Abstract
In two H215O PET scan experiments, we investigated the cerebral correlates of explicit and implicit knowledge in a serial reaction time (SRT) task. To do so, we used a novel application of the Process Dissociation Procedure, a behavioral paradigm that makes it possible to separately assess conscious and unconscious contributions to performance during a subsequent sequence generation task. To manipulate the extent to which the repeating sequential pattern was learned explicitly, we varied the pace of the choice reaction time task—a variable that is known to have differential effects on the extent to which sensitivity to sequence structure involves implicit or explicit knowledge. Results showed that activity in the striatum subtends the implicit component of performance during recollection of a learned sequence, whereas the anterior cingulate/mesial prefrontal cortex (ACC/MPFC) supports the explicit component. Most importantly, we found that the ACC/MPFC exerts control on the activity of the striatum during retrieval of the sequence after explicit learning, whereas the activity of these regions is uncoupled when learning had been essentially implicit. These data suggest that implicit learning processes can be successfully controlled by conscious knowledge when learning is essentially explicit. They also supply further evidence for a partial dissociation between the neural substrates supporting conscious and nonconscious components of performance during recollection of a learned sequence.
In cognitive neuroscience, dissociating the brain networks that subtend conscious and nonconscious memories constitutes a very complex issue, both conceptually and methodologically. Specifically, it remains unclear whether implicit and explicit learning processes recruit different brain structures or share, either in part or in totality, the same neural mechanisms. Today, many behavioral, brain imaging, and modeling studies (e.g. Rauch et al. 1995; Willingham and Goedert-Eschmann 1999; Wallach and Lebiere 2003) have explored this issue through a simple paradigm that involves asking participants to respond to successive visual stimuli in the context of a choice reaction time task. Unknown to them, the material contains sequential structure, obtained, for instance, by the repetition of an invariant pattern across the experiment. The extent to which subjects become sensitive to the sequential regularities is established by modifying the repeating pattern during some experimental blocks, without informing subjects. Typically (e.g., Reed and Johnson 1994), reaction times slow down considerably during these transfer blocks, thus suggesting that subjects' responses are influenced by their knowledge of the sequential regularities. Even so, many participants typically fail to be able to verbalize knowledge about the material—a dissociation finding that has led many authors to characterize sequence learning as involving essentially implicit knowledge. This paradigm—sequence learning—has thus become one of the best empirical situations through which to study the mechanisms of implicit learning, because it offers truly incidental learning conditions as well as the possibility for tight control over the material's structure. However, consensus has not yet been reached on the nature of sequence learning mechanisms and on the extent to which they recruit conscious or nonconscious knowledge.
The latter point has proven particularly challenging. Even cursory overviews of the literature reveal enduring conceptual and methodological debate about the possibility for human learning to be unconscious (Shanks et al. 1994; Cleeremans et al. 1998). Insofar as sequence learning studies are concerned, while no one disputes the fact that subjects often find themselves unable to verbalize relevant knowledge despite showing clear sensitivity to sequential structure, as numerous early reports have indicated (Willingham et al. 1989), a substantial number of recent studies have also clearly demonstrated associations between SRT task performance and subsequent forced-choice tasks involving, for instance, recognition of sequence fragments or sequence generation. These later findings (e.g., Perruchet and Amorim 1992; Shanks and Johnstone 1999) thus clearly suggest that sequence learning also involves the acquisition of explicit knowledge. Note, however, that many relevant studies share an assumption that does not necessarily hold, namely, that performance on the SRT task taps exclusively implicit knowledge, and that performance on the subsequent forced-choice tasks tap exclusively explicit knowledge. This so-called exclusiveness assumption (Reingold and Merikle 1988) is a methodological cornerstone of the dissociation logic (Erderlyi 1986) most commonly used to assess the extent to which performance involves implicit knowledge. Conceptually, following the dissociation logic also naturally yields dichotomous characterizations of the contrast between implicit and explicit learning.
However, studies based on this logic overlook the fact that even carefully designed learning and testing conditions can hardly be considered as “process-pure” (Reingold and Merikle 1988; Jacoby 1991). In other words, the exclusivity assumption seldom holds, and it instead appears much more plausible to endorse the idea that any task will always tend to involve both implicit and explicit processes, to different degrees determined by a host of factors ranging from the complexity of the material to the subject's orientation to learn (e.g., Jiménez et al. 1996). This mandates a different experimental approach, one in which it is sought to explore qualitative differences between the consequences of learning with or without full conscious awareness of what is learned.
For instance, it has been frequently proposed that explicit—but not implicit—learning subtends intentional control (Jacoby 1991; Merikle and Reingold 1991). Based on this idea, recent studies have revealed that the degree to which sequence learning results in implicit or explicit knowledge may depend on various training conditions, including variations in the amount of attentional resources devoted to the task (Goschke 1997), or modifications in the pace of the SRT task obtained by manipulating the value of the time interval that separates manual responses from the onset of the next visual target (the response-to-stimulus interval, RSI). As a case in point, Destrebecqz and Cleeremans (2001, 2003) have shown that decreasing the length of the RSI, indeed, tends to selectively impair explicit sequence learning. In this study, to assess the implicit/explicit nature of sequence knowledge acquired through practice on the SRT task, Jacoby's Process Dissociation Procedure (Jacoby 1991) was adapted by asking subjects to generate sequences of key presses, first under so-called Inclusion instructions, and then under so-called Exclusion instructions. In the Inclusion condition, subjects were simply asked to try to reproduce the sequence practiced during the SRT task. Successful performance in this condition might depend on explicit processes (i.e., when participants consciously recollect the training sequence), but it can also be influenced by implicit knowledge (i.e., when participants feel that they are guessing the next stimulus location but can nevertheless reproduce the sequence accurately). Importantly, both conscious recollection as well as intuition-based responding will contribute to increasing performance in the Inclusion condition: Both implicit and explicit influences contribute to successful generation. In contrast, in the Exclusion condition, subjects were specifically asked to generate a sequence that does not contain the regularities of the training sequence. Here, implicit and explicit knowledge work in opposition. Indeed, since conscious knowledge of the sequential regularities makes it possible for subjects to generate a different sequence and hence perform the Exclusion task successfully, continued generation of the practiced sequential transitions in the Exclusion task can only be due to implicit influences, which cannot be controlled by conscious knowledge. Failure to exclude is thus construed, in this paradigm, as an indication that people lack control over their knowledge.
When a standard 250-msec RSI was used during training in the Destrebecqz and Cleeremans experiments (2001, 2003), participants were able both to reproduce the training sequence under Inclusion instructions and to avoid reproducing it under Exclusion instructions. This suggests that learning had resulted in explicit knowledge. In contrast, when the RSI had been reduced to 0 msec in the SRT task, subsequent generation results indicated that subjects' Exclusion scores failed to differ from Inclusion scores. This suggests that learning had resulted in essentially implicit knowledge, since subjects were unable to refrain from producing familiar sequence regularities under Exclusion conditions, despite being explicitly instructed to avoid doing so. Based on these results, Destrebecqz and Cleeremans concluded that sequence learning tended to be explicit when participants have time to develop conscious expectations about the location at which the next stimulus will appear in the SRT task. Awareness, however, is not always necessary for sequence learning to occur.
Turning now to the neural correlates of sequence learning, an open issue remains, to determine whether implicit and explicit processes depend on different brain systems and how they interact in subtending performance. Previous brain imaging studies of sequence learning have addressed this issue by various means. One approach consisted in contrasting brain activity during practice of the SRT task before and after the moment subjects became conscious of the presence of a repeated sequential pattern in the practiced material (Rauch et al. 1995; Honda et al. 1998). Others compared patterns of brain activity during SRT practice with or without an additional distraction task that prevented subjects from realizing the presence of the repeated sequence (Grafton et al. 1995; Hazeltine et al. 1997). Despite large methodological differences, these early studies similarly concluded that implicit and explicit components of performance activate different, and even nonoverlapping, regions. According to some reports, explicit sequence learning is associated with activation in the frontoparietal cortex, while implicit sequence learning is essentially associated with activity in the contralateral primary sensorimotor cortex (SM1) and supplementary motor area (SMA) (Grafton et al. 1995; Hazeltine et al. 1997; Honda et al. 1998). Others showed that implicit learning involves the right ventral premotor cortex, right ventral caudate, right thalamus, and bilateral visual association cortex, while explicit learning involves the primary visual and inferior parietal cortex (Rauch et al. 1995).
