Abstract
Moraxella osloensis is a gram-negative bacterium associated with Phasmarhabditis hermaphrodita, a slug-parasitic nematode that has prospects for biological control of mollusk pests, especially the grey garden slug, Deroceras reticulatum. This bacterium-feeding nematode acts as a vector that transports M. osloensis into the shell cavity of the slug, and the bacterium is the killing agent in the nematode-bacterium complex. We discovered that M. osloensis produces an endotoxin(s), which is tolerant to heat and protease treatments and kills the slug after injection into the shell cavity. Washed or broken cells treated with penicillin and streptomycin from 3-day M. osloensis cultures were more pathogenic than similar cells from 2-day M. osloensis cultures. However, heat and protease treatments and 2 days of storage at 22°C increased the endotoxin activity of the young broken cells but not the endotoxin activity of the young washed cells treated with the antibiotics. This suggests that there may be a proteinaceous substance(s) that is structurally associated with the endotoxin(s) and masks its toxicity in the young bacterial cells. Moreover, 2 days of storage of the young washed bacterial cells at 22°C enhanced their endotoxin activity if they were not treated with the antibiotics. Furthermore, purified lipopolysaccharide (LPS) from the 3-day M. osloensis cultures was toxic to slugs, with an estimated 50% lethal dose of 48 μg per slug, thus demonstrating that the LPS of M. osloensis is an endotoxin that is active against D. reticulatum. This appears to be the first report of a biological toxin that is active against mollusks.
Moraxella osloensis is a gram-negative aerobic bacterium related to other members of the Moraxellaceae in the gamma subdivision of the purple bacteria. This bacterium produces oxidase and catalase but not indole and pigment. It is sensitive to penicillin and can grow in mineral media with acetate and ammonium salts (1). M. osloensis is an opportunistic human pathogen, as it has been found to cause several human diseases (2, 7, 15, 16). This bacterium has also been isolated from xenic foam chip cultures of a lethal nematode parasite of slugs, Phasmarhabditis hermaphrodita (Rhabditida: Peloderinae) (22).
Phasmarhabditis hermaphrodita has potential for biological control of mollusk pests (10, 21), especially the grey garden slug, Deroceras reticulatum (Stylommatophora: Agriolimacidae), which is the most common and serious slug pest of agricultural and horticultural plants worldwide (8, 13). P. hermaphrodita has been found to be associated with several different species of bacteria. However, the nematode yield in in vitro culture and the pathogenicity for slugs differ with different bacterial species (22). Wilson et al. (23) selected M. osloensis as the preferred associated bacterium to rear P. hermaphrodita in monoxenic culture. A commercial product, NemaSlug, based on monoxenic culture of P. hermaphrodita with M. osloensis, has been developed in England.
The parasitic cycle of P. hermaphrodita is initiated by the third-stage infective juveniles (IJs). Each of these IJs is enclosed in a retained second-stage cuticle with closed mouth and anus (21). The IJs enter the shell cavity of D. reticulatum through the posterior mantle region. Once inside the slug host, the IJs release the associated bacteria (18), resume normal growth, and develop into self-fertilizing hermaphrodites, which finally leads to death of the host (18, 21). When the food source is depleted, the nematodes form the next generation of IJs that search for new hosts (21). Wilson et al. (23) reported that a 24-h culture of M. osloensis that was injected into D. reticulatum hemocoel was not pathogenic. However, we (17) discovered that aged cultures of M. osloensis were actually pathogenic to D. reticulatum both in the shell cavity and in the hemocoel. Moreover, axenic J1 and J2 forms of P. hermaphrodita were nonpathogenic after injection into the shell cavity, and the pathogenicity of the IJs depended on the number of viable M. osloensis carried by the IJs (17). Therefore, it was concluded that P. hermaphrodita acts as a vector which transports the associated bacterium M. osloensis into the shell cavity of D. reticulatum and that the bacterium is the main killing agent in the nematode-bacterium complex (17). The mutualism between P. hermaphrodita and M. osloensis seems to be parallel to the association between the entomopathogenic nematodes in the genera Heterorhabditis and Steinernema and their associated bacteria in the genera Photorhabdus and Xenorhabdus, respectively (9).
