Abstract
The influence exerted by nonpathogenic Fusarium oxysporum strain Fo47 in triggering cucumber protection against infection by Pythium ultimum was investigated ultrastructurally. Macroscopic and microscopic observations of the pathogen colony in dual cultures revealed that reduction of Pythium growth was associated with marked disorders, including generalized disorganization of the host cytoplasm, retraction of the plasmalemma, and complete loss of the protoplasm. Cytochemical labeling of cellulose with an exoglucanase-gold complex showed that the cellulose component of the host cell walls was structurally preserved at a time when the host cytoplasm had undergone complete disorganization. A similar antagonistic process was observed at the root cell surface. Most striking and interesting was the finding that mycoparasitism, as evidenced by the frequent occurrence of Fo47 hyphae within nearly empty cells of the pathogen, occurred not only at the root surface but also within the invaded root tissues. The specific labeling pattern obtained with the exoglucanase-gold complex confirmed that Fo47 successfully penetrated cells of the pathogen, both in the rhizosphere and inside the root tissues. Pythium cells that could evade the first defensive line in the rhizosphere could penetrate the root epidermis, but their growth was restricted to the outermost tissues. Positive correlations between Fo47 treatment and induced resistance to infection by P. ultimum in cucumber were confirmed by (i) the reduction of pathogen viability; (ii) the elaboration of newly formed barriers, a phenomenon which was not seen in Fo47-free plants, where the pathogen proliferated in all root tissues within a few days; and (iii) the occlusion of intercellular spaces with a dense material likely enriched in phenolics. Taken together, our observations provide the first convincing evidence that Fo47 exerts a direct inhibitory effect on P. ultimum through a combination of antibiosis and mycoparasitism, in addition to being a strong inducer of plant defense reactions.
Research over the last 2 decades has provided convincing evidence that root health and vigor are directly related to plant productivity. As a consequence, root disease control has become one of the most challenging research areas in the context of plant productivity improvement. This has led to the concept that manipulating the rhizosphere in such a way that beneficial microorganisms with antagonistic and/or eliciting properties are favored would protect roots from the deleterious effect of soilborne pathogens (28, 44). Support for this concept came from the discovery of suppressive soils in which the active microflora naturally controls the disease-causing activities of pathogen populations (3, 4). Soils naturally suppressive to fusarium wilt (3), pythium damping-off (34), and Thielaviopsis basicola- and Gaeumannomyces graminis-incited diseases (43, 44) have been reported in different areas of the world and have been the focus of intensive studies dealing mainly with the identification of the physicochemical and biological factors involved in biological control (1, 30). Fusarium wilt control in suppressive soils from ChÂteaurenard (France) has been attributed mainly to the occurrence of nonpathogenic Fusarium strains such as Fo47, as demonstrated by Rouxel et al. (39), who found that eradication of nonpathogenic Fusarium populations by heat treatment coincided with a disease increase.
The actual mechanisms underlying Fusarium-mediated induced protection in suppressive soils are not completely understood, although a number of possibilities have been raised, including microbial competition for nutrients (20), competition for infection sites and root colonization (26), and, more recently, plant-induced resistance through the rapid stimulation of a general cascade of nonspecific defense responses, including structural barriers (12) and pathogenesis-related proteins such as chitinases and β-1,3-glucanases (23). By using root-inducing transferred-DNA-transformed pea roots, Benhamou and Garand (12) recently demonstrated that pea root inoculation with nonpathogenic Fusarium strain Fo47 triggered a set of plant defense reactions that resulted in the elaboration of permeability barriers and in the creation of a fungitoxic environment that protected the roots by barricading the fungus in the outermost root tissues.
An interaction that has been the focus of much interest as a model pathosystem in both basic and applied research is that between cucumber and Pythium ultimum Trow (35). Over the last few years, a number of approaches, including chemical (9, 18), biological (25), and microbial (10, 14, 31, 45) treatments, have been proposed for the control of Pythium-incited diseases in cucumber. However, the value of these options in agriculture and horticulture has yet to be realized, mainly because serious limitations, such as phytotoxicity and variability, have delayed any transfer of technology from the laboratory to the field. Since biofungicides are usually composed of living organisms, their efficacy may vary under a wide range of environmental, cultural, and biotic conditions. Because of such difficulties, integrated pest management strategies, involving more than one biocontrol product or agent, offer bright opportunities for success, provided that the mode of action of each component is understood. An approach that is gaining increasing interest in the context of integrated biological control of soilborne pathogens concerns the potential value of nonpathogenic fungal strains, such as strain Fo47, in association with bacterial and fungal antagonists (23, 24).
As a complement to previous investigations of the potential use of Fo47 for control of tomato crown and root rot (12), the present study was undertaken to determine whether Fo47 is capable of protecting cucumber plants from P. ultimum infection. Up to now, suppressiveness associated with Fusarium oxysporum strain Fo47 was thought to be specifically effective, operating on diseases incited by formae speciales of pathogenic F. oxysporum (2) but not, apparently, against diseases caused by other plant pathogens (38). In order to determine whether Fo47, which is known to protect tomato plants against F. oxysporum attack through the synergistic action of several mechanisms (1), is also effective in controlling infection by P. ultimum, the effect of this nonpathogenic F. oxysporum strain on P. ultimum in vitro and in vivo on greenhouse-grown cucumber plants was examined. We present the first conclusive evidence that cucumber plants previously inoculated with nonpathogenic Fusarium strain Fo47 have increased resistance to Pythium attack. This protection is mainly associated with a strong antagonistic activity in the rhizosphere and in planta, as well as with the induction of structural and biochemical barriers that adversely affect pathogen growth and development.
MATERIALS AND METHODS
Fungal culture and growth conditions.
Nonpathogenic F. oxysporum strain Fo47, initially isolated by Alabouvette et al. (3, 5) from a suppressive soil located at Châteaurenard (France), was supplied by Natural Plant Production (Noguères, France) as a liquid formulation termed Fusaclean and containing spores at a concentration of 1011 CFU/liter. It was grown on Difco potato dextrose agar (PDA; Difco Laboratories, Detroit, Mich.) at 24°C in the dark and subcultured weekly. The root pathogen P. ultimum Trow, isolate BARR 447 (Center for the Land and Resources Research, Ottawa, Ontario, Canada), which is known to be virulent to several crops, including cucumber, was routinely grown on PDA in darkness at 26°C.
Dual-culture tests.
The effect of strain Fo47 on the growth, morphology, and ultrastructure of P. ultimum was assayed in dual-culture tests by using a procedure previously described by Chérif and Benhamou (17). Briefly, 5-mm mycelial disks collected from the edges of actively growing colonies of Fo47 and P. ultimum were placed 3 cm apart on the surface of PDA medium and allowed to grow at 25°C under continuous light. As the first apparent contact between the antagonist and the pathogen occurred by 2 days after inoculation, mycelial samples from the interaction region were collected at 2, 3, and 4 days and processed for electron microscopy. The experiment was repeated twice with three petri dishes per sampling time.
Plant treatment with F. oxysporum strain Fo47 and fungal inoculation with P. ultimum.
Seeds of cucumber, (Cucumis sativus L., cv. Corona; De Ruiter Seeds Inc.) were surface sterilized by immersion in 1% aqueous sodium hypochlorite for 60 min and thoroughly rinsed in sterile distilled water prior to being allowed to germinate in petri dishes containing sterile, moist cotton. Plates were incubated at 25°C in the dark for 48 h. Germinated seeds were carefully removed from the cotton and sown in a mixture of peat-perlite-vermiculite (1:1:1) at a density of four seedlings per 6-cm pot. Plants were fertilized every other day with a nutrient solution containing (in milliequivalents) NO3 (12.0), PO4 (1.0), K (1.7), Mg (1.5), Ca (2.8), and S (0.5) and (in microequivalents) Fe (70.0), Mn (18.0), Zn (7.7), Cu (1.5), B (27.5), and Mo (0.5). The pH of the solution was adjusted to 6.2, and the electrical conductivity was adjusted to 2.4 mS. Seedlings were grown on a greenhouse bench at 24 to 26°C with 16 h of light supplemented by high-pressure sodium lamps (100 microeinsteins · m−2 · s−1).
Three-week old cucumber seedlings were inoculated with a microconidial suspension of Fo47 (105 conidia/g of dry substrate) carefully introduced into the soil (as close as possible to the root system) with a sterile syringe. Control plants were treated similarly but with sterile deionized water only. Four days later, plants were challenge inoculated by introducing two plugs (5-mm diameter) of actively growing mycelium of P. ultimum as close as possible to the root system. Controls were treated with fungus-free PDA disks The experimental design included the following treatments: (i) no Fo47 or P. ultimum (healthy controls), (ii) Fo47 but no P. ultimum, (iii) P. ultimum but no Fo47, and (iv) Fo47 and P. ultimum. Twenty plants were used for each treatment (Fo47 alone, P. ultimum alone, and a combination Fo47 and P. ultimum), and the experiment was repeated twice. The roots were pulled out of the substrate and examined daily for fungal infection (visible necrotic lesions). For electron microscope investigations, samples from the main roots were collected 3 to 5 days after pathogen inoculation and either deposited on PDA in sterile petri dishes or processed for electron microscopy.
Tissue processing for light and transmission electron microscopy.
