Abstract
Viruses were characterized by their adsorption to DEAE-Sepharose or by their elution from octyl-Sepharose by using buffered solutions of sodium chloride with different ionic strengths. Viruses whose adsorption to DEAE-Sepharose was reduced most rapidly by an increase in the sodium chloride concentration were considered to have the weakest electrostatic interactions with the solids; these viruses included MS2, E1, and φX174. Viruses whose adsorption to DEAE-Sepharose was reduced least rapidly were considered to have the strongest electrostatic interactions with the column; these viruses included P1, T4, T2, and E5. All of the viruses studied adsorbed to octyl-Sepharose in the presence of 4 M NaCl. Viruses that were eluted most rapidly following a decrease in the concentration of NaCl were considered to have the weakest hydrophobic interactions with the column; these viruses included φX174, CB4, and E1. Viruses that were eluted least rapidly from the columns after the NaCl concentration was decreased were considered to have the strongest hydrophobic interactions with the column; these viruses included f2, MS2, and E5.
Since enteric viruses are of public health concern, studies on their adsorption to solids have been conducted for three main reasons: (i) to understand their fate during water and wastewater treatment, where adsorption may affect their removal from the water; (ii) to understand their migration through soil following accidental or deliberate application; and (iii) to develop adsorption-elution procedures for their recovery from natural environments.
Virus adsorption to solids has been examined in many studies, and these studies have been reviewed (2, 8, 9). In most cases, the adsorption of viruses with protein coats rather than the adsorption of viruses with lipid envelopes has been studied. The main reason for the use of viruses with protein exteriors for studies is the fact that many enteric viruses (rotaviruses, reoviruses, enteroviruses) are this type. Therefore, virus adsorption resembles the adsorption of proteins to solids. Since the side chains of amino acids may have charged or hydrophobic groups attached, electrostatic and hydrophobic interactions have been found to influence virus adsorption to solids (4, 7, 14, 18).
Although animal viruses are of concern because of their possible effects on human health, the adsorption of bacterial viruses (bacteriophages) has also been studied. These viruses may provide another method for detecting fecal pollution of water, soil or other natural environments and may serve as models for human viruses in tests of barrier materials (12, 15).
In some studies, the adsorption characteristics of different viruses to solids were found to be similar (6, 18). In other studies, different patterns of adsorption were observed. In an earlier study, we found that the elution patterns of two viruses that had similar sizes and shapes (poliovirus 1 and bacteriophage MS2) and were adsorbed to microporous filters were affected differently by the solutions used for elution (17). As shown in Table 1, a solution containing a detergent (Tween 80) was found to elute 80% of the adsorbed MS2 but only 12% of the adsorbed poliovirus. The adsorption of viruses to solids has been found to depend on the virus type in other studies (13, 14). Since the association of viruses with solids has been shown to depend on the virus type, we decided to study the adsorption of several animal and bacterial viruses to defined solids. We chose a solid with charged groups (DEAE-Sepharose) and a solid with hydrophobic groups (octyl-Sepharose). It was hoped that the results of this study would be useful for selecting viruses with different and contrasting adsorption characteristics for future studies.
TABLE 1.
Comparative elution profiles of poliovirus 1 and MS2 adsorbed to microporous filtersa
| Solution | % of viruses elutedb
|
|
|---|---|---|
| Poliovirus 1 | MS2 | |
| Buffer | 0 | 0 |
| Buffer + 1.0 M NaCl | 2 ± 1 | 1 ± 1 |
| Buffer + 0.1% Tween 80 | 12 ± 3 | 80 ± 11 |
| Buffer + 0.1% Tween 80 + 1.0 M NaCl | 96 ± 4 | 98 ± 4 |
Data modified from the data in reference 17.
Fifty milliliters of buffer (0.05 M potassium hydrogen phthalate, pH 4.0) was seeded with approximately 107 viruses and passed through Millipore HA filters in 25-mm holders at a rate of 1 ml/s. Next, 50-ml portions of the solutions were passed through the filters in the order shown. The cumulative number of viruses eluted was determined, and the results are expressed as percentages of the number of viruses adsorbed to the filters. The values are the means ± standard deviation for triplicate determinations.
MATERIALS AND METHODS
Viruses.
