Abstract
We investigated the regulation of the psbA and pvdA pyoverdine biosynthesis genes, which encode the l-ornithine N5-oxygenase homologues in Pseudomonas strain B10 and Pseudomonas aeruginosa PAO1, respectively. We demonstrate that pyoverdineB10, as the end product of its biosynthetic pathway, is a key participant of the control circuit regulating its own production in Pseudomonas strain B10. In P. aeruginosa PAO1, however, pyoverdinePAO1 has no apparent role in the positive regulation of the pvdA gene.
The competition for iron is a main determinant for the dynamics of microbial populations in natural ecosystems. Aerobic bacteria living in neutral environments are normally faced with the nutritional iron deficit resulting from the low solubility of iron in its oxidized state (13). Likewise, bacterial parasites, which multiply within higher organisms, must counteract the iron deficiency imposed by the activity of the host's iron binding proteins (15). Iron withholding also affects the interactions between microbial populations in the rhizosphere (14).
Pyoverdines (also referred to as pseudobactins), the fluorescent siderophores produced by the rRNA group I species of genus Pseudomonas, constitute a large family of iron chelators that differ in terms of the length and composition of a hydroxamate-containing oligopeptide joined to a structurally conserved dihydroxyquinoline chromophore (1). Due to their high affinity for ferric iron, pyoverdines can suppress the growth of deleterious rhizomicroorganisms, which lack uptake specificities for ferripyoverdine complexes, thereby contributing to the plant defense from parasitic infection (2, 14).
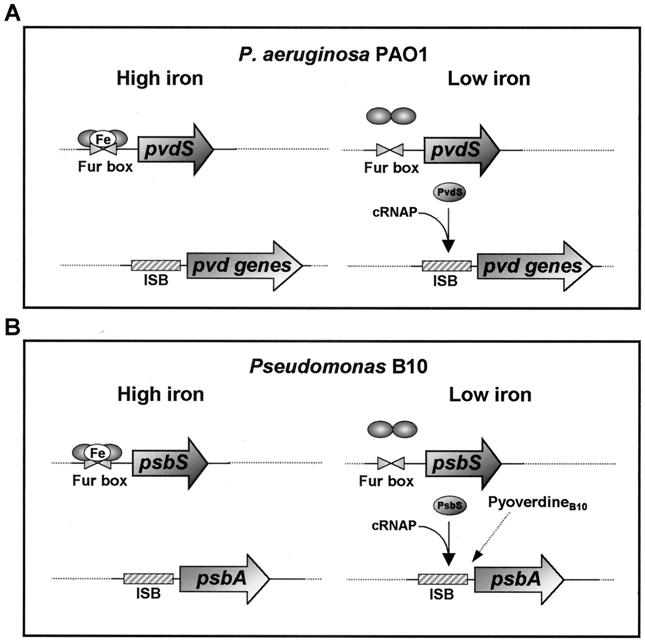
In Pseudomonas spp. and many other bacteria, regulation of iron transport is primarily controlled by the Fur repressor protein (22). When the level of intracellular iron is high, transcription of iron-repressible genes is blocked through the binding of Fur-Fe(II) complexes to a highly conserved sequence (the Fur box) located within the promoters of these genes (5). However, Fur is not the only regulator of iron uptake in fluorescent pseudomonads (24). In Pseudomonas aeruginosa, the rRNA group I type species, expression of pyoverdinePAO1 (pvd) genes is controlled by iron through a cascade of negative and positive regulatory proteins (Fig. 1). Briefly, Fur acts as a repressor of the pvdS gene, encoding an extra cytoplasmic function (ECF) sigma factor, called PvdS, which is required for transcription initiation at the promoters of pvd genes (4, 9). A DNA sequence element, the iron starvation box (ISB), has been identified in PvdS-dependent promoters and has been shown to participate in sequence-specific promoter recognition by PvdS, acting as a −35-like sequence element (27). Several Fur-controlled alternative sigma factors, structurally related to PvdS, have been identified in fluorescent Pseudomonas spp. and have been found to be involved in pyoverdine synthesis and/or uptake (7, 20, 23). An additional level of complexity of the siderophore regulatory pathway in these species is inferred by the positive effect of ferric siderophore complexes on the expression of cognate biosynthesis genes. Siderophore-regulated responses have previously been demonstrated for the pyochelin-salicylate operons of P. aeruginosa and for the pyoverdine gene systems of Pseudomonas putida WCS358 and Pseudomonas fluorescens M114 (3, 16, 23, 24).
