Abstract
Background
A subset of COVID-19 patients develops post-COVID-19 condition (PCC). This condition results in disability in numerous areas of patients’ lives and a reduced health-related quality of life, with societal impact including work absences and increased healthcare utilization. There is a scarcity of models predicting PCC, especially those considering the severity of the initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and incorporating long-term follow-up data. Therefore, we developed and internally validated a prediction model for PCC 2 years after SARS-CoV-2 infection in a cohort of COVID-19 patients.
Methods
Data from the CORona Follow-Up (CORFU) study were used. This research initiative integrated data from multiple Dutch COVID-19 cohort studies. We utilized 2-year follow-up data collected via the questionnaires between October 1st of 2021 and December 31st of 2022. Participants were former COVID-19 patients, approximately 2-year post-SARS-CoV-2 infection. Candidate predictors were selected based on literature and availability across cohorts. The outcome of interest was the prevalence of PCC at 2 years after the initial infection. Logistic regression with backward stepwise elimination identified significant predictors such as sex, BMI and initial disease severity. The model was internally validated using bootstrapping. Model performance was quantified as model fit, discrimination and calibration.
Results
In total 904 former COVID-19 patients were included in the analysis. The cohort included 146 (16.2%) non-hospitalized patients, 511 (56.5%) ward admitted patients, and 247 (27.3%) intensive care unit (ICU) admitted patients. Of all participants, 551 (61.0%) participants suffered from PCC. We included 20 candidate predictors in the multivariable analysis. The final model, after backward elimination, identified sex, body mass index (BMI), ward admission, ICU admission, and comorbidities such as arrhythmia, asthma, angina pectoris, previous stroke, hernia, osteoarthritis, and rheumatoid arthritis as predictors of post-COVID-19 condition. Nagelkerke’s R-squared value for the model was 0.19. The optimism-adjusted AUC was 71.2%, and calibration was good across predicted probabilities.
Conclusions
This internally validated prediction model demonstrated moderate discriminative ability to predict PCC 2 years after COVID-19 based on sex, BMI, initial disease severity, and a collection of comorbidities.
Keywords: Post-COVID-19 condition, Long COVID, Post-acute sequelae of COVID-19, Clinical prediction model, Prognostic factors
Introduction
COVID-19, currently having transitioned from a pandemic to an endemic phase, is likely to have a continued disease burden on society [1]. A subset of patients that survived their illness experience a continuation or the development of new symptoms after the acute phase of their severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has ended. This is referred to as the post-COVID-19 condition (PCC) [2–4]. The exact number of patients experiencing and living with PCC is unclear. In our previous study, we found that the prevalence of PCC 2 years after acute COVID-19 disease ranged between 42.5 and 65.0% depending on the severity of the disease during the acute phase [5]. Patients can experience many different symptoms, affecting various organ systems [2]. PCC can result in a reduced quality of life and be the cause of major disability in many aspects of patients’ daily lives. Patients report cognitive dysfunction, including a below average attention span, fatigue, trouble with executive functioning, and challenges with problem solving. This in turn affects among many other domains in their lives, their ability to work, and their social lives [6–8]. At 2 to 3 months after hospital discharge, rates of absence are between 9 and 40% of working time [7]. Among patients who were not hospitalized, 12–23% of former COVID-19 patients are reported to be absent from work between 3 to 7 months after the acute phase [7]. Besides work absence, PCC patients are also reported to work less hours 2 years after their infection. Only 27% of people were able to work the same number of hours as before their illness [6, 9]. Another effect is a higher healthcare utilization among PCC patients, leading to an increase in healthcare costs for either society, or patients, depending on insurance coverage [10–12].
Early identification of patients who are at increased risk of developing PCC could justify changes in disease management during the acute phase. Evidence suggests that certain treatments and interventions employed during, and right after the acute phase, may reduce the risk of developing PCC [13–16]. A recent study has identified risk factors for PCC in COVID-19 patients. The most important risk factors associated with PCC include hospitalization during SARS-CoV-2 infection, a high body mass index (BMI), sex, and the presence of comorbidities [17].
Several prediction models have been developed for diagnosing COVID-19, prediction of mortality risk, progression to severe disease, admission to the intensive care unit (ICU), intubation, and length of hospital stay [18]. However, prediction models for PCC are scarce, and the models that are available ignore the severity of the acute COVID-19 disease as a predictor and have not yet incorporated long term follow-up data available at this time [19–21]. In this current study, we aimed to develop and internally validate a prediction model for PCC 2 years after SARS-CoV-2 infection in a cohort of non-hospitalized, ward admitted, and ICU admitted COVID-19 patients.
