Abstract
Hepatocellular carcinoma (HCC) ranks fifth in frequency of cancers worldwide. The main aetiological factor is hepatitis B virus (HBV) although the importance of hepatitis C virus (HCV) is growing. The most important tumour marker for HCC is alpha-fetoprotein (AFP). The common method of screening high risk patients by AFP and ultrasonography has been shown to result in earlier detection and consequently more easily treatable tumours and longer survival. Proposed screening interval varies from once every 3 months to annually to “as indicated’ but, most commonly, is once every 6 months.
AFP is a fairly specific but insensitive marker for HCC. Sensitivity of HCC detection by blood markers is improved by combining various other markers with AFP. Of the other markers, the newer high sensitivity des-gamma-carboxy-prothrombin (DCP) has been found to be useful. In addition the AFP fractions L3, P4/5 and the +II band are highly specific for HCC.
Among routinely assayed tumour markers in the laboratory, CA 125 is more sensitive for HCC than AFP but far less specific. Various other enzymes, isoenzymes, growth factors, adhesion molecules, other proteins such as interleukin-2 receptor (IL-2R), human cervical cancer oncogene protein (HCCR) and glypican-3 (GPC3), p15 and p16 hypermethylation and nitrite/nitrate ratio have been tested; some of these show promise but none is presently in routine use.
The value of other newer markers such as the HBx protein that is produced by HBV, and what are thought to be specific proteins and signatures identified by proteomics remain to be determined.
Introduction
Liver cancer ranks fifth in frequency of cancers in the world. The geographic areas at highest risk, with age-adjusted incidence rates (AAIRs) of >20 per 100,000, are located in China and eastern Asia, Middle Africa and some countries of Western Africa.1 Moderately high incidences (AAIR of 10–20 per 100,000) are encountered in Japan, southern Europe, Switzerland and Bulgaria while areas at lowest risk are Northern Europe, Australia, New Zealand, and the Caucasian populations in North and Latin America.1,2
The preponderance of males among patients with HCC is universal. The male:female ratio is far more striking in African and Oriental patients (4 to 8:1) than in patients in low incidence regions (2 to 3:1) while populations with an intermediate risk of HCC generally have a ratio of about 4:1.2 While HCC is commonest in persons over the age of 40, there is considerable geographical variation in the peak age of onset.3
The prognosis of HCC is generally grave and is especially so in Africans and Chinese. In these populations the mean survival time may be as short as 11 weeks from the onset of symptoms and 6 weeks from the time of diagnosis.4 By contrast, the disease runs a more benign course in patients in low risk regions, although even they have a mean survival of only about 6 months.5
Aetiology of HCC
The role of chronic infection with HBV and HCV in the aetiology of liver cancer is well established.1 The most convincing evidence that HBV infection and HCC are causally linked has come from the classical prospective studies of 22,707 men in Taiwan by Beasley and colleagues.6 HBV accounts for 60–80% of HCC globally. Some 360 million people are chronically infected and are at risk of death from cirrhosis and HCC. Five hundred thousand to 700,000 people die each year from HBV-related liver diseases.7,8
More than 170 million people worldwide are chronically infected with the HCV, which is responsible for more than 100,000 cases of liver cancer per year.9 Prospective studies similar to the size of the study by Beasley et al.6 are not available for HCV infection. Nevertheless, population studies have indicated a correlation between the seroprevalence of HCV antibodies and the prevalence of HCC.10
Together HBV and HCV account for well over 80% of liver cancer cases worldwide. However, the attributable risk estimates for liver cancer for each of HBV and HCV vary among countries and their relative importance in the causation of HCC is changing. Studies undertaken in the United States and in Greece, for example, indicate that the role of HBV in both countries has significantly decreased in recent years, while that of HCV has significantly increased.11,12 One study from Japan showed that only 25% of HCC cases were hepatitis B surface antigen (HBsAg) positive whereas 76% were HCV antibody positive.13 HCV infection seems to be the major risk factor for HCC in the US, particularly among individuals of white and black ethnicity, whereas HBV remains the main risk factor among patients of Asian ethnicity.14 Co-infection in HCC with both hepatitis B or C viruses results in synergism, with the odds ratio being greater than the sum and lower than the product of those for each infection alone, which is indication of an independent effect and of interference between the two viruses in the carcinogenic process.15
More recently, it has been suggested that chronic infection with hepatitis G (HGV) may play a role in the development of HCC.16,17 In a study of non-Asians in Los Angeles, California, it was found that HGV infection was associated with a 5.4-fold risk of HCC and may account for up to 8% of the HCC cases.17
Other documented risk factors for HCC include aflatoxin exposure in diet, cigarette smoking, alcohol consumption, oral contraceptives and metabolic and familial diseases such as haemochromatosis, tyrosinaemia, hypercitrullinaemia, α-1-antitrypsin deficiency, type I and II glycogenesis, Wilson’s disease and hereditary fructose intolerence.1,18
Screening for HCC
Screening refers to the performance of a diagnostic test for a particular target disorder in patients without symptoms of the disorder in the hope of improving clinical outcomes.19 As patients with chronic hepatitis or cirrhosis may develop HCC only after many years, emphasis has been placed on the early detection of HCC when it is small, asymptomatic and potentially curable, through the screening of patients at high risk.20
Benefits and Limitations of Screening
The screening of subjects at risk, such as asymptomatic patients with chronic hepatitis B infection, for the early detection of subclinical HCC by AFP measurement and/or ultrasonography (US) has been proposed since the 1980s. It has been implemented in many countries and is commonly performed.20–25 However, large, randomised trials that clearly document the effectiveness of such screening are not available.25
Several trials in areas of high and low HCC incidence have demonstrated that screening can detect patients in the early stages, increase the resection rate and prolong survival time. Randomised, prospective studies in China on high risk patients which compared bi-annual AFP plus US screening with no screening for five years showed that most HCCs detected in the screened group but none in the control group, were at an early stage. The survival rate of patients with resected HCC in the screened group reached 52.7% after three and five years, but was 0% for those in the control group.26,27 Similarly, a prospective 16-year, population-based cohort study of 1,487 HBsAg-positive Alaska native carriers demonstrated that screening of such carriers with semi-annual AFP and evaluation of those with an elevated AFP by US examination was effective in detecting most HCC tumours at a resectable stage and significantly prolonged survival rates.28 Two recent studies from Italy have shown that patients detected with liver tumours in surveillance programmes had longer survival than controls.29,30 Nevertheless, one of these studies concluded that the surveillance of patients with liver cirrhosis offered little overall benefit in terms of cost effectiveness.29
A critical appraisal of screening for HCC is given in a recent meta-analysis of the subject.31 The important measure of screening is reduced all-cause mortality and mortality from the disease under study, not merely the number of HCC patients detected or apparent increased survival. It is important to have an evidence-based answer to the question of whether or not such screening does diminish mortality (total and/or HCC-specific). So far, there is not enough evidence to indicate that screening people with persistent HBsAg for HCC is effective.
An apparent increased survival time after screening may be due to over-diagnosis (non-life-threatening cancers that may never have surfaced clinically in the absence of screening, are diagnosed and treated), lead-time bias (the time between the early diagnosis and usual clinical diagnosis), and length-time bias (less aggressive cancers with long preclinical duration are more likely to be screen-detected than those with short preclinical duration). Generally, these biases favour screening. Accordingly, patients whose disease is detected by screening may appear to survive longer because they are detected earlier than those without screening, not necessarily because their death from the disease is postponed.
None of the cited papers by this meta-analysis review gave information on the stress and inconvenience of being under surveillance and, more importantly, being subjected to further tests due to false positive results.31 Its authors note that while people with positive HBsAg are at a higher risk of developing HCC than those without HBsAg, most of them would die in old age from other causes. Additionally, there were no papers that also considered the harm done by screening or the quality of life of those people screened. The quality of life of those screened is just as or more important than the extra cost of unnecessary tests or treatment. People in screening programs often develop some degree of anxiety or worry. False positive results cause further physical and psychological harm by leading to more unnecessary investigations or even treatment.
