Abstract
A pathway was metabolically engineered to produce poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), a biodegradable thermoplastic with proven commercial applications, from a single, unrelated carbon source. An expression system was developed in which a prpC strain of Salmonella enterica serovar Typhimurium, with a mutation in the ability to metabolize propionyl coenzyme A (propionyl-CoA), served as the host for a plasmid harboring the Acinetobacter polyhydroxyalkanoate synthesis operon (phaBCA) and a second plasmid with the Escherichia coli sbm and ygfG genes under an independent promoter. The sbm and ygfG genes encode a novel (2R)-methylmalonyl-CoA mutase and a (2R)-methylmalonyl-CoA decarboxylase, respectively, which convert succinyl-CoA, derived from the tricarboxylic acid cycle, to propionyl-CoA, an essential precursor of 3-hydroxyvalerate (HV). The S. enterica system accumulated PHBV with significant HV incorporation when the organism was grown aerobically with glycerol as the sole carbon source. It was possible to vary the average HV fraction in the copolymer by adjusting the arabinose or cyanocobalamin (precursor of coenzyme B12) concentration in the medium.
Polyhydroxyalkanoate (PHA) biopolymers are widespread among microbes and are used for storage of carbon and reducing equivalents in intracellular granules (45). These natural polyesters have attracted considerable attention because they have properties similar to those of common thermoplastics or elastomers, depending on the monomeric composition. PHA bioplastics, which are biodegradable and made from renewable resources, offer an exciting alternative to petrochemical-derived plastics.
Biopol [poly(3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV)], a copolymer of monomers of 3-hydroxybutyrate (HB) and 3-hydroxyvalerate (HV), is a biodegradable PHA thermoplastic that was produced by Imperial Chemical Industries, Zeneca Bio Products, and then Monsanto (3, 7, 33). The latter company terminated production at the end of 1998 because of high production costs. Biodegradable shampoo bottles, razors, and food trays were made out of this material, and a number of other products were in development (8).
PHBV is synthesized from its precursors, acetyl coenzyme A (acetyl-CoA) and propionyl-CoA, by three enzymes (58) (Fig. 1). The first step involves either a condensation of two molecules of acetyl-CoA or a condensation of acetyl-CoA and propionyl-CoA by a 3-ketothiolase encoded by phaA. The resulting intermediate, either acetoacetyl-CoA or 3-ketovaleryl-CoA, is reduced by the phaB gene product, an NADPH-dependent acetoacetyl-CoA reductase. This reaction generates 3-hydroxybutyryl-CoA and 3-hydroxyvaleryl-CoA, which are incorporated into the growing polymer chain by PHA synthase or polymerase, encoded by phaC, as four- and five-carbon monomers, respectively.
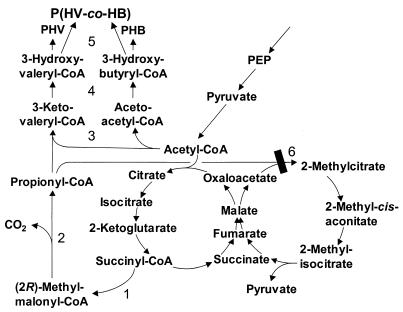
FIG. 1.
Novel metabolic pathway for PHBV production from an unrelated carbon source. 1, Sbm; 2, methylmalonyl-CoA decarboxylase (YgfG); 3, 3-ketothiolase (PhaA); 4, acetoacetyl-CoA reductase (PhaB); 5, PHA synthase (PhaC); 6, 2-methylcitric acid synthase (PrpC). The citric acid cycle (left) and the 2-methylcitric acid cycle (right), which compete for acetyl-CoA and for propionyl-CoA, respectively, are shown. The 2-methylcitric acid cycle is blocked in the expression system described in this paper, as indicated. PHV, poly(3-hydroxyvalerate); PEP, phosphoenolpyruvate.
When PHBV was produced industrially, glucose and propionate were supplied to a fed-batch fermentation of Ralstonia eutropha, a natural PHA accumulator. Adjusting the ratio of these carbon sources in the feed controlled the copolymer composition (9). A major factor in the prohibitively high price of Biopol was that propionate, which was activated to form the propionyl-CoA precursor of HV, is expensive to produce industrially and is considerably more costly than glucose (45). A more economical alternative is to produce propionyl-CoA from an inexpensive, unrelated carbon source.
Many organisms produce propionate (and propionyl-CoA) by the fermentation of a variety of organic compounds (56). In the well-studied methylmalonyl-CoA pathway, succinyl-CoA is an important intermediate. The conversion of succinyl-CoA to (2R)-methylmalonyl-CoA proceeds through the action of coenzyme B12-dependent methylmalonyl-CoA mutase (20). Next, (2R)-methylmalonyl-CoA is converted to (2S)-methylmalonyl-CoA via methylmalonyl-CoA epimerase. To convert (2S)-methylmalonyl-CoA to propionyl-CoA, two alternative enzymes are involved. For example, in Propionigenium modestum, a biotin-dependent, sodium ion-translocating methylmalonyl-CoA decarboxylase complex converts (2S)-methylmalonyl-CoA to propionyl-CoA and uses the energy of decarboxylation to transport sodium (14). In Propionibacterium shermanii, a biotin-dependent transcarboxylase complex catalyzes the reversible transfer of a carboxylate group from (2S)-methylmalonyl-CoA to pyruvate, generating propionyl-CoA and oxaloacetate (68).
