Abstract
Further understanding of its endocrine mechanisms and increased evidence for autocrine/paracrine actions has recently enhanced our knowledge of the biological activities of the vitamin D metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D). The recognition of the contribution of vitamin D depletion to increased risk of osteoporosis, and most importantly the risk of hip fracture in the elderly, has increased the clinical significance of clinical laboratory testing for vitamin D status. Research has revealed that at least three genes contribute to vitamin D activity within tissues. These are the vitamin D receptor as well as two major vitamin D metabolising enzymes, CYP27B1, responsible for synthesis of 1,25(OH)2D and CYP24, responsible for catabolism of vitamin D metabolites. Current research focuses on the contribution of vitamin D metabolism to increasing vitamin D activity. This is of particular interest in bone forming cells where increased 1,25(OH)2D activity has been proposed to contribute to strengthening the skeleton. As well, solid tumours such as prostate, breast and colon cancers are another increasing area of vitamin D research. The major issues for the clinical laboratory in vitamin D testing include defining clinical decision limits for the interpretation of serum 25-hydroxyvitamin D (25OHD) levels and improving the precision and accuracy of this assay.
Introduction
Rickets is a bone disease in children, producing weak bones that are easily bent because of a defect in bone mineralisation. During the 19th century it was recognised that the incidence of rickets was increasing particularly with the industrialisation of cities at the higher latitudes. Already at this time sunlight and cod-liver oil were established anti-rachitic agents and were used to treat such patients if their families could afford the cost of consulting a medical practitioner or the treatment. In adults this disease is known as osteomalacia and the weak bones are due to an inability to adequately mineralise the bone matrix proteins, known as osteoid, during bone formation. The key feature for the diagnosis of rickets, or osteomalacia is the bone mineralisation defect, which is indicated by a markedly increased amount of unmineralised osteoid and an increased mineralisation lag time.1
It was not until 1919 that vitamin D was identified as the key anti-rachitic agent and became available for treating children and adults alike.2 Large doses were often used during the 1930s and 1940s, a period when nutritional deficiencies were rampant as a result of economic and political upheavals. Research on the mechanism of action of vitamin D continued and by 1969 the biologically active metabolite of vitamin D - 1,25(OH)2D - was identified. 3 1,25(OH)2D is a steroid hormone activating a nuclear transcription factor, the vitamin D receptor (VDR), which regulates the transcription of vitamin D responsive genes.4 Despite the original misnaming of vitamin D (since it is actually a prehormone) this term has continued to be used.
Vitamin D Toxicity
During the period between the 1930s and 1950s considerable experience was gained with vitamin D toxicity when overdoses of vitamin D were often provided to patients being treated for hypocalcaemic disorders such as hypoparathyroidism. Incidences of vitamin D over-dosing also occurred with the general public when batches of vitamin D-supplemented foods were poorly mixed. Clinical biochemistry studies of patients receiving large doses of vitamin D demonstrated that the first adverse side effect of vitamin D to develop was hypercalcaemia. Following the development of an assay for serum 25OHD to quantitatively assess vitamin D status, it was demonstrated that hypercalcaemia did not develop until 25OHD levels were over at least 500 nmol/L and most commonly above 750 nmol/L5 (Figure 1). These levels have been confirmed with more recent studies.6,7 This experience has left a strong clinical concern regarding vitamin D supplementation and the risk of vitamin D toxicity. Consequently the medical profession takes a very conservative approach today. However in current clinical practice it is very rare to observe levels of 25OHD approaching one fifth of the levels required for toxicity to occur.
Figure 1.
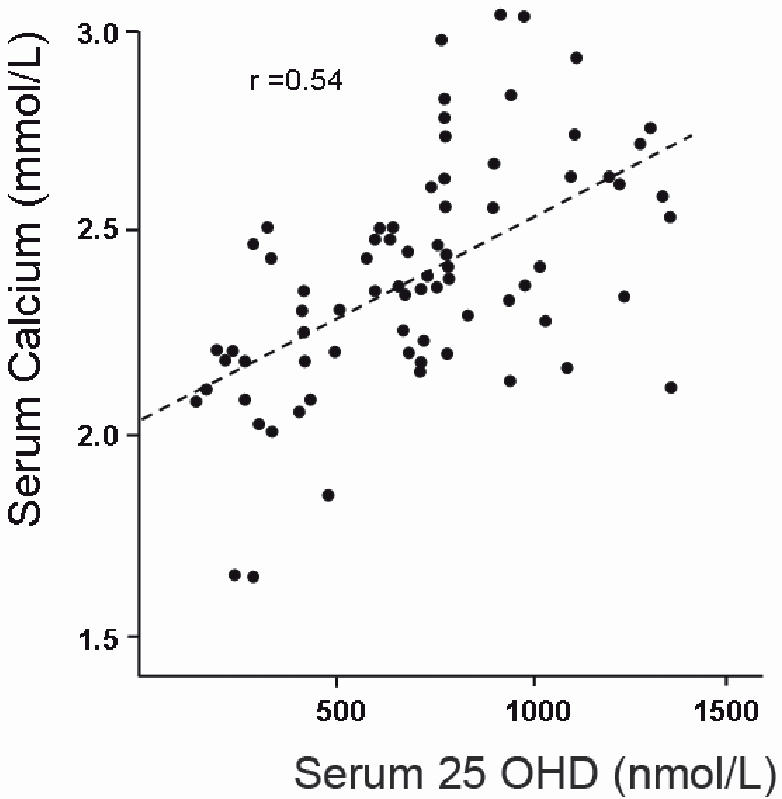
The relationship between serum 25OHD levels and serum calcium in patients receiving vitamin D supplementation. Toxic levels of vitamin D are indicated by hypercalcaemia at levels greater than 2.55 mmol/L. The data are reproduced by permission, from reference 5.
This experience provided the basis for a concept that there existed at least three levels of vitamin D status, which were found useful in clinical practice. These are listed in Table 1.
Table 1.