The meta-analysis concluded that there are not enough quality trials to support or refute the screening of HBsAg-positive patients for HCC. It further noted that screening patients with hepatitis C in the absence of cirrhosis was not universally recommended.31
Who Should be Screened?
Though screening increases survival, it requires a large amount of resources. The decision on whether to adopt a screening policy towards HCC would depend on the extent of the prevalence of the disease in the population and on the resources of a particular country.29 Two consensus development conferences, in Anchorage, Alaska, USA and Milan, Italy have identified chronic carriers of HBsAg and HCV, patients with cirrhosis and patients with rare metabolic liver diseases as candidates for periodic screening.22,32,33
Asymptomatic male patients aged above 40 years with chronic hepatitis B infection are also at high risk of HCC and it may be most cost-effective to screen this subgroup.34–36 Screening all liver cirrhosis patients, however, is a questionable approach because it is very expensive and its benefit in terms of patient survival is poor. While patients with cirrhosis have a higher risk of HCC, they also have a greater chance of being unsuitable for liver resection.3,25,37–39
APF, US or Both?
The use of AFP measurement alone as a screening tool has been successful in detecting early treatable tumours in populations with a high prevalence and incidence of HCC such as Asia and Sub-Saharan Africa.28,40,41 However, the screening for HCC with AFP alone in populations with a lower incidence of HCC is associated with very low predictive values.42,43 Trials in China have shown that the combination of US and AFP is better than either alone for the screening of HBsAg positive subjects; otherwise, US alone is the method of choice since it is better than AFP.44,45
The superiority of US to AFP was further demonstrated in a 7-year prospective surveillance study to determine the optimal test for detection of early HCC. It studied both AFP and US and concluded that US examination was more accurate than AFP. Thirty-one cases of HCC were detected in 602 patients with chronic viral hepatitis; the positive predictive value (PPV) for AFP to detect HCC was only 12% or less for all AFP cut-off values, and the maximum joint sensitivity and specificity were approximately 65 and 90%, respectively. Abdominal US on the other hand identified all 31 cases of HCC. The PPV for US examinations to detect HCC was 78%, while the sensitivity and specificity were 100 and 98%, respectively.45
Screening Frequency
The optimal frequency of screening has not been firmly established. The surveillance interval in different studies has varied from 3 months to annually to “as indicated” or “6 to 12 months”.46–51 The shorter screening intervals of 3 months is based on the estimate that tumours greater than 1 cm may double every 2 months.47 Semi-annual screening with AFP and US appears to be the commonly recommended procedure and frequency, including that of the consensus development conference in Milan.33,35,52,53
Screening Tools for HCC
Imaging Modalities
Imaging of HCC is complicated because the tumour has a varied radiologic appearance and frequently coexists with regenerative and dysplastic nodules in the cirrhotic liver. The sensitivity of US imaging for HCC ranges widely from 34 to 100%.19,45,48,53,54 Significant sensitivity for hepatic lesions has been reported with the use of new US modalities such as tissue harmonic imaging, US angiography and specialised Doppler techniques. However, their early developmental stage and limited availability do not make them appropriate screening tools at this time.19
Computed tomography (CT) is an attractive imaging modality for HCC screening because it can detect lesions in the cirrhotic liver, allow lesion characterisation and also assist in clinical staging. Its sensitivity and specificity for HCC are variable.19 One study in the United States of established cirrhosis found that CT scan exhibited higher sensitivity for detecting HCC than US or AFP while another found that sensitivity and specificity for US and CT were comparable.54,55 Sensitivity of CT for HCC detection can be enhanced with the use of new helical techniques and the dynamics of intravenous contrast agents.19
Magnetic resonance imaging (MRI) has, in recent years, led to significantly better detection of liver lesions following improvements in technology and techniques.19 Lesion detection rates of 80% for nodules >2 cm, 50% for nodules 1–2 cm and 33% for lesions <1 cm have been reported in a study which concluded that MRI was insensitive for the detection of HCC nodules <2 cm in patients with cirrhosis.56 However, a more recent study of patients with cirrhosis reported that the presence of delayed hypointensity in patients with arterially-enhancing liver lesions had a sensitivity of 80% and specificity of 95%, for small HCC (<2 cm).57
Given its lower cost , US is preferable to CT and MRI for the routine screening of HCC.55
AFP
AFP is the most established tumour marker in HCC and the gold standard by which other markers for the disease are judged. A serum AFP <10 ng/mL is expected in healthy men and non-pregnant women.
Transient increases and fluctuations in serum AFP may occur in chronic liver disease and cirrhosis, especially during exacerbations of hepatitis. Such increases are misleading and a patient should be retested for AFP after a fortnight if a raised result is obtained in the absence of positive imaging. Nonetheless, a persistently elevated serum AFP must alert the clinician to the possible presence of a tumour.20 While most symptomatic HCC are associated with AFP >1000 ng/mL, two-thirds of patients with small asymptomatic tumours will have an AFP <200 ng/mL.20,58 In general, an AFP value above 500 ng/mL in patients with chronic liver disease in the presence of a radiologically detected mass are virtually diagnostic of HCC.19
Approximately 66–80% of HCC are associated with AFP above the upper reference limit (positivity rate). Certain factors affect serum AFP and thus, positivity rates. Some HCC do not produce AFP and will remain this way for the remainder of the natural history of the tumour; therefore the positivity rate is not stage-dependent. Though small tumours tend to produce lower levels of AFP, there is not a direct relationship between serum AFP and tumour size. Younger patients and men tend to have higher levels compared to older patients and women, respectively.59,60
The sensitivity and specificity of AFP for HCC are highly dependent on the cut-off value above which AFP is considered positive. Values ranging from 10 to 500 ng/mL have been used in the literature as diagnostic cut-off to detect HCC.61 While the threshold abnormal level for serum AFP is often taken as 20 ng/mL, the cut-point for the suggestion or diagnosis of HCC varied among different studies especially the earlier ones, e.g. 10.5 μg/L, 25 μg/L, 50 μg/L, 100 μg/L, 200 μg/L, 400 μg/L and 500 μg/L.37,42,50,51,61–69
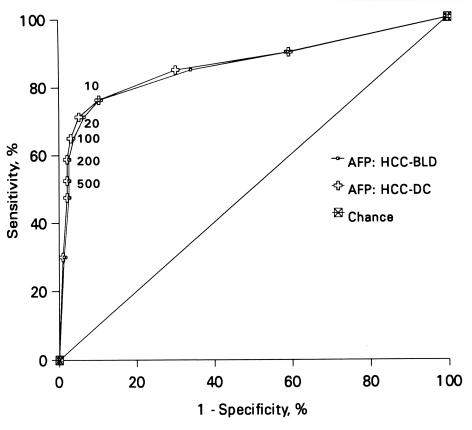
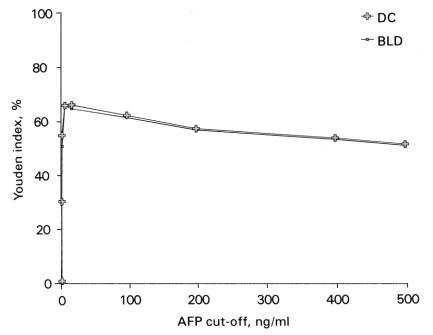
The cut-point used for diagnosis affects the calculation of sensitivity and specificity of a test; as sensitivity decreases with increasing cut-off values, the specificity increases. In a study of 80 HCC patients from Malaysia, the sensitivity of AFP in HCC was found to vary from about 76% at the cutoff of 10 ng/mL to about 53% at the cut-off of 500 ng/mL (Figures 1 and 2).61 The study reported that any AFP result >10 ng/mL, and especially >20 ng/mL (Figures 1 and 2), should raise a suspicion of HCC, while an AFP of >200 ng/mL, particularly in the presence of HBsAg is highly suggestive of it.61 Others have proposed that serum AFP >20 ng/mL should be considered elevated whereas an AFP >100 ng/mL is highly suggestive of HCC.70 A comparison of AFP with several other commonly available tumour markers such as CA 125, ferritin, CA 19-9, beta-2-microglobulin, CEA and CA 72-4 found AFP to be the most efficient marker for HCC (Figure 3).71
Figure 1.