The animal viruses used and their sources are as follows: poliovirus 1 (LSc strain; ATCC VR-59), coxsackievirus B3 (Nancy; ATCC VR-30), coxsackievirus B4 (ATCC VR-184), coxsackievirus B5 (ATCC VR-689), echovirus 1 (ATCC VR-31), echovirus 4 (ATCC VR-34), echovirus 5 (ATCC VR-35), and echovirus 7 (Wallace; ATCC VR-36). The bacteriophages used and their hosts and sources are as follows: MS2 (ATCC 15597-B1), Escherichia coli C-3000 (ATCC 15597); f2, E. coli K-13 (Charles Gerba, University of Arizona); φX174 (ATCC 13706-B1), E. coli C (ATCC 13706); and T2 (ATCC 11303-B1), T3, T4, and T7 (Department of Microbiology & Cell Science Collection), E. coli B (ATCC 11303). Animal viruses were assayed by determining the number of PFU with BGM cells (19). Bacterial viruses were assayed by determining the number of PFU by a soft agar overlay procedure (20).
Adsorption studies.
DEAE-Sepharose CL-6B and octyl-Sepharose CL-4B were obtained from Pharmacia Fine Chemicals, Piscataway, N.J. Both Sepharose derivatives were washed at least 20 times with the solutions used for adsorbing viruses before use. The solutions were 10 mM imidazole-4 M NaCl (pH 7.0) for octyl-Sepharose and 1.0 mM imidazole (pH 7.0) for DEAE-Sepharose.
One-milliliter portions of rinsed DEAE-Sepharose were placed in 50-ml centrifuge tubes. Next, 3 ml of adsorbing solution with approximately 105 PFU of virus was added to each tube, and the tubes were placed on a rotary shaker for 2 h. After this adsorption period, the tubes were centrifuged for 10 min at 12,000 × g. A portion of each supernatant fraction was removed and placed in a 13-mm tube. The tubes were centrifuged for 5 min at high speed in a Whisperfuge (Fisher Scientific Co.) to remove any remaining Sepharose derivative. The numbers of viruses in the supernatant fractions were compared with the numbers in control solutions without DEAE-Sepharose to determine the percentage of virus adsorbed. For experiments with DEAE-Sepharose, virus adsorption in solutions containing increasing concentrations of NaCl was determined.
Hydrophobic interactions were studied by using octyl-Sepharose (16). After rinsing, 3 ml of octyl-Sepharose was poured into glass columns (0.7 by 10.0 cm; Bio-Rad, Rockville Center, N.Y.). Next, 5 ml of adsorbing solution (10 mM imidazole, 4 M NaCl; pH 7) containing approximately 105 PFU of virus was passed through the column at a rate of 2.5 ml/h. The column was rinsed with 2 bed volumes of adsorbing solution, and the void volume and rinse solutions were assayed to confirm virus adsorption. Virus elution was studied by passing 3 bed volumes of buffer solutions with decreasing amounts of NaCl through the columns. Finally, 3 bed volumes of a solution of buffer with 0.1% Tween 80 was passed through the columns. The cumulative amount of virus eluted as the concentration of NaCl in the rinse solutions was decreased was determined and compared to the amount initially adsorbed.
Analyses.
The percentages of virus adsorbed to DEAE-Sepharose were plotted against the ionic strengths of the solutions used for adsorption. The cumulative percentages of viruses eluted from octyl-Sepharose were plotted against the ionic strengths of the solutions used for rinsing the samples. The values presented below are the means and standard deviations for triplicate determinations.
Regression coefficients (slopes and intercepts and their standard deviations) were determined by using ProStat software (Poly Software International, Salt Lake City, Utah). The significance of differences between slopes of lines was determined by using a t test (21).
RESULTS
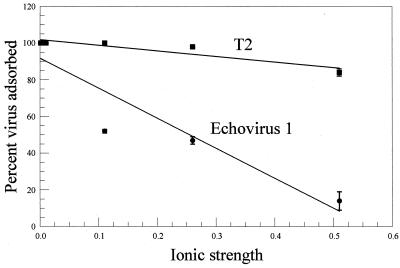
The adsorption of two viruses to DEAE-Sepharose as a function of ionic strength is shown in Fig. 1. The viruses were chosen to represent a virus whose adsorption decreased relatively rapidly as ionic strength increased (E1) and a virus whose adsorption was less affected by increases in ionic strength (T2). The slope of the best-fit straight line through the data points was calculated for these and other viruses, and the results are presented in Table 2. In most cases the correlation between the data points and a straight line was fair (r > 0.80). Three of the viruses (MS2, E1, and φX174) whose adsorption rapidly decreased as ionic strength increased were found to be in the group of viruses that Gerba and Goyal (10) found to weakly adsorb to soil. Also, three of the viruses (P1, T4, and T2) whose adsorption decreased relatively slowly as ionic strength increased were found to be in the group of viruses that strongly adsorbed to soil described by Gerba and Goyal (10).