Mycelial samples from dual-culture tests, as well as root samples from treated and untreated cucumber plants, were processed for electron microscope studies. Root samples (2 mm3) were carefully excised from colonized cucumber roots at sites of potential fungal penetration (16). They were pre-embedded in 2% aqueous Bacto Agar in order to preserve the rhizosphere microbial populations. Mycelial and root samples were then immersed in a mixture of 3% (vol/vol) glutaraldehyde and 3% (wt/vol) paraformaldehyde in 0.2 M sodium cacodylate buffer, pH 7.2, for 2 h at room temperature prior to being postfixed with 1% (wt/vol) osmium tetroxide in the same buffer for 1 h at 4°C and dehydrated in a graded ethanol series prior to being embedded in Epon 812 (J. B. E. M. Chemical Co., Pointe-Claire, Quebec, Canada). Thin (0.7-μm) sections cut from the Epon-embedded material with glass knives were mounted on glass slides and stained with 1% aqueous toluidine blue prior to examination with a Zeiss Axioskop microscope (Carl Zeiss Canada, Don Mills, Ontario, Canada). Ultrathin (0.1-μm) sections were collected on Formvar-coated nickel grids and either contrasted with uranyl acetate and lead citrate for direct examination with a JEOL 1200 EX electron microscope (JEOL, Tokyo, Japan) at 80 kV or processed for cytochemical labeling. An average of five samples from three different plants per sampling time were investigated. For each sample, 10 to 15 ultrathin sections were examined under the electron microscope.
Preparation of the gold-complexed probes and cytochemical labeling.
Colloidal gold particles (BDH Chemicals, Montreal, Quebec, Canada) averaging 12 nm in diameter were prepared as described by Frens (27) by using sodium citrate as a reducing agent.
The β-1,4-exoglucanase-gold complex (β-1,4-D-glucan cellobiohydrolase; EC 3.2.1.21) used for localization of cellulosic β-1,4-glucans was prepared as described by Benhamou et al. (13). N-Acetylglucosamine residues (chitin) were localized by a two-step procedure (7) using wheat germ agglutinin (WGA; Sigma Chemical Co., St Louis, Mo.) as a first-step reagent and gold-complexed ovomucoid (Sigma Chemical Co.) as a second-step reagent. The exoglucanase was complexed to gold at pH 9.0, and the ovomucoid was complexed to gold at pH 5.4.
Labeling with gold-complexed exoglucanase was performed by incubating first the ultrathin root sections for 5 to 10 min on a drop of phosphate-buffered saline (PBS), pH 6.0, containing 0.02% (wt/vol) polyethylene glycol 20000 at pH 6.0. Sections were then transferred to a drop of the enzyme-gold complex for 30 min at room temperature in a moist chamber.
For the labeling of N-acetylglucosamine residues, sections were first floated on a drop of PBS, pH 7.4, for 5 min and then transferred to a drop of WGA (12 μg/ml in PBS, pH 7.4) and incubated for 60 min at room temperature in a moist chamber. After washing with PBS, pH 7.4, sections were incubated on a drop of the ovomucoid-gold complex (1:30 in PBS-polyethylene glycol, pH 6.0) for 30 min at room temperature.
After careful washing with PBS, pH 7.2, and rinsing with distilled water, all sections were contrasted with uranyl acetate and lead citrate and observed with a JEOL 1200 EX transmission electron microscope.
Labeling specificity was assessed by incubating the sections with (i) gold-complexed exoglucanase to which were previously added β-1,4-glucans from barley (1 mg/ml in PBS); (ii) WGA to which was previously added an excess of N,N′,N′-triacetylchitotriose (1 mg/ml in PBS); (iii) WGA, followed by unlabeled ovomucoid and finally by ovomucoid-gold complex; and (iv) a stabilized or an unstabilized gold suspension.
RESULTS
In vitro interactions between F. oxysporum strain Fo47 and P. ultimum.
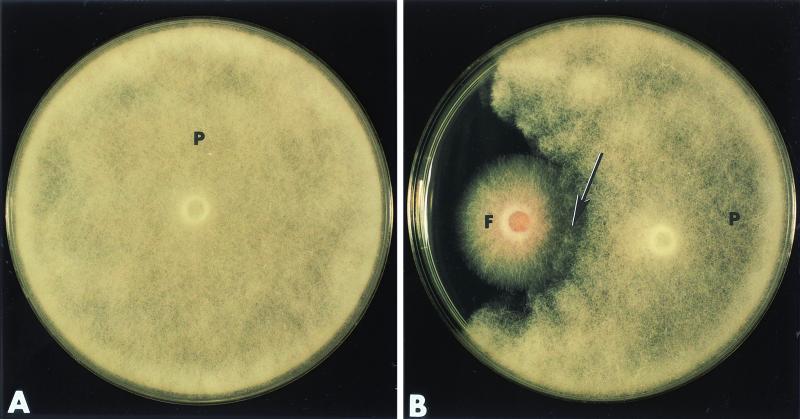
In single cultures, P. ultimum grew actively and colonized the entire agar surface within 2 days after inoculation (Fig. 1A). Confrontation experiments (dual-culture assays) provided evidence that Fo47 substantially reduced the growth of P. ultimum (Fig. 1B). Close examination of the petri dishes by stereoscopy revealed that the first apparent contact between the hyphae of the two fungi occurred within 2 days after inoculation (Fig. 1B, arrow). In the following days, a halo was visible around the P. ultimum colony, indicating that Fo47 was producing some fungal inhibitors. Overgrowth of Fo47 on P. ultimum was not observed.
FIG. 1.
In vitro interactions between F. oxysporum strain Fo47 and P. ultimum 2 days after inoculation of the fungi on PDA medium. (A) P. ultimum (P) grown in single culture. (B) P. ultimum (P) grown in the presence of Fo47 (F). Growth of the pathogen is delayed, and changes in hyphal density at the edge of the colony are evident (arrow).
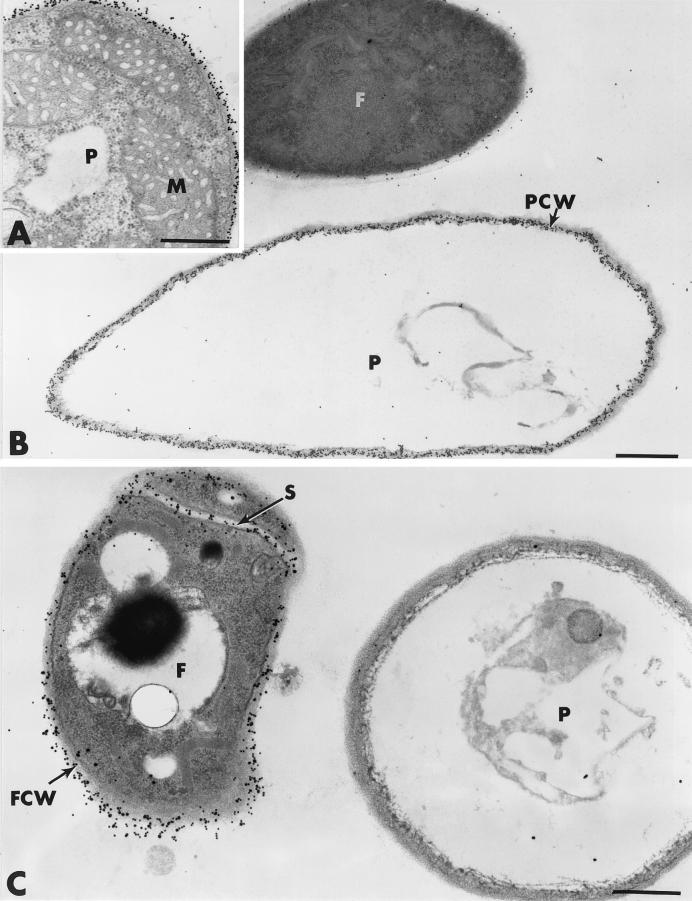
Ultrastructural investigations of mycelial samples from the interaction region between Fo47 and P. ultimum confirmed the macroscopic observations and brought further insights into the events leading to pathogen growth inhibition. Pythium hyphae, grown in single cultures, showed a typical ultrastructure characterized by a thin, cellulose-containing cell wall surrounding a dense polyribosome-enriched cytoplasm in which a large number of organelles, including mitochondria, were visible (Fig. 2A). Examination of ultrathin sections from the interaction region of 2-day-old dual cultures showed that cells of Fo47, readily discernible by the higher density of their cytoplasm and their smaller diameter, neighbored hyphae of P. ultimum without, however, encircling them in a way similar to that observed with Trichoderma harzianum (10) (Fig. 2B and C). Although intimate contact between the two fungi was not detected, P. ultimum hyphae exhibited marked ultrastructural changes characterized mainly by drastic alterations of the cytoplasm, which was, in most cases, reduced to fibrillar and/or vesicular remnants (Fig. 2B and C). Organelles were no longer discernible in such altered fungal cells. Labeling with the gold-complexed exoglucanase for localization of cellulosic β-1,4-glucans, which are known to be the main cell wall-bound compounds in oomycetes such as Pythium spp. (11, 14, 18), resulted in regular deposition of gold particles over the walls of damaged Pythium cells (Fig. 2B). When sections were treated with the WGA-ovomucoid-gold complex, Fo47 cell walls were specifically labeled whereas Pythium cell walls were free of labeling (Fig. 2B).
FIG. 2.
Transmission electron micrographs of mycelial samples collected 2 days after inoculation in the region of interaction between Fo47 and P. ultimum. (A) Cell of P. ultimum (P) grown in single culture. Upon labeling with gold-complexed exoglucanase, regular deposition of gold particles occurs over the cell wall whereas the cytoplasm and the organelles, such as mitochondria (M), are unlabeled. (B) In dual-culture tests, hyphae of P. ultimum (P) are severely altered at a time when the P. ultimum cell wall (PCW) is regularly labeled by the exoglucanase-gold complex. Cells of Fo47 (F) are not specifically labeled. (C) Labeling with the WGA-ovomucoid-gold complex results in labeling of Fo47 cell walls (FCW). Altered Pythium (P) cells are unlabeled. S, septum. Bars, 0.5 μm.
