Abstract
Human Tissue Banks (BTH) must validate the storage of collected/processed tissues ensuring physical integrity, sterility, and microbial protection for up to 5 years. (1) Is it safe to use bone tissue for transplants collected by a BTH after 5 years of storage? (2) Do the packaging of stored tissues present physical integrity, sterility, and microbial protection after 5 years? (3) What are the morphological results of bone tissues after 5 years?. 20 femoral heads were used with a storage time of between 9 and 10 years at −80 °C. From each femoral head, the following were carried out: microbiological tests for aerobic and anaerobic bacteria and fungi, collecting 3 fragments of bone tissue, 3 Stuart swabs from the inner surface of the packaging, and a sample of 0.9% SF for Bioburden examination; tissue histology; the quantitative and qualitative mechanical resistance test and pyrogenicity/cytotoxicity test were used on the packaging, pre and post storage. No bone tissue samples showed pathogenicity. Histological findings showed morphologically preserved osteocytes with points of bone degeneration and necrotic adipose tissue. No packaging showed contamination, cytotoxicity, or pyrogenicity. The mechanical properties of these packages demonstrated uniformity in thickness, high tension, and relative stiffness even after storage (p = 0.001). It is concluded that the packaging used in the study presented physical integrity, sterility, and microbial protection for bone tissues after 9 and 10 years of storage. A possible increase in the shelf life of fabrics is contemplated to up to 10 years. Such results expand future research directions to continuously improve the quality of products and services offered by BTH.
Keywords: Packaging validation, Tissue storage, Tissue validity, Biological quality control, Human tissue bank
Introduction
Resolution of the Collegiate Board (RDC) No. 707/2022, which revoked RDC No. 55/2015, aimed at good practices in Human Tissue Banks (BTH), determines that these specialized services validate their storage systems for collected, processed tissues, stored and distributed. These guidelines determine that the packaging used must be registered/authorized by the National Health Surveillance Agency (ANVISA), approved, non-pyrogenic and non-toxic (Resolução da Diretoria Colegiada [RDC] 2015; RDC 2022).
For collection, processing, and storage, the packaging used must present proven sterility, being triple sealed and capable of withstanding deep-freezing, re-sterilization, and freeze-drying processes to guarantee physical integrity, sterility, and microbial protection of tissues for up to 5 years on the shelf life validity determined by current legislation (RDC 2015; RDC 2022).
According to the Brazilian National Transplant System (SNT), for the preparation of RDC 55/2015 (currently RDC 707/22), the main references used to determine the five-year shelf life of tissues were RDC 220/2006 (first sanitary standard that regulated musculoskeletal and skin BTH in the country) and the guide from the American Association of Tissue Banks (13th edition) (RDC 2006; RDC 2022; Heng et al. 2020). Furthermore, the SNT Technical Chamber uses these validations within the active services, in addition to the opinion of experts, international guidelines, and other available scientific evidence, seeking a common consensus regarding the expiration date of the tissues.
It is worth noting that RDC 707/2022 (Art. 152) allows BTH to establish storage times and temperatures different from those presented in Table 1 of Annex II of the resolution, as long as they are duly validated, being: 1) Refrigerated (from 2 to 8 °C): 14 to 42 days; 2) Frozen or cryopreserved (from − 20 to − 40 °C): 6 months; 3) Frozen or cryopreserved (less than or equal to − 40 °C): 5 years and; 4) Freeze-dried (room temperature): 5 years (RDC 2022). However, in the current literature, there are no scientific validation studies and/or that prove specific expiration dates that precisely substantiate the period proposed by legislation (Sychterz et al. 2005; Weimin et al. 2009; Heng et al. 2020; Corsi et al. 2020; Corsi et al. 2023a).
Table 1.