FIG. 1.
Adsorption of T2 and echovirus 1 to DEAE-Sepharose as a function of the ionic strengths of solutions at pH 7.0.
TABLE 2.
Adsorption of viruses to DEAE-Sepharosea
| Virus | Slopeb | Correlation (r) | Adsorption groupc |
|---|---|---|---|
| MS2 | −170 ± 26 A | 0.88 | I |
| E1 | −160 ± 17 A | 0.87 | I |
| φX174 | −130 ± 26 AB | 0.71 | I |
| CB4 | −115 ± 22 B | 0.82 | |
| T3 | −110 ± 21 B | 0.81 | |
| T7 | −110 ± 21 B | 0.81 | |
| CB5 | −105 ± 15 B | 0.89 | |
| E4 | −100 ± 19 B | 0.82 | |
| f2 | −95 ± 15 BC | 0.87 | |
| P1 | −65 ± 20 C | 0.80 | II |
| T4 | −65 ± 10 C | 0.88 | II |
| T2 | −30 ± 4 D | 0.91 | II |
| E5 | −6 ± 8 E | 0.08 |
The amount of virus adsorbed as the ionic strength of the solution was increased was determined and plotted as a percentage of the number of viruses in the portion of the solution that was not mixed with DEAE-Sepharose.
The slope was determined by dividing the percentage of virus adsorbed by the ionic strength of the solution. The values are means ± standard deviations for triplicate determinations. Values followed by the same letter are not significantly different at a P value of >0.05. The weakest electrostatic interaction is represented by the slope of −170 ± 26 (MS2), and the strongest electrostatic interaction is represented by the slope of −6 ± 8 (E5).
Virus groups described by Gerba and Goyal (10).
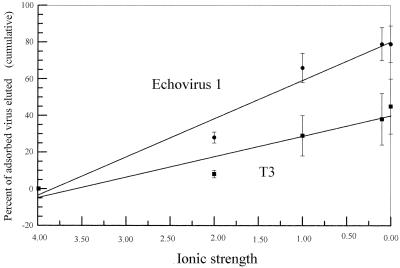
All of the viruses studied adsorbed to octyl-Sepharose in the presence of 4 M NaCl. The elution of two viruses from octyl-Sepharose in the presence of decreasing concentrations of salt is shown in Fig. 2. The viruses studied differed in the rate of elution of adsorbed virus and the maximum percentage eluted. The slopes of the best-fit straight lines through the data points for each virus were calculated and are shown in Table 3. The viruses studied differed in the rates that they were eluted from the octyl-Sepharose. Viruses found to be relatively weak or strong adsorbers by Gerba and Goyal (10) could not be placed in one category on the basis of their rates of elution from octyl-Sepharose. The viruses could be placed into two main groups that differed in terms of elution from octyl-Sepharose. The members of the first group, the first nine viruses listed in Table 3 (φX-174 through T3), were eluted in greater numbers as the ionic strength of the rinsing solution was decreased (average slope, −18.3 ± 4.4). The members of the second group, which consisted of three viruses (f2, E5, and MS2) (Table 3), were more likely to remain adsorbed as the ionic strength of the rinsing solution was decreased (average slope, −0.7 ± 0.5). The correlations between the data points and a straight line were relatively high for viruses in the first group (average r, 0.90 ± 0.04), but they were much lower for the second group (average r, 0.53 ± 0.18). The fact that the values for the viruses in the second group did not fit a straight line very likely reflects the fact that elution was observed only for the lowest concentrations of NaCl. Also, the percentage of the total viruses eluted by rinsing solutions consisting of buffer and salts and a solution of 0.1% Tween 80 was higher for the first group (80% ± 11%) than for the second group (40% ± 33%).
FIG. 2.
Elution of echovirus 1 and T3 adsorbed to octyl-Sepharose as a function of the ionic strengths of eluting solutions at pH 7.
TABLE 3.