Methods
Study
This current study is part of the CORona Follow-Up (CORFU) research initiative, in which multiple previously established COVID-19 retrospective cohort studies were included. The protocol for CORFU has previously been described elsewhere [22]. In summary, CORFU integrated data of seven Dutch COVID-19 cohorts. Data from the following cohorts were combined: the Maastricht Intensive Care COVID (MaastrICCht) cohort [23, 24], the Bernhoven early detection of vascular damage after COVID-19 cohort (COVAS) cohort [25], the ZuydErLand COVID-19 regiStry (ELVIS) cohort [26] and the Cardiac complications in patients with COVID-19 (CAPACITY-COVID) cohort [27], and the community-based POPulation health impact of the COVID-19 pandemic (POPCORN) cohort [28]. The latter cohort predominantly consisted of participants without a known SARS-CoV-2 infection and were subsequently regarded as non-COVID controls. POPCORN participants who reported to have suffered from (mild) COVID-19 were counted as cases. The different cohorts collected data from five academic hospitals, 18 teaching hospitals, 13 non-teaching hospitals, and from non-hospitalized participants.
The cohort-specific follow-up measurements were complemented by repeatedly administered CORFU patient questionnaires from 3 months to 2 years after SARS-CoV-2 infection. The questionnaires covered a large array of symptoms related to the PCC, health-related quality of life effects, and their key determinants. In the present study, the 2-year follow-up data were used. The CORFU questionnaire was developed in collaboration with the POPCOrn research group [28]. The study was registered at ClinicalTrials.gov (NCT05240742) and ethical approval was obtained from the medical research ethics committee azM/UM (METC2021-2990) and from the local committees of the participating cohorts. Participants provided informed consent prior to self-administering the CORFU questionnaire. Data was collected between 1 October 2021 and 31 December 2022. This manuscript has been reported according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Statement [29].
Participants
The study population consists of former COVID-19 patients who had been included in one of the CORFU cohort studies, and who were approximately (between 21 and 27 months) 2 years after their SARS-CoV-2 infection during the period of questionnaire data collection. Patients were divided into distinct groups based on severity of COVID-19 disease: non-hospitalized, ward admitted, or ICU admitted. All participants were aged 18 years or above. Patients had a confirmed SARS-CoV-2 infection or were included as suspected cases based on clinical presentation due to testing limitations. Patients were also required to have sufficient understanding of the Dutch language to complete the CORFU questionnaire. No additional exclusion criteria were used.
Candidate predictors
We only selected candidate predictors that could be easily obtained outside a hospital setting to improve usability of the model both regardless of setting. The predictors were selected based on expertise, literature, our own previous work [5], and availability in the contributing cohorts, regardless of the setting (non-hospitalized, ward admitted, or ICU admitted). Patients were classified as ICU admitted if they were admitted to the ICU at any point during their hospital stay. Those classified as ward admitted were never admitted to the ICU. In this way, the setting was used as a proxy for initial disease severity and added to the prediction model. We did not stratify on this variable as the aim was to develop a single model applicable to all former COVID-19 patients. The self-reported candidate predictors were admitted to the ward, admitted to the ICU, sex, age, BMI (continuous variable), education level (based on the Dutch educational system, but converted using the International Standard Classification of Education [ISCED] into basic, intermediate and advanced) [30], severity of acute COVID-19 disease based on these three groups, and the presence and number of comorbidities prior to the initial infection. The following self-reported comorbidities were considered for the analysis: asthma, chronic bronchitis, lung emphysema, diabetes, angina pectoris, heart failure, previous stroke, hernia or other severe back problems, osteoarthritis, prior knee or hip replacement, chronic rheumatoid arthritis, and prior or current malignancy.
Outcome
The following post-COVID symptoms were measured: fatigue, headache, dizziness, muscle weakness or pain, coughing, dyspnea, pain when breathing, angina pectoris, palpitations, cognitive problems, loss of smell or taste, sleep problems, loss of appetite, and swollen ankles or feet.
A participant was defined as having PCC when they reported at least one post-COVID symptom that was either new or that worsened post-infection with a severity level reported as three or higher on the five-point Likert scale ranging from “no” to “extreme.” In case a participant had reported that a symptom pre-existed and had not worsened, that symptom was not counted toward having PCC.
Statistical analysis
The sample size was determined pragmatically; with all cohort participants that responded with a completed CORFU-questionnaire two years after SARS-CoV-2 infection. The number of patients who experienced the event of interest (that is any PCC symptom) and those who did not was used to ascertain the number of candidate predictors that could be included in the multivariable analysis, according to the method described by Riley et al. [31]. In case of this study, we allowed a maximum shrinkage of coefficients (i.e., a measure of overfitting the model to the data) of 0.9 and assumed a minimal c-statistic (i.e., a measure of discriminative ability) of 0.75 as in previously developed models without COVID-19 severity.
Baseline characteristics of the combined cohorts were described as mean and standard deviation (SD) or median and first and third quartile, or as count and percentage, depending on the distribution of the variable. Missing data were imputed using stochastic regression imputation with fully conditional specification and based on baseline characteristics and the outcome.