Concepts of Vitamin D status from the 1970s.
| Vitamin D deficiency | - Osteomalacia/Rickets/Mineralisation defect |
| Vitamin D sufficiency | - Skeletal health/Eucalcaemia |
| Vitamin D toxicity | - Hypercalcaemia |
Vitamin D Depletion and Hip Fracture
More recently considerable information about the effect of low levels of vitamin D on bone health has come from studies of the hip fracture syndrome. Hip fracture is the most devastating complication of osteoporosis. A bone histology study of hip fracture patients from Leeds, UK in the early 1970s reported a significant incidence of osteomalacia suggesting that vitamin D deficiency might be involved in the aetiology of this fracture.8 It was subsequently reported that the osteomalacia demonstrated a seasonal variation again implying vitamin D deficiency was involved.9 When serum 25OHD assays became available in the late 1970s they confirmed that hip fracture patients often have a low vitamin D status.10 However reports of histological evidence for osteomalacia in hip fracture patients has remained controversial. For example in Adelaide, South Australia no osteomalacia was observed in hip fracture patients, although significant osteoporosis was detected.11 Most interestingly the low vitamin D status in hip fracture patients in Adelaide was reported shortly thereafter.12 The mean 25OHD level in hip fracture patients was 39 nmol/ L compared with 72 nmol/L in age and sex-matched control subjects.
Since that time low vitamin D status has been confirmed throughout the world to be associated with an increased risk of hip fracture in the elderly, irrespective of latitude and economic status.13 However this observation was simply an association until the double-blind, placebo-controlled clinical trial of vitamin D and calcium supplementation in elderly women in France.14,15 The authors showed that when their data were analysed after three years on an intention to treat basis, 800 IU vitamin D and 1.2 g calcium daily reduced the incidence of hip fracture by some 30% (Table 2). This study clearly demonstrated that a low, but not deficient vitamin D status, significantly increased the risk of hip fracture.
Table 2.
Effect of Daily Calcium and Vitamin D Supplements for 3 years on Hip Fracture.
| 800 IU Vit D + 1.2 g Ca | Placebo | P | |
|---|---|---|---|
| Total number of women | 1176 | 1127 | |
| Number with hip fracture | 137 | 178 | <0.02 |
| Number with peripheral fracture | 255 | 308 | <0.02 |
Data derived from reference 15
However there is still the outstanding question as to the mechanism by which a low vitamin D status adversely affects the status of the skeleton. A number of studies had been unable to detect any evidence of osteomalacia in bone biopsies from hip fracture patients. Recently histological studies conducted in a laboratory rat model of vitamin D insufficiency (serum 25OHD levels at 13 nmol/L) demonstrated evidence of osteomalacia when these animals were fed a low calcium diet (0.1%). Vitamin D-depleted animals fed a high calcium diet (1%) showed evidence of osteoporosis (Iida S, Anderson PH, Morris HA, unpublished data). In summary this animal model study provides evidence that vitamin D depletion on a low dietary calcium intake produces a mineralisation defect, that is osteomalacia, whereas vitamin D depletion on a high dietary calcium intake produces osteoporosis. The low vitamin D status however compromises bone architecture and is likely to increase the risk of fracture.
Thus the identification of a causal role for a low vitamin D status in the hip fracture syndrome suggests that our views on the levels of vitamin D should be modified to include a contribution of a low vitamin D status to osteoporosis (Table 3). This view has been presented previously.1
Table 3.
Current concepts of Vitamin D status.
| Vitamin D deficiency | - Osteomalacia |
| Vitamin D depletion | - Osteoporosis/Secondary Hyperparathyroidism/Calcium malabsorption |
| Vitamin D sufficiency | - Skeletal health/ Eucalcaemia |
| Vitamin D toxicity | - Hypercalcaemia |
Bio-activation and Metabolism of Vitamin D, the Endocrine System
Vitamin D is obtained from sunlight exposure of the skin where the UVA and UVB light converts 7-dehydrocholesterol to vitamin D, which is then subject to sequential hydroxylation reactions for bio-activation16 (Figure 2). The first hydroxylation is at the carbon 25 position to produce 25OHD. Circulating 25OHD levels are the best indicator of vitamin D status because of the increased solubility of this metabolite in blood compared with vitamin D. Circulating 25OHD levels are considered to arise from the liver activity of the cytochrome P450 enzyme CYP27. However, at least for humans, this enzyme has not been unequivocally identified and until that is accomplished, it is not possible to identify all tissues, which may convert vitamin D to 25OHD. The major source of the circulating levels of the biologically active metabolite of vitamin D, 1,25(OH)2D arises from hydroxylation at the carbon 1 of 25OHD in the kidney.16 This step is catalysed by the P450 enzyme CYP27B1. A third hydroxylation of the vitamin D metabolites, which has a major effect on vitamin D activity, is the hydroxylation at carbon 24. It is catalysed by the P450 enzyme CYP24. While this reaction is responsible for measurable levels of the 24,25-dihydroxyvitamin D and 1,24,25-trihydroxyvitamin D metabolites in blood, it is the first step in the inactivation of vitamin D. This is a very important step in vitamin D metabolism, protecting organisms from vitamin D toxicity as demonstrated by generation of a mouse line with the CYP24 gene ablated.17 All cells that express the vitamin D receptor and therefore are biologically responsive to 1,25(OH)2D are thought to express the CYP24 gene. Serum 1,25(OH)2D acts at the small intestine to control active intestinal calcium absorption and serum 1,25(OH)2D levels correlate with active intestinal absorption of calcium in humans and rats.18,19
Figure 2.