However, the actual mechanism of pathogenicity of M. osloensis for D. reticulatum is still unknown. We (17) reported that injection of penicillin and streptomycin along with aged M. osloensis cultures reduced the pathogenicity of the bacterium for the slug and suggested that M. osloensis may produce a toxin(s) that kills the slug. Information on the pathogenicities of related bacteria in the genus Moraxella for their individual hosts inspired us to explore the mechanism of the virulence of M. osloensis against D. reticulatum. Moraxella catarrhalis, the third most common pathogen of the respiratory tract of humans, is thought to liberate endotoxin for its pathogenicity (3). Moreover, lipopolysaccharide (LPS), outer membrane proteins, pili or fimbriae, and a possible capsule have been considered virulence factors of M. catarrhalis (19). Furthermore, LPS, outer membrane proteins, pili, and secretion of a hemolysin and/or cytotoxin seem to contribute to the virulence of Moraxella bovis, which is the most common etiological agent isolated in acute and chronic cases of infectious bovine keratoconjunctivitis (12). As endotoxin usually plays a major role in the pathogenesis of gram-negative infections (6), we hypothesized that M. osloensis produces an endotoxin(s) that kills the slug. We further hypothesized that the LPS of M. osloensis is an endotoxin that is active against the slug.
MATERIALS AND METHODS
Sources of bacteria and slugs.
A pure culture of M. osloensis was supplied by MicroBio Ltd. (Cambridge, United Kingdom). All D. reticulatum adults were collected from the field and fed pieces of fresh carrots and cabbage leaves at 18°C for at least 12 days. Only healthy adult slugs were then used in the following experiments.
Endotoxin activity of M. osloensis from 3-day cultures.
A pure culture of M. osloensis was inoculated onto nutrient agar plates and incubated at 25°C for 3 days until it reached the stationary phase. The bacteria were then washed off the plates into a sterile petri dish by using sterile saline solution (0.85% NaCl). The total number of bacteria in the resulting suspension was measured with a spectrophotometer at 600 nm, and the concentration was estimated to be 1.58 × 1010 CFU/ml by using a standard curve for the bacteria. Part of the bacterial suspension, which was designated intact cells, was divided into aliquots, placed in 2-ml sterile microcentrifuge tubes, and then centrifuged at 16,000 × g for 5 min by using an Eppendorf microcentrifuge (model 5415 C). The supernatant obtained was transferred into a sterile petri dish and was filtered through a 0.2-μm-pore-size filter. The final filtrate was designated the cell-free suspension. The bacterial pellet was then washed with the sterile saline solution and was gently pipetted up and down in the tubes to obtain complete suspension following centrifugation under the same conditions. The washing process was repeated three times. The final bacterial pellet was resuspended in the same amount of sterile saline solution as the cell-free suspension and was designated the washed cells. A portion of the washed cells was then broken twice with a French pressure cell and press (Thermo Spectronic, Rochester, N.Y.), and the resulting preparation was designated the broken cells. Portions (80 μl) of the intact cells, washed cells with and without penicillin (500 U/ml) and streptomycin (500 μg/ml), broken cells with the antibiotics, and cell-free suspension were injected into the shell cavities of D. reticulatum slugs as described previously (18). The antibiotics were used to inhibit the multiplication and metabolism of the washed cells or of any unbroken cells in the broken cell preparation in order to eliminate the potential production of toxin(s) after injection. Eighteen slugs were used for each treatment and were then separated into three petri dishes (six slugs per dish) as three replicates for calculation of slug mortality. Slugs injected with the saline solution or the antibiotics served as controls. All slugs were fed pieces of fresh carrots and cabbage leaves and were incubated at 18°C. The numbers of dead slugs were recorded every day for 12 days.