A novel pathway which offers original conversion of succinyl-CoA to propionyl-CoA for recombinant PHBV production was discovered (Fig. 1). A previously unelucidated Escherichia coli operon (sbm-ygfD-ygfG-ygfH), encoding a pathway for the conversion of succinate to propionate, was discovered by conducting a sequence analysis of members of a superfamily of crotonases (enoyl-CoA hydratases) encoded on the E. coli genome (18). Sleeping beauty mutase (Sbm) catalyzes the coenzyme B12-dependent conversion of succinyl-CoA to (2R)-methylmalonyl-CoA. The next step in the pathway involves a novel methylmalonyl-CoA decarboxylase (YgfG), which catalyzes the conversion of (2R)-methylmalonyl-CoA to propionyl-CoA. This activity had never been reported before [the previously studied decarboxylase and transcarboxylase act on (2S)-methylmalonyl-CoA], and it is not biotin dependent. Finally, propionyl-CoA is converted back to succinyl-CoA by propionyl-CoA:succinyl-CoA transferase (YgfH). There is also a fourth gene in the operon, ygfD, located between sbm and ygfG, which encodes a putative protein kinase. This enzyme may be a regulatory enzyme, but it has not been thoroughly investigated. Interestingly, the genome of Salmonella enterica serovar Typhimurium, a close relative of E. coli, does not contain genes homologous to the genes in the operon (37).
The 2-methylcitric acid cycle (Fig. 1) is responsible for the catabolism of propionate as a sole carbon and energy source in many gram-negative bacteria (5, 6, 32, 60) and has been studied extensively in S. enterica (23-28, 32, 43, 62). It has been proposed that this cycle is a detoxification pathway for propionyl-CoA (23).
In an analogous manner to which citrate synthase condenses a molecule of acetyl-CoA and oxaloacetate to make citrate for the citric acid (tricarboxylic acid [TCA]) cycle, 2-methylcitrate synthase condenses a molecule of propionyl-CoA and oxaloacetate to synthesize 2-methylcitrate for the 2-methylcitric acid cycle (Fig. 1). These two cycles compete for the acetyl-CoA and propionyl-CoA precursors, respectively, necessary for PHBV production.
A mutant strain of Burkholderia sacchari, defective in prpC, accumulated a copolyester with a higher HV content than that of its parent when it was fed propionate (precursor of propionyl-CoA), presumably because of decreased propionyl-CoA catabolism in the mutant strain (5, 52). The prpC mutation should also eliminate competition for propionyl-CoA derived from an unrelated substrate, and this could be manipulated for recombinant PHBV production. Therefore, a well-characterized S. enterica prpC mutant (62) was chosen as the host strain for this study. Like E. coli, S. enterica does not naturally accumulate PHA, and it has a number of advantages as a recombinant PHBV producer (1, 31). Furthermore, preliminary results indicated that S. enterica is a better host for reconstituting Sbm and YgfG activities than E. coli.
In this paper, we describe reconstitution of the novel propionyl-CoA biosynthesis pathway (Sbm and YgfG activities) in a recombinant strain of S. enterica that has a mutation in prpC and harbors the Acinetobacter PHA synthesis operon (phaBCA). This heterologous pathway converts succinyl-CoA to propionyl-CoA, the essential precursor of HV in PHBV, without addition of propionate to the culture medium.
MATERIALS AND METHODS
Bacterial strains and plasmids, culture media, and growth conditions.
The E. coli and S. enterica strains and plasmids used are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara,leu)7697 galU galK λ−rpsL nupG | Gibco |
| Salmonella enterica serovar Typhimurium JR501 | hsdSA29 hsdSB121 hsdL6 trpC2 metA22 metE551 ilv452 leu3121 rpsL120 galE719 xyl-404 H1-b H2-e,n,x nml (Fels2)−fla-66 | 61 |
| LT2 derivatives | ||
| TR6583 | metE205 ara-9 | 62 |
| JE4199 | metE205 ara-9 prpC::MudJ | 62 |
| Plasmids | ||
| pBAD24 | Apr, PBAD, araC, pBR322 ori | 17 |
| pMMB206 | Cmr, PtaclacUV5, lacIq, RSF1010 ori | 39 |
| pTrc99A | Apr, Ptrc, lacIq, pBR322 ori | Amersham Pharmacia |
| pBAD-Ae-pha | Apr, R. eutropha PHA synthesis operon (phaCAB) in pBAD24 | 1 |
| pISA1 | Apr, Acinetobacter sp. strain RA3849 PHA operon (phaBCA) in pBAD24 | 1 |
| pMMB-pha | Cmr, Acinetobacter PHA synthesis operon from pISA1 in pMMB206 | This study |
| pTrc-Cro | Apr, operon including sbm, ygfD, and ygfG in pTrc99A | This study |
| pBAD-Cro | Apr, operon including sbm, ygfD, and ygfG in pBAD24 | This study |
To construct pBAD-Cro, a 4.4-kb fragment including sbm, ygfD, and ygfG was amplified by PCR from E. coli W3110 genomic DNA, which had been extracted with a genomic DNA extraction kit (Qiagen). The forward primer 5′-TGA ACT GAT TGA CTT AAC GCC-3′ and the reverse primer 5′-AAA GCC GCT AAA TGC CAC-3′ were designed by using GCG SeqWeb Primer Selection. The product was first cloned into the EcoRV site of pBluescriptII SK+ (Stratagene) in a blunt-end ligation. Next, the 3.9-kb operon was PCR amplified from the clone by using forward primer 5′-G GAA TTC ATG TCT AAC GTG CAG GAG TGG C-3′ and reverse primer 5′-CTC TAG ATT AAT GAC CAA CGA AAT TAG GTT TAC-3′. These primers contained appropriate restriction sites for cloning and facilitated the ligation of the operon as an EcoRI-XbaI fragment into pTrc99A to construct pTrc-Cro. The operon was subsequently subcloned from pTrc-Cro as an NcoI-XbaI fragment into pBAD24 to form pBAD-Cro.
