Abstract
When analyzed by fluorescent amplified fragment length polymorphism and repetitive extragenic palindrome-PCR fingerprinting, a total of 47 Vibrio halioticoli strains isolated from four Japanese abalone species and one turban shell species formed three clusters that roughly reflect the different species of host abalone from which they were isolated. The V. halioticoli isolates from turban shells were distributed evenly among the clusters. Representative isolates from two clusters were deemed separate species or subspecies by DNA-DNA hybridization.
Gut microbial ecology studies of human, mouse, and ruminant systems are quite advanced due to the development of tools for examining host-microbe interactions in an evolutionary context (time scale) and for identifying the interactions (7, 16). Host characteristics such as innate immunity and nutrition suggest that gut microbes have coevolved with their hosts to develop various symbiotic, commensal, and pathogenic associations (7). With the exception of the Vibrio halioticoli-abalone relationship, these relationships in marine herbivores have not been well studied (17, 18, 22). Abalone have had conserved herbivorous feeding behavior (on algae) throughout their long evolutionary history. In one abalone species (Haliotis discus hannai), a unique alginolytic bacterium has been found in abundances of 105 to 109 CFU/g of gut tissue, and both the abalone species and the bacterium have unique substrate specificities and alginate degradation activities, suggesting that the bacterium may contribute significantly to the host's digestion of alginate (17). This novel alginolytic and facultatively anaerobic bacterium was classified as Vibrio halioticoli (18). Recently, V. halioticoli-like strains have been found in the gut of three other species of Haliotidae abalone and one species of Turbinidae shell in Japan (T. Sawabe, N. Setoguchi, R. Tanaka, O. Setoguchi, M. Yoshimizu, and Y. Ezura, abstract from the Annual Scientific Meeting of the Australian Society for Microbiology 2000, Microbiol. Aust. 21:A119, 2000). V. halioticoli may be a key symbiotic microbe for digesting and converting alginate to available energy sources for the host like volatile short-chained fatty acids.
Genetic diversity among bacterial strains can be assessed by using Box, enterobacterial repetitive intergenic consensus, and repetitive extragenic palindrome (REP)-PCR genomic fingerprinting techniques (14, 15, 24). Another fingerprinting technique, amplified fragment length polymorphism (AFLP), has been used in bacterial taxonomy (8, 9, 23) and diversity studies of pathogenic bacteria for epidemiological purposes (1, 2, 10, 11). AFLP fingerprinting has a higher discriminating power than the Box, enterobacterial repetitive intergenic consensus, and REP-PCR fingerprinting techniques (15). However, results of both AFLP and REP-PCR fingerprinting techniques are in close agreement with those of DNA-DNA hybridization studies, and these techniques are regarded as the best tools available to date for determining the taxonomic and phylogenetic structures of bacterial populations (3, 13, 15). Recent studies comparing the similarity coefficients of genomic fingerprinting results and DNA-DNA hybridization values for Stenotrophomonas and Xanthomonas strains found that AFLP similarity values (Dice coefficients [SDs]) above 55 to 65% correlated with DNA homology values of 70 to 75% (6, 15).
Gut microbes, which play an important role in the host digestion system, may have coevolved with their hosts. To examine whether the host-gut microbe association of V. halioticoli-like strains is host specific, the genetic diversity of these V. halioticoli-like strains from abalone and turban shells collected from various locations along coastal Japan were analyzed by both AFLP and REP-PCR fingerprinting techniques. Relatedness was further examined by DNA-DNA hybridization.
AFLP and REP-PCR genomic fingerprinting of V. halioticoli-like strains.
The V. halioticoli-like strains used in this study were isolated from five host animals (Table 1): 17 strains were from the abalone species H. discus hannai, 14 were from Haliotis discus discus, 10 were from Haliotis diversicolor aquatilis, 1 was from Haliotis diversicolor diversicolor, and 3 were from the turban shell species Turbo cornutus. Two strains were collected from seawater around abalone farms (22; Sawabe et al., Microbiol. Aust. 21:A119, 2000). All isolates except confirmed V. halioticoli strains IAM14569T, IAM14597, IAM14598, and IAM14599 (18) were identified as V. halioticoli-like by using V. halioticoli-specific colony hybridization (21) and 16S ribosomal DNA PCR/restriction fragment length polymorphism analysis (20). Isolates used for fingerprint analysis were geographically distributed in Japan as follows: H. discus hannai isolates from the three Hokkaido sites and one Iwate site were 500 km apart, and H. discus discus isolates from Kanagawa and Izu Ohshima were 100 km apart (Table 1). All strains were maintained on ZoBell 2216E agar containing 0.5% sodium alginate (17).