However, the observation that different brain networks are involved in each experimental condition might merely reflect differences in training regimens rather than differences between implicit and explicit learning processes. For instance, order effects on brain activations between implicit then explicit learning cannot be excluded in the previous studies in which implicit and explicit learning were measured sequentially (Rauch et al. 1995; Honda et al. 1998), whereas the use of an additional task could have increased difficulty levels and modified the learning context in studies that made use of this procedure (Grafton et al. 1995; Hazeltine et al. 1997).
Recent studies have attempted to circumvent these methodological problems. Willingham et al. (2002) trained volunteers during a pre-scan learning session on two different sequences. One sequence (appearing in red) was learned explicitly, while the other one (appearing in black among random trials) was learned implicitly. Subjects were then scanned using block-design fMRI during further practice of the task in the same implicit or explicit (explicit-overt) condition as during learning, except for one condition (explicit-covert) during which the red sequence that was initially learned explicitly appeared disguised as black circles in the test phase (i.e., participants were unwittingly exposed to material that they thought was random but that they had previously learned explicitly). Hence, task requirements and sequential material were similar in the explicit-overt and explicit-covert conditions, that differed only by the presence or absence of awareness of the sequence. Interestingly, the authors found activations in largely overlapping cerebral networks independently of whether sequence learning had been implicit or explicit, and independently of whether subjects were aware or not of the sequence during scanning. This finding was later supported by another fMRI study using two different sequences for the implicit and explicit learning conditions. This study showed overlaps in caudate, prefrontal, and medial temporal areas during implicit and explicit sequence learning (Schendan et al. 2003). Partial overlapping of cerebral activations was also found during simultaneous implicit (sequences of color) and explicit (sequences of shapes) learning (Aizenstein et al. 2004).
These recent studies, which controlled for previously identified potential methodological problems, therefore challenge prior conclusions that strictly nonoverlapping brain networks are dedicated to implicit and explicit sequence learning. Nevertheless, an assumption shared by all these studies is that implicit and explicit processes are a priori associated with different experimental conditions that are further assumed to specifically involve implicit or explicit learning. As discussed above, however, this exclusiveness assumption does not necessarily hold. Furthermore, the experimental procedures used in these studies do not ensure that implicit and explicit components of learning were effectively completely dissociated during the different training conditions. In other words, one cannot exclude the possibility that explicit knowledge partially supports performance in a priori designed implicit learning conditions, and vice versa. As already pointed out by Honda et al. (1998), the “contamination and complicated interaction between two learning processes make the interpretation of the results from the subtraction analysis [...] somewhat problematic”.
Based on different methodological approaches, two of our previous studies favor the hypothesis that the striatum and the medial prefrontal cortex play a distinct role in implicit and explicit sequence learning. It is worth pointing out that all of the studies described above have reported consistent activation in these structures, suggesting that frontal and striatal areas play an executive key role in sequence learning. However, the exact nature of their role, and how their respective activity modulates implicit and explicit contributions to performance in a segregated or overlapping manner, remains a matter of debate. In our first study, we reported increased activation of the striatum when subjects' reaction times in the SRT task demonstrated their knowledge of a complex probabilistic sequence of stimuli regulated by an artificial grammar (Peigneux et al. 2000). Given the complexity of the embedded sequential regularities in this material and the inability of participants to report these regularities, learning can be described as essentially implicit (Jiménez et al. 1996). We therefore hypothesized that the striatum plays a central role in implicit sequence learning. However, no attempt was made in this study to evidence the neural correlates of potential explicit contributions to performance. Also, it was not clear whether the striatum was involved only in the implicit acquisition of sequential regularities in the SRT task, or also plays a role in the implicit retrieval of sequence knowledge. Therefore, further demonstration was awaited to delineate and confirm the implicit nature of the processes subtended by the striatum in sequence learning. In our second study, using an application of the Process Dissociation Procedure (PDP) to sequence learning (Destrebecqz and Cleeremans 2001), we reported that regional cerebral blood flow (rCBF) in the anterior cingulate/medial prefrontal cortex (ACC/MPFC) region specifically correlates with performance scores thought to reflect the conscious control of sequence knowledge in a generation task administered after the end of SRT practice (Destrebecqz et al. 2003). This result was in line with the proposal that some brain areas might be specifically involved in the conscious treatment of sequential material (Clegg et al. 1998). In that study, however, behavioral data demonstrated that most of the knowledge gained by the participants during the learning phase was explicit, which prevented us from probing the role of the striatum in the implicit component of sequence knowledge using the PDP paradigm.
The present study was therefore specifically designed to further explore the respective roles of ACC/MPFC and striatum in subtending explicit and implicit contributions to performance in a sequence generation task. Just as in our prior study (Destrebecqz et al. 2003), we adapted the Process Dissociation Procedure to sequence learning in an H215O PET scan experiment. The main difference was that the RSI was reduced to 0 msec (RSI0) in order to prevent explicit preparation to the apparition of the next stimulus, whereas in our prior study the RSI was set to 250 msec (RSI250), a value known to promote explicit learning, as discussed above. In both experiments, after completing the SRT task, participants were scanned under two successive conditions. They were first instructed to reproduce the training sequence or, failing recollection, to guess the location of the next stimulus (Inclusion condition). In the subsequent Exclusion condition, participants were instructed to avoid, on each trial, giving the response that would reproduce a part of the training sequence. According to the logic of the PDP, explicit (E) and implicit (I) processes both contribute to improve performance in the Inclusion condition, while they act in opposition to each other in the Exclusion condition. It may therefore be posited that Exclusion generation scores reflect the influence of implicit knowledge (I), to the extent that explicit knowledge of the sequence (E) could only result in successful exclusion (Richardson-Klavehn et al. 1996). In contrast, Inclusion scores reflect the contribution of both explicit (i.e., recollection) and implicit (i.e., guessing) processes to performance (E + I). An evaluation of explicit influences on performance (E) can be obtained by computing the difference between Inclusion and Exclusion scores.
Using a similar reasoning to identify the neural correlates of explicit processes in sequence learning, we therefore looked for brain regions in which the correlation between rCBF and generation scores was higher in the Inclusion than in the Exclusion condition, irrespective of the duration of the RSI. Indeed, rCBF variations related to the generation score obtained in the Inclusion condition will, by assumption, reflect both implicit and explicit contributions, whereas rCBF variations related to the generation score obtained in the Exclusion condition should only reflect implicit contributions. Note that, in line with the assumption that no task is process-pure, explicit sequence learning might not be completely suppressed in the RSI0 condition. Therefore, Inclusion scores (E + I) might still be higher than Exclusion (I) scores in that condition.
We expected this analysis to confirm the role of the ACC/MPFC in subtending the conscious component of performance in both RSI0 and RSI250 conditions. In contrast, we expected implicit sequence learning and, therefore, Exclusion scores, to be increased in the RSI0 condition as compared to the RSI250 condition. Hence, to identify the neural correlates of implicit sequence knowledge, we looked for regions where the correlation between CBF and Exclusion scores was modulated by the training condition, that is, was higher in the RSI0 than in the RSI250 condition.
Results
Data from the RSI250 group have been reported elsewhere (Destrebecqz et al. 2003). Data from the RSI0 group and analyses comparing RSI250 to RSI0 data are original contributions in the present report.
Behavioral data
Sequence learning (SRT) task
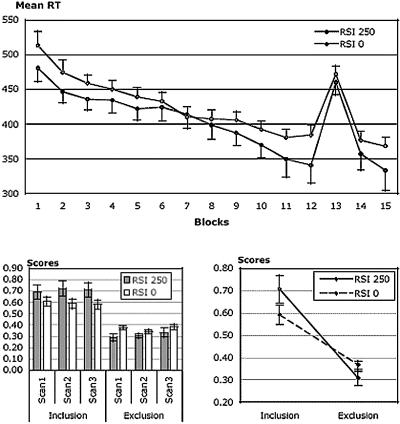
Before PET scans acquisition, participants were trained on the four-choice serial reaction time (SRT) task during 15 blocks of 96 successive trials. Unknown to them, each block contained eight repetitions of the same 12-element sequence, except block 13, which contained a different sequence. Figure 1 (upper panel) shows the average reaction time (RT) observed over the 15 blocks of practice, plotted separately for the two conditions. An analysis of variance (ANOVA) with Blocks (15 levels) as a within-subject variable and Condition (2 levels; RSI0 vs. RSI250) as a between-subjects variable revealed a significant effect of Blocks (F(14,504) = 45.542, p < 0.0001). Neither the main effect of Condition nor the Blocks by Condition interaction reached significance (both Fs < 1). To measure sequence learning, we computed a transfer effect as the RT difference between Block 13 (new sequence) and the mean RT between adjacent Block 12 and Block 14 (learned sequence). Significant transfer effects of 111 msec and 91 msec were observed in the RSI 250 (one-sample t-test, t = 8.024, p < 0.0001) and RSI 0 condition (one-sample t-test, t = 11.091, p < 0.0001), respectively. The cost in RT for the new sequence did not differ between both conditions (F(1,36) = 1.602, p > 0.2). These results indicate that participants learned the training sequence in both conditions. Based on these RT measures only, sequence learning appears to be quantitatively equivalent in both RSI conditions.
