Abstract
The biological process for phosphate (Pi) removal is based on the use of bacteria capable of accumulating inorganic polyphosphate (polyP). We obtained Escherichia coli mutants which accumulate a large amount of polyP. The polyP accumulation in these mutants was ascribed to a mutation of the phoU gene that encodes a negative regulator of the Pi regulon. Insertional inactivation of the phoU gene also elevated the intracellular level of polyP in Synechocystis sp. strain PCC6803. The mutant could remove fourfold more Pi from the medium than the wild-type strain removed.
Inorganic phosphate (Pi) is recognized as one of the major nutrients causing eutrophication of lakes, bays, and waterways (18). Considerable attention has been paid to effective Pi removal from wastewater (6). Many bacteria are capable of accumulating excess Pi in the form of inorganic polyphosphate (polyP), which is a linear polymer of hundreds of Pi residues linked by high-energy phosphoanhydride bonds (7, 9, 10). Improvement of the ability to accumulate polyP contributes to increased Pi removal from wastewater (18).
Previously, we have demonstrated genetic improvement of polyP accumulation in Escherichia coli (8). High levels of accumulated polyP were achieved by increasing the dosage of the E. coli genes encoding polyP kinase (ppk) and the Pi-specific transport system (pstSCAB). The E. coli recombinant accumulated approximately 16% of its dry weight as phosphorus (P) (49% as Pi) (8). Over 60% of cellular P was stored in the form of polyP in the genetically engineered E. coli strain. However, growth of the E. coli recombinant was severely limited in minimal medium (8). In addition, this recombinant released polyP back into the medium when it accumulated high levels of polyP. In this paper, we report that a mutation in the phoU gene, which encodes a negative regulator of the Pi regulon (3, 21), led to high levels of accumulated polyP in E. coli. phoU mutants could be easily screened on agar plates containing 5-bromo-4-chloro-3-indolylphosphate (XP) after N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis. Therefore, isolating phoU mutants seems to be a useful way to improve the ability of bacteria to accumulate polyP. To show whether this method is effective in another bacterium, we performed insertional inactivation of the phoU gene in Synechocystis sp. strain PCC6803 and showed that the intracellular level of polyP increased in the mutant.
Isolation of E. coli mutants.
The levels of polyP in E. coli MG1655 were very low (less than 1 nmol of Pi residues/mg of protein) when the organism was grown on a rich medium (11). PolyP was recovered with silicate glass from cells lysed with guanidine isothiocyanate, and the polyP content was determined by a two-enzyme assay (4). PolyP was first converted to ATP by polyphosphate kinase, and then the amount of ATP was measured by a bioluminescence assay. We first selected alkaline phosphatase constitutive mutants, which could form blue colonies on agar plates containing XP (50 mg/liter) under Pi-sufficient conditions after N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis (17). One of 150 mutants, designated MT4, accumulated a large amount of polyP (approximately 100 nmol of Pi residues/mg of protein). The levels of polyP were at least 100-fold higher than the levels in the wild-type strain, MG1655.
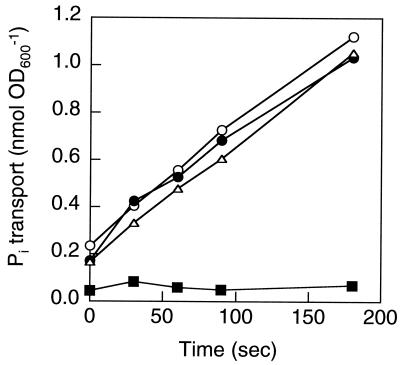
To assess Pi transport (14), the E. coli mutants were grown overnight in MOPS (morpholinepropanesulfonic acid) medium (12) containing either 0.1 mM Pi (Pi limiting) or 2 mM Pi (Pi sufficient). Cells were harvested by centrifugation at 8,000 × g for 5 min and washed with HEPES buffer (13). Assays were started by adding 32Pi to a final concentration of 2.5 μM, and the amount of 32Pi incorporated into the cells was determined by using a scintillation counter (Packard). Only MT4 exhibited Pi-specific transport even when the organisms were grown with excess Pi (Fig. 1).
FIG. 1.
Pi transport as assessed by using Pi-sufficient MG1655 (▪), Pi-limited MG1655 (•), Pi-sufficient MT4 (▵), and Pi-limited MT4 (○) cultures. Cells were grown in MOPS medium containing either 2 mM Pi (Pi sufficient) or 0.1 mM Pi (Pi limited). OD, optical density at 600 nm.
Analysis of the mutation of MT4.
