Abstract
Increasing evidence has shown the role of small extracellular vesicles (sEVs) in autoimmune rheumatic diseases (ARDs). This systematic literature review aims to evaluate the role of sEVs as biomarkers in ARDs, focusing on their molecular cargo and their utility for disease diagnosis, monitoring, and treatment response. A systematic search was conducted in MEDLINE/PubMed and Scopus from inception until July 2025, using the search terms; [(small extracellular vesicles) or exosomes) and ((rheumatic disease) or (rheumatoid arthritis) or (psoriatic arthritis) or (axial spondylarthritis) or (ankylosing spondylitis) or (systemic lupus erythematosus) or (Sjögren’s syndrome) or scleroderma or (systemic sclerosis) or myositis or polymyositis)]. Eligible studies were those reporting on sEV isolation from patient samples and comparing it with healthy individuals or controls with non-inflammatory conditions. The initial search yielded 1593 results, and 46 studies met the inclusion criteria. Literature reviews revealed that miRNAs and long non-coding (lnc)RNAs isolated from sEVs might serve as potential biomarkers for disease activity and treatment response in ARDs, mainly in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). In addition, sEV miRNA-21 and miRNA-146a have been often described in studies in SLE patients, indicating their potential role in differentiating SLE from healthy individuals. Although proteomic studies identified disease-specific proteins within sEVs, there is no consensus among the limited studies reporting sEV proteins. Although numerous studies have examined the role of sEVs in ARDs, there is a lack of consensus in the findings. Further research using well-standardized methodologies is needed to ensure reliable and reproducible results.
PROSPERO 2025 CRD420250646438. Available from https://www.crd.york.ac.uk/PROSPERO/view/CRD420250646438.
Keywords: Rheumatic diseases, Extracellular vesicles, Rheumatoid arthritis, Systemic lupus erythematosus, Differential diagnosis, Prognosis
Introduction
Autoimmune Rheumatic Diseases (ARDs) are characterized by dysregulated immune responses, chronic inflammation and a wide range of clinical symptoms and signs from several organs [1]. While the exact pathogenic mechanism of ARDs remains widely unclear, it is now understood that a variety of factors, such as genetic profile, environmental triggers, and hormonal factors, contribute to their initiation and perpetuation [2]. The overlapping clinical manifestations of these diseases often lead to diagnostic challenges, requiring more specific diagnostic tests.
Developing novel diagnostic tools is crucial, as some ARDs, such as psoriatic arthritis (PsA), lack specific laboratory tests and rely primarily on clinical and imaging evaluation for diagnosis [3]. The use of biomarkers is pivotal not only for diagnosing ARDs but also for enabling personalized treatment approaches. As novel biological therapies continue to emerge, the ability to predict treatment response through biomarker profiling is becoming increasingly important, paving the way for more targeted and effective treatment plans.
Accumulating evidence suggests the important role of small extracellular vesicles (sEVs) as critical mediators of intercellular communication and modulators of cellular responses [4]. sEVs are saucer-shaped structures produced by almost all human cell types, with a size between 30 and 200 nm. They consist of a lipid bilayer membrane and contain a cargo of macromolecules such as proteins, lipids, and nucleic acids [5]. The nomenclature of extracellular vesicles has long been a source of debate in literature. The term “exosome” has been widely used interchangeably with sEVs. According to the Minimal Information for Studies of Extracellular Vesicles (MISEV 2023), the term exosome implies the biogenetic process that leads to the creation of an extracellular vesicle, while the term sEV only refers to the size of the examined vesicle, making exosomes a subpopulation of sEVs [4, 5].
Concerning ARDs, emerging data accentuates the important role of sEVs not only as contributors to the pathogenesis but also as promising biomarkers and potential therapeutic targets for these diseases [6]. The molecular cargo of sEVs consists of lipids, proteins, and RNAs. Among these molecules, non-coding RNAs (ncRNA) have been mostly investigated as potential biomarkers for various diseases. These molecules’ short half-life and chemical instability create discrepancies in studies examining ncRNAs, especially miRNAs [7]. Exosomal miRNAs are promising biomarkers as they are encapsulated in a lipid bilayer membrane, providing protection and enabling more reliable analysis [8].
This review aims to evaluate the existing literature on the isolation of sEV from the biological fluids of patients with ARDs and the comparison of their cargo to that of healthy individuals and controls of non-inflammatory conditions.
Search strategy
The protocol for this review was registered in PROSPERO (CRD420250646438). A literature search was conducted in MEDLINE/PubMed and Scopus from inception until 15/07/2025 using the key terms: ((small extracellular vesicles) or exosomes) and ((rheumatic disease) or (rheumatoid arthritis) or (psoriatic arthritis) or (axial spondyloarthritis) or (ankylosing spondylitis) or (systemic lupus erythematosus) or (Sjögren’s syndrome) or (scleroderma) or (systemic sclerosis) or (myositis) or (polymyositis) following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. References of relevant articles were also screened for additional eligible studies not identified in the initial search. The eligibility of studies was assessed independently by two investigators (MKC and E-KV). Disagreements were discussed and resolved after consensus with the other authors (GEF and PPS).
Inclusion criteria: studies on isolating sEVs and their cargo from biological fluid samples from patients with ARD, in comparison to healthy individuals or controls with noninflammatory conditions (e.g., osteoarthritis). Exclusion criteria: non-English studies and those using animal models or cell cultures. Studies not adequately describing the method of sEV isolation and characterization are excluded.
Results of literature selection and data extraction
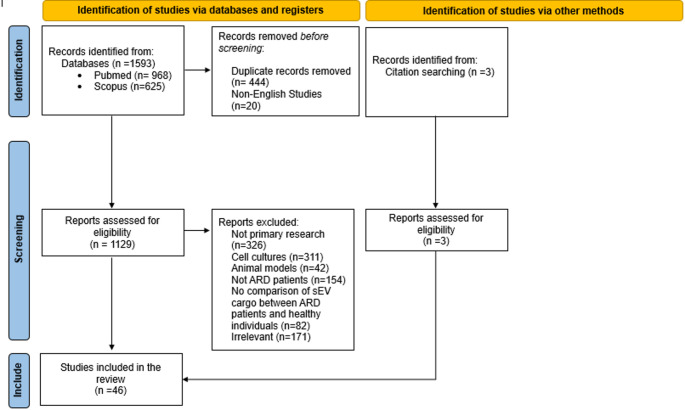
The initial literature search yielded 1593 publications (968 from PubMed and 625 from the SCOPUS database), of which 1129 were found to be non-duplicate records in English. Among these results, 803 consisted of primary research studies. A total of 154 studies were considered ineligible as they did not examine patients with ARDs. Another 311 publications were excluded because they reported on isolated sEVs from cell cultures, and 42 studies on isolated sEVs from animal models. Eighty-two studies did not compare the cargo of sEVs between healthy individuals and ARD patients, while 141 studies were deemed irrelevant to the topic of this review. Three additional studies were identified through the snowball approach by searching the references. Full texts of 46 studies were finally included in this review. A graphical representation of the above can be found in Fig. 1.