Figure 1.
Behavioral data. (Upper panel) Mean reaction times for each training block in the SRT task plotted separately for RSI0 and RSI250 participants. RT decreases across blocks through practice, but returns back to initial RT values when a different sequence (block 13) is presented, demonstrating learning of the repeated sequence. (Lower left panel) Mean generation scores measured in both conditions for the three Inclusion and Exclusion scans in RSI0 and RSI250 participants. Performance remains stable over replications/scans in all conditions. (Lower right panel) Mean generation scores averaged over the three Inclusion or Exclusion scans in the Inclusion or Exclusion task in RSI0 and RSI250 participants. Higher generation scores under the Inclusion than the Exclusion condition in RSI250 and RSI0 conditions indicates the presence of explicit knowledge in both RSI conditions. Under Inclusion instruction, higher generation score in the RSI250 than the RSI0 condition shows that participants in the RSI250 condition gained more sequence knowledge irrespective of its implicit/explicit status. Under Exclusion instruction, a higher generation score in RSI0 than RSI250 indicates higher implicit knowledge in the former condition.
Sequence generation task
After the SRT task, we tested whether participants in the RSI0 and RSI250 conditions differ in their ability to generate or to avoid generating the training sequence in the Inclusion and Exclusion tasks. Our hypothesis was that reducing the RSI should impair explicit learning, as assessed by participants' ability to control their sequential knowledge. In other words, we expected the difference between Inclusion and Exclusion performance to be reduced in the RSI0 condition as compared to the RSI250 condition.
Before the onset of the generation task, participants were informed that the sequence had followed a repeating pattern. They were then introduced to the Inclusion task, in which they had to reproduce the training sequence, during three consecutive PET scans. After completion of the Inclusion task, they were given Exclusion instructions specifying that they had now to avoid, on each trial, reproducing the sequential regularities of the training sequence. The Exclusion task also involved three consecutive scans. Performance was measured, for each Inclusion and Exclusion scan, by the number of generated chunks of three elements that were part of the training sequence, divided by the total number of triplets produced during that scan (Destrebecqz et al. 2003). Mean generation scores are shown in Figure 1 (lower left panel).
We performed an ANOVA with Scans (3 levels) and Instructions (2 levels; Inclusion vs. Exclusion) as within-subject variables, and Condition (2 levels; RSI250 vs. RSI0) as a between-subjects variable. The main effect of Scans and every interaction including this factor were nonsignificant, indicating that generation scores did not differ from one another for the three Inclusion or Exclusion scans in both RSI conditions (all Fs < 1). In other words, generation performance remained stable across the three scans in each condition. The analysis also revealed a significant effect of Instructions (F(1,36) = 76.286, p < 0.0001) as well as a significant Instruction by Condition interaction (F(1,36) = 6.093, p < 0.05). The main effect of Condition was not significant (F < 0.5). As shown in Figure 1 (lower right panel), participants reproduced more training triplets under Inclusion instructions than under Exclusion instructions. Planned comparisons revealed that Inclusion scores were higher than Exclusion scores in both RSI250 (F(1,16) = 39.284, p < 0.0001) and RSI0 (F(1,20) = 33.768, p < 0.0001) conditions. This result suggests that participants acquired explicit sequence knowledge in both conditions. Accordingly, the estimate of explicit learning (E) obtained from subtracting Exclusion scores from Inclusion scores is significantly higher than zero in both RSI250 [mean = 0.401; t(16) = 6.284; p < 0.0001] and RSI0 [mean = 0.224; t(20) = 5.811; p < 0.0001] conditions. However, the estimate of E was significantly higher in the RSI250 condition than in the RSI0 condition [t(36) = 2.468; p < 0.05], suggesting that participants gained more explicit sequence knowledge in the RSI250 than in the RSI0 condition. Post hoc tests also revealed that Inclusion scores were on average higher in the RSI250 (mean = 0.71, SE = 0.06) condition than in the RSI0 condition (mean = 0.59, SE = 0.04; mean difference = 0.12; crit. diff. = 0.082; p < 0.01; Fischer's PLSD). This result indicates that RSI0 participants were less successful in reproducing the training sequence, when asked to do so, than RSI250 participants—suggesting that the latter acquired more sequence knowledge irrespective of its implicit or explicit status. Exclusion scores, however, were on average higher in the RSI0 (mean = 0.37, SE = 0.02) than in the RSI250 condition (mean = 0.31, SE = 0.03; mean difference = 0.06; crit. diff. = 0.040; p < 0.01; Fischer's PLSD), indicating that RSI0 participants reproduced more often the training sequence under Exclusion instructions than RSI250 participants. This result, therefore, suggests an increased influence of implicit knowledge (I) when the RSI is reduced to 0 msec.
To further analyze Exclusion performance, one-tailed t-tests were used to compare Exclusion scores to the chance level. As participants were told not to produce repetitions, the chance level is 0.33. If participants lack control over their sequential knowledge, we expect Exclusion scores to be above what could be expected by chance. This pattern of results was observed in the RSI0 condition [t(20) = 1.83, p < 0.05] but not in the RSI250 [t(16) < 0.6] condition, confirming the influence of implicit knowledge on generation performance in the former but not the latter condition.
We also performed an ANOVA on the number of responses produced during each scan with Scans (3 levels) and Instructions (2 levels) as within-subject variables, and Condition (2 levels) as a between-subjects variable. This analysis revealed significant main effects of Instructions (F(1,35) = 15.124, p < 0.001) and of Scans (F(2,70) = 32.566, p < 0.0001). The Scans by Condition interaction was also significant (F(2,70) = 5.293, p < 0.01). The main effect of Condition and all the other interactions did not reach significance (all Fs < 3). In both RSI conditions, participants produced an increasing number of responses between the first and the third scan within both Inclusion and Exclusion tasks. They also produced more responses in the Inclusion than in the Exclusion task. Therefore, regression analyses between rCBF and performance (i.e., generation score in Inclusion and Exclusion conditions; see next section) incorporated the number of generated responses for each scan as a confounding covariate to control for the mere effect of modifications of response frequency on brain activity.
Brain imaging data
Neural bases of explicit processes in sequence generation
In a first analysis, we aimed to identify the brain regions that are specifically in charge of the explicit component of performance. To do so, in line with the logic of the PDP approach presented above, we considered the brain areas in which the regression between CBF and performance scores was modulated by generation instructions in the RSI0 and RSI250 groups. These interaction analyses looked for brain areas in which the correlation between rCBF and performance scores was significantly different between the Inclusion and Exclusion conditions. Results were further restricted to a priori locations in striatum and ACC/MPFC target brain areas, after correction for multiple comparisons in a small spherical volume (psvc; radius 20 mm).
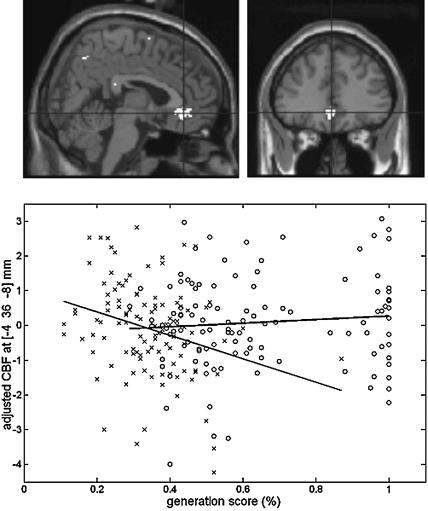
A conjunction analysis showed a common interaction effect in RSI250 and RSI0 groups in the anterior cingulate area (coordinates -436 -8 mm; Z = 3.87, psvc = 0.035) (Fig. 2, top panel). The analysis also revealed a nearly significant interaction effect in the putamen (-18 4 0 mm; Z = 3.74, psvc = 0.06). Data inspection revealed a significant negative correlation between the Exclusion generation scores and rCBF in the ACC/MPFC (Pearson correlation coefficient; r = -0.24, p = 0.009) (Fig. 2, lower panel). Correlation between the Inclusion generation scores and rCBF was nonsignificant (r = 0.012, p = 0.22). A two-tailed homogeneity test confirmed that correlations between rCBF and performance significantly differ between the Inclusion and Exclusion conditions (Fisher's Z comparison; p = 0.006). Interaction effects in other brain areas did not survive the statistical correction threshold. Uncorrected activations are reported Table 1A for completeness. This analysis indicates that higher activity in the ACC/MPFC corresponds to lower generation scores in the Exclusion condition (i.e., a better behavioral performance with regard to the Exclusion instructions) irrespective of the RSI. This result further supports the proposed role of the ACC/MPFC in the conscious component of performance in the sequence generation task.
