Abstract
The impact of a previously successful or unsuccessful experience on the subsequent acquisition of a related task is not well understood. The nature of past experience may have even greater impact in individuals with learning deficits, as their cognitive processes can be easily disrupted. Mice with a targeted disruption of the α and δ isoforms of the cAMP-response element-binding protein (CREB) gene (CREBαδ--deficient mice) have a genetic vulnerability to impaired learning and memory that is highly influenced by experimental conditions. Thus, we studied the impact of prior successful and unsuccessful experiences on the degree to which CREBαδ--deficient mice exhibit impaired spatial learning and memory in the Morris water maze (MWM). In Experiment 1, we replicated the cognitive deficit of CREBαδ--deficient mice when given two trials per day with a 1-min intertrial interval (MWM2), and labeled this experience as a “failure.” We rescued the deficit using four trials per day with a 3- to 5-min intertrial interval (MWM4) and labeled this experience a “success.” In Experiment 2, a new, naive set of wild-type (WT) and CREBαδ--deficient mice were randomly assigned to one of two sequence protocols to assess the influence of a success or a failure on subsequent performance. In Group 1, mice were first exposed to the MWM4 condition, followed by the more difficult MWM2 task. As expected, CREBαδ--deficient mice performed well in the MWM4; they also performed well during reversal testing (MWM4R) where the goal location is changed. With this initial successful learning experience, the CREBαδ--deficient mice then performed as well as WT mice in the MWM2, the condition in which they are known to be impaired. In contrast, CREBαδ--deficient mice in Group 2 had an unsuccessful experience when first exposed to the MWM2 condition, and then also showed impairment in the MWM4, the condition in which they would normally perform well. This deficit was amplified when CREBαδ--deficient mice were then tested in the reversal test. Sex differences in learning among CREBαδ--deficient mice were amplified upon exposure to an unsuccessful learning experience. These data indicate that, under conditions of cognitive impairment, past experience can—depending on its nature—significantly facilitate or hinder future performance.
The impact of past history on learning and memory across the lifespan is complex and largely unknown. In particular, a previous learning experience may be crucial in individuals with mild cognitive deficit, and the nature of that experience might influence whether that deficit is amplified or abrogated. Experience is likely relevant whether the deficit is due to a genetic problem, environmental insult, or aging. Here we explore the effect of a past learning experience on subsequent performance in a mouse genetic model known to cause impairment in learning and memory. The behavioral testing paradigm described can be useful in a number of other situations where cognitive impairment is present.
Learning theory states that prior training on an easy discrimination task facilitates learning of a difficult discrimination task more than the equivalent amount of training on the difficult discrimination task alone (Lawrence 1952). This phenomenon, called easy-hard learning, or transfer along a continuum, is well known in the experimental literature on animal learning (Lawrence 1952; Riley 1968). One factor in easy-hard learning is stimulus generalization (Gluck and Myers 1993). After learning, generalization of learned responses to nearby stimuli might account for some of the facilitation of learning. More recent studies in rodents have also shown that prior learning experience facilitates performance when the same task is subsequently tested (Dellu et al. 1997; Vicens et al. 1999, 2002; Markowska and Savonenko 2002).
In contrast, the effects of exposure to a hard-learning task followed by an easy-learning task are largely unreported. If the hard task is learned, then through stimulus generalization, the subsequent easier task will likely be learned very quickly. However, if the hard task is not learned, stimulus generalization would not occur and the subsequent easier task may not be learned. Would this lack of success be neutral or would it negatively impact future learning? We hypothesized that previous exposure to stimuli that do not become associated with successful learning may, in fact, hinder learning in a subsequent easier task. This would result from a phenomenon termed latent inhibition, which occurs when the pre-exposed stimuli have lost associability or salience (Chamizo 2003). Such initial unreinforced stimulus exposure (e.g., unsuccessful learning) may impair learning of a new task because an animal must now respond to features it previously was unable to use successfully. However, the negative impact of a failed experience may not be evident under all conditions and more sensitive models may be needed to uncover it.
The studies on learning transfer were conducted in intact animals, and little is known about the role of past experience with either a hard or an easy task in animals that are cognitively impaired. We hypothesized that under conditions of cognitive impairment, the role of prior experience may be more pronounced. Thus, we sought to use an animal model of genetic vulnerability to cognitive impairment to examine the effect of previous success or failure on learning and memory performance.
One prominent molecule involved in learning and memory is the cAMP-response element-binding protein (CREB). CREB activates transcription of genes required for long-term synaptic changes involved in memory formation (Dash et al. 1990; Bourtchuladze et al. 1994; Yin et al. 1994; Guzowski and McGaugh 1997). Mice with a targeted disruption of the α and δ isoforms of the CREB gene (CREBαδ--deficient mice) are impaired in the Morris water maze (MWM), a test of spatial learning and memory (Bourtchuladze et al. 1994) and have an altered ability to code space, as demonstrated by decreased spatial selectivity and stability of hippocampal place cells (Cho et al. 1998). This impairment, however, is not evident under all testing conditions or genetic backgrounds. CREBαδ--deficient mice are impaired when given two trials per day with a 1-min intertrial interval (ITI), but perform normally with spaced training (Kogan et al. 1997). The memory deficit in CREBαδ--deficient mice is, therefore, not absolute. Under highly demanding conditions, the mice are unable to acquire or retain adequate information for learning and memory and are impaired. Under less demanding conditions, the mice are unimpaired. This may be due to related transcription factors (e.g., CREM or the CREB β isoform which is up-regulated; Blendy et al. 1996) and may make up for the deficit. Spatial learning and memory performance of CREBαδ--deficient mice in different genetic backgrounds have been reported to show either a genetically dose-dependent effect (Gass et al. 1998) or no impairment at all (Graves et al. 2002). Further, various CREB mutants (in the 129SvEv × C57BL/6 background) with either a marked reduction or complete loss of hippocampal CREB exhibit only modestly impaired water maze learning (Balschun et al. 2003).
Thus, mice with a CREBαδ- mutation on a C57BL/6 × 129SvJ background, that show test-condition-dependent spatial learning and memory, can be considered an animal model of mild cognitive impairment. These mice have a genetic vulnerability to impaired learning and memory that is highly influenced by environmental conditions. Since the learning and memory deficit of CREBαδ--deficient mice is not absolute, we asked whether their performance is determined by the intrinsic difficulty of the task or by other variables (e.g., emotional or psychological). In Experiment 1, we sought to replicate the learning and memory deficit of CREBαδ- homozygous-deficient mice in the two trials per day with a 1-min ITI protocol (Kogan et al. 1997), assess their performance in a protocol different from that already published, which may rescue the deficit, and assess the learning and memory ability of CREBαδ- heterozygous-deficient mice. In Experiment 2, we examined the influence of previous experience on subsequent learning and memory performance by randomly assigning wild-type (WT) and CREBαδ- homozygous-deficient mice to one of two MWM sequence protocols. We asked the following questions:
If given success in a learning task, how do CREBαδ--deficient mice then perform in a more demanding task?
If impaired in a highly demanding task, how do these mice then perform in a less demanding task?
Results
Single-trial analysis of swim time and distance traveled during the regular trials of Experiments 1 and 2 did not reveal any different patterns from that of the reported mean trial analysis. The time course of behavioral performance during the probe trials was analyzed in 10-sec epochs. Sometimes rodents search accurately for the goal during the early portion of a 60-sec probe trial, and then began to search other locations for the goal during the latter portion of the trial (Hebda-Bauer 1998). This time-dependent search pattern did not occur in WT or CREBαδ--deficient mice. Since all variables measured during the probe trials, percent time and distance and number of platform crossings in the four quadrants revealed a similar pattern throughout the 60-sec trial, data from each probe trial are reported as a single 60-sec unit.
Experiment 1
The purpose of this experiment was to replicate the learning and memory deficit of CREBαδ- homozygous-deficient mice in the MWM2 protocol (Kogan et al. 1997). In addition, we assessed their performance in a different protocol, which may rescue the deficit. We also examined the learning and memory ability of CREBαδ- heterozygous-deficient mice. CREBαδ--deficient mice used in this study have been reported to show impaired learning and memory in the MWM (see genetic background in the Materials and Methods section; Bourtchuladze et al. 1994; Kogan et al. 1997).