Receiver-operating characteristic (ROC) curves for AFP in HCC when compared to benign liver diseases (AFP: HCC-BLD) and all diseased controls (AFP: HCC-DC) Reproduced with permission, from reference 61.
Figure 2.
Plot of Youden indices [(sensitivity + specificity) – 100], in percent, at various cut-offs, for AFP in HCC when compared with benign liver diseases (BLD) and all diseased controls (DC). Reproduced with permission, from reference 61.
Figure 3.
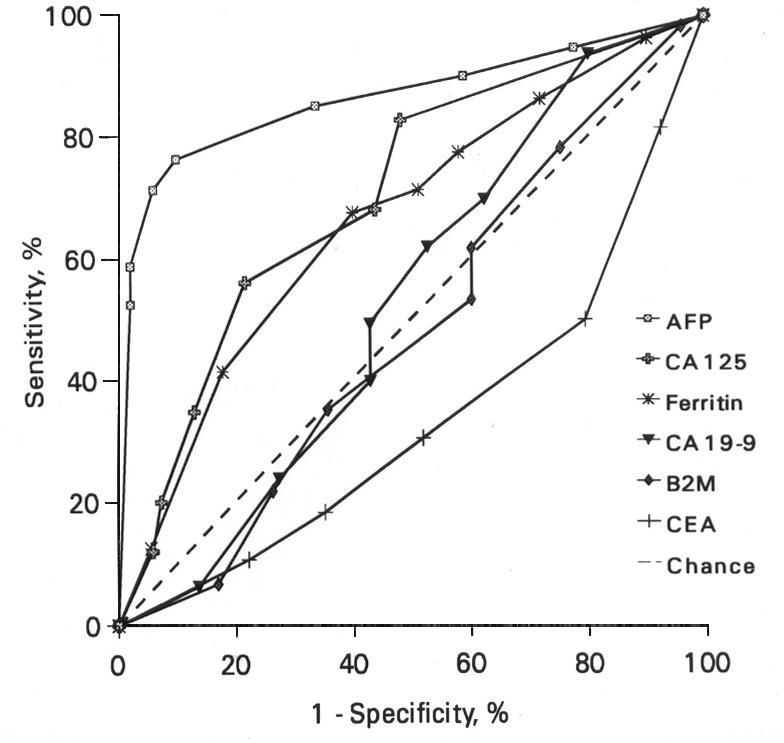
ROC curves for various tumour markers in HCC, when compared with benign liver diseases. Reproduced with permission, from reference 71.
Additional Plasma Markers Used in HCC
As the sensitivity of AFP is not good enough for it to serve as a marker for the screening of HCC, several other biomarkers have been studied for possible use. These are discussed below and the sensitivities and specificities of some of these and comparisons with AFP are summarised in the Table.
Table.
Sensitivities and specificities of some recent markers used for HCC and comparison with AFP.
| Markera (reference)
|
Cut-off
|
Marker
|
AFP
|
Marker when combined with AFP
|
|||
|---|---|---|---|---|---|---|---|
| Sensitivity % | Specificity % | Sensitivity % (cut-off in ng/mL) | Specificity % | Sensitivity % | Specificity % | ||
| Overall: 60 | Overall: 86.7 | ||||||
| Des-gamma- carboxy- prothrombin, sensitive kit73 | 40 mAU/mL | 35 (HCC <2 cm)
50 (HCC 2–3 cm) 78.1 (HCC >3 cm) |
92.3 | 71.7 (20) | 78.3 (for solitary HCC) | ||
| Des-gamma- carboxy- prothrombin76 | 125 mAU/mL | 89 | 95a, 91b | 77 (11) | 79b, 71c | 88 | 95a, 95b |
| AFP-L378 | >13% | 67.1 | 96.2 | 57.5 (250) | 92.3 | ||
| CA 12590 | Male: 12 U/mL
Female: 55 U/mL |
92 | 48.5 | 58.8 (200)
71.3 (20) |
97.4 (cut-off 200ng/mL) | 96 | 48.5 |
| Albumin-GGT isoenzyme94 | 67 | 76 | 54 (20) | 85 | 84 | ||
| Alpha-L- fucosidase96 | 870 nmol/mL/hr | 81.7 | 70.7 | 39.1 (400) | 99.3 | 82.6 | |
| Transforming growth factor-β199 | 50 μg/g creatinine | 53.1 | 99 | 55.3 (100) | 99 | 84 | 98 |
| Insulin-like growth factor-II101,d | 4.1 mg/g prealbumin | 63 | 90 | 44 (50) | 95 | 80 | 90 |
| Insulin-like growth factor-II102 | 4.5 mg/g prealbumin | 42 | 73 (100) | 97.9 | 95.1 | ||
| Interleukin-2 receptor106 | 99 | 80 | |||||
| Human cervical cancer oncogene protein110 | 15 μg/mL | 78.2 | 95.7 | 64.6 | |||
| Glypican-3111 | 50 | 72 | |||||
| Glypican-3112 | 53 | 95e, 100f | 82 | ||||
| Glypican-3113 | 40 | 100g | |||||
| Nitrite/nitrate114 | 79.5 | 72.0 | 74.4 (18) | 76.0 | 92.3 | 58 | |
with the exception of transforming growth factor-β1 which is a urinary marker, all the others are blood markers
vs. chronic liver diseases
vs. cirrhosis
for HCC ≤ 3cm
vs. patients with hepatitis plus liver cirrhosis
vs. healthy donors and patients with hepatitis
vs. liver cirrhosis (0/13), chronic hepatitis (0/34), and healthy donors (0/60)
Serum DCP
One of the most useful of these biomarkers is abnormal prothrombin DCP or prothrombin induced by vitamin K absence or antagonist II (PIVKA-II), which is an inactive prothrombin deficient in γ-carboxyglutamic acid. It is produced by malignant hepatocytes. DCP results from an acquired post-translational defect in the carboxylase system, independent of vitamin K deficiency.72
The first generation DCP assay (Eitest MONO, P-II, Eisai, Tokyo, Japan) was able to detect advanced HCC with high specificity but had low sensitivity for tumours <3 cm in size. Thus it was not sensitive enough for the early diagnosis of HCC.70,73 A revised EIA kit for DCP (Eitest PIVKA-II, Eisai) showed higher sensitivity and superior performance, especially in combination with AFP, and was found to be useful for the detection of early or small HCC.73,74
DCP has been shown to add significantly to the ability to discriminate between benign liver disease and HCC in European and American patients.75,76 The latter study found DCP alone to be more sensitive and specific than AFP for this purpose.76 The determination of both DCP and AFP have been demonstrated to be superior as a marker for HCC compared to either marker alone.70,73,75,77
AFP Fractions
The chemical basis for the heterogeneity of AFP is related to the degree of fucosylation of the asparagine-linked sugar chain of AFP which comprises about 5% of the molecular weight of the known total sequence of AFP. The differing affinities of the carbohydrate chain for lectins, in addition to mere isoelectric focusing (IEF), has been used in the differentiation of AFP source and disease.70,78
In 1990 Taketa et al. reported on lectin reactive profiles of AFP in HCC and related conditions, and proposed a simple nomenclature numbering the major AFP bands consecutively from the anode (1 = nonreactive) to the cathode as suffix to the capitalised initial letter of the lectin.79 In accordance with this definition, HCC was characterised by an increase in L3 (Lens culinaris agglutinin [LCA]-reactive band 3) and P4/5 (erythroagglutinating phytohaemeagglutinin, [E-PHA]-reactive bands 4, 5) compared with patients with benign liver disease.18,78
AFP-L3 is highly specific for HCC and, indeed, one study reported a specificity of 100% (and sensitivity of 73%) for the disease.18,80 Malignant liver cells produce AFP-L3 even when HCC is at its early stages. AFP-L3 can be detected in the serum of approximately 35% of the patients with small HCC (<2 cm). Compared to imaging techniques, it has been shown to add 9–12 months of lead-time to HCC detection.81 The combination of the highly sensitive DCP assay and AFP-L3 has been effective for the early determination of HCC and improved the detection rate of small HCC (2 cm or less).82,83 HCC patients who are AFP-L3 positive have tumours with potential for rapid growth, worse liver function, larger tumours, more advanced cancer with poor tumour histology and early and distant metastasis significantly more often compared to AFP-L3 negative ones.81,84 HCC patients who are seropositive for AFP-L3 and seronegative for DCP have demonstrated clinicopathologic features of more advanced HCC compared with those who were seropositive for DCP alone.85
However, there are some limitations to the usefulness of AFP-L3 for HCC screening: firstly, the assay is extremely cumbersome; secondly, in those patients in whom serum AFP remains low (<30 ng/mL), fractionation of AFP is not useful; and thirdly, no randomised trial comparing total AFP with fractionated AFP at the time of diagnosis of HCC has been carried out.18
IEF has identified the presence of disease-specific isoforms: three major bands are apparent: +I (associated with ‘benign liver diseases’), +II (associated with HCC) and +III (associated with non-seminomatous germ cell tumours, NSGCT).86 Characterisation of the predominant glycans of human AFP has shown that HCC-associated isoforms (Band +II) represent a group of glycoproteins whose carbohydrate structures are all characterised by being monosialylated (msAFP), whereas those associated with benign liver diseases and NSGCT are di- and a-sialo species, respectively.87 The same group measured msAFP by IEF, Western blot and densitometry and by a novel glycosylation immunosorbent assay and found msAFP to be potentially a diagnostic marker for HCC with non-diagnostic AFP (<500 ng/mL).88
Other Plasma Markers Studied in HCC
Besides AFP, its fractions and DCP, several other potential markers have been tested for possible use in HCC.