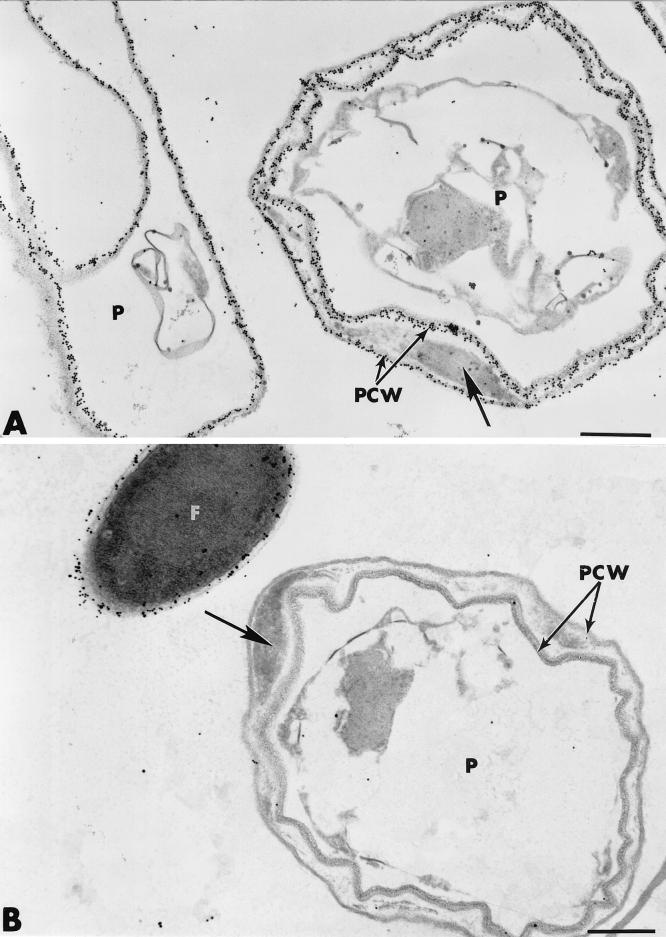
By 3 to 4 days after inoculation, most (nearly 90%) of the P. ultimum cells appeared to have been highly altered, as evidenced by the appearance of abnormally shaped, empty pleiomorphic shells (Fig. 3A and B). Structural changes, usually accompanied by local splitting of the wall layers and deposition of vesicular material between the split layers, were frequently observed (Fig. 3A, arrow). Such changes did not affect the intensity of labeling with the exoglucanase-gold complex since gold particles were evenly distributed over the split wall layers (Fig. 3A). By contrast, the amorphous material accumulating between the circonvoluted layers was unlabeled. Labeling with the WGA-ovomucoid-gold complex did not result in any deposition of gold particles over both the split layers and the filling material of Pythium hyphae, while Fo47 cell walls were labeled (Fig. 3B). All control tests, including previous adsorption of the probes with their corresponding substrate molecules, yielded negative results (not shown).
FIG. 3.
Transmission electron micrographs of mycelial samples collected 3 to 4 days after inoculation in the region of interaction between Fo47 and P. ultimum. (A and B). Local splitting of Pythium (P) cell wall (PCW) layers, accompanied by the deposition of vesicular material between the split layers (arrows), is evident. The split wall layers are labeled with the exoglucanase-gold complex (A) but not with the WGA-ovomucoid-gold complex (B). F, F. oxysporum strain Fo47. Bars, 0.5 μm.
Effect of F. oxysporum strain Fo47 on symptomatology and incidence of disease caused by P. ultimum.
In the absence of Fo47 preinoculation, cucumber seedlings infected with P. ultimum exhibited typical root symptoms as early as 3 days after inoculation (Fig. 4A). By 4 to 5 days postinoculation, plants showed severe symptoms of root rot and wilting and most of them were dead by 10 days after fungal inoculation (Table 1). In all of the symptomatic cucumber plants examined, a positive correlation was established between root damage and the presence of P. ultimum because the fungus was readily isolated from tissue samples collected in and around the brownish root areas.
FIG. 4.
Effect of F. oxysporum strain Fo47 on incidence of disease caused by P. ultimum. (A) In the absence of Fo47 preinoculation, cucumber seedlings infected with P. ultimum exhibit typical root symptoms, as well as leaf wilting. (B) Plants treated with Fo47 prior to Pythium inoculation have a more vigorous root system, on which only a few tiny lesions (arrow) are detectable.
TABLE 1.
Effect of nonpathogenic F. oxysporum strain Fo47 on the number of root lesions induced by P. ultimum Trow
| Time (days) after inoculation with P. ultimum | Mean no. of root lesions induced by P. ultimum ± SEMa
|
|
|---|---|---|
| Nontreated cucumber plants (control) | Fo47-treated cucumber plants | |
| 1 | 0 | 0 |
| 2 | 2 ± 0.50 | 0 |
| 3 | 5 ± 1.5 | 0 |
| 4 | 8 ± 0.5 | 0 |
| 5 | 9 ± 0.5 | 2 ± 0.5 |
| 6 | 11 ± 1.5 | 2 ± 0.5 |
The number of root lesions was determined from observations of 10 main roots per day after inoculation with P. ultimum.
Treatment with Fo47 prior to Pythium inoculation resulted in less seedling disease development than that which occurred in nontreated plants (Fig. 4B; Table 1). Fo47-treated plants harbored a more vigorous root system and were free of apparent symptoms such as wilting, chlorosis, or senescence. Although some small, brownish lesions could be seen on the main root (Fig. 4B, arrow), their frequency and severity never reached levels similar to those observed in control plants (Table 1).
Histological observation of effect of F. oxysporum strain Fo47 on the rate and extent of root cell colonization by P. ultimum.
In cucumber plants that were not preinoculated with Fo47 prior to infection with P. ultimum, the root tissues were massively invaded as early as 3 days after fungal inoculation. Pythium hyphae propagated through much of the epidermis, the cortex, the endodermis, and the paratracheal parenchyma of cells (Fig. 5A; Table 2) by inter- and intracellular modes of growth (Fig. 5B). Host cell penetration by local rupture of the cell wall was frequently recorded (Fig. 5B, arrow). Such a massive fungal colonization, which apparently prevented the elaboration of host defense reactions, coincided with cytological disorders such as cell wall alterations as estimated by the noticeable decrease in wall staining intensity.
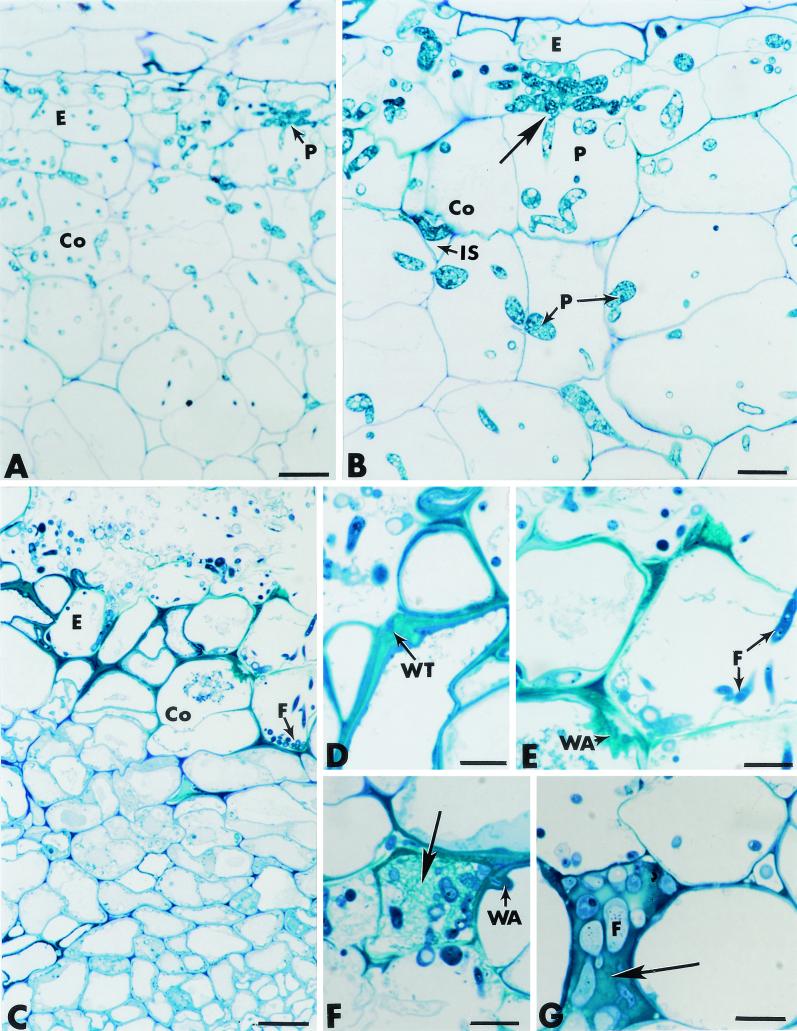
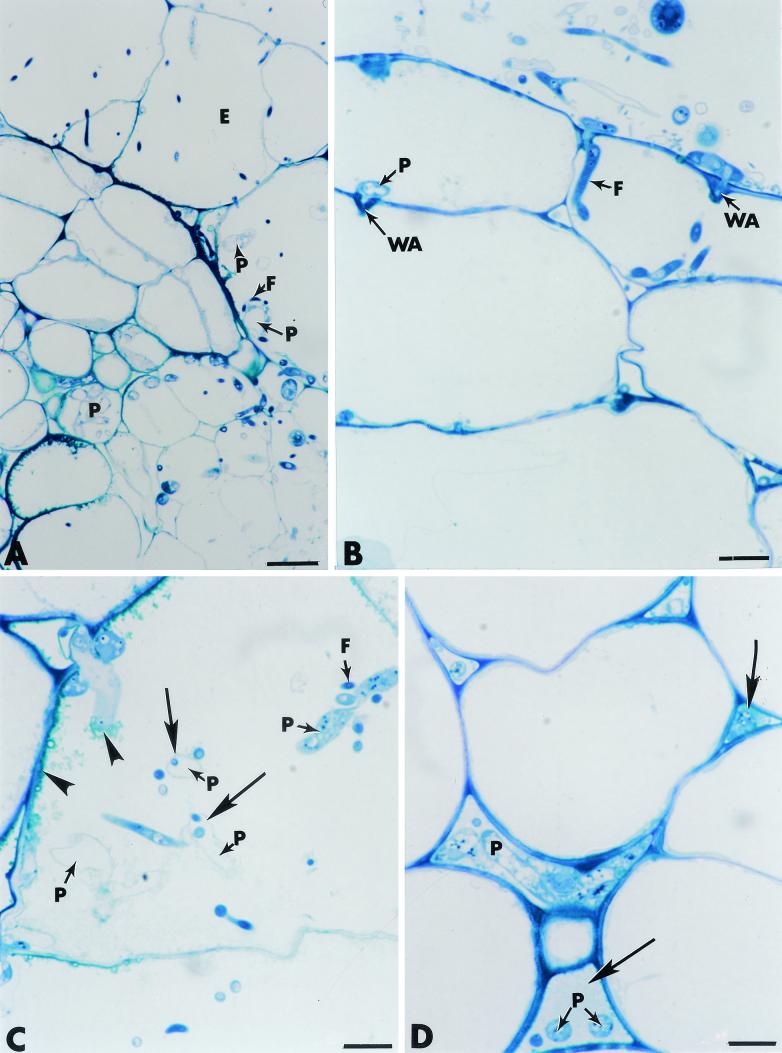
FIG. 5.