First, all candidate predictors were entered into a logistic regression model. Using backward stepwise elimination with a more liberal p value of 0.10, candidate predictors that did not contribute sufficiently to the model were omitted. Results are expressed as odds ratio (OR) including 95% confidence interval (CI). Subsequently, the model was internally validated using bootstrap resampling. The number of bootstraps was set to 1000. The bootstrap internal validation was used to estimate a shrinkage factor (i.e., a constant between 0 and 1) to account for overfitting, and to compute measures of optimism (i.e., the expected degree to which future performance will be lower compared to apparent performance). The shrinkage factor was used to multiply regression coefficients, and the model intercept was subsequently re-estimated. Together, these can be used to compute an individual’s probability of having at least one PCC symptom 2 years after SARS-CoV-2 infection.
Performance was evaluated as model fit, discriminative ability, and calibration. We quantified model fit as Nagelkerke’s R-squared. The area under the receiver operating characteristic (ROC) curve was computed to assess the ability of the model to discriminate between those who will develop PCC or not. Prediction model calibration was assessed by visual inspection of the calibration plot. For both model fit and discriminative ability, estimates adjusted for optimism, resulting from the internal validation step, were reported as well.
All analyses were performed using R version 4.3.2 (The R Foundation for Statistical Computing, Vienna University of Economics and Business, Vienna, Austria).
Results
In total, 904 former COVID-19 patients were included, of whom 146 (16.2%) were non-hospitalized, 511 (56.5%) were ward admitted, and 247 (27.3%) were ICU admitted (Table 1). Before data imputation, 1.3% of values were missing for education, up to 11.4% for some of the comorbidity questions, and 21.7% for BMI. About one in three participants (33.4%) had at least one missing value on any of the candidate predictors.
Table 1.
Baseline characteristics of included former COVID-19 patients
| Total cohort N = 904 |
|
|---|---|
| Sex (male) | 573 (63.4%) |
| Age (year) | 62.9 (11.6) |
| BMI (kg/m2) | 28.1 (5.0) |
| Education, n (%) | |
| Basic | 225 (25.2%) |
| Intermediate | 398 (44.6%) |
| Advanced | 269 (30.2%) |
| Living situation, n (%) | |
| Alone | 162 (18.0%) |
| Alone, but with children | 38 (4.2%) |
| With partner | 534 (59.3%) |
| With partner and children | 149 (16.5%) |
| With parents | 2 (0.2%) |
| Other | 16 (1.8%) |
| Comorbidities, n (%) | |
| None | 372 (41.2%) |
| Arrhythmia/palpitations | 134 (14.8%) |
| Asthma | 102 (12.0%) |
| Chronic bronchitis | 52 (6.5%) |
| DM type 1 or 2 | 125 (13.8%) |
| Lung emphysema | 27 (3.4%) |
| Angina pectoris | 44 (5.5%) |
| Heart failure | 33 (3.7%) |
| Prior stroke or CVA | 46 (5.4%) |
| Hernia or severe back pain | 112 (14.0%) |
| Osteoarthritis | 116 (14.5%) |
| Prior knee or hip replacement | 51 (6.4%) |
| Chronic rheumatoid arthritis | 41 (5.1%) |
| Prior or current malignancy | 51 (5.6%) |
Data are presented as mean (standard deviation) or count (percentage). BMI Body-mass index, DM Diabetes mellitus, CVA Cerebrovascular accident
Based on the prevalence of post-COVID symptoms that were either not pre-existing or were pre-existing but had worsened since the SARS-CoV-2 infection (Table 2), we determined that 551 (61.0%) of all participants met the definition for having PCC. Of those who were classified as such, the median number of complaints was 2 [IQR 1, 4]. Based on the event-rate, approximately 20 candidate predictors could be included in the multivariable analysis, as described in the sample size computation. Lung emphysema and heart failure were excluded from the analyses, as these comorbidities were the least prevalent (both less than 4%).
Table 2.
Prevalence of post COVID-related symptoms
| Symptoma | Total cohort (N = 904) |
|---|---|
| Fatigue | 268 (31.3%) |
| Headacheb | 33 (11.9%) |
| Dizziness | 81 (9.0%) |
| Muscle weakness/pain | 211 (24.6%) |
| Coughing | 92 (10.3%) |
| Shortness of breath | 164 (18.6%) |
| Pain when breathing | 14 (1.6%) |
| Chest pain | 23 (2.6%) |
| Heart palpitations | 44 (4.9%) |
| Cognitive problems | 161 (17.9%) |
| Loss of smell or taste | 96 (10.6%) |
| Problems with sleep | 194 (21.5%) |
| Loss of appetite | 47 (5.2%) |
| Swollen ankles or feet | 109 (12.2%) |
aSymptoms that were known to be pre-existing and hadnot worsened were disregarded
bHeadache was not available in all questionnaires and hence, denominators may differ from those of other symptoms
After backward elimination, the following 12 predictors for developing PCC 2 years after a SARS-CoV-2 infection remained: sex, BMI, admittance to the ward, admittance to the ICU, and the following comorbidities: arrhythmia/palpitations, chronic bronchitis, asthma, angina pectoris, previous stroke or CVA, hernia or other severe back problems, osteoarthritis, and chronic rheumatoid arthritis. Table 3 shows the prediction model parameters. Nagelkerke’s R-squared was 0.19. Figures 1 and 2 show the ROC curve and the calibration plot, respectively. The apparent AUC was 72.7% (95% CI: 69.4–76.0%). The calibration plot shows good calibration over the whole range of predicted probabilities.
