratory CV of 22% established for several methods performed in many laboratories reflected that an unacceptably high inter-laboratory imprecision existed amongst the assay types and individual laboratories”.30
In 1994 the Dutch group distributed firstly six lyophilised haemolysates and then six months later the same haemolysates with three lyophilised calibrators with values assigned by the BioRad Diamat HPLC.31 The Diamat reference values were obtained from nine laboratories.
Three point calibration with assigned values improved mean intra-laboratory CV from 6.6% to 3.5%. For samples with low (5.5%) and high (14.1%) HbA1c percentages, calibration decreased inter-laboratory variation per method from 10% to 4% and from 6% to 3%, inter-method variation from 18% to 4% and from 16% to 3%, and overall inter-laboratory variation from 25% to 7% and from 15% to 4% respectively for the two levels. Assays that did not use a calibrator supplied by the manufacturer benefited most from calibration.31
In 1995 they further studied whole blood comparisons in 103 laboratories using 20 methods, again with lyophilised calibrators.32 It was noted that lyophilised calibrators were not suitable for some assays, e.g. for the Abbott IMX and Vision (Abbott Laboratories, Abbott Park, Illinois). Lyophilisation increased the HbA1c values by 1 – 1.5% over the original whole blood.
The external calibrators produced distinct improvements in precision for all samples. At low HbA1c levels the CV decreased from 17% to 4% in the lyophilised sample and from 14% to 6% in the whole blood sample. At high HbA1c levels, the decrease was from 12% to 4% in the lyophilised sample and from 12% to 5% in whole blood.
They noted that the beneficial effect of calibration was somewhat less in the whole blood samples compared to lyophilised samples, which they stated was probably due to the artefacts introduced by the storage and transport of whole blood, and that lyophilised harmonisation may be more beneficial for multi-centre multinational studies, than the use of frozen haemolysates as is used in the Missouri studies.
Under the auspices of the International Federation of Clinical Chemistry (IFCC), both the Missouri DCCT / NGSP (National Glycohaemoglobin Standardisation Programme) laboratory and the Dutch SKZL, Winterswyck laboratory, along with that of the IFCC Chairman, Kor Miedema from Zwolle (Netherlands) were instrumental and pivotal in the establishment and ongoing progress of the IFCC Working Group on Standardisation of HbA1c.
In 1996 twenty seven laboratories in the State of Victoria, Australia were each distributed with four whole bloods from patients with significantly different HbA1c values. Reference target values were established by the Primus CLC 330 HPLC assay at Austin Health, Heidelberg, Victoria. The target values were (a) Non-diabetic 5.3% HbA1c, (b) Good Control 6.7% HbA1c, (c) Moderate Control 8.5% HbA1c, and (d) Poor Control 11.4% HbA1c.
The range of values reported in the 27 laboratories was as follows:
| (a) | Non-diabetic | 5.3% | target, |
| Reported laboratory range from | 4.1% to | 5.8% | |
| (b) | Good control | 6.7% | target, |
| Reported laboratory range from | 5.1% to | 8.2% | |
| (c) | Moderate control | 8.5% | target, |
| Reported laboratory range from | 6.7% to | 9.3% | |
| (d) | Poor control | 11.4% | target, |
| Reported laboratory range from | 10.1% to | 14.7% |
These different reported values had significant overlap between the non-diabetic and diabetic control levels.
There was inter-method variation between HbA1c values and also differences in method principle group CVs. CE HPLC assays produced the lowest imprecision.
CE HPLC assays had a mean CV of 2.8%
Microcolumn CE assays had a mean CV of 5.9%
Immunoassay assays had a mean CV of 4.5%
Manual AC assays had a mean CV of 7.6%
The conclusion was that a substantial degree of variability for HbA1c measurement existed amongst laboratories in Victoria.33
The South Australian AACB QC Subcommittee reported in 2004 a trial using a lyophilised calibrator (ERL) obtained from the supplier of the Australian Glycohaemoglobin External Quality Control Programme, SKZL in Winterswyck in the Netherlands. The calibrator was assigned to twelve participating laboratories in the South Australian Group QC Subcommittee. The ERL sample was run four times in one day and then four times each day for four days.
A subset of patient samples with values from 6 – 14% HbA1c was also assayed using each laboratory’s normal routine calibrator, and then repeating the assays using the ERL sample as calibrator. This was done in one day and then for four successive days.
Using this protocol they noted that for a HbA1c level of 9.0%, the reported HbA1c level ranged from 8.24 – 9.66% (CV 3.94%) and for a HbA1c level of 7.0%, the reported HbA1c ranged from 6.42 – 7.58% (CV 4.14%).
For one particular analyser this would raise the routine manufacturer calibrator standardisation CV of approximately 2.0 – 2.5% up to levels of 4.14% and, therefore, represent a significant backward step and they noted “that this could quite considerably inflate individual within laboratory CVs to undesirable levels”.
The authors also noted that where changes of lot numbers of reagent, columns and calibrators occurs as frequently as 3 – 4 times per year, this would require that such calibrator reassignment would need to occur rather frequently and that this realignment process must necessarily be subject to extreme vigilance and close supervision.34
Methods using one point calibration could only be compensated for in the area of slope bias. Only methods that employ true two point calibrators would be able to achieve intercept corrections by such protocols.
DCCT
The DCCT study commenced in 1983 and the final report was published in 1993.35 This landmark study involved 1441 patients with type 1 diabetes with zero or minimal diabetic complications subjected to two different treatment regimes. The first group was treated with conventional type 1 diabetes medication, 1–2 insulin injections daily and normal general practitioner supervision. The second group were treated by intensive insulin therapy, 3–4 insulin injections per day and intensive medical and nursing supervision.
The trial showed conclusively that in persons with Insulin Dependent Diabetes Mellitus the degree of metabolic control, as measured by glycohaemoglobins, was closely related to the risk of developing chronic diabetic complications. The risk of development or progression of retinopathy, nephropathy and neuropathy was reduced by 50–75% by intensive treatment regimes compared to conventional regimes.35
The difference in HbA1c between the two groups was a mean of 8.9% HbA1c in the conventional treatment group versus a mean of 7.1% HbA1c in the intensive treatment group.