TABLE 1.
V. halioticoli-like strains used for fingerprinting analysis
| V. halioticoli strain (synonym)a | Host animal of isolateb
|
|||
|---|---|---|---|---|
| Species | Date collected | Place of collectionc | Statusd | |
| IAM 14596 | H. discus hannai | July 1991 | Kumaishi | Cultured |
| IAM 14597 | H. discus hannai | Apr. 1993 | Taisei | Cultured |
| IAM 14598 | H. discus hannai | Jan. 1994 | Shiriuchi | Cultured |
| IAM 14599 | H. discus hannai | Jan. 1994 | Shiriuchi | Cultured |
| 1Y2-20 | H. discus hannai | May 1997 | Kumaishi | Cultured |
| 1Y2-26 (LMG19964) | H. discus hannai | May 1997 | Kumaishi | Cultured |
| 2Y1-13 (LMG19965) | H. discus hannai | May 1997 | Kumaishi | Cultured |
| 2Y2-23 | H. discus hannai | May 1998 | Kumaishi | Cultured |
| 25Y1-10 | H. discus hannai | May 1997 | Kumaishi | Cultured |
| 25Y2-25 | H. discus hannai | May 1997 | Kumaishi | Cultured |
| KN1Y1-9 | H. discus hannai | July 1997 | Ofunato | Cultured |
| KN1Y2-9 | H. discus hannai | July 1997 | Ofunato | Cultured |
| KL1Y1-12 | H. discus hannai | July 1997 | Ofunato | Cultured |
| KL1Y1-18 | H. discus hannai | July 1997 | Ofunato | Cultured |
| KL1Y2-10 | H. discus hannai | July 1997 | Ofunato | Cultured |
| KLA1Y1-10 | H. discus hannai | July 1997 | Ofunato | Cultured |
| KLA1Y2-23 | H. discus hannai | July 1997 | Ofunato | Cultured |
| HDD1-1 (LMG19972) | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD1-2 | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD2-1 (LMG19971) | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD2-2 | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD3-1 (LMG19973) | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD3-2 | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD4-1 (LMG19974) | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD4-2 | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD5-1 (LMG19975) | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD5-2 | H. discus discus | July 1999 | Kanagawa | Cultured |
| HDD6-1 (LMG19977) | H. discus discus | Dec. 1999 | Izu Ohshima | Cultured |
| HDD6-2 (LMG19976) | H. discus discus | Dec. 1999 | Izu Ohshima | Cultured |
| HDD7-1 | H. discus discus | Dec. 1999 | Izu Ohshima | Wild |
| HDD7-2 (LMG19978) | H. discus discus | Dec. 1999 | Izu Ohshima | Wild |
| HDS1-1 (LMG19970) | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS1-2 | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS2-1 (LMG19969) | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS2-2 | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS3-1 (LMG19968) | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS3-2 | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS4-1 (LMG19967) | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS4-2 | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS5-1 (LMG19966) | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDS5-2 | H. diversicolor aquatilis | July 1999 | Kanagawa | Cultured |
| HDV1-1 (LMG19979) | H. diversicolor diversicolor | Dec. 1999 | Izu Ohshima | Wild |
| TC2-1 | T. cornutus | Dec. 1999 | Izu Ohshima | Wild |
| TC2-3 | T. cornutus | Dec. 1999 | Izu Ohshima | Wild |
| TC4-2 (LMG19963) | T. cornutus | Dec. 1999 | Izu Ohshima | Cultured |
| KSW-5 | Seawater | May 1997 | Kumaishi | |
| COSW-2 | Seawater | July 1999 | Kumaishi | |
LMG, BCCMTM/LMG Bacteria Collection, Laboratorium voor Microbiologie, Gent Universitiet, Ghent, Belgium; IAM, Institute of Applied Microbiology, Tokyo University, Tokyo, Japan.
All isolates were from host animals, except for KSW-5 and COSW-2, which were isolated from seawater.
Kumaishi, Taisei, and Shiriuchi are located in Hokkaido, Japan; Ofunato is located in Iwate Prefecture, Japan; Kanagawa is located in Kanagawa Prefecture, Japan; and Izu Ohshima is located in Tokyo, Japan.