Overview of the research results, demonstrating the identification of the samples, date of collection, and pre and post-storage tests of the tissues at − 80 °C
| ID | Sample | Date of capture | Microbiology uptake | Mechanical resistance test | Pyrogenicity cytotoxicity test | Test date | Microbiology after storage | Bioburden | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerobic | Anaerobic | Fungi | Aerobic | Anaerobic | Fungi | |||||||
| 1 | 02/2013 | 06/07/2013 | − | − | − | OK | OK | 09/06/2022 | − | − | – | – |
| 2 | 14/2013 | 08/30/2013 | − | − | − | OK | OK | 09/06/2022 | – | − | - | - |
| 3 | 17/2013 | 10/04/2013 | OK | OK | 09/06/2022 | |||||||
| 4 | 19/2013 | 10/18/2013 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 5 | 21/2013 | 10/25/2013 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 6 | 22/2013 | 10/25/2013 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 7 | 25/2013 | 11/08/2013 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 8 | 27/2013 | 11/29/2013 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 9 | 28/2013 | 12/06/2013 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 10 | 29/2013 | 12/06/2013 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 11 | 30/2013 | 12/20/2013 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 12 | 32/2014 | 01/24/2014 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 13 | 33/2014 | 02/07/2014 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 14 | 34/2014 | 02/07/2014 | − | − | − | OK | OK | 09/06/2022 | – | – | – | – |
| 15 | 35/2014 | 02/14/2014 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 16 | 36/2014 | 02/14/2014 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 17 | 37/2014 | 02/21/2014 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 18 | 38/2014 | 02/21/2014 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 19 | 39/2014 | 03/21/2014 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
| 20 | 52/2015 | 01/16/2015 | − | − | − | OK | OK | 06/16/2023 | – | – | – | – |
Therefore, this study aims to evaluate whether the musculoskeletal tissues captured by a BTH are suitable for use after 5 years of storage, comparing their packaging in terms of quality control determined by legislation, with the following questions: 1) Is it safe to use bone tissues for transplants captured by a BTH after 5 years of storage? 2) Does the packaging of stored tissues present physical integrity, sterility, and microbial protection after 5 years? 3) What are the morphological results of bone tissues after 5 years?
Method
This is an observational study with interventions and analyses of bone tissue samples subdivided into specific groups, with analyses of processes and determined periods. Patients were selected by convenience sampling (non-probability), and authorization for inclusion of samples in the study was carried out after authorization from the patient himself, through the Free and Informed Consent Form (TCLE) / Quality Registry (RQ—BCO—030) (Corsi et al. 2023a).
We used 20 femoral heads from living patients undergoing hip arthroplasty surgery in a tertiary hospital located in the interior of the state of São Paulo, Brazil, where these patients donated human bone tissue (femoral head) to the BTH located in the same center. The femoral heads were captured between 01/2013 and 08/2014, stored in a Thermo Scientific ultra freezer, model 706 (Thermo Fisher Scientific, Waltham, Massachusetts, USA), and kept at − 80 °C until the date of this study. The experiments were carried out between 09/2022 and 06/2023.
To store the fabrics, plastic packaging from the brand FlexPack® “Soluções de Embalagens Ltda.”, batch 7502/2011 and manufactured on 07/13/2011, was purchased and used, with its compositions being 70 µm polyethylene; 10 µm of nylon and 10 µm of coextrusion adhesive; and with properties of 15 cm wide; 25 cm long and approximately 18 µm thick. The packages were previously sterilized in a sterilizing gas chamber (130 LF), with a combination of 2% formaldehyde solution and a temperature between 50 and 78 °C, with a cycle lasting a maximum of three hours. For each femoral head, pre- and post-storage microbiological examinations were carried out to detect aerobic and anaerobic bacteria and fungi; Bioburden and tissue histology after storage; mechanical resistance test and pyrogenicity/cytotoxicity test of pre and post-storage packaging (Corsi et al. 2023a; Corsi et al. 2023b).
The results of the procedures were coded and tabulated in a Microsoft Excel spreadsheet with double entries performed by 2 independent researchers. Statistical analyses were performed using Student’s t-test (parametric) and Mann–Whitney test (non-parametric) with a significance level of p < 0.05%, using the GraphPad Prism version 8.0 program (Graphpad Software Inc., San Diego, California, USA). Approval of the research project was authorized by the Technical Board of this BTH (Corsi et al. 2023b).