Table 3.
Predictors of at least one post COVID symptom 2 years after infection
| Predictor | Odds ratio (95% CI) |
Shrunk regression coefficienta |
|---|---|---|
| Intercept | Na | − 1.789 |
| Sex (Female) | 2.31 (1.68–3.19) | 0.753 |
| BMI (kg/m2) | 1.03 (1.00–1.06) | 0.025 |
| Admitted to ward | 2.88 (1.91–4.37) | 0.951 |
| Admitted to ICU | 3.24 (2.04–5.20) | 1.058 |
| Arrhythmia/palpitations | 1.47 (0.96–2.28) | 0.348 |
| Chronic bronchitis | 2.03 (1.04–4.18) | 0.639 |
| Asthma | 2.12 (1.27–3.63) | 0.675 |
| Angina pectoris | 2.54 (1.27–5.44) | 0.838 |
| Prior stroke or CVA | 1.96 (0.99–4.05) | 0.604 |
| Hernia or severe back pain | 2.75 (1.71–4.57) | 0.911 |
| Osteoarthritis | 2.43 (1.50–4.05) | 0.798 |
| Chronic rheumatoid arthritis | 2.27 (1.05–5.47) | 0.737 |
ICU intensive care unit, CI confidence interval, DM diabetes mellitus, CVA cerebrovascular accident
aThe prediction model intercept was subsequently re-estimated
Fig. 1.
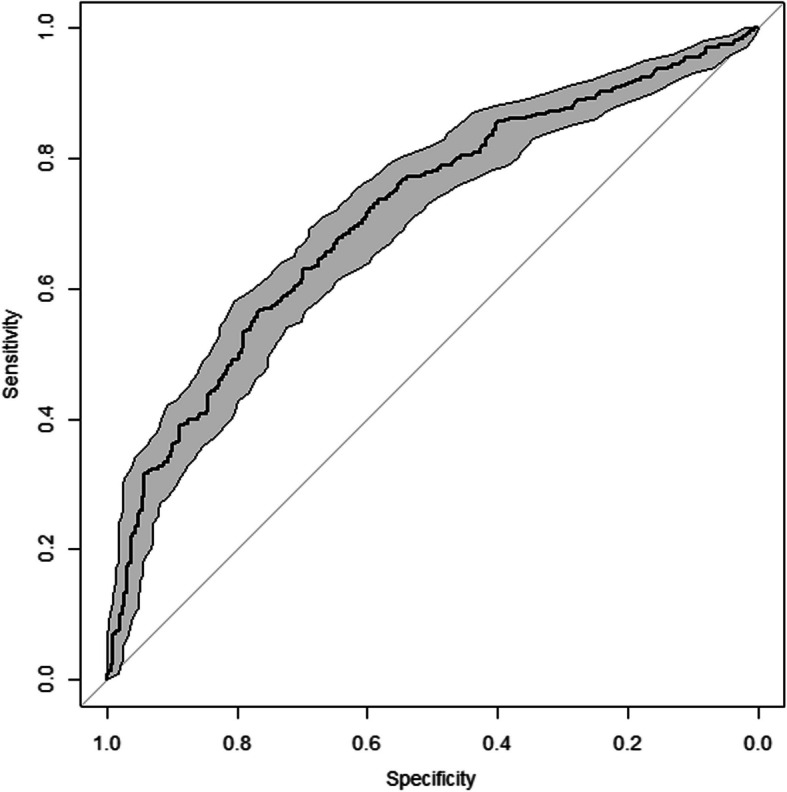
Receiver operating characteristic curve of the prediction model, including 95% confidence band
Fig. 2.