Fig. 1.
Flowchart
In the first section, we will summarize the bibliographic findings of studies comparing the sEVs cargo between patients with Rheumatoid Arthritis (RA) and controls. In this section, we will initially examine the ncRNA cargo of sEVs, followed by an analysis of their proteomic cargo. Next, we will present data from studies involving the exosomal ncRNAs and proteins in patients with Systemic Lupus Erythematosus (SLE). Finally, we will outline the data concerning the cargo of sEVs in patients with other ARDs.
Rheumatoid arthritis (RA)
Exosomal/sEV RNAs
To our knowledge, the first report of exosomal ncRNA isolation in patients with RA was published by Song et al. in 2014. In a cohort of 28 RA patients, they found that the sEVs long non-coding RNA (lncRNA) HOTAIR (HOX transcript antisense RNA) was significantly upregulated in serum compared to healthy individuals [10]. Recently, Wu et al. have shown a differential expression of sEV lncRNAs between patients with active RA and healthy controls, as well as among patient subgroups classified by their disease activity score 28 (DAS28), demonstrating that sEV lncRNA cargo analysis can provide valuable insights into disease activity [11]. Similarly, Shuai et al. showed a correlation between sEV lncRNAs RPS18P9 and SNGH31 with DAS28 [12], further supporting the potential of sEV lncRNAs as markers of disease activity.
Among exosomal ncRNAs, most research has focused on microRNAs (miRNAs). Various studies have documented differences in sEV miRNA expression between RA patients and healthy individuals. As shown by Lu et al., plasma-derived sEV miR-144-3p and miR-30b-5p were downregulated in RA patients, and these levels were inversely correlated with DAS28 score and anti-CCP antibody levels, indicating their potential as biomarkers for monitoring disease activity in RA [13].
Interestingly, in a large cohort of 231 RA patients and 185 healthy controls, another sEV miRNA molecule, miR-885-5p, could effectively distinguish between healthy controls and anti-CCP-negative RA patients, while miR-1268a appears to be expressed at lower levels, following methotrexate treatment initiation [14]. Moreover, clinical remission after methotrexate administration has also been correlated with sEV miRNA-1915-3p [15]. In the largest patient cohort identified in the present review, comprising 306 individuals with RA and an equal number of healthy controls, significant findings emerged regarding the expression of microRNAs in serum-derived sEVs. Specifically, miRNA-103a-3p, miRNA-10a-5p, miRNA-204-3p, miRNA-330-3p, and miRNA-19b were found to be significantly downregulated in the sEVs of patients compared to healthy controls [16]. These results highlight the potential role of circulating miRNAs within sEV to serve as diagnostic tools in RA, especially when assessed in well-powered studies [13]. Moreover, miR-204-5p and miR-204-3p, though different strands derived from the same precursor miRNA, are both reported to be downregulated in RA patients. Wu et al. observed reduced levels of miR-204-5p [17], while Hussain et al. reported a decreased expression of miR-204-3p [16]. This recurring pattern of downregulation within the miR-204 family may suggest its potential relevance as a biomarker in RA [13, 14]. Beyond ncRNAs, limited research has been conducted on sEV mRNAs in patients with chronic arthritis. Xue et al. demonstrated that the mRNA of the chemotactic molecule CCL5, which plays a significant role in the pathogenesis of inflammatory arthritis, is downregulated in the sEVs of RA patients [18]. A detailed summary of all relevant studies is provided in Table 1.
Table 1.
Nucleic acid cargo of small extracellular vesicles in patients with rheumatoid arthritis
| A/A | Study | Sample | No. of patients | No of comparators | sEV’s size (nm) |
ncRNA | Major findings |
|---|---|---|---|---|---|---|---|
| 1 | Wu et al. [11] | Serum | 13 | 5 | N/A | TCONS_I2_00013502 ENST00000363624 |
lncRNA TCONS_I2_00013502 (elevated) and lncRNA ENST00000363624 (decreased), in RA patients compared to HC Potential biomarkers for RA diagnosis Combined with ACPA improved the accuracy of RA diagnosis |
| 2 | Lu et al. [13] | Plasma | 24 | 24 | 50–150 |
miR-144-3p miR-30b-5b |
Reduced levels in RA patients Negatively correlated with disease activity and ACPA levels Effectively distinguish RA patients from HC |
| 3 | Gong et al. [14] | Serum | 231 | 185 | 90 |
miR-885-5p miR-6894-3p miR-1268a |
Upregulated in RA patients Combined with ACPA, demonstrate high diagnostic accuracy for RA miR-885-5p can distinguish ACPA-negative RA patients from HC Decreased miR-1268a expression following methotrexate treatment, suggests its potential as a treatment marker |
| 4 | Shuai et al. [12] | Peripheral Blood | 48 | 48 | N/A | SNHG6, RPS18P9, RPL21P28, EBLN3P, FAM153CP, RPL23P8, SNHG31, NORAD, H3P6, DLEU2, TUG1, OIP5-AS1, CXXC4-AS1, OLMALINC, and NPHP3-AS1 |
12 lncRNAs (SNHG6, RPS18P9, RPL21P28, EBLN3P, FAM153CP, RPL23P8, SNHG31, NORAD, H3P6, DLEU2, TUG1, and OIP5-AS1) were upregulated, while three lncRNAs (CXXC4-AS1, OLMALINC, and NPHP3-AS1) were downregulated in RA patients. SNHG6 exhibited a negative correlation with CRP and ESR, while RPS18P9 and SNHG31 showed negative and positive effects on DAS28, respectively. |
| 5 | Xue et al. [18] | Serum | 19 | 19 | 30–200 | CCL5 mRNA NONHSAT193357.1 |
Decreased in RA patients compared to HC The expression level of lncRNA NONHSAT193357.1 was markedly reduced in RA patients compared to HC |
| 6 | Yang et al. [49] | Plasma | 46 | 38 | N/A |
let-7a-5p, let-7b-5p, let-7d-5p, let-7f-5p, let-7 g-5p, let-7i-5p, miR-128-3p, miR-25-3p |
Increased levels of let-7a-5p, let-7b-5p, let-7d-5p, let-7f-5p, let-7 g-5p, let-7i-5p, miR-128-3p, and miR-25-3p in RA patients let-7a-5p and miR-25-3p levels were associated with a rheumatoid factor-positive phenotype in RA patients |
| 7 | Hussain et al. [16] | Serum | 306 | 306 | 30–150 |
miRNA-103a-3p miRNA-10a-5p miRNA-204-3p miRNA-330-3p miRNA-19b |
Downregulated in RA patients compared to HC. |
| 8 | Yu et al. [50] | Peripheral Blood | 13 | 10 | N/A |
has-miR-335-5 hsa-miR-486-5p |
Elevated expression in RA compared to HC |