The learning and memory deficit of CREBαδ--deficient mice is replicated in one protocol and rescued in another
Statistical analysis of learning curves during MWM2 testing reveals a significant effect of time (F = 3.69, df 5/75, P < 0.005) and genotype (F = 17.28, df 2/15, P < 0.001; Fig. 1A). In contrast to the WT mice, CREBαδ- homozygous deficient mice demonstrate little learning across test days. These mice swam greater than twice the distance to the goal as the WT mice on most test days. CREBαδ- heterozygous-deficient mice performed as well as WT mice on all MWM measures during Experiment 1 and, thus, are not discussed further. Post hoc tests show that the distance the CREBαδ- homozygous-deficient mice traveled to reach the goal was significantly longer than WT mice on five of the six testing days, thus revealing their spatial learning impairment when given two trials per day with a 1-min ITI. In contrast, when given four trials per day with a 3- to 5-min ITI (MWM4), CREBαδ- homozygous-deficient mice exhibited similar swim distances to those of WT mice across all testing days (Fig. 1B). All animals learned the MWM4 task equally well, as revealed by a significant effect of time (F = 20.61, df 5/75, P < 0.001), but not genotype (F = 0.10, df 2/15, P > 0.05). Thus, WT mice exhibit spatial learning under both conditions, but spatial learning in CREBαδ- homozygous-deficient mice is test-condition dependent.
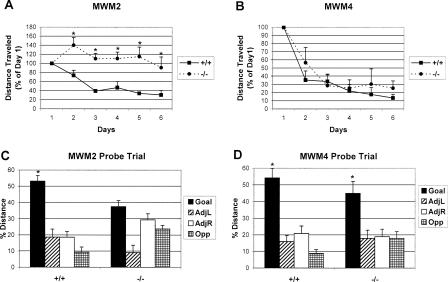
Figure 1.
The learning and memory deficit of CREBαδ--deficient mice is replicated in one protocol (MWM2) and rescued in another (MWM4). Data show the mean ±SEM. WT and CREBαδ- homozygous-deficient mice are designated as +/+ and -/-, respectively. (A) Percent distance traveled, compared with day 1, to reach the goal during the regular MWM2 trials. CREBαδ- homozygous-deficient mice swam significantly longer distances to reach the goal than WT mice on five of the six test days (P < 0.05) when given two trials per day with a 1-min ITI. (B) Percent distance traveled, compared to day 1, to reach the goal during the regular MWM4 trials. CREBαδ- homozygous-deficient mice have similar path lengths to that of the WT mice when given four trials per day with a 3- to 5-min ITI. (C) Comparison of percent distance traveled in the goal vs. left adjacent, right adjacent, and opposite quadrants during the MWM2 probe trial. CREBαδ- homozygous-deficient mice do not show a preference for the goal quadrant, unlike the preference shown by WT mice (P < 0.001). (D) Comparison of percent distance traveled in the goal vs. left adjacent, right adjacent, and opposite quadrants during the MWM4 probe trial. Both WT and CREBαδ- homozygous-deficient mice show a preference for the goal quadrant (P < 0.001). (*) P < 0.05.
Mean swim time (i.e., the mean of two or four trials on each testing day for MWM2 or MWM4, respectively) and swim speed (i.e., a measure of the distance swam, in centimeters, divided by the swim time) were compared among the genotypes in each of the two MWM protocols. CREBαδ- homozygous-deficient mice swam faster than their WT counterparts across all six testing days in the MWM2 protocol (F = 0.81, df 2/15, P = 0.001), but no genotype differences in swim speed were found in the MWM4 protocol. To ensure that CREBαδ- homozygous-deficient mice in the two protocols were not inherently different from each other, swim speed during the first 60 sec on day 1 was compared. This time is equivalent to the first trial in the MWM2 protocol and the first half of the first trial in the MWM4 protocol. Mice of all genotypes across protocols demonstrated similar swim speeds during the first 60 sec on day 1 (data not shown). Swim time was not considered a reliable measure of spatial learning in Experiment 1, however, because CREBαδ- homozygous-deficient mice had a faster swim speed than the other mice beginning with trial 2 in the MWM2 protocol. Percent distance traveled to reach the goal during the regular trials, with day 1 equal to 100%, is therefore reported here to normalize differences in swim speed.
Analysis of the probe trials reveals that WT mice showed a clear preference for the goal quadrant in both protocols, with >50% of the distance traveled in the goal quadrant alone (MWM2: F = 14.57, df 3/15, P < 0.001; MWM4: F = 18.75, df 3/15, P < 0.001; Fig. 1C,D). In contrast, CREBαδ- homozygous-deficient mice exhibited a preference in the MWM4 but not in the MWM2 (MWM4: F = 3.89, df 3/15, P < 0.05; MWM2: F = 2.20, df 3/15, P > 0.05; Fig. 1C,D). Number of crossings over the goal location versus hypothetical goal locations in the other three quadrants revealed a similar pattern (data not shown). As expected, WT mice exhibited a significantly higher number of platform crossings in the goal quadrant compared with the other quadrants in both protocols (MWM2: F = 11.06, df 3/15, P < 0.001; MWM4: F = 19.48, df 3/15, P < 0.001). CREBαδ- homozygous-deficient mice demonstrated more platform crossings in the goal quadrant in the MWM4, but not the MWM2, protocol (MWM4: F = 4.55, df 3/15, P = 0.06; MWM2: F = 1.21, df 3/15, P > 0.05). Thus, consistent with the regular trial performance, WT mice perform well in the probe trials of both protocols and CREBαδ- homozygous-deficient mice perform well in the MWM4 but not the MWM2.
Experiment 2
This study tested the effects of previous successful or unsuccessful learning experience on subsequent spatial learning and memory performance. Sex differences in learning and memory were also examined. Only new WT and CREBαδ- homozygous-deficient mice were tested in Experiment 2 because CREBαδ- heterozygous-deficient mice performed similarly to WT mice under both test conditions in Experiment 1.
CREBαδ---deficient mice exhibited similar swim speeds to those of WT mice under all testing conditions. Thus, swim time and distance traveled, along with other measures, were examined. Analysis of distance traveled shows results that parallel the swim time data reported in this work. The data reflect the mean of all trials given on each testing day.
CREBαδ--deficient mice, especially males, demonstrate flexibility in learning after an initial successful learning experience
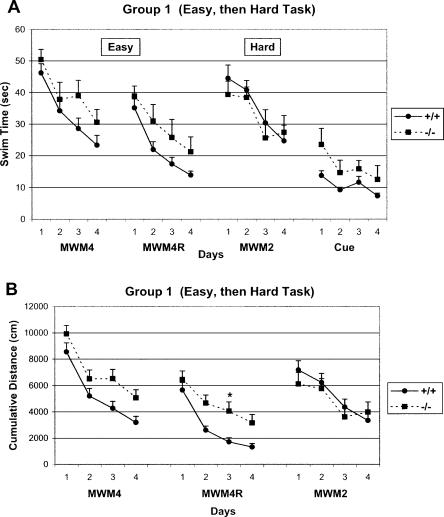
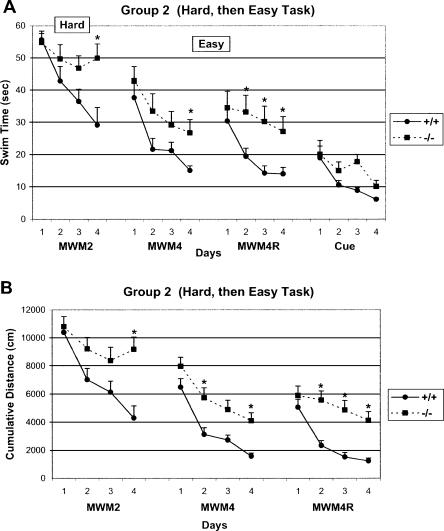
As expected, CREBαδ--deficient mice demonstrated similar swim times and degree of search error (i.e., cumulative distance) to those of WT mice, when first given the MWM4 condition (Group 1, Fig. 2A,B). Repeated measures ANOVAs revealed significant effects of time, but not genotype (Swim Time, Time: F = 16.12, df 3/66, P < 0.001; Genotype: F = 2.99, df 1/22, P > 0.05 and Cumulative Distance Time: F = 20.11, df 3/333, P < 0.001; Genotype: F = 3.46, df 1/21, P > 0.05), showing that mice of both genotypes learned the task equally well. CREBαδ--deficient mice, especially males, continued to perform as well as the WT mice during reversal testing when only the platform was moved (MWM4R). Analysis of mean swim times revealed a significant effect of time, but not of genotype for mice in the MWM4R condition (Time: F = 18.16, df 3/66, P < 0.001; Genotype: F = 3.76, df 1/22, P > 0.05; Fig. 2A). Analysis of cumulative distance across test days showed significant effects of time and genotype (Time: F = 14.00, df 3/333, P < 0.001; Genotype: F = 4.82, df 1/21; P < 0.05; Fig. 2B). The genotype difference on day 3 is due to only the female CREBαδ--deficient mice that showed more search error (see Fig. 5A, below). The purpose of the MWM4R task was to determine the animals' degree of flexibility in learning a new goal location. A trial-by-trial analysis during the reversal test revealed that all mice in Group 1 swam over the old goal location with decreasing frequency across trials, showing similar rates of shifting to the new location (data not shown). Thus, CREBαδ--deficient mice, especially males, demonstrate flexibility in learning a new goal location after an initial successful spatial learning experience.