In both cases, the PCR was conducted with Platinum Pfx DNA polymerase (Invitrogen) by using a model PTC-200 thermal cycler (MJ Research). Samples were incubated at 94°C for 2 min (for initial denaturation), and this was followed by 25 cycles of denaturing at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 68°C for 5 min. There was a final extension period of 7 min at 68°C.
pMMB-pha was constructed by subcloning a 4.6-kb EcoRI-SalI fragment, harboring the Acinetobacter PHA synthesis operon from pISA1 (1), into vector pMMB206 (39).
All cloning steps were performed by using standard procedures (49). In most cases plasmid preparation was performed by using kits from Qiagen; in the case of preparations involving pMMB206-derived plasmids, boiling lysis was used to facilitate restriction digestion. Electromax E. coli DH10B (Invitrogen) was used to screen for correct clones, and enzymes and molecular biology reagents were purchased from Roche and Invitrogen, respectively.
Transformation into S. enterica was performed by using electroporation, after cells were made electrocompetent with a series of washes in 10% glycerol (49). Plasmids were first transformed into the hsdSB121 (restriction-negative and modification-positive) strain JR501 and then isolated and transformed into the desired S. enterica strain.
PHBV accumulation.
PHA synthesis experiments were performed in morpholinopropanesulfonate (MOPS)-buffered medium (40) with 274 mM glycerol under aerobic conditions by using the method described previously (1). Propionic acid (8 mM) was added in experiments involving exogenous propionate but was omitted in experiments involving pMMB-pha and pBAD-Cro, which tested the propionate-independent pathway for HV accumulation. Expression of the plasmid-encoded genes was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) and arabinose when the culture reached an optical density at 600 nm (OD600) of 0.15. For the experiments in which plasmid-encoded genes were fully induced, 0.1% arabinose and 500 μM IPTG were added. For the arabinose induction study, a 200-ml culture was cultivated in a 1-liter shake flask, induced with 500 μM IPTG, and then split into 40-ml cultures in 250-ml flasks with different concentrations of arabinose, ranging from 0 to 0.1%. When other medium components were added, their final concentrations were 0.1% succinate, 0.2 mM methionine, 100 μg of ampicillin per ml, and 50 μg of chloramphenicol per ml. Exogenous cyanocobalamin (CN-B12) (Sigma), a precursor of coenzyme B12, was added, in general, at a concentration of 1 μM from a 1-mg/ml stock solution, which was stored in a plastic container in the dark. The CN-B12 concentration was varied for the coenzyme B12 dependence study.
For the studies involving external propionate, in general, experiments were performed in triplicate, and the averages and standard deviations of the results are presented below. The other data reported below are representative of trends observed in repeated experiments.
PHBV analysis.
Following a 40-h PHA synthesis phase, cells were harvested by centrifugation, washed, and dried as previously described (1). PHBV was quantified following propanolysis by using a Varian 3800 gas chromatograph with a Silco-steel-packed column (custom packing was with liquid-phase 10% RT-1000 on a solid support of Chromosorb W-AW [Restek Corporation]) (1, 47). PHBV copolymer (Aldrich) was used to generate a standard curve. The polymer content was defined as the ratio of PHA mass to dry cell mass (DCW) in a given sample, expressed as a percentage. The HV fraction was defined as the ratio of HV to HV plus HB in the copolymer, expressed in mole percent.
RESULTS
Propionate-dependent PHBV synthesis in JE4199, an S. enterica prpC mutant.
S. enterica JE4199 harboring pISA1 clearly showed enhanced HV incorporation in the copolymer compared to the incorporation in the parent when propionate was added to the medium, indicating that when the 2-methylcitric acid cycle was blocked, more propionyl-CoA was shunted to HV biosynthesis (Table 2). In these experiments, the parent (TR6583) harboring the R. eutropha PHA synthesis operon yielded an HV fraction considerably lower than the fraction when it harbored the Acinetobacter operon. However, the HV fraction when the R. eutropha operon was used markedly increased when the operon was introduced into the prpC mutant host, JE4199. The total polymer content varied from run to run for a given expression system, while the molar fraction of HV remained relatively constant, so the HV accumulation effects were very reproducible.
TABLE 2.
PHBV production in S. enterica strains, demonstrating the effects of prpC mutation and Acinetobacter and R. eutropha PHA synthesis constructsa
| Plasmid | Host | Total polymer (% DCW) | PHBV (% DCW) | HV in total polymer (mol%) |
|---|---|---|---|---|
| pISA1 | S. enterica serovar Typhimurium TR6583 | 40.7 ± 18.0 | 34.2 ± 15 | 14.2 ± 1.4 |
| S. enterica serovar Typhimurium JE4199 | 41.4 ± 9.9 | 27.0 ± 8.0 | 30.6 ± 1.9 | |
| pBAD-Ae-pha | S. enterica serovar Typhimurium TR6583 | 32.2 ± 6.6 | 30.3 ± 7.5 | 5.6 ± 3.7 |
| S. enterica serovar Typhimurium JE4199 | 24.1 ± 8.1 | 18.2 ± 6.0 | 21.7 ± 0.7 |
Cells were grown in MOPS-buffered minimal medium containing 274 mM glycerol and 8 mM propionic acid. Expression of the plasmid-encoded genes was fully induced with 0.1% arabinose when the cell density reached an OD600 of 0.15. The values are means ± standard errors for triplicate experiments.