Effect of heat pretreatment on the endotoxin activities of different types of M. osloensis cells.
M. osloensis was cultured on nutrient agar plates at 25°C for 2 days (in the log phase) and for 3 days (in the stationary phase). The bacterial concentrations were estimated and adjusted to 1.0 × 1010 CFU/ml. Washed cells or broken cells from the 2- or 3-day cultures were then prepared as described above. Portions of the washed or broken cells were then heated at 60 or 100°C in a water bath for 15 min. The heated bacterial cells were allowed to settle at 22°C for 30 min. An 80-μl suspension of a heated or unheated preparation of cells (at 22°C) with the antibiotics was injected into the shell cavity of each D. reticulatum used. The antibiotics were used to inhibit the multiplication and metabolism of the washed cells or of any unbroken cells in the broken cell preparation in order to eliminate the potential production of toxin(s) after injection. The rest of experimental design was as described above, except that slugs injected only with the antibiotics served as the controls.
Effect of protease pretreatment on the endotoxin activities of different types of M. osloensis cells.
Washed or broken cells from 2- or 3-day M. osloensis cultures were prepared as described above for the first experiment. Three milliliters of the washed or broken cell preparation was then used to dissolve three trypsin tablets (1 mg of porcine trypsin type II per tablet) containing a small amount of chymotrypsin (Sigma Chemical Co., St. Louis, Mo.), which yielded a ready-to-use buffered solution (pH 8.0). The reaction mixture was incubated at 25°C in a water bath for 15 min. An 80-μl portion of the suspension containing washed or broken cells with the antibiotics was then injected into the shell cavity of each D. reticulatum used. The rest of the experimental design was as described above for the first experiment, except that slugs injected with the antibiotics alone or with the proteases plus the antibiotics served as the controls.
Effect of 2 days of storage at different temperatures on the endotoxin activities of different types of M. osloensis cells.
Washed or broken cells from 2- or 3-day M. osloensis cultures were prepared as described above for the first experiment. The washed or broken cells were treated with the antibiotics and then divided into 1.5-ml portions, placed into sterile prelabeled microcentrifuge tubes, and stored for 2 days at 22, 4, or −20°C. The tubes that were stored at 4 or −20°C were taken out, and the contents were allowed to settle at 22°C for 30 min. An 80-μl suspension of a fresh washed or broken cell preparation or a preparation stored at one of the three temperatures for 2 days with the antibiotics was then injected into the shell cavity of each D. reticulatum used. The antibiotics were used to inhibit the multiplication and metabolism of the washed cells or of any unbroken cells in the broken cell preparation in order to eliminate the potential production of toxin(s) during the experimental period. The rest of experimental design was as described above for the second experiment.
Effect of 2 days of storage at 22°C on the endotoxin activity of washed cells from 2-day M. osloensis cultures with or without the antibiotics.
Washed cells from 2-day M. osloensis cultures were prepared as described above for the first experiment. The bacterial concentration was estimated to be 1.32 ×1010 CFU/ml before the washing process. The washed cells were divided into two parts, and only one part was treated with the antibiotics. Both of the parts were stored at 22°C for 2 days. Eighty microliters of the fresh washed cells or the cells stored at 22°C for 2 days with or without the antibiotics was then injected into the shell cavity of each D. reticulatum used. The rest of experimental design was as described above for the first experiment.
Purification of LPS from 3-day M. osloensis cultures.