Cross sections of pea roots stained with toluidine blue. (A and B) Roots inoculated with P. ultimum (controls). Hyphae of P. ultimum (P) propagate through much of the root tissues, including the epidermis (E) and the cortex (Co) (panel A). Fungal growth occurs in intercellular spaces (IS), as well as intracellularly (panel B). Direct host cell wall penetration is visible (panel B, arrow). Bars: A, 25 μm; B, 10 μm. (C through G) Roots inoculated with F. oxysporum strain Fo47. The fungus (F) develops actively at the root surface and penetrates the epidermis (E) and the outer cortex (Co) (panel C). Colonization of the outermost root tissues correlates with marked thickening of the host cell walls (panel D), formation of wall appositions (panel E), deposition of a fibrillar matrix in which fungal cells (F) are trapped (panel F, arrow), and occlusion of intercellular spaces with amorphous material in which fungal cells are also captured (panel G, arrow). Bars: C, 25 μm; D through G, 10 μm.
TABLE 2.
Effect of nonpathogenic F. oxysporum strain Fo47 on the rate and extent of P. ultimum colonization of cucumber root tissues 4 days after inoculation
| Root tissue | Mean no. of fungal hyphae/host plant cell ± SEMa
|
|
|---|---|---|
| Nontreated cucumber plants (control) | Fo47-treated cucumber plants | |
| Epidermis | 7.8 ± 1.5 | 3.5 ± 1.5 |
| Outer cortex | 5.5 ± 0.5 | 1.2 ± 0.5 |
| Inner cortex | 5.0 ± 0.8 | 0 |
| Endodermis | 4.8 ± 0.4 | 0 |
| Vascular stele | 3.5 ± 0.7 | 0 |
The number of fungal hyphae was determined from observations of 40 light microscope photographs of 10 transversally sectioned samples from five cucumber roots per treatment.
As previously reported in the case of root-inducing transferred-DNA-transformed pea roots (12), hyphae of Fo47 displayed the ability to penetrate and colonize the outermost cucumber root tissues (Fig. 5C) without causing the extensive cell damage that is known to be caused by pathogenic F. oxysporum (15). Close examination of the invaded root areas revealed that Fo47 colonization was associated with various structural changes that were apparently made to restrict fungal ingress toward the inner tissues. Such changes included marked thickening of the host cell walls adjacent to invaded cells (Fig. 5D), formation of polymorphic wall appositions at sites of potential fungal penetration (Fig. 5E), deposition in several host cells of a fibrillar matrix in which fungal cells were trapped (Fig. 5F, arrow), and occlusion of some intercellular spaces with an amorphous material that was stained densely by toluidine blue and in which fungal cells were also captured (Fig. 5G, arrow).
In Fo47-inoculated cucumber roots that were challenged with P. ultimum, the pattern of colonization by the pathogen differed markedly from that observed in control roots grown in the absence of Fo47 (Fig. 6). Both fungi, easily recognizable by their different electron density and diameter, were restricted to the outermost root cell layers, including the epidermis and the outer cortex (Fig. 6A; Table 2). Growth of the pathogen in planta was associated with cytological changes characterized mainly by elaboration of wall appositions in the regions proximal to potential fungal penetration (Fig. 6B), deposition of densely stained material along the host cell walls and the pathogen cell surface (Fig. 6C, arrowhead), and filling of most intercellular spaces with matrices varying in texture and density (Fig. 6D, arrows). A large percentage (70%) of Pythium hyphae was reduced to empty shells in which the only recognizable structure was the cell wall (Fig. 6C). Close examination of the colonized areas suggested that hyphae of Fo47 could penetrate and grow within cells of the pathogen, as indicated by the occurrence of empty fungal shells containing some dense inclusions resembling Fusarium cells (Fig. 6C, arrows). Although such light microscope observations gave some indications about the mechanisms by which Fo47 could protect cucumber plants against infection by P. ultimum, more detailed ultrastructural investigations were needed to accurately evaluate the antagonistic process in planta and to provide additional information about the functional significance of the host defense reactions in restricting pathogen growth and development.
FIG. 6.
Effect of F. oxysporum strain Fo47 on the rate and extent of root cell colonization by P. ultimum. Staining was done with toluidine blue. (A through D) Both fungi, easily recognizable by their different electron densities and diameters, are restricted to the outermost root cell layers, including the epidermis (E) (panel A). Growth of P. ultimum (P) in planta is associated with formation of wall appositions (WA) at sites of potential fungal penetration (panel B), deposition of densely stained material along the host cell walls and the pathogen cell surface (panel C, arrowhead), and filling of intercellular spaces with fibrillar matrices (panel D, arrows). Features of potential mycoparasitism exerted by Fo47 (F) are visible (panel C, arrows). Bars: A, 25 μm; B to D, 10 μm.
Cytology of infection of cucumber root tissues by P. ultimum.
In plants grown in the absence of Fo47, features of root colonization by P. ultimum were essentially the same as those previously described (14). By 3 to 4 days after inoculation, massive intra- and intercellular fungal invasion was observed in all root tissues, including the vascular stele. Parasitized host cells were depleted of their cytoplasm, and host cell wall breakdown was frequently seen (Fig. 7A). Host defense reactions, including wall appositions, were seldom detected (Table 3).
FIG. 7.
Transmission electron micrographs of cross sections of cucumber roots inoculated with either P. ultimum alone (A) or F. oxysporum strain Fo47 (B through E). (A) Pythium hyphae (P) develop abundantly in all root tissues, causing some host cell wall (HCW) alterations. (B through E) Attempts of Fo47 hyphae to penetrate the epidermis are often halted by the formation of hemispheric wall appositions (WA) at sites of potential penetration (B and C). Elongated and stratified wall appositions are also seen in areas proximal to attempted fungal penetration (D, arrows). Electron-dense deposits (E, arrows) surround an Fo47 cell (F), which is distorted. Bars: A through C, 2 μm; D, 0.5 μm; E, 1 μm.
TABLE 3.
Effect of nonpathogenic F. oxysporum strain Fo47 on the number and size of wall appositions formed at sites of P. ultimum entry in cucumber root tissues 4 days after inoculation
| Root tissue | Mean no. of wall appositions/root section ± SEM
|
|
|---|---|---|
| Nontreated cucumber plants (control) | Fo47-treated cucumber plants | |
| Epidermis | 1 ± 0.5 | 8.5 ± 1.5 |
| Outer cortex | 0 | 5.2 ± 0.5 |
The number of wall appositions was determined from observations of 40 transverse root sections from three tomato roots per treatment.
Cytology of infection of cucumber root tissues by F. oxysporum strain Fo47.