With its advantageous behavior established for propionate-dependent PHBV biosynthesis, JE4199 harboring the Acinetobacter PHA synthesis operon was used as a host for the propionate-independent pathway involving Sbm and YgfG, encoded on pBAD-Cro. pMMB-pha was used instead of pISA1 in order to provide a PHA synthesis plasmid compatible with pBAD-Cro. The Acinetobacter operon cloned into pMMB206 showed the same advantageous behavior, relative to the behavior of the R. eutropha operon cloned into this vector, as when pBAD24 was used (data not shown).
HV formation in JE4199 harboring pMMB-pha and pBAD-Cro.
Initial experiments with pBAD-Cro were conducted in MOPS medium with glycerol and succinate (precursor of succinyl-CoA) in order to provide optimal conditions for succinyl-CoA production and thus HV incorporation. JE4199 harboring pMMB-pha and pBAD-Cro accumulated significant amounts of HV compared to the amounts accumulated byJE4199 harboring pMMB-pha and pBAD24, a vector control for pBAD-Cro (16.2 mol% HV in total polymer that accounted for 30.8% DCW versus no HV in total polymer that accounted for 29% DCW).
Induction study.
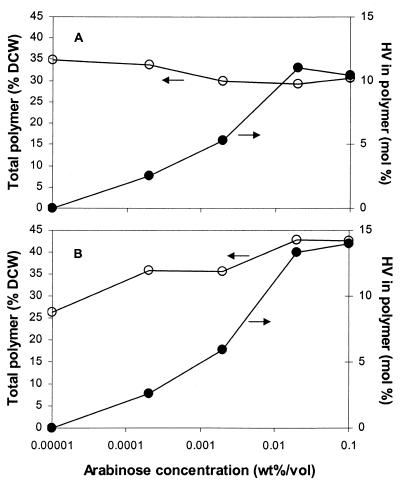
The induction study (Fig. 2) showed that both with and without exogenous succinate, HV incorporation is dependent on pBAD-Cro expression. Increasing the level of arabinose between 0 and 0.02% led to increased HV fractions in the copolymer, while the total polymer level was not adversely affected. Apparently, at arabinose concentrations above 0.02%, expression becomes saturated or there is a bottleneck in the pathway, and further addition of inducer does not lead to significantly more HV incorporation. Pellets resulting from centrifugation of cultures expressing pBAD-Cro were pink (data not shown), and as the arabinose concentration was increased, the color darkened, presumably due to more incorporation of coenzyme B12 in Sbm. The induction study shows that the operon harboring the genes encoding Sbm and YgfG was removed successfully from native control, which involves a putative σ70 promoter (GenBank accession number AE000375). Surprisingly, succinate supplementation did not enhance propionyl-CoA formation, and a high HV fraction could be obtained with glycerol alone.
FIG. 2.
Variation of total polymer content (○) and polymer composition (•) in JE4199 harboring pMMB-pha and pBAD-Cro with arabinose induction. Cells were grown in MOPS-buffered minimal medium containing 274 mM glycerol with 0.1% succinate (A) and without succinate (B). Expression of the plasmid-encoded genes was induced with 500 μM IPTG and different arabinose concentrations when the cell density reached an OD600 of 0.15. The points obtained when no arabinose was added are plotted at 0.00001% (wt/vol) arabinose because zero arabinose could not be plotted on the logarithmic scale.
Vitamin B12 dependence.
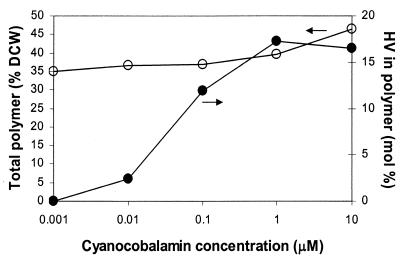
The vitamin B12 dependence study (Fig. 3) demonstrated that HV incorporation is dependent on CN-B12 supplementation of the medium and that it is saturated at CN-B12 concentrations above 1 μM. As observed with arabinose additions, the cell pellets became a darker pink as more CN-B12 was added.
FIG. 3.
Variation of total polymer content (○) and polymer composition (•) in JE4199 harboring pMMB-pha and pBAD-Cro with external CN-B12 (precursor of coenzyme B12). Cells were grown in MOPS-buffered minimal medium containing 274 mM glycerol. Expression of the plasmid-encoded genes was induced with 500 μM IPTG and 0.1% arabinose when the cell density reached an OD600 of 0.15.
DISCUSSION
In this study, endogenous propionyl-CoA catabolism was blocked by using JE4199, a strain of S. enterica with a mutation in prpC, as a host for PHBV biosynthesis. This was very effective for shunting propionyl-CoA to HV (Table 2) and was consistent with results obtained with B. sacchari IPT101T producing PHBV (5) and with recombinant E. coli producing polyketides that require propionyl-CoA (13, 44).
The Acinetobacter PHA synthesis operon (phaBCA) produced a higher HV fraction than the R. eutropha operon (phaCAB) in a separate study (1), but no clear explanation was provided. PHA synthesis experiments with JE4199 and its parent (TR6583) harboring the R. eutropha operon indicated that a larger intracellular pool of propionyl-CoA leads to a significantly higher HV fraction in the copolymer (Table 2). The affinities for propionyl-CoA of the two operon-encoded ketothiolases (PhaA), which catalyze the condensation of propionyl-CoA and acetyl-CoA in PHBV biosynthesis, may differ. It would be interesting to determine whether the in vivo Km of R. eutropha PhaA is higher than that of Acinetobacter PhaA.