Based on the DCCT outcome results the American Diabetes Association (ADA) recommended treatment aimed at keeping blood glucose levels as close as possible to normal. This set the scene for the recommendation of a specific HbA1c target of <7.0% and an action limit >8.0% for changing to more intensive antidiabetic therapy and treatment.36
All the HbA1c assays in the DCCT study were performed in one Central Laboratory, the University of Missouri, Department of Child Health, Columbia, Missouri USA under the direction of Professor David Goldstein and Dr Randie Little. The DCCT HbA1c method comprised a HPLC system using BioRex 70 CE resin column.37 Specimen throughput was only approximately two specimens per hour, and compared to modern HPLC HbA1c assays the DCCT assay had poor separation of peaks with no baseline separation. HbA1a and HbA1b were eluted in one peak and the HbA1c peak contained HbA1c, HbF and uraemic adduct. The assay was interfered with by abnormal haemoglobin variant specimens.
The method is very non-specific and some non-glycated haemoglobin appears in the glycated peak, and some of the glycated haemoglobin is included in the leading edge of the non-glycated HbA0 peak.21
Besides the medical implications of the DCCT, the study also established the necessity of an accurate precise HbA1c assay, and for all laboratories, independent of which HbA1c assay they were using, to be able to achieve similar HbA1c values so that all HbA1c results can be directly related to the DCCT outcome risks based on stated levels of HbA1c.
USA Standardisation Scheme
The USA “standardisation” scheme is actually a scheme which uses a designated comparison method. The American Association for Clinical Chemistry (AACC) established a Glycohaemoglobin Standardisation Subcommittee in April 1993. The aim was to develop a plan for Glycohaemoglobin standardisation and to prepare a pure calibrator. Initial results showed that the results of fresh blood comparisons were comparable between two laboratories using the same BioRex 70 assay in different institutions, however “purified” standards were more variable. The AACC committee recommended that at least two laboratories establish the DCCT candidate reference assay. The preparation of a pure calibrator was not achieved.
The NGSP was formed in July 1996 to implement the plan developed by the AACC Glycohaemoglobin Standardisation Subcommittee. The plan was to establish a system of reference laboratories which would support each other in a network which would be used to calibrate and standardise commercial HbA1c assays and analytical systems using all three basic principles of analysis for glycated haemoglobins. These are CE, AC and immunological based assays.
The BioRex 70 HPLC System, established in the DCCT Central Laboratory, and used to perform all the HbA1c assays for the nine year DCCT Study, was chosen as the NGSP “reference standard method”. The Missouri DCCT laboratory was established as the Central Primary Reference Laboratory (CPRL) for the NGSP. This laboratory sets the initial calibration for the NGSP Certification Programme as the “set point” as used in the DCCT. Precision must be documented to be <3% following NCCLS EP5-A Guidelines.38
Back-up Primary Reference Laboratories (PRL) were established to support the CPRL and ensure that the CPRL function continues if the CPRL cannot meet the NGSP programme needs. All PRLs must establish the CPRL BioRex 70 HPLC Method and use a single haemolysate calibrator prepared by the CPRL with an established target value based on the mean of at least 50 CPRL runs. This calibrator is to be used to perform daily calibration, and PRL assays must be quality controlled by QC haemolysate samples prepared and value assigned by the CPRL. Each PRL then accepts or rejects assays based on the CPRL guidelines.
To be traceable to the CPRL, each PRL must partake in a 100 sample fresh blood comparison for estimation of bias using a modified NCCLS EP9-T guideline.39
To be considered traceable to the CPRL, each PRL must have
Total imprecision (CV) that is not statistically significantly >3%
No more than one within method outlier and no more than one between method outlier.
The 95% confidence interval (CI) for predicted bias must overlap the ±3% range of the CPRL at two levels (6 and 9% HbA1c).
PRLs are regularly monitored by the CPRL using 10 fresh or fresh frozen whole blood samples prepared monthly by the CPRL over the range 4–14% HbA1c and analysed in duplicate over two separate runs over two days.
To maintain certification each PRL must fulfil each month the requirements for bias and precision which are
The mean of the difference (n=10) between the Network Laboratory and the CPRL must not exceed 0.35% HbA1c
The estimate of the SD of the difference in sample replicates must not exceed 0.229 % HbA1c (99th percentile of the sampling distribution around a target SD of 0.15).
A number of Secondary Reference Laboratories (SRL) were established to work directly with manufacturers to assist them in standardising commercial methods for Certification of Traceability to the CPRL BioRex 70 assay.
The SRLs use established precise routine automated assays but must have these calibrated against the Central Primary Reference CPRL calibrator. The initial SRL methods were the BioRad Diamat (Hercules, California) CE HPLC analyser and the Primus CLC 330 (Kansas City, Missouri) AC HPLC analyser.
Currently the SRLs utilize either the Primus CLC 330, Tosoh 2.2 (Tokyo, Japan) CE HPLC, or the Roche Tinaquant (Penzberg, Germany) Immuno HbA1c assay.
In the establishment of each SRL, the SRL must partake in a 100 sample fresh blood exchange as do the PRL laboratories, and must meet the same initial criteria and same monthly monitoring as that of a PRL to be considered traceable to the NGSP.
The NGSP has an excellent website www.ngsp.org which details all aspects of the NGSP processes, important HbA1c/ diabetic data, and has excellent links to diabetes information sites. The latest list of NGSP Certified Manufacturer Methods and Laboratories (updated November 2004) details 61 combinations of manufacturers, analysers and methods, and 37 individually certified laboratories.
NGSP Manufacturer Annual Certification
Almost 100% of USA manufacturers have all their analytical HbA1c assays and systems NGSP certified. Manufacturers must perform precision testing according to NCCLS EP5-A Guidelines38 and must partake in a fresh blood sample comparison (n=40) for estimation of bias from the SRL.39
To be certified as traceable to the DCCT, manufacturers must meet the following criteria:
Total imprecision (CV) must not be statistically significantly >4%
The 95% CI of the differences between methods (test method and SRL method) must fall within the clinically significant limits of ±1% HbA1c.40
There is no monthly monitoring for manufacturers. Manufacturers may need to re-assign their own calibrator values, or use conversion equations to re-assign standard values.