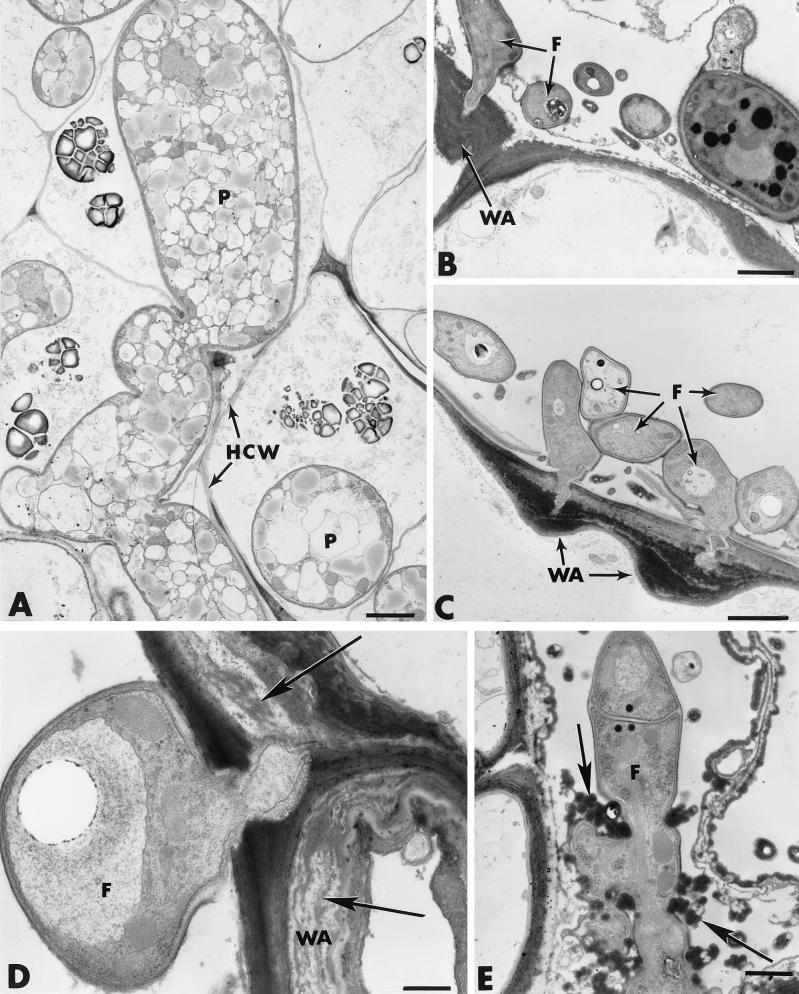
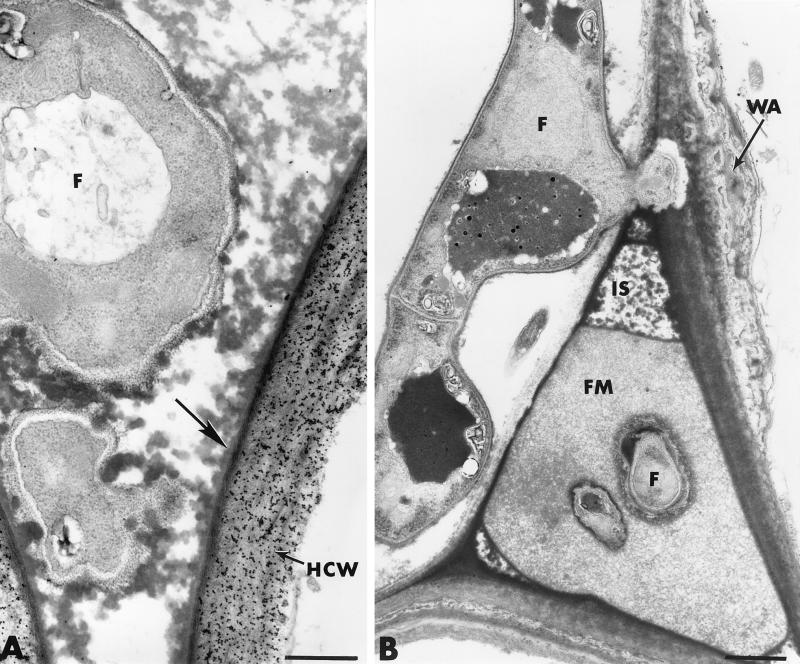
In cucumber plants treated with Fo47 but not challenged with P. ultimum, Fusarium hyphae developed abundantly on the root surface and established intimate contact with the host exodermis (Fig. 7B). Observation of about 70 sections from 10 different roots provided evidence that the hyphae displayed the ability to penetrate the epidermis even though most attempts failed because of the rapid formation of hemispheric wall appositions (papillae) at sites of potential penetration (Fig. 7B and C; Table 3). Besides the frequent occurrence of papillae in the epidermis, another structural reaction concerned the deposition, along large portions of the host cell wall, of elongated and stratified wall appositions bordered by compact and amorphous layers of cytoplasmic material (Fig. 7D, arrows). Host reactions in the outer cortex were characterized mainly by the accumulation of electron-dense deposits in some infected host cells (Fig. 7E). Fusarium hyphae, which could escape the first line of defense in the epidermis, were frequently found to be trapped by this material through an apparent physical interaction that resulted in marked hyphal distortion (Fig. 7E, arrows). Similar osmiophilic material was detected in a large number of colonized intercellular spaces (Fig. 8A). This dense material, which lined the primary walls (Fig. 8A, arrow), usually extended toward the inside to coat Fo47 hyphae. Labeling with gold-complexed exoglucanase resulted in heavy deposition of gold particles over the host cell wall but not over the osmiophilic material (Fig. 8A). Other intercellular spaces, neighboring colonized areas, were filled with a fibrillar matrix in which fungal cells were trapped (Fig. 8B).
FIG. 8.
Transmission electron micrographs of cross sections of cucumber roots inoculated with F. oxysporum strain Fo47. (A) Osmiophilic material lining the primary walls of invaded intercellular spaces (arrow) extends toward the inside to coat Fo47 hyphae (F). The host cell wall (HCW) is labeled with gold-complexed exoglucanase. Bar, 0.5 μm. (B) Occlusion of an intercellular space (IS) with a fibrillar matrix (FM) in which fungal cells (F) are trapped. WA, wall apposition. Bar, 1 μm.
Effect of F. oxysporum strain Fo47 on the cytology of infection by P. ultimum in cucumber root tissues.
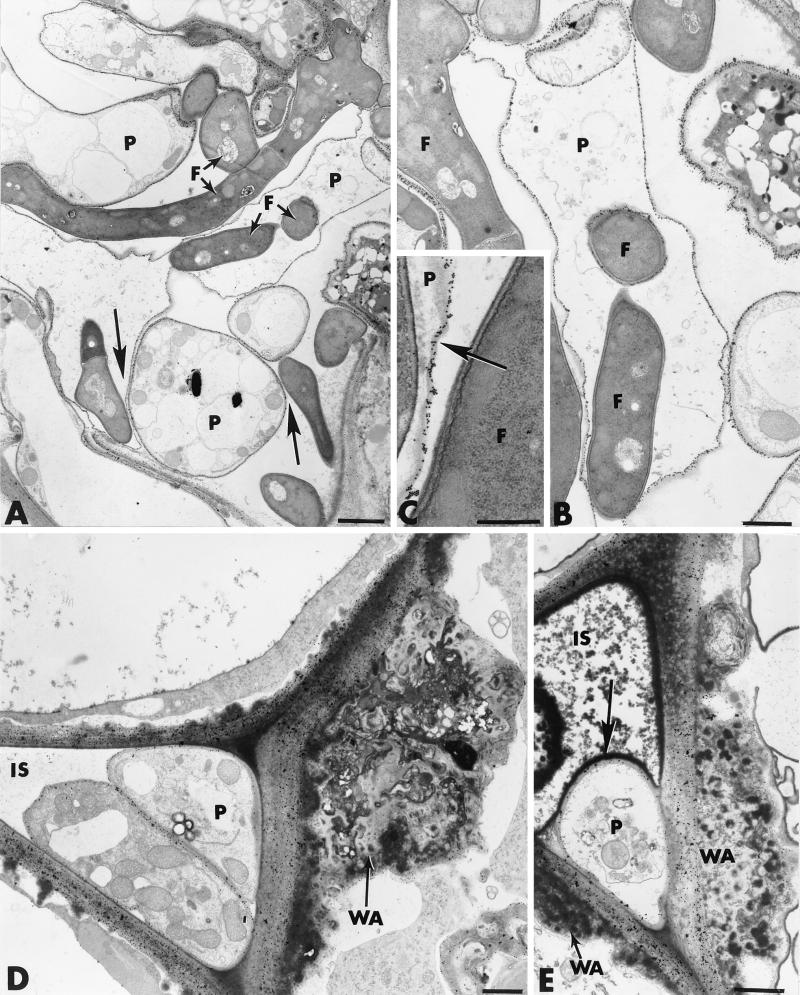
In Pythium-infected root samples collected 3 to 4 days after inoculation from cucumber plants grown in the presence of Fo47, the intensity of colonization by the pathogen was appreciably reduced compared to that observed in control inoculated plants. In order to determine whether a direct interaction between Fo47 and P. ultimum occurred in the rhizosphere, we examined the fungal populations at the root cell surface (Fig. 9A). Pre-embedding of the root samples in Bacto Agar prior to tissue processing allowed precise visualization of the rhizosphere populations along the root. Examination of the root surface revealed that Fo47 developed massively at the root surface and closely interacted with Pythium hyphae (Fig. 9A). Most cells of P. ultimum appeared to have been altered, as estimated by their abnormal shape (Fig. 9 B), which was associated with increased vacuolation (Fig. 9A). Interestingly, features of mycoparasitism, which could not be detected in vitro, were frequently observed in the rhizosphere (Fig. 9A, arrow). Labeling with gold-complexed exoglucanase for localization of cellulose subunits confirmed that Fo47 cells, recognized by their size and cytoplasmic density, displayed the ability to penetrate and grow within hyphae of P. ultimum, the cell walls of which were labeled (Fig. 9B and C, arrow). Fo47 growth in the Pythium hyphae was always associated with marked pathogen cell damage involving gradual disorganization of the cytoplasm, eventually leading to complete loss of the protoplasm (Fig. 9B).
FIG. 9.
Cytological and cytochemical observations of the effect of F. oxysporum strain Fo47 on the rate and extent of root cell colonization by P. ultimum. (A through C) Colonization of the root surface by Fo47 and P. ultimum. Fusarium cells (F) develop abundantly at the root surface and interact with the pathogen either through intimate contact (A, arrows) or through mycoparasitism (B and C). The wall of penetrated Pythium hyphae is labeled with gold-complexed exoglucanase (C, arrow). (D and E) Colonization of intercellular spaces (IS) by Pythium hyphae (P) is associated with host defense reactions, including formation of wall appositions (WA) and deposition of osmiophilic material that forms a coating band around invading fungal cells (E, arrow). Bars: A, 2 μm; B, D, and E, 1 μm; C, 0.5 μm.
Despite the antagonistic effect exerted by Fo47 on P. ultimum in the rhizosphere, successful penetration of the root epidermis was achieved by a few hyphae of P. ultimum (Fig. 9D and E). Colonization of some intercellular spaces by Pythium hyphae was always associated with strong host defense reactions, including the elaboration of wall appositions containing electron-dense inclusions (Fig. 9D) and the deposition of an osmiophilic material that formed a coating band around the invading fungal cells (Fig. 9E, arrow). Pythium cells surrounded by this material were severely damaged and, most often, halted in their potential ingress by the deposition of wall appositions on both sides of the colonized intercellular spaces (Fig. 9E).