FIG. 1.
Regulatory models of pyoverdine genes in P. aeruginosa PAO1 and in Pseudomonas strain B10. (A) In iron-replete P. aeruginosa PAO1 cells, transcription of pvdS is prevented by the binding of dimeric Fur-Fe(II) complexes to Fur boxes overlapping the pvdS promoter. In the absence of sufficient iron, release of Fur from DNA allows pvdS to be transcribed. The ECF sigma factor PvdS mediates recognition of the ISB, thereby conferring specificity to core RNA polymerase (cRNAP) for transcription initiation at the pvd promoters. (B) In Pseudomonas strain B10, expression of the psbA gene is similarly regulated by the Fur-controlled ECF sigma factor PsbS. An additional level of regulation is provided by exogenous pyoverdineB10, which exerts a positive induction effect on expression of the psbA biosynthetic gene.
The pyoverdineB10 biosynthesis gene psbA, encoding the enzyme l-ornithine (l-Orn) N5-oxygenase (PsbA) in the plant growth-promoting Pseudomonas strain B10, has previously been characterized in our laboratory (2). PsbA expression is regulated at the transcriptional level by a Fur-controlled ECF sigma factor, designated PsbS (8). Herein, we provide evidence that pyoverdineB10 itself plays a crucial role in the expression of the psbA gene. The comparison of autogenous regulation of pyoverdine biosynthesis in Pseudomonas strain B10 and P. aeruginosa PAO1 highlighted relevant differences between the two species.
The bacterial strains and plasmids used in this study are listed in Table 1. The media and growth conditions have been described previously (6, 9, 25).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Pseudomonas | Prototroph | 12 |
| B10 | ||
| B10CA1 | psbA::cat site-specific insertion mutant | 2 |
| P. aeruginosa | ||
| PAO1 (ATCC 15692) | Prototroph | American Type Culture Collection |
| PALS124 | pvdA | 26 |
| PALS106 | pvdC1 | 26 |
| PALS125 | pvdC3 | 26 |
| Plasmids | ||
| pMP220 | Broad-host-range, low-copy-number promoter-probe vector, IncP replicon, lacZ Tcr Tra− | 21 |
| pMP220::PpvdA | pvdA promoter directionally cloned in pMP220 (formerly designated pPV51) | 9 |
| pMP220::PpsbA | psbA promoter from pMP190::PpsbA directionally cloned in pMP220 | This study |
| pMP190 | Broad-host-range, low-copy-number promoter-probe vector, IncQ replicon, lacZ Cmr Tra− | 21 |
| pMP190::PpsbA | psbA promoter directionally cloned in pMP190 | 8 |
| pMP190::PpsbS | 470-bp PCR-generated fragment encompassing the psbS promoter directionally ligated to the KpnI-SalI sites of pMP190 | 8 |
| pMP220::PpsbS | DNA fragment of ca. 530 bp excised from pMP190::PpsbS by HindIII digestion and directionally ligated to the HindIII site of pMP220 | This study |
| pMP220::PpvdS | 587-bp PCR-generated fragment encompassing the pvdS promoter directionally ligated to the BglII-EcoRI sites of pMP220 | This study |
| pUCP18 | Escherichia coli-Pseudomonas shuttle vector derived from pUC18, pMB1, replicon pRO1600 lacZα bla Mob+ Tra− Apr Cbr | 19 |
| pCAΔSh | pUCP18-derivative containing a 2.7-kb PstI-SphI fragment encompassing the entire psbA gene | 2 |
Characterization of the psbA promoter in Pseudomonas strain B10.