| 9 | Wu et al. [17] | Plasma | 100 | 104 | 50–200 | miR-204-5p | Downregulated in RA patients |
| 10 | Rodríguez-Muguruza et al. [51] | Peripheral Blood | 28 | 28 | 30–150 |
miR-144-3p miR-451a miR-25-3p miR-15a-5p miR-107 |
Upregulated in RA patients |
| 11 | Lim et al. [15] | Serum |
20 RA non remission |
22 RA in remission |
NA | hsa-miR-1915-3p | Negatively associated with the disease activity of RA |
| 12 | Wang et al. [52] | Plasma | 25 | N/A | 89.5 | miR-17 | Increased and inversely correlates with Treg frequency in RA patients |
| 13 | Song et al. [10] | Plasma | 28 | 5 | N/A | HOTAIR | Upregulated in RA patients with RA |
sEV, small extracellular vesicles; No, number; ncRNA, non-coding RNA; RA, rheumatoid arthritis; HC, healthy control; miRNA, micro-RNA; lncRNA, long-noncoding RNA; ACPA, anti-citrullinated protein antibodies; CRP, C-reactive proteins; ESR, erythrocyte sedimentation rate; DAS, Disease Activity Score; N/A, not available
Exosomal/sEV proteins
In 2006, Skriner et al. were the first to conduct a proteomic analysis in synovial fluid-derived sEVs from RA patients, discovering that citrullinated proteins are present in these vesicles, contributing to the pathogenesis of the disease [7]. A more recent study by Alghamdi et al. in 2025 confirmed the presence of citrullinated proteins in the serum of RA patients [19]. Further research focused on the common protein identification markers of sEVs CD9, CD63, and CD81 tetraspanins in RA. Rydland et al. examined the proteomic profile of tetraspanin molecules on sEVs from patients with RA and healthy individuals, observing a distinct pattern of tetraspanin expression in the sEVs of RA patients (Table 2). They also found that responders to methotrexate had a unique high relative proportion of CD81 single-positive sEV compared to non-responders, suggesting tetraspanin expression in sEVs as a potential marker of response to methotrexate treatment [20].
Table 2.
Proteomic cargo of small extracellular vesicles in patients with inflammatory arthritis
| A/A | Study | Disease | Sample | No. of patients | No. of comparators | sEV’s size (nm) |
Proteins | Major findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Brenis Gómez et al. [25] | RA | Serum | 9 | 8 OA 5 HC | 119.4-219.9 | 45 differentially expressed sEV proteins |
Higher levels of Annexin A2 (ANXA2), filaggrin (FLG), and fatty acid-binding protein 5 (FABP5) in OA sEV Higher levels of alpha-2-macroglobulin (A2M), apolipoprotein B (APOB), and fibronectin (FN1) in RA sEV |
| 2 | Alghamdi et al. [19] | RA | Serum | 116 | 35 | 30–153 | Citrullinated fibrinogen |
Patients sEVs were more enriched in protein Half of the sEV samples that tested positive for anticitrullinated protein autoantibodies contained citrullinated fibrinogen (cFBG), a protein that plays a significant role in the development of rheumatoid arthritis (RA) |
| 3 | Rydland et al. [20] | RA | Plasma | 8 | 5 | 50–200 |
CD9 CD63 CD81 |
CD9- or CD81-positive sEVs were more abundant in RA patients compared to HC, but double-positive sEVs were scarcer MTX-responders exhibited a distinctive pattern of tetraspanin expression |
| 4 | Huang et al. [21] |
RA axSpA |
Synovial Fluid |
9 RA 9 axSpA |
9 OA | 60–120 | 1,678 detected |
39 sEV proteins were highly expressed in axSpA, while 28 proteins were highly expressed in RA Lysozyme C levels in RA and axSpA were higher than in OA Sec24C was highly expressed in axSpA compared to other groups High PZP levels in RA compared to other groups. Complement and coagulation cascade proteins were upregulated in RA synovial sEV. |
| 5 | Qin et al. [22] | RA | Plasma | 6 | 6 | N/A | 278 detected |
32 upregulated and 5 downregulated proteins in RA patients compared to HC Among upregulated proteins, TTR, AGT, CD14, LBP, SAP/APCS, TNC, and COMP interact and are involved in inflammatory pathways related to RA |
| 6 | Tsuno et al. [23] | RA | Serum | 33 | 10 | 50–100 | TLR3 | Upregulated in active RA patients |
| 7 | Yoo et al. [24] | RA | Serum |
30 active |
30 inactive |
NA |
AA LYVE-1 |
AA concentrations were higher in patients with high disease activity LYVE-1 concentration was lower in patients with higher disease activity |
| 8 | Skriner et al. [7] | RA | Synovial Fluid | 5 RA | 5 OA | 30–200 | Citrullinated proteins | Synovial sEV contained citrullinated proteins which act as autoantigens in RA |
No, number; RA, rheumatoid arthritis; axSpA, axial spondyloarthritis; OA, osteoarthritis; HC, healthy controls; sEV, small extracellular vesicles; MTX, methotrexate; SF, synovial fluid; PZP, Pregnancy zone protein; TTR, transthyretin; AGT, angiotensinogen; LBP, lipopolysaccharide-binding protein; SAP/APCS, serum amyloid P-component; TNC, tenascin; COMP, cartilage oligomeric matrix protein; TLR3, Toll-like receptor 3; AA, amyloid A; LYVE-1; lymphatic vessel endothelial hyaluronan receptor 1; N/A, not available
Proteomic profiling of synovial fluid-derived sEVs from RA, axial spondyloarthritis (axSpA) and osteoarthritis (OA; comparators) patients revealed that sEV proteins could distinguish inflammatory from non-inflammatory arthritis. Additionally, the study showed that sEV proteins, such as Sec24C and pregnancy zone protein (PZP), could be used for diagnosing SpA and RA, respectively [21]. Another group of researchers confirmed the distinct proteomic cargo in plasma sEV of RA patients, discovering that proteins closely related to inflammatory or autoimmune diseases were upregulated in RA plasma-derived sEV [22]. Tsuno et al., analyzed the protein cargo of serum-derived sEV from RA patients and showed that the expression of some exosomal proteins, such as Toll-like receptor 3 (TLR3), can distinguish patients with active from those with inactive disease [23]. Additionally, other proteins, such as serum amyloid A (AA) and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) within sEV, also appear to be promising biomarkers as they significantly correlate well with disease activity in patients with RA [24]. In a more recent study, Brenis Gómez et al. compared the sEV protein cargo between OA and RA patients, finding distinct differences [25]. A detailed summary of the aforementioned studies is provided in Table 2.