As the endotoxin activity in gram-negative bacteria is generally associated with LPS, we purified the LPS from 3-day M. osloensis cultures. Intact cells from the cultures were prepared, centrifuged, and washed once as described above for the first experiment. The bacterial pellet obtained was resuspended in distilled water, placed in a sterile beaker, and then lyophilized. LPS was purified from the resulting bacterial powder by classical phenol-water extraction (20), with the modification described by Gu et al. (11). About 0.5 g of the bacterial powder was suspended in 35 ml of distilled water at 65 to 68°C in a water bath. The same amount of 90% phenol, preheated to 65 to 68°C, was then added with vigorous stirring. The mixture was kept at 65°C for 15 min and cooled to about 10°C in an ice bath. The resulting emulsion was centrifuged at 3,000 rpm (1,470 × g) for 45 min by using an IEC clinical centrifuge (model 428). The upper aqueous phase was aspirated off, and the remainder was reextracted with distilled water as described above. Sodium acetate (5 mg/ml) was added to the combined aqueous phase, and LPS was precipitated with 2 volumes of acetone to reduce phospholipid contamination. The pellet was washed twice with 70% ethanol to reduce the trace phenol content and then suspended in distilled water. RNase and DNase (80 μg/ml; Sigma Chemical Co.) were added, and the digestion mixture was incubated at 37°C for 4 h. Proteinase K (0.5 mg/ml; Shelton Scientific, Shelton, Conn.) was then added. The digestion mixture was incubated at 60°C overnight. The digested mixture was ultracentrifuged twice at 150,000 × g for 3 h. Gel-like LPS was dissolved in distilled water, lyophilized, and then stored at −20°C. The yield of LPS was about 0.7% of the dry weight of the bacteria.
Toxicity of purified LPS from 3-day M. osloensis cultures.
The purified LPS powder from the 3-day M. osloensis cultures was dissolved and diluted with distilled water to produce a series of concentrations of LPS (0.1, 0.4, 0.6, 1.0, 1.6, and 2.0 mg/ml). A 50-μl portion of each concentration was injected into the shell cavity of a D. reticulatum. Eighteen slugs were treated with each concentration as described above for the first experiment. At the same time, slugs injected with distilled water served as controls. All slugs were fed pieces of fresh carrots and cabbage leaves and were incubated at 18°C. The numbers of dead slugs were recorded after 4 days.
Statistical analyses.
All data presented below as percentages were arcsine transformed and subjected to a one-way analysis of variance by using the statistical software STATISTICA Kernel, release 5.5 (StatSoft Inc., Tulsa, Okla.). The significance of differences among treatments was determined by using Tukey's honestly significant difference tests and a P value of 0.05.
RESULTS
Endotoxin activity of M. osloensis from 3-day cultures against D. reticulatum.
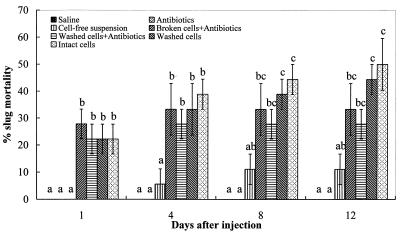
The percentages of mortality of D. reticulatum following injection of different components from 3-day M. osloensis cultures into the shell cavity are shown in Fig. 1. Compared with the two groups of controls, all preparations except the cell-free suspension had significant effects (P < 0.05) on slug mortality during the entire experimental period. The broken cells with antibiotics caused the highest level of slug mortality at 1 day after treatment, and the peak mortality was reached on day 4. The washed cells with antibiotics had a similar effect on slug mortality, and there was no additional mortality after 4 days. However, the levels of slug mortality continued to increase with the intact cells and the washed cells without antibiotics, reaching 50 and 45%, respectively, by day 12. These results indicate that M. osloensis produces an endotoxin(s) that is active against D. reticulatum.
FIG. 1.
Percentages of mortality (means ± standard errors; n = 3) of D. reticulatum following injection of different components of 3-day M. osloensis cultures into the shell cavity. Different letters above the bars indicate that values differed significantly at P < 0.05.
Effect of heat pretreatment on the endotoxin activities of different types of M. osloensis cells.