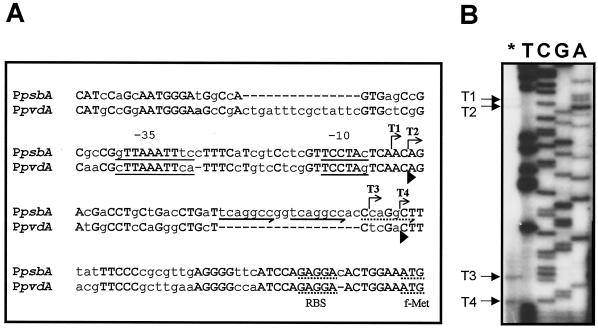
To define the structure of the psbA promoter (PpsbA), the initiation sites of the psbA transcript were determined by primer extension analysis. The primer PEpsbARV (5′-GGGCGATGCAATTGCCGTTGTCAT-3′) was end labeled with [γ-32P]ATP and used for reverse transcription of total RNA from iron-deprived Pseudomonas strain B10 cells as described elsewhere (9). The unlabeled primer was used to sequence the DNA region upstream from the psbA gene from plasmid pCAΔSh with a T7 sequencing kit (Pharmacia) and [α-32P]dATP. The 5′ ends of the psbA mRNA were mapped at four distinct sites (Fig. 2). The most abundant transcripts, T3 and T4, originate at positions −44 and −48 relative to the psbA start codon, respectively, while two minor transcripts, T1 and T2, originate at positions −87 and −89, respectively. No primer extension product was detected with total RNA from bacteria grown in iron-rich medium (data not shown), consistent with the observation that psbA is not transcribed in iron-rich cells (2). The initiation pattern of psbA mRNA is consistent with the presence of multiple transcription start sites in the homologous pvdA promoter (PpvdA) and in P. aeruginosa iron-regulated promoters directly or indirectly controlled by the PvdS sigma factor, including pvdD, regAB, and toxA (9, 17, 22). As shown in Fig. 2, the 158-bp DNA sequence region preceding the psbA start codon is 68% identical to the corresponding PpvdA region (9). Both sequences contain regulatory motifs shared by ECF-dependent promoters, namely the ISB and the TCCTA element, which is also present in the corresponding region of algD and algU promoters of P. aeruginosa (18). It was also noticed that the psbA T2 and T4 transcripts correspond to the pvdA T1 and T2 transcripts, respectively. Interestingly, in Pseudomonas strain B10, the most abundant psbA transcript was T4, while, under the same experimental conditions, the major pvdA transcript was T1 (9). The alignment highlights the presence of a 14-nucleotide (nt) deletion and an 18-nt insertion at positions −137 and −49 upstream of the psbA start codon, respectively. Due to the presence of the 18-nt insertion in PpsbA, the T4 start point is located at a longer distance from the ISB compared with the T2 start point of pvdA (Fig. 2). This insertion is endowed with remarkable features, consisting of two heptameric direct repeats (TCAGGCC), followed by a third partially conserved repeat (cCAGGCt).
FIG. 2.
Structural analysis of the psbA promoter. (A) Alignment of the 158-nt region preceding the Pseudomonas strain B10 psbA start codon with the P. aeruginosa pvdA promoter. Identical nucleotides are capitalized and in boldface. The bent arrows indicate the minor (T1 and T2) and major (T3 and T4) transcription start sites of the psbA gene and the direction of transcription. The ISB and the TCCTA conserved motifs, which overlap the −35 and −10 regions, respectively, are underlined. The potential ribosome binding sites (RBS) and the transcriptional start codons (f-Met) of psbA and pvdA are underlined with dots. The locations of the conserved (solid arrows) and partially conserved (dotted arrow) direct repeats are indicated below the psbA sequence. Arrowheads below the pvdA sequence mark the positions of the 5′ ends of the pvdA T1 and T2 transcripts (9). (B) Localization of the transcriptional start sites of psbA. The asterisk indicates primer extension analysis of the psbA mRNA carried out with the 5′-end-labeled oligonucleotide PEpsbARV. Lanes T, C, G, and A represent sequencing ladders of pCAΔSh with the same oligonucleotide. These reactions were run in parallel with primer extension products to determine exactly the 5′ ends of the transcripts. The arrows on the left indicate the origins of psbA transcripts T1, T2, T3, and T4.
Activity of psbA and pvdA promoters in Pseudomonas strain B10 and P. aeruginosa.
The structural similarity between the PpsbA and PpvdA, combined with previous evidence that PsbS and PvdS are functionally interchangeable (8), led us to investigate promoter activity in Pseudomonas strain B10 and P. aeruginosa PAO1. For this purpose, the β-galactosidase levels expressed by pMP220::PpsbA and pMP220::PpvdA fusions were measured in P. aeruginosa PAO1 and Pseudomonas strain B10 cells grown under low- and high-iron conditions. Both promoters showed similar activities in iron-poor cultures of P. aeruginosa PAO1 (Table 2). Comparable levels of β-galactosidase (ca. 10,000 U) were also expressed by iron-limited cultures of Pseudomonas strain B10 carrying the homologous promoter fusion (pMP220::PpsbA), while a dramatic (>90%) reduction in reporter gene activity was observed for the heterologous pMP220::PpvdA fusion (Table 2). These results suggest that the transcriptional apparatus of Pseudomonas strain B10 discriminates between the homologous promoter (PpsbA) and the heterologous promoter (PpvdA), the latter being less efficiently recognized.