Microbiological and bioburden exams
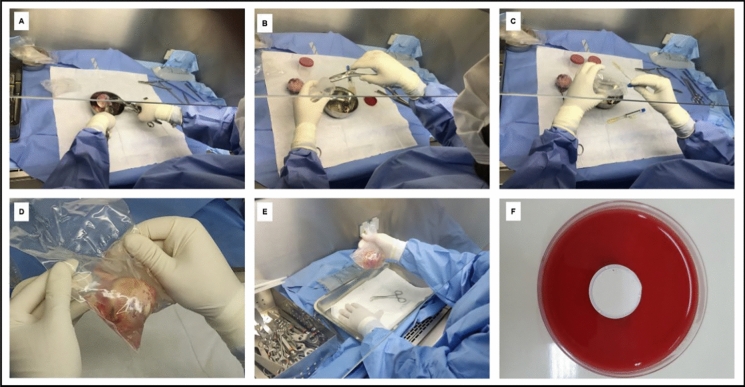
Femoral heads were exposed separately inside a Bio Seg 12—Class II laminar flow chamber (Grupo VECO, Campinas, São Paulo, Brazil), using an aseptic technique to collect the exams. From each femoral head, three fragments of bone tissue and three Stuart swabs were collected from the inner surface of the packages. Additionally, 20 ml of 0.9% Physiological Serum (SF) was instilled and shaken inside each package, and these samples (washing solutions) were then stored in sterile multipurpose cups and sent to the Clinical Analysis Laboratory within the same institution, following Anvisa’s recommendations for the transport of biological materials (Fig. 1).
Fig. 1.
Methodological procedures for collecting microbiological and Bioburden tests, where: A and B Collection of bone fragments; C Collection of material (swab) from the inner surface of the packaging; D and E Preparation and collection of packaging washing solution and; F Membrane on the surface of a 5% Sheep Blood agar plate from the Bioburden test
To perform the Bioburden exam, a fragment of bone tissue was stored for 24 h at 37 °C in 50 ml of sterile 0.9% SF. Subsequently, the liquid was passed through a closed system containing a sterile membrane/filter (K18-230 / lot: 210,410–338), with a 0.22 µm pore size (Kasvi, São José dos Pinhais, PR, Brazil). The membrane was removed from the system and placed on the surface of a 5% Sheep Blood agar plate (Plastilabor, Rio de Janeiro, RJ, Brazil), identified and sent to the same laboratory, where they were incubated in a bacteriological oven for 24—48 h at 35 °C (Hentz 2009). (Fig. 1F).
The microbiological analysis of the samples sent was carried out by searching for the growth of aerobic and anaerobic bacteria and fungi. The bone tissue fragments and the packaging washing solutions were placed in tubes containing Brain Hearth Infusion (BHI) broth (Plastilabor, Rio de Janeiro, RJ, Brazil) and incubated for 24 h in an oven at 37 °C with 5% CO2. After this period, they were sown on 5% Sheep Blood agar, Mac Conkey agar, and Mannitol agar plates (Plastilabor, Rio de Janeiro, RJ, Brazil) and incubated for 24 to 48 h in a bacteriological greenhouse.
The search for anaerobic bacteria was carried out by inoculating the material in Tioglycolato Fluid broth (Plastilabor, Rio de Janeiro, RJ, Brazil), which was incubated in an oven for 24 h and after this period, seeded on Brucella agar plates (Plastilabor, Rio de Janeiro, RJ, Brazil), and again incubated in an oven for 48 h in an anaerobic jar with an oxygen inhibitor. Regarding the research on yeast-like and filamentous fungi, during the processing of the material, these were sown directly on Sabouraud agar in a tube, remaining incubated for a period of up to 42 days.
For cultures of bacteria or fungi that showed growth of microorganisms, the identification and quantification of colonies of microorganisms was carried out using mass spectrometry using the Microflex LT MALDI TOF equipment (Bruker Maldi Biotyper™) and the sensitivity test by microdilution in broth was carried out using the VITEK® 2 Compact equipment (bioMérieux Brasil) and sensitivity tests were carried out using a diffusion disc. The interpretation of possible results for antimicrobial susceptibility was standardized according to the Brazilian Committee on Antimicrobial Susceptibility Testing/European Committee on Antimicrobial Susceptibility Testing guidelines (Arendrup et al. 2020; EDQM-Guide 2022).