An E. coli DNA library based on the SuperCos plasmid (Stratagene) was introduced into MT4 by transformation (17). Approximately 200 transformants were examined for the ability to accumulate polyP in 2×YT medium (17). One transformant reduced the levels of polyP to the level in the wild-type strain. This transformant carried a recombinant plasmid containing a 30-kb DNA fragment of the E. coli chromosome. Nucleotide sequence analysis of the 30-kb insert revealed that this fragment contained the pst and bgl operons which were located at 84 min on the E. coli linkage map (5). Subcloning and complementation analysis revealed that a 3.0-kb EcoRI fragment, carrying the entire phoU gene, could complement the mutation of MT4. The chromosomal phoU gene of MT4 was amplified by PCR with primers EU1 (5′-ATTGGGATTTGTCTGGTGAA-3′) and EU2 (5′-AGAAGACTACATCACCGGTC-3′) and cloned into the pGEM-T vector (Promega). Nucleotide sequence analysis showed that the 29th codon of the phoU gene was changed from a glycine codon to an aspartic acid codon in MT4. We again selected a polyP-accumulating mutant from the mutants producing alkaline phosphatase constitutively. In this mutant, the 83rd codon of the phoU gene was changed from an alanine codon to a threonine codon. These results indicated that mutation of the phoU gene resulted in elevated intracellular levels of polyP in E. coli.
To further confirm that phoU mutants could accumulate high levels of polyP, the chromosomal phoU gene of MG1655 was disrupted by inserting a kanamycin resistance (Kmr) gene cassette into the wild-type gene. A 0.8-kb DNA fragment containing the entire phoU gene was amplified by PCR with the EU1 and EU2 primers and inserted into the pGEM-T vector. The resultant plasmid was digested with ClaI and ligated with the Kmr gene cassette of pUC4K (Amersham-Pharmacia), and an EcoRI fragment containing a disrupted phoU gene was inserted into an EcoRI site of pGP704 containing the sacB gene (16). This plasmid was introduced into E. coli S17-1 and then transferred into MG1655 by transconjugation (22). Transconjugants were selected on agar plates containing 5% sucrose and kanamycin (50 mg/liter). Disruption of the chromosomal phoU gene was confirmed by Southern hybridization. As expected, the insertional phoU mutant accumulated high levels of polyP (approximately 400 nmol of Pi residues/mg of protein).
One might assume that ppk is a member of the Pi regulon in E. coli and that derepressed expression of this gene in the phoU mutants results in polyP accumulation. However, no significant increase in the level of polyphosphate kinase was observed under Pi-limited conditions in E. coli (Morohoshi and Kuroda, unpublished data). Furthermore, expression of a single-copy ppk-lacZ transcriptional fusion did not increase under Pi limitation conditions (L. Zhou and B. L. Wanner, personal communication). Pi transport is rate limiting for polyP accumulation in E. coli (8). A phoU mutant exhibited Pi-specific transport even under Pi-excess conditions (Fig. 1). We transferred a Δ(pstSCAB-phoU)::km mutation from BW17335 (20) to the wild-type strain, MG1655, by using P1 phage. This mutant failed to accumulate polyP (0.5 nmol/mg of protein). Consequently, it is likely that constitutive expression of Pi-specific transport (PstSCAB) is responsible for the elevated levels of polyP in the phoU mutants.
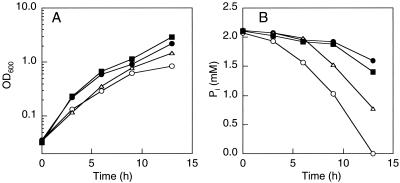
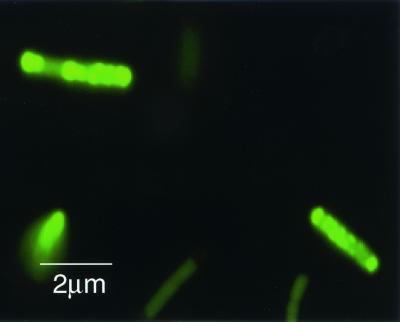
Although MT4 grew relatively slowly, it removed twofold more Pi from the medium than the wild type removed (Fig. 2). Introduction of multicopy plasmid pBC29, which contains the E. coli ppk gene (1), into the wild-type strain, MG1655, did not increase Pi removal significantly (Fig. 2). By contrast, MT4(pBC29) removed about twofold more Pi than MT4 removed (Fig. 2). The P content of MT4(pBC29) reached approximately 9% on a dry weight basis (27% as Pi) and was about sixfold greater than that of the wild-type strain, MG1655. PolyP granules were detected in MT4(pBC29) when the cells were observed by fluorescent microscopy after they were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (2) (Fig. 3).