CA 125 and Other Tumour Markers of its Genre
AFP and markers of its genre are commonly measured in the laboratory. Many have been tested for their value as HCC markers and of these, CA 125 appears to be the marker that could be most useful.71
Sensitivities for CA 125 in HCC have been reported to range from 43% to more than 90%.89,90 However, the specificity of CA 125 in relation to benign liver diseases was poor. Together, AFP (cut-off: 200 ng/mL) and CA 125 have been reported to give a combined sensitivity of 96%. The use of AFP (sensitivity 58.8%, specificity of 97.4%) and CA 125 (sensitivity of 92%, specificity 48.5%) together as screening blood markers for HCC has been suggested, a negative result for both markers would most likely rule out HCC.90 Despite its excellent sensitivity for HCC, it should be noted that raised CA125 occurs in both benign and malignant liver disease. It is closely associated with the presence of ascites and is especially elevated when this condition is present, in both malignant and benign liver disease.91,92
Enzymes and Isoenzymes
Several enzymes and isoenzymes have been tested for use as possible markers of HCC.
Serum gamma-glutamyltransferase isoenzyme II (GGT II) band which is hepatoma-specific is a useful tumour marker complementary to AFP for diagnosis of HCC. Combining the information from DCP, AFP, and GGTII significantly increases the sensitivity over AFP alone.93
The conjugate albumin-GGT has been found in patients with cirrhosis with and without HCC and in patients with liver metastases but not in chronic active hepatitis patients or control subjects. The combined use of albumin-GGT and AFP (cut-off >20 ng/mL) was associated with greater sensitivity and accuracy than those observed with either of the two markers considered alone.94
Serum alpha-L-fucosidase (AFU) activity is useful in the early detection of HCC, especially in conjunction with AFP and US, and particularly in patients with underlying viral hepatitis and cirrhosis. Its activity in patients with HCC was significantly higher than that found in cirrhosis, chronic hepatitis or other tumours and in controls. Patients with cirrhosis, who have a marked increase in serum AFU s during follow-up should be closely monitored for signs of HCC development.95,96
Serum thioredoxin, a reductase enzyme, has been shown to be significantly higher at 147.4 ng/mL (125.5–173.0 ng/mL) in patients with HCC compared to normal volunteers, 81.8 ng/mL (74.6–89.6 ng/mL) and patients with chronic hepatitis or liver cirrhosis without HCC, 80.9 ng/mL (69.7–93.9 ng/mL).97
Serum angiotensin-converting enzyme (SACE) is low in patients with advanced HCC and significantly decreased in patients with HCC, whose AFP was not altered. SACE may also be helpful in detecting HCC in patients with cirrhosis, where it can be difficult to differentiate between small HCC tumours and regeneration nodules.98
Growth Factors
Cytokines are small secreted proteins which mediate and regulate immunity, inflammation and haematopoiesis. They are produced de novo in response to an immune stimulus. Growth factors are cytokines, and a number of these have been tested for use as markers for HCC.
Transforming growth factor-β1 (TGF-β1) is involved in the regulation and differentiation of normal and malignant cells. Plasma and urine TGF-β1 are elevated in patients with HCC compared with healthy controls and patients with benign liver diseases. In one study, the sensitivity of urine TGF-β1 was 53% and the specificity 99%, which is comparable to serum AFP (cut-off 100 ng/mL), while the combination of both markers resulted in a sensitivity and specificity of 84 and 98 %, respectively and a diagnostic accuracy of 90.1%.99 Elevated urinary TGF-β1 has been proposed as a complementary marker to AFP to discriminate HCC from cirrhosis and for the detection of HCC with low AFP production.99,100
A recently published study of small HCC, showed serum insulin-like growth factor-II (IGF–II) to have a sensitivity, specificity and diagnostic accuracy of 63%, 90% and 70%, respectively, while the figures for AFP were 44%, 95% and 70% respectively. The determination of both markers in parallel significantly increased the diagnostic accuracy and sensitivity while maintaining a high specificity.101 IGF-II may be used as an independent marker for the diagnosis of small HCC or as a complementary marker together with AFP to both diagnose small HCC and to discriminate HCC from cirrhosis.101,102
The elevation of serum hepatocyte growth factor has been shown to be a most important risk factor for the occurrence of HCC in patients with C-viral chronic hepatitis and cirrhosis. It may be useful as a tumour marker for HCC detection and follow-up therapy.103
Adhesion Molecules
Serum vascular-cell adhesion molecules (VCAM-1) was significantly higher in HCC patients with cirrhosis compared with those without cirrhosis. A significantly better disease-free survival was observed in HCC patients with low serum VCAM-1.104
High serum intercellular adhesion molecule-1 has been demonstrated to be associated with severe liver disease, such as liver cirrhosis and HCC. It tends to increase with deteriorating hepatic function and tumour size.105
IL-2R
Izzo and colleagues have shown that in chronic HBV or HCV infected patients, serum IL-2R, in comparison with AFP, is (i) abnormal with a significantly greater frequency in the presence of HCC, and (ii) a more sensitive marker of successful treatment and recurrence of HCC.106 They have used IL-2R measurements both to screen high-risk patients and to monitor treatment responses in patients with hepatitis who develop HCC.