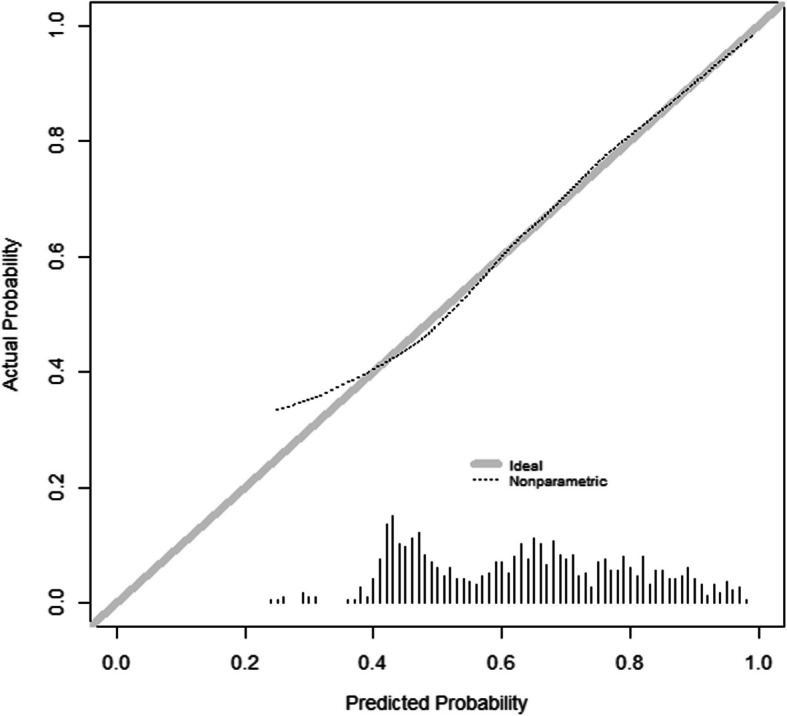
Calibration plot of the prediction model with a histogram of predicted probabilities on the x-axis
The internal validation step yielded a shrinkage factor of 0.90, which was subsequently used to penalize regression coefficients (see Table 3 for further details). The optimism-adjusted R-squared resulting from internal validation was 0.16. The estimated optimism in the AUC was 1.5% and hence, the expected AUC in future COVID-19 patients is 71.2%. The probability of PCC can be computed based on the internally validated model as:
Linear predictor (LP) = − 1.789 + 0.753*Female = 1 + 0.025*BMI + 0.951*Ward = 1 + 1.058*ICU = 1 + 0.348*Arrhythmia/palpitations = 1 + 0.639*Chronic bronchitis = 1 + 0.675*Asthma = 1 + 0.838*Angina pectoris = 1 + 0.604*Prior stroke or CVA = 1 + 0.911*Hernia or severe back pain = 1 + 0.798*Osteoarthritis = 1 + 0.737*Chronic rheumatoid arthritis = 1.
Probability of PCC = 1/(1 + exponent(-LP)).
Discussion
We developed a prediction model to predict PCC, based on the following 12 variables: sex, BMI, ward- or ICU admitted, previous arrhythmia/palpitations, chronic bronchitis, asthma, previous angina pectoris, previous stroke, hernia or other severe back problems, osteoarthritis, and chronic rheumatoid arthritis. The model’s performance can be considered moderate, with an estimated AUC of approximately 70% for future patients.
Clinicians can use this model to identify patients at high risk of PCC, regardless of setting. When the model identifies a patient as being high-risk during the acute phase, antivirals could be considered. It should be noted however, that evidence regarding the effectiveness of administering antivirals during the acute phase for the reduction of PCC, is conflicting [32]. Early analyses of recently developed antivirals show promising results; however, no definitive treatment strategy has been put in place [33–37]. After a patient recovers, the importance of reducing the risk of re-infection needs to be emphasized. Re-infections create a cumulative risk increase for developing PCC [38]. One way for patients to avoid re-infection is by getting (booster)vaccinations [15, 16]. These are effective in reducing the chances of developing PCC, even if administered after the first infection [15]. Lastly, clinicians should familiarize themselves with healthcare services in their areas for patients that end up developing PCC, despite efforts to prevent it [37].
The 61.0% prevalence of PCC at two years post infection in our cohort was relatively high compared to other studies. One meta-analysis pooling data of 16 studies shows that more than 12 months after the initial infection, the pooled prevalence was 41% [39]. However, one of the studies included in this meta-analysis reported a PCC prevalence of 71% at 24 months [40]. Another meta-analysis provides a pooled prevalence at 24 months of 28% [41]. Furthermore, a study analyzing both pre- and post-COVID symptoms, found that after accounting for pre-pandemic symptoms, 13% of participants with COVID-19 developed symptoms attributable to COVID-19, therefore qualifying them as having PCC [42]. The wide range of reported prevalence rates shows that comparing the prevalence of PCC in our study to other studies is difficult. This is most likely due to a large heterogeneity in definitions, follow-up times, and disease severities across studied populations.
The performance of our prediction model was inferior to that of the model proposed by Antony et al. (2023), with AUCs of 75 and 76% depending on the type of statistical model employed [21]. This discrepancy may be attributed to the inclusion of a vast initial set of 86 candidate predictors, some of which pertain to domains that are unavailable within our cohort (e.g., medication- each single medication as a predictor either during or after acute infection). It should be noted, however, that the definition of PCC used in this study differs from the definition used in our study. They used the ICD-10 coding to define the presence of PCC. This resulted in a PCC prevalence of only 0.3%, which is markedly lower than the prevalence found in literature. This is most likely explained by many institutions not having adopted the use of the ICD-10 code yet. Another difference is that 94% of these participants were non-hospitalized, which was significantly more than the 16% in our cohort.