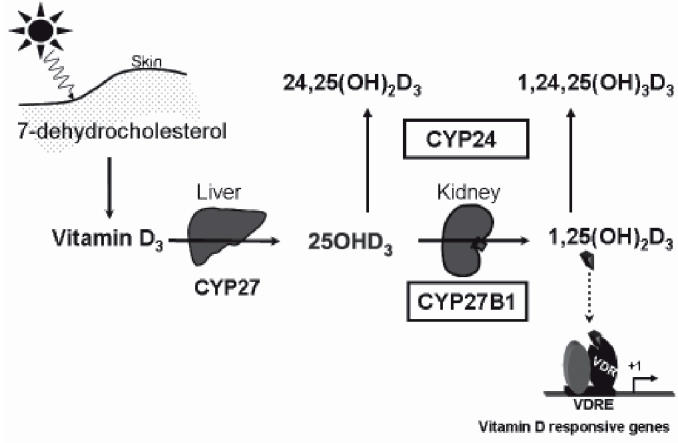
The synthesis and metabolism of vitamin D. Abbreviations: CYP27, vitamin D-25-hydroxylase; CYP27B1,25-hydroxyvitamin D-1alpha-hydroxylase; CYP24, 25-hydroxyvitamin D-24-hydroxylase; VDR, vitamin D receptor; VDRE, vitamin D responsive element.
Serum levels of 1,25(OH)2D are highest in the newborn and decrease exponentially throughout life. The implications of the fall in serum 1,25(OH)2D and decreased intestinal calcium absorption with increased incidence of osteoporosis are currently unclear although there is evidence that such a relationship is an important risk factor for fracture in postmenopausal women.20 Recent evidence has demonstrated that in adult rats, the expression of both the CYP27B1 and CYP24 genes in the kidney contribute to the serum level of 1,25(OH)2D. 21,22 Data suggest that during growth and early adulthood, the synthesis of 1,25(OH)2D is a major determinant of serum levels. However in the mature and ageing animals the increased expression of kidney CYP24 is the major cause of the decreased serum 1,25(OH)2D levels with ageing.
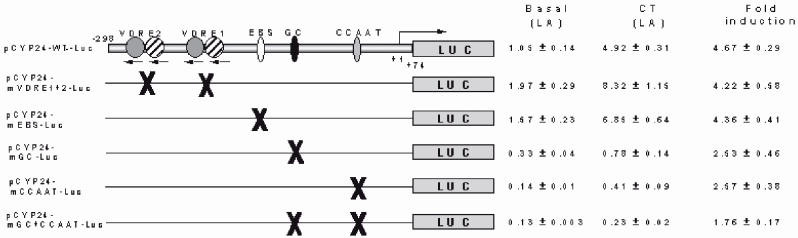
Factors controlling the expression of CYP24 gene in the kidney are of potential clinical interest because up-regulation of kidney CYP24 may be responsible for decreased levels of serum 1,25(OH)2D particularly in the elderly. 21,22 One potential factor is the calciotropic hormone calcitonin. Calcitonin is secreted by the follicular cells of the thyroid gland in response to hypercalcaemia. This hormone can lower blood calcium levels by reducing both osteoclastic bone resorption and renal tubular reabsorption of calcium.23 Recent data obtained from rodent studies have demonstrated a positive correlation between serum calcitonin levels and the renal expression of CYP24.21,22 These data confirm in vitro molecular studies of the 5’-proximal promoter region of the CYP24 gene that have identified specific transcription factors activated by calcitonin to up-regulate CYP24 gene expression24 (Figure 3).
Figure 3.
Transient transfection of wild type and mutated CYP24 promoter linked to the firefly luciferase gene in human kidney cells (HEK-293). Calcitonin (CT) stimulates the CYP24 promoter activity some 4-fold in all constructs except those in which the GC and CCAAT sites have been mutated indicating that calcitonin acts on this gene through transcription factors that bind to these sites. Data are reproduced with permission, from reference 24. (Gao XH, Dwivedi PP, Omdahl JL, Morris HA, May BK. 2004 J Molec Endocrinol 32:87-98)
This newly identified biological activity of calcitonin is consistent with the calcium-lowering activity of calcitonin. Future studies are required to unequivocally demonstrate that calcitonin is responsible for up-regulation of kidney CYP24 levels with ageing in humans.
What are the Biological Activities of Vitamin D?
The most powerful evidence for the physiological role of vitamin D arises from inactivation of genes necessary for biological activity of vitamin D. There are two genes that may be inactivated, the CYP27B1 and VDR. These inactivated genes occur in humans and mouse models have been generated in which these genes have been knocked out. Two human diseases occur, vitamin D-dependent rickets type I (VDDR type I) when the CYP27B1 gene is inactivated, and vitamin D-dependent rickets type II (VDDR type II) when the VDR is inactivated. Both these conditions demonstrate a phenotype including hypocalcaemia, hypophosphataemia, secondary hyperparathyroidism and the bone-mineralising defect of rickets in the young.25–27 Both humans and mice also show effects on the skin with areas of marked hair loss. They are also infertile and the immune system is adversely affected.
The phenotype appears to be rescued by a high calcium and high phosphate diet, which normalises blood calcium and phosphate levels. This treatment has a marked effect on stimulating bone mineralisation, restores fertility and apparently corrects the dysfunction of the immune system.27,28 The effects on skin and alopecia are not corrected by normalisation of blood calcium levels. These data indicate that 1,25(OH)2D is essential for intestinal absorption of dietary calcium and phosphate from normal diets. Normalisation of blood calcium and phosphate levels apparently corrects a number of systems that are disrupted by inactivation of vitamin D although it appears that 1,25(OH)2D and VDR are absolute requirements in keratinocytes for normal cellular development.
The VDR is expressed widely throughout the body and this distribution suggests that vitamin D biological activity extends further than maintaining normal calcium and phosphate homeostasis. However to date it has been difficult to obtain data that unequivocally demonstrate any such other activities in vivo. This is a major current area of research. Recently a requirement for active 1,25(OH)2D for the full development of the skeleton has been demonstrated in adult, combined VDR and CYP27B1 gene knockout mice, in whom blood calcium and phosphate levels have been normalised.29 It is interesting to note that a requirement for the expression of CYP27B1 was identified in these mice for normal maturation of the growth plate. Thus current data suggest that with continuing research it is likely that further novel activities of 1,25(OH)2D will be identified.