FIG. 2.
Growth (A) and Pi uptake (B) of E. coli MG1655, MG1655(pBC29), MT4, and MT4(pBC29). Strains MG1655 (▪), MG1655(pBC29) (•), MT4 (▵), and MT4(pBC29) (○) were grown in MOPS medium containing 2 mM Pi at 28°C. OD600, optical density at 600 nm.
FIG. 3.
PolyP granules in MT4(pBC29) detected by DAPI fluorescence. Strain MT4(pBC29) was grown overnight on 2×YT medium, stained with DAPI at a final concentration of 50 μg/ml, and observed with a fluorescence microscope (Olympus BX-40).
phoU mutant of Synechocystis sp. strain PCC6803.
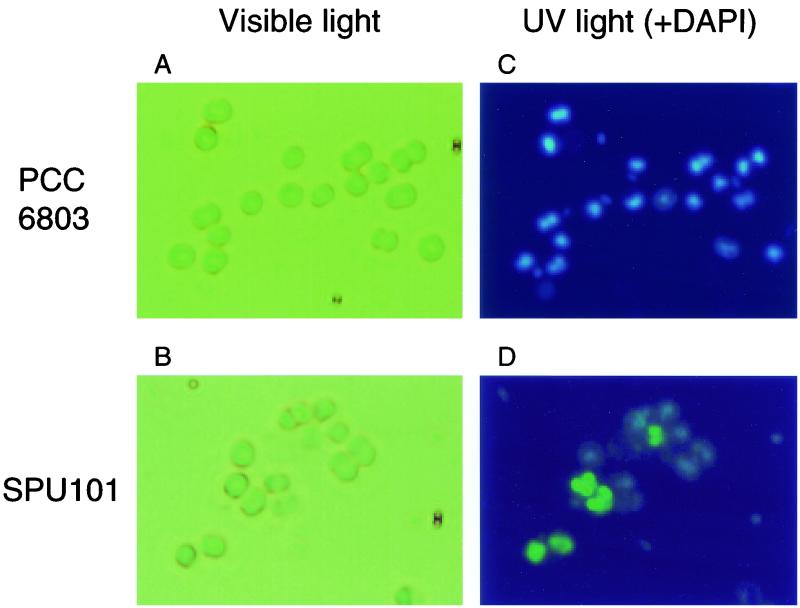
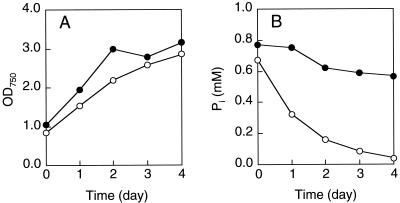
A chromosomal phoU mutant of Synechocystis sp. strain PCC6803 was also constructed by inserting a Kmr gene cassette into the wild-type gene. A 2.0-kb DNA fragment, which carried the entire phoU gene, was amplified by PCR with primers SU1 (5′-GGTACCATCAACCTGATCGCCTAT-3′) and SU2 (5′-GCTACTGCTCCAGTCGACCCGAGT-3′) and cloned into pUC119. The resultant plasmid was digested with BglII, ligated to the Kmr gene cassette of pUC4K, and introduced into Synechocystis sp. strain PCC6803 by electroporation (17). Disruption of the chromosomal phoU gene was confirmed by Southern hybridization (data not shown). The Synechocystis phoU mutant, designated SPU101, accumulated about 15% as much polyP (on a dry weight basis) as Pi. As in E. coli MT4(pBC29), polyP particles were detected in SPU101 by DAPI fluorescence (Fig. 4). The total P content of the mutant strain reached a maximum of 6% on a dry weight basis. Pi uptake experiments were performed with growing cultures of PCC6803 and SPU101 (Fig. 5). Cultures were grown in BG-11 medium (19) with 1% CO2 at 30°C under light (5,000 lx). Strain SPU101 removed about fourfold more Pi from the medium than the parental strain removed. The growth of SPU101 was almost equivalent to that of PCC6803.
FIG. 4.
PolyP granules in Synechocystis sp. strain PCC6803 and phoU mutant SPU101 detected by DAPI fluorescence. Strains PCC6803 and SPU101 were grown in BG-11 medium at 30°C under light (5,000 lx) with 1% CO2 for 3 days and stained with DAPI. Cells were observed with either visible light (A and B) or UV light (C and D).
FIG. 5.