Histology:
Samples collected from ten femoral heads were fixed in a 10% buffered formalin solution after the longitudinal section for better penetration of the fixative liquid. Macroscopic evaluation was then carried out and fragments of cancellous and cortical bone tissue were collected for decalcification with EDTA and conventional histological processing with dehydration, clearing, and paraffin embedding. After 24 h of mounting, 4-micron histological sections were made (Leica CM 1.850) and stained with hematoxylin and eosin (HE). In this staining, the acidic structures, such as the nucleus and cartilaginous matrix, are stained purple by hematoxylin (basic dye), and the basic structures, such as collagen and generally the cytoplasm, are stained pink by eosin (acidic dye). Morphological analyses were performed by a pathologist experienced in histological evaluations of bone tissues and photographed under a conventional optical microscope using CellSens software (Olympus®), and image editing was performed using Fiji software (ImageJ) (Corsi et al. 2023b).
Quantitative and qualitative mechanical strength test:
For the qualitative test of packaging resistance, 100 ml of SF0.9% was diluted and 2 ml of 1% Methylene blue was added, completing and sealing the packaging. Then, using manual force, he checked whether they remained properly sealed or punctured through physical maneuvers. For the quantitative packaging resistance test, 5 samples of packaging belonging to the same batch and manufacturer were obtained and distributed into specific groups, as follows: A) Non-sterilized packaging; B) Sterile and non-stored packaging; C) Sterilized packaging, with 10 years of storage at − 80 °C and subsequently thawed at room temperature and; D) Sterilized packaging with 10 years of storage, frozen at − 80 °C.
Mechanical properties were determined using an Analyzer TA TX Plus texturometer (TA Instrument, England). The average thickness of the packages was determined by measuring the thicknesses at 3 different points of the material using a digital electronic micrometer (ZAAS Precision). Stress (TS) was obtained according to the ASTM D882-18 method (EL-Bagory 2019). The packages were subjected to traction at a speed of 1.0 mm/s, with an initial separation of 80 mm, until the film ruptured. Young’s modulus (MY) was calculated as the slope of the initial linear portion of the stress versus elongation curve using texturometer software V.1.22 (SMS).
Pyrogenicity and cytotoxicity test:
The in vivo pyrogen and cytotoxic test evaluated the variation in body temperature in rabbits after intravenous injection of a solution containing plastic from packaging, taking as a reference the initial and final temperature of the animals to verify possible febrile reactions caused by samples of plastic packaging (Protocol nº 24,496–1). 3 rabbits of the species Oryctolagus cuniculus, New Zealand lineage, with healthy presentation and no detectable clinical changes, were used (Granja RG, Suzano, SP, Brazil). The animals were allocated to a specific location and acclimatized for a minimum period of 5 days before the start of the test in a 12/12 h photoperiod. The animals’ diet consisted of conventional feed for the species and drinking water. During the test period, the test system was kept in a room with a temperature between 20.1 and 20.9 °C and relative humidity between 69.4 and 76.9%.
The sample was applied intravenously to the marginal vein of the ear of the specimens and extracted at 37 °C for 1 h in the proportion of each representative part of the sample to 40 mL of 0.9% sodium chloride solution. Total prepared: 4 representative parts of the sample for 160 mL of 0.9% sodium chloride solution. Injected dose: 10 mL/kg of animal weight. The animals were placed in restraints for a minimum period of 30 minutes. After the acclimatization period, the control temperature of the animals was measured. The test solution was then applied according to the route and dose described above. After applying the test solution, the temperature was measured again at intervals of 30 min for 3 h. The animal was observed during this test period for the presence of clinical changes, and the measured temperatures were recorded.
Interpretation of the results: satisfactory, when no animal shows an increase in temperature of up to 0.5 °C, indicating that the product meets the requirements for being pyrogenic and cytotoxic free (the test must be redone if one or more animals show an increased temperature of at least 0.5 °C, making it necessary to redo the test using another five animals); unsatisfactory, if more than one animal presents an increase in temperature after 0.5 °C, and/or if the sum of the temperature increases exceeds 3.3 °C, indicating that the product under test does not meet the requirements for being pyrogenic and cytotoxic free.
Results and discussion
None of the 20 femoral heads showed any contamination by aerobic and anaerobic bacteria or fungi, as shown in Table 1.
Histological findings showed morphologically preserved osteocytes with points of bone degeneration and necrotic adipose tissue (Fig. 2).
Fig. 2.