Since Fo47 hyphae could also colonize the epidermis and the outer cortex, it was of interest to determine whether or not the antagonistic activity observed at the root cell surface also occurred in the root tissues. Examination of a large number of sections from different roots provided evidence that Fo47 hyphae could physically interact with the pathogen, causing cytological damage similar to that observed in vitro and in the rhizosphere (Fig. 10A). Such altered Pythium cells were usually devoid of cytoplasm and organelles, and cell wall labeling with the gold-complexed exoglucanase was the only indication of a previously living entity. Close examination of the labeling pattern, however, revealed that gold particles were irregularly distributed, especially in areas adjacent to Fo47 cells (Fig. 10A, arrows).
FIG. 10.
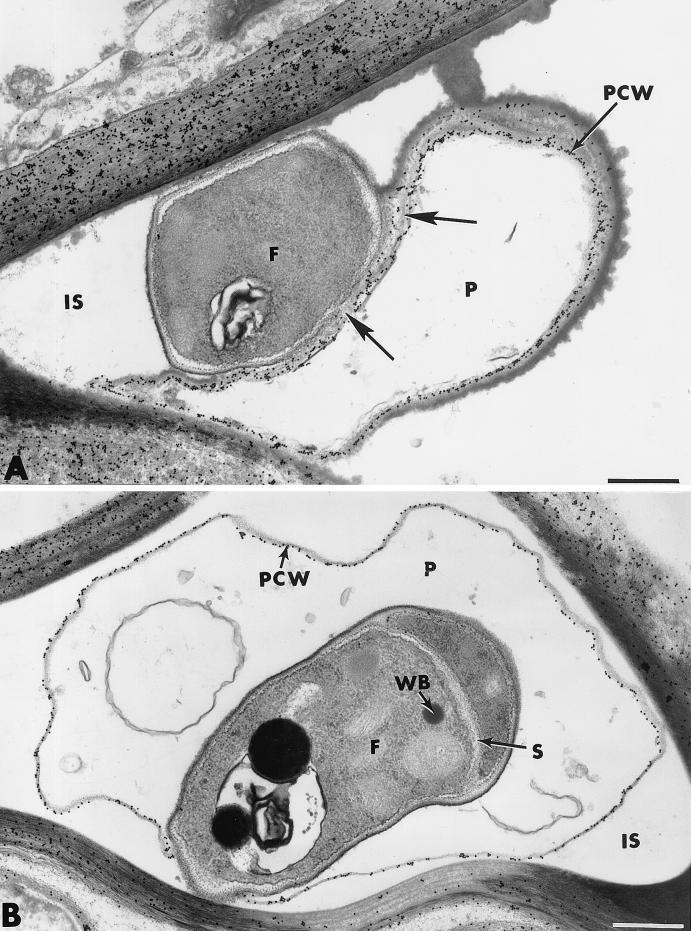
Cytological and cytochemical observations of effect of F. oxysporum strain Fo47 on the rate and extent of root cell colonization by P. ultimum. (A) Interactions between Fo47 (F) and P. ultimum (P) causing cytological damage similar to that observed at the root surface are evident in some intercellular spaces (IS). Incubation of sections with gold-complexed exoglucanase results in regular labeling of Pythium cell walls (PCW), except in areas adjacent to the F047 cell wall (arrows). (B) Feature of mycoparasitism observed in an intercellular space (IS). The penetrated Pythium cell wall (PCW) is labeled with gold-complexed exoglucanase. Labeling is absent over the wall of the invading Fusarium cell (F), in which a septum (S) and Woronin bodies (WB) are visible. Bars, 0.5 μm.
On the basis of our histological observations, one could suspect that Fo47 displayed the ability to penetrate and grow within hyphae of the pathogen. Such a feature of mycoparasitism was, indeed, frequently observed in intercellular spaces in which both fungi were present (Fig. 10B). Upon incubation with the exoglucanase-gold complex, irregular deposition of gold particles occurred over the walls of penetrated Pythium cells, which were reduced to empty shells (Fig. 10B). By contrast, no labeling was detected over the walls of the invading hyphae parasitizing the altered fungal cells. Such invading hyphae displayed a normal ascomycete appearance with a thin wall and a dense cytoplasm containing numerous organelles, as well as Woronin bodies in the septum area (Fig. 10B). When sections were treated with the WGA-ovomucoid complex, an inverse labeling pattern (not shown) was obtained. Penetrated Pythium cells were unlabeled, while gold particles were specifically associated with the walls of invading hyphae (data not shown).
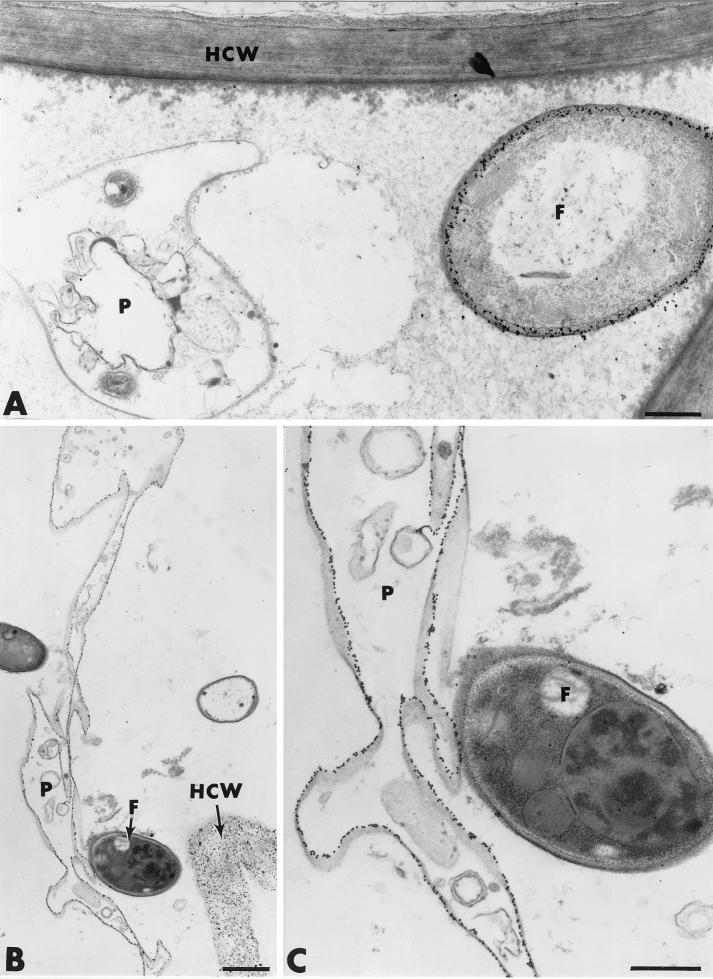
Pathogen cell alteration did not occur only upon contact with Fo47. Indeed, marked changes in both the morphology and ultrastructure of Pythium hyphae were frequently observed at a distance from Fo47 hyphae (Fig. 11A). Such changes usually coincided with the deposition of an unusual fibrillar network in some intercellular spaces. By 5 days after pathogen inoculation, most Pythium hyphae were so morphologically and structurally altered that labeling of cellulose with gold-complexed exoglucanase was the only way to detect their presence (Fig. 11B and C).
FIG. 11.
Cytological and cytochemical observations of effect of F. oxysporum strain Fo47 on the rate and extent of root cell colonization by P. ultimum. (A) Pythium hyphae (P) are altered even at a distance from Fusarium cells (F). (B and C) By 5 days after pathogen inoculation, Pythium hyphae (P) are morphologically and structurally altered. Labeling of cellulose with gold-complexed exoglucanase confirms their presence. F, Fo47 hypha; HCW, host cell wall. Bars: B, 1 μm; A and C, 0.5 μm.
All control tests, including previous adsorption of the probes with their target substrate molecules, resulted in a near absence of labeling over plant and fungal cell walls (data not shown).
DISCUSSION
Recent years have witnessed the discovery of several new approaches to enhancement of the resistance of plants to disease through biotechnology. Besides the use of potential microbial antagonists (19) and beneficial microorganisms such as plant growth-promoting rhizobacteria (31) to control pathogen populations, the possibility of stimulating a plant's immune system by using nonpathogenic organisms has opened novel avenues for plant disease management (1). While cucumber has been frequently used as a model of induced resistance (42), the use of nonpathogenic Fusarium spp. as inducers of resistance to root diseases has not been widely explored and the specific mechanisms involved in their suppressive effect remain unclear.
Results of the present study confirm and further document the ability of F. oxysporum strain Fo47 to stimulate resistance genes leading to the production and accumulation of plant defense molecules (12). Most striking and interesting was the observation that preinoculation of strain Fo47 conferred increased resistance of cucumber plants to infection by P. ultimum. This stands in contrast to the general conceptual framework in which strain Fo47 exhibits biocontrol capabilities against a very narrow spectrum of root diseases, mainly those caused by pathogenic F. oxysporum strains (4, 38). Since nonpathogenic Fusarium spp. can occupy overlapping ecological niches and compete with phytopathogenic microorganisms for available nutrients (i.e., carbon and iron) (26, 32), in addition to inducing systemic resistance (23), one would expect these fungi to be highly versatile and exhibit little specificity as to the targets they control. Our results are thus of particular relevance since they highlight, for the first time, the potential of this rhizosphere-competent fungus as a promising biocontrol agent of Pythium-incited diseases.