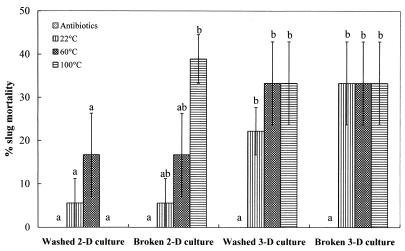
The levels of D. reticulatum mortality caused by preheated washed or broken M. osloensis cells from the 2- or 3-day cultures are shown in Fig. 2. Compared with the control (antibiotic treatment), all treatments with the washed or broken cells from 3-day M. osloensis cultures caused significant slug mortality (P < 0.05). However, the treatments with the washed cells from 2-day M. osloensis cultures, regardless of whether the cells were preheated or not preheated, did not cause significant slug mortality (P > 0.05) compared with the control. In contrast, the heat pretreatment did have a significant effect on the endotoxin activity of broken cells from the 2-day bacterial cultures. The broken cells from the 2-day cultures did not cause significant slug mortality (P > 0.05) when they were kept at 22°C but had a significant effect (P < 0.05) on slug mortality after they were heated at 100°C for 15 min compared with the results for the control.
FIG. 2.
Percentages of mortality (means ± standard errors; n = 3) of D. reticulatum following injection of different types of M. osloensis cells pretreated with heat into the shell cavity. All types of cells were treated with the antibiotics before injection. Different letters above the bars indicate that values differed significantly at P < 0.05. 2-D, 2-day; 3-D, 3-day.
Effect of protease pretreatment on the endotoxin activities of different types of M. osloensis cells.
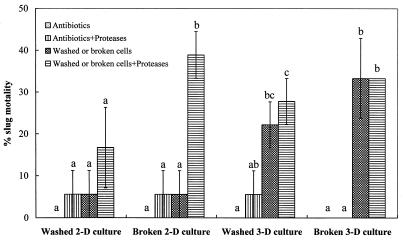
Protease pretreatment had an effect on the endotoxin activities of different types of M. osloensis cells with antibiotics against D. reticulatum similar to the effect of the heat treatment described above (Fig. 3). Compared with the two groups of controls (treatment with antibiotics and treatment with antibiotics plus protease), the washed or broken cells from 3-day M. osloensis cultures had a significant effect (P < 0.05) on slug mortality, but the level of mortality did not increase significantly (P > 0.05) after protease pretreatment. However, the washed cells from 2-day M. osloensis cultures did not result in significant slug mortality (P > 0.05) during the entire experimental period, regardless of whether the cells were pretreated with the proteases. In contrast, either the nonbroken cells or the less-toxic broken cells from same young cultures that were pretreated with the proteases had a significant effect (P < 0.05) on slug mortality compared with the controls.
FIG. 3.
Percentages of mortality (means ± standard errors; n = 3) of D. reticulatum following injection of different types of M. osloensis cells pretreated with proteases into the shell cavity. All types of cells were treated with the antibiotics before injection. Different letters above the bars indicate that values differed significantly at P < 0.05. 2-D, 2-day; 3-D, 3-day.
Effect of 2 days of storage at different temperatures on the endotoxin activities of different types of M. osloensis cells.
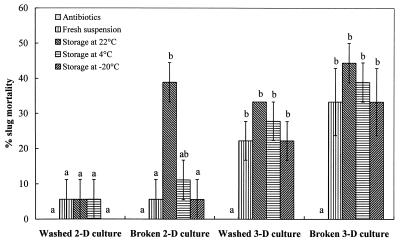
Similar to the heat and protease treatments described above, 2 days of storage at different temperatures did not have a significant effect (P > 0.05) on the endotoxin activity of the washed or broken cells from 3-day M. osloensis cultures against the slugs. However, all treatments with these old bacterial cells resulted in significantly (P < 0.05) higher slug mortality than the control treatment (Fig. 4). Treatment with the washed cells from the 2-day M. osloensis cultures, including the fresh washed cells and the cells stored at different temperatures for 2 days, did not have an observable effect on slug mortality compared with the results for the control. However, the broken bacterial cells from the 2-day cultures stored at 22°C for 2 days resulted in significant slug mortality (P < 0.05) compared with the control.