p15, p16 Methylation
Hypermethylation of p16, a cyclin-dependent kinase inhibitor gene that regulates cell cycling, has been detected frequently in human cancers including HCC. The p15 gene is a cyclin-dependent kinase inhibitor gene adjacent top16 on chromosome 9p21. It is aberrantly methylated in several human neoplasms and especially among haematopoietic malignancies. Work by Wong and colleagues in Hong Kong has shown aberrantly methylated p16 to occur in the plasma/serum of HCC patients where such changes had occurred in HCC tissue; healthy controls and patients with chronic hepatitis/cirrhosis did not show these changes.107,108 Using methylation-specific PCR, they demonstrated p15 promoter methylation in 64% of tumours and 25% (4 of 16) of patients’ plasma and serum samples. They also found that among the 92% (23 of 25) of HCC patients with tumour p15/p16 methylation, circulating tumour DNA and HCC cells were detected in the peripheral blood of 87% (20 of 23). None of the control samples were methylation positive.108 A combination of these epigenetic markers may prove valuable for non-invasive HCC diagnosis and monitoring.107–109
HCCR
HCCR appears to function as a negative regulator of p53 gene. HCCR proteins are over-expressed in tumorous cirrhosis tissues compared with non-tumorous cirrhosis tissues but are undetected in normal liver tissue.110 Serological studies in 570 subjects revealed that HCCR had a significantly higher sensitivity than AFP (P = 0.0098) for HCC at the specificity of 95.7%. Forty of 52 (76.9%) AFP negative patients with carcinoma showed positive values for HCCR. The positive rate of 69.2% seen in carcinoma patients with tumour sizes <2 cm was higher than the rate for AFP. HCCR may have an advantage over AFP in being elevated according to disease progression from liver cirrhosis to carcinoma. It was also more frequently positive in patients with early, small HCC.110
GPC3
GPC3, a heparan sulphate proteoglycan anchored to the plasma membrane, is a good candidate marker of HCC because it is an oncofetal protein that is over-expressed in HCC at both the mRNA and protein levels. Its soluble NH2-terminal portion (sGPC3) is cleaved between Arg(358) and Ser(359) of GPC3 and sGPC3 can be specifically detected in the sera of patients with HCC.111 Tissue GPC3 is specifically over-expressed in most HCCs and this is reflected in the excellent specificities of the blood marker (Table).112 Serum GPC3 has been shown to be significantly higher in HCC than in liver cirrhosis or in healthy controls.111 The simultaneous determination of GPC3 and AFP has been shown to increase the sensitivity for diagnosis of HCC.111,112 In another study, though the GPC3 protein was positive in the sera of only 40% (16/40) of HCC patients, it was negative in sera from subjects with liver cirrhosis, chronic hepatitis, and healthy donors. Although 12 of 40 HCC patients were negative for both AFP and PIVKA-II, four of these were GPC3-positive.113 GPC3 is thus a novel serological marker for the early detection of HCC.111
Nitrite/Nitrate Concentrations
Plasma concentrations of nitrite/nitrate, the stable end-products of nitric oxide, increase in proportion to tumour volume in patients with HCC. Moriyama and colleagues showed that the clinical utility of plasma nitrite/nitrate as a tumour marker approximated that of serum AFP, but exceeded it in AFP-negative patients.114 Indeed, nitrite/nitrate was positive in 70% of AFP-negative HCC patients. The simultaneous determination of serum AFP and plasma nitrite/nitrate concentrations gave significant improvement compared with serum AFP alone in detection of HCC in chronic liver disease patients.
Comment on the Newer Markers
AFP remains the gold-standard blood marker for HCC despite its poor sensitivity at the higher cut-off values (where the specificity is most useful) and the promising diagnostic performance of some of the newer markers. The reasons for this include its long history, reasonable cost compared to the newer markers, easy availability and wide application in assay systems. AFP has relatively high diagnostic specificity for HCC and good organ-specificity compared to the other markers that have been proposed.
Of the newer markers, some such as GPC3 show great potential. Although some of these have been found to perform better than AFP on their own and when used in conjunction with it, rigorous clinical evaluation of promising markers and modalities in different environments will be required before they are deemed acceptable at the population level, even as complementary markers.
The sensitive DCP assay is the probably the most widely accepted among the newer markers. For the present, the use of a combination of US, AFP and sensitive DCP constitute the best strategy for the early first detection of HCC. More studies on the other newer markers are required before they can be accepted for routine use in patients.
HCC Markers of the Future?
Developmental research to identify new markers and modalities for HCC is needed. Among the possible markers for the future are newly discovered proteins and signatures identified by proteomics.
HBx
The small, 3.2-kb DNA genome of HBV contains four open reading frames called S, C, P, and X.115 The X gene is the smallest with 465 nucleotides and the HBx protein is 154 amino acids long. During the natural course of HBV infection, the HBx gene expresses the HBx polypeptide that is implicated in HBV-mediated HCC. Anti-HBx antibodies have been found in the sera of HCC patients (70%) and in the sera of a far lower proportion (5%) of patients with chronic hepatitis who were HBV DNA positive. The results also showed that the liver tissues of 85% of these HCC patients contained the HBx protein. All who were negative for the antibody were also negative for tissue protein, thus giving full correlation between anti-HBx Ab positivity in serum and HBx protein in HCC tissues.115 However, the usefulness of HBx protein or its antibody as a prognostic marker for the development of HCC of HBV origin has been questioned116 and the significance of the serological data remains to be established.
Possible Markers Identified by Proteomics
Proteomic analysis involves the systematic separation, identification and characterisation of proteins present in a biological sample. By comparing the proteins present in diseased samples with those present in normal samples, it is possible to identify changes in expression of proteins that potentially provide opportunities for earlier detection. The combination of 2-dimensional polyacrylamide gel electrophoresis and mass spectrometry is the most widely used technique for proteomics, although other more automated high-throughput techniques are being developed.117
Proteins eliciting a humoral response in HCC have been identified and the occurrence of autoantibodies to specific proteins have possible utility for HCC screening and diagnosis.118 In addition, two relatively abundant features that were reduced in patients with HCC of HBV origin as compared to the healthy subjects have been demonstrated: tryptic fragment mass-fingerprinting identified these features as a carboxy-terminal fragment of complement C3 and an isoform of apolipoprotein A1.119
Markers Proven to be Not Useful
Although serum tumour necrosis factor-alpha has been demonstrated to be increased in patients with HCC, and associated with disease severity and nutrition status, it has not been recommended for use as a marker for the early diagnosis of HCC in cirrhotic patients.120 Several other proteins markers in serum have been studied for possible use in the detection of HCC but have not proven to be useful because of poor sensitivity and/or specificity. These include C-reactive protein, matrix metalloproteinase-2 and -9, tissue polypeptide specific antigen, beta2-microglobulin, CA 19-9, carcinoembryonic antigen, CA 72-4 and ferritin.71,121–123
Concluding Remarks
HCC is an important cancer with a dismal outcome and a high mortality rate. HBV and, increasingly, HCV are the main aetiological agents.
Despite lack of evidence that screening results in improved patient outcomes, it will continue to be carried out as long as the tools are available and affordable. The screening of patients at risk at 6 monthly intervals using AFP and US is a common screening procedure. US on its own has been shown to be superior to AFP alone.
Newer markers have been tested because of the poor sensitivity of AFP for HCC at the higher cut-off values where its specificity is most useful. Some of these markers have been shown to perform better than AFP. DCP is probably the best known of these markers. Detection of HCC is improved when AFP is used in conjunction with these markers.
While several newer markers have been proposed, there is a need for developmental research and rigorous clinical evaluation of these newer markers and diagnostic modalities before their application at the population level.
Acknowledgments
The author wishes to thank the Director of the Institute for Medical Research, Kuala Lumpur, for permission to publish this review.
The contents of articles or advertisements in The Clinical Biochemist – Reviews are not to be construed as official statements, evaluations or endorsements by the AACB, its official bodies or its agents. Statements of opinion in AACB publications are those of the contributors. Print Post Approved - PP255003/01665.
No literary matter in The Clinical Biochemist – Reviews is to be reproduced, stored in a retrieval system or transmitted in any form by electronic or mechanical means, photocopying or recording, without permission. Requests to do so should be addressed to the Editor. ISSN 0159 – 8090.
Footnotes
Competing Interests: None declared.
References
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–85. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.Kew MC. The development of hepatocellular cancer in humans. Cancer Surveys. 1986;5:719–39. [PubMed] [Google Scholar]
- 3.Hoofnagle JH. The natural history of hepatocellular carcinoma, pp393–5. In: Di Bisceglie AM, moderator. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- 4.Kew MC, Geddes EW. Hepatocellular carcinoma in rural southern African Blacks. Medicine. 1982;61:98–108. doi: 10.1097/00005792-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kew MC, Dos Santos HA, Sherlock S. Diagnosis of primary cancer of the liver. Br Med J. 1971;vi:408–11. doi: 10.1136/bmj.4.5784.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–33. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 7.Website of the World Health Organization http://www.who.int/mediacentre/factsheets/fs204/en/accessed 2 February 2005.
- 8.Website of Advanced Immunization Management (AIM) http://aim-e-learning.stanford.edu/en/vaccines/hepb/assessBurden/burden/2.html accessed 2 February 2005.