Cultured, abalone farmed and cultured using artificial fertilization; wild, abalone caught in the wild.
Cells used for DNA extraction were cultured in ZoBell 2216E broth at 25°C for 24 h, harvested, and extracted with a Promega (Madison, Wis.) Wizard genomic DNA extraction kit according to the manufacturer's instructions.
Fluorescent AFLP patterns of the 47 strains were generated and analyzed as described previously (23). Briefly, 1 μg of high-molecular-weight DNA was digested with TaqI and HindIII, followed by ligation of restriction half-site-specific adapters, and amplified by performing PCR twice with primers H00/T00 and H01-6FAM/T03 (9). PCR products were separated on 36-cm denaturing polyacrylamide gels on an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, Calif.). GeneScan 3.1 software (Applied Biosystems) was used to track and normalize the lanes. Tables of data from normalized peaks containing fragments of 50 to 536 bp were transferred into BioNumerics 2.0 software (Applied Maths, Sint-Martens-Latem, Belgium) for numerical analysis. Clustering of the patterns was done by use of the Dice coefficient (SD) and the Ward algorithm (19).
REP-PCR fingerprinting was performed using the PCR conditions described previously by Rademaker et al. (14) and reaction mixtures containing 1 μl of DNA (50 ng μl−1), 5 μl of 5× Gitschier buffer (14), 0.4 μl of bovine serum albumin (10 mg ml−1), 2.5 μl of dimethyl sulfoxide (10 mg ml−1), 1.25 μl of a deoxynucleoside triphosphate mixture (100 mM each deoxynucleoside triphosphate), 1 μl of GTG5 primer (0.3 μg μl−1; Amersham Pharmacia Biotech, Uppsala, Sweden) (24), and 0.4 μl of Taq DNA polymerase (5 U μl−1; Goldstar Red). PCR products were separated on a 1.5% (wt/vol) agarose gel with TAE buffer [1.21 g of Tris 2-amino-2 (hydroxymethyl)-1,3-propandiol liter−1, 0.2 ml of 0.5 M EDTA liter−1 (pH 8)] at a constant voltage of 55 V for 900 min at 4°C. Molecular markers (45.5% [vol/vol] 100-bp ruler; 36.5% [vol/vol] 500-bp ruler, and 18% [vol/vol] loading buffer) were loaded in the first and every sixth lane. After the gels were stained with ethidium bromide, the visualized patterns were digitalized. Normalization, recognition, and assignment of bands on the gel were performed using BioNumerics software (Applied Maths), and a dendrogram based on the Pearson similarity coefficient (γ) was constructed(5).
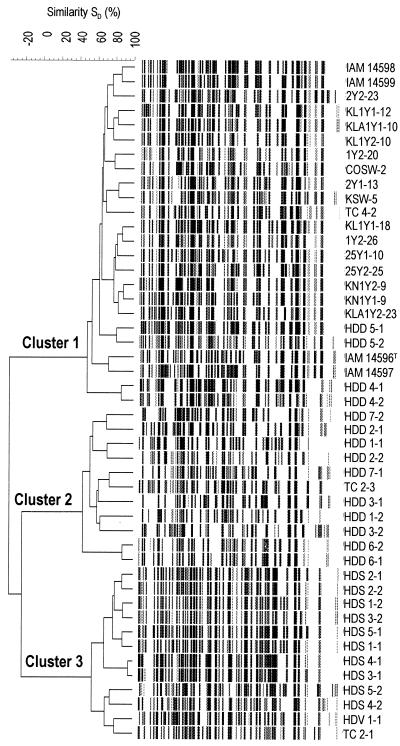
AFLP analysis grouped the 47 V. halioticoli-like strains into three main clusters (Fig. 1). Cluster 1 (SD, 48.6%) included the four V. halioticoli type strains, all V. halioticoli-like strains isolated from H. discus hannai, two isolates from seawater from abalone farms, one isolate (TC4-2 [=LMG19963]) from T. cornutus, and four isolates (HDD4-1 [=LMG19974], HDD4-2, HDD5-1 [=LMG19975], and HDD5-2) from H. discus discus. Cluster 2 (SD, 54.6%) included 10 isolates from H. discus discus and 1 isolate (TC2-3) from T. cornutus. Cluster 3 (SD, 54.6%) included all isolates from H. diversicolor aquatilis and H. diversicolor diversicolor and isolate TC2-1 from T. cornutus. The low SDs (<20%) among these three groups indicate that they consist of isolates with divergent genomes (Fig. 1).