Based on the evidence presented here, it seems fairly clear that succinyl-CoA from the TCA cycle was converted to propionyl-CoA for HV production (Fig. 2 and 3). However, it is slightly counterintuitive that this strategy for producing propionyl-CoA works in S. enterica. First, propionyl-CoA is a competitive inhibitor of E. coli citrate synthase (35), and thus, feedback inhibition of synthesis of this critical precursor was a possibility. Inhibiting citrate synthase with propionyl-CoA would decrease flux through the TCA cycle for succinyl-CoA formation, limiting the pool of this intermediate available for the conversion of succinyl-CoA to propionyl-CoA. Second, both HB biosynthesis and HV biosynthesis require acetyl-CoA, which also feeds the TCA cycle, and there is competition among these pathways for the central metabolic intermediate.
Perhaps S. enterica citrate synthase behaves differently than E. coli citrate synthase, although this seems unlikely. Also, possibly because pMMB-pha is a low-copy-number plasmid, the PHA biosynthetic enzymes encoded on it do not drain all the acetyl-CoA from the TCA cycle, and sufficient succinyl-CoA is produced to make propionyl-CoA.
In a previous study, succinyl-CoA, derived from glucose, was converted to 4-hydroxybutyrate for poly(3-hydroxybutyrate-co-4-hydroxybutyrate) production in recombinant E. coli harboring the R. eutropha PHA operon and the Clostridium kluyveri succinate degradation genes (65). Thus, the TCA cycle has proven to be a viable source of abundant precursors for recombinant PHA copolymer production despite the competition of citrate synthase with the PHA biosynthesis enzymes for acetyl-CoA. Furthermore, the pathway from succinyl-CoA to propionyl-CoA is thought to function in a number of natural PHBV accumulators (30, 42, 50, 64, 67).
The functions of Sbm and YgfG were identified in vitro by purifying the relevant proteins, but initial attempts to find physiological conditions that yielded in vivo enzyme activity were unsuccessful (18). Based on the HV accumulation reported here, in vivo activity of each of these enzymes under aerobic conditions is inferred. The role (if any) of the enzyme encoded by ygfD is unclear, but its expression was not necessary for HV incorporation in the copolymer (data not shown). Interestingly, previous in vitro studies indicated that Sbm functions better with the N-terminal His tag used for its purification, as after cleavage the mutase activity was less than 7% of that measured with the tag present (18). However, the native form of the enzyme is active in vivo in our expression system, as it was in an expression system recently developed for recombinant polyketide production in E. coli (13).
HV incorporation was dependent on external CN-B12, which is consistent with the activity of a coenzyme B12-dependent methylmalonyl-CoA mutase (Fig. 3). In previous work, CN-B12 was nonlimiting at concentrations above ∼35 nM for coenzyme B12-dependent growth of S. enterica on (1,2)-propanediol (19) and at concentrations above 10 nM for the production of (1,3)-propanediol with a plasmid-encoded, coenzyme B12-dependent glycerol dehydratase in recombinant E. coli (53). However, apparently, more coenzyme B12 is needed for Sbm in this expression system.
The physical and mechanical properties of PHBV depend strongly on the HV fraction in the copolymer. Therefore, to obtain plastics suitable for different applications, it is critical to be able to control copolymer composition (12). This is difficult when PHBV is made from a single carbon source (42), as varying the external carbon concentration affects both HB and HV production. A “dial-a-composition” system was described previously that decoupled exogenous propionate concentration from copolymer composition (1). The arabinose induction studies (Fig. 2) indicated that a similar dial-a-composition system is possible with the controlled expression of sbm-ygfD-ygfG at a fixed carbon concentration. However, the present two-plasmid system likely suffers from the all-or-none phenomenon associated with the arabinose promoter (51) and could not be used to obtain a homogenous copolymer (1). Interestingly, the vitamin B12 dependence study suggested that controlled CN-B12 addition is another way to adjust copolymer composition, although this would clearly be uneconomical.
While methylmalonyl-CoA mutase activity was detected in cell extracts of Rhodococcus ruber, the enzyme responsible for conversion of methylmalonyl-CoA to propionyl-CoA for the production of PHBV from an unrelated carbon source in this organism was not identified in a previous study (67); neither methylmalonyl-CoA decarboxylase nor methylmalonyl-CoA:oxaloacetate transcarboxylase activity was detected. Also, a methylmalonyl-CoA mutase was implicated in HV production in Nocardia corallina when mutB strains accumulated copolymers with insignificant amounts of HV compared to the amounts in the wild-type strain (64), but the enzyme(s) responsible for converting methylmalonyl-CoA to propionyl-CoA was not identified. A YgfG homolog may catalyze propionyl-CoA formation in these microbes, and perhaps we have reconstituted a pathway present in organisms that naturally make PHBV from unrelated carbon sources. Unfortunately, the genomes of R. ruber and N. corallina have not been sequenced, and therefore, the Basic Local Alignment Search Tool (BLAST) (2) cannot be used to confirm whether such a homolog is found in these bacteria.
S. enterica may be an excellent host for recombinant PHBV production (although its Biosafety Class 2 designation is a major practical disadvantage). In this study, CN-B12 was added to the medium to provide the essential precursor of coenzyme B12. However, while E. coli cannot synthesize vitamin B12, S. enterica is able to generate this cofactor de novo under anaerobic conditions (29, 48). Interestingly, anaerobiosis should also generate succinyl-CoA fermentatively through the reverse TCA cycle (41), and much of the strain engineering for the bio-based overproduction of succinate in E. coli (10, 21, 22, 38, 59, 66) could be applied to propionyl-CoA synthesis of PHBV in S. enterica.