The NGSP Certification Process can also be obtained by individual laboratories to be traceable to the DCCT. This type of certification is recommended for large laboratories involved in research studies or clinical trials where long term precision is critical. The certification process is similar to that of manufacturers but the certification criteria are more stringent (a) CV must not be statistically significantly >3%, and (b) the 95% CI of the differences between methods must fall within ± 0.75% HbA1c.
Level One laboratories are monitored quarterly using 10 fresh blood specimens, analysed in two separate runs on separate days. Monitoring criteria are the same as for the NGSP network laboratories.
The NGSP Certification and DCCT values are not traceable to a “reference” method and do not use purified standards. The %HbA1c unit is traceable only to a peak on the non-specific BioRex 70 chromatogram using specific HPLC elution conditions, column resin and reagents, and using whole blood comparison values that have been assigned by multiple analytical runs on the same “reference” method.37
College of American Pathologists (CAP) Surveys
All USA laboratories are asked as part of Certification for Quality to enrol in an external quality assurance programme for HbA1c using whole blood specimens.
The CAP Survey established a monitoring programme covering Glycohaemoglobinsin 1985 using lyophilised material initially as the Electrophoresis – Chromatography Survey.41 This was trialled with whole blood in 1996 in a Glycohaemoglobin pilot programme for 500 laboratories. From the start of 1998 all laboratories in the CAP Glycohaemoglobin Programme, more than 2000 laboratories, used whole blood samples, initially in 1998 using five different subsets, and in 1999 using only one subset for the entire 2000 laboratories.
Typically three whole blood samples are distributed each six months. One sample is a normal range level, one a mid-range level, typically 8–9% HbA1c, and one a high level, typically 10–12% HbA1c.
Despite ten years of NGSP standardisation, there are still significant differences between manufacturers’ medians as documented in the NGSP website (www.ngsp.org) for CAP specimens which are distributed twice per year.
In a Five Year Progress Report on the NGSP Programme it is noted that in 2000, 78% of laboratories were using an NGSP Certified Method and for most certified methods, between laboratory CVs were <5%.42 For all certified methods, the mean % HbA1c was within 0.8% of the NGSP target at all HbA1c concentrations, being ±0.6% at the low level, ±0.5% at the middle level and ±0.8% at the high level respectively for the CAP 2000 GH2-B samples.
However, assuming these are ±2SD ranges, these ranges represent CVs of 5.35% at a CAP / NGSP target of 5.6% HbA1c, 3.0% CV at a target of 8.3% HbA1c, and 3.3% CV at a target of 12.2% HbA1c.
In terms of true percentages of HbA1c measured by laboratories, these values therefore represent ±10.7% at the low level (5.1 – 6.1% HbA1c), ±6.0% at the middle level (7.8 – 8.8%% HbA1c), and ±6.6% at the high level (11.4 – 13.0% HbA1c).
The most recently completed CAP surveys for NGSP certified methods are detailed in the Table. These are calculated from the three most recent six monthly CAP surveys available from retrospective NGSP website data supplied by the NGSP Director, Dr Randie Little, a member of the IFCC Working Group on Standardisation of HbA1c.
Table.
CAP Data from NGSP website. 2003-2004 samples.
| 2003 GH2A | 2003 GH2B | 2004 GH2A | ||||
|---|---|---|---|---|---|---|
| Low Level | Target | 5.2% | Target | 5.3% | Target | 5.2% |
| Range | ±0.4% | Range | ±0.4% | Range | ±0.3% | |
| CV | 3.8% | CV | 3.8% | CV | 2.9% | |
| Actual | ±7.6% | Actual | ±7.8% | Actual | ±5.8% | |
| Mid Level | Target | 8.7% | Target | 8.0% | Target | 9.3% |
| Range | ±0.35% | *Data deleted by CAP | Range | ±0.5% | ||
| CV | 2.0% | CV | 2.7% | |||
| Actual | ±4.0% | Actual | ±5.4% | |||
| High Level | Target | 11.5% | Target | 9.2% | Target | 12.0% |
| Range | ±0.45% | Range | ±0.6% | Range | ±0.65% | |
| CV | 2.0% | CV | 3.3% | CV | 2.7% | |
| Actual | ±4.0% | Actual | ±6.6% | Actual | ±5.4% | |
Mid level 2003 GH2B data deleted by CAP, due to this sample being a heterozygous HbAS variant which resulted in very significant bias with some NGSP manufacturer certified methods.
For the low level CAP sample over the three cycles the range for NGSP certified manufacturers’ medians have varied from ±0.3% to ±0.4%, representing true variations between ±5.8% and ±7.8%.
For the mid level CAP samples, the range has varied from ±0.35% to ±0.5%, representing true variations between ±4.0% and ±5.4%.
For the high level CAP samples, the range has varied from ±0.45% to ±0.65% representing true variations of ±4.0% and ±6.6%.
The values reported in the Table are from approximately 2000 laboratories in each cycle and are calculated as the manufacturers’ medians for each NGSP Certified manufacturers’ analytical systems. The overall variation between individual laboratories using these NGSP Certified methods would therefore be significantly greater than the differences between NGSP Certified manufacturers’ medians stated in CAP and NGSP / CAP GHb Programme Reports.
Japanese Standardisation Scheme
In 1995 the Japanese Diabetes Society (JDS) in collaboration with the Japan Society of Clinical Chemistry (JSCC) developed a National Standardisation Scheme. Two calibrators (JDS Calibrator Lot 1) were prepared as lyophilised haemolysates. The HbA1c values were assigned as the mean of the two most common HPLC analysers, Tosoh (Tokyo, Japan) and Kyoto Daiichi (Kyoto, Japan), both very precise CE based assays.43 At the time most Japanese laboratories were using one of these methods. These two point calibrators were recommended to be used for the calibration of all routine HbA1c assays in Japan.