FIG. 4.
Percentages of mortality (means ± standard errors; n = 3) of D. reticulatum following injection into the shell cavity of different types of M. osloensis cells after storage at different temperatures for 2 days with antibiotics. Different letters above the bars indicate that values differed significantly at P < 0.05. 2-D, 2-day; 3-D, 3-day.
Effect of 2 days of storage at 22°C on the endotoxin activity of washed cells from 2-day M. osloensis cultures with or without the antibiotics.
If the washed cells from the 2-day cultures were treated with the antibiotics, they had no observable effect on slug mortality after 2 days of storage at 22°C. In contrast, the same washed cells without the antibiotics did not result in significant slug mortality (P = 0.15) when they were freshly prepared, but they caused 39% ± 6% (mean ± standard error; n = 3) slug mortality after storage at 22°C for 2 days, which is significantly different (P < 0.05) from the results obtained for the controls (data not shown).
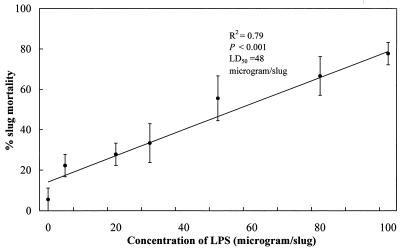
Toxicity of purified LPS from 3-day M. osloensis cultures.
The levels of D. reticulatum mortality caused by different concentrations of LPS from 3-day M. osloensis cultures are summarized in Fig. 5. There is a significant linear relationship (R2 = 0.79) between slug mortality and the concentration of injected LPS. The highest concentration of LPS (100 μg per slug) caused about 80% slug mortality, whereas less than 6% of the slugs died in the control (distilled water treatment). Furthermore, the 50% lethal dose of the LPS was estimated to be 48 μg per slug by using a log-probit analysis in the statistical software MINITAB, release 13.1 (Minitab Inc., State College, Pa.).
FIG. 5.
Percentages of mortality (means ± standard errors; n = 3) of D. reticulatum following injection into the shell cavity of different concentrations of purified LPS from 3-day M. osloensis cultures. LD50, 50% lethal dose.
DISCUSSION
The results described here demonstrate that M. osloensis produces an endotoxin(s) that kills D. reticulatum adults after injection into the shell cavity. The cell-free supernatant of the bacterial suspension was not pathogenic to the slug; thus, no exotoxin activity was detected. However, the washed cells with or without the antibiotics and the broken cells with the antibiotics could kill the slug after injection into the shell cavity, indicating that an endotoxin(s) was produced by the bacterium before injection. Furthermore, the broken cells with the antibiotics resulted in the highest level of slug mortality just 1 day after treatment. It is possible that the intact and washed cells had to be broken by the slug host to liberate the endotoxin(s). In addition, slug mortality caused by the intact cells or the washed cells without the antibiotics increased over time, perhaps due to the continued production of the endotoxin(s) resulting from replication of the bacterial cells inside the slug. We (17) reported previously that 6 and 6.15 × 108 CFU of M. osloensis per slug resulted in similar significant levels of slug mortality (around 80%) at 12 days after injection into the shell cavity, implying that the live cells of M. osloensis may multiply inside the slug after injection.
The present results further demonstrate that the LPS of M. osloensis is an endotoxin that is active against D. reticulatum. This appears to be first report of a biological toxin that is active against mollusks. LPS is an important constituent of the outer membranes of gram-negative bacteria and is resistant to heat and protease treatments. The purified LPS from 3-day M. osloensis cultures was toxic to the slug after injection into the shell cavity. Furthermore, dead slugs killed by the LPS exhibited symptoms similar to those exhibited by slugs killed by the intact, washed, or broken M. osloensis cells. Enright and McKenzie (6) reported that three serotypes of lipooligosaccharide, fimbriae, and a possible capsule might be related to the pathogenicity of M. catarrhalis. The LPS of M. catarrhalis was found to have an effect similar to that of the enterobacterial LPS in mice and in the Limulus amoebocyte lysate assay (14). Doyle (4) reported that formalin-killed isolates of M. catarrhalis caused effusions in the middle ear of chinchilla, suggesting the involvement of an endotoxin(s) in the disease process. In addition, the endotoxin produced by Xenorhabdus nematophilus, a bacterial symbiont of the entomopathogenic nematode Steinernema carpocapsae, is also an LPS that is toxic to the hemocytes of larvae of the wax moth Galleria mellonella (5).