Both the coenzyme B12 biosynthetic pathway and the novel pathway from succinyl-CoA to propionyl-CoA should function in a recombinant E. coli host. Such an organism, harboring the S. enterica vitamin B12 biosynthetic cluster, produced the cofactor under anaerobic conditions (46). However, trials involving CN-B12 addition with E. coli DH10B harboring pBAD-Cro and pMMB-pha yielded insignificant HV incorporation, in contrast to experiments performed with S. enterica TR6583 (data not shown). This hurdle must be overcome to generate an analogous E. coli expression system.
There has been considerable work towards synthesizing PHBV in planta, a plan which could produce large volumes of PHAs at low cost from carbon dioxide and sunlight (55). This has involved converting intracellular threonine to propionyl-CoA, another strategy used to produce HV from unrelated carbon sources in natural and recombinant microbes (16, 57, 63). However, when a research group at Monsanto moved this pathway into Arabidopsis thaliana leaves and Brassica napus seeds in a complex feat of plant metabolic engineering, the total copolymer content reached only 3% DCW (54), far below the 15% DCW deemed sufficient to make extraction and processing economical (63). The pathway from succinyl-CoA to propionyl-CoA probably is inappropriate for transgenic plants because these organisms neither synthesize nor use vitamin B12 in their metabolism (15, 48; http://www.nature.com/nbt/journal/v17/n10/suppinfo/nbt1099_1011_S1.html).
Copolymer composition would be difficult to control in plants, however, and is best manipulated in a microbial system. To make such production economical, inexpensive substrates must be used. Glycerol is more expensive than glucose, but it was used here to avoid catabolite repression of the PBAD promoter by glucose (17). Presumably, succinyl-CoA derived from glucose could also be used for HV production, and probably the PHA yield would be higher with this substrate. Furthermore, the reconstitution of this new pathway is a major step towards making PHBV with a desired composition and thus specific properties using recombinant microbes fed an inexpensive, carbon-laden waste stream (e.g., effluent from a food-processing plant). If PHBV were made from an abundant TCA cycle intermediate and copolymer composition were controlled genetically, the carbon composition of such a stream would not have to be tightly specified.
A direct comparison of the utility of the pathway from succinyl-CoA to propionyl-CoA with that of the pathway from threonine to propionyl-CoA for making PHBV from an unrelated carbon source in a recombinant microbe is difficult. An early study of the threonine pathway in recombinant E. coli yielded a very low HV fraction unless exogenous valine was added (16). A later study did not report total polymer values and indicated that threonine supplementation was necessary for high HV fractions (63).
1H nuclear magnetic resonance indicated that the extracted copolymer produced in this study was composed of HB and HV units and was similar to that produced by Monsanto in plants (63) (data not shown). Furthermore, the HV fractions obtained were similar to those used in Biopol (0 to 20%) (34). An important next step is to develop fed-batch culture methods for high-level PHBV production that use the pathway from succinyl-CoA to propionyl-CoA, like the methods involving exogenous propionate that were established for recombinant E. coli producing PHBV (11). As described previously, constitutive promoters of different strengths could be used instead of expensive inducers to create strains capable of producing PHBV copolymers with desired compositions at low cost (1).
In addition, the novel pathway for propionyl-CoA synthesis from an unrelated substrate is exciting because of its broad application. The pathway from succinyl-CoA to propionyl-CoA is useful not only for PHBV copolymer production but also for recombinant polyketide antibiotic biosynthesis, which utilizes propionyl-CoA and (2S)-methylmalonyl-CoA as critical precursors. By using Sbm and YgfG, as well as methylmalonyl-CoA epimerase (to convert the R form to the S form), whose cloning from a number of organisms was recently reported (4, 13, 18, 36), both these polyketide precursors may be synthesized from a simple, low-cost carbon source (13).
Acknowledgments
We thank Alexander Horswill and Jorge Escalante-Semerena for the generous gift of the S. enterica strains.
This work was supported by grants BES-9912472, BES-9612840, and BES-9502495 from the National Science Foundation.
REFERENCES
- 1.Aldor, I., and J. D. Keasling. 2001. Metabolic engineering of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonella enterica serovar Typhimurium. Biotechnol. Bioeng. 76:108-114. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1999. End of Biopol. Chem. Br. 35:8. [Google Scholar]
- 4.Bobik, T. A., and M. E. Rasche. 2001. Identification of the human methylmalonyl-CoA racemase gene based on the analysis of prokaryotic gene arrangements. J. Biol. Chem. 276:37194-37198. [DOI] [PubMed] [Google Scholar]
- 5.Bramer, C. O., L. F. Silva, J. G. Gomez, H. Pierfert, and A. Steinbuchel. 2002. Identification of the 2-methylcitrate pathway involved in the catabolism of propionate in the polyhydroxyalkanoate-producing strain Burkholderia sacchari IPT101T and analysis of a mutant accumulating a copolyester with higher 3-hydroxyvalerate content. Appl. Environ. Microbiol. 68:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramer, C. O., and A. Steinbuchel. 2001. The methylcitric acid pathway in Ralstonia eutropha: new genes identified involved in propionate metabolism. Microbiology 147:2203-2214. [DOI] [PubMed] [Google Scholar]
- 7.Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. [DOI] [PubMed] [Google Scholar]
- 8.Byrom, D. 1994. Polyhydroxyalkanoates, p. 5-33. In D. P. Mobley (ed.), Plastics from microbes: microbial synthesis of polymers and polymer precursors. Hanser/Gardner Publications, New York, N.Y.