In 2000 the calibration based on the mean of the Tosoh and Kyoto Daiichi HPLC calibration methods was replaced by a more specific high resolution CE HPLC analyser KO500, developed by the JSCC44 and a second set of National Calibrators (deep frozen blood) called JDS/JSCC Calibrator Lot 2 were prepared and assigned HbA1c values. The values of these new calibrators were assigned using the KO500 analyser in four Reference laboratories of the JDS/JSCC. To keep consistency with the Japanese Calibrator Lot 1 values, the Calibrator Lot 2 values were adjusted to those of the Calibrator Lot 1.
The JDS/JSCC calibrators are used by approximately 1800 laboratories in Japan. The effect of the calibrators has been a significant improvement in HbA1c results between laboratories and a progressive decrease of mean inter-laboratory variation from values of more than 12% CV to approximately 5%. More precise CVs were found amongst HPLC methods.45,46
Three Japanese reference laboratories are included in the IFCC Reference Laboratory Network. For all IFCC comparative studies which were used to establish the IFCC – JDS Master Equations the KO500 method was calibrated with JDS Calibrator Lot 2.47
The JDS/JSCC values are on average approximately 0.2% HbA1c below the NGSP values.
The JDS/JSCC Scheme has a non-diabetic reference range of 4.3–5.8% HbA1c.
The JDS/JSCC Standardisation Scheme is not traceable to a “reference” method and does not use purified calibrators. Like the NGSP/DCCT BioRex 70 method it is only traceable to a peak on a chromatogram using defined HPLC elution conditions, column resin and reagents, and using “standards” that have values designated by multiple analytical runs on the same “reference” method but actually referenced to the HbA1c values from the original JDS calibrator 1 assigned from the Tosoh and Kyoto Daiichi systems. The KO500 is not a commercially available assay.
Swedish Standardisation Scheme
The Swedish standardisation scheme uses as its “reference” method the analysis of HbA1c using Mono S HPLC48 (a strong methylsulphonate cation exchanger on monobeads, Pharmacia LKB Biotechnology, Uppsala, Sweden).
Mono S is a relatively specific HbA1c assay separating HbA1c from all known minor endogenous components except carbamylated (uraemic adduct) haemoglobin and α chains. HbF is well separated.
Attempts at national External Quality Assurance Scheme (EQAS) inter-laboratory surveys initially commenced in 1991. Five Swedish laboratories assigned values for lyophilised samples and distributed them with a recommended value. Initial inter-laboratory CVs were 15–20% but these included two laboratories using AC and producing total GHb values.49 From 1998, common standardisation throughout Sweden was introduced. Five selected Swedish reference laboratories using Mono S HPLC assays assign values to pooled whole blood samples. These laboratories are used for calibration of all hospital and point of care instruments in Sweden every second year. In Sweden all laboratories must subscribe to the External Quality Assurance in Laboratory Medicine in Sweden (EQALIS). Samples are distributed each month. Inter-laboratory CVs have appreciably improved from around 10–12% CV to less than 6%, but the recommended limit of <3% has not been achieved. HPLC assays show inter-laboratory CVs <3%, and immunochemical assays CV’s of 4 – 6%.46
The non-diabetic reference range for the Swedish Mono S standardisation scheme is 3.6 – 5.0% HbA1c with a treatment target of <6.5% HbA1c.
Yearly comparisons between the Swedish Mono S laboratories and the USA NGSP reference laboratories show 1.1% lower values for HbA1c with Mono S over the whole range compared to the NGSP values.46
Standardisation of HbA1c Methods in Australia and New Zealand
There is no national standardisation in Australia or New Zealand. Almost 100% of methods used in both countries, with the exception of in-house CE HPLC assays, are standardised against the USA based NGSP scheme. This is by virtue of all major multinational manufacturers’ methods and instrument applications used in both countries being NGSP Certified.
It is difficult to envisage further significant improvements between manufacturers’ assays unless the current NGSP manufacturer certification requirements of inter-run imprecision <4% CV and 95% CI <± 1.0% HbA1c, detailed in the NGSP website www.ngsp.org are significantly tightened.
Tighter limits on imprecision and inter-sample accuracy have been recommended. A consensus Position Statement published by the Australian Diabetes Society, Royal College of Pathologists of Australasia and the Australasian Association of Clinical Biochemists recommended that “between run CVs of <3% are far more clinically useful than CVs of <5% and are therefore desirable.50
At the international workshop held in Dusseldorf, Germany on 17 January 2002, under the auspices of the European Association for the Study of Diabetes (EASD) and representatives of the EASD, ADA, IDF (International Diabetes Federation), IFCC and CEN (European Committee for Standardisation) the consensus statement agreed to was that the “clinical requirements of a HbA1c test include (a) precision that justifies clinicians acting on differences of 0.35% to 0.5% as being significant and (b) HbA1c assays with CV less than or equal to 2% for combined, between and within run precision.”51
Incorporation of tighter criteria for NGSP manufacturer certification would result in a considerable number of current manufacturers’ methods and instruments being unable to meet such criteria, but would ensure that the manufacturers that did meet such criteria would have much closer comparative HbA1c patient values between all NGSP certified manufacturers’ methods.
IFCC Global Standardisation
In 1994 the IFCC established a Working Group (WG) on Standardisation of HbA1c. The overall aim was to have one worldwide Reference System and to develop an International Reference Method and purified HbA1c standards/calibrators.52 The IFCC group was, and still comprises HbA1c specialists with particular knowledge of glycohaemoglobins/HbA1c. The WG does not represent particular countries or regions, but in essence the WG members are all representing member countries of the IFCC and covering widespread parts of the globe. The WG has met regularly since 1995.