Histology of bone tissue from adult individuals, analyzed between nine and ten years of storage, stained in HE, where: A Necrotic adipose tissue with membrane degeneration and lipid cyst (100x). B Some morphologically preserved osteocytes and necrotic adipose tissue, with degeneration of membranes (400x). C Joint cartilage with chondrocyte morphology, covered by fibrous tissue due to osteoarthrosis (100x). D Necrosis of osteocytes (or empty lacunae) and medullary adipose tissue (degeneration of cell membranes) (200x). E Remains of osteocyte nuclei with loss of cellular structure (necrosis) medullary fibrosis secondary to osteoarthrosis (200x). F Remains of osteocyte nuclei, but apparently with loss of cellular structure (necrosis) (400x) (n = 10)
Collagen bone lamellae containing morphologically preserved osteocyte lacunae were found; however, in this case, this does not mean that the cells were alive before histological processing, as they may have undergone apoptosis before or after freezing at − 80 °C. The histological findings are in line with studies that showed equivalent morphology between the layers of bone tissue with the same storage conditions (Tang et al. 2016; Roseti et al. 2017; Winkler et al. 2018; Rahman et al. 2019; Buffrénil et al. 2021).
As for testing the mechanical properties of packaging before and after storage, all samples showed mechanical integrity in both the qualitative test (Fig. 3) and the quantitative test (Table 2; Fig. 4).
Fig. 3.
Qualitative mechanical test of packaging resistance with the addition of 100 ml of SF0.9% + 2 ml of 1% Methylene blue
Table 2.
Qualitative mechanical properties of packaging
| Group | Thickness (mm) | Voltage (MPa) | Young’s modulus (MPa) |
|---|---|---|---|
| A | 0,075 ± 0,003 | 4478,125 ± 343,282 | 69,018,867 ± 44,127 |
| B | 0,079 ± 0,004 | 2972,949 ± 158,079 | 64,053,900 ± 25,960 |
| C | 0,082 ± 0,001 | 3025,775 ± 253,450 | 54,443,433 ± 20,514 |
| D | 0,079 ± 0,001 | 3110,131 ± 615,307 | 64,354,667 ± 80,555 |
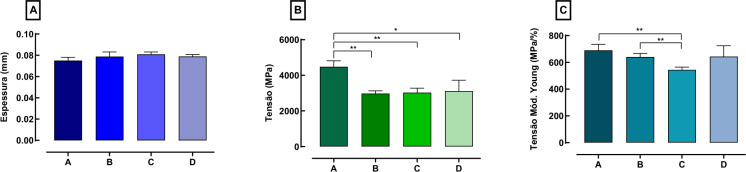
Fig. 4.
Quantitative mechanical resistance test of packaging represented by specific groups (n = 8), where it is observed: A measurement of the average thickness of the material (mm); B tensile stress (TS) of the packaging ‘surface’ and C Young’s modulus (MY) = material stiffness. Data is presented as the mean and standard error of the mean. The results were statistically analyzed using the T and Mann–Whitney test, with a significance level of p < 0.05
According to Fig. 3, this qualitative test of packaging resistance shows the preservation of the integrity of the plastic material, even after 10 years. The integrity of the material is of paramount importance in tissue conservation and storage, as tissue contamination is avoided (Dziedzic-Goclawska et al. 2005; Dhabale et al. 2016). If there were holes in the packaging, this could indicate the entry of microorganisms and contaminants into the protected fabric, which would make the function of this material unfeasible. In this way, the material demonstrated good mechanical resistance and good preservation of its physical integrity over time. Quantitative mechanical properties are presented in Table 2 and Fig. 4.
The mechanical properties of these packages demonstrated uniformity in thickness, with a total average of 0.078 ± 0.003 mm. According to the manufacturer, the material that makes up these packages is 70 µm polyethylene and 10 µm nylon (polymers that make up the packaging), presenting high tension and relative rigidity. According to Table 2, it is discreetly noted that when the packages were passed through a sterilization process in a 2% formaldehyde solution and temperatures between 50 and 78 °C, there was a small reduction in their mechanical properties, thus demonstrating there is a negative effect on these temperature conditions and/or the solution used in the sterilization process (Table 3).
Table 3.