Figure 2.
Neural correlates of conscious sequence learning. (Top panel) Conjunction analysis showing ACC/MPFC area [-436 -8 mm] in which correlation between sequence generation score and rCBF is modulated by the generation condition [Inclusion vs. Exclusion] both in the RSI0 and RSI250 conditions, psvc < 0.05. Data are displayed at the uncorrected p < 0.001 threshold, on an averaged T-1-weighted MRI. (Bottom panel) Scatterplot (and regression lines) of the correlations between rCBF changes (at coordinate indicated above) and generation scores in the Inclusion (circles, black line) and Exclusion (crosses, gray line) conditions. Generation performance in the Exclusion condition (i.e., low generation score reflects successful exclusion) significantly correlates with ACC/MPFC activity. Correlations with generation performance in the Inclusion condition is nonsignificant.
Table 1.
Correlations of regional cerebral activity with implicit and/or explicit components of performance in sequence generation
| Brain area | x | y | z | Z | ||||
|---|---|---|---|---|---|---|---|---|
| A. [Inclusion vs. Exclusion] by performance (RS1250 and RS10) | ||||||||
| Anterior cingulate gyrus | −4 | 36 | −8 | 3.87a | ||||
| Putamen | −18 | 4 | 0 | 3.74b | ||||
| 30 | 2 | 4 | 3.29 | |||||
| Cingulate gyrus | 22 | −22 | 44 | 3.46 | ||||
| Inferior frontal gyrus | 26 | 16 | −12 | 3.60 | ||||
| Superior frontal gyrus | 2 | 4 | 66 | 3.34 | ||||
| Insula | −44 | −18 | 16 | 3.48 | ||||
| Cuneus | −24 | −80 | 6 | 3.90 | ||||
| Precuneus | −4 | −60 | 50 | 3.38 | ||||
| Inferior parietal lobule | −40 | −28 | 40 | 3.68 | ||||
| Cerebellum, anterior lobe | −12 | −58 | −10 | 3.59 | ||||
| B. [RS1250 vs. RS10] × [Inclusion vs. Exclusion] by performance | ||||||||
| Medial frontal gyrus | −14 | 46 | 12 | 3.96a | ||||
| Caudate nucleus (striatum) | 14 | 14 | 16 | 3.17a | ||||
| Middle frontal gyrus | −28 | 12 | 34 | 3.48 | ||||
| Anterior cingulate | −14 | 42 | 0 | 3.29 | ||||
| C. [RS10 vs RS1250] by performance in Exclusion condition | ||||||||
| Caudate nucleus (striatum) | 14 | 12 | 18 | 3.64a | ||||
| 16 | 12 | 14 | 3.63a | |||||
| Superior frontal gyrus | −24 | 62 | −20 | 3.39 | ||||
| Cingulate gyrus | 2 | 24 | 34 | 3.32 | ||||
x, y, z are standard stereotactic coordinates (mm). Z is the Z-score. Results are significant at the voxel level p (uncorrected) <0.001 in clusters >5 voxels, excepted.
pSVC < 0.05 (radius 20 mm).
Statistical trend pSVC = 0.06.
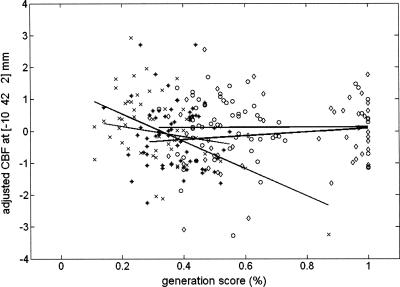
Nevertheless, the fact that the ACC was commonly activated in the above contrast does not preclude that reliable activation differences may exist between interactions conducted separately in the RSI250 and RSI0 groups. To assess this possibility, a factorial analysis looked for brain areas in which correlation between rCBF and generation score was higher in the Inclusion than the Exclusion condition, and more so in the RSI250 than in the RSI0 group. This analysis revealed a significant [Group × Condition] interaction effect in the medial prefrontal cortex (-14 46 12 mm; Z = 3.96, p = 0.016) and caudate nucleus (14 14 16 mm; Z = 3.17, p = 0.033) (Table 1B). In addition, we compared correlation patterns at the previously published coordinate associated with explicit processing in the RSI250 condition (-10 42 2 mm) (Destrebecqz et al. 2003) with RSI0 data. Scatterplots (Fig. 3) indicate a negative correlation between the Exclusion generation scores and rCBF both in the RSI250 (r = -0.50, p = 0.001) and the RSI0 (correlation coefficient r = -0.14, p = 0.29) conditions, but the correlation was significantly higher in the former than in the latter case (p = 0.038). Inclusion scores do not significantly differ (p = 0.51) between RSI0 (r = 0.008, p = 0.95) and RSI250 (r = 0.13, p = 0.34) conditions. Inclusion and Exclusion scores significantly differ in the RSI250 (p = 0.001) but not in the RSI0 (p = 0.43) condition. These results indicate that the ACC/MPFC is more involved in the conscious component of performance in the sequence generation task after essentially explicit (RSI250) than implicit (RSI0) learning during the SRT task, and that this increased involvement in conscious sequence processing is also accompanied by higher activation of the caudate nucleus.
Figure 3.
Scatterplot (and regression lines) of the correlations between rCBF changes in the ACC/MPFC and generation scores under Inclusion (black regression lines) and Exclusion (gray regression lines) instructions separately in the RSI0 and RSI250 conditions at the previously reported coordinate for the explicit contribution to sequence knowledge (coordinate taken from Destrebecqz et al. 2003). Generation performance in the Exclusion condition (i.e., low generation score reflects successful exclusion) significantly correlates with ACC/MPFC activity in the RSI250 (crosses) condition. The correlation did not reach significance in the RSI0 (asterisks) condition. Correlations with generation performance in the Inclusion condition are nonsignificant both in the RSI250 (diamonds) and RSI0 (circles) conditions.
Neural bases of implicit processes in sequence generation
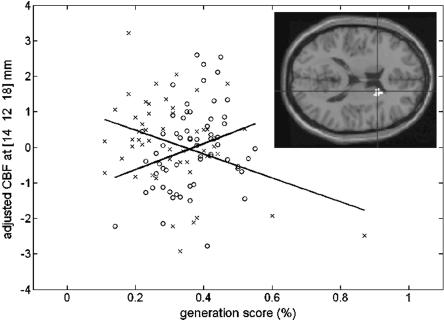
In another analysis, we aimed to identify the brain areas that are exclusively in charge of the implicit component of performance. Behavioral data indicated higher generation scores in the Exclusion condition in the RSI0 than the RSI250 group, suggesting less explicit control (or higher implicit influences) on performance in the former than in the latter group. Therefore, we looked for regions where the correlation between rCBF and generation scores in the Exclusion condition was modulated by the RSI (0 vs. 250 msec). This analysis yielded a significant interaction effect in the caudate nucleus of the striatum (14 12 18 and 16 12 14 mm; pssvc < 0.05) (Fig. 4). Interaction effects in other brain areas did not survive the statistical correction threshold. Uncorrected values are reported in Table 1C for completeness.
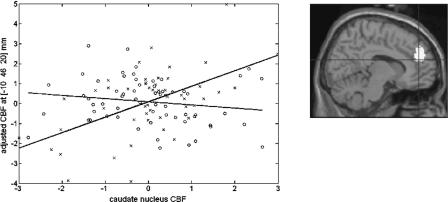
Figure 4.
Neural correlates of nonconscious sequence learning. Scatterplot of the interaction effect between RSI condition [RSI0 (circles) vs. RSI250 (crosses)] and rCBF correlation with generation scores under Exclusion instructions (psvc < 0.05). A positive significant correlation between generation score and caudate nucleus activity is found in the RSI0 condition (black regression line), whereas a significant negative correlation is found in the RSI250 condition (gray regression line). (Insert) Image shows activation in the caudate nucleus of the striatum (peak voxel [14 12 18] mm in standard anatomical space), displayed at uncorrected p < 0.001 on averaged T-1-weighted MRI.