Our results suggest that a proteinaceous substance(s) may be structurally associated with the endotoxin(s), which masks the toxicity in the young cells from 2-day M. osloensis cultures. Washed or broken cells treated with the antibiotics from the 3-day M. osloensis cultures were more pathogenic to the slugs than similar cells from the 2-day M. osloensis cultures. Furthermore, heat and protease treatment and storage for 2 days at 22°C increased the endotoxin activity of the young broken cells but not the endotoxin activity of the young washed cells treated with the antibiotics. As the heat treatments are able to inactivate all proteins in both the young washed cells and the broken cells, the endotoxin activity is unlikely to be related to the biological activity (e.g., enzyme activity) of the proteinaceous substance(s). Therefore, it is possible that the proteinaceous substance(s) structurally associated with the endotoxin(s) may have a physical effect (e.g., steric hindrance) on the endotoxin activity but is disassociated from the endotoxin(s) by heat treatment, protease treatment, or storage for 2 days at 22°C in the young broken cells. However, this did not occur in the young washed cells, perhaps because (i) the endotoxin(s) and the proteinaceous substance(s) were protected from disassociation by other substances around them in the washed cells when they were treated with heat or stored for 2 days at 22°C or (ii) the proteases could not locate and digest the proteinaceous substance(s), which may not be exposed on the cell surface of M. osloensis. In addition, since the LPS is an endotoxin that is active against the slug, the proteinaceous substance(s) may be an outer membrane protein(s) in the cell wall of M. osloensis. The enhanced endotoxin activity of the young washed bacterial cells during storage for 2 days at 22°C in the absence of the antibiotics indicates that the young viable bacterial cells are able to increase their endotoxin activity both on plates and in a sterile saline solution. It is possible that the aged bacteria enhance their pathogenicity for the slug by reducing the amount of the proteinaceous substance(s), by decreasing the intensity of association between the endotoxin(s) and the proteinaceous substance(s), or by increasing the amount of LPS per bacterium. Therefore, the increase in the endotoxin activity of M. osloensis cells over time may be related to a structural change in the bacterial cell wall.
Acknowledgments
This work was supported by a matching fund grant from the Ohio Agricultural Research and Development Center (OARDC) and MicroBio Ltd. to P. S. Grewal and by an OARDC graduate research competitive grant to L. Tan.
REFERENCES
- 1.Bovre, K. 1984. Genus II. Moraxella Lwoff 1939, 173 emend, Henriksen and Bovre 1986, 391AL, p. 296-303. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 2.Buchman, A. L., M. J. Pickett, and M. E. Ament. 1993. Central venous catheter infection caused by Moraxella osloensis in a patient receiving home parenteral-nutrition. Diagn. Microbiol. Infect. Dis. 17:163-166. [DOI] [PubMed] [Google Scholar]
- 3.Cullmann, W. 1997. Moraxella catarrhalis: mechanisms of virulence and antibiotic resistance. Med. Klin. 92:162-166. [DOI] [PubMed] [Google Scholar]
- 4.Doyle, W. J. 1989. Animal models of otitis media: other pathogens. Pediatr. Infect. Dis. J. 81:S45-S47. [PubMed] [Google Scholar]
- 5.Dunphy, G. B., and J. M. Webster. 1988. Lipopolysaccharides of Xenorhabdus nematophilus (Enterobacteriaceae) and their haemocyte toxicity in non-immune Galleria mellonella (Insecta: Lepidoptera) larvae. J. Gen. Microbiol. 134:1017-1028. [Google Scholar]
- 6.Enright, M. C., and H. McKenzie. 1997. Moraxella (Branhamella) catarrhalis: clinical and molecular aspects of a rediscovered pathogen. J. Med. Microbiol. 46:360-371. [DOI] [PubMed] [Google Scholar]
- 7.Fijen, C. A. P., E. J. Kuijper, H. G. Tjia, M. R. Daha, and J. Dankert. 1994. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin. Infect. Dis. 18:780-784. [DOI] [PubMed] [Google Scholar]
- 8.Godan, D. 1983. Pest slugs and snails: biology and control. Springer-Verlag, Berlin, Germany.