Comparing our study to Deforth et al. (2022), they find a much lower PCC prevalence of approximately 22% at a median follow-up time of 162 days and have a better model performance [19]. However, also in this study, another definition was used for PCC, using only three symptoms (reduced exercise tolerance/reduced resilience, shortness of breath, and tiredness). Another difference was that 80% of their cohort consisted of non-hospitalized participants, while this was 16% in our cohort. This most likely explains their much lower prevalence of PCC. Yet another difference was their use of 12 candidate predictors compared to our 20. Some candidate predictors were similar, like disease severity, sex, and BMI. Differences were their inclusion of the number of acute COVID-19 symptoms, and gender related factors. The model by Sudre et al. (2021) reported a prevalence of PCC of 13.3% and an average AUC at 76.8%. However, the cut-off time for PCC was 28 days or longer, which is much shorter than the two years used in our study [20]. It is also unclear what proportion of their population was hospitalized, since only information was presented on hospital visits, not hospital admission. Their model consisted only of age, sex, and the number of symptoms experienced during the first week.
Yet another study by Cervia et al. (2022) reports a PCC prevalence between 54 and 82% based on severity [43]. The hospitalization rate in their derivation cohort was 60% compared to our 84%, this most likely explains why their PCC prevalence is higher than the other studies discussed here and closer to our PCC prevalence. Their prediction model has a reported AUC of 77%. It consists of symptoms during primary SARS-CoV-2 infection and laboratory measurements as predictors in the model. Their analysis, however, was also performed with a cut-off value of 28 days for PCC. We hypothesize that the discriminatory ability of our model could be better when adding certain laboratory values to the candidate predictors, but this information is not available for a large proportion of our participants.
A major strength of this study was the inclusion of a diverse group of formerly COVID-19 patients. We included individuals who, because of mild disease, were non-hospitalized, while we also included participants that were admitted to the ICU due to severe disease, and in many cases received prolonged mechanical ventilation. The combination of multiple cohorts enhances the generalizability of the model beyond the Netherlands, hospital type (i.e., academic university hospitals and secondary care hospitals) and disease severity (non-hospitalized, ward admitted, or ICU admitted). Another strength of this study is the clear timeframe in which it was conducted. All patients in our cohort had an approximate follow-up time of 2 years after initial SARS-CoV-2 infection. To the best of our knowledge, this is also the only model that predicts PCC 2 years after SARS-CoV-2 infection. Lastly, despite our model being slightly less accurate than some of the other previously published models, it is easier to use. The variables necessary to predict PCC can easily be obtained by clinicians simply taking a patient’s history. There is no need for any diagnostics to be or to have been performed.
Our study also has some limitations. For one, participants who had been hospitalized (ward admitted or ICU admitted) due to COVID-19 were overrepresented in our sample, which could have resulted in an elevated prevalence of PCC. However, the severity of the acute phase of infection was included in our prediction model as a predictor variable (with categorical variables for ward-admitted or ICU admitted). Consequently, future predictions for patients who were not hospitalized are unlikely to be overestimated. In other words, if all predictors remain the same, the predicted probability of PCC will be lower. Another limitation of the study is that the outcome was based on the prevalence of post-COVID related symptoms, which are not specific to PCC. Some participants may have developed new symptoms or experienced a worsening of pre-existing ones due to causes unrelated to COVID-19. There is currently no definitive way to fully differentiate post-COVID symptoms from those caused by other conditions. Finally, the inclusion of only participants that completed the questionnaire may have introduced some self-selection bias, potentially affecting the generalizability of our results.
In conclusion, our internally validated prediction model demonstrated moderate discriminative ability in predicting PCC 2 years after SARS-CoV-2 infection and showed very good calibration over the whole range of predictions. The prediction model was based on sex, BMI, initial disease severity and a collection of comorbidities.
Acknowledgements
Not applicable.
Author Contribution
GJB, EB, CGD, BH, BCTB, FWA, BLJHK, JH, SMJK conceived and designed the study. DOK, NW, SFW, and SMJK drafted the manuscript. DOK, NW, SFW, GJB, EB, MSJNW, LMCJ, BH, JAV, BCTB, SS, BLJHK, GJ, FWA, ML, JAH , and SMJK critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by The Netherlands Organization for Health Research and Development (ZonMw), grant number 10430302110005, and an unrestricted grant that was received from the EuroQol Research Foundation. Individual COVID-19 cohorts contributing to the CORFU study may have been funded independently from this grant. The CAPACITY-COVID registry is supported by the Dutch Heart Foundation (2020B006 CAPACITY), and The Netherlands Organization for Health Research and Development (ZonMw grant number 10430102110006 DEFENCE), the EuroQol Research Foundation, Novartis Global, Sanofi Genzyme Europe, Novo Nordisk Nederland, Servier Nederland, and Daiichi Sankyo Nederland. Permission was obtained from the EuroQol Research Foundation to use their questionnaires and the bolt-on questions on post-COVID condition. The according permission to modify agreement number was 163467.