- 9.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 10.Resnick R, Koff R. Hepatitis C related hepatocellular carcinoma. Arch Intern Med. 1993;153:1672–7. [PubMed] [Google Scholar]
- 11.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol 2002;35:266–9. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Raptis I, Koskinas J, Emmanouil T, Hadziyannis S. Changing relative roles of hepatitis B and C viruses in the aetiology of hepatocellular carcinoma in Greece. Epidemiological and clinical observations. J Viral Hepat. 2003;10:450–4. doi: 10.1046/j.1365-2893.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 13.Nishioka K, Watanabe J, Furuta S, et al. A high prevalence of antibody to the hepatitis C virus in patients with hepatocellular carcinoma in Japan. Cancer. 1991;67:429–33. doi: 10.1002/1097-0142(19910115)67:2<429::aid-cncr2820670218>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Liver Cancer Network. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol 2003;98:2060–3. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 15.Donato F, Boffetta P, Puotti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–54. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez Agullo JL, Suarez A, Ladero JM, Lopez-Alonso G, Picazo JJ, Diaz-Rubio M. Hepatitis G virus infection in Spanish patients with hepatocellular carcinoma. Liver. 1998;18:255–8. doi: 10.1111/j.1600-0676.1998.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 17.Yuan JM, Govindarajan S, Ross RK, Yu MC. Chronic infection with hepatitis G virus in relation to hepatocellular carcinoma among non-Asians in Los Angeles County, California. Cancer. 1999;86:936–43. doi: 10.1002/(sici)1097-0142(19990915)86:6<936::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Haydon GH, Hayes PC. Screening for hepatocellular carcinoma. European J Gastroenterol Hepatol. 1996;8:856–60. [PubMed] [Google Scholar]
- 19.Arguedas MR. Screening for hepatocellular carcinoma: why, when, how? Curr Gastroenterol Rep 2003;5:57–62. doi: 10.1007/s11894-003-0010-1. [DOI] [PubMed] [Google Scholar]
- 20.Dusheiko GM. Diagnosis of hepatocellular carcinoma, pp395-7.In: Di Bisceglie AM, moderator. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- 21.Tang ZY, Yang BH, Tang CL, et al. Evaluation of population screening for hepatocellular carcinoma. Chinese Med J. 1980;93:795–9. [PubMed] [Google Scholar]
- 22.Colombo M. Screening for hepatocellular carcinoma. Digestion. 1998;59(suppl):70–1. doi: 10.1159/000051428. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki N, Yoshino M, Yoshida T, et al. Early diagnosis of hepatocellular carcinoma. Hepatogastroenterology. 1990;37:480–3. [PubMed] [Google Scholar]
- 24.Yuen MF, Cheng CC, Lauder IJ, et al. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–5. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 25.Sherman M. Surveillance for hepatocellular carcinoma. Semin Oncol. 2001;28:450–9. doi: 10.1016/s0093-7754(01)90138-1. [DOI] [PubMed] [Google Scholar]
- 26.Yang B, Zhang B, Xu Y, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol 1997;123:357–60. doi: 10.1007/BF01438313. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Yang B. Combined alpha-fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–10. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 28.McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–6. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 29.Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trevisani F, Cantarini MC, Labate AM, et al. Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol. 2004;99:1470–6. doi: 10.1111/j.1572-0241.2004.30137.x. [DOI] [PubMed] [Google Scholar]
- 31.Wun YT, Dickinson JA. Alpha-fetoprotein and/or liver ultrasonography for liver cancer screening in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2003;(2):CD002799. doi: 10.1002/14651858.CD002799. [DOI] [PubMed] [Google Scholar]
- 32.McMahon BJ, London T. Workshop on screening for hepatocellular carcinoma. J Natl Cancer Inst. 1991;83:916–9. doi: 10.1093/jnci/83.13.916. [DOI] [PubMed] [Google Scholar]
- 33.Colombo M. Early diagnosis of hepatocellular carcinoma in Italy. A summary of a Consensus Development Conference held in Milan, 16 November 1990 by the Italian Association for the Study of the Liver (AISF) J Hepatol. 1992;14:401–3. [PubMed] [Google Scholar]
- 34.Popper H, Shafritz DA, Hoofnagle JH. Relation of the hepatitis B virus carrier state to hepatocellular carcinom a. Hepatology. 1987;7:764–72. doi: 10.1002/hep.1840070425. [DOI] [PubMed] [Google Scholar]
- 35.McMahon B, Lanier A, Wainwright R, Kilkenny S. Hepatocellular carcinoma in Alaska Eskimos: epidemiology, clinical features, and early detection. Prog in Liver Dis. 1990;9:643–55. [PubMed] [Google Scholar]
- 36.Okuda K. Hepatocellular carcinoma: recent progress. H epatology. 1992;15:948–63. doi: 10.1002/hep.1840150532. [DOI] [PubMed] [Google Scholar]
- 37.Liaw YF, Tai DI, Chu CM, et al. Early detection of hepatocelluar carcinoma in patients with chronic type B hepatitis, a prospective study. Gastroenterology. 1986;90:263–7. doi: 10.1016/0016-5085(86)90919-4. [DOI] [PubMed] [Google Scholar]
- 38.Bottelli R, Tibballs J, Hochhauser D, et al. Ultrasound screening for hepatocellular carcinoma (HCC) in cirrhosis: the evidence for an established clinical practice. Clinical Radiology. 1998;53:713–6. doi: 10.1016/s0009-9260(98)80311-5. [DOI] [PubMed] [Google Scholar]
- 39.Ebara M, Ohto M, Shinagawa T. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. Gastroenterology. 1986;90:289–98. doi: 10.1016/0016-5085(86)90923-6. [DOI] [PubMed] [Google Scholar]
- 40.Lok AS, Lai CL. Alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110–5. doi: 10.1002/hep.1840090119. [DOI] [PubMed] [Google Scholar]
- 41.Chen CJ, Lu SN, You SL, et al. Community-based hepatocellular carcinoma screening in seven townships in Taiwan. J Formos Med Assoc. 1995;94 (Suppl 2):S94–102. [PubMed] [Google Scholar]
- 42.Cottone M, Turri M, Caltagirone M, et al. Screening for hepatocellular carcinoma in patients with Child’s A cirrhosis: an 8-year prospective study by ultrasound and alpha-fetoprotein. J Hepatol. 1994;21:1029–34. doi: 10.1016/s0168-8278(05)80613-0. [DOI] [PubMed] [Google Scholar]
- 43.Colombo M. Screening for cancer in viral hepatitis. Clin Liver Dis. 2001:109–22. doi: 10.1016/s1089-3261(05)70156-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–10. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 45.Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553–9. doi: 10.1046/j.1440-1746.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 46.Oka H, Kurioka N, Kim K, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12(4 Pt 1):680–7. doi: 10.1002/hep.1840120411. [DOI] [PubMed] [Google Scholar]
- 47.Murakami T, Mochizuki K, Nakamura H. Imaging evaluation of the cirrhotic liver. Semin Liver Dis. 2001;21:213–24. doi: 10.1055/s-2001-15497. [DOI] [PubMed] [Google Scholar]
- 48.Okano H, Shiraki K, Inoue H, et al. Comparison of screening methods for hepatocellular carcinomas in patients with cirrhosis. Anticancer Res. 2001;21:2979–82. [PubMed] [Google Scholar]
- 49.Milne A, Moyes C, Pearce N. Liver disease and hepatitis B infection in a large New Zealand family. N Z Med J. 1989;102:318–20. [PubMed] [Google Scholar]
- 50.Colombo M, De Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. New Engl J Med. 1991;325:675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 51.Larcos G, Sorokopud H, Berry G, Farrell G. Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation. Am J Radiol. 1998;171:433–5. doi: 10.2214/ajr.171.2.9694470. [DOI] [PubMed] [Google Scholar]