- 9.Grewal, P. S., and R. Georgis. 1999. Entomopathogenic nematodes, p. 271-299. In F. R. Hall and J. J. Menn (ed.), Methods in biotechnology, vol. 5. Biopesticides: use and delivery. Humana Press Inc., Totowa, N.J.
- 10.Grewal, P. S., S. K. Grewal, R. A. J. Taylor, and R. B. Hammond. 2001. Application of molluscicidal nematodes to slug shelters: a novel approach to economic biological control of slugs. Biol. Control 22:72-80. [Google Scholar]
- 11.Gu, X., C. Tsai, M. A. Apicella, and D. J. Lim. 1995. Quantitation and biological properties of released and cell-bound lipooligosaccharides from nontypeable Haemophilus influenzae. Infect. Immun. 63:4115-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prieto, C. I., O. M. Aguilar, and O. M. Yantorno. 1999. Analyses of lipopolysaccharides, outer membrane proteins and DNA fingerprints reveal intraspecies diversity in Moraxella bovis isolated in Argentina. Vet. Microbiol. 70:213-223. [DOI] [PubMed] [Google Scholar]
- 13.South, A. 1992. Terrestrial slugs: biology, ecology and control. Chapman & Hall, London, England.
- 14.Storm Fomsgaard, J., A. Fomsgaard, N. Hoiby, B. Bruun, and C. Galanos. 1991. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect. Immun. 59:3346-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stryker, T. D., W. J. Stone, and A. M. Savage. 1982. Renal-failure secondary to Moraxella osloensis endocarditis. Johns Hopkins Med. J. 151:217-219. [PubMed] [Google Scholar]
- 16.Sugarman, B., and J. Clarridge. 1982. Osteomyelitis caused by Moraxella osloensis. J. Clin. Microbiol. 15:1148-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan, L., and P. S. Grewal. 2001. Pathogenicity of Moraxella osloensis, a bacterium associated with the nematode Phasmarhabditis hermaphrodita, to the slug Deroceras reticulatum. Appl. Environ. Microbiol. 67:5010-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan, L., and P. S. Grewal. 2001. Infection behavior of the rhabditid nematode Phasmarhabditis hermaphrodita to the grey garden slug Deroceras reticulatum. J. Parasitol. 87:1349-1354. [DOI] [PubMed] [Google Scholar]
- 19.Verduin, C. M., C. Hol, A. Fleer, H. V. Dijk, and A. V. Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides: extraction with phenol-water and future applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 21.Wilson, M. J., D. M. Glen, and S. K. George. 1993. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Sci. Technol. 3:503-511. [Google Scholar]
- 22.Wilson, M. J., D. M. Glen, J. D. Pearce, and P. B. Rodgers. 1995. Monoxenic culture of the slug parasite Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) with different bacteria in liquid and solid phase. Fundam. Appl. Nematol. 18:159-166. [Google Scholar]
- 23.Wilson, M. J., D. M. Glen, S. K. George, and J. D. Pearce. 1995. Selection of a bacterium for the mass production of Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) as a biocontrol agent for slugs. Fundam. Appl. Nematol. 18:419-425. [Google Scholar]