Vitamin D and Bone Cell Activity
One major focus of activity in this field is to identify the activities of 1,25(OH)2D in skeletal tissue. Approximately 60 genes are known to respond to vitamin D.30 Many of these vitamin D-responsive genes are expressed by the bone forming cells (osteoblasts) including type I collagen, alkaline phosphatase, osteocalcin and the tumour necrosis factor ligand member (RANKL), which is central to osteoclastogenesis. The bone resorbing cells (osteoclasts) also express vitamin D-responsive genes. It is evident that 1,25(OH)2D plays a major role in controlling osteoclastogenesis and bone resorption through its modulation of the RANKL gene in osteoblasts.31
Evidence has now been reported that increasing vitamin D activity solely within the mature, mineralising osteoblasts increases the amount of mineralised bone laid down by these cells. Gardiner et al increased the level of the vitamin D receptor solely in mature osteoblasts by generating a transgenic mouse line containing the complementary DNA for the human vitamin D receptor under the control of the osteocalcin promoter.32 The human vitamin D receptor gene was expressed only in mineralising tissues in a manner identical to osteocalcin expression, thus increasing the level of vitamin D receptor protein in these tissues. These transgenic mice have increased trabecular bone in their vertebrae as a result of reduced resorption. Interestingly the cortical bone in these vertebrae is increased as a result of increased bone formation.
Increased activity of the 1,25(OH)2D synthetic enzyme CYP27B1 would also increase vitamin D activity inside cells. It has been well known for over 20 years that osteoblasts express this enzyme and can synthesise 1,25(OH)2D. However the physiological significance of this activity remains to be elucidated. Recent studies on bone tissue levels of CYP27B1 mRNA have found a positive relationship in mature rats between bone CYP27B1 and trabecular thickness.22 Furthermore similar studies in laboratory rats, which have a low vitamin D status, have demonstrated a positive relationship between bone CYP27B1 mRNA levels and trabecular bone volume (Iida S, Anderson PH, Morris HA, unpublished data). These observations suggest that under certain circumstances, the 1,25(OH)2D activity in bone cells can stimulate formation of bone or preserve bone mineral. These types of results are increasing interest in the view that the modulation of vitamin D activity within bone cells, either through the vitamin D receptor or metabolism of vitamin D, has effects on the levels of bone mineral. It is through such a mechanism that higher levels of vitamin D in the elderly may provide protection against hip fractures.
The synthesis of 1,25(OH)2D occurs in tissues other than kidney and bone. Recent studies using another transgenic mouse model have clearly demonstrated the brain and testis are major areas of 1,25(OH)2D synthesis as well as many other tissues.33 In this mouse model the DNA for the promoter region of the human CYP27B1 gene was linked to the complementary DNA for the non-mammalian luciferase reporter gene and was inserted into a mouse line. These mice demonstrated that the CYP27B1 promoter activity is strongest in the kidney, brain, testes, skin, bone and bone marrow and spleen with lower levels of expression in muscle, lung and liver (Figure 4). In the kidney the expression of the reporter gene occurs in the same cells as the endogenous CYP27B1 protein and expression is regulated in a similar manner to the endogenous gene by dietary calcium and vitamin D status.34 Importantly these data demonstrate that the major transcriptional regulatory elements for the CYP27B1 gene are located within the −1497 to +44 bp of this gene. This is a relatively small region and provides strong evidence for the physiological significance of studies on the molecular mechanism of transcriptional control of the CYP27B1 gene expression conducted in this region of DNA.35,36
Figure 4.
Relative luciferase activity in tissue extracts from a transgenic mouse line in which the gene for firefly luciferase had been inserted under the control of the human CYP27B1 promoter. It is evident that the CYP27B1 activity is expressed in a range of tissues. Notably the small intestine, a major target organ for 1,25(OH)2D, does not demonstrate CYP27B1 promoter activity. The data are reproduced with permission, from reference 34. (Hendrix I, Anderson PH, Omdahl JL, May BK, Morris HA. 2005 J Molec Endocrinol 34: 237-245). pr small intestine: pr= proximal, di small intestine: di= distal
The local tissue-specific synthesis of 1,25(OH)2D throughout many tissues suggests that the endocrine paradigm for vitamin D biological activity is insufficient to explain all biological activity of vitamin D. It is probable that vitamin D exerts paracrine or autocrine activities, that is the 1,25(OH)2D synthesised by cells only acts on either the synthesising cells or cells in close proximity. Most, if not all, of the circulating 1,25(OH)2D arises from the kidney metabolism of vitamin D metabolites.21,22 However the physiology of such local synthesis is poorly understood at this time. This local activity of vitamin D poses further challenges for determining the vitamin D requirement for optimal health. Initially the physiological role of these activities will need to be identified before such basic information can be derived.
Vitamin D and Cancer
An area of particular interest for novel vitamin D activities is the regulation of cell growth and differentiation. It has been recognised for over 20 years that the addition of 1,25(OH)2D to culture media for cancer cell lines produced a strong inhibition of growth. Initially studies included breast cancer and other solid tumour cells lines.37 Particular progress has been made with the study of human prostate cancer cell lines as well as normal prostate epithelial tissue and primary prostate cancer cell cultures. The prostate functions as a vitamin D-target organ in that normal epithelial cells express the VDR and display regulation of numerous genes by 1,25(OH)2D. A recent complementary DNA microarray analysis of primary human prostatic epithelial cells revealed that 1,25(OH)2D up-regulated at least 38 genes and 9 were significantly down-regulated.38 The highest induction of expression was the gene for the vitamin D catabolic enzyme CYP24. The expression of similar but not identical genes was observed in primary prostate cancer cultures. Some of these genes modulate the mitogen-activated kinase (MAPK) pathways associated with growth factor signally while others induce apoptosis or reduce cell cycling activity necessary for cell division and replication.