TABLE 2.
Activity of PpsbA::lacZ and PpvdA::lacZ transcriptional fusions in wild-type Pseudomonas strain B10 and P. aeruginosa PAO1 and in isogenic pyoverdine-defective mutants
| Strain | Plasmid | LacZ activity (Miller units)a
|
||
|---|---|---|---|---|
| −Fe(III) | +Fe(III) | −Fe(III) + pyoverdineB10 | ||
| Pseudomonas strain B10 | pMP220 | 38 | 31 | 40 |
| pMP220::PpsbA | 9,828 | 23 | 9,614 | |
| pMP220::PpvdA | 897 | 29 | 941 | |
| P. aeruginosa PAO1 | pMP220 | 58 | 50 | |
| pMP220::PpsbA | 10,015 | 54 | ||
| pMP220::PpvdA | 10,317 | 57 | ||
| B10CA1 | pMP220 | 42 | 35 | 36 |
| pMP220::PpsbA | 107 | 23 | 6,922 | |
| pMP220::PpvdA | 48 | 30 | 646 | |
| PALS124 | pMP220 | 62 | 55 | |
| pMP220::PpsbA | 9,624 | 51 | ||
| pMP220::PpvdA | 10,186 | 67 | ||
| PALS106 | pMP220 | 50 | 43 | |
| pMP220::PpsbA | 8,968 | 31 | ||
| pMP220::PpvdA | 9,075 | 57 | ||
| PALS125 | pMP220 | 47 | 50 | |
| pMP220::PpsbA | 9,920 | 39 | ||
| pMP220::PpvdA | 10,080 | 35 | ||
Determined in Pseudomonas culture lysates grown for 12 h in DCAA [−Fe(III)] and DCAA supplemented with 100 μM FeCl3 [+Fe(III)] (25). LacZ activity is represented by units of β-galactosidase as defined by Miller (11) and correspond to means of four determinations. Standard deviations are <16% of the given value.
PyoverdineB10 was purified from culture supernatants of Pseudomonas strain B10 according to a previously described procedure (26) and added at 50 μM.
Pyoverdine-dependent regulation of pvdA and psbA promoters.
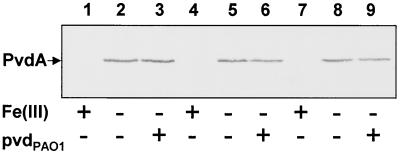
Preliminary reports from independent laboratories have related the down-regulation of pyoverdine promoters in pyoverdine biosynthetic mutants to pyoverdine-mediated induction of cognate siderophore genes (3, 23). On this basis, we compared the pyoverdine-regulated responses in wild-type Pseudomonas strain B10 and P. aeruginosa PAO1 and in their isogenic pyoverdine-defective derivatives, B10CA1 (psbA::cat) and PALS124 (pvdA) (2, 26). Under low-iron conditions, the activities of both PpsbA::lacZ and PpvdA::lacZ fusions were strongly reduced in Pseudomonas strain B10CA1 compared with those in the parental strain, and this effect was reversed by addition of 50 μM pyoverdineB10 to the culture medium (Table 2). In contrast, the activities of both promoter fusions were similar in P. aeruginosa PAO1 and in the PALS124 mutant. The pyoverdine-insensitive behavior of the PpvdA promoter was confirmed in P. aeruginosa mutants blocked at later steps of pyoverdine biogenesis, including PALS106 (pvdC1) and PALS125 (pvdC3) (Table 2). These results were supported by Western blot analysis of PvdA expression in the pvd mutants (Fig. 3). Under conditions of iron deficiency, comparable levels of PvdA were synthesized by P. aeruginosa PAO1, PALS106, and PALS125, irrespective of the presence of exogenous pyoverdinePAO1 in the culture medium (Fig. 3). As expected, addition of 100 μM FeCl3 to the iron-poor medium completely repressed PpvdA::lacZ activity (Table 2) and PvdA expression (Fig. 3). Therefore, it can be deduced that homologous pyoverdine is involved in the activation of the psbA promoter in the Pseudomonas strain B10 system, while it plays no apparent role in positive regulation of the pvdA biosynthesis gene in P. aeruginosa PAO1. This effect cannot be ascribed to pyoverdineB10-mediated induction of the psbS gene, since both pvdS::lacZ and psbS::lacZ promoter fusions (as in plasmids pMP220::PpvdS and pMP220::PpsbS) were found to be equally expressed and iron regulated in P. aeruginosa PAO1 and Pseudomonas strain B10 wild-type strains and in their l-Orn N5-oxygenase-defective mutants (data not shown).