There was a significant positive correlation between rCBF in the caudate nucleus and Exclusion generation score in the RSI0 condition (r = 0.28, p = 0.03). Correlation in the RSI250 condition was also significant but in the opposite (negative) direction (r = -0.36, p = 0.009). Homogeneity test confirmed correlation differences (p = 0.0006). Given that behavioral data indicated an increased influence of implicit knowledge in the RSI0 Exclusion condition, this result suggests that caudate nucleus activity supports implicit contribution to performance in sequence generation.
Dialogue between striatum and prefrontal areas in explicit and implicit sequence learning
Finally, a psychophysiological interaction analysis yielded a significant correlation between caudate nucleus activity (at coordinates identified above, 14 12 18 mm) and medial prefrontal cortex rCBF (-10 46 20 mm; psvc < 0.05) in the RSI250 (r = 0.54, p < 0.001) but not in the RSI0 (r = -0.16, p = 0.2) condition in the Exclusion task (Fig. 5). A homogeneity test confirmed significant correlation differences (p < 0.007). Interaction effects in other brain areas did not survive the statistical correction threshold. Uncorrected values are reported in Table 2 for completeness. The converse interaction (i.e., [RSI0 vs. RSI250] by caudate activity) failed to evidence any significant result. These data therefore suggest that the ACC/MPFC area is able to efficiently control the activity of the striatum during the Exclusion task, but only in the RSI250 condition, in which sequence learning was mostly explicit. In the RSI0 condition, in which explicit learning was impaired, ACC/MPFC activity does not prevent implicit influences on performance, which is reflected by increased erroneous generation of training triplets in the Exclusion task.
Figure 5.
Medial prefrontal cortex exerts control on the striatum in explicit learning condition. (Right panel) Psychophysiological interaction analysis showing medial prefrontal area [-10 46 20 mm] in which rCBF correlation with caudate nucleus activity is modulated by the RSI condition [RSI0 vs. RSI250] under Exclusion instructions (psvc < 0.05). Data are displayed at the uncorrected p < 0.001 threshold on averaged T-1-weighted MRI. (Left panel) Scatterplot of the correlations between rCBF changes in the medial prefrontal cortex and the caudate nucleus in RSI0 (circles, gray regression line) and RSI250 (crosses, black regression line) conditions under the Exclusion instruction. Activity of these two regions is coupled in the RSI250 condition only.
Table 2.
Explicit but no implicit modulation of caudate nucleus connectivity
| Brain area | x | y | z | Z | |||
|---|---|---|---|---|---|---|---|
| A. (explicit) [RS1250 vs. RS10] by caudate in Exclusion | |||||||
| Medial frontal gyrus | −10 | 46 | 20 | 4.02a | |||
| Middle frontal gyrus | −50 | 4 | 50 | 3.34 | |||
| Superior frontal gyrus | −14 | 26 | 56 | 3.28 | |||
| 32 | 44 | 30 | 3.89 | ||||
| Cuneus | 30 | −78 | 32 | 3.88 | |||
| −20 | −76 | 6 | 4.43 | ||||
| Cingulate gyrus | 12 | −36 | 34 | 4.11 | |||
| −22 | −36 | 44 | 3.47 | ||||
| Parahippocampal gyrus | 28 | −8 | −16 | 3.48 | |||
| Superior temporal gyrus | −60 | −48 | 18 | 3.44 | |||
| B. (implicit) [RS10 vs. RS1250] by caudate in Exlcusion | |||||||
| No activation found | |||||||
x,y,z are standard stereotactic coordinates (mm). Z is the Z-score. Results are significant at the voxel level p (uncorrected) <0.001 in clusters > 5 voxels, excepted.
psvc < 0.05 (radius 20 mm).
Discussion
In this paper, we have compared sequence learning in two training conditions differing only by the pace (RSI) of the choice reaction time task. To identify the brain regions subtending conscious and nonconscious sequence knowledge, participants were subsequently scanned during a generation task performed under Inclusion and Exclusion instructions.
We were not interested in a direct comparison between regional cerebral activities elicited in these conditions. The validity of such a comparison might, indeed, be undermined by several differences between the Inclusion and Exclusion tasks (see Destrebecqz et al. 2003, for a methodological discussion). First, as every participant performed the Inclusion task before the Exclusion task, time order is a confounding factor. Second, the response selection process differs in both tasks, for the Inclusion task requires choosing one response among three possible successors, whereas, in the Exclusion task, one element must be first excluded and the response then selected between the two remaining possibilities (recall that repetition responses were forbidden by the instructions). As a consequence, a simple rCBF difference between scans obtained under Inclusion and Exclusion conditions might be related to time order or task modality effects. However, given that Inclusion and Exclusion scores, respectively, reflect either the contribution of both explicit and implicit influences, or the contribution of implicit influences only, our main interest rested in the differences in the regression of regional cerebral activity on the performance measure.
We predicted that reducing the RSI would impair explicit knowledge acquisition during training and, therefore, participants' ability to successfully exclude trained sequence fragments in the Exclusion task. Behavioral results showed that participants learned the training sequence during the SRT task in both conditions. The magnitude of the transfer effect was, indeed, comparable for RSI0 and RSI250 participants—suggesting that sequence learning was, at least quantitatively, equivalent in both conditions irrespective of the value of the RSI. Generation results indicated, however, that learning differed qualitatively between the two conditions: Participants in the RSI250 condition reproduced the training sequence in the Inclusion task more frequently than RSI0 participants did. Importantly, Inclusion scores exceeded Exclusion scores in both conditions, but the difference between Inclusion and Exclusion scores was higher in the RSI250 condition. Within the Process Dissociation Procedure, the difference between Inclusion and Exclusion performance reflects participants' ability to control the expression of the acquired knowledge, and can therefore be used as an estimate of subjects' ability to consciously access this information. Under these assumptions, generation results thus suggest that subjects acquired conscious knowledge in both conditions, but that explicit learning was improved in the RSI250 condition. In the Exclusion task, RSI0 participants reproduced the training sequence more frequently than RSI250 participants. Exclusion scores were also above chance level in the former but not in the latter condition. Larger Exclusion scores are suggestive of increased implicit influences in the RSI0 as compared to the RSI250 condition.
The difference between Inclusion and Exclusion scores, reflecting explicit learning, was more important in our RSI0 condition as compared to previous behavioral reports using the same procedure (Destrebecqz and Cleeremans 2001, 2003). Different aspects of the task may be at the origin of this discrepancy: the specific procedural requirements of the PET scan measurements; the financial compensation provided to participants; and the fact that motor responses involved, ampler, forearm instead of finger movements may have fostered the acquisition of explicit knowledge about the sequence. However, our generation results are, in essence, in line with previous behavioral studies indicating that reducing the pace of the SRT task increases the acquisition of explicit sequence knowledge (Destrebecqz and Cleeremans 2001, 2003; see also Stadler 1997), and also improves the processes necessary for conscious and deliberate choice or error-signaling responses and for subsequent explicit recall of errors (Rabbitt 2002). It must be noted, however, that the influence of the pace of the SRT has received different interpretations. Willingham et al. (1997), for instance, have argued that this factor only influences the expression of knowledge rather than learning per se. Wilkinson and Shanks (2004) consider that the acquired knowledge is always conscious regardless of the value of the RSI used during training. We argue, however, that our behavioral results tend to confirm the influence of temporal factors on the explicit/implicit nature of the knowledge acquired in the SRT task. In this context, it is worth mentioning that evidence from classical conditioning studies also suggests that the temporal relationship between successive events profoundly modifies availability to consciousness (e.g., Clark and Squire 1998). We come back to this point below in the Discussion, but here summarize our PET results concerning the identity of the neural correlates of implicit and explicit sequence processing.
What are the brain regions that subtend the implicit and explicit components of performance? Our results confirm our initial hypothesis that the ACC/MPFC and the striatum are differentially involved in these components of sequence processing. These regions, among others, have been previously related with sequence learning in many functional brain imaging studies, but they have not been systematically associated with the explicit or implicit components of sequence processing. While the striatum has been generally associated with implicit sequence learning (Grafton et al. 1995; Rauch et al. 1995, 1997; Peigneux et al. 2000), activation in this region has also been found concurrently in both implicit and explicit sequence learning conditions (Willingham et al. 2002; Schendan et al. 2003; Aizenstein et al. 2004). In the same way, anterior cingulate and mesiofrontal activation has also been associated with both conscious and unconscious learning modes (Willingham et al. 2002; Aizenstein et al. 2004).