A study of the effect of 1,25(OH)2D on growth of a number of human prostate cancer cell lines indicated varied responses to 1,25(OH)2D with the LNCaP line being most sensitive while the DU145 cell line was unresponsive39 (Figure 5). Further studies on the expression of the genes that determine vitamin D activity in these cell lines as well as normal prostate epithelial cells and benign prostate hyperplastic cells indicate a gradation of decreasing CYP27B1 activity as prostate epithelial cells move from normal epithelium with the highest activity through benign prostate hyperplastic epithelium with moderate activity to cancer cells with markedly repressed activity (Table 4). Neither the expression of VDR or CYP24 demonstrates such a relationship with the development of cancer. It is interesting that when the DU145 cancer cell, which is unresponsive to 1,25(OH)2D was treated with an inhibitor of CYP24 activity, the growth inhibition by 1,25(OH)2D was demonstrated.43 A recent immunohistochemical study of a human prostate cancer series indicated that the CYP27B1 protein was present in a significant number of these specimens. Their data suggest that the increased expression of CYP24 or some inactivation of the CYP27B1 enzyme may be important mechanisms for reducing 1,25(OH)2D activity in many clinical prostate cancers.44
Figure 5.
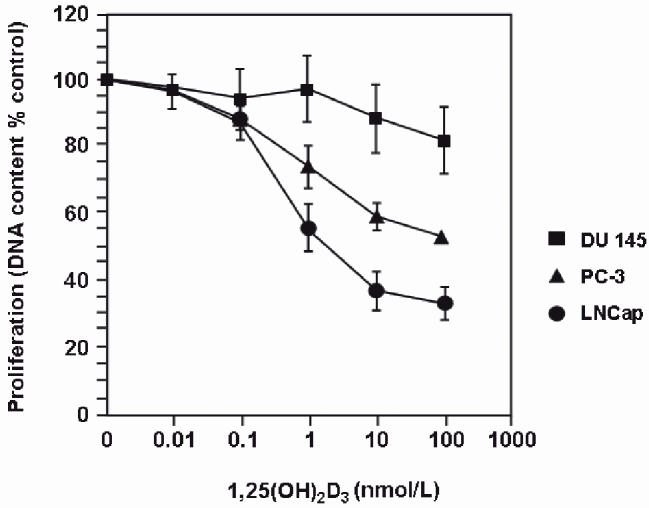
The differential effects of increasing concentrations of 1,25(OH)2D on the growth of three human prostate cell lines in culture. The data are reproduced with permission from reference 39.
Table 4.
VDR Expression, CYP27B1 and CYP24 enzyme activities, and inhibition of growth by 1,25(OH)2D in Human Prostate Epithelial Cells.
These findings all suggest that modulation of vitamin D activity through disruption of vitamin D metabolism within prostate cells may play a permissive role in the development of prostate cancer. There is considerable epidemiological evidence that either decreased sunlight exposure or decreased vitamin D status is associated with increased risk of many cancers including prostate. In the USA rates of cancer mortality vary inversely with exposure to sunlight (reviewed45). A study in Finland demonstrated that men with an initial low vitamin D status were at greater risk for earlier onset prostate cancer and tumours were generally more aggressive suggesting vitamin D status may be critical during the earlier stages of prostate cancer development. These observations have been confirmed in the United Kingdom. Thus if a low vitamin D status is confirmed to increase the risk of prostate or any cancers, the maintenance of an adequate vitamin D status and assessment of vitamin D levels are very simple procedures that could be adopted at the population level. Thus clinical laboratory vitamin D testing would further markedly increase. Such a public health policy will require the identification of the level of vitamin D required to reduce the risk of cancer.
Determinants of Serum 25OHD levels
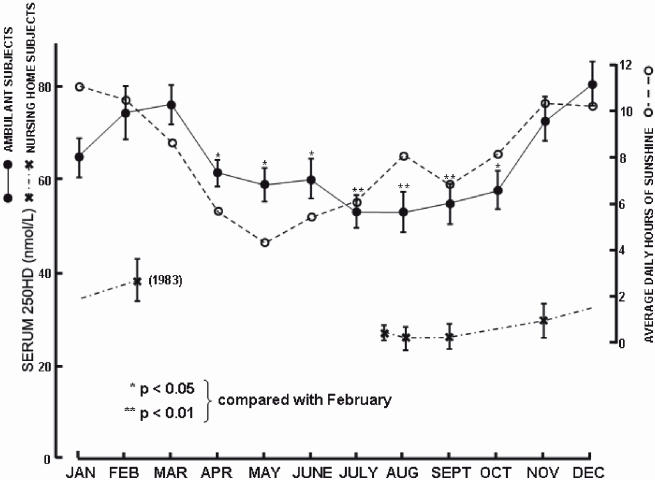
Sunlight exposure is clearly the major determinant of the vitamin D status of the individual and for populations. When vitamin D levels are measured across a 12 month period serum 25OHD levels lag about 1 to 2 months behind the average daily hours of sunlight12 (Figure 6). There appears to be little effect of vitamin D-supplemented food on vitamin D status possibly because such supplements are at a low level.46 Skin thickness contributes positively to vitamin D status.47 This is a clinically important observation since treatments such as the use of glucocorticoid steroids markedly reduce skin thickness and it is highly likely that such patients are at increased risk of low vitamin D status. Thinning of the skin occurs with ageing but there is also an independent effect of age to decrease vitamin D levels.47 Apparently the efficiency of skin to synthesise vitamin D decreases with age.45 However ageing is not always associated with a fall in vitamin D levels48 and if elderly people have an outdoor lifestyle they can maintain similar vitamin D levels as younger people. Increased body mass index reduces vitamin D status,47 presumably due to sequestration of vitamin D, a fat-soluble prohormone, into fat stores. These data provide a plausible explanation for the anecdotal evidence that the vitamin D status of Australians is decreasing. One would expect serum 25OHD levels to decrease in a population in which obesity and a sedentary lifestyle are increasing.
Figure 6.
The relationship between mean serum 25OHD levels in ambulant subjects and average daily hours of sunlight in Adelaide, Australia (latitude 35 degrees south) in 1982. Note that 25OHD levels obtained from nursing home subjects did not demonstrate the seasonal variation indicating their housebound and low vitamin D status. The data are reproduced with permission from reference 12.