Exosomal/sEV ncRNAs and proteins in patients with systemic lupus erythematosus (SLE)
A substantial body of research on sEV cargo in patients with ARDs focuses on SLE, particularly in the discovery of ncRNAs as biomarkers for assessing disease activity. Ji et al. reported that the exosomal miR-122-5p levels in SLE patients were strongly correlated with disease activity (Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score) [26]. In a cohort of 20 SLE patients, two exosomal lncRNA molecules, the LINC00667 and DANCR, have been correlated with the SLEDAI score [27]. Similarly, Somparn et al. found an inverse correlation between the exosomal miR-146a concentration and SLEDAI score in patients with Juvenile Proliferative Lupus Nephritis (JPLN) [28]. miR-146a has also been investigated as a potential biomarker by Li et al., who reported its downregulation in exosomes of SLE patients, a finding consistent with the results of Dong et al. [29, 30]. The same molecule, miR-146a, has been highlighted by Perez-Hernandez et al. as an exosomal marker in urine, with a potential role in assessing the severity and the activity of Lupus Nephritis (LN) [31, 32]. Additionally, Tan et al. focused on the sEV miRNA miR-451a, another promising molecule that appears to correlate with disease activity and renal damage in SLE inversely [33].
sEV tRNA-derived small non-coding RNAs (tsRNAs) have emerged as a promising biomarker for diagnosing SLE and distinguishing SLE patients with and without LN. In one of the largest cohorts identified, including 253 SLE patients, tRF-Thr-TGT-4-M3 and tRF-Tyr-GTA-1-M2 were highly expressed in patients with LN compared to those without, while tRF-iMet-CAT-1, tRF-Ala-AGC-2-M4, and tRF-Tyr-GTA-1-M2 were highly expressed in patients without LN compared to healthy controls [34]. The same group of researchers previously demonstrated that tsRNAs were found to be upregulated in urine-derived sEVs of SLE patients with LN compared to those without LN and healthy controls, suggesting their potential role as urine biomarkers in LN [35].
As highlighted in the aforementioned studies, the urgent need for non-invasive methods to diagnose LN has driven research toward identifying molecular biomarkers for liquid biopsies. For instance, hsa-miR-4796-5p and hsa-miR-7974 have been proposed as potential biomarkers for diagnosing LN [36]. Similarly, Li et al. highlighted miR-21 and miR-155 as sEV biomarkers for the same purpose [29]. In addition, miR-31, miR-107, and miR-135b-5p have been reported as potential urine exosomal biomarkers for the prediction of treatment response in patients with LN [37]. A panel of three sEV miRNAs in urine, including miR-21, miR-150, and miR-29c, was found by Solé et al. to predict disease progression in LN patients [38]. Detailed results are summarized in Table 3. Although non-coding RNAs in the sEV of patients with SLE have been extensively studied, there is a paucity of data regarding sEV proteins in these patients. Therefore, this represents a promising area for future research.
Table 3.
ncRNA and proteins in small extracellular vesicles of patients with systemic lupus erythematosus
| A/A | Study | Disease | Sample | No. of patients | No. of comparators | sEV’s size (nm) | ncRNA | Major findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Peng et al. [27] | SLE | Plasma | 20 | 20 | 100 | LINC00667 DANCR |
Upregulated in the patients Positively correlated with disease activity |
| 2 | Ji et al. [26] | SLE | Plasma | 43 | 19 | 107 |
miR-151a-3p miR-1180-3p miR-1246 miR-122-5p |
miR-151a-3p was lower in SLE patients, while miR-1180-3p, miR-1246, miR-122-5p were higher miR-122-5p was correlated with SLEDAI activity, positively correlated with dsDNA level, while negatively correlated with C3 and C4 level |
| 3 | Yang et al. [34] | SLE | Serum | 253 | 80 | NA |
tRF-iMet-CAT-1 tRF-Ala-AGC-2-M4 tRF-Tyr-GTA-1-M2 tRF-Thr-TGT-4-M3 tRFTyr-GTA-1-M2 |
tRF-iMet-CAT-1, tRF-Ala-AGC-2-M4 and tRF-Tyr-GTA-1-M2 showed high expression in SLE group without LN than in HC tRF-Thr-TGT-4-M3 and tRFTyr-GTA-1-M2 were higher expressed in LN group than in SLE without LN |
| 4 | Somparn et al. [28] |
SLE (Juvenile Proliferative LN) |
Plasma | 12 | NA | 100–150 | miR-146a | Upregulated following 12 months of treatment and inversely correlated with lupus disease severity, anti-double-stranded DNA antibody concentration and proteinuria (urine protein–creatinine ratio) |
| 5 | Chen et al. [36] | SLE | Serum |
116 SLE-LN |
116 SLE |
127 | hsa-miR-4796-5p hsa-miR-7974 | Upregulated in LN patients compared to SLE patients |
| 6 | Flores-Chova et al. [53] | SLE | Plasma | 96 | 25 | NA |
miR-101-3p miR-16-5p LINC01986 AC087257 AC022596.1 LINC01015 |
Upregulated in LN patients |
| 7 | Chen et al. [35] | SLE | Urine | 173 | 24 | 129 | tRF3-Ile-AAT-1 tiRNA5-Lys-CTT-1 | Increased in the urine of patients with LN compared to SLE patients without LN and HC |
| 8 | Song et al. [54] | SLE | Plasma | 10 | 20 | 30–200 |
Various miRNAs and proteins |
407 upregulated and 596 downregulated miRNAs 84 upregulated (CRP, LGALS3BP, IGHG1, C1QB, C4BPA, etc.) and 13 downregulated (IGHV3OR15-7, HPR and HYDIN) proteins |
| 9 | Tan et al. [33] | SLE | Serum | 42 | 21 | 30–110 |
miR-451a miR-16 miR-451a |
miR-451a and miR-16 were downregulated miR-451a expression correlated with disease activity and renal damage |
| 10 | Li et al. [29] | SLE | Serum | 38 | 18 | NA |
miR-21 miR-155 miR-146a miR-155 |
miR-21 and miR-155 were elevated miR-146a levels were decreased Higher levels of miR-21 and miR-155 in LN patients compared to non-LN SL? patients |
| 11 | Peres Hernandez et al. [32] | SLE | Urine | 41 | 20 | 70–170 | miR-146a |
Increased sEV miR-146a levels compared to urine microvesicles in SLE patients, especially in patients with LN Association with disease activity |
| 12 | Garcia-vives et al. 2020 [37] | SLE | Urine | 22 LN Responders |
21 LN non-responders |
93.4 ± 36.6 |
miR-31 miR-107 miR-135b-5p MiR-135b |
Responders expressed increased levels of sEV miR-31, miR-107, and miR-135b-5p in urine and renal tissue compared to non-responders miR-135b exhibited the best predictive value to discriminate responders |