Figure 2.
The learning curves of WT and CREBαδ--deficient mice during the Easy to Hard Task Sequence (Group 1) show successful learning. Data show the mean swim time or cumulative distance ± SEM. WT and CREBαδ--deficient mice are designated as +/+ and -/-, respectively. (A) CREBαδ--deficient mice without previous MWM experience demonstrate similar swim times to that of WT mice, as expected, when exposed to the MWM4 condition and continued to perform as well as WT mice during the reversal when the goal location was changed (MWM4R). These CREBαδ--deficient mice with a previous successful learning experience (MWM4/MWM4R) learned as well as their WT counterparts in a changed environment when tested in the hard condition in which they are known to be impaired (MWM2). CREBαδ--deficient mice also learned as well as WT mice during cued-platform testing performed at the end of the testing sequence. (B) Analysis of search error or cumulative distance also show that CREBαδ--deficient mice exposed to the Easy to Hard Task Sequence performed as well as WT mice with one exception. The temporary impairment of CREBαδ--deficient mice during one day of reversal testing (MWM4R) was due to the female mice (P < 0.05). (*) P < 0.05.
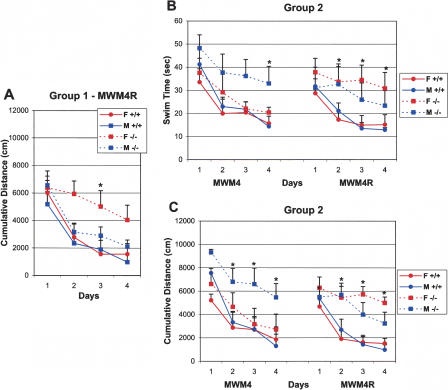
Figure 5.
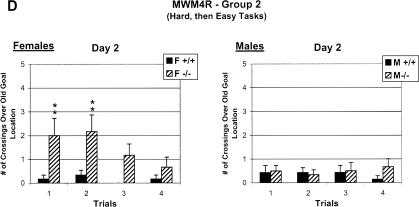
Sex differences in learning of CREBαδ--deficient mice exposed to the Easy to Hard Task Sequence (Group 1) were only found in one spatial learning measure during reversal testing, but CREBαδ--deficient mice exposed to the Hard to Easy Task Sequence (Group 2) displayed sex differences in several measures of learning following their failure in the hard MWM2 condition. Data show the mean cumulative distance (A,C), swim time (B), or number of crossings (D) ±SEM. WT and CREBαδ--deficient mice are designated as +/+ and -/-, respectively. Female and male mice are designated as F and M, respectively. (A) Female CREBαδ--deficient mice of Group 1 showed significantly greater search error than WT and male CREBαδ--deficient mice on the third test day (P < 0.05), but later performed as well as all other mice during the hard MWM2 condition. (B,C) In contrast, in a changed environment, male CREBαδ--deficient mice of Group 2 were again impaired in swim time by the fourth day and cumulative distance on three of the four test days when next exposed to the condition in which they normally perform well (MWM4), but female CREBαδ--deficient mice performed as well as WT mice. However, both male and female CREBαδ--deficient mice performed poorly when the goal location was changed (MWM4R). CREBαδ--deficient mice of Group 2 demonstrated significantly longer swim times and greater search error than their WT counterparts over three of the four test days (P < 0.05). (D) Female CREBαδ--deficient mice of Group 2 crossed over the old goal location significantly more times than the other mice during the first two trials of the second day of reversal testing. WT mice had learned the new goal location, but male CREBαδ--deficient mice did not cross over the old goal location because they had not learned the task in the first place. (*) P < 0.05 (**) P < 0.01.
A successful learning experience enhances subsequent learning and memory in CREBαδ--deficient mice
CREBαδ--deficient mice that performed well in the MWM4/MWM4R condition (Group 1) continued to learn as well as their WT counterparts when next given the MWM2 condition in a novel environment, the condition in which they are known to be impaired (Fig. 2A,B). Repeated measures ANOVAs revealed significant effects of time, but not genotype (Swim Time, Time: F = 8.19, df 3/63, P < 0.001; Genotype: F = 0.38, df 1/21, P > 0.05 and Cumulative Distance, Time: F = 7.66, df 3/153, P < 0.001; Genotype: F = 0.29, df 1/21, P > 0.05). In contrast, CREBαδ--deficient mice of Group 2 did not have previous MWM experience and were impaired in the MWM2 condition as expected (Swim Time, Time: F = 6.80, df 3/69, P < 0.001; Genotype: F = 6.69, df 1/23, P < 0.05; Time × Genotype: F = 2.83, df 3/69, P < 0.05 and Cumulative Distance, Time: F = 8.69, df 3/167, P < 0.001; Genotype: F = 6.65, df 1/23, P < 0.05; Fig. 3A,B). As shown in Figure 3, A and B, WT mice learned across testing days by taking less time to reach the goal and decreasing their search error each day. In contrast, CREBαδ--deficient mice of Group 2 showed little learning by taking significantly longer to reach the goal and exhibiting more search error than their WT counterparts by the fourth testing day (P < 0.05). These data show that, when first given a successful learning experience, CREBαδ--deficient mice perform well in a subsequent test condition in which they are normally impaired.
Figure 3.
The learning curves of mice during the Hard to Easy Task Sequence (Group 2) show impairment of CREBαδ--deficient mice. Data show the mean swim time or cumulative distance ± SEM. WT and CREBαδ--deficient mice are designated as +/+ and -/-, respectively. (A) CREBαδ--deficient mice without any previous MWM experience exhibited little learning across test days, as expected, when exposed to the hard MWM2 condition. In a changed environment, CREBαδ--deficient mice with previous unsuccessful MWM2 experience, continued to show impairment when tested in the condition in which they normally perform well (MWM4). The impairment of these mice was amplified when the goal location was changed during reversal testing (MWM4R). CREBαδ--deficient mice demonstrated significantly longer swim times than their WT counterparts over three of the four test days (P < 0.05). In contrast, CREBαδ--deficient mice of Group 2 performed as well as WT mice during cued-platform testing. (B) CREBαδ--deficient mice exposed to the Hard to Easy Task Sequence also exhibited significantly greater search error (i.e., cumulative distance) than WT mice by the fourth MWM2 test day, the second and fourth MWM4 test days, and three of the four MWM4R test days (P < 0.05). (*) P < 0.05.