Another novel finding of the present study concerns the antagonistic activity mediated by Fo47 to control pathogen density. The main mechanisms by which fungal antagonists may exert activity against pathogens have long been attributed to direct effects such as mycoparasitism, antibiosis, competition for nutrients and space, and indirect effects such as plant-induced resistance. These mechanisms may operate either alone or synergistically (2). Recent investigations of the involvement of microbial antagonism in the suppression of fusarium wilts have disclosed that reduction of pathogenic F. oxysporum populations in suppressive soils was greatly enhanced when Fo47 was associated with fluorescent Pseudomonas spp. (24, 32). Evidence was provided that the antagonistic process relies mainly on iron competition mediated by siderophores such as the pseudobactin produced by P. putida (32) and on competition for carbon and for infection sites at the root surface (37). That antibiosis and/or mycoparasitism could be involved in the antagonistic process has been a poorly explored research hypothesis. With the exception of the study by Anjaiah et al. (6), who reported that toxic metabolites produced by fluorescent Pseudomonas spp. (i.e., phenazines) could directly affect pathogen viability in soils suppressive to fusarium wilts, the possibility that Fo47 itself may exert direct antimicrobial activity has been ignored, although expectations with regard to the ability of a specific microorganism to function as a successful biocontrol agent are partly conditioned by deep knowledge of its mechanisms of action (29). Confrontation experiments between Fo47 and P. ultimum in vitro revealed that a substantial reduction of P. ultimum growth was achieved by Fo47. At the ultrastructural level, the observation that the viability of Pythium hyphae was disrupted in the absence of intimate contact with Fo47 cells was taken as an indication that antimicrobial substances were produced by Fo47. Similar cellular alterations (i.e., plasmalemma retraction and disruption, cytoplasm aggregation and/or dissolution, and organelle disintegration) have often been reported to occur upon exposure of fungal pathogens to antibiotics or hydrolytic enzymes produced by antagonistic organisms (10, 11, 33). In the interaction under study, the regular pattern of cellulose distribution over the cell walls of highly altered P. ultimum hyphae excludes the option that cell wall-degrading enzymes may contribute to the observed cell disturbances. More realistic is the possibility that Fo47 produced antibiotics that diffused in the medium prior to reaching their potential receptors at the plasma membrane of Pythium cells. Cell wall splitting and deposition of amorphous material between the split wall layers were other prominent facets of the interaction between Fo47 and P. ultimum. Although the mechanisms underlying such a process are unknown, one may suggest that such changes in cell wall structure reflect a defense strategy elaborated by Pythium hyphae to protect themselves from the action of toxic metabolites secreted by Fo47.
Taken together, our results open the way to a better understanding of the role played by antibiosis in Fo47-mediated antagonism against P. ultimum. These results, obtained from in vitro bioassays, must, however, be viewed with caution since physiological and cellular events observed in culture media do not necessarily indicate that similar phenomena occur in the presence of the host plant. Data corroborating this concept come from the finding that mycoparasitism, which was never observed in dual-culture tests, was a major determinant of the biocontrol activity exerted by Fo47 in planta. Observations of the root surface showed that Fo47 cells established close contact with hyphae of P. ultimum, leading to a series of cellular alterations similar, in most respects, to those observed in dual-culture tests. Most striking and interesting was the finding that mycoparasitism, as evidenced by the frequent occurrence of Fo47 hyphae within nearly empty cells of the pathogen, occurred not only at the root surface but also within the invaded root tissues. Successful penetration and colonization of Pythium hyphae by Fo47 cells imply that cellulolytic enzymes were produced. Data favoring the concept of increased production of cellulases include the labeling pattern of cellulose, which clearly showed that the level of integrity of this wall-bound compound was greatly affected in penetrated Pythium hyphae. The production of extracellular lytic enzymes by Fo47 is not a well-documented phenomenon, although Benhamou and Garand (12) recently concluded that the ability of Fo47 to colonize the outer root tissues is likely associated with the production of cell wall-hydrolytic enzymes such as pectinases and cellulases. The possibility that Fo47 may use the cellulases produced to breach the plant cell wall as part of its biocontrol activity against P. ultimum appears to be a realistic option that would explain why features of mycoparasitism were not observed in dual cultures. According to our observations, it seems reasonable to speculate that cellulase production by Fo47 is a phenomenon induced upon perception of signals produced by the plant. In a number of biological systems, molecular signaling has been shown to play a crucial role in the process of host recognition, leading to a series of events resulting in penetration and invasion of the host cells (8, 22). Because mycoparasitism always occurred at a time when cells of the pathogen were depleted of their protoplasm, one may speculate that cellulolytic enzymes are not the only critical traits involved in the antagonistic process. Although it is clear that such enzymes play a key role in breaching the pathogen cell walls, it seems likely that cellulase activity is preceded by or coincides with the action of toxic compounds, as recently shown by Di Pietro et al. (21) and Schirmböck et al. (41), in the biocontrol process mediated by fungal antagonists. All of these results suggest that a specific enzyme-antibiotic balance may be required for optimal antagonistic activity in the rhizosphere. Within this framework, the apparent contradictions between the present results and the earlier reports should be reconsidered on the basis that the ability of Fo47 to produce hydrolytic enzymes requires an inducing signal originating from the plant. Since plants probably do not generate chitinase-inducing signals, it is unlikely that mycoparasitism against pathogenic F. oxysporum spp., which are known to contain chitin as a major cell wall component, occurs in the rhizosphere. On the contrary, the production of cellulases, used by Fo47 for colonizing root tissues, may lead to an important reduction of oomycete population densities through mycoparasitism. By providing food sources, such a process may, in turn, contribute to an increase in Fo47 biomass, thus conferring obvious advantages in terms of competition for space and nutrients.
Even though direct microbial interactions appear to be a major determinant in restricting Pythium invasion of cucumber root tissues, induction of host defense reactions is another facet of the mechanisms involved in the protection mediated by Fo47. In line with previous reports (12, 37, 40), our ultrastructural work confirmed the rhizosphere competence of Fo47 and the subsequent penetration of the outer root tissues. The consequences of root colonization by Fo47 were striking in terms of host physiological changes, as evidenced by the reinforcement of cell walls beyond the infection sites and the deposition of fibrillar and/or amorphous matrices in the invaded areas. Similar barriers, likely laid down to prevent extensive fungal spreading and potential fungal pathogenesis, have been described in cucumber and tomato roots inoculated with other mycoparasites such as T. harzianum (45) and Pythium oligandrum (15). Such a rapid induction of plant defense reactions may represent a process by which nonpathogenic microorganisms coexist within root tissues without causing the extensive cell damage frequently observed in compatible host-pathogen interactions. Although a large number of wall appositions were formed at sites of potential entry, Fo47 hyphae displayed the ability to penetrate the epidermis and the outer root cortex, indicating its ability to produce the required cell wall-degrading enzymes.
Positive correlations between Fo47 treatment and induced resistance to infection by P. ultimum in cucumber were confirmed by at least three main observations: (i) reduction of pathogen viability, (ii) elaboration of newly formed barriers, and (iii) occlusion of intercellular spaces. Restriction of pathogen growth to the outer root tissues coincided with a substantial decrease in pathogen viability, suggesting the creation of a fungitoxic environment in the invaded areas. The mechanisms involved in pathogen cell alteration in planta have not been clearly defined, although it is reasonable to postulate that direct inhibitory effects, mediated by the synergistic action of phenolic compounds produced by the plant and antifungal metabolites produced by Fo47, are responsible for the observed Pythium cell damage. Strengthening of the host cell walls by either incrustation of newly formed compounds or deposition of numerous heterogeneous wall appositions was a prominent facet of the plant response to Pythium infection. This phenomenon, which was not detected in Fo47-free plants, where the pathogen proliferated in all root tissues within a few days, correlates well with the concept that barricading the pathogen through physical exclusion to limit extensive host tissue invasion is a genetically programmed resistance response that involves the de novo synthesis of various structural compounds, including phenolics and lignin (36). Filling of most intercellular spaces with a dense material likely enriched in phenolics frequently coincided with marked pathogen cell alterations. Since intercellular spaces are known to be preferential sites for pathogen ingress, it is thus not surprising that one of the earliest host defense reactions consists of accumulation of fungitoxic substances at these strategic areas (14, 15).
In contrast to the view summarized by Djuiff et al. (23, 24) regarding the secondary importance of plant-induced resistance in the fusarium wilt suppression achieved by Fo47, our cytological observations clearly demonstrate that Fo47-mediated induced resistance is a key complementary mechanism in the protection of cucumber against infection by P. ultimum. The present study brings novel insights into the multifaceted mechanisms by which Fo47 operates to trigger plant protection and highlights the potential of cytological approaches in evaluating the functional activity of plant and microbial cells during specific biological processes. Although such results are difficult to generalize since they may be linked to the particular interaction under study, our observations demonstrate for the first time that: (i) Fo47 has the ability to control more root pathogens than previously thought, (ii) Fo47 exerts a direct inhibitory effect on P. ultimum, and (iii) Fo47 is a strong inducer of plant defense reactions, mainly structural barriers laid down to protect the inner root tissues from massive invasion. Today, an improved protection program implies the availability of highly efficient biofungicides. Because of its remarkable biological properties, nonpathogenic F. oxysporum strain Fo47 offers very good prospects for integrated management of root disease in greenhouse crops.
Acknowledgments
This work was supported by grants from the Fonds Québécois pour la Formation de Chercheurs et l'Aide à la Recherche and the Natural Sciences and Engineering Council of Canada.
REFERENCES
- 1.Alabouvette, C. 1999. Fusarium wilt suppressive soils: an example of disease-suppressive soils. Aust. J. Plant Pathol. 28:57-64. [Google Scholar]
- 2.Alabouvette, C., and Y. Couteaudier. 1992. Biological control of fusarium wilts with nonpathogenic fusaria, p. 415-426. In E. C. Tjamos, G. C. Papavizas, and R. Cook (ed.), Biological control of plant diseases: progress and challenges for the future. Plenum Press, New York, N.Y.
- 3.Alabouvette, C., Y. Couteaudier, Y., and J. Louvet. 1985. Soils suppressive to fusarium wilt: mechanisms and management of suppressiveness, p. 101-106. In C. A. Parker, A. D. Rovira, K. J. Moore, P. T. W. Wong, and J. F. Kollmorgen (ed.), Ecology and management of soilborne plant pathogens. American Phytopathological Society, St. Paul, Minn.
- 4.Alabouvette, C., D. De la Broise, P. Lemanceau, Y. Couteaudier, and J. Louvet. 1987. Utilisation de souches non pathogènes de Fusarium spp. pour lutter contre les fusarioses: situation actuelle dans la pratique. EPPO Bull. 17:665-674. [Google Scholar]
- 5.Alabouvette, C., P. Lemanceau, and C. Steinberg. 1996. Biological control of Fusarium wilts: opportunities for developing a commercial product, p. 192-212. In R. Hall (ed.), Principles and practice of managing soilborne plant pathogens. American Phytopathological Press, St. Paul, Minn.