FIG. 3.
Western blot analysis of iron-regulated PvdA expression in wild-type and pvd mutants of P. aeruginosa PAO1. Immunodetection of the 50-kDa PvdA protein in crude lysates (≅20 μg of proteins per lane) of exponentially grown bacteria (A600 ≅ 0.5) probed with an anti-PvdA mouse antiserum was performed as previously described (2). Cultures were performed in KB medium supplemented or not with either 100 μM FeCl3 [Fe(III)] or 50 μM pyoverdinePAO1 (pvdPAO1), as indicated below each lane. Lanes: 1 to 3, P. aeruginosa PAO1 (wild type); 4 to 6, PALS106 (pvdC1); 7 to 9, PALS125 (pvdC3). The position of PvdA is indicated on the left.
Conclusions.
The data just presented demonstrate that pyoverdineB10, as the end product of its biosynthesis pathway, is a key participant in the control circuit regulating its own production in Pseudomonas strain B10. In P. aeruginosa PAO1, however, extracellular pyoverdinePAO1 has no apparent role in the positive regulation of the homologous pvdA gene see Fig. 1 for a comparison between the two systems). The lack of pvdA response to exogenous pyoverdine is consistent with the observation that pvd mutants blocked in late steps of pyoverdinePAO1 biogenesis produce wild-type levels of hydroxamate nitrogen in iron-poor medium (26). This implies that PvdA is equally expressed in the pyoverdinePAO1-proficient and -deficient backgrounds, and immunoblot analysis of PvdA expression in the wild-type and pvd mutants corroborates this conclusion.
Despite the differences in individual activity between PpsbA and PpvdA, which are likely to reflect their structural diversity, both promoters were found to respond positively to homologous pyoverdine in the Pseudomonas strain B10 system, but not in P. aeruginosa. The mechanism by which pyoverdineB10 increases psbA expression does not involve up-regulation of psbS transcription, implying that sensing of pyoverdineB10 could result in posttranscriptional activation of the PsbS sigma factor. Siderophore-dependent induction has been elucidated in the pupIR-pupB system of P. putida WCS358. The Fur-controlled pupIR operon encodes a surface-signaling system. PupI is an ECF sigma factor required for the expression of pupB, encoding the receptor for heterologous pyoverdineBN8. PupR up-regulates pupB expression through activation of PupI in the presence of ferripyoverdineBN8, but it prevents the PupI-dependent transcription of pupB in its absence (7). Sequencing of the complete genomes of P. fluorescens and P. putida (available at http://www.jgi.doe.gov/and http://www.ncbi.nlm.nih.gov/, respectively) disclosed the presence in these species of multiple genes encoding putative PupR-like proteins. Whether a similar regulatory device also exists in Pseudomonas strain B10, it would account for modulation of PsbS activity through signaling of ferripyoverdineB10 binding to its outer membrane receptor (10). This would ensure up-regulation of psbA expression under conditions in which pyoverdineB10 is effective in delivering iron to the cell: namely, when ferripyoverdineB10 is engaged with its receptor.
Acknowledgments
This investigation was supported by grants from the Department of Biology, University of Roma Tre, and from the Italian Ministry of Health (targeted projects 1999 and 2000) to P.V.
REFERENCES
- 1.Abdallah, M. A. 1991. Pyoverdins and pseudobactins, p. 139-153. In G. Winkelman (ed.), CRC handbook of microbial iron chelates. CRC Press, Inc., Boca Raton, Fla.