- 9.Byrom, D. 1987. Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol. 5:246-250. [Google Scholar]
- 10.Chatterjee, R., C. S. Millard, K. Champion, D. P. Clark, and M. I. Donnelly. 2001. Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl. Environ. Microbiol. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, J., and S. Y. Lee. 1999. High-level production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by fed-batch culture of recombinant Escherichia coli. Appl. Environ. Microbiol. 65:4363-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, M. K. 1994. Properties and applications of polyhydroxyalkanoates, p. 120-135. In Y. Doi and K. Fukuda (ed.), Biodegradable plastics and polymers. Proceedings of the 3rd International Scientific Workshop on Biodegradable Plastics and Polymers. Elsevier Science, Amsterdam, The Netherlands.
- 13.Dayem, L. C., J. R. Carney, D. V. Santi, B. A. Pfeifer, C. Khosla, and J. T. Kealey. 2002. Metabolic engineering of a methylmalonyl-CoA mutase-epimerase pathway for complex polyketide biosynthesis in Escherichia coli. Biochemistry 41:5193-5201. [DOI] [PubMed] [Google Scholar]
- 14.Dimroth, P., and B. Schink. 1998. Energy conservation in the decarboxylation of dicarboxylic acid by fermenting bacteria. Arch. Microbiol. 170:69-77. [DOI] [PubMed] [Google Scholar]
- 15.Duda, J., Z. Pedziwilk, and K. Zodrow. 1967. Studies on the vitamin B12 content of the leguminous plants. Acta Microbiol. Pol. 6:233-238. [PubMed] [Google Scholar]
- 16.Eschenlauer, A. C., S. K. Stoup, F. Srienc, and D. A. Somers. 1996. Production of heteropolymeric polyhydroxyalkanoate in Escherichia coli from a single carbon source. Int. J. Biol. Macromol. 19:121-130. [DOI] [PubMed] [Google Scholar]
- 17.Guzman, L., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller, T., T. Buckel, J. Retey, and J. A. Gerlt. 2000. Discovering new enzymes and metabolic pathways: conversion of succinate to propionate by Escherichia coli. Biochemistry 39:4622-4629. [DOI] [PubMed] [Google Scholar]
- 19.Havemann, G. D., E. M. Sampson, and T. A. Bobik. 2002. PduA is a shell protein of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 184:1253-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilpert, W., B. Schink, and P. Dimroth. 1984. Life by a new decarboxylation-dependent energy conservation mechanism with Na+ as coupling ion. EMBO J. 3:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong, S. H., and S. Y. Lee. 2002. Importance of redox balance on the production of succinic acid by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 58:286-290. [DOI] [PubMed] [Google Scholar]
- 22.Hong, S. H., and S. Y. Lee. 2001. Metabolic flux analysis for succinic acid production by recombinant Escherichia coli with amplified malic enzyme activity. Biotechnol. Bioeng. 74:89-95. [DOI] [PubMed] [Google Scholar]
- 23.Horswill, A. R., A. R. Dudding, and J. C. Escalante-Semerena. 2001. Studies of propionate toxicity in Salmonella enterica identify 2-methylcitrate as a potent inhibitor of cell growth. J. Biol. Chem. 276:19094-19101. [DOI] [PubMed] [Google Scholar]
- 24.Horswill, A. R., and J. C. Escalante-Semerena. 2002. Characterization of the propionyl-CoA synthetase (PrpE) enzyme of Salmonella enterica: residue Lys592 is required for propionyl-AMP synthesis. Biochemistry 41:2379-2387. [DOI] [PubMed] [Google Scholar]
- 25.Horswill, A. R., and J. C. Escalante-Semerena. 2001. In vitro conversion of propionate to pyruvate by Salmonella enterica enzymes: 2-methylcitrate dehydratase (PrpD) and aconitase enzymes catalyze the conversion of 2-methylcitrate to 2-methylisocitrate. Biochemistry 40:4703-4713. [DOI] [PubMed] [Google Scholar]
- 26.Horswill, A. R., and J. C. Escalante-Semerena. 1997. Propionate catabolism in Salmonella typhimurium LT2: two divergently transcribed units comprise the prp locus at 8.5 centisomes, prpR encodes a member of the sigma-54 family of activators, and the prpBCDE genes constitute an operon. J. Bacteriol. 179:928-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horswill, A. R., and J. C. Escalante-Semerena. 1999. The prpE gene of Salmonella typhimurium LT2 encodes propionyl-CoA synthetase. Microbiology 145:1381-1388. [DOI] [PubMed] [Google Scholar]
- 28.Horswill, A. R., and J. C. Escalante-Semerena. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. J. Bacteriol. 181:5615-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeter, R. M., B. M. Olivera, and J. R. Roth. 1984. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J. Bacteriol. 159:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, E. Y., S. H. Kang, and C. Y. Choi. 1995. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by newly isolated Agrobacterium sp. SH-1 and GW-014 from structurally unrelated single carbon substrates. J. Ferment. Bioeng. 79:328-334. [Google Scholar]
- 31.Lee, S. Y. 1997. E. coli moves into the plastic age. Nat. Biotechnol. 15:17-18. [DOI] [PubMed] [Google Scholar]
- 32.London, R. E., D. L. Allen, S. A. Gabel, and E. F. DeRose. 1999. Carbon-13 nuclear magnetic resonance study of metabolism of propionate by Escherichia coli. J. Bacteriol. 181:3562-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubove, S. 1999. Good intentions. Forbes 163:62-64. [Google Scholar]
- 34.Luzier, W. D. 1992. Materials derived from biomass/biodegradable materials. Proc. Natl. Acad. Sci. USA 89:839-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man, W.-J., Y. Li, D. O'Connor, and D. C. Wilton. 1995. The binding of propionyl-CoA and carboxymethyl-CoA to Escherichia coli citrate synthase. Biochim. Biophys. Acta 1250:69-75. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy, A. A., H. M. Baker, S. C. Shewry, T. F. Kagawa, E. Saafi, and M. L. Patchett. 2001. Expression, crystallization and preliminary characterization of methylmalonyl coenzyme A epimerase from Propionibacterium shermanii. Acta Crystallogr. Sect. D 57:706-708. [DOI] [PubMed] [Google Scholar]
- 37.McClelland, M., K. E. Sanderson, J. Spieth, S. Clifton, P. Latreille, L. Courtney, S. Powollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 38.Millard, C. S., Y. P. Chao, J. C. Liao, and M. I. Donnelly. 1996. Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl. Environ. Microbiol. 62:1808-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 40.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neidhardt, F. C., J. L. Ingraham, and M. Schaechtar. 1990. Physiology of the bacterial cell. Sinauer Associates, Inc., Sunderland, Mass.