Elution of viruses adsorbed to octyl-Sepharosea
| Virus | Slopeb | Correlation (r) | Adsorption groupc |
|---|---|---|---|
| φX174 | −25 ± 2.0 A | 0.96 | I |
| CB4 | −22 ± 2.8 AB | 0.90 | |
| E1 | −21 ± 1.7 AB | 0.96 | I |
| CB5 | −20 ± 2.5 B | 0.92 | |
| E4 | −19 ± 2.3 BC | 0.92 | |
| P1 | −18 ± 2.8 BC | 0.87 | II |
| CB3 | −16 ± 3.0 CD | 0.82 | |
| T7 | −13 ± 1.8 DE | 0.89 | |
| T3 | −11 ± 1.6 E | 0.89 | |
| f2 | −1.2 ± 0.33 F | 0.70 | |
| E5 | −0.64 ± 0.28 GH | 0.54 | II |
| MS2 | −0.26 ± 0.19 H | 0.35 | I |
The cumulative amount of virus eluted as the ionic strength of the solutions used for elution was reduced was measured and compared with the amount initially adsorbed.
The slope was determined by dividing the cumulative percentage of virus eluted by the ionic strength of the solution used for elution. The values are means ± standard deviations for triplicate determinations. Values followed by the same letter are not significantly different at a P value of >0.5. The weakest hydrophobic interaction is represented by the slope of −25 ± 2.0 (φX174), and the strongest hydrophobic interaction is represented by the slope of −0.26 ± 0.19 (MS2).
Virus groups described by Gerba and Goyal (10).
DISCUSSION
The viruses examined in this study showed significant differences in adsorption to DEAE-Sepharose and octyl-Sepharose. Based on the rates at which the adsorption to and elution from Sepharose derivatives were changed by changes in the ionic strengths of solutions, the viruses could be ranked on the basis of the strengths of their electrostatic and hydrophobic interactions. For different reasons, it was not possible to use all viruses in both sets of experiments. For example, high concentrations of NaCl greatly reduced the titers of T2 phage (possibly by aggregating the viruses) so the interaction of this virus with octyl-Sepharose could not be studied.
Increasing the ionic strength of the solution by increasing the concentration of NaCl decreased the adsorption of viruses to DEAE-Sepharose. The interpretation of this finding is that the effect of electrostatic interactions was decreased by shielding of the charges on the virus and the solid by the charged ions (3). In previous studies, we and others have shown that salts can interfere with virus adsorption to certain solids (13, 18).
In general, it has been shown that certain salts can influence hydrophobic interactions (11). In particular, the influence of salts on virus adsorption to certain solids has been shown to be related to the influence of the salts on hydrophobic interactions (7). In this study, all of the viruses adsorbed to octyl-Sepharose in the presence of 4 M NaCl. As the concentration of NaCl was decreased, the different viruses studied were eluted at different rates.
The ranking of the viruses on the basis of the relative strengths of their electrostatic interactions showed some similarity to the grouping of viruses on the basis of their adsorption to soil described by Gerba and Goyal (10). Three of the viruses that were placed in the group of relatively strongly adsorbing viruses in the study of Gerba and Goyal (10) were found to be among the viruses with relatively strong electrostatic interactions in this study. Similarly, three of the viruses that were placed in the relatively weakly adsorbing group by Gerba and Goyal (10) were among the viruses that had the weakest electrostatic interactions in this study. No such correlations were found with the viruses ranked on the basis of the strengths of their hydrophobic interactions.
The ranking of viruses on the basis of the strengths of their hydrophobic interactions has similarities to the ranking of Lytle and Routson (14). These authors found the following order for strength of hydrophobic interactions based on elution of viruses adsorbed to nitrocellulose filters using serum: φX174 ≃ T7 < MS2. In this study, we found the order for strength of hydrophobic interactions to be φX174 < T7 < MS2.
Bales et al. (1) found that hydrophobic interactions were important in the attachment of MS2 to solids. These authors suggested that hydrophobic interactions might be orders of magnitude more important than electrostatic interactions for adsorption of MS2. Our finding that MS2 had the strongest hydrophobic and weakest electrostatic interactions of the viruses tested is consistent with their findings.
Virus adsorption to soils was found to be influenced by the isoelectric points of the viruses by Dowd et al. (5). Isoelectric points were available for only a few of the viruses used in this study so no correlations between virus adsorption and isoelectric point could be made.
It may not be possible to study hydrophobic and electrostatic interactions separately since solids and viruses are capable of both types of interactions. However, we believe that the DEAE-Sepharose adsorbed viruses mainly by electrostatic interactions and that the octyl-Sepharose adsorbed them mainly by hydrophobic interactions. Using these Sepharose derivatives, we can rank viruses based on the relative strengths of their electrostatic and hydrophobic interactions. Our rankings show some similarities to the rankings found in other studies and may be useful for selecting viruses for future adsorption studies.