FIG. 1.
Dice coefficient-Ward algorithm cluster analysis of AFLP fingerprinting patterns of V. halioticoli-like strains isolated from the gut of Japanese abalone, turban shell, or seawater from abalone farms.
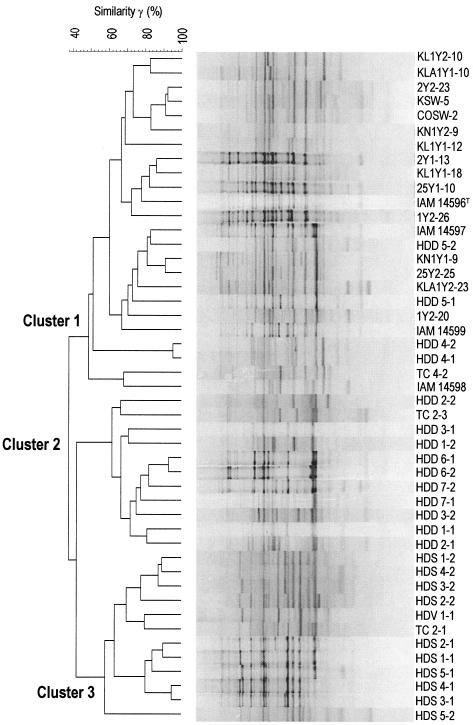
REP-PCR fingerprinting of these 47 strains resulted in a delineation of the three main clusters identical to the AFLP grouping (Fig. 2.). V. halioticoli-like strains grouped in the same clusters according to their host abalone species, except for the rearrangement within cluster 1 of isolates HDD4-1 (=LMG19974), HDD4-2, HDD5-1 (=LMG19975), and HDD5-2. As with the AFLP clustering, isolates from seawater and T. cornutus were found in the same clusters. Clusters 1, 2, and 3 had inner r values of 48.7, 60.6, and 59.0%, respectively, and the r value among the clusters was lower than 45%. The correlation between the fluorescent AFLP and REP-PCR groupings was found to be high. In addition, differences in geographical distribution, for example, of the strains from H. discus hannai from Hokkaido and Iwate and of the strains from H. discus discus from Kanagawa and Izu Ohisima, were not found to be distinct subgroups by either AFLP fingerprinting or REP-PCR fingerprinting analysis (Fig. 1 and 2). Therefore, the only criteria for the grouping of the V. halioticoli-like strains by both fingerprinting techniques appears to be the abalone host species from which the strains were isolated.
FIG. 2.
Cluster analysis by the Pearson unweighted-pair group method using average linkages of the REP-PCR fingerprinting patterns of V. halioticoli-like strains isolated from the gut of Japanese abalone, turban shell, or seawater from abalone farms.
V. halioticoli-like isolates from abalone were divided into three clusters by both AFLP and REP-PCR fingerprinting analyses based on their abalone host species. However, V. halioticoli-like strains from turban shells were not grouped in a single cluster (Fig. 1 and 2). In fact, the nearest neighbors of isolates TC2-1 and TC2-3 from wild turban shells were wild abalone isolates HDV1-1 (from H. diversicolor diversicolor) and HDD7-1 (from H. discus discus), with 79.4 and 73.4% SDs, respectively, by the AFLP analysis (Fig. 1). Alternatively, the host species of strain TC4-2 was cultured at Izu Oshima, and the nearest neighbors of TC4-2 were isolates from H. discus hannai (Fig. 1). These data lead to the hypothesis that V. halioticoli-like isolates can be taken up into the gut of turban shells without strong specificity in the symbiotic associations between V. halioticoli and their turban shell hosts. Rather low populations (below 20%) of V. halioticoli-like strains in the gut of the turban shell (unpublished data) compared to the abundant populations (40 to 60%) in the gut of abalone suggests a transient or neutral relationship between turban shells and the bacterium.
Results of DNA-DNA hybridization experiments with representative V. halioticoli-like strains divide into the fingerprinting clusters.