| 13 | Dong et al. 2019 [30] | SLE | Serum | 10 | 10 | 100 | miR-146a | Downregulated in SLE patients |
| 14 | Sole et al. [38] | SLE | Urine | 45 | 20 |
85.5 ± 33.4 |
miR-21 miR-150 miR-29c |
Detected early renal fibrosis and predict disease progression in LN patients |
| 15 | Tangtanatakul et al. [48] | SLE | Urine |
13 SLE-LN |
18 SLE inactive-LN |
40–200 |
Let-7a miR-21 |
Downregulated in active LN patients |
| 16 | Peres Hernandez et al. [31] | SLE | Urine | 38 | 12 | 21–140 |
miR-146a miR-335 miR-302d miR-200c |
Increased levels in active LN patients. sEVmiR-146a indicates the presence of active LN miR-302d elevated in inactive LN |
| 17 | Sole et al. [55] | SLE | Urine | 32 | 35 | 65.35 ± 34.7 | miR-29c | Correlated with the degree of renal chronicity but not with the creatinine levels |
SLE, Systemic Lupus Erythematosus; sEV, small extracellular vesicles; LN, Lupus Nephritis; HC, healthy controls; SLEDI, Systemic Lupus Erythematosus Disease Activity Index; dsDNA, double stranded; NA, not available
Sjögren’s syndrome (SS), polymyositis/dermatomyositis (PM/DM), systemic sclerosis (SSc)
Few studies have explored the differences in sEVs in patients with other ARDs, beyond RA and SLE, compared to healthy controls. We mention these studies for the completeness of the current review.
Regarding Sjögren’s Syndrome (SS), researchers have identified sEV miRNAs in mouth rinse-derived sEV as potential diagnostic biomarkers [39]. In a cohort of 18 patients, Peng et al. discovered that ferroptosis-related proteins, such as ceruloplasmin and transferrin, are downregulated in plasma-derived sEV of SS patients compared to healthy individuals. Apart from the discovery of a distinct pattern of protein expression in sEV of patients with SS, the results of this study highlight that ferroptosis may play a role in the pathogenesis of SS [40].
In 2021, Li et al. examined the RNA profile in plasma sEV of patients with Dermatomyositis (DM) and identified three differently expressed miRNAs and three lncRNAs participating in autophagy, which might serve as clinical biomarkers [41]. Zhong et al. suggested that the sEV miRNA profile in patients with DM can distinguish patients from HC as well as patients with different clinical profiles of the disease [42]. Proteomic analysis performed by Meng and colleagues revealed a unique sEV protein signature in Polymyositis/Dermatomyositis (PM/DM) patients, including proteins of the complement system and the coagulation cascade, suggesting their pathogenic role [43]. Finally, Wermuth et al. reported differences in the levels of profibrotic and antifibrotic miRNAs in sEVs of limited and diffuse Systemic Sclerosis (SSc) patients and healthy individuals [44]. Detailed results are summarized in Table 4.
Table 4.
Cargo of small extracellular vesicles in patients with Sjögren’s syndrome, Systemic Sclerosis, Polymyositis, and Dermomyositis
| A/A | Study | Disease | Sample | No. of patients | No. of comparators | sEV’s size (nm) |
Proteins/ncRNAs | Major findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Guiot et al. [56] | SSc | Plasma | 91 | 43 | 90–200 | miR-584-5p, miR-744-5p, miR-1307-3p and miR-10b-5p | These four sEV miRNAs can serve as a biomarker to differentiate between SSc patients with and without ILD |
| 2 | Peng et al. [40] | SS | Plasma | 18 | 18 | 131.8 | Ferroptosis related proteins | 24 differentiated expressed proteins in pSS compared to HC, 17 of which were upregulated proteins, while seven were downregulated and some of these promote ferroptosis |
| 3 | Yamashiro et al. [39] | SS | Mouth rinse | 24 | 24 | 145 |
let-7b-5p miR-1290 |
In combination, can be used for diagnosing SS. |
| 4 | Meng et al. [43] | PM/DM | Plasma | 24 | 9 | 116.4 | Various proteins (coagulation and complement proteins) |
50 and 34 differentially expressed in DM and PM patients, respectively 16 proteins were common to DM and PM patients Complement components C1QB and C1QC were significantly upregulated in DM/PM patients compared to HC Coagulation-associated proteins (FGA, FGB, FGC, and VWF) were significantly higher in DM/PM patients compared to HC |
| 5 | Li et al. [41] | DM | Plasma | 32 | 22 | 120 |
hsa-miR-1246 hsa-miR-125a-3p hsa-miR3614-5p ENST00000584157.1 ENST00000523380.1 ENST00000560054.1 |
44 upregulated and 9 downregulated miRNAs in DM patients compared with HC hsa-miR-1246, hsa-miR-125a-3p and hsa-miR3614-5p were decreased in DM patients after treatment. 313 upregulated lncRNAs and 139 downregulated lncRNAs in DM patients compared with HC The 3 miRNAs and the 3 lncRNAs formed a network and participated in autophagy. |
| 6 | Zhong et al. [42] | DM | Plasma | 10 | 5 | 122 | Various miRNAs |
38 miRNAs were significantly upregulated in sEV from patients with DM-ILD-MDA5 Ab+ compared to HC 21 miRNAs were significantly downregulated 73 sEV miRNAs were differentially expressed between DM-nonILD-MSA16- and HC |
| 7 | Wermuth et al. [44] | SSc | Serum | 6 | 12 | 75–200 | Various miRNAs |
Increased expression of let-7 g, miR17, miR23b and miR29a (profibrotic) in diffuse SSc patients Decreased expression of let-7a, miR125b, miR140, and miR146a (antifibrotic) in diffuse SSc patients Differential antifibrotic miRNA profile between diffuse SSc patients and limited SSc patients |
sEV, small extracellular vesicles; SS, Sjögren’s Syndrome; SSc, Systemic Sclerosis; PM, polymyositis; DM, dermomyositis; HC, healthy controls; ILD, interstitial lung disease
Discussion
Our literature review revealed an imbalance in the number of studies between ncRNA and proteomics analysis on sEVs in ARDs, with the greatest emphasis on nc RNAs. Among ARDs, RA and SLE are the most extensively investigated in this context, yet studies on sEV proteins remain relatively low. In this review, a key observation is the increasing use of non-invasive methods for sEV isolation, such as saliva from SS patients and urine from LN patients. These non-invasive approaches, by allowing repeated non-invasive sampling, have the potential to provide valuable insights not only for diagnosis but also for prognosis and treatment response in these diseases.