- 52.Heyward W, Lanier A, Bender T, et al. Early detection of primary hepatocellular carcinoma by screening for alpha-fetoprotein in high-risk families. A case-report. Lancet. 1983;2 :1161–2. doi: 10.1016/s0140-6736(83)91214-x. [DOI] [PubMed] [Google Scholar]
- 53.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–8. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 54.Chalasani N, Horlander JC, Sr, Said A, et al. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988–93. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 55.Gambarin-Gelwan M, Wolf DC, Shapiro R, et al. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol 2000;95:1535–8. doi: 10.1111/j.1572-0241.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 56.Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explanation correlation. Radiology. 2001;219:445–54. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 57.Marrero JA, Hussain HK, Nghiem HV, et al. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl. 2005;11:281–9. doi: 10.1002/lt.20357. [DOI] [PubMed] [Google Scholar]
- 58.Dusheiko GM. Hepatocellular carcinoma associated with chronic viral hepatitis – aetiology, diagnosis and treatment. Brit Med Bull. 1990;46:492–511. doi: 10.1093/oxfordjournals.bmb.a072412. [DOI] [PubMed] [Google Scholar]
- 59.Kew MC. Hepatocellular cancer: A century of progress. Clin Liver Dis. 2000;4:257–68. doi: 10.1016/s1089-3261(05)70107-0. [DOI] [PubMed] [Google Scholar]
- 60.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–60. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 61.Lopez JB, Thambyrajah V, Balasegaram M, Timor J. Appropriate cut-off levels for serum alpha-fetoprotein in hepatocellular carcinoma. Diag Oncol. 1994;4:287–91. [Google Scholar]
- 62.Sherman M, Peltekian K, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–8. [PubMed] [Google Scholar]
- 63.Johnson PJ, Protman B, Willimas R. Alpha-fetoprotein concentrations measured by radioimmunoassay in diagnosing and excluding hepatocellular carcinoma. Br Med J. 1978;ii:661–3. doi: 10.1136/bmj.2.6138.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heyward WL, Lanier AP, McMahon BJ, et al. Early detection of primary hepatocellular carcinoma. JAMA. 1985;254:3052–4. [PubMed] [Google Scholar]
- 65.Mima S, Sekiya C, Kanagawa H, et al. Mass screening for hepatocellular carcinoma: experience in Hokkaido, Japan. J Gastroenterol Hepatol. 1994;9:361–5. doi: 10.1111/j.1440-1746.1994.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 66.Okuda K the Liver Cancer Study Group of Japan. Primary liver cancers of Japan. Cancer. 1980;6:2663–9. [Google Scholar]
- 67.Solmi L, Primerano A, Gandolfi L. Ultrasound follow-up of patients at risk for hepatocellular carcinoma: results of a prospective study on 360 cases. Am J Gastr oenterol. 1996;91:1189–94. [PubMed] [Google Scholar]
- 68.Sheu JC, Sung JL, Chen DS, et al. Early detection of hepatocellular carcinoma by real-time ultrasonography, a prospective study. Cancer. 1985;56:660–6. doi: 10.1002/1097-0142(19850801)56:3<660::aid-cncr2820560338>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 69.Johnson PJ. Tumour markers in the diagnosis and management of patients with hepatocellular carcinoma. Recent Results Cancer Res. 1986;100:68–72. doi: 10.1007/978-3-642-82635-1_8. [DOI] [PubMed] [Google Scholar]
- 70.Trojan J, Raedle J, Zeuzem S. Serum tests for diagnosis and follow-up of hepatocellular carcinoma after treatment. Digestion. 1998;59 (Suppl 2):72–4. doi: 10.1159/000051429. [DOI] [PubMed] [Google Scholar]
- 71.Lopez JB, Balasegaram M, Timor J, Thambyrajah V. Comparison of alpha-fetoprotein with some other tumour markers in Malaysians with hepatocellular carcinoma. Malays J Pathol. 1997;19:53–8. [PubMed] [Google Scholar]
- 72.Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology. 1993;18:990–7. doi: 10.1002/hep.1840180434. [DOI] [PubMed] [Google Scholar]
- 73.Okuda H, Nakanishi T, Takatsu K, et al. Measurement of serum levels of des-γ-carboxy prothrombin in patients with hepatocellur carcinoma by a revised enzyme immunoassay kit with increased senstivity. Cancer. 1999;85:812–8. [PubMed] [Google Scholar]
- 74.Mita Y, Aoyagi Y, Yanagi M, et al. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643–8. doi: 10.1002/(sici)1097-0142(19980501)82:9<1643::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 75.Lamerz R, Runge M, Stieber P, Meissner E. Use of serum PIVKA-II (DCP) determination for differentiation between benign and malignant liver diseases. Anticancer Res. 1999;19:2489–94. [PubMed] [Google Scholar]
- 76.Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37:1114–21. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 77.Grazi GL, Mazziotti A, Legnani C, et al. The role of tumour markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver Transpl Surg. 1995;1:249–55. doi: 10.1002/lt.500010410. [DOI] [PubMed] [Google Scholar]
- 78.Lamerz R. AFP isoforms and their clinical significance (overview) Anticancer Res. 1997;17:2927–30. [PubMed] [Google Scholar]
- 79.Taketa K, Sekiya C, Namiki M, et al. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology. 1990;99:508–18. doi: 10.1016/0016-5085(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 80.Sato Y, Nakata K, KatoY, et al. Early recognition of hepatocellular carcinoma based on altered profiles of AFP. N Engl J Med. 1993;328:1802–6. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- 81.Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313:15–9. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- 82.Sassa T, Kumada T, Nakano S, Uematsu T. Clinical utility of simultaneous measurement of serum high-sensitivity des-gamma-carboxy prothrombin and Lens culinaris agglutinin A-reactive alpha-fetoprotein in patients with small hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1999;11:1387–92. doi: 10.1097/00042737-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Shimauchi Y, Tanaka M, Kuromatsu R, et al. A simultaneous monitoring of Lens culinaris agglutinin A-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin as an early diagnosis of hepatocellular carcinoma in the follow-up of cirrhotic patients. Oncol Rep. 2000;7:249–56. doi: 10.3892/or.7.2.249. [DOI] [PubMed] [Google Scholar]
- 84.Yamashiki N, Seki T, Wakabayashi M, et al. Usefulness of Lens culinaris agglutinin A-reactive fraction of alpha-fetoprotein (AFP-L3) as a marker of distant metastasis from hepatocellular carcinoma. Oncol Rep. 1999;6:1229–32. doi: 10.3892/or.6.6.1229. [DOI] [PubMed] [Google Scholar]
- 85.Okuda H, Nakanishi T, Takatsu K, et al. Clinicopathologic features of patients with hepatocellular carcinoma seropositive for α-fetoprotein-L3 and seronegative for des-gamma-carboxy prothrombin in comparison with those seropositive for des-gamma-carboxy prothrombin alone. J Gastroenterol Hepatol. 2002;17:772–8. doi: 10.1046/j.1440-1746.2002.02806.x. [DOI] [PubMed] [Google Scholar]
- 86.Johnson PJ, Leung N, Cheng P, et al. ‘Hepatoma-specific’ alphafetoprotein may permit preclinical diagnosis of malignant change in patients with chronic liver disease. Br J Cancer. 1997;75:236–40. doi: 10.1038/bjc.1997.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson PJ, Poon TC, Hjelm NM, Ho CS, Blake C, Ho SK. Structures of disease-specific serum alpha-fetoprotein isoforms. Br J Cancer. 2000;83:1330–7. doi: 10.1054/bjoc.2000.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poon TCW, Mok TSK, Chan ATC, et al. Quantification and utility of monosialylated α-fetoprotein in the diagnosis of hepatocellular carcinoma with nondiagnostic serum total α-fetoprotein. Clin Chem. 2002;48:1021–7. [PubMed] [Google Scholar]
- 89.Elias J, Kew MC. Evaluation of CA 125 as a serum marker of hepatocellular carcinoma. Int J Cancer. 1990;46:805–7. doi: 10.1002/ijc.2910460510. [DOI] [PubMed] [Google Scholar]