An unsuccessful learning experience hinders subsequent learning and memory in CREBαδ--deficient mice
A naive group of mice (Group 2) was assigned to a sequence where they would first have a hard task, which would likely produce failure, before being exposed to the easy task. Thus, they were first given the MWM2 experience, a condition in which CREBαδ--deficient mice are impaired, and were then tested with a changed environment in the MWM4/MWM4R condition. The results showed that CREBαδ--deficient mice were not only impaired in MWM2, but continued to be impaired even in the usually easy MWM4 condition. This impairment is revealed by repeated measures ANOVAs showing significant effects of time and genotype (Swim Time, Time: F = 16.47, df 3/69, P < 0.001; Genotype: F = 4.86, df 1/23, P < 0.05 and Cumulative Distance, Time: F = 22.41, df 3/363, P < 0.001; Genotype: F = 4.83, df 1/23, P < 0.05; Fig. 3A,B). Although mice of both genotypes learned over time, CREBαδ--deficient mice still swam significantly longer to reach the goal than WT mice by the fourth testing day (P < 0.05) and demonstrated significantly greater search error than WT mice on the second and fourth test days (P < 0.05).
When the goal location was changed during reversal testing (MWM4R), CREBαδ--deficient mice showed more pronounced impairment, as revealed by significant effects of time and genotype (Swim Time, Time: F = 12.25, df 3/66, P < 0.001; Genotype: F = 5.43, df 1/22, P < 0.05 and Cumulative Distance, Time: F = 16.05, df 3/363, P < 0.001; Genotype: F = 5.53, df 1/23, P < 0.05; Time × Genotype Interaction: F = 3.71, df 3/363, P < 0.05; Fig. 3A,B). Post hoc analyses showed that CREBαδ--deficient mice took significantly longer to reach the goal and exhibited significantly greater search error than WT mice on three of the four testing days (P < 0.05). An initial unsuccessful learning experience can, therefore, hinder subsequent learning performance of CREBαδ--deficient mice in a test condition in which they normally perform well, and amplify this impairment when flexibility in learning is important.
Indices of success vs. failure in learning in CREBαδ--deficient mice
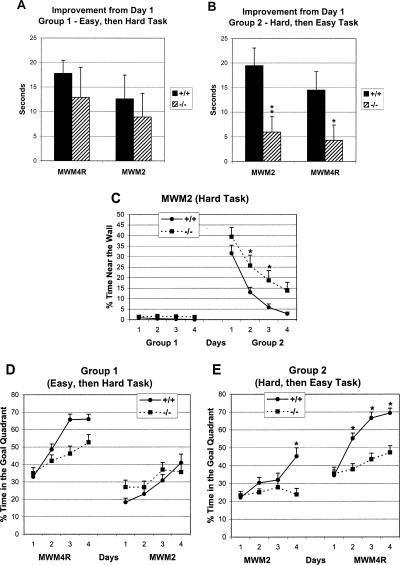
The effect of past success or failure on subsequent learning in CREBαδ--deficient mice is highlighted in Figure 4. CREBαδ--deficient mice showed similar improvement of learning (as measured in seconds to reach the goal location) in the easy and hard MWM conditions, like that found in WT mice, when given the easy to hard task sequence (Group 1; Fig. 4A). In contrast, CREBαδ--deficient mice showed little improvement of learning in all MWM conditions when given the hard to easy task sequence (Group 2; Fig. 4B). Thus, in CREBαδ--deficient mice, success in learning leads to more success in a harder task and failure in learning leads to more failure in an easier task.
Figure 4.
The effect of past success or failure on subsequent learning in CREBαδ--deficient mice. Data show mean improvement in time to reach the goal from day one of a given task (A,B) or the mean percent time ± SEM (C-E). WT and CREBαδ--deficient mice are designated as +/+ and -/-, respectively. (A) CREBαδ--deficient mice show similar improvement of learning in the MWM4R and MWM2 conditions, like that found in WT mice, when given the Easy to Hard Task Sequence (Group 1). (B) CREBαδ--deficient mice show little improvement of learning in all MWM conditions when given the Hard to Easy Task Sequence (Group 2). (C) Thigmotaxic behavior in the hard MWM2 task. CREBαδ--deficient mice that are successful in learning (Group 1) exhibit very little thigmotaxic behavior in all testing conditions. In contrast, CREBαδ--deficient mice that fail to learn (Group 2) exhibit significantly greater thigmotaxic behavior than WT mice in the MWM2 condition, but this behavior decreases over time. (D) Successful CREBαδ--deficient mice (Group 1) exhibit increasing search time for the goal in the goal quadrant across test days like that of WT mice in the easy and hard MWM conditions. (E) In contrast, unsuccessful CREBαδ--deficient mice (Group 2) do not increase percent time in the goal quadrant during the hard MWM2 condition. Although these unsuccessful mice later exhibit more percent time in the goal quadrant during the easy MWM condition, they are still significantly impaired compared with WT mice on three of four MWM4R test days (P < 0.05). (*) P < 0.05; (**) P < 0.01.
The Morris water-maze data were further analyzed to get a better understanding of the learning strategies used by CREBαδ--deficient mice that were successful versus those that failed in the MWM conditions. Percent time spent near a 10-cm zone along the tub wall gives an estimate of the degree of thigmotaxic behavior, which is most prevalent during the first trials of water maze learning (Lipp and Wolfer 1998). In normal animals, thigmotaxic behavior quickly subsides as they learn more efficient strategies for locating the escape platform. CREBαδ--deficient mice (Group 1), like WT mice, exposed to the easy to hard task sequence successfully learned the hard MWM2 task and spent very little time near the wall of the tub during all the MWM conditions (MWM4: F = 2.11, df 1/21, P > 0.05; MWM4R: F = 0.12, df 1/21, P > 0.05; MWM2: F = 3.52, df 1/21, P > 0.05, Fig. 4C for MWM2). In contrast, CREBαδ--deficient mice (Group 2) first exposed to the MWM2 were impaired and showed significantly more percent time near the wall than WT controls on the second and third test days (F = 6.05, df 1/23, P < 0.05; Fig. 4C). However, the unsuccessful CREBαδ--deficient mice of Group 2 showed significantly decreasing time spent near the wall in the MWM2 (F = 24.58, df 3/167, P < 0.001) such that, by the subsequent MWM4/MWM4R condition, the percent time they spent near the wall did not differ from that of WT mice (MWM4: F = 1.65, df 1/23, P > 0.05; MWM4R: F = 1.34, df 1/23, P > 0.05; data not shown). This dramatic decrease in percent time spent near the wall reveals that the CREBαδ--deficient mice attempted alternate strategies to find the goal as opposed to just swimming until picked up by the experimenter.
Increase in the percent time spent in the goal quadrant across test days is an indicator of the development of such alternate strategies. CREBαδ--deficient mice of Group 1, that were successful in the easy to hard task sequence, showed similar increases in percent time swimming in the goal quadrant to that of their WT counterparts in the easy and hard conditions (MWM4R, Time: F = 19.59, df 3/332, P < 0.001; Genotype: F = 3.05, df 1/21, P > 0.05 and MWM2, Time: F = 7.88, df 3/153, P < 0.001, Genotype: F = 0.67, df 1/21, P > 0.05; Fig. 4D). Although the unsuccessful CREBαδ--deficient mice of Group 2 showed little change in percent time spent in the goal quadrant across test days in the MWM2 condition, in contrast to an increase in percent time by WT mice (Time: F = 4.33, df 3/167, P < 0.01; Genotype: F = 5.48, df 1/23, P < 0.05; post hoc: day 4, P < 0.05), they did show an increase in the MWM4R (Fig. 4E). This increase in goal quadrant time, however, was not nearly as much as that found in the WT mice (Time: F = 31.59, df 3/363, P < 0.001; Genotype: F = 7.43, df 1/23, P = 0.01). Post hoc analyses revealed that CREBαδ--deficient mice spent significantly less percent time in the goal quadrant than their WT counterparts on three of the four MWM4R test days (P < 0.05) lending to their failure in the task. Thus, the alternate strategies developed by the unsuccessful CREBαδ--deficient mice of Group 2 were not sufficient enough to overcome their initial failure in the hard MWM2 condition.