National Institute of Health Research University College London Hospitals Biomedical Research Centre,Dutch Heart Foundation,2020B006 CAPACITY,2020B006 CAPACITY,The Netherlands Organization for Health Research and Development (ZonMW),10430102110006 DEFENCE,10430102110006 DEFENCE
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. All R code used for the statistical analyses will be made publicly available via a GitHub repository, after all CORFU projects are finalised.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT05240742) and ethical approval was obtained from the medical research ethics committee azM/UM (METC2021-2990) and from the local committees of the participating cohorts. Participants provided informed consent prior to self-administering the CORFU questionnaire.
Consent for publication
Not applicable.
Competing interests
DOK, NW, SFW, GJB, EB, MSJNW, SCMH, EBNJJ, CGD, MCW, LMCJ, BH, JAV, BCTB, SS, BLJHK, GJ, and JAH declare no competing interests. FWA is supported by the National Institute of Health Research University College London Hospitals Biomedical Research Centre. For the CAPACITY-COVID cohort participating in CORFU, FWA and ML received support from Dutch Heart Foundation (2020B006 CAPACITY) and The Netherlands Organization for Health Research and Development (ZonMW) (10430102110006 DEFENCE).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID is here to stay. countries must decide how to adapt. Nature. 2022;601(7892):165. [DOI] [PubMed] [Google Scholar]
- 2.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, Condition WHOCCDWGoP-C-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102-e7. [DOI] [PMC free article] [PubMed]
- 5.Klein DO, Waardenburg SF, Janssen EBNJ, Wintjens MSJN, Imkamp M, Heemskerk SCM. Two years and counting: unraveling the scope and severity of post-COVID symptoms across diverse patient groups—insights from the CORFU study. Under review 2024.
- 6.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38: 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nittas V, Gao M, West EA, Ballouz T, Menges D, Wulf Hanson S, et al. Long COVID through a public health lens: an umbrella review. Public Health Rev. 2022;43:1604501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demko ZO, Yu T, Mullapudi SK, Varela Heslin MG, Dorsey CA, Payton CB, et al. Post-acute sequelae of SARS-CoV-2 (PASC) impact quality of life at 6, 12 and 18 months post-infection. medRxiv. Open Forum Infectious Diseases. 2022;11(3).
- 9.Brus I.; Biere-Rafi S.; Ter Wolbeek M.; Tieleman P.; Rijssenbeek L.; Polinder S. HS. PostCOVID: Impact op gezondheid, het dagelijks leven en zorggebruik. Uitkomsten van 2 jaar onderzoek. Erasmus Medical Center 2024.
- 10.McNaughton CD, Austin PC, Sivaswamy A, Fang J, Abdel-Qadir H, Daneman N, et al. Post-acute health care burden after SARS-CoV-2 infection: a retrospective cohort study. CMAJ. 2022;194(40):E1368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koumpias AM, Schwartzman D, Fleming O. Long-haul COVID: healthcare utilization and medical expenditures 6 months post-diagnosis. BMC Health Serv Res. 2022;22(1):1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartof SY, Malden DE, Liu IA, Sy LS, Lewin BJ, Williams JTB, et al. Health care utilization in the 6 months following SARS-CoV-2 infection. JAMA Netw Open. 2022;5(8): e2225657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebo KA, Heath SL, Fukuta Y, Zhu X, Baksh S, Abraham AG, et al. Early antibody treatment, inflammation, and risk of post-COVID conditions. mBio. 2023;14(5):e0061823. [DOI] [PMC free article] [PubMed]
- 14.Bramante CT, Buse JB, Liebovitz DM, Nicklas JM, Puskarich MA, Cohen K, et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023;23(10):1119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao P, Liu J, Liu M. Effect of COVID-19 vaccines on reducing the risk of long COVID in the real world: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(19):12422. [DOI] [PMC free article] [PubMed]
- 16.Richard SA, Pollett SD, Fries AC, Berjohn CM, Maves RC, Lalani T, et al. Persistent COVID-19 symptoms at 6 months after onset and the role of vaccination before or after SARS-CoV-2 infection. JAMA Netw Open. 2023;6(1): e2251360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183(6):566–580. [DOI] [PMC free article] [PubMed]
- 18.Update to living systematic review on prediction models for diagnosis and prognosis of covid-19. BMJ. 2022;378:o2009. [DOI] [PubMed]