Acknowledgments
We acknowledge the financial contributions of the Engineering Research Center for Particle Science and Technology at the University of Florida, National Science Foundation grant EEC-94-02989, the industrial partners of the Engineering Research Center, and U.S. Environmental Protection Agency grant R810126-01-0.
Footnotes
Florida Agricultural Experiment Station Journal Series no. R-08863.
REFERENCES
- 1.Bales, R. C., S. Li, K. M. Maguire, M. T. Yahya, and C. P. Gerba. 1993. MS-2 and poliovirus transport in porous media: hydrophobic effects and chemical perturbations. Water Resour. Res. 29:957-963. [Google Scholar]
- 2.Bitton, G. 1975. Adsorption of viruses onto surfaces in soil and water. Water Res. 9:473-484. [Google Scholar]
- 3.Cantor, C. R., and P. R. Schimmel. 1980. Biophysical chemistry, p. 676-678. W. H. Freeman & Co., San Francisco, Calif.
- 4.Dizer, H., A. Nasser, and J. M. Lopez. 1984. Penetration of different human pathogenic viruses into sand columns percolated with distilled water, groundwater, or wastewater. Appl. Environ. Microbiol. 47:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowd, S. E., S. D. Pillai, S. Wang, and M. Y. Corapcioglu. 1998. Delineating the specific influence of virus isoelectric point and size on virus adsorption and transport through sandy soils. Appl. Environ. Microbiol. 64:405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrah, S. R., D. R. Preston, G. A. Toranzos, M. Giard, G. A. Erdos, and V. Vasuhdivan. 1991. Use of modified diatomaceous earth for removal and recovery of viruses in water. Appl. Environ. Microbiol. 57:2502-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrah, S. R., D. O. Shah, and L. O. Ingram. 1981. Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc. Natl. Acad. Sci. USA 78:1229-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuhs, G. W. 1987. Mechanisms of adsorption and elution of viruses to solids in the natural environment, p. 139-178. In G. Berg (ed.), Methods for recovering viruses from the environment. CRC Press, Boca Raton, Fla.
- 9.Gerba, C. P. 1984. Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 30:133-168. [DOI] [PubMed] [Google Scholar]
- 10.Gerba, C. P., and S. M. Goyal. 1981. Quantitative assessment of the adsorptive behavior of viruses to soils. Environ. Sci. Technol. 15:940-944. [DOI] [PubMed] [Google Scholar]
- 11.Hatefi, Y., and W. G. Hanstein. 1969. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc. Natl. Acad. Sci. USA 62:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havelaar, A. H., M. Butler, S. R. Farrah, J. Joffre, E. Marques, A. Ketratanakul, M. T. Martins, S. Ohgaki, M. D. Sobsey, and U. Zaiss. 1991. Bacteriophages as model viruses in water quality control. Water Res. 25:529-545. [Google Scholar]
- 13.Lukasik, J., T. M. Scott, D. Andryshak, and S. R. Farrah. 2000. Influence of salts on virus adsorption to microporous filters. Appl. Environ. Microbiol. 66:2914-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lytle, C. D., and L. B. Routson. 1995. Minimized virus binding for tests of barrier materials. Appl. Environ. Microbiol. 61:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lytle, C. D., W. Truscott, A. P. Budacz, L. Venegas, L. B. Routson, and W. H. Cyr. 1991. Important factors for testing barrier materials with surrogate viruses. Appl. Environ. Microbiol. 57:2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Påhlman, S., J. Rosengren, and S. Hjertén. 1977. Hydrophobic interaction chromatography on uncharged Sepharose derivatives. J. Chromatogr. 131:99-108. [DOI] [PubMed] [Google Scholar]
- 17.Shields, P. A. 1982. Contributions of electrostatic and hydrophobic interactions in virus-filter associations. M.S. thesis. The University of Florida, Gainesville.
- 18.Shields, P. A., and S. R. Farrah. 1983. Influence of salts on electrostatic interactions between poliovirus and membrane filters. Appl. Environ. Microbiol. 45:526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, E. M., and C. P. Gerba. 1982. Laboratory methods for the growth and detection of animal viruses, p. 15-47. In C. P. Gerba and S. M. Goyal (ed.), Methods in environmental virology. Marcel Dekker, Inc., New York, N.Y.
- 20.Snustad, S. A., and D. S. Dean. 1971. Genetic experiments with bacterial viruses, p. 1-5. W. H. Freeman & Co., San Francisco, Calif.
- 21.Sokal, R. R., and F. J. Rohlf. 1969. Biometry, p. 448-458. W. H. Freeman & Co., San Francisco, Calif.