Members and countries of origin are as follows:
| Kor Miedema (Chairman) | Zwolle | Netherlands |
| Andrea Mosca (Secretary) | Milan | Italy |
| Cas Weykamp (Network Coordinator) | Winterswyck | Netherlands |
| Ian Goodall | Melbourne | Australia |
| Tadao Hoshino | Ono | Japan |
| Jan-Olof Jeppsson | Malmo | Sweden |
| Garry John | Norwich | UK |
| Randie Little | Columbia | USA |
| Gary Myers | Atlanta | USA |
| David Sacks | Boston | USA |
The following members have retired from the IFCC WG.
| David Goldstein | Columbia | USA |
| Wieland Hoelzel (ex WG Secretary) | Penzberg | Germany |
| Theo Penders | Winterswyck | Netherlands |
| Kenji Shima | Tokushima City | Japan |
Specific aims for the Working Group were developed and established.
These were:
To define the heterogenous HbA1c molecule
To prepare pure HbAO and HbA1c
To develop a Reference Method
To establish a Reference Laboratory Network
To prepare secondary reference calibrators and controls
All previous methods of widely used inter-laboratory HbA1c calibration schemes had been based on either (a) harmonisation by calibration using a single or multiple calibrators with values assigned by a particular method or group of laboratories e.g. Japan JDS/JSCC; or (b) harmonisation by a designated method comparison (DCM) whereby all assays are standardised based on comparisons to a selected reference method or system and then the method standards are recalibrated by linear regression analysis e.g. NGSP and Sweden.53
The IFCC WG has subsequently developed a global HbA1c Reference system and Network of Reference Laboratories supporting newly developed HbA1c Reference Methodology and pure HbA1c standards to calibrate the IFCC system.54
IFCC WG Progress to Date
HbA1c has been defined as β N-valine glycated Hb (β-N(1-deoxy) fructosyl Hb), a hexapeptide. This represents the major glycation site of the HbA1c molecule.
-
Purified calibrators have been prepared after extensive preparative chromatography steps. Briefly, whole blood is washed with saline to remove aldimine and then purified through CE chromatography followed by AC followed by a further CE chromatography step.54
Following this method, HbAO is >99.5% pure and HbA1c >98.5% pure. Batches are prepared annually and six linearly related standard mixtures covering the range of 0–15% HbA1c are used to standardise the IFCC reference methods. This process has produced stable and consistent calibrators over the past five years.
Two IFCC Reference methods have been developed and have been published by Kobold et al.55 and Jeppsson et al.56 Whole blood is washed and lysed and the haemoglobin is cleaved into peptides using a proteolytic enzyme endoproteinase Glu-C. The resulting glycated and non-glycated N-terminal hexapeptides are then separated by reversed phase HPLC. Final quantitation is by mass spectrometry or by capillary electrophoresis with ultra violet detection. Both IFCC reference methods yield identical % HbA1c values in all samples and mixtures analysed.
-
A Network of 13 IFCC Reference Laboratories has been established in Europe, USA and Japan. European Reference Laboratories are established in Belgium, Germany, Italy, the Netherlands and Sweden.
Each IFCC Reference Laboratory must establish one or both of the IFCC Reference Methods and be approved and certified by the IFCC. Twice yearly pooled sample exchanges are distributed to all IFCC Network Laboratories and National Standardisation Programmes (Designated Comparison Methods [DCM]).
IFCC Network rules are detailed as Standard Operating Procedures for the IFCC Network of Reference Laboratories. The procedure manual is available on the IFCC WG website www.ifcchba1c.com.57
The criteria for IFCC Reference Laboratory precision was tightened in 2002 from “average CV <3%” to “average CV 2.5%”. The Network Laboratories consistently produce CVs of approximately 1% CV in the twice yearly inter-comparison studies. The mean deviation of each IFCC laboratory from the overall mean of IFCC laboratories must be <2.5%.
Secondary reference materials have been prepared with values assigned by the IFCC Network Laboratories.
Twice yearly comparison studies have been performed for many years. Samples have been prepared at the IFCC Network Coordinator, Cas Weykamp’s, laboratory at SKZL Queen Beatrix Hospital, Winterswyck, The Netherlands, and distributed to IFCC Reference Laboratories, candidate IFCC Reference Laboratories, DCM Reference laboratories in Japan, Sweden and the USA, and multinational manufacturers in Europe (4), Japan (2) and the USA (6). Comparison studies have been performed using IFCC standards, and mixtures of patient samples and lyophilised material.
The mean intra-laboratory CV performed in the five comparisons reported (2001–2003) was 1.0 – 1.2%, the intra-laboratory CV varied from 0.5% to 2.2% and the inter-laboratory CVs were in the range 1.4 – 1.9%.47
Significant differences were observed between the HbA1c values of the IFCC Network laboratories and the three DCM networks, and significant differences between each of the DCMs, but in each individual case the relationship between each DCM and the IFCC was linear.
The relationship of each DCM with the IFCC method is described by the following Master Equations:
 |
 |
 |
These links have been stable and reproducible between all DCMs and IFCC.47
All past, present and future studies and research trials can be linked and converted to IFCC units or to old DCM units by the use of the Master Equations. All previous clinical trials such as the DCCT and United Kingdom Prospective Diabetes Study (UKPDS) can be readily converted into IFCC units or to any other internationally agreed unit scale and numerical value of HbA1c.
Discussions between the IFCC-WG, DCMs and clinicians (e.g. ADA) have been ongoing for several years regarding the choice of the worldwide unit, whether to report in new IFCC % HbA1c or to back convert to % HbA1c – NGSP/DCCT in the USA. Due to the inability to reach a consensus, discussions on this matter have been referred to International Diabetes Associations, IDF, EASD and the ADA.
International Diabetes Associations Working Group Meeting
A meeting was held in London on 20 January 2004 between the IDF, EASD and ADA with the Chairman of the IFCC HbA1c WG and a representative of the NGSP.58, 59
The Meeting Objective was to make recommendations on the implementation of the IFCC reference method.
The International Diabetes Workgroup agreed that the IFCC Reference Method should become the global reference standard (anchor) and that all manufacturers should now calibrate to the new method. Such a change would imply that % HbA1c numbers would be 1–2% lower than those currently reported, as would the reference range, target for good control and change of therapy level.