- 19.Deforth M, Gebhard CE, Bengs S, Buehler PK, Schuepbach RA, Zinkernagel AS, et al. Development and validation of a prognostic model for the early identification of COVID-19 patients at risk of developing common long COVID symptoms. Diagn Progn Res. 2022;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antony B, Blau H, Casiraghi E, Loomba JJ, Callahan TJ, Laraway BJ, et al. Predictive models of long COVID EBioMedicine. 2023;96: 104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghossein-Doha C, Wintjens M, Janssen E, Klein D, Heemskerk SCM, Asselbergs FW, et al. Prevalence, pathophysiology, prediction and health-related quality of life of long COVID: study protocol of the longitudinal multiple cohort CORona Follow Up (CORFU) study. BMJ Open. 2022;12(11): e065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tas J, van Gassel RJJ, Heines SJH, Mulder MMG, Heijnen NFL, Acampo-de Jong MJ, et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort (MaastrICCht). BMJ Open. 2020;10(9): e040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiertz CMH, Hemmen B, Sep SJS, van Santen S, van Horn YY, van Kuijk SMJ, et al. Life after COVID-19: the road from intensive care back to living—a prospective cohort study. BMJ Open. 2022;12(11): e062332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willems LH, Nagy M, Ten Cate H, Spronk HMH, Groh LA, Leentjens J, et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res. 2022;209:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leijte WT, Wagemaker NMM, van Kraaij TDA, de Kruif MD, Mostard GJM, Leers MPG, et al. [Mortality and re-admission after hospitalization with COVID-19]. Ned Tijdschr Geneeskd. 2020;164:D5423. [PubMed]
- 27.Linschoten M, Asselbergs FW. CAPACITY-COVID: a European Registry to determine the role of cardiovascular disease in the COVID-19 pandemic. Eur Heart J. 2020;41(19):1795–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long D, Haagsma JA, Janssen MF, Yfantopoulos JN, Lubetkin EI, Bonsel GJ. Health-related quality of life and mental well-being of healthy and diseased persons in 8 countries: does stringency of government response against early COVID-19 matter? SSM Popul Health. 2021;15: 100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350: g7594. [DOI] [PubMed] [Google Scholar]
- 30.CBS. Standaard Onderwijsindeling 2021. Available from: https://www.cbs.nl/, Editie 2020/21.
- 31.Riley RD, Snell KI, Ensor J, Burke DL, Harrell FE Jr, Moons KG, et al. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med. 2019;38(7):1276–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-de-Las-Penas C, Torres-Macho J, Catahay JA, Macasaet R, Velasco JV, Macapagal S, et al. Is antiviral treatment at the acute phase of COVID-19 effective for decreasing the risk of long-COVID? A systematic review Infection. 2024;52(1):43–58. [DOI] [PubMed] [Google Scholar]
- 33.Yotsuyanagi H, Ohmagari N, Doi Y, Yamato M, Fukushi A, Imamura T, et al. Prevention of post COVID-19 condition by early treatment with ensitrelvir in the phase 3 SCORPIO-SR trial. Antiviral Res. 2024;229: 105958. [DOI] [PubMed] [Google Scholar]
- 34.Al-Aly Z, Topol E. Solving the puzzle of Long Covid. Science. 2024;383(6685):830–2. [DOI] [PubMed] [Google Scholar]
- 35.Al-Aly Z, Davis H, McCorkell L, Soares L, Wulf-Hanson S, Iwasaki A, et al. Long COVID science, research and policy. Nat Med. 2024;30(8):2148–64. [DOI] [PubMed] [Google Scholar]
- 36.Antar AAR, Peluso MJ. CROI 2023: acute and post-acute COVID-19. Top Antivir Med. 2023;31(3):493–509. [PMC free article] [PubMed] [Google Scholar]
- 37.Greenhalgh T, Sivan M, Perlowski A, Nikolich JZ. Long COVID: a clinical update. Lancet. 2024;404(10453):707–24. [DOI] [PubMed] [Google Scholar]
- 38.Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28(11):2398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sk Abd Razak R, Ismail A, Abdul Aziz AF, Suddin LS, Azzeri A, Sha'ari NI. Post-COVID syndrome prevalence: a systematic review and meta-analysis. BMC Public Health. 2024;24(1):1785. [DOI] [PMC free article] [PubMed]
- 40.Kim Y, Bae S, Chang HH, Kim SW. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. 2023;13(1):11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-de-Las-Penas C, Notarte KI, Macasaet R, Velasco JV, Catahay JA, Ver AT, et al. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J Infect. 2024;88(2):77–88. [DOI] [PubMed] [Google Scholar]
- 42.Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines corona research I. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–61. [DOI] [PMC free article] [PubMed]
- 43.Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. All R code used for the statistical analyses will be made publicly available via a GitHub repository, after all CORFU projects are finalised.