In our study, AFLP fingerprinting similarities (measured as SDs) between cluster 1, which includes the V. halioticoli type strains, and the other groups and those between cluster 2 and cluster 3 were below 20%. DNA-DNA hybridization experiments were performed with microdilution wells by a fluorometric direct binding methodology as previously described (4, 18). DNAs of V. halioticoli IAM14596T (representative of AFLP cluster 1), HDD3-1 (representative of AFLP cluster 2), and HDS1-1 (representative of AFLP cluster 3) were labeled with photobiotin (Vector Laboratories, Burlingame, Calif.). Unlabeled single-stranded DNA from each of these strains was immobilized in microdilution wells (Immuron 200, FIA/LIA plate, black type; Greiner Labotechnik, Frichenhausen, Germany). Hybridization was performed at 45°C (18).
DNA-DNA relatedness levels between the biotinylated strain IAM14596T and unlabeled strains HDD3-1 and HDS1-1 were 97 and 70%, respectively (Table 2). However, the DNA relatedness values between labeled HDD3-1 and HDS1-1 and the other unlabeled strains were all below 70% (Table 2). The results indicate that isolates belonging to AFLP clusters 2 and 3 (HDD3-1 and HDS1-1, respectively) can be defined as a species or subspecies that is distinct from authentic V. halioticoli by using a DNA relatedness of greater than 70% as the criterion for defining a bacterial species(25).
TABLE 2.
DNA relatedness among V. halioticoli and related isolates
| Strain | AFLP cluster | DNA relatedness (%) with biotinylated DNA from:
|
||
|---|---|---|---|---|
| IAM14596T | HDD3-1 | HDS1-1 | ||
| IAM14596T | 1 | 100 | 43 | 32 |
| HDD3-1 | 2 | 97 | 100 | 67 |
| HDS1-1 | 3 | 70 | 48 | 100 |
Conclusion.
The genomic fingerprinting analysis reveals that host-driven (or host-dependent) DNA polymorphism rather than geographical or environmental factors accounts for the groupings observed in the V. halioticoli-like strains isolated from Japanese abalone (Fig. 1 and 2). Furthermore, cospeciation of V. halioticoli-related species and Japanese abalone may have occurred (Table 2). There are, however, about 70 abalone species in the world that populate coastal areas along France, South Africa, Australia, New Zealand, United States, and Taiwan. In the one regional example described in the present report, we have shown the strong role of host species specificity in the abalone-gut microbe relationship.
One proposed ancestral abalone species (Haliotis iris, a New Zealand species) split into two main lineages according to molecular phylogenetic analysis using a sequence of abalone sperm lysine and morphological comparisons to the oldest abalone fossil records (12). A study of the distribution of the gut microbe V. halioticoli or related species in modern abalone species should be conducted to clarify the coevolution of abalone and V. halioticoli.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (no. 09460081) from the Ministry of Education, Science and Culture of Japan. F.L.T. has a Ph.D. scholarship (no. 2008361/98-6) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasilia, Brazil.
We thank Johan Vandenberghe for useful comments.
REFERENCES
- 1.Duim, B., T. M. Wassenaar, A. Rigter, and Wagenaar, J. 1999. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 65:2369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duim, B., C. W. Ang, A. van Belkum, A. Rigter, N. W. J. van Leeuwen, H. P. Endtz, and J. Wagenaar. 2000. Amplified fragment length polymorphism analysis of Campylobacter jejuni strains isolated from chickens and from patients with gastroenteritis or Guillain-Barré or Miller Fisher syndrome. Appl. Environ. Microbiol. 66:3917-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duim, B., P. A. R. Vandamme, A. Rigter, S. Laevens, J. R. Dijkstra, and J. A. Wagenaar. 2001. Differentiation of Campylobacter species by AFLP fingerprinting. Microbiology 147:2729-2737. [DOI] [PubMed] [Google Scholar]
- 4.Ezaki, T., Y. Hashimoto, N. Takeuchi, H. Yamamoto, S.-L. Liu, H. Miura, K. Matsui, and E. Yabuuchi. 1988. Simple genetic method to identify viridans group Streptococci by colorimetric dot hybridization and fluorometric hybridization in microdilution wells. J. Clin. Microbiol. 26:1708-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häne, B. G., K. Jäger, and H. G. Drexler. 1983. The Pearson product-moment correlation coefficient is better suited for identification of DNA fingerprint profiles than band matching algorithms. Electrophoresis 14:967-972. [DOI] [PubMed] [Google Scholar]