The Workgroup discussed the advantages and disadvantages of reporting the new IFCC numerical range or maintaining the current ranges. The main advantage of reporting the new IFCC range is that these are the true actual HbA1c values. Disadvantages are (a) the high cost, and prolonged timeline for re-education of patients and clinicians and other diabetes support staff, (b) the risk of deterioration of glycaemic control as experienced in a Swedish study when new low Mono S values were introduced and overall diabetic control worsened initially as many patients adjusted their lower Mono S HbA1c values to the old higher levels,60 and (c) that lower numbers make it even more difficult to convince patients that small changes in %A1c have a big impact on health.
The main advantage of reporting the old values would be that they are familiar to clinicians and patients, and relate values to evidence based trials such as DCCT/UKPDS. Disadvantages are that they are not the “pure” result and are frequently confused with mmol/L glucose values, and this would be a missed opportunity to reinforce the importance of the test. There is also not just one universal “old’ value, but several.
Of paramount importance was the agreement that the whole world should be using and reporting the same units and reference values.
The European and American WG members suggestions at the meeting that the name of the assay be changed to something that reflects the mean blood glucose (MBG) was enthusiastically and universally supported.
This relationship between HbA1c and MBG established from the DCCT is:
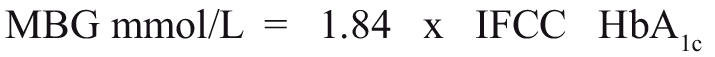 |
This would give the test a new name, a new range in familiar blood glucose units and a more direct link to glucose levels in people with diabetes and their health care professionals.
The disadvantages are that there are scientific reservations about the use of MBG equivalent values rather than the HbA1c value itself, the simple HbA1c / MBG proportionally may not apply to all populations, and that more clinical data is considered to be needed for populations with different ethnic/ racial backgrounds.
The Workgroup voted unanimously to endorse the MBG suggestion and outlined the following steps.58, 59
There should be immediate adoption of the IFCC reference method as the new calibration standard. Lead responsibility IFCC/NGSP.
International Certification processes should be immediately anchored within current existing DCMs to the IFCC Reference Method. Lead responsibility NGSP/IFCC.
The IFCC and NGSP must immediately direct manufacturers NOT to change the reporting units until further work on links between MBG / HbA1c is researched. Lead responsibility IFCC/NGSP.
The IFCC should determine if other retrospective data linking HbA1c and MBG is available especially for non-Caucasian patients. Time frame 4–6 months.
ADA, EASD and IDF should design and conduct prospective studies on populations worldwide to further establish the MBG / HbA1c relationship. Time frame 2004–2007.
The current workgroup should plan public and professional education programmes about the new reporting units and system. Time frame 2005–2007.
Despite the seeming acceptance and choice of MBG proposed by the International Diabetes Associations Working Group, the choice of other units for HbA1c is still open. The Joint IFCC Committee on Nomenclature, Properties and Units and IUPAC (International Union of Pure and Applied Chemistry) Subcommittee on Nomenclature, Properties and Units (C-PNU) have proposed mmol / mol as being the true SI unit, representing mmol HbA1c / mol Hb. This would give an approximate reference range of 28–40 mmol / mol with a target of 50 mmol / mol and a change of therapy at >60 mmol / mol.
True % HbA1c – IFCC Units or “old % HbA1c” units (DCMs) are still possible, but unlikely.
Conclusion
HbA1c has over the past twenty five years become the major assay used in the long-term monitoring of diabetes. The successful establishment of true reference methodology and pure HbA1c calibration material, when internationally used, together with an agreement between the IFCC, IUPAC and International Diabetes Associations on the choice of one unit and one numerical value throughout the world, will be the final significant stage in the global use and true value of the glycohaemoglobin assay.
Irrespective of the name of the assay or the numerical unit, the concept and use of long-term monitoring of diabetes by the HbA1c assay will ultimately depend on the accuracy and precision of the HbA1c assay itself.
It is certainly the author’s strong belief that a HbA1c target expressed as a MBG level of <9.5 mmol/L, and a change of therapy at a MBG level >11.5 mmol/L will not be regarded by clinicians or patients with diabetes, to the same degree of importance and definition, as currently is the target of <7% HbA1c, and the change of therapy at >8% HbA1c. The upper limit of the non-diabetic HbA1c range of 6.0% HbA1c is equivalent to a MBG of 7.5 mmol/L.
The contents of articles or advertisements in The Clinical Biochemist – Reviews are not to be construed as official statements, evaluations or endorsements by the AACB, its official bodies or its agents. Statements of opinion in AACB publications are those of the contributors. Print Post Approved - PP255003/01665.
No literary matter in The Clinical Biochemist – Reviews is to be reproduced, stored in a retrieval system or transmitted in any form by electronic or mechanical means, photocopying or recording, without permission. Requests to do so should be addressed to the Editor. ISSN 0159 – 8090.