- 90.Lopez JB, Balasegaram M, Thambyrajah V. Serum CA 125 as a marker of hepatocellular carcinoma. Int J Biol Markers. 1996;11:178–82. doi: 10.1177/172460089601100307. [DOI] [PubMed] [Google Scholar]
- 91.Bergmann JF, Beaugrand M, Labadie H, et al. CA 125 (ovarian tumour-associated antigen) in ascitic liver diseases. Clin Chim Acta. 1986;155:163–6. doi: 10.1016/0009-8981(86)90278-0. [DOI] [PubMed] [Google Scholar]
- 92.Devarbhavi H, Kaese D, Williams AW, et al. Cancer antigen 125 in patients with chronic liver disease. May Clin Proc. 2002;77:538–41. doi: 10.4065/77.6.538. [DOI] [PubMed] [Google Scholar]
- 93.Cui R, He J, Zhang F, et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88:1878–82. doi: 10.1038/sj.bjc.6601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pompili M, Addolorato G, Pignataro G, et al. Evaluation of the albumin-gamma-glutamyltransferase isoenzyme as a diagnostic marker of hepatocellular carcinoma-complicating liver cirrhosis. J Gastroenterol Hepatol. 2003;18:288–95. doi: 10.1046/j.1440-1746.2003.02962.x. [DOI] [PubMed] [Google Scholar]
- 95.Giardina MG, Matarazzo M, Morante R, et al. Serum alpha-L-fucosidase activity and early detection of hepatocellular carcinoma: a prospective study of patients with cirrhosis. Cancer. 1998;83:2468–74. [PubMed] [Google Scholar]
- 96.Tangkijvanich P, Tosukhowong P, Bunyongyod P, et al. Alpha-L-fucosidase as a serum marker of hepatocellular carcinoma in Thailand. Southeast Asian J Trop Med Public Health. 1999;30:110–4. [PubMed] [Google Scholar]
- 97.Miyazaki K, Noda N, Okada S, et al. Elevated serum level of thioredoxin in patients with hepatocellular carcinoma. Biotherapy. 1998;11:277–88. doi: 10.1023/a:1008032703468. [DOI] [PubMed] [Google Scholar]
- 98.Kardum D, Huskic J, Fabijanic D, et al. Activity of serum angiotensin-converting enzyme as a tumour marker of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1999;11:1209–13. doi: 10.1097/00042737-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 99.Tsai JF, Jeng JE, Chuang LY, et al. Clinical evaluation of urinary transforming growth factor-beta-1 and serum alpha-fetoprotein as tumour markers of hepatocellular carcinoma. Br J Cancer. 1997;75:1460–6. doi: 10.1038/bjc.1997.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsai JF, Jeng JE, Chuang LY, et al. Urinary transforming growth factor-beta-1 in relation to serum alpha-fetoprotein in hepatocellular carcinoma. Scand J Gastroenterol. 1997;32:254–60. doi: 10.3109/00365529709000203. [DOI] [PubMed] [Google Scholar]
- 101.Tsai JF, Jeng JE, Chuang LY, et al. Serum insulin-like growth factor-II as a serologic marker of small hepatocellular carcinoma. Scand J Gastroenterol. 2005;40:68–75. doi: 10.1080/00365520410009311. [DOI] [PubMed] [Google Scholar]
- 102.Tsai JF, Jeng JE, Chuang LY, et al. Serum insulin-like growth factor-II and alpha-fetoprotein as tumour markers of hepatocellular carcinoma. Tumour Biol. 2003;24:291–8. doi: 10.1159/000076461. [DOI] [PubMed] [Google Scholar]
- 103.Yamagamim H, Moriyama M, Matsumura H, et al. Serum concentrations of human hepatocyte growth factor is a useful indicator for predicting the occurrence of hepatocellular carcinomas in C-viral chronic liver diseases. Cancer. 2002;95:824–34. doi: 10.1002/cncr.10732. [DOI] [PubMed] [Google Scholar]
- 104.Ho JW, Poon RT, Tong CS, Fan ST. Clinical significance of serum vascular cell adhesion molecule-1 levels in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:2014–8. doi: 10.3748/wjg.v10.i14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soresi M, Cervello M, Lipani G, et al. Circulating intercellular adhesion molecule-1 in patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1997;9:805–9. doi: 10.1097/00042737-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 106.Izzo F, Cremona F, Delrio P, et al. Soluble interleukin-2 receptor levels in hepatocellular cancer: a more sensitive marker than alfa fetoprotein. Ann Surg Oncol. 1999;6:178–85. doi: 10.1007/s10434-999-0178-1. [DOI] [PubMed] [Google Scholar]
- 107.Wong IH, Lo YM, Zhang J, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–3. [PubMed] [Google Scholar]
- 108.Wong IH, Lo YM, Yeo W, Lau WY, Johnson PJ. Frequent p15 promoter methylation in tumour and peripheral blood from hepatocellular carcinoma patients. Clin Cancer Res. 2000;6:3516–21. [PubMed] [Google Scholar]
- 109.Wong IH, Zhang J, Lai PB, et al. Quantitative analysis of tumor-derived methylated p16INK4a sequences in plasma, serum, and blood cells of hepatocellular carcinoma patients. Clin Cancer Res. 2003;9:1047–52. [PubMed] [Google Scholar]
- 110.Yoon SK, Lim NK, Ha SA, et al. The human cervical cancer oncogene protein is a biomarker for human hepatocellular carcinoma. Cancer Res. 2004;64:5434–41. doi: 10.1158/0008-5472.CAN-03-3665. [DOI] [PubMed] [Google Scholar]
- 111.Hippo Y, Watanabe K, Watanabe A, et al. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res 2004;64:2418–23. doi: 10.1158/0008-5472.can-03-2191. [DOI] [PubMed] [Google Scholar]
- 112.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and photochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 113.Nakatsura T, Yoshitake Y, Senju S, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 114.Moriyama A, Tabaru A, Unoki H, et al. Plasma nitrite/nitrate concentrations as a tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2000;296:181–91. doi: 10.1016/s0009-8981(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 115.Hwang G-Y, Lin C-Y, Huang L-M, et al. Detection of the hepatitis B virus X protein (HBx) antigen and anti-HBx antibodies in cases of human hepatocellular carcinoma. J Clin Microbiol. 2003;41:5598–603. doi: 10.1128/JCM.41.12.5598-5603.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu M, London WT, Duan LX, Feitelson MA. The value of hepatitis B X antigen as a prognostic marker in the development of hepatocellular carcinoma. Int J Cancer. 1993;55:571–6. doi: 10.1002/ijc.2910550409. [DOI] [PubMed] [Google Scholar]
- 117.Poon TC, Johnson PJ. Proteome analysis and its impact on the discovery of serological tumor markers. Clin Chim Acta. 2001;313:231–9. doi: 10.1016/s0009-8981(01)00677-5. [DOI] [PubMed] [Google Scholar]
- 118.Shalhoub P, Kern S, Girard S, Beretta L. Proteomic-based approach for the identification of tumor markers associated with hepatocellular carcinoma. Dis Markers. 2001;17:217–23. doi: 10.1155/2001/210580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steel LF, Shumpert D, Trotter M, et al. A strategy for the comparative analysis of serum proteomes for the discovery of biomarkers for hepatocellular carcinoma. Proteomics. 2003;3:601–9. doi: 10.1002/pmic.200300399. [DOI] [PubMed] [Google Scholar]
- 120.Wang YY, Lo GH, Lai KH, et al. Increased serum concentrations of tumor necrosis factor-alpha are associated with disease progression and malnutrition in hepatocellular carcinoma. J Chin Med Assoc. 2003;66:593–8. [PubMed] [Google Scholar]
- 121.Lin ZY, Wang LY, Yu ML, et al. Role of serum C-reactive protein as a marker of hepatocellular carcinoma in patients with cirrhosis. J Gastroenterol Hepatol 2000;15:417–21. doi: 10.1046/j.1440-1746.2000.02149.x. [DOI] [PubMed] [Google Scholar]
- 122.Kwon OS, Lim do Y, Kwon KA, et al. Clinical usefulness of plasma activities of gelatinase (matrix metalloproteinase-2 and 9) in chronic liver disease Taehan Kan Hakhoe Chi 2003;9:222–30. [PubMed] [Google Scholar]
- 123.Tu DG, Wang ST, Chang TT, Chiu NT, Yao WJ. The value of serum tissue polypeptide specific antigen in the diagnosis of hepatocellular carcinoma. Cancer. 1999;85:1039–43. [PubMed] [Google Scholar]