Some interesting observations on the effects of the variation of vitamin D metabolism through ageing have come from animal studies. Kidney CYP27B1 activity is highest in the newborn and declines exponentially throughout the remainder of life both in rodents and humans. In rats followed from 3 weeks of age until 2 years during which they were fed a constant level of vitamin D, the mRNA level of kidney CYP27B1 was negatively related to their serum 25OHD level in the early weeks of life between 3 and 15 weeks. In contrast, serum 25OHD levels were negatively related to kidney CYP24 mRNA levels in adulthood and old age between 15 and 104 weeks of age.22 These data suggest that renal synthesis of 1,25(OH)2D is a significant consumer of vitamin D stores during the earlier stages of life and that during the later stages when renal CYP24 catabolism of vitamin D metabolites is increased, catabolism plays a significant role in reducing vitamin D stores. If these data are reproduced in humans then factors that up-regulate the expression of kidney CYP24 expression may be important in reducing vitamin D status in the elderly.
How much 25OHD do we need?
As with most clinical laboratory testing, when plasma 25OHD tests were introduced into routine clinical laboratories in the late 1970s and early 1980s reference intervals were obtained from testing levels in a reference population. In Adelaide, the population lives at latitude 35 degrees south and the reference interval was 40 to 160 nmol/L. However in other cities at different latitudes the reference interval was often different. For instance in Hobart at latitude 42 degrees south the reference interval was 25 to 100 nmol/L. Around the world the reference interval, particularly the lower limit, was inversely related to the latitude of the city in which the reference population was living.49 However there is no evidence that the minimal requirement for vitamin D is dependent on latitude. Therefore testing of vitamin D status in a reference population is inappropriate for identifying the minimum or toxic levels of plasma 25OHD levels.
It is necessary to identify objective measures from which we can derive clinical decision limits for vitamin D requirement. Currently the only known in vivo requirement for vitamin D metabolites is to maintain calcium homeostasis. Thus the first task in this field is to define the vitamin D requirement, in terms of plasma levels of 25OHD, necessary to maintain calcium homeostasis and optimal skeletal health. A number of studies have been published reporting plasma 25OHD levels to reduce plasma parathyroid hormone (PTH) levels and biochemical bone turnover markers.50 It is clear from clinical data that an inverse relationship exists between plasma 25OHD levels and plasma PTH and urine bone resorption markers indicating that as vitamin D status declines PTH secretion and bone resorption are increased. Jesudason et al reported that plasma 25OHD levels of 60 nmol/L or greater were required to minimise plasma PTH and urine deoxypyridinoline levels in postmenopausal women.50
Others have reported minimal requirements for plasma 25OHD levels ranging from 30 to 122 nmol/L. However a number of the most recent publications approach a minimum level of 50 or 60 nmol/L. Currently only surrogate markers of calcium homeostasis and bone metabolism have been used and ultimately clinical decision limits for skeletal health will require definition based on bone strength or fracture data. Of course if vitamin D status is confirmed to affect human health through other systems such as cell growth and proliferation, and therefore impact on cancer risk for example, then other assessments of clinical decision limits for vitamin D status will have to be defined. Provisional critical levels for 25OHD for the determination of vitamin D status are presented in Table 5.
Table 5.
Current suggested decision limits for Vitamin D status.
| Vitamin D deficiency | - < 20 nmol/L |
| Vitamin D depletion | - 20 to 59 nmol/L |
| Vitamin D sufficiency | - 60 to 200 nmol/L |
| Vitamin D toxicity | - > 500 nmol/L |
How good are we at measuring 25OHD?
Clinical requirements for measuring plasma 25OHD levels include assessing vitamin D status and monitoring vitamin D supplementation. A number of radioimmunoassays are used in routine laboratories as well as an automated system that utilises competitive-protein binding technology.51 The data from a recent major external quality assurance program indicate that 50% of enrolled laboratories that include most routine clinical laboratories in Australia achieve a coefficient of variation of 13%.52 This value rises to 22.6% for 90% of laboratories. Such data indicate that for a plasma specimen with a 25OHD level of 50 nmol/L, 50% of laboratories would report values between 37 and 63 nmol/L with 90% of laboratories reporting values between 27 and 73 nmol/L. Although the range achieved by 50% of laboratories may be clinically acceptable, clearly the larger range of values is clinically unacceptable. Thus clinical laboratories and assay kit manufacturers must work together to improve the precision of this assay.
Furthermore these data indicate a significant bias between the various methods being used in clinical laboratories, a bias that is more important at lower 25OHD levels.52 Such variation has been described by a number of studies comparing the performance of these assay methods.51 As discussed previously, optimising the clinical utility for 25OHD assays requires defining clinical decision limits that are transferable across all clinical assays. Currently assay performance does not achieve such a criterion. An international 25OHD assay standardisation will be necessary before clinical laboratories can provide such a service.
Monitoring vitamin D supplementation provides further difficulties for the clinical laboratory. In many countries including Australia the major supplement available is ergocalciferol or vitamin D2 which is derived from plants. Mammals produce cholecalciferol or vitamin D3. While it is currently considered that there are no known differences with regard to biological activity between the two forms of vitamin D, it is clear that our routine assays do not measure them identically. All current assays are standardised to measure 25OHD3 and the cross-reactivity between 25OHD2 and 25OHD3 for the various assays is controversial.51 It is generally agreed that there is not 100% cross-reactivity. Thus an important development in 25OHD assay technology is the availability of assays that can measure 25OHD2 and 25OHD3 equally.