References
- 1.Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and foetal haemoglobins. A study of the effects of crystallisation and chromatography in the heterogeneity and isoleucine content. J Am Chem Soc. 1958;80:1628–34. [Google Scholar]
- 2.Clegg MD, Schroeder WA. A chromatographic study of the minor components of normal adult haemoglobin including a comparison of haemoglobin from normal and phenylketamine individuals. J Am Chem Soc. 1959;81:6065–9. [Google Scholar]
- 3.Schneck AG, Schroeder WA. The relation between the minor components of normal adult haemoglobin as isolated by chromatography and starch block electrophoresis. J Am Chem Soc. 1961;83:1472–8. [Google Scholar]
- 4.Huisman THJ, Dozy AM. Studies on the heterogeneity of haemoglobin. V. Binding of haemoglobin with oxidised glutathione. J Lab Clin Med. 1962;60:302–19. [PubMed] [Google Scholar]
- 5.Rahbar S. An abnormal haemoglobin in red cells of diabetics. Clin Chim Acta. 1968;22:296–8. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- 6.Rahbar S, Blumanfeld O, Ranney HM. Studies of an unusual haemoglob in inpatients with diabetes mellitus. Biochem Biophys Res Commun. 1969;36:838–45. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- 7.Trivelli LA, Ranney HM, Lai HT. Haemoglobin components in patients with diabetes mellitus. N Eng J Med. 1971;284:353–7. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- 8.Koenig RJ, Cerami A. Synthesis of HbA1c and diabetic mice. Potential model of basement membrane thickening. Proc Natl Acad Sci USA 1985;72:9:3687–91. [DOI] [PMC free article] [PubMed]
- 9.Koenig RJ, Peterson CM, Kilo C, Cerami A, Williamson JR. Haemoglobin A1c as an indicator of the degree of glucose intolerance in diabetics. Diabetes. 1976;25:230–2. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- 10.Bunn HF, Haney DN, Gabbay KH, Gallop PM. Further identification of the nature and linkage of the carbohydrate in haemoglobin A1c. Biochem Biophys Res Commun. 1975;67:103–9. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- 11.Gabbay KH, Hasty K, Breslow JL, Ellison RC, Bunn HF, Gallop PM. Glycosylated haemoglobins and long term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977;44:859–64. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- 12.Gonen B, Rubenstein AH, Rockman M, Tanega S, Horwitz DL. Haemoglobin A1: An indicator of the metabolic control of diabetics. Lancet. 1977;11:734–7. doi: 10.1016/s0140-6736(77)90237-9. [DOI] [PubMed] [Google Scholar]
- 13.Kynoch PAM, Lehman H. Rapid estimation (2½ hour) of glycosylated haemoglobin for routine purposes. Lancet. 1977;2:16. doi: 10.1016/s0140-6736(77)90007-1. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal MA. The effect of temperature on the fast haemoglobin test system. Haemoglobin. 1979;3:215–7. doi: 10.3109/03630267908998917. [DOI] [PubMed] [Google Scholar]
- 15.Hankins WD, Holloway L. A temperature conversion nomogram for glycosylated haemoglobin analysis. Clin Chim Acta. 1980;104:251–7. doi: 10.1016/0009-8981(80)90203-x. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan LA, Cline D, Gartside P, Burstein S, Sperling M, Stein EA. Haemoglobin A1 in haemolysates from healthy and insulin dependent diabetic children, as determined with temperature controlled minicolumn assay. Clin Chem. 1982;28:13–8. [PubMed] [Google Scholar]
- 17.Haemoglobin A1c by column test for measuring the percentage of HbA1c in whole blood. Bulletin 4237 BioRad Laboratories, Richmond, California. July 1984.
- 18.Hanson NQ, Anderson ML, Freier EF. Temperature control better than calibrators for precision in the microcolumn method for haemoglobin A1c. Clin Chem. 1985;31:339–40. [PubMed] [Google Scholar]
- 19.Mallia AK, Hermanson GT, Krohn RI, Fujimoto EK, Smith PK. Preparation and use of a boronic acid affinity support for separation and quantitation of glycosylated haemoglobin. Anal Lett. 1981;14:649–61. [Google Scholar]
- 20.Weykamp CW, Penders TJ, Muskiet FAJ, van der Slik W. Influence of haemoglobin variants and derivatives on glycohaemoglobin determinations, as investigated by 102 laboratories using 16 methods. Clin Chem. 1993;39:1717–23. [PubMed] [Google Scholar]
- 21.Garlick RL, Mazer JS, Higgin PJ, Bunn HG. Characteristics of glycosylated haemoglobins. Relevance to monitoring diabetic control and analysis of other proteins. J. Clin Invest. 1983;71:1062–72. doi: 10.1172/JCI110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham EC, Perry RE, Stallings M. Application of affinity chromatography for separation and quantitation of glycosylated haemoglobin. J Lab Clin Med. 1983;102:187–97. [PubMed] [Google Scholar]
- 23.Fluckiger R, Winterhalter KH. In vitro synthesis of haemoglobin A1c. FEBS Lett. 1976;71:356–60. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- 24.Parker KM, England JD, Da Costa J, Hess R, Goldstein DE. Improved colorimetric assay for glycosylated haemoglobin. Clin Chem. 1981;27:669–72. [PubMed] [Google Scholar]
- 25.Javid J, Pettis PK, Koenig RJ, Cerami A. Immunological characterisation and quantitation of HbA1c. Br J Haematol. 1978;38:329–37. doi: 10.1111/j.1365-2141.1978.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 26.Boucher BJ, Burrin JM, Gould BJ, et al. A collaborative study of the measurement of glycosylated haemoglobin by several methods in the United Kingdom. Diabetologia. 1983;24:265–71. doi: 10.1007/BF00282711. [DOI] [PubMed] [Google Scholar]
- 27.Peterson CM, Jovanovic L, Raskin P, Goldstein DE. A comparative evaluation of glycosylated haemoglobin assays : feasibility of references and standards. Diabetologia. 1984;26:214–7. doi: 10.1007/BF00252410. [DOI] [PubMed] [Google Scholar]
- 28.Little RR, England JD, Wiedmeyer H-M, et al. Interlaboratory standardisation of glycated haemoglobin determinations. Clin Chem. 1986;32:358–60. [PubMed] [Google Scholar]
- 29.Bodor GS, Little RR, Garrett N, Brown W, Goldstein DE, Nahm MH. Standardisation of glycohaemoglobins in the clinical laboratory. 3 years of experience. Clin Chem. 1992;38:2414–8. [PubMed] [Google Scholar]
- 30.Weykamp CW, Penders TJ, Muskiet FAJ, van der Slik W. Glycohaemoglobin : Comparison of 12 analytical methods, applied to lyophilised haemolysates by 101 laboratories in an external quality control programme. Ann Clin Biochem. 1993;30:169–74. doi: 10.1177/000456329303000210. [DOI] [PubMed] [Google Scholar]
- 31.Weykamp CW, Penders TJ, Muskiet FAJ, van der Slik W. Effect of calibration on dispersion of glycohaemoglobin values determined by 111 laboratories using 21 methods. Clin Chem. 1994;40:138–44. [PubMed] [Google Scholar]
- 32.Weykamp CW, Penders TJ, Miedema K, Muskiet FAJ, van der Slik W. Standardisation of glycohaemoglobin results and reference values in whole blood studied in 103 laboratories using 20 methods. Clin Chem. 1995;41:82–6. [PubMed] [Google Scholar]
- 33.Gilbert RE, Goodall I, Young V, Jerums G. Interlaboratory variation of GHb assays in Victoria, Australia. Diabetes Care. 1996;19:730–4. doi: 10.2337/diacare.19.7.730. [DOI] [PubMed] [Google Scholar]
- 34.Calleja J, Gill J, Booth J, et al. An investigation into the standardisation of HbA1c assays using a common calibrator. AACB Website “www.aacb.asn.au” Accessed November 2004. PDF File. Resource Documents. HbA1c Standardisation.