Since their discovery, sEVs have been attributed to numerous physiological and pathological roles. Their structure and biological composition make them ideal messengers for cell-to-cell communication and efficient carriers of information, traveling through biological fluids and transferring molecules away from their cellular origin. A growing body of knowledge implicates these structures in the pathogenesis of various diseases, and increasing research focuses on identifying sEV molecules that can serve as biomarkers or liquid biopsies, providing new, efficient methods for diagnosing and tracking disease progression. While new techniques for accurately identifying different types of sEVs have been proposed, questions remain regarding the optimal strategies for their isolation and characterization [45].
Efforts to use the cargo of sEVs to distinguish ARD patients from healthy individuals have consistently shown promising results. In this review, we aimed to compile studies comparing the sEV cargo between patients and healthy individuals. Reviewing the current bibliography, we compiled 46 scientific papers reporting on the cargo of sEV in patients with ARDs, with most research focused on miRNAs contained within sEVs. These small ncRNAs can alter gene expression and have been extensively investigated as potential biomarkers or therapeutic targets in ARDs [46]. In this review, we analyzed thirty-three studies that highlighted sEV miRNAs as promising biomarkers to diagnose ARDs or assess the activity and the response to treatment. Despite the relatively large number of studies, there seemed to be no universal consensus on the miRNA molecules that differ in various ARDs and healthy individuals.
Among all the exosomal miRNAs examined, miR-146a emerged as a promising biomarker in SLE in multiple studies [28, 29, 32]. miR-146a has been proposed as a biomarker in various ARDs in the past [47]. Additionally, based on findings from multiple studies, sEV miR-21 appears to play a complex and context-dependent role in SLE and LN. Elevated levels of miR-21 have been reported in the serum of SLE with high SLEDAI scores and in LN patients, suggesting a potential association with disease activity and renal involvement. Conversely, urinary sEV studies indicate that miR-21 may be downregulated in patients with active LN, while also serving as a biomarker for early renal fibrosis and disease progression. These contrasting patterns highlight the importance of biological source and disease stage in interpreting miR-21’s diagnostic and prognostic value [29, 38, 48].
Beyond RNA cargo, proteomic analysis of sEVs has provided compelling evidence of their involvement in ARDs. Several studies have identified disease-specific protein signatures in sEVs, with implications for the pathogenesis and diagnosis of these diseases. For example, citrullinated proteins within sEVs in rheumatoid arthritis patients suggest a direct role in propagating autoimmunity [7]. Additionally, proteomic analyses of sEVs in SS and PM/DM revealed key differences in immune-related and ferroptosis-associated proteins, further emphasizing their relevance in disease pathology [40, 43].
It is important to note that although there are only a limited number of studies concerning the proteomic profile in sEVs in the studied populations, discrepancies in methodology exist. Different techniques, which are not always well-standardized, continue to be employed. To address this issue, it is essential to adopt standardized methods that can precisely detect proteins. Techniques such as Liquid Chromatography Mass Spectrometry (LC-MS) are highly recommended, as they allow for the detection of a wide array of proteins with great accuracy and reproducibility. This approach would ensure more reliable and consistent results, facilitating the identification of protein biomarkers in small extracellular vesicles across autoimmune rheumatic diseases.
Another important point from the reviewed literature is the significant heterogeneity in the number of participants across studies. Some studies include a substantial number of patients and comparators, which strengthens the findings. However, some studies include a very small number of patients, which represents a limitation. This variability in sample sizes should be considered when interpreting the results.
We acknowledge certain limitations in the present review. As mentioned before, the studies included exhibit heterogeneity both in terms of study design and sample size. Despite these limitations, we believe our review is comprehensive, encompassing a broad range of articles from the existing literature, both older and more recent, and highlighting the current knowledge gap regarding the molecular content of small extracellular vesicles. To our knowledge, this is the first review to systematically summarize findings on both miRNAs and proteins contained within these vesicles in the context of autoimmune rheumatic diseases.
We believe that our systematic review offers readers of a rheumatology journal a roadmap for a new biomarker in rheumatic diseases, which is currently under investigation. It is noteworthy that our review focuses on small extracellular vesicles rather than extracellular vesicles in general, and it summarizes the existing knowledge while highlighting the gaps for future research.
Conclusion
sEVs appear to be promising biomarkers for autoimmune rheumatic diseases. While non-coding RNAs, such as miR-146a, miR-21, and miR-204, demonstrate potential for diagnosing and monitoring ARDs, variability in study methodologies highlights the need for standardized techniques. Proteomic profiles also hold diagnostic promise but require further validation. Non-invasive sampling methods are particularly promising for improving patient care. Further research with larger, standardized cohorts is essential to translate these findings into clinical practice and fully realize the potential of sEVs.