Pyrogenicity and cytotoxicity test: Body weight, injected volume, and readings
| Rabbit No | Weight (g) | Injected volume (mL) | Temperature readings (minutes) | Temperature variation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 30 | 60 | 90 | 120 | 150 | 180 | ||||
| 017 | 3506 | 35,1 | 39,5 | 39,5 | 39,5 | 39,5 | 39,5 | 39,5 | 39,4 | 0,0 |
| 019 | 4122 | 41,2 | 39,3 | 39,4 | 39,3 | 39,3 | 39,3 | 39,2 | 39,2 | 0,1 |
| 025 | 3958 | 39,6 | 39,0 | 39,1 | 39,1 | 39,1 | 39,1 | 39,0 | 39,2 | 0,2 |
| Total increases | 0,3 | |||||||||
As shown in Fig. 4, there was no statistical difference between the groups regarding the average thickness of the materials (Fig. 4-A). There were significant statistical differences in TS between groups: A–B (p = 0.0023); A–C (p = 0.0041) and; A–D (p = 0.0282) (Fig. 4-B). There were also statistical differences in MY between groups: A–C (p = 0.0066) and B–C (p = 0.0073) (Fig. 4-C).
Regarding the effect of time on the sterilized packaging, a significant loss of tension was noticed only after sterilization due to the use of high temperatures for the material, leaving it less elastic and, consequently, more rigid, to the point of showing the loss of tension and stretching of the packages, observed between the groups, mainly between A and B. The storage time and the low temperatures that elapsed did not significantly affect the properties of TS and MY in groups C and D. However, it noted lower relative rigidity of the material between groups B and C, which can be explained by the sum of sterilization and contact of the packaging at very low temperatures (− 80 °C), as well as its return to room temperature in the freezing and thawing process, presenting a change in temperature and possible loss of its rigidity, but without affecting the material. No statistically significant intragroup differences were observed in mean thickness (mm).
No abnormalities were observed in the animals during the test period, and no animal showed an individual increase in temperature equal to or greater than 0.5 °C. The sample was considered satisfactory, following the methodology adopted with expanded uncertainty (U) average = 0.1 °C regarding temperature sensors. Therefore, there was no cytotoxicity and pyrogenicity in the packaging samples used before and after storage.
The data demonstrates the good performance of fabrics and packaging stored after 5 years in deep freezing. Bone is a mineralized connective tissue that performs important functions in the body, such as locomotion, support, protection of medullary tissues, and storage matrix containing calcium and phosphate (Roseti et al. 2017). Due to this complex and hierarchical structure, they are made from inorganic nanocrystallines such as hydroxyapatite and organic components such as collagens and water. Such proteins together form a nanostructured extracellular matrix that directly influences the adhesion, proliferation, and differentiation of various types of cells, such as osteoblasts, osteocytes, and osteoclasts, important in the formation and regeneration of this tissue through inherent bone defects (Tang et al. 2016; Roseti et al. 2017; Vivacqua et al. 2020; Corsi et al. 2023c; Corsi et al. 2024a). In transplants, this mineralized matrix will function as an adhesion base, favoring the genesis and migration of the recipient patient’s bone lining cells through osteogenesis, osteoconduction, and osteoinduction (Tang et al. 2016; Corsi et al. 2024a, 2024b).
The histological findings of this study showed a drop in cell viability and necrotic spots in the tissue, which are justifiable due to the donor’s previous pathology, basic physiology, and freezing (−80 °C), but without significant concerns, since the bone tissue used for Transplantation does not require cell viability (Winkler et al. 2018; Rahman et al. 2019; Corsi et al. 2023c; Corsi et al. 2024c). Furthermore, BTH, when preparing bone tissue for transplantation, processes it through washing and physical–chemical sterilization, thus removing all cellular structure, adipose tissue, and necrotic tissues contained in the captured structures, ultimately leaving only the mineralized bone matrix that will be sent for grafting in patients who present large bone losses of various etiologies (tumors, prosthesis replacement, trauma, congenital and spinal deformities, dental problems, infection, osteolysis, aseptic loosening etc.) (Corsi et al. 2024a; Corsi et al. 2024b; de Freitas Filho et al. 2024), thus favoring, better short- and long-term results with a good post-surgical prognosis for the recipient patient (Tang et al. 2016; Corsi et al. 2024b; Corsi et al. 2024c).
Finally, the basic morphology of the cells remained unchanged with time and storage in the histological analysis of layer constructs. Furthermore, the composition of the packaging favored microbiological protection, sealing, and durability, consequently allowing the fabric to be transported to other regions of the country and even to other parts of the world when kept in good storage conditions, following current regulations.