Growth (A) and Pi uptake (B) of Synechocystis sp. strain PCC6803 and phoU mutant SPU101. Strains PCC6803 (•) and SPU101 (○) were grown in BG-11 medium at 30°C under light (5,000 lx) with 1% CO2. OD750, optical density at 750 nm.
We showed that a mutation of the phoU gene, which encodes a negative regulator of the Pi regulon, led to high levels of accumulated polyP in both E. coli and Synechocystis sp. Rao et al. previously reported that a phoU mutation had no effect on polyP accumulation (15). The phoU mutant of these authors probably had a secondary mutation in the Pst system, as described previously (20).
In enhanced biological P removal, sludge microorganisms accumulate 3 to 5% of their dry weight as P (18). Similar levels of accumulated polyP were observed with the phoU mutant of Synechocystis sp. strain PCC6803. In general, the levels of carbon sources in wastewater are relatively low. This is likely to limit Pi removal from wastewater by sludge microorganisms (18). Use of the Synechocystis mutant, which is able to accumulate polyP if light is present, may improve biological removal of Pi from wastewater.
Acknowledgments
We thank B. L. Wanner for a gift of the phoU and pstSCAB mutant and M. Ikeuchi for a gift of strain PCC6803.
This work was supported in part by the New Energy and Industrial Technology Development Organization (NEDO) and by the Research Institute of Innovative Technology for the Earth (RITE).
REFERENCES
- 1.Akiyama, M., E. Crooke, and A. Kornberg. 1992. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J. Biol. Chem. 267:22556-22561. [PubMed] [Google Scholar]
- 2.Allan, R. A., and J. J. Miller. 1980. Influence of S-adenosylmethionine on DAPI-induced fluorescence of polyphosphate in the yeast vacuole. Can. J. Microbiol. 26:912-920. [DOI] [PubMed] [Google Scholar]
- 3.Amemura, M., K. Makino, H. Shinagawa, A. Kobayashi, and A. Nakata. 1985. Nucleotide sequence of the genes involved in phosphate transport and regulation of the phosphate regulon in Escherichia coli. J. Mol. Biol. 20:241-250. [DOI] [PubMed] [Google Scholar]
- 4.Ault-Riché, D., C. D. Fraley, C.-M. Tzeng, and A. Kornberg. 1998. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 180:1841-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlyn, M. K. B., K. B. Low, and K. E. Rudd. 1996. Linkage map of Escherichia coli K-12, p. 1715-1902. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 6.Hammond, A. E. 1971. Phosphate replacements: problems with the washday miracles. Science 172:361-364. [DOI] [PubMed] [Google Scholar]
- 7.Harold, F. M. 1966. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol. Rev. 30:772-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato, J., K. Yamada, A. Muramatsu, Hardoyo, and H. Ohtake. 1993. Genetic improvement of Escherichia coli for enhanced biological removal of phosphate from wastewater. Appl. Environ. Microbiol. 59:3744-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornberg, A. 1995. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J. Bacteriol. 177:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulaev, I. S. 1979. The biochemistry of inorganic polyphosphates. Wiley, New York, N.Y. [DOI] [PubMed]
- 11.Kuroda, A., H. Murphy, M. Cashel, and A. Kornberg. 1997. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 272:21240-21243. [DOI] [PubMed] [Google Scholar]
- 12.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikata, T., Y. Sakai, K. Shibata, J. Kato, A. Kuroda, and H. Ohtake. 1996. Molecular analysis of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol. Gen. Genet. 250:692-698. [DOI] [PubMed] [Google Scholar]
- 14.Poole, K., and R. E. Hancock. 1984. Phosphate transport in Pseudomonas aeruginosa: involvement of a periplasmic phosphate-binding protein. Eur. J. Biochem. 144:607-612. [DOI] [PubMed] [Google Scholar]
- 15.Rao, N. N., S. Liu, and A. Kornberg. 1998. Inorganic polyphosphate in Escherichia coli: the phosphate regulon and the stringent response. J. Bacteriol. 180:2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyrat, J. M., V. Pelicic, B. Gicquel, and R. Rappuoli. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Sedlak, R. I. 1991. Phosphorus and nitrogen removal from municipal wastewater, 2nd ed. Lewis Publishers, Boca Raton, Fla.
- 19.Stanier, R. Y., R. Kunisawa, M. Mandel, and G. Cohen-Bazire. 1971. Purification and properties of unicellular blue-green algae (order Chroococales). Bacteriol. Rev. 35:171-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steed, P. M., and B. L. Wanner. 1993. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J. Bacteriol. 175:6797-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 22.Young, G. M., M. J. Smith, S. A. Minnich, and V. L. Miller. 1999. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 181:2823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]