Author contributions
All author contributions are in line with the ICMJE 4 authorship criteria. Study conception and design: EKV, PPS; Data collection and review of the literature: MKC, EKV; interpretation of data: MKC, EKV, MS, GEF, PPS; drafting of the manuscript: MKC, EKV, GEF; Supervision: EKV. All authors reviewed and approved the final version of the manuscript to be published. All authors take full responsibility for the integrity and accuracy of all aspects of the work.
Funding
Open access funding provided by HEAL-Link Greece. No funding was received to assist with the preparation of this manuscript.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
No part of this review has been copied or published elsewhere in whole or in part.
AI declaration
AI was not used in manuscript preparation; no editing services were used.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Szekanecz Z, McInnes IB, Schett G et al (2021) Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol 17:585–595. 10.1038/S41584-021-00652-9 [DOI] [PubMed] [Google Scholar]
- 2.Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH (2017) Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31:306–320. 10.1016/j.berh.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gialouri CG, Fragoulis GE (2021) Disease activity indices in psoriatic arthritis: current and evolving concepts. Clin Rheumatol 40:4427–4435. 10.1007/S10067-021-05774-9 [DOI] [PubMed] [Google Scholar]
- 4.Paolicelli RC, Bergamini G, Rajendran L (2019) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157. 10.1016/j.neuroscience.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Welsh JA, Goberdhan DCI, O’Driscoll L et al (2024) Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles 13. 10.1002/JEV2.12404
- 6.Zhang B, Zhao M, Lu Q (2021) Extracellular vesicles in rheumatoid arthritis and systemic lupus erythematosus: functions and applications. Front Immunol. 10.3389/FIMMU.2020.575712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skriner K, Adolph K, Jungblut PR, Burmester GR (2006) Association of citrullinated proteins with synovial exosomes. Arthritis Rheum 54:3809–3814. 10.1002/ART.22276 [DOI] [PubMed] [Google Scholar]
- 8.Song Q, Yu H, Han J et al (2022) Exosomes in urological diseases - biological functions and clinical applications. Cancer Lett. 10.1016/J.CANLET.2022.215809 [DOI] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. 10.1136/BMJ.N71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Kim D, Han J et al (2015) PBMC and exosome-derived HOTAIR is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med 15:121–126. 10.1007/S10238-013-0271-4 [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Chen Q, Wang S et al (2024) Serum exosomes lncrnas: TCONS_I2_00013502 and ENST00000363624 are new diagnostic markers for rheumatoid arthritis. Front Immunol 15:1419683. 10.3389/FIMMU.2024.1419683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuai ZQ, Wang ZX, Ren J, Le et al (2024) Differential expressions and potential clinical values of LncRNAs in the plasma exosomes of rheumatoid arthritis. Int Immunopharmacol 128. 10.1016/J.INTIMP.2024.111511
- 13.Lu J, Wu J, Zhang X et al (2024) Characterization of the MicroRNA profile in rheumatoid arthritis plasma exosomes and their roles in B-cell responses. Clinics 79:100441. 10.1016/J.CLINSP.2024.100441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong J, Zhang X, Khan A et al (2024) Identification of serum exosomal miRNA biomarkers for diagnosis of rheumatoid arthritis. Int Immunopharmacol. 10.1016/J.INTIMP.2024.111604 [DOI] [PubMed] [Google Scholar]
- 15.Lim MK, Yoo J, Sheen DH et al (2020) Serum Exosomal miRNA-1915-3p ?s correlated with disease activity of Korean rheumatoid arthritis. Vivo (Brooklyn) 34:2941. 10.21873/INVIVO.12124 [Google Scholar]
- 16.Hussain MZ, Haris MS, Rizwan M et al (2023) Deregulation of exosomal miRNAs in rheumatoid arthritis patients. PLoS One 18:e0289301. 10.1371/JOURNAL.PONE.0289301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu LF, Zhang Q, Mo XB et al (2022) Identification of novel rheumatoid arthritis-associated MiRNA-204-5p from plasma exosomes. Exp Mol Med 54:334. 10.1038/S12276-022-00751-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue L, Wang B, Li X et al (2023) Comprehensive analysis of serum exosome-derived LncRNAs and mRNAs from patients with rheumatoid arthritis. Arthritis Res Ther 25:201. 10.1186/S13075-023-03174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alghamdi MA, Bahlas SM, Alamry SA et al (2025) Exploring anticitrullinated antibodies (ACPAs) and serum-derived exosomes cargoes. Antibodies (Basel) 14:10. 10.3390/ANTIB14010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rydland A, Heinicke F, Flåm ST et al (2023) Small extracellular vesicles have distinct CD81 and CD9 tetraspanin expression profiles in plasma from rheumatoid arthritis patients. Clin Exp Med 23:2867. 10.1007/S10238-023-01024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Liu Y, Huang Q et al (2022) TMT-based quantitative proteomics analysis of synovial fluid-derived exosomes in inflammatory arthritis. Front Immunol. 10.3389/FIMMU.2022.800902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Q, Song R, Du P et al (2021) Systemic Proteomic Analysis Reveals Distinct Exosomal Protein Profiles in Rheumatoid Arthritis. J Immunol Res 2021. 10.1155/2021/9421720
- 23.Tsuno H, Arito M, Suematsu N et al (2018) A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol. 10.1186/s41927-018-0041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo J, Lee SK, Lim M et al (2017) Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Res Ther 19:119. 10.1186/S13075-017-1334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenis Gómez CM, Plasencia-Rodríguez C, Novella-Navarro M et al (2025) Exosomal protein biomarkers in arthritis: deciphering the inflammatory profiles of RA and OA. Biomedicines 13:1283. 10.3390/BIOMEDICINES13061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji J, He Q, Xia Y et al (2024) Circulating plasma derived exosomes from systemic lupus erythematosus aggravate lupus nephritis through miR-122-5p/FOXO3-mediated macrophage activation. J Nanobiotechnol 22:779. 10.1186/S12951-024-03063-6 [Google Scholar]
- 27.Peng X, chen, Ma L, li, Miao Jyu et al (2024) Differential LncRNA profiles of blood plasma-derived exosomes from systemic lupus erythematosus. Gene 927. 10.1016/J.GENE.2024.148713