In tissue banking practice, the storage time of frozen bone at − 80 °C is often arbitrarily defined, as there is a lack of scientific evidence supporting precise limits. Our study helps to fill this gap, demonstrating that even after 10 years of storage, bone tissue remains free from microbiological contamination and preserves its spongy architecture, as evidenced by histological analyses. This information is particularly relevant for tissues used as fillers for bone defects, where maintaining trabecular structure and sterility are far more important than mechanical properties—hence, mechanical testing was not performed.
Moreover, although most grafts are used within a few years, there are situations where prolonged storage may be necessary, such as in cases of rare grafts or logistical difficulties. It is also important to highlight that tissue donation is a voluntary practice in Brazil, in which the patient and/or their family decide whether to donate to tissue banks. This context can result in challenges and shortages in the prompt supply of tissues, making the ability to safely extend storage periods an important tool for tissue bank management. Our findings provide evidence that such practice is safe and feasible, increasing flexibility in managing tissue bank inventories.
Conclusion
The present study concludes that the samples of bone tissue and plastic packaging used in this research, after 9 and 10 years of storage at − 80 °C, present physical integrity, sterility, and microbial protection. This reflects the safety of the processing used by BTH, and, in addition, a possible increase in the shelf life of the tissues is contemplated to up to 10 years.
Finally, it is safe to use bone tissue for transplants, harvested after 5 years of storage. The packaging of stored fabrics presents physical integrity, sterility, and microbial protection after 5 years. The results of histological analyses of the tissues after 5 years show the morphological structure unchanged, with preserved layer constructions. Such results expand future research directions to continuously improve the quality of products and services offered by BTH.
Acknowledgements
This study is a multicenter study between the Human Tissue Bank of the Hospital das Clínicas of the Faculty of Medicine of Ribeirão Preto/SP—Brazil (HCFMRP/USP), the Faculty of Philosophy, Sciences, and Letters of Ribeirão Preto (USP-RP) and the NOVA National School of Public Health, NOVA University Lisbon, Lisbon, Portugal. The authors thank these institutions for their contributions.
Author contributions
Each author contributed individually and significantly to the development of this article: CACC, KCGS, RLB, AVAL, and ASC: Planned and carried out all stages and activities of the study, participating in the review, discussion, and results process; CACC, JDBP, LFZB, and DAFSL: Participated in the experimental studies; CACC, FLG, CHFP, and LGGM: Participated in the preparation of the study, correction, and final approval.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- Arendrup MC, Meletiadis J, Mouton JW, et al (2020) EUCAST. DEF. 7.3. 2–Abril 2020. DEF. 7:2.
- Buffrénil V, Quilhac A (2021) Bone tissue types: a brief account of currently used categories. Vertebr Skelet Histol Paleohistol 24:147–190 [Google Scholar]
- Corsi CA, Shoji M, Scarpelini KC et al (2020) Implementation and certification of ISO 9001: 2015 seal in human tissue bank HCFMRP-USP. Cell Tissue Bank 21:563–571 [DOI] [PubMed] [Google Scholar]
- Corsi CA, Sares CT, Mestriner F et al (2023) Isolation and primary culture of human abdominal aorta smooth muscle cells from brain-dead donors: an experimental model for vascular diseases. Cell Tissue Bank 5:1–8 [Google Scholar]
- Corsi CA, Luiz AV, Cintra ÁS et al (2023) The significance of the nucleic acid test (NAT) to prevent viral contamination in musculoskeletal tissue transplantation. Revista Brasileira De Ortopedia 12(58):23–29 [Google Scholar]
- Corsi CAC, Scarpelini KCG, Bento RL et al (2024a) Uso de técnicas para controle de qualidade microbiológico adotadas em um banco de tecidos humanos. Braz J Transplant 27:e0924 [Google Scholar]