- 2.Ambrosi, C., L. Leoni, L. Putignani, N. Orsi, and P. Visca. 2000. Pseudobactin biogenesis in the plant growth-promoting rhizobacterium Pseudomonas strain B10: identification and functional analysis of the l-ornithine N5-oxygenase (psbA) gene. J. Bacteriol. 182:6233-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callanan, M., R. Sexton, D. N. Dowling, and F. O'Gara. 1996. Regulation of the iron uptake genes in Pseudomonas fluorescens M114 by pseudobactin M114: the pbrA sigma factor does not mediate the siderophore regulatory response. FEMS Microbiol. Lett. 144:61-66. [DOI] [PubMed] [Google Scholar]
- 4.Cunliffe, H. E., T. R. Merriman, and I. L. Lamont. 1995. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa. PvdS is probably an alternative sigma factor. J. Bacteriol. 177:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escolar, L., J. Pérez-Martìn, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanine and fluorescin. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 7.Koster, M., W. van Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role of the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leoni, L., C. Ambrosi, A. Petrucca, and P. Visca. 2002. Transcriptional regulation of pseudobactin synthesis in the plant growth-promoting Pseudomonas B10. FEMS Microbiol. Lett. 208:219-225. [DOI] [PubMed] [Google Scholar]
- 9.Leoni, L., A. Ciervo, N. Orsi, and P. Visca. 1996. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J. Bacteriol. 178:2299-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magazin, M. D., J. C. Moores, and J. Leong. 1986. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J. Biol. Chem. 261:795-799. [PubMed] [Google Scholar]
- 11.Miller, J. H. 1972. Experiments in molecular genetics, p. 252-255. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Moores, J. C., M. Magazin, G. S. Ditta, and J. Leong. 1984. Cloning of genes involved in the biosynthesis of pseudobactin, a high-affinity iron transport agent of a plant growth-promoting Pseudomonas strain. J. Bacteriol. 157:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neilands, J. B., K. Konopka, B. Schwyn, M. Coy, R. T. Francis, B. H. Paw, and A. Bagg. 1987. Comparative biochemistry of microbial iron assimilation, p. 3-33. In G. Winkelmann, D. van der Helm, and J. B. Neilands (ed.), Iron transport in microbes, plants and animals. VCH Verlagsgesellschaft mbH, Weinheim, Germany.
- 14.O'Sullivan, D. J., and F. O'Gara. 1992. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 56:662-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 16.Reimmann, C., L. Serino, M. Beyeler, and D. Haas. 1998. Dihydroaeruginoic acid synthetase and pyochelin synthetase, products of the pchEF genes, are induced by extracellular pyochelin in Pseudomonas aeruginosa. Microbiology 144:3135-3148. [DOI] [PubMed] [Google Scholar]
- 17.Rombel, I. T., B. J. McMorran, and I. L. Lamont. 1994. Identification of a DNA sequence motif required for expression of iron-regulated genes in pseudomonads. Mol. Gen. Genet. 246:519-528. [DOI] [PubMed] [Google Scholar]
- 18.Schurr, M. J., H. Yu, M. Martinez-Salazar, N. S. Hibler, and V. Deretic. 1995. Biochemical characterization and posttranslational modification of AlgU, a regulator of stress response in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 216:874-880. [DOI] [PubMed] [Google Scholar]
- 19.Schweizer, H. P. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109-112. [DOI] [PubMed] [Google Scholar]
- 20.Sexton, R., P. R. Gill, Jr., M. J. Callanan, D. J. O'Sullivan, D. N. Dowling, and F. O'Gara. 1995. Iron-responsive gene expression in Pseudomonas fluorescens M114: cloning and characterization of a transcription-activating factor, PbrA. Mol. Microbiol. 15:297-306. [DOI] [PubMed] [Google Scholar]
- 21.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 22.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 23.Venturi, V., C. Ottewanger, M. Bracke, and P. J. Weisbeek. 1995. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol. Microbiol. 15:1081-1093. [DOI] [PubMed] [Google Scholar]
- 24.Venturi, V., P. Weisbeek, and M. Koster. 1995. Gene regulation of siderophore mediated iron acquisition in Pseudomonas: not only the Fur repressor. Mol. Microbiol. 17:603-610. [DOI] [PubMed] [Google Scholar]
- 25.Visca, P., G. Colotti, L. Serino, D. Verzili, N. Orsi, and E. Chiancone. 1992. Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Appl. Environ. Microbiol. 58:2886-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visca, P., L. Serino, and N. Orsi. 1992. Isolation and characterization of Pseudomonas aeruginosa mutants blocked in the synthesis of pyoverdin. J. Bacteriol. 174:5727-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson, M. J., B. J. McMorran, and I. L. Lamont. 2001. Analysis of promoters recognized by PvdS, an extracytoplasmic-function sigma factor protein from Pseudomonas aeruginosa. J. Bacteriol. 183:2151-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]