An unsuccessful learning experience amplifies sex differences in subsequent learning performance of CREBαδ--deficient mice
The testing conditions of Experiment 2 uncovered sex differences in spatial learning performance of CREBαδ--deficient mice for the first time. A hint for sex differences in CREBαδ--deficient mice undergoing the easy to hard MWM task sequence (Group 1) was found in one spatial learning measure (i.e., cumulative distance) during reversal testing. Female CREBαδ--deficient mice exhibited greater search error than all other mice on the second MWM4R test day (F = 4.53, df 1/19, P < 0.05; Fig. 5A). They did, however, show decreasing search error across days like that of WT and male CREBαδ--deficient mice (F = 13.71, df 3/326, P < 0.001). Importantly, female CREBαδ--deficient mice subsequently performed as well as WT and male CREBαδ--deficient mice in all measures during testing in the MWM2 condition.
In contrast, sex differences in learning and memory performance were amplified in CREBαδ--deficient mice after an unsuccessful learning experience. After a previous unsuccessful learning experience (MWM2), male CREBαδ--deficient mice of Group 2 took significantly longer to reach the goal by the fourth test day and exhibited greater search error on three of the four test days than WT mice in the MWM4 condition, but female CREBαδ--deficient mice performed as well as all WT mice across test days (Swim Time: F = 3.19, df 3/21, P < 0.05 and Cumulative Distance: F = 5.38, df 1/21, P < 0.05; Fig. 5B,C). When the goal location was changed during reversal testing (MWM4R), female CREBαδ--deficient mice now showed impairment in swim time and cumulative distance like that of their male counterparts, as revealed by significant effects of time and genotype but not sex (Swim Time, Time: F = 12.25, df 3/66, P < 0.001; Genotype: F = 5.43, df 1/22, P < 0.05; Sex: F = 0.23, df 1/22, P > 0.05 and Cumulative Distance, Time: F = 16.22, df 3/357, P < 0.001; Genotype: F = 5.16, df 1/21, P < 0.05; Sex: F = 0.13, df 1/21, P > 0.05; Genotype × Day: F = 3.76, df 3/357, P = 0.01; Fig. 5B,C). Post hoc analyses showed that both male and female CREBαδ--deficient mice took significantly longer to reach the goal and exhibited greater search error than WT mice on three of the four testing days (P < 0.05).
Female CREBαδ--deficient mice first given an unsuccessful learning experience (Group 2) learned a subsequent task, but then swam significantly more times over the old goal location than all other mice during the first two trials on the second reversal day (MWM4R, Genotype: F = 8.08, df 1/21, P < 0.01; Sex: F = 2.43, df 1/21, P > 0.05; Genotype × Sex: F = 5.25, df 1/21, P < 0.05; Fig. 5D). Although swimming over the old goal location highlights intact memory of the female CREBαδ--deficient mice from the MWM4 task, the persistence of this behavior into day 2 of reversal testing shows their inflexibility and difficulty in learning the MWM4R task. Male CREBαδ--deficient mice in Group 2 exhibited fewer crossings than all other mice over the old goal location during the first trial on the first day (data not shown). Fewer crossings were not surprising, however, because male CREBαδ--deficient mice showed impairment in the previous MWM4 condition and, therefore, would most likely not remember the old goal location. Since male CREBαδ--deficient mice were impaired in the MWM4 after an unsuccessful learning experience; it was not possible to assess their degree of flexibility in learning a new goal location. Interestingly, female CREBαδ--deficient mice performed well in a learning task (MWM4) after an unsuccessful learning experience, but then had difficulty learning the new location of the goal when it was moved. An unsuccessful learning experience can therefore amplify sex differences in subsequent learning performance of CREBαδ--deficient mice.
Cued-platform learning is unimpaired in CREBαδ--deficient mice
Following the given sequence of the testing conditions, all mice were given four trials per day for 4 d in a cued-platform task. CREBαδ--deficient mice from Groups 1 and 2 demonstrated similar swim times to that of their WT counterparts across all four testing days. (Figs. 2A and 3A). All mice learned the task as revealed by a significant effect of time but not genotype (Group 1, Time: F = 8.99, df 3/63, P < 0.001 and Genotype: F = 3.01, df 1/21, P > 0.05; Group 2, Time: F = 10.96, df 3/69, P < 0.001 and Genotype: F = 3.30, df 1/23, P > 0.05). Thus, learning and memory deficits due to mobility or vision problems can be ruled out.
Discussion
In this study, we have shown a profound effect of past experience on subsequent performance in animals that have a genetically induced cognitive impairment. In Experiment 1, we replicated the learning and memory deficit initially reported in CREBαδ--deficient mice (Bourtchuladze et al. 1994; Kogan et al. 1997), which is at odds with more recent reports of intact or only mildly impaired (i.e., on one measure) learning and memory in these mice (Gass et al. 1998; Graves et al. 2002). The discrepancy in results is likely due to the different genetic backgrounds of the mice used in these studies, suggesting that the role CREB plays in learning and memory is sensitive to genetic background. CREBαδ--deficient mice of the current study clearly demonstrated minimal learning with only two trials per day and a 1-min ITI, but learned as well as WT mice when given longer trials, a larger ITI (3-5 min), and more trials per day. Kogan et al. (1997) attenuated and rescued the deficit with 10- and 60-min ITIs, respectively. We found that increasing the ITI by only 2 min is sufficient to rescue the deficit if the maximum time and number of trials are also increased. Intact spatial memory of CREBαδ--deficient mice given more than two trials per day with a longer ITI in this study is also consistent with the results reported by Gass et al. (1998). The CREBαδ--deficient mice in the current study are an ideal model of a mild cognitive impairment from which to examine how various test conditions affect spatial learning and memory.
Interestingly, CREBαδ- homozygous-deficient mice swam faster than the other mice in the two trial, 1-min ITI protocol in Experiment 1. This finding, however, was not replicated in Experiment 2. The swim speed difference may be cohort dependent, in spite of our efforts to test them under equivalent conditions. Other groups have reported that locomotor activity differences are not consistent from one group of mice to the next, even when great efforts are taken to equate experimental conditions (Crabbe et al. 1999). It should be noted that faster swimming in the CREBαδ- homozygous-deficient mice did not reflect good performance, since they displayed little learning across test days. We have corrected for this basal difference in Experiment 1 by normalizing the distance traveled across the learning day against the initial distance traveled on day 1, to show within group patterns of learning or failure to learn.
In Experiment 2, we demonstrated that the spatial learning and memory deficit of CREBαδ- homozygous-deficient mice is not absolute; rather, it is highly dependent on past experience. The memory phenotype in mice with various CREB mutations is sensitive to training parameters (Bourtchuladze et al. 1994; Kogan et al. 1997; Gass et al. 1998; Balschun et al. 2003); however, this is the first study to show that different sequences of Morris water-maze training parameters also play a role in this memory phenotype. In the current study, CREBαδ--deficient mice first exposed to a successful learning experience, (i.e., MWM4/MWM4R) continued to learn well under more demanding test conditions in which they are known to be impaired. Previous experience with the spatial and procedural aspects of the Morris water maze, however, did not help CREBαδ--deficient mice to perform well if they initially exhibited poor learning. Indeed, an unsuccessful experience significantly disrupted their ability to perform a task that would otherwise be easy for them.
One may argue that CREBαδ--deficient mice that failed in the Hard to Easy Task Sequence demonstrated continued primitive thigmotaxic behavior without developing alternative strategies for locating the escape platform. It has been reported that mice with reduced CREB levels (i.e., four CREB mutant strains) exhibit a large amount of thigmotaxic behavior throughout acquisition, including reversal learning, by swimming around and around the edge of the tub (Gass et al. 1998; Balschun et al. 2003). Substantial thigmotaxic behavior was observed in CREBαδ--deficient mice that performed poorly in the hard two trial/day, 1-min ITI task (MWM2) of this study. Such time spent near the wall of the water maze, however, diminished across days with search time in the goal quadrant increasing and cumulative distance to the goal decreasing across days during the subsequent easy MWM4/MWM4R condition. Thus, these CREBαδ--deficient mice did not just swim around and around the edge of the water maze waiting to be picked up at the 60-sec mark. They began to develop alternative strategies for finding the escape platform, which enabled them over time to search closer to the actual goal. Importantly, however, the strategies they used were not sufficient enough for them to perform as well as WT mice.