- Corsi CAC, Sares CTG, Mestriner F et al (2024b) Isolation and primary culture of human abdominal aorta smooth muscle cells from brain-dead donors: an experimental model for vascular diseases. Cell Tissue Bank 25(1):187–194 [DOI] [PubMed] [Google Scholar]
- Corsi CAC, Jordani MC, Michelon-Barbosa J et al (2024c) Role of preservation solution in human aneurysmatic aorta harvest and transport: a comparative analysis of different solutions for tissue injury protection. Braz J Cardiovasc Surg 39(5):e20230434 [Google Scholar]
- Corsi CA, Scarpelini KCG, Assunção-Luiz AV, et al (2023) Temperature monitoring of two different thermal boxes for the transport of biological samples. In Transplantation Proceedings b. vol 55, no 6, pp 1368–1372. Elsevier, Amsterdam
- de Freitas Filho LH, Neves CDCS, Silva NP et al (2024) Challenges of implementing a human multi-tissue bank in a public hospital in the interior of São Paulo: under the light of the quality management system. Transplant Proc 56(5):1041–1047 [DOI] [PubMed] [Google Scholar]
- Dhabale R, Jatti VS (2016) A bio-material: mechanical behavior of LDPE-Al2O3-TiO2. InIOP Mater Sci Eng 149(1):012043 [Google Scholar]
- Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I et al (2005) Irradiation as a safety procedure in tissue banking. Cell Tissue Bank 6:201–219 [DOI] [PubMed] [Google Scholar]
- EDQM-Guide. Guide to the quality and safety of tissues and cells for human application, 5th Edition, 2022 | Freepub [Internet]. [zitiert 6. Februar 2025]. in: https://freepub.edqm.eu/publications/AUTOPUB_17/detail
- EL-Bagory TM, Alarifi IM, Younan MY (2019) Prediction of mechanical properties for curved dumbbell-shaped specimen at different orientation angles of ring hoop tension test. Adv Eng Mater 21(9):1900191 [Google Scholar]
- Heng WL, Wang QW, Sornarajah R et al (2020) A review of skin banking guidelines and standards worldwide: towards the harmonization of guidelines for skin banking in therapeutic applications for the regions under the Asia Pacific Burn Association (APBA). Burns Trauma 8:tkaa019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentz NG (2009) Pharmaceutical bioburden testing. Encycl Ind Biotechnol Bioprocess Biosep Cell Technol 16:757–774 [Google Scholar]
- Rahman MS, Akhtar N, Hasan MZ et al (2019) Human tissue banking in Bangladesh: hope for the patients of massive burns, surgical wound and bone associated complications. Int J Burns Trauma 9(2):23 [PMC free article] [PubMed] [Google Scholar]
- Resolução da Diretoria Colegiada (RDC) nº 220/2006. Agência nacional de vigilância sanitária (ANVISA) de 2006. Available at: http://antigo.anvisa.gov.br/documents/10181/2718376/RDC_220_2006_COMP.pdf/7ada9eb3-37a8-451d-95e2-a827314fd725. Accessed 20 July 2024.
- Resolução da Diretoria Colegiada (RDC) nº 55/2015. Agência nacional de vigilância sanitária (ANVISA) de 2015. Available at: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2015/rdc0055_11_12_2015.pdf. Accessed 29 Jan 2025.
- Resolução da Diretoria Colegiada (RDC) nº 707/2022. Agência nacional de vigilância sanitária (ANVISA) de 2022. Available at: http://antigo.anvisa.gov.br/documents/10181/2718376/RDC_707_2022_.pdf/cb3640e6-bd0f-46d3-a8be-64484c3e6c37. Accessed 15 Dec 2025.
- Roseti L, Parisi V, Petretta M et al (2017) Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C 1(78):1246–1262 [Google Scholar]
- Sychterz CJ, Young AM, Orishimo K et al (2005) The relationship between shelf life and in vivo wear for polyethylene acetabular liners. J Arthroplast 20(2):168–173 [Google Scholar]
- Tang D, Tare RS, Yang LY et al (2016) Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 1(83):363–382 [Google Scholar]
- Vivacqua TA, Prinz RD, Cavanellas N et al (2020) Protocol for harvest, transport and storage of human osteochondral tissue. Revista Brasileira De Ortopedia 15(55):163–169 [Google Scholar]
- Weimin F, Huanghe S, Xiang L et al (2009) The impact of storage time on the wear rates of ultrahigh-molecular-weight polyethylene acetabular liners in hip simulators. J Arthroplast 24(4):543–548 [Google Scholar]
- Winkler T, Sass FA, Duda GN et al (2018) A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: the unsolved challenge. Bone Joint Res 7(3):232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.