- 6.Hauben, L., L. Vauterin, E. R. B. Moore, B. Hoste, and J. Swings. 1999. Genomic diversity of the genus Stenotrophomonas. Int. J. Syst. Bacteriol. 49:1749-1760. [DOI] [PubMed] [Google Scholar]
- 7.Hooper, L. K., and J. I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115-1118. [DOI] [PubMed] [Google Scholar]
- 8.Huys, G., R. Coopman, P. Janssen, and K. Kersters. 1996. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int. J. Syst. Bacteriol. 46:572-580. [DOI] [PubMed] [Google Scholar]
- 9.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, S. C., V. Louis, N. Choopun, A. Sharma, A. Huq, and R. R. Colwell. 2000. Genetic diversity of Vibrio cholerae in Chesapeake Bay determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang, S. C., M. Matte, G. Matte, A. Huq, and R. R. Colwell. 2000. Genetic diversity of clinical and environmental isolates of Vibrio cholerae determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, Y.-H., and V. D. Vacquire. 1995. Evolution and systematics in Haliotidae (Mollusca: Gastropoda): inferences from DNA sequences of sperm lysine. Mar. Biol. 124:267-278. [Google Scholar]
- 13.Nick, G., M. Jusilla, B. Hoste, R. M. Niemi, S. Kaijalainen, R. de Lajudie, M. Gillis, F. J. de Bruijn, and K. Lindström. 1999. Rhizobia isolated from root nodules of tropical leguminous trees characterized using DNA-DNA dot-blot hybridization and rep-PCR genomic fingerprinting. Syst. Appl. Microbiol. 22:287-299. [Google Scholar]
- 14.Rademaker, J. L. W., F. J. Louws, and F. J. de Brujin. 1998. Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting, p. 1-27. In J. D. Van Elsas et al. (ed.), Molecular microbial ecology manual, vol. 3.4.3. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 15.Rademaker, J. L. W., B. Hoste, F. J. Louws, K. Kersters, J. Swings, L. Vauterin, P. Vauterin, and F. J. de Brujin. 2000. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA-DNA homology studies: Xanthomonas as a model system. Int. J. Syst. E vol. Microbiol. 50:665-677. [DOI] [PubMed] [Google Scholar]
- 16.Russel, J. B., and J. L. Rychlik. 2001. Factors that alter rumen microbial ecology. Science 292:1119-1122. [DOI] [PubMed] [Google Scholar]
- 17.Sawabe, T., Y. Oda, Y. Shiomi, and Y. Ezura. 1995. Alginate degradation by bacteria isolated from the gut of sea urchins and abalones. Microb. Ecol. 30:192-202. [DOI] [PubMed] [Google Scholar]
- 18.Sawabe, T., I. Sugimura, M. Ohtsuka, K. Nakano, K. Tajima, Y. Ezura, and R. Christen. 1998. Vibrio halioticoli sp. nov., a nonmotile alginolytic marine bacterium isolated from the gut of abalone Haliotis discus hannai. Int. J. Syst. Bacteriol. 48:573-580. [DOI] [PubMed] [Google Scholar]
- 19.Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy. W. H. Freeman and Company, San Francisco, Calif.
- 20.Tanaka, R., T. Sawabe, K. Tajima, J. Vandenberghe, and Y. Ezura. 2001. Identification of Vibrio halioticoli using 16S rDNA PCR/RFLP (restriction fragment length polymorphism) analysis. Fish. Sci. (Tokyo) 67:185-187. [Google Scholar]
- 21.Tanaka, R., M. Ootsubo, T. Sawabe, K. Tajima, J. Vandenberghe, and Y. Ezura. 2002. Identification of Vibrio halioticoli by colony hybridization with non-radioisotope labeled genomic DNA. Fish. Sci. (Tokyo) 68:227-229. [Google Scholar]
- 22.Tanaka, R., T. Sawabe, M. Yoshimizu, and Y. Ezura. 2002. Distribution of Vibrio halioticoli around an abalone farming center in Japan. Microb. Environ. 17:6-9. [Google Scholar]
- 23.Thompson, F. L., B. Hoste, K. Vandemeulebroecke, and J. Swings. 2002. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism. Syst. Appl. Microbiol. 24:520-538. [DOI] [PubMed] [Google Scholar]
- 24.Versalovic, J., M. Schneider, F. J. de Brujin, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5:25-40. [Google Scholar]
- 25.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. L. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Trüper. 1987. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]