- 6.Anjaiah, V., N. Koedam, B. Nowak-Thompson, E. Loper, M. Höfte, J. T. Tambong, and P. Cornelis. 1998. Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 and Tn5 derivatives toward Fusarium spp. and Pythium spp. Mol. Plant-Microbe Interact. 9:847-854. [Google Scholar]
- 7.Benhamou, N. 1989. Preparation and applications of lectin-gold complexes, p. 95-143. In M. A. Hayat (ed.), Colloidal gold: principles, methods and applications, vol. 1. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 8.Benhamou, N. 1996. Elicitor-induced plant defense pathways. Trends Plant Sci. 1:233-240. [Google Scholar]
- 9.Benhamou, N., and R. R. Bélanger. 1998. Induction of systemic resistance to Pythium damping-off in cucumber plants by benzothiadiazole: ultrastructure and cytochemistry of the host response. Plant J. 14:13-21. [DOI] [PubMed] [Google Scholar]
- 10.Benhamou, N., and J. Brodeur. 2000. Evidence for antibiosis and induced host defense reactions in the interaction between Verticillium lecanii and Penicillium digitatum, the causal agent of green mold. Phytopathology 90:932-943. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou, N., and I. Chet. 1997. Cellular and molecular mechanisms involved in the interaction between Trichoderma harzianum and Pythium ultimum. Appl. Environ. Microbiol. 63:2095-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benhamou, N., and C. Garand. 2001. Cytological analysis of defense-related mechanisms induced in pea root tissues in response to colonization by the non-pathogenic Fusarium oxysporum, strain Fo47. Phytopathology 91:730-740. [DOI] [PubMed] [Google Scholar]
- 13.Benhamou, N., H. Chamberland, G. B. Ouellette, and F. J. Pauzé. 1987. Ultrastructural localization of β-1,4-d-glucans in two pathogenic fungi and in their host tissues by means of an exoglucanase-gold complex. Can. J. Microbiol. 33:405-417. [Google Scholar]
- 14.Benhamou, N., S. Gagné, D. LeQuéré, and L. Dehbi. 2000. Bacterial-mediated induced resistance in cucumber: beneficial effect of the endophytic bacterium, Serratia plymuthica, on the protection against infection by Pythium ultimum. Phytopathology 90:45-56. [DOI] [PubMed] [Google Scholar]
- 15.Benhamou, N., P. Rey, M. Chérif, J. Hockenhull, and Y. Tirilly. 1997. Treatment with the mycoparasite, Pythium oligandrum, triggers the induction of defense-related reactions in tomato roots upon challenge with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 87:108-122. [DOI] [PubMed] [Google Scholar]
- 16.Charest, P. M., G. B. Ouellette, and F. J. Pauzé. 1984. Cytological observations of early infection process of Fusarium oxysporum f. sp. radicis-lycopersici. Can. J. Bot. 62:1232-1244. [Google Scholar]
- 17.Chérif, M., and N. Benhamou. 1990. Cytochemical aspects of chitin breakdown during the parasitic action of a Trichoderma sp. on Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 80:1406-1414. [Google Scholar]
- 18.Chérif, M., N. Benhamou, J. G. Menzies, and R. R. Bélanger. 1993. Silicon induced resistance in cucumber plants against Pythium ultimum. Physiol. Mol. Plant Pathol. 41:411-425. [Google Scholar]
- 19.Chet, I., and J. Inbar. 1994. Biological control of fungal pathogens. Appl. Biochem. Biotechnol. 48:37-43. [DOI] [PubMed] [Google Scholar]
- 20.Couteaudier, Y. 1992. Competition for carbon in soil and rhizosphere, a mechanism involved in biological control of fusarium diseases, p. 99-104. In E. C. Tjamos, G. C. Papavizas, and R. J. Cook (ed.), Biological control of plant diseases. Plenum Press, New York, N.Y.
- 21.Di Pietro, A., M. Lorito, C. K. Hayes, R. M. Broadway, and G. E. Harman. 1993. Endochitinases from Gliocladium virens: isolation, characterization, and synergistic antifungal activity in combination with gliotoxin. Phytopathology 83:308-313. [Google Scholar]
- 22.Downie, J. A. 1994. Signalling strategies for nodulation of legumes by rhizobia. Trends Microbiol. 2:318-324. [DOI] [PubMed] [Google Scholar]
- 23.Duijff, B. J., D. Poulain, C. Olivain, C. Alabouvette, and P. Lemanceau. 1998. Implication of systemic induced resistance in the suppression of fusarium wilt of tomato by Pseudomonas fluorescens WCS417r and by nonpathogenic Fusarium oxysporum Fo47. Eur. J. Plant Pathol. 104:903-910. [Google Scholar]
- 24.Duijff, B. J., G. Recorbet, P. A. H. M. Bakker, J. E. Loper, and P. Lemanceau. 1999. Microbial antagonism at the root level is involved in the suppression of fusarium wilt by the combination of nonpathogenic Fusarium oxysporum Fo47 and Pseudomonas putida WCS358. Phytopathology 89:1073-1079. [DOI] [PubMed] [Google Scholar]
- 25.El Ghaouth, A., J. Arul, J. Grenier, N. Benhamou, A. Asselin, and R. R. Bélanger. 1994. Effect of chitosan on cucumber plants: suppression of Pythium aphanidermatum and induction of defense reactions. Phytopathology 84:313-320. [Google Scholar]
- 26.Eparvier, A., and C. Alabouvette. 1994. Use of ELISA and GUS-transformed strains to study competition between pathogenic and non-pathogenic Fusarium oxysporum for root colonization. Biocontrol Sci. Technol. 4:35-47. [Google Scholar]
- 27.Frens, G. 1973. Controlled nucleation for the regulation of the particle size in monodisperse gold solutions. Nat. Phys. Sci. 241:20-22. [Google Scholar]
- 28.Handelsman, J., and E. V. Stabb. 1996. Biocontrol of soilborne plant pathogens. Plant Cell 8:1855-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harman, G. E. 2000. Myths and dogma of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 84:377-393. [DOI] [PubMed] [Google Scholar]
- 30.Hoeper, H., and C. Alabouvette. 1996. Importance of physical and chemical soil properties in the suppressiveness of soils to plant diseases. Eur. J. Soil Biol. 32:41-58. [Google Scholar]
- 31.Kloepper, J. W. 1993. Plant growth-promoting rhizobacteria as biological control agents, p. 255-274. In B. Metting (ed.), Soil microbial technologies. Marcel Dekker Inc., New York, N.Y.
- 32.Lemanceau, P., P. A. H. M. Bakker, W. J. De Kogel, C. Alabouvette, and B. Schippers. 1993. Antagonistic effect of nonpathogenic Fusarium oxysporum strain Fo47 and pseudobactin 358 upon pathogenic Fusarium oxysporum f. sp. dianthi. Appl. Environ. Microbiol. 59:74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis, J. A., and G. C. Papavizas. 1987. Permeability changes in hyphae of Rhizoctonia solani induced by germling preparations of Trichoderma and Gliocladium. Phytopathology 77:699-703. [Google Scholar]
- 34.Lumsden, R. D., E. R. Garcia, J. A. Lewis, and G. A. Frías-T. 1987. Suppression of damping-off caused by Pythium spp. in soil from the indigenous Mexican Chinampa agricultural system. Soil Biol. Biochem. 19:501-508. [Google Scholar]
- 35.Martin, F. N., and J. E. Loper. 1998. Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology and prospects for biological control. Crit. Rev. Plant Sci. 18:111-191. [Google Scholar]
- 36.Nicholson, R. L., and R. Hammerschmidt. 1992. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 30:369-389. [Google Scholar]
- 37.Olivain, C., and C. Alabouvette. 1997. Colonization of tomato root by a non-pathogenic strain of Fusarium oxysporum. New Phytol. 137:481-494. [DOI] [PubMed] [Google Scholar]
- 38.Paulitz, T. C., and A. Matta. 1999. The role of the host in biological control of diseases, p. 394-410. In R. Alabajes, M. L. Gullino, J.C. van Lenteren, and Y. Elad (ed.), Integrated pest and disease management in greenhouse crops. Kluwer Academic Press, New York, N.Y.
- 39.Rouxel, F., C. Alabouvette, and J. Louvet. 1979. Recherches sur la résistance des sols aux maladies. IV. Mise en évidence du rôle des Fusarium autochtones dans la résistance d'un sol à la fusariose vasculaire du melon. Ann. Phytopathol. 11:199-207. [Google Scholar]
- 40.Salerno, M. I., S. Gianinazzi, and V. Gianinazzi-Pearson. 2000. Effects on growth and comparison of root tissue colonization patterns of Eucalyptus viminalis by pathogenic and nonpathogenic strains of Fusarium oxysporum. New Phytol. 146:317-324. [DOI] [PubMed] [Google Scholar]
- 41.Shirmböck, M., M. Lorito, Y. L. Wang, C. K. Hayes, I. Arisan-Atac, F. Scala, G. E. Harman, and C. P. Kubicek. 1994. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 60:4364-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegrist, J., W. Jeblick, and H. Kauss. 1994. Defense responses in infected and elicited cucumber (Cucumis sativus L.) hypocotyl segments exhibiting acquired resistance. Plant Physiol. 105:1365-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stutz, E. W., G. Défago, and H. Kern. 1986. Naturally occurring fluorescent pseudomonas involved in suppression of black root rot of tobacco. Phytopathology 76:181-185. [Google Scholar]
- 44.Weller, D. M. 1988. Biological control of soilborne pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26:379-407. [Google Scholar]
- 45.Yedidia, I., N. Benhamou, and I. Chet. 1999. Induction of defense responses in cucumber by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 65:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]