In contrast, CREBαδ--deficient mice given the Easy to Hard Task Sequence showed dramatic improvement in all indices of spatial learning measured. Successful mice showed dramatic improvement in swim time, taking less time to find the goal each day. This improvement was accomplished by using strategies that allowed them to search nearer to the goal each day as shown by negligible thigmotaxic behavior, decreasing cumulative distance to the goal, and increasing time spent in the goal quadrant. It is possible that exposure to the easy condition first enabled CREBαδ--deficient mice of Group 1 to get a head start on learning how to efficiently find the goal so that they were not adversely affected by the later presentation of the hard MWM2 condition.
Previous experience and consistent testing conditions have been reported to have a beneficial influence on learning and memory performance in other models of cognitive impairment, particularly aging (Dellu et al. 1997; Vicens et al. 1999, 2002; Markowska and Savonenko 2002). For example, in a combined longitudinal and cross-sectional study, previous experience in the MWM prevented age-related impairments of Sprague-Dawley rats in the same task (Dellu et al. 1997). The benefits of previous experience on water-maze performance have also been reported in mice (Vicens et al. 1999, 2002), but the extent of the benefit is strain dependent.
A successful learning experience may act as an activator of neuroplasticity, and thus enhance subsequent performance. Indeed, learning in the MWM has been shown to result in increased mossy fiber synaptogenesis within the hippocampus (Ramirez-Amaya et al. 1999, 2001). Various growth factors are also up-regulated after learning. Notably, brain-derived neurotrophic factor (BDNF) mRNA is up-regulated in the hippocampus with MWM training (Kesslak et al. 1998; Schaaf et al. 2001), and this up-regulation is specific to MWM learners as opposed to nonlearners (Schaaf et al. 2001) and to swimming and sedentary controls (Kesslak et al. 1998). MWM testing with four or more trials per day has also been found to increase cell proliferation (Lemaire et al. 2000) and survival in the dentate gyrus (Gould et al. 1999; Ambrogini et al. 2000) of rat learners. Note, however, that C57BL/6 mice tested in the MWM2 protocol did not show an increase in cell survival (van Praag et al. 1999). It is reasonable to hypothesize that CREBαδ--deficient mice exposed to an initial successful learning experience may exhibit greater experience-dependent neural plasticity than their counterparts with an initial unsuccessful learning experience. This enhanced plasticity may serve to facilitate subsequent learning in a highly demanding task.
Little research to date has described the effects of previous unsuccessful learning on subsequent spatial learning performance. It is reasonable to hypothesize that the CREBαδ--deficient mice in Group 2 were more stressed than either WT mice or CREBαδ--deficient mice in Group 1 as a result of previous impaired performance. One can further hypothesize that this stress can disrupt hippocampal plasticity and hinder learning in less-demanding spatial tasks. Note, however, that the CREBαδ--deficient mice do not exhibit any differences in basal or stress-induced levels of corticosterone from those of WT mice (Hebda-Bauer et al. 2004). Moreover, the MWM exposure, no matter its difficulty level, produces significant increases in circulating glucocorticoids in all the animals. Thus, glucocorticoid levels alone may not be sufficient to explain the observed effects of unsuccessful learning; other neural concomitants of stress or “frustration” may need to be evaluated to assess the differential impact of failure or success on the animals. We would suggest that an unsuccessful or negative experience in a given environment results in specific neural and molecular changes that can be elicited again upon re-exposure to that environment and would interfere with the individual's ability to learn in that context. For example, negative regulators of learning and memory such as calcineurin or protein phosphatase 1 (Malleret et al. 2001; Genoux et al. 2002) may remain up-regulated for longer than normal amounts of time in CREBαδ--deficient mice following an unsuccessful experience, and this inhibition may interfere with further attempts to learn. It would be of great interest to uncover these molecular correlates and understand how they hinder subsequent learning and memory.
Finally, this study represents the first report of sex differences in learning and memory of CREBαδ--deficient mice. Sex differences in spatial learning and memory tasks have been reported for normal humans and rodents, with males often performing better than females (Perrot-Sinal et al. 1996; Astur et al. 1998; Moffat et al. 1998; Sandstrom et al. 1998; LaBuda et al. 2002). Male and female WT mice in the current study performed equally well in all of the MWM conditions in both sequence protocols, and sex differences were only found in mice with a genetic vulnerability to impaired learning and memory.
Interestingly, sex differences in learning curves of CREBαδ--deficient mice were amplified after initial exposure to an unsuccessful learning experience. When next exposed to a less-demanding condition in a changed environment, female CREBαδ--deficient mice learned as well as WT mice, but male CREBαδ--deficient mice continued to be impaired. It should be recalled that the transition from one task to the next (MWM4/MWM4R to MWM2 and vice versa) involves changing the extramaze environment without changing the room itself. This environmental change in extra-maze cues may have more adversely affected the learning of male CREBαδ--deficient mice. In contrast, the movement of the goal location (reversal) in the MWM4R more adversely affected female CREBαδ--deficient mice. This is evidenced by the fact that they continued swimming to the old goal location more frequently than other mice, no longer performing as well as WT female mice. Female CREBαδ--deficient mice in the Easy to Hard Task Sequence also showed some difficulty during reversal testing as suggested by increased search error, but not any other measures. This impairment, however, was only temporary, since they later performed well in the hard MWM2 condition. Importantly, after an unsuccessful learning experience, male CREBαδ--deficient mice have difficulty learning less-demanding tasks in a changed environment; in contrast, female CREBαδ--deficient mice appear less impacted at first, but have difficulty revising their strategy for finding a new goal location.
The sex-specific impairment in spatial learning following an unsuccessful experience may reflect differences in the way female and male CREBαδ--deficient mice encode space. It is known that CREBαδ--deficient mice have an altered ability to code space, as demonstrated by decreased spatial selectivity and stability of hippocampal place cells (Cho et al. 1998), but sex differences in place cell activity of these mice have not been reported. Data from human and rodent studies show that females and males use different navigational strategies (Williams et al. 1990, Williams and Meck 1991; Sandstrom et al. 1998). Females are more likely to rely primarily on landmark information (e.g., objects or cues in the room), while males more readily use geometric information (Sandstrom et al. 1998). Although they experienced initial unsuccessful learning, female CREBαδ--deficient mice in Group 2 may have been able to encode the changed extra-maze cues during the MWM4 trials due to a combination of their tendency to focus on landmarks and the increased trial number and ITI. In contrast, the increased trial number and ITI did not benefit male CREBαδ--deficient mice with an initial unsuccessful learning experience because they may have more readily relied on geometric information (e.g., room shape) that remained constant between the MWM2 to the MWM4 protocol. The consistency in the geometric information between the two phases may have served as unreinforced stimuli (since they did not learn in the initial MWM2 phase) that hindered subsequent learning, a phenomenon known as latent inhibition (Chamizo 2003). During the reversal, female CREBαδ--deficient mice may have become impaired because of the additional alteration in geometric relationship of the extra-maze cues resulting from the change in goal location. Thus, although female and male WT mice performed equally well under all testing conditions in the current study, an unsuccessful learning experience most clearly uncovered sex differences in spatial learning ability in the cognitively impaired animals.
In conclusion, the results from this study reveal that the nature of previous experience in mice with a genetic vulnerability to impaired learning and memory strongly influences subsequent cognitive performance. Of particular interest is the demonstration that failure to learn in a given environment can increase the chances of failure in learning a related, but easy task undertaken in a similar setting. Thus, there is a significant cost of past failure, and this cost is particularly evident when cognitive ability is impaired. Uncovering the neuronal and molecular mechanisms underlying these phenomena in CREBαδ--deficient mice may represent a useful animal model for defining the role of experience, both success and failure, in determining future performance in other types of cognitive impairment, including childhood learning disabilities, early Alzheimer disease, and other forms of age-related dementias.