We believe that this apparent discrepancy with our results, which offer a more stringent delineation of implicit and explicit components, is due to the fact that both types of processes may have influenced performance in some previous imaging sequence learning experiments. Indeed, given that any task can involve both implicit and explicit components, different learning conditions aimed at improving either implicit or explicit learning may result in differential levels of activation of the same brain areas rather than in the activation of different regions assumed to be specifically in charge of the implicit or explicit component of processing. Willingham et al. (2002), for instance, have found greater prefrontal activation in an explicit sequence learning condition than under implicit conditions. Although our study specifically targeted the role of the striatum and the ACC/MPFC region in explicit and implicit learning, it is worth pointing out that many other regions have been described as being concurrently associated with implicit and explicit learning, including cuneus and precuneus, cingulate, parietal and frontal areas, thalamus, insula, media temporal regions, and cerebellum (Willingham et al. 2002; Schendan et al. 2003; Aizenstein et al. 2004). Interestingly, those various structures have been found to be activated in the present study, but in association with the explicit component of performance in the generation task (Table 1), suggesting that some explicit processes were also subtending performance in the implicit condition of those recent studies. Hence, the use of a sensitive methodology such as the Process Dissociation Procedure makes it possible to disentangle between the brain regions that specifically subtend one or the other type of processes.
As mentioned earlier, our results suggest that temporal factors influence the implicit versus explicit status of the acquired knowledge, a result that is reminiscent of a similar phenomenon described in the field of classical eye-blink conditioning (Clark and Squire 1998). In classical eye-blink conditioning, an air-puff directed toward the participant's cornea (the unconditioned stimulus, US) is systematically preceded by a tone (the conditioned stimulus, CS). After repeated CS-US pairings, participants will tend to produce a conditioned eye-blink response (CR) after the CS, even before the onset of the air-puff. In one form of classical conditioning, called delay conditioning, conditioned (CS) and unconditioned (US) stimuli are concomitant for a few hundred milliseconds before the CS terminates. In this case, awareness of the relationship between both types of stimuli is not necessary for participants to learn to associate them and to produce the conditioned response in response to the CS. In trace conditioning, in contrast, the CS and US are separated by a temporal delay (typically 1000 msec), and only the participants who become aware of the CS-US association can be successfully conditioned. At the neuroanatomical level (for a thorough overview, see Woodruff-Pak and Steinmetz 2002), it has been shown that learning depends on the cerebellum in both trace (Woodruff-Pak et al. 1985) and delay conditioning (Thompson and Krupa 1994). In trace conditioning, however, the neocortex and hippocampus are involved to maintain information over time, making it possible for the cerebellum to process the CS and US at the same time (Clark and Squire 1998).
An interesting possibility is that a similar kind of time-dependent mechanism applies in sequence learning. Indeed, the SRT task can be considered as a complex form of conditioning in which the successive and systematic presentation of two events in close succession results in their association in memory (Keele et al. 2003). Accordingly, Graybiel (1998) proposed that the basal ganglia may chunk the representations of motor and cognitive action sequences so that they can be implemented as performance units. As discussed elsewhere (Peigneux et al. 2000), such striatum-related chunking mechanisms are, for at least two reasons, interestingly compatible with the slow kinetics of learning without awareness. First, slow learning is a necessary condition to avoid collapsing all temporally ordered acts into chunks. Indeed, only relevant sequences must be selected, because once formed, one of the characteristics of a chunk is that it will be difficult to break apart. Second, the absence of awareness is an advantageous property for a chunking mechanism because it entails that chunks will be processed as encapsulated units rather than as chains of separate elements. Once the information is acquired and automated, we have suggested (Peigneux et al. 2000) that the striatum is particularly active for the selection of the most appropriate response in the context created by both the current stimulus and sequences of encapsulated previous stimuli, a process that would not only lead to higher efficiency and faster response preparation in the SRT task but may also support accurate predictions in the subsequent sequence generation task. Hence, this analysis suggests that the striatum plays the role of an automated association system for temporally successive events, a mechanism that might support generation performance both in the RSI0 and RSI250 conditions and does not necessarily need to be associated with conscious processing. However, when longer time delays are used between the to-be-associated events in the SRT task, medial prefrontal and cingulate areas should come into play in order to maintain information relative to the temporal context of the current target, making it possible for the striatum to process temporally disjointed events. The involvement of medial prefrontal and cingulated areas, whose activity tends to be systematically accompanied by awareness, will incidentally promote conscious processing when a slower pace is adopted in the SRT task. This interpretation is also consistent with (1) animal reports showing that the prefrontal cortex contributes to the association of events separated in time (McLaughlin et al. 2002; Runyan et al. 2004), and (2) modeling studies suggesting that this cortical region may provide the timing information required for organizing various behaviors (Kitano et al. 2003). It also fits with our finding that rCBF in the ACC/MPFC specifically correlates with the explicit component of performance in the generation task (Destrebecqz et al. 2003; this study).
Most importantly, the correlated activity of the ACC/MPFC and of the striatum in the RSI250 but not the RSI0 group in the Exclusion condition suggests that RSI250 participants use their explicit knowledge of the sequence to inhibit striatum-related implicit response preparation and successfully master Exclusion instructions. As shown in Figure 4, the caudate is active in both the RSI0 and RSI250 Exclusion condition, but in opposite directions. In the RSI250 Exclusion condition, caudate activity is negatively correlated with performance scores, likewise the ACC/MPFC. Hence, higher activity in these areas is associated with lower generation scores in the Exclusion condition, that is, a better performance with regard to the instruction to avoid generating the learned sequence. This association is further reflected in the psychophysiological analysis in which a significant coupling of activity was observed between striatum and ACC/MPFC during Exclusion in the RSI250 condition (Fig. 5). In the RSI0 Exclusion condition, however, there was a positive correlation between CBF in the striatum and generation score (Fig. 4). Here, the higher the striatum activity, the more the subjects generated elements from the learned sequence. Moreover, no coupling between striatum and ACC/MPFC was observed anymore in the RSI0 Exclusion condition in the psychophysiological analysis, suggesting stochastic independence between the activities of these two structures. Hence, striatum activity positively correlates with generation score when learning is essentially implicit (RSI0), while uncoupled with ACC/MPFC activity, but the direction of the correlation is opposite and parallels ACC/MPFC activity when learning is essentially explicit (RSI250). Our results therefore suggest that the striatum and the ACC/MCPF interact in such a way that the ACC/MPFC exerts control on the activity of the striatum during retrieval of the sequence when learning had been mostly explicit, whereas the activity of these regions is uncoupled when learning had been essentially implicit. An alternative interpretation would be that, rather than a unidirectional modulation of the ACC/MPFC on caudate nucleus activity in explicit conditions, reciprocal connections are created between striatal and ACC/MPFC regions and define an interactive functional circuit that is selectively engaged when learning has been essentially explicit. However, this interpretation cannot be easily reconciled with the fact that caudate nucleus activity correlates with behavioral performance scores positively in the implicit condition, but negatively in the explicit condition during which positive relationships are observed between ACC/MPFC and performance.
To sum up, this study provides further evidence for dissociation between the neural substrates that support conscious and nonconscious components of performance during recollection of a learned sequence. It must be stressed, however, that we do not argue for the existence of two separate and independent learning systems but, rather, for the functional interaction between different brain regions that subtend different computational objectives, and in which information processing appears to be either accompanied by awareness (the ACC/MCPF) or not (the striatum).
Materials and Methods
Participants
This study was approved by the Ethics Committee of the Faculty of Medicine of the University of Liège. Subjects in the RSI0 group were 21 young (range 20-26 yr), healthy, right-handed males (n = 14) and females (n = 7), recruited through advertisement. Data in the RSI250 group were obtained from a prior experiment (Destrebecqz et al. 2003) in which 17 right-handed male volunteers (range 18-32 yr) participated. Written informed consent was obtained from all subjects. Data from the RSI250 group were fully reprocessed and reanalyzed for the purpose of the present study.