- 35.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. N Eng J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association Clinical Practice recommendations: standards of medical care for patients with diabetes mellitus. Diabetes Care 1999;22 (Suppl 1).S32– S41. [PubMed]
- 37.Goldstein DE, Little RR, England JD, Wiedmeyer HM, McKenzie EM. Methods for quantitating glycosylated haemoglobins : high performance liquid chromatography and thiobarbituric acid colorimetry. In : Clarke WL, Larner J, Pohl SL eds Methods in diabetes research Vol 2: Clinical Methods, New York: John Wiley, 1986. pp 475–504.
- 38.NCCLS evaluation of precision performance of clinical chemistry devices. Approved Guidelines NCCLS publication EP5-A, Villanova PA, NCCLS 1999.
- 39.NCCLS method comparisons and bias estimation using patient samples. Approved Guidelines NCCLS publication EP9-T, Villanova PA, NCCLS 1993.
- 40.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 41.Little RR, Wiedmeyer H-S, England JD, Naito HK, Goldstein DE. Interlaboratory comparison of glycohaemoglobin results. College of American Pathologists Survey Data. Clin Chem. 1991;37:1725–9. [PubMed] [Google Scholar]
- 42.Little RR, Rofling CL, Wiedmeyer H-M, Myers GL, Sacks DB, Goldstein DE. The national glycohaemoglobin standardisation programme : A five year progress report. Clin Chem. 2001;47:1985–92. [PubMed] [Google Scholar]
- 43.Shima K, Endo J, Oimomi M, et al. Interlaboratory differences in HbA1c measurement in Japan, an interim report of the committee on an interlaboratory standardisation of HbA1c determination, the Japan Diabetes Society. J Jap Diab Soc. 1994;37:233–43. [Google Scholar]
- 44.Hoshino T, Nakayama T, Kuwa K, et al. Reference method for St-GHbA1c determination. Standard Operating Procedure version 1.4 – 2000 JSCC Working Group on SOP for St-GHbA1c Determination.
- 45.Shima K, Endo J, Oimomi M, et al. Interlaboratory differences in GHb measurement in Japan, the fifth report of the GHb standardisation committee, the Japan Diabetes Society. J Jap Diab Soc. 1998;41:317–23. [Google Scholar]
- 46.Mosca A, Paleari R. Standardisation schemes for haemoglobin A1c determination. In: John WG (ed). Monitoring glycaemic control in the diabetic patient, Harcourt Health Communications, London (pub) pp137–150.
- 47.Hoelzel W, Weykamp C, Jeppsson J-O, et al. IFCC Reference System for measurement of haemoglobin A1c in human blood and the National Standardisation Schemes in the United States, Japan and Sweden : A Method Comparison Study. Clin Chem. 2004;50:166–74. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 48.Jeppsson J-O, Jerntorp P, Sundkvist G, Englund, Nylind V. Measurement of haemoglobin A1c by a new liquid chromatography assay : methodology, clinical utility and relation to glucose intolerance evaluated. Clin Chem. 1986;32:1867–72. [PubMed] [Google Scholar]
- 49.Eckerbom S, Bergqvist Y, Jeppsson J-O. Improved method for analysis of glycated haemoglobin by ion exchange chromatography. Ann Clin Biochem. 1994;31:355–60. doi: 10.1177/000456329403100409. [DOI] [PubMed] [Google Scholar]
- 50.Colman P, Goodall I, Garcia-Webb P, Williams P, Dunlop M. Glycohaemoglobin – a crucial measurement in modern diabetes management. Progress towards standardisation and improved precision of measurement. Med J Aust. 1997;167:96–8. doi: 10.5694/j.1326-5377.1997.tb138790.x. [DOI] [PubMed] [Google Scholar]
- 51.Boulton AJM, Saudek CD. The need for standardisation of glycated haemoglobin measurements. Diabetologia. 2002;45:R19–21. [Google Scholar]
- 52.Hoelzel W, Miedema K. Development of a reference system for the international standardisation of HbA1c / glycohaemoglobin determinations. JIFCC. 1996;9:62–7. [PubMed] [Google Scholar]
- 53.Miedema K. Towards worldwide standardisation of HbA1c determination. Diabetologia. 2004;47:1143–8. doi: 10.1007/s00125-004-1453-0. [DOI] [PubMed] [Google Scholar]
- 54.Finke A, Kobold U, Hoelzel W, Weykamp C, Jeppsson J-O, Miedema K. Preparation of a candidate primary reference material for the international standardisation of HbA1c determinations. Clin Chem Lab Med. 1998;36:299–308. doi: 10.1515/CCLM.1998.051. [DOI] [PubMed] [Google Scholar]
- 55.Kobold U, Jeppsson J-O, Dulffer T, Hoelzel W, Miedema K. Candidate reference methods for HbA1c based on peptide methods. Clin Chem. 1997;43:1944–51. [PubMed] [Google Scholar]
- 56.Jeppsson J-O, Kobold U, Barr J, et al. Approved IFCC in reference method for the measurement of HbA1c human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 57.IFCC WG website www.ifcchba1c.org, last accessed November 2004.
- 58.EASD Website www.easd.org listed under Current News 5/2004, last accessed November 2004.
- 59.Report of the ADA / EASD / IDF Working Group of the HbA1c Assay. Diabetologia 2004;47/5:R53–54 News Section.
- 60.Hanas R. Psychological impact of changing the scale of reported HbA1c results affects metabolic control. Diabetes Care. 2002;25:2110–1. doi: 10.2337/diacare.25.11.2110. [DOI] [PubMed] [Google Scholar]