- 28.Somparn P, Srichaimongkol A, Jungjing S et al (2024) Potential involvement of circulating exosomal miRNA-146a in disease activity and TRAF6 gene expression in juvenile proliferative lupus nephritis. Lupus Sci Med 11:e001078. 10.1136/LUPUS-2023-001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Liu S, Chen Y et al (2020) Circulating exosomal microRNAs as biomarkers of systemic lupus erythematosus. Clinics 75:e1528. 10.6061/clinics/2020/e1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong C, Zhou Q, Fu T et al (2019) Circulating exosomes Derived-miR-146a from systemic lupus erythematosus patients regulates senescence of mesenchymal stem cells. Biomed Res Int 2019:6071308. 10.1155/2019/6071308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Hernandez J, Forner MJ, Pinto C et al (2015) Increased urinary exosomal micrornas in patients with systemic lupus erythematosus. PLoS One. 10.1371/JOURNAL.PONE.0138618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Hernandez J, Martinez-Arroyo O, Ortega A et al (2021) Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J Nephrol 34:1157–1167. 10.1007/S40620-020-00832-Y [DOI] [PubMed] [Google Scholar]
- 33.Tan L, Zhao M, Wu H et al (2021) Downregulated serum exosomal miR-451a expression correlates with renal damage and its intercellular communication role in systemic lupus erythematosus. Front Immunol 12:630112. 10.3389/FIMMU.2021.630112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang P, Sun Y, Wang C et al (2024) Serum exosomal TsRNA biomarkers: a novel strategy for identifying lupus nephritis. Clin Transl Med 14:e1677. 10.1002/CTM2.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Zhang X, Meng K et al (2023) Urinary exosome TsRNAs as novel markers for diagnosis and prediction of lupus nephritis. Front Immunol 14:1077645. 10.3389/FIMMU.2023.1077645/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Shi B, Liu W et al (2023) Circulating Exosomal MicroRNAs as biomarkers of lupus nephritis. Front Immunol 14:1326836. 10.3389/FIMMU.2023.1326836/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-vives E, Solé C, Moliné T et al (2020) The urinary exosomal miRNA expression profile is predictive of clinical response in lupus nephritis. Int J Mol Sci 21:1372. 10.3390/IJMS21041372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solé C, Moliné T, Vidal M et al (2019) An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells 8:773. 10.3390/CELLS8080773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashiro K, Hamada T, Mori K et al (2022) Exosome-Derived MicroRNAs from mouthrinse have the potential to be novel biomarkers for Sjögren syndrome. J Pers Med 12:1483. 10.3390/JPM12091483/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng X, Hou L, Wu X et al (2023) The plasma exosomes from patients with primary Sjögren’s syndrome contain epithelial cell–derived proteins involved in ferroptosis. J Mol Med (Berl) 101:1289. 10.1007/S00109-023-02361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Zuo X, Liu D et al (2022) Plasma exosomal RNAs have potential as both clinical biomarkers and therapeutic targets of dermatomyositis. Rheumatology (Oxford) 61:2672–2681. 10.1093/RHEUMATOLOGY/KEAB753 [DOI] [PubMed] [Google Scholar]
- 42.Zhong D, Wu C, Xu D et al (2021) Plasma-Derived Exosomal hsa-miR-4488 and hsa-miR-1228-5p: novel biomarkers for Dermatomyositis-Associated interstitial lung disease with Anti-Melanoma Differentiation-Associated protein 5 Antibody-Positive subset. Biomed Res Int 2021:6676107. 10.1155/2021/6676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng S, Wang T, Zhao Q et al (2023) Proteomics analysis of Plasma-Derived exosomes unveils the aberrant complement and coagulation cascades in dermatomyositis/polymyositis. J Proteome Res 22:123–137. 10.1021/ACS.JPROTEOME.2C00532/SUPPL_FILE/PR2C00532_SI_001.XLSX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wermuth PJ, Piera-Velazquez S, Jimenez SA (2017) Exosomes isolated from serum of systemic sclerosis patients display alterations in their content of profibrotic and antifibrotic microRNA and induce a profibrotic phenotype in cultured normal dermal fibroblasts. Clin Exp Rheumatol 35:21 [PMC free article] [PubMed] [Google Scholar]
- 45.Jia Y, Yu L, Ma T et al (2022) Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics 12:6548. 10.7150/THNO.74305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prajzlerová K, Šenolt L, Filková M (2022) Is there a potential of Circulating MiRNAs as biomarkers in rheumatic diseases? Genes Dis 10:1263. 10.1016/J.GENDIS.2022.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae SC, Lee YH (2018) Mir-146a levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Int J Rheum Dis 21:1335–1342. 10.1111/1756-185X.13338 [DOI] [PubMed] [Google Scholar]
- 48.Tangtanatakul P, Klinchanhom S, Sodsai P et al Allergy and immunology Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. 10.12932/AP-130318-0280
- 49.Yang X, Wang Z, Zhang M, Shuai Z (2023) Differential expression profiles of plasma exosomal MicroRNAs in rheumatoid arthritis. J Inflamm Res 16:3687–3698. 10.2147/JIR.S413994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Park S, Lee H et al (2023) Exosomal hsa-miR-335-5p and hsa-miR-483-5p are novel biomarkers for rheumatoid arthritis: a development and validation study. Int Immunopharmacol 120:110286. 10.1016/J.INTIMP.2023.110286 [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Muguruza S, Altuna-Coy A, Castro-Oreiro S et al (2021) A serum biomarker panel of exomiR-451a, exomiR-25-3p and soluble TWEAK for early diagnosis of rheumatoid arthritis. Front Immunol 12:790880. 10.3389/FIMMU.2021.790880/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Wang C, Jia X, Yu J (2018) Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem 50:1754–1763. 10.1159/000494793 [DOI] [PubMed] [Google Scholar]
- 53.Flores-Chova A, Martinez-Arroyo O, Riffo-Campos AL et al (2023) Plasma Exosomal Non-Coding RNA profile associated with renal damage reveals potential therapeutic targets in lupus nephritis. Int J Mol Sci 24:7088. 10.3390/IJMS24087088/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song W, Li C, Qiu J et al (2023) Differential expression of exosomal miRNAs and proteins in the plasma of systemic lupus erythematous patients. Heliyon. 10.1016/j.heliyon.2023.e13345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sole C, Cortes-Hernandez J, Felip ML et al (2015) MIR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol Dial Transplant 30:1488–1496. 10.1093/NDT/GFV128 [DOI] [PubMed] [Google Scholar]
- 56.Guiot J, André B, Potjewijd J et al (2025) Association of fibrotic-related extracellular vesicle microRNAs with lung involvement in systemic sclerosis. Eur Respir J 65:2400276. 10.1183/13993003.00276-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]