As discussed above the medical profession currently takes a very conservative approach to prescribing dosages of vitamin D. This is partly a result of the adverse experiences with vitamin D toxicity in earlier times and partly because of the paucity of quantitative data regarding the plasma 25OHD levels achieved with various doses in humans. Recent studies on healthy men in Omaha, USA (latitude 41 degrees north) indicate 12.5 μg (500 IU) per day of vitamin D3 is required to prevent the seasonal fall in plasma 25OHD levels. 25 μg (1000 IU) of vitamin D3 per day is required to raise plasma 25OHD levels by 12.5 nmol/L in healthy young men.53 The authors calculated that the steady state input of vitamin D3 required to maintain plasma 25OHD levels is 0.7 nmol/L/μg vitamin D3 per day. This is in close agreement with another value published.7
Conclusions
Knowledge of the physiology and pathology of vitamin D is currently increasing at a rapid rate. The realisation that vitamin D can act in a paracrine and autocrine manner in addition to its well-described endocrine action opens up considerable opportunities for the development of new understanding of the requirement for an adequate vitamin D status for optimal health. It is encouraging that the relatively simple and cheap practice of maintaining an adequate vitamin D status has the potential to provide health benefits in a number of areas, which afflict an increasing proportion of the population, as well as consume an increasing proportion of the healthcare budget to provide treatment. The reduction of the risk of hip fracture in the elderly is one such area. A high priority of research must be to identify the critical 25OHD values required to maintain a healthy skeleton in the elderly. As well research must determine whether a low vitamin D status influences the development of cancer, whether it increases the absolute risk of cancer or whether it modulates the growth or invasiveness of cancers. Clinical laboratory professionals have a responsibility to improve the precision and accuracy of current 25OHD assays in clinical use. This work will require the collaboration between the profession and instrument and reagent manufacturers. The International Federation of Clinical Chemistry and Laboratory Medicine is in an optimal position to coordinate such a project.
The contents of articles or advertisements in The Clinical Biochemist – Reviews are not to be construed as official statements, evaluations or endorsements by the AACB, its official bodies or its agents. Statements of opinion in AACB publications are those of the contributors. Print Post Approved - PP255003/01665.
No literary matter in The Clinical Biochemist – Reviews is to be reproduced, stored in a retrieval system or transmitted in any form by electronic or mechanical means, photocopying or recording, without permission. Requests to do so should be addressed to the Editor. ISSN 0159 – 8090.
References
- 1.Parfitt AM. Osteomalacia and related disorders. In: Alvioli LV, Krane SM, (eds) Metabolic Bone Disease and Clinical Related Disorders, 2nd edition. WB Saunders, Philadelphia. 1990.pp 329–96.
- 2.Mellanby E. An experimental investigation of rickets. Lancet. 1919;1:407–12. doi: 10.1111/j.1753-4887.1976.tb05815.x. [DOI] [PubMed] [Google Scholar]
- 3.Lawson DE, Fraser DR, Kodicek E, Morris HR, William DH. Identification of 1,25-dihydroxychole-calciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971;230:228–30. doi: 10.1038/230228a0. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 5.Mason RS, Posen S. The relevance of 25-hydroxycalciferol measurements in the treatment of hypoparathyroidism. Clin Endocrinol. 1979;10:265–9. doi: 10.1111/j.1365-2265.1979.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 6.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 7.Vieth R, Chan P-CR, MacFarlane G. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–94. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 8.Aaron J, Gallagher JC, Nordin BEC. Osteomalacia and femoral fractures. Lancet. 1974;1:572. doi: 10.1016/s0140-6736(74)92767-6. [DOI] [PubMed] [Google Scholar]
- 9.Aaron JE, Gallagher JC, Nordin BEC. Seasonal variation of histological osteomalacia in femoral-neck fractures. Lancet. 1974;2:84–5. doi: 10.1016/s0140-6736(74)91640-7. [DOI] [PubMed] [Google Scholar]
- 10.Baker MR, McDonnell H, Peacock M, Nordin BEC. Plasma 25-hydroxyvitamin D concentrations in patients with fractures of the femoral neck. Br Med J. 1979;1:589. doi: 10.1136/bmj.1.6163.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wicks M, Garrett R, Vernon-Roberts B, Fazzalari N. Absence of metabolic bone disease in the proximal femur in patients with fracture of the femoral neck. J Bone Joint Surg Br. 1982;64:319–22. doi: 10.1302/0301-620X.64B3.7096397. [DOI] [PubMed] [Google Scholar]
- 12.Morris HA, Morrison GW, Burr M, Thomas DW, Nordin BEC. Vitamin D and femoral neck fractures in elderly South Australian women. Med J Aust. 1984;141:144–5. doi: 10.5694/j.1326-5377.1984.tb108222.x. [DOI] [PubMed] [Google Scholar]
- 13.Feskanich D, Willett WC, Colditz GA. Calcium, vitamin D, milk consumption, and hip fracture: a prospective study among postmenopausal women. Am J Clin Nutr. 2003;77:504–11. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 14.Chapuy MC, Arlot ME, Duboef F, et al. Vitamin D3 and calcium prevent hip fractures in elderly women. N Eng J Med. 1992;327:1637–42. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 15.Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. Br Med J. 1994;308:1081–2. doi: 10.1136/bmj.308.6936.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Ann Rev Nutr. 2002;22:139–66. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 17.St Arnaud R, Arabian A, Travers R, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24, 25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–66. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 18.Morris HA, Need AG, Horowitz M, O’Loughlin PD, Nordin BE. Calcium absorption in normal and osteoporotic postmenopausal women. Calcif Tissue Int. 1991;49:240–3. doi: 10.1007/BF02556211. [DOI] [PubMed] [Google Scholar]
- 19.Moore AJ, Morris HA, Larik R, O’Loughlin PD. Effects of dietary calcium restriction on radiocalcium absorption and trabecular bone structure in the rat. In: Norman AW, Bouillon R, Thomasset M (eds) Vitamin D Endocrine System, Structural, Biological, Genetic, and Clinical Aspects. University of California, Riverside Cal. 2000.pp 653–6.
- 20.Nordin BE, O’Loughlin PD, Need AG, Horowitz M, Morris HA. Radiocalcium absorption is reduced in postmenopausal women with vertebral and most types of peripheral fractures. Osteoporos Int. 2004;15:27–31. doi: 10.1007/s00198-003-1493-1. [DOI] [PubMed] [Google Scholar]
- 21.Anderson PH, O’Loughlin PD, May BK, Morris HA. Determinants of circulating 1,25-dihydroxyvitamin D3 levels: the role of renal synthesis and catabolism of vitamin D. J Steroid Biochem Mol Biol 2004;89–90:111–3. [DOI] [PubMed]
- 22.Anderson PH, O’Loughlin PD, May BK, Morris HA. Modulation of CYP27B1 and CYP24 mRNA expression in bone is independent of circulating 1,25(OH)2D3 levels. Bone (in press). [DOI] [PubMed]
- 23.Deftos LJ. Calcitonin. In: Favus MJ (ed) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism 5th edition. American Society for Bone and Mineral Research, Washington DC 2003. pp 137–41.