Materials and Methods
Subjects
CREBαδ--deficient mice were originally generated in the laboratory of Gunther Schutz (Hummler et al. 1994). They were initially obtained for our laboratory from Alcino Silva as F2 progeny derived from a cross between CREBαδ--deficient heterozygotes in the C57BL/6 background (>87%) and wild-type 129SvJ mice. Thus, the genetic background of the wild-type and mutant mice subsequently bred and used for Experiments 1 and 2 consists of approximately a 50% contribution of genes from each of the C57BL/6 and 129SvJ strains. Approximately 15% of the newborn pups are homozygous for the CREBαδ- mutation, consistent with that of Silva's laboratory (J.H. Kogan, pers. comm.).
All of the mice used in the experiments were 3-6 mo of age. The wild-type (WT; +/+) mice were age and sex matched to the CREBαδ- heterozygous (+/-) and homozygous (-/-) deficient mice. Mice were group housed in a temperature and humidity-controled room with free access to food and water. They were maintained on a 14:10 light/dark cycle (lights on at 0600, lights off at 2000 h). All behavioral testing was conducted between 0800 and 1600 h. The experiments were conducted in accordance with the guidelines of the University Committee on the Use and Care of Animals at the University of Michigan.
CREBαδ- PCR genotyping
Mice were genotyped by PCR analysis. Tail biopsies were obtained at weaning and digested in 600 μL of TNES (10 mM Tris at pH 7.5, 400 mM NaCl, 100 mM EDTA, and 0.6% SDS) and 35 μL of Proteinase K (10 mg/mL) overnight at 57°C. The next day, 166.7 μL of saturated NaCl was added and mixed. After centrifugation (14,000 rpms for 5 min) and recovery of the supernatant were performed twice, equal volume of 100% EtOH was added and the DNA was spooled, dipped briefly in 70% EtOH, allowed to dry, and then resuspended in TE (10 mM Tris, 1 mM EDTA). One microliter of the DNA was used directly in a PCR reaction. The following PCR primers were used for genotyping CREBαδ--deficient mice: CREB1, 5′-CCATATTATTGTAGGTAACTAAATGA-3′, CREB2, 5′-ATGTATTTTTATACCTGGGC-3′, and NEO, 5′-ATGATGGATACTTTCTCGGCAAGG-3′. The following PCR conditions were used in a Peltier Thermal Cycler (PTC-2000, MJ Research): 4°C for 180 sec; 94°C for 90 sec; 40 cycles of 93°C for 45 sec, 47°C for 45 sec, and 72°C for 90 sec; then 72°C for 600 sec.
Behavioral testing
The Morris water maze (MWM) was used to test for spatial learning and memory. The inside of the 91-cm diameter tub was painted white and filled with 26°C ± 2°C water made white with powdered milk to control for intramaze cues. Animals must use a stationary array of cues outside of the tub to find a submerged platform that they cannot see, hear, or smell. These cues, and the location of the platform, remained constant during testing.
All mice were given a preliminary trial the day before regular testing began to acclimate them to water and let them know that a platform can be found. For this trial, a mouse was placed in the water for 10 sec, allowed to swim around, and then placed on the platform for only 1-2 sec. The hidden platform was put in a different location from where it was located for the regular trials. If a mouse found the platform before the 10-sec mark, it was removed immediately.
A regular trial began by placing a mouse in the water at one of two (MWM2) or four (MWM4 and MWM4R) randomly assigned starting positions. After a mouse located the platform, it was allowed to remain on the platform for 30 sec. If a mouse had not located the platform within 60 sec, it was removed from the water and placed on the platform for 30 sec. MWM4R trials were conducted like the MWM4 trials, except that the location of the platform was moved to the opposite quadrant. Changing the goal location serves to assess animals' flexibility in learning the new goal location. Animals that are inflexible tend to continue swimming to the old goal location. Mice received two (MWM2) or four (MWM4 and MWM4R) trials per day with a 1 (MWM2) or 3- to 5- (MWM4 and MWM4R) min intertrial interval (ITI). The types and locations of cues outside of the tub and the goal location were changed to provide a novel environment for spatial learning when changing from the MWM4/MWM4R to the MWM2 protocol and vice versa. A videotracking system (Ethovision, Noldus Technology) was used to measure swim time, distance traveled, swim speed, number of crossings over the old vs. the new goal location, search error (i.e., cumulative distance: sum of distances to goal taken every second minus the value obtained for an ideal direct swim; Gallagher et al. 1993), percentage of time in a 10-cm wide wall zone, and percentage of time in goal quadrant. Data are expressed as the mean of trials per day unless otherwise specified.
Twenty-four hours after the last regular trial, the platform was removed for the probe trial and mice were allowed to swim for 60 sec to assess their memory for the platform location. Time spent and distance traveled in the four quadrants were measured. The number of platform crossings in the goal quadrant and arbitrary platform locations in the other three quadrants were measured to assess the accuracy toward the trained submerged platform location.
After completion of regular testing, all mice received four cued-platform trials per day for 4 d to rule out impairments in vision and mobility. For these trials, the platform was moved to a different quadrant and marked with a black cube.
Experimental design
In Experiment 1, mice were randomly assigned to either the MWM2 or MWM4 protocol (n = 12 +/+, 12 +/-, and 12 -/-). Each protocol consisted of 6 d of regular trial testing with a single probe trial given on the seventh day. Each regular trial in the MWM2 and MWM4 protocols lasted a maximum of 60 and 120 sec, respectively.
In Experiment 2, a new, naive set of WT and CREBαδ--deficient mice were randomly assigned to one of two MWM sequence protocols to assess the influence of previous experience on subsequent learning and memory performance (n = 24 +/+ and 24 -/-). Mice in Group 1 were exposed to the Easy to Hard Sequence of Tasks. For the easy tasks, Group 1 mice first received four trials per day with a 3- to 5-min ITI (MWM4), and then received the same protocol, except that the goal location was changed to the opposite quadrant (MWMR, R = reversal). For the hard task, Group 1 mice received two trials per day with a 1-min ITI MWM2 in a changed environment. Group 2 mice were exposed to the Hard to Easy Sequence of Tasks. The mice in Group 2 first received the hard MWM2 task, then the MWM4/MWM4R easy tasks (with a changed environment). All mice in Groups 1 and 2 received cued-platform trials during the last phase of testing. Each protocol consisted of 4 d of regular trial testing with a single probe trial given on the fifth day, with the exception of cued-platform testing, which did not include a probe trial. Each regular trial in all protocols for Experiment 2 lasted a maximum of 60 sec.
Data analysis
Data were analyzed using SAS statistical software. Two-way analysis of variance (ANOVA) with repeated measures using the Mixed and General Linear Model procedures in SAS were used to analyze regular trial performance across days. Tukey's post hoc comparisons were used to determine genotype and sex differences on each day. ANOVAs were also used to analyze probe-trial performance. Sex differences were examined for all data in Experiment 2 and are indicated when statistically significant. Otherwise, male and female data for each genotype are combined. The sample size was not large enough in Experiment 1 to examine sex differences.
Acknowledgments
This research was supported by NIMH PO1MH42251, NIDA RO1DA3386, and NIDA T32-DA07268.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.94105.