SRT and sequence generation tasks
Before the SRT task, participants were simply told that the goal of the experiment was to study, with PET, the cerebral effects of sustained practice on a simple motor task. On each trial of the four-choice SRT task, a black circle appeared on a computer screen 2 cm above one out of four permanent black squares spaced 4 cm apart. Participants (using the right hand) were instructed to press as fast and as accurately as possible on the key corresponding to the location of the target on the screen. The target was erased after each response and appeared at another location after a 0-msec or 250-msec delay depending on the RSI condition. Errors were indicated by a short beep. A short break occurred after each block. Unknown to participants, each block contained eight repetitions of one of the two following 12-element sequences: 342312143241 (S1) or 341243142132 (S2). These sequences are identical insofar as stimulus locations and transition frequencies are concerned, but differ in terms of which subsequences of three elements they contain. To assess the extent to which subjects learned the training sequence, the other sequence was presented on block 13 (if a participant was trained on S1, S2 was used on block 13 and vice versa). Assuming that reaction time improvement reflects motor response preparation and anticipation of the next stimulus, reaction times should increase on block 13 only if participants have acquired specific knowledge about the sequential regularities characteristic of the training sequence presented over blocks 1-12 (Reed and Johnson 1994). Participants practiced the SRT task lying in the scanner, but were not scanned at this time.
After completion of the SRT task, participants were told that the sequence of stimuli contained regularities, and were then introduced to the generation task, during which they were scanned in both the Inclusion and Exclusion conditions (three scans each). In both Inclusion and Exclusion conditions, each scan began with the presentation of a randomly selected stimulus. Participants then had to indicate, throughout the 90-sec duration of the scan, the location of the next stimulus by pressing on the corresponding key. Participants were also explicitly told that the generated sequence, as the training sequence, could not contain repetition. The stimulus moved according to the participant's responses after a 0-msec or a 250-msec delay depending on the condition (i.e., RSI0 or RSI250, respectively). To avoid further learning during generation, no feedback was given in this task. During the first three scans, participants were asked to perform the Inclusion task, that is, to try to reproduce the training sequence or, failing recollection, to guess the location of the next stimulus. During the next three scans, they performed the Exclusion task, where they had to try, on each trial, to avoid reproducing the sequential regularities of the training sequence. In the Exclusion condition, to ensure that participants, indeed, performed Exclusion on each trial, they were specifically instructed not to systematically repeat a sequence they believed to be different from the training one, and not to apply particular strategies such as repeating the same response throughout the task. In both tasks, participants were also instructed not to produce repetitions (which never occurred in the training material).
PET data acquisition and analysis
PET data were acquired on a Siemens CTI 951 R 16/31 scanner in 3D mode using an identical procedure in the RSI0 and RSI250 conditions. The subject's head was stabilized by a thermoplastic facemask secured to the head holder (Truscan Imaging), and a venous catheter was inserted in a left antebrachial vein. A transmission scan was performed to allow measured attenuation corrections. Regional CBF was estimated during six 90-sec emission scans obtained successively in Inclusion then Exclusion (three scans each) conditions. Each scan consisted of two frames: a 30-sec background frame and a 90-sec active frame. The slow intravenous water (H215O) infusion began frame. 10 sec before the second For each scan, 6 mCi (222 MBq) was injected, in 5 cc of saline, over a period of 20 sec. Data were reconstructed using a Hanning filter (cut-off frequency: 0.5 cycle/pixel) and corrected for attenuation and background activity.
PET data were analyzed using the statistical parametric mapping software SPM2 (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks Inc.). For each subject, all scans were realigned together, then normalized to a standard PET template and smoothed using a Gaussian kernel of 16 mm full-width at half-maximum (FWHM). Group (RSI0 or RSI250) and scan (Inclusion or Exclusion) conditions, covariate of interest (generation score at each scan), condition by covariate, and subject (block) effects were estimated at each and every voxel according to the general linear model (Frackowiak et al. 1997). Global flow adjustment was performed by subject-specific analysis of covariance, and the number of responses generated during each scan was entered as a confounding covariate to control for individual differences in response frequency and motor-related activations. The resulting set of voxel values for each contrast constituted a map of the t-statistic {[SPM(T)] thresholded at p < 0.001 uncorrected (T ≤ 3.14)}. The reported results are significant at psvc < 0.05 after a small volume correction (radius 20 mm) around previously published coordinates of interest (see below). In essence, these analyses in which within-subject and between-subject variability are combined rely on a fixed effect model. The results therefore only pertain to the sampled subjects, and will need to be confirmed at the population level.
The first analysis aimed to show that the ACC/MPFC region specifically subtends the conscious, explicit, component of performance, both in the RSI0 and RSI250 conditions. To do so, we looked for interaction effects in regions in which rCBF correlation with performance (i.e., the generation score) was modulated by the generation condition [Inclusion vs. Exclusion], separately in the RSI0 and RSI250 condition groups. A subsequent conjunction analysis identified the common plots of interaction in both conditions. This analysis was further complemented by a Condition [Inclusion vs. Exclusion] × RSI [0 vs. 250 msec] by generation score interaction analysis to highlight the brain areas in which this [Inclusion vs. Exclusion] by performance modulation was higher in the RSI250 than in the RSI0 group. Reported results are significant at psvc < 0.05 around previously published coordinates of conscious sequence knowledge-related activation in the ACC/MPFC (-16 42 2 mm), thalamus (6 -18 16 mm), and superior temporal gyrus (-50 -34 8 mm) (Destrebecqz et al. 2003).
The second analysis aimed to probe the role of the striatum in subtending the implicit, automatic, component of performance. This analysis looked for brain areas in which rCBF correlation with performance (i.e., generation score) during the Exclusion condition was modulated by the duration of the RSI during learning ([RSI0 vs. RSI250] condition). Reported results are significant at psvc < 0.05 around a previously published coordinate of implicit learning-related activation in the caudate nucleus of the striatum (-16 8 10 mm) (Peigneux et al. 2000).
Finally, we aimed to probe the nature of the relationships between ACC/MPFC and caudate nucleus rCBF in [RSI0 vs. RSI250] Exclusion conditions. To do so, a psychophysiological interaction analysis (PPI) (Friston et al. 1997) aimed to assess if rCBF in the ACC/MPFC regions differentially correlates with the caudate nucleus rCBF (as identified in the second analysis) in the RSI0 versus RSI250 groups. For the PPI analysis, three regressors were included in a novel design matrix. The first two regressors included the main two components of the PPI. One was the time series from the source region (i.e., caudate nucleus activity at [14 12 18 mm]) across scans in the Exclusion condition. The second was the psychological variable in which each scan was weighted (+1/-1) according to the RSI250 or RSI0 condition. The third regressor was the product of the interaction between the time series and the psychological variable. The PPI was assessed by looking to see if the regression coefficient for the third regressor (i.e., the interaction variable) is significantly nonzero, in other terms, if significant differences exist in functional connectivity between the caudate nucleus and any other region in one RSI condition with respect to the other RSI condition. Reported results are significant at psvc < 0.05 around the above-mentioned previously published coordinates of interest (Destrebecqz et al. 2003).
Acknowledgments
We thank D.B. Willingham and two anonymous reviewers for their insightful and helpful comments on previous versions of this manuscript. A.D., S.L., A.C., and P.M. are with the Fonds National de la Recherche Scientifique (FNRS; Belgium): A.D. is Scientific Research Worker, S.L. is Research Associate, A.C. is Senior Research Associate, and P.M. is Research Director. This research was supported by FNRS grant number 3.4537.00, by the Reine Elisabeth Medical Foundation, by Interuniversity Attraction Poles Programme-Belgian Science Policy and special funds for scientific research of the Université de Liège, by grant HPRN-CT-1999-00065 from the European Commission, and by an institutional grant from the Université Libre de Bruxelles to A.C.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.95605.
References
- Aizenstein, H.J., Stenger, V.A., Cochran, J., Clark, K., Johnson, M., Nebes, R.D., and Carter, C.S. 2004. Regional brain activation during concurrent implicit and explicit sequence learning. Cereb. Cortex 14: 199-208. [DOI] [PubMed] [Google Scholar]
- Clark, R.E. and Squire, L. 1998. Classical conditioning and brain systems: The role of awareness. Science 280: 77-81. [DOI] [PubMed] [Google Scholar]
- Cleeremans, A., Destrebecqz, A., and Boyer, M. 1998. Implicit learning: News from the front. Trends Cogn. Sci. 2: 406-416. [DOI] [PubMed] [Google Scholar]
- Clegg, B.A., DiGirolamo, G.J., and Keele, S.W. 1998. Sequence learning. Trends Cogn. Sci. 2: 275-281. [DOI] [PubMed] [Google Scholar]
- Destrebecqz, A. and Cleeremans, A. 2001. Can sequence learning be implicit? New evidence with the process dissociation procedure. Psychonom. Bull. Rev. 8: 343-350. [DOI] [PubMed] [Google Scholar]
- ____. 2003. Temporal factors in sequence learning. In Attention and implicit learning (ed. L. Jiménez), pp. 181-213. John Benjamins, Amsterdam and Philadelphia.