- 24.Gao XH, Dwivedi PP, Omdahl JL, Morris HA, May BK. Calcitonin stimulates expression of the rat 25-hydroxyvitamin D3-24-hydroxylase (CYP24) promoter in HEK-293 cells expressing calcitonin receptor: identification of signalling pathways. J Molec Endocrinol. 2004;32:87–98. doi: 10.1677/jme.0.0320087. [DOI] [PubMed] [Google Scholar]
- 25.Liberman UA, Marx SJ. Vitamin D-dependent rickets. In: Favus MJ (ed) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism 5th edition. American Society for Bone and Mineral Research, Washington DC 2003. pp 407–13.
- 26.Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–41. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 27.Li YC, Amling M, Pirro AR, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–6. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 28.Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1) Bone. 2003;32:332–40. doi: 10.1016/s8756-3282(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 29.Panda DK, Miao D, Boliver I, et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–66. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 30.Atkins GJ, Findlay DM, Anderson PH, Morris HA. Target genes: bone proteins. In: Feldman D, Pike JW, Glorieux FH. Vitamin D, 2nd edition. Academic Press, San Diego, 2005 (in press).
- 31.Atkins GJ, Kostakis P, Pan B, et al. RANKL expression is related to the differentiation state of osteoblasts. J Bone Miner Res. 2003;18:1088–98. doi: 10.1359/jbmr.2003.18.6.1088. [DOI] [PubMed] [Google Scholar]
- 32.Gardiner EM, Baldock PA, Thomas GP, et al. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000;14:1908–16. doi: 10.1096/fj.99-1075com. [DOI] [PubMed] [Google Scholar]
- 33.Hendrix I, Anderson P, May B, Morris H. Regulation of gene expression by the CYP27B1 promoter – study of a transgenic mouse model. J Steroid Biochem Mol Biol 2004;89–90:139–42. [DOI] [PubMed]
- 34.Hendrix I, Anderson PH, Omdahl JL, May BK, Morris HA. Response of the 5’-flanking region of the human 25-hydroxyvitamin D 1α-hydroxylase gene to physiological stimuli using a transgenic mouse model. J Molec Endocrinol 2005 (in press).
- 35.Gao XH, Dwivedi PP, Choe SS, et al. Basal and parathyroid hormone induced expression of the human 25-hydroxyvitamin D 1alpha-hydroxylase gene promoter in kidney AOK-B50 cells: role of Sp1, Ets and CCAAT box protein binding sites. Int J Biochem Cell Biol. 2002;34:921–30. doi: 10.1016/s1357-2725(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 36.Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab. 2003;14:423–30. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Frampton RJ, Suva LJ, Eisman JA, et al. Presence of 1,25-dihydroxyvitamin D3 receptors in established human cancer cell lines in culture. Cancer Res. 1982;42:1116–9. [PubMed] [Google Scholar]
- 38.Peehl DM, Shingal R, Nonn L, et al. Molecular activity of 1,25-dihydroxyvitamin D(3) in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol. 2004;92:131–41. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132:1952–60. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 40.Peehl DM, Skowronski RJ, Leung GK, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805–10. [PubMed] [Google Scholar]
- 41.Miller GJ, Stapleton GE, Hedlund TE, Moffatt KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1 alpha, 25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1:997–1003. [PubMed] [Google Scholar]
- 42.Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP. 1 alpha, 25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 1997;6:727–32. [PubMed] [Google Scholar]
- 43.Ly LH, Zhao X-Y, Holloway L, Feldman D. Liarozole acts synergistically with 1alpha, 25-dihydroxyvitamin D3 to inhibit growth of DU 145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology. 1999;140:2071–6. doi: 10.1210/endo.140.5.6698. [DOI] [PubMed] [Google Scholar]
- 44.Ma JF, Nonn L, Campbell MJ, Hewison M, Feldman D, Peehl DM. Mechanisms of decreased vitamin D 1alpha-hydroxylase activity in prostate cancer cells. Mol Cell Endocrinol. 2004;221:67–74. doi: 10.1016/j.mce.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 46.Omdahl JL, Garry PJ, Hunsaker LA, Hunt WC, Goodwin JS. Nutritional status in a healthy elderly population: vitamin D. Am J Clin Nutr. 1982;36:1225–33. doi: 10.1093/ajcn/36.6.1225. [DOI] [PubMed] [Google Scholar]
- 47.Need AG, Morris HA, Horowitz M, Nordin BEC. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–5. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 48.Morris HA, Chatterton BE, Ross PD, Durbridge TC. Diagnostic procedures. In: Nordin BEC, Need AG, Morris HA (eds) Metabolic Bone and Stone Disease, Third Edition. Churchill Livingstone, Edinburgh 1993. pp 339–79.
- 49.McKenna MJ, Freaney R, Meade A, Muldowney FP. Hypovitaminosis D and elevated serum alkaline phosphatase in elderly Irish people. Am J Clin Nutr. 1985;41:101–9. doi: 10.1093/ajcn/41.1.101. [DOI] [PubMed] [Google Scholar]
- 50.Jesudason D, Need AG, Horowitz M, O’Loughlin PD, Morris HA, Nordin BE. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone. 2002;31:626–30. doi: 10.1016/s8756-3282(02)00866-9. [DOI] [PubMed] [Google Scholar]
- 51.Glendenning P, Noble JM, Taranto M, et al. Issues of methodology, standardization and metabolite recognition for 25-hydroxyvitamin D when comparing the DiaSorin radioimmunoassay and the Nichols Advantage automated chemiluminescence protein-binding assay in hip fracture cases. Ann Clin Biochem. 2003;40:546–51. doi: 10.1258/000456303322326470. [DOI] [PubMed] [Google Scholar]
- 52.RCPA AACB Chemical Pathology QAP Group. Endocrine Program Vitamin D3 Summary Data July-November 2003.
- 53.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-cholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]