References
- Ambrogini, P., Cuppini, R., Cuppini, C., Ciaroni, S., Cecchini, T., Ferri, P., Sartini, S., and Del Grande, P. 2000. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. 286: 21-24. [DOI] [PubMed] [Google Scholar]
- Astur, R.S., Ortiz, M.L., and Sutherland, R.J. 1998. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behav. Brain Res. 93: 185-190. [DOI] [PubMed] [Google Scholar]
- Balschun, D., Wolfer, D.P., Gass, P., Mantamadiotis, T., Welzl, H., Schutz, G., Frey, J.U., and Lipp, H.P. 2003. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 23: 6304-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendy, J.A., Kaestner, K.H., Schmid, W., Gass, P., and Schutz, G. 1996. Targeting of the CREB gene leads to up-regulation of a novel CREB mRNA isoform. EMBO J. 15: 1098-1106. [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze, R., Frenguelli, B., Blendy, J., Cioffi, D., Schutz, G., and Silva, A.J. 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59-68. [DOI] [PubMed] [Google Scholar]
- Chamizo, V.D. 2003. Acquisition of knowledge about spatial location: Assessing the generality of the mechanism of learning. Q. J. Exp. Psychol. B. 56: 102-113. [DOI] [PubMed] [Google Scholar]
- Cho, Y.H., Giese, K.P., Tanila, H., Silva, A.J., and Eichenbaum, H. 1998. Abnormal hippocampal spatial representations in aCaMKITT286A and CREBad- Mice. Science 279: 867-869. [DOI] [PubMed] [Google Scholar]
- Crabbe, J.C., Wahlsten, D., and Dudek, B.C. 1999. Genetics of mouse behavior: Interactions with laboratory environment. Science 284: 1670-1672. [DOI] [PubMed] [Google Scholar]
- Dash, P.K., Hochner, B., and Kandel, E.R. 1990. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345: 718-721. [DOI] [PubMed] [Google Scholar]
- Dellu, F., Mayo, W., Vallee, M., Le Moal, M., and Simon, H. 1997. Facilitation of cognitive performance in aged rats by past experience depends on the type of information processing involved: A combined cross-sectional and longitudinal study. Neurobiol. Learn. Mem. 67: 121-128. [DOI] [PubMed] [Google Scholar]
- Gallagher, M., Burwell, R., and Burchinal, M. 1993. Severity of spatial learning impairment in aging: Development of a learning index for performance in the morris water maze. Behav. Neurosci. 107: 618-626. [DOI] [PubMed] [Google Scholar]
- Gass, P., Wolfer, D.P., Balschun, D., Rudolph, D., Frey, U., Lipp, H.P., and Schutz, G. 1998. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn. Mem. 5: 274-288. [PMC free article] [PubMed] [Google Scholar]
- Genoux, D., Haditsch U., Knobloch, M., Michalon, A., Storm, D., and Mansuy, I.M. 2002. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418: 970-975. [DOI] [PubMed] [Google Scholar]
- Gluck, M.A. and Myers, C.E. 1993. Hippocampal mediation of stimulus representation: A computational theory. Hippocampus 3: 491-516. [DOI] [PubMed] [Google Scholar]
- Gould, E., Beylin, A., Tanapat, P., Reeves, A., and Shors, T.J. 1999. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2: 260-265. [DOI] [PubMed] [Google Scholar]
- Graves, L., Dalvi, A., Lucki, I., Blendy, J.A., and Abel, T. 2002. Behavioral analysis of CREB mutation on a B6/129 F1 hybrid background. Hippocampus 12: 18-26. [DOI] [PubMed] [Google Scholar]
- Guzowski, J.F. and McGaugh, J.L. 1997. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. 94: 2693-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebda-Bauer, E.K. 1998. The effects of aging and corticosterone levels on spatial learning. Ph.D. thesis, University of Michigan, Ann Arbor, MI.
- Hebda-Bauer, E.K., Watson, S.J., and Akil, H. 2004. CREB deficient mice show inhibition and low activity in novel environments without changes in stress reactivity. Eur. J. Neurosci. 20: 503-513. [DOI] [PubMed] [Google Scholar]
- Hummler, E., Cole, T.J., Blendy, J.A., Ganss, R., Aguzzi, A., Schmid, W., Beermann, F., and Schutz, G. 1994. Targeted mutation of the CREB gene: Compensation within the CREB/ATF family of transcription factors. Proc. Natl. Acad. Sci. 91: 5647-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesslak, J.P., So, V., Choi, J., Cotman, C.W., and Gomez-Pinilla, F. 1998. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: A mechanism to facilitate encoding and circuit maintenance? Behav. Neurosci. 112: 1012-1019. [DOI] [PubMed] [Google Scholar]
- Kogan, J.H., Frankland, P.W., Blendy, J.A., Coblentz, J., Marowitz, Z., Schutz, G., and Silva, A.J. 1997. Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. 7: 1-11. [DOI] [PubMed] [Google Scholar]
- LaBuda, C.J., Mellgren, R.L., and Hale, R.L. 2002. Sex differences in the acquisition of a radial maze task in the CD-1 mouse. Physiol. Behav. 76: 213-217. [DOI] [PubMed] [Google Scholar]
- Lawrence, D.H. 1952. The transfer of a discrimination along a continuum. J. Comp. Physiol. Psychol. 45: 511-516. [DOI] [PubMed] [Google Scholar]
- Lemaire, V., Koehl, M., Le Moal, M., and Abrous, D.N. 2000. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. 97: 11032-11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp, H.P. and Wolfer, D.P. 1998. Genetically modified mice and cognition. Curr. Opin. Neurobiol. 8: 272-280. [DOI] [PubMed] [Google Scholar]
- Malleret, G., Haditsch, U., Genoux, D., Jones, M.W., Bliss, T.V.P., Vanhoose, A.M., Weitlauf, C., Kandel, E.R., Winder, D.G., and Mansuy, I.M. 2001. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104: 675-686. [DOI] [PubMed] [Google Scholar]
- Markowska, A.L. and Savonenko, A.V. 2002. Protective effect of practice on cognition during aging: Implications for predictive characteristics of performance and efficacy of practice. Neurobiol. Learn. Mem. 78: 294-320. [DOI] [PubMed] [Google Scholar]
- Moffat, S.D., Hampson, E., and Hatzipantelis, M. 1998. Navigation in a “virtual” maze: Sex differences and correlation with psychometric measures of spatial ability in humans. evolution and human behavior. Evol. Hum. Behav. 19: 73-87. [Google Scholar]
- Perrot-Sinal, T.S., Kostenuik, M.A., Ossenkopp, K.P., and Kavaliers, M. 1996. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav. Neurosci. 110: 1309-1320. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya, V., Escobar, M.L., Chao, V., and Bermudez-Rattoni, F. 1999. Synaptogenesis of mossy fibers induced by spatial water maze overtraining. Hippocampus 9: 631-636. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya, V., Balderas, I., Sandoval, J., Escobar, M.L., and Bermudez-Rattoni, F. 2001. Spatial long-term memory is related to mossy fiber synaptogenesis. J. Neurosci. 21: 7340-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, D. 1968. Discrimination learning. Allyn & Bacon, Boston, MA.
- Sandstrom, N.J., Kaufman, J., and Huettel, S.A. 1998. Males and females use different distal cues in a virtual environment navigation task. Brain Res. Cogn. Brain Res. 6: 351-360. [DOI] [PubMed] [Google Scholar]
- Schaaf, M.J., Workel, J.O., Lesscher, H.M., Vreugdenhil, E., Oitzl, M.S., and de Kloet, E.R. 2001. Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res. 915: 227-233. [DOI] [PubMed] [Google Scholar]
- van Praag, H., Kempermann, G., and Gage, F.H. 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2: 266-270. [DOI] [PubMed] [Google Scholar]
- Vicens, P., Bernal, M.C., Carrasco, M.C., and Redolat, R. 1999. Previous training in the water maze: Differential effects in NMRI and C57BL mice. Physiol. Behav. 67: 197-203. [DOI] [PubMed] [Google Scholar]
- Vicens, P., Redolat, R., and Carrasco, M.C. 2002. Effects of early spatial training on water maze performance: A longitudinal study in mice. Exp. Gerontol. 37: 575-581. [DOI] [PubMed] [Google Scholar]
- Williams, C.L. and Meck, W.H. 1991. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology 16: 155-176. [DOI] [PubMed] [Google Scholar]
- Williams, C.L., Barnett, A.M., and Meck, W.H. 1990. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav. Neurosci. 104: 84-97. [DOI] [PubMed] [Google Scholar]
- Yin, J.C., Wallach, J.S., Del Vecchio, M., Wilder, E.L., Zhou, H., Quinn, W.G., and Tully, T. 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49-58. [DOI] [PubMed] [Google Scholar]