- Destrebecqz, A., Peigneux, P., Laureys, S., Degueldre, C., Del Fiore, G., Aerts, J., Luxen, A., van der Linden, M., Cleeremans, A., and Maquet, P. 2003. Cerebral correlates of explicit sequence learning. Brain Res. Cogn. Brain Res. 16: 391-398. [DOI] [PubMed] [Google Scholar]
- Erderlyi, M.H. 1986. Experimental indeterminacies in the dissociation paradigm of subliminal perception. Behav. Brain Sci. 9: 30-31. [Google Scholar]
- Frackowiak, R.S.J., Friston, K.J., Frith, C.D., Dolan, R.J., and Mazziotta, J.C. 1997. Human brain function. Academic Press, San Diego, CA.
- Friston, K.J., Buechel, C., Fink, G.R., Morris, J., Rolls, E., and Dolan, R.J. 1997. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218-229. [DOI] [PubMed] [Google Scholar]
- Goschke, T. 1997. Implicit learning and unconscious knowledge: Mental representation, computational mechanism, and brain structures. In Knowledge, concepts and categories (eds. K. Lambert and D. Shanks), Vol. 8, pp. 247-333. Psychology Press, London. [Google Scholar]
- Grafton, S.T., Hazeltine, E., and Ivry, R. 1995. Functional mapping of sequence learning in normal humans. J. Cogn. Neurosci. 7: 497-510. [DOI] [PubMed] [Google Scholar]
- Graybiel, A.M. 1998. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 70: 119-136. [DOI] [PubMed] [Google Scholar]
- Hazeltine, E., Grafton, S.T., and Ivry, R. 1997. Attention and stimulus characteristics determine the locus of motor-sequence encoding—A PET study. Brain 120: 123-140. [DOI] [PubMed] [Google Scholar]
- Honda, M., Deiber, M.P., Ibanez, V., Pascual-Leone, A., Zhuang, P., and Hallett, M. 1998. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain 121: 2159-2173. [DOI] [PubMed] [Google Scholar]
- Jacoby, L.L. 1991. A process dissociation framework: Separating automatic from intentional uses of memory. Mem. Lang. 30: 513-541. [Google Scholar]
- Jiménez, L., Mendéz, C., and Cleeremans, A. 1996. Comparing direct and indirect measure of sequence learning. J. Exp. Psych. Learn. Mem. Cogn. 22: 948-969. [Google Scholar]
- Keele, S.W., Ivry, R., Mayr, U., Hazeltine, E., and Heuer, H. 2003. The cognitive and neural architecture of sequence representation. Psychol. Rev. 110: 316-339. [DOI] [PubMed] [Google Scholar]
- Kitano, K., Okamoto, H., and Fukai, T. 2003. Time representing cortical activities: Two models inspired by prefrontal persistent activity. Biol. Cybern. 88: 387-394. [DOI] [PubMed] [Google Scholar]
- McLaughlin, J., Skaggs, H., Churchwell, J., and Powell, D.A. 2002. Medial prefrontal cortex and Pavlovian conditioning: Trace versus delay conditioning. Behav. Neurosci. 116: 37-47. [PubMed] [Google Scholar]
- Merikle, P.M. and Reingold, E.M. 1991. Comparing direct (explicit) and indirect (implicit) measures to study unconscious memory. J. Exp. Psych. Learn. Mem. Cogn. 17: 224-233. [Google Scholar]
- Peigneux, P., Maquet, P., Meulemans, T., Destrebecqz, A., Laureys, S., Degueldre, C., Delfiore, G., Aerts, J., Luxen, A., Franck, G., et al. 2000. Striatum forever, despite sequence learning variability: A random effect analysis of PET data. Hum. Brain Mapp. 10: 179-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchet, P. and Amorim, M.A. 1992. Conscious knowledge and changes in performance in sequence learning: Evidence against dissociation. J. Exp. Psych. Learn. Mem. Cogn. 18: 785-800. [DOI] [PubMed] [Google Scholar]
- Rabbitt, P. 2002. Consciousness is slower than you think. Q. J. Exp. Psychol. A 55: 1081-1092. [DOI] [PubMed] [Google Scholar]
- Rauch, S.L., Savage, C.R., Brown, H.D., Curran, T., Alpert, N.M., Kendrick, A., Fischman, A.J., and Kosslyn, S.M. 1995. A PET investigation of implicit and explicit sequence learning. Hum. Brain Mapp. 3: 271-286. [Google Scholar]
- Rauch, S.L., Whalen, P.J., Savage, C.R., Curran, T., Kendrick, A., Brown, H.D., Bush, G., Breiter, H.C., and Rosen, B.R. 1997. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum. Brain Mapp. 5: 124-132. [PubMed] [Google Scholar]
- Reed, J. and Johnson, P. 1994. Assessing implicit learning with indirect tests: Determining what is learned about sequence structure. J. Exp. Psychol. Learn. Mem. Cogn. 20: 585-594. [Google Scholar]
- Reingold, E.M. and Merikle, P.M. 1988. Using direct and indirect measures to study perception without awareness. Percept. Psychophys. 44: 563-575. [DOI] [PubMed] [Google Scholar]
- Richardson-Klavehn, A., Gardiner, J.M., and Java, R.I. 1996. Memory: Task dissociations, process dissociations, and dissociation of consciousness. In Implicit cognition (ed. G. Underwood), pp. 85-158. Oxford University Press, Oxford.
- Runyan, J.D., Moore, A.N., and Dash, P.K. 2004. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosc. 24: 1288-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan, H.E., Searl, M.M., Melrose, R.J., and Stern, C.E. 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37: 1013-1025. [DOI] [PubMed] [Google Scholar]
- Shanks, D.R. and Johnstone, T. 1999. Evaluating the relationship between explicit and implicit knowledge in a serial reaction time task. J. Exp. Psychol. Learn. Mem. Cogn. 25: 1435-1451. [DOI] [PubMed] [Google Scholar]
- Shanks, D.R., Green, R.E.A., and Kolodny, J.A. 1994. A critical examination of the evidence for unconscious (implicit) learning. In Attention and performance XV (eds. C. Umiltà and M. Moscovitch), pp. 837-860. MIT Press, Cambridge, MA.
- Stadler, M.A. 1997. Distinguishing implicit and explicit learning. Psychon. Bull. Rev. 4: 56-62. [Google Scholar]
- Thompson, R.F. and Krupa, D.J. 1994. Organization of memory traces in the mammalian brain. Annu. Rev. Neurosci. 17: 519-549. [DOI] [PubMed] [Google Scholar]
- Wallach, D. and Lebiere, C. 2003. Implicit and explicit learning in a unified architecture of cognition. In Attention and implicit learning (ed. L. Jiménez), pp. 215-250. John Benjamins, Amsterdam and Philadelphia.
- Wilkinson, L. and Shanks, D.R. 2004. Intentional control and implicit sequence learning. J. Exp. Psychol. Learn. Mem. Cogn. 30: 354-369. [DOI] [PubMed] [Google Scholar]
- Willingham, D.B. and Goedert-Eschmann, K. 1999. The relation between implicit and explicit learning: Evidence for parallel development. Psychol. Sci. 10: 531-534. [Google Scholar]
- Willingham, D.B., Nissen, M.J., and Bullemer, P. 1989. On the development of procedural knowledge. J. Exp. Psychol. Learn. Mem. Cogn. 15: 1047-1060. [DOI] [PubMed] [Google Scholar]
- Willingham, D.B., Greenberg, A.R., and Cannon Thomas, R. 1997. Response-to-stimulus interval does not affect implicit motor sequence learning, but does affect performance. Mem. Cogn. 25: 534-542. [DOI] [PubMed] [Google Scholar]
- Willingham, D.B., Salidis, J., and Gabrieli, J.D.E. 2002. Direct comparison of neural systems mediating conscious and unconscious skill learning. J. Neurophysiol. 88: 1451-1460. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak, D. and Steinmetz, A. 2002. Eyeblink classical conditioning. Volume 1: Applications in humans. Kluwer Academic Publisher, New York.
- Woodruff-Pak, D.S., Lavond, D.G., and Thompson, R.F. 1985. Trace conditioning: Abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 348: 249-260. [DOI] [PubMed] [Google Scholar]