- 42.Page, W. J., N. Bhanthumnavin, J. Manchak, and M. Ruman. 1997. Production of poly(β-hydroxybutyrate-β-hydroxyvalerate) copolymer from sugars by Azotobacter salinestris. Appl. Microbiol. Biotechnol. 48:88-93. [Google Scholar]
- 43.Palacios, S., and J. C. Escalante-Semerena. 2000. prpR, ntrA, and ihf functions are required for expression of the prpBCDE operon, encoding enzymes that catabolize propionate in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 182:905-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeifer, B. A., S. J. Admiraal, H. Gramajo, D. E. Cane, and C. Khosla. 2001. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291:1790-1792. [DOI] [PubMed] [Google Scholar]
- 45.Poirier, Y., C. Nawrath, and C. Somerville. 1995. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Bio/Technology 13:142-150. [DOI] [PubMed] [Google Scholar]
- 46.Raux, E., A. Lanois, F. Levillayer, M. J. Warren, E. Brody, A. Rambach, and C. Thermes. 1996. Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J. Bacteriol. 178:753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riis, V., and W. Mai. 1988. Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J. Chromatogr. 445:285-298. [Google Scholar]
- 48.Roth, J. R., J. G. Lawrence, and T. A. Bobik. 1996. Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol. 50:137-181. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 50.Satoh, H., T. Mino, and T. Matsuo. 1999. PHA production by activated sludge. Int. J. Biol. Macromol. 25:105-109. [DOI] [PubMed] [Google Scholar]
- 51.Siegele, D. A., and J. C. Hu. 1997. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. USA 94:8168-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva, L. F., J. G. C. Gomez, M. S. Oliveira, and B. B. Torres. 2000. Propionic acid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV) production by Burkholderia sp. J. Biotechnol. 76:165-174. [DOI] [PubMed] [Google Scholar]
- 53.Skraly, F. A., B. L. Lytle, and D. C. Cameron. 1998. Construction and characterization of a 1,3-propanediol operon. Appl. Environ. Microbiol. 64:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slater, S., T. Mitsky, K. L. Houmiel, M. Hao, S. E. Reiser, N. B. Taylor, M. Tran, H. E. Valentin, D. J. Rodriguez, D. A. Stone, S. R. Padgette, G. Kishore, and K. J. Gruys. 1999. Metabolic engineering of Arabidopsis and Brassica for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nat. Biotechnol. 17:1011-1016. [DOI] [PubMed] [Google Scholar]
- 55.Snell, K. D., and O. P. Peoples. 2002. Polyhydroxyalkanoate polymers and their production in transgenic plants. Metab. Eng. 4:29-40. [DOI] [PubMed] [Google Scholar]
- 56.Stams, A. J. M., C. Dijkema, C. M. Plugge, and P. Lens. 1998. Contribution of 13C-NMR spectroscopy to the elucidation of pathways of propionate formation and degradation in methanogenic environments. Biodegradation 9:463-473. [Google Scholar]
- 57.Steinbuchel, A., and U. Pieper. 1992. Production of a copolyester of 3-hydroxybutyric acid and 3-hydroxyvaleric acid from single unrelated carbon sources by a mutant of Alcaligenes eutrophus. Appl. Microbiol. Biotechnol. 37:1-6. [Google Scholar]
- 58.Steinbuchel, A., and H. G. Schlegel. 1991. Physiology and molecular genetics of poly(β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol. Microbiol. 5:535-542. [DOI] [PubMed] [Google Scholar]
- 59.Stols, L., and M. I. Donnelly. 1997. Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl. Environ. Microbiol. 63:2695-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Textor, S., V. F. Wendisch, A. A. De Graaf, U. Muller, M. I. Linder, D. Linder, and W. Buckel. 1997. Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch. Microbiol. 168:428-436. [DOI] [PubMed] [Google Scholar]
- 61.Tsai, S. P., R. J. Hartin, and J. Ryu. 1989. Transformation of restriction-deficient Salmonella typhimurium LT2. J. Gen. Microbiol. 135:2561-2567. [DOI] [PubMed] [Google Scholar]
- 62.Tsang, A. W., A. R. Horswill, and J. C. Escalante-Semerena. 1998. Studies of regulation of expression of the propionate (prpBCDE) operon provide insights into how Salmonella typhimurium LT2 integrates its 1,2-propanediol and propionate catabolic pathways. J. Bacteriol. 180:6511-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valentin, H. E., D. L. Broyles, L. A. Casagrande, S. M. Colburn, W. L. Creely, P. A. DeLaquil, H. M. Felton, K. A. Gonzalez, K. L. Houmiel, K. Lutke, D. A. Mahadeo, T. A. Mitsky, S. R. Padgette, S. E. Reiser, S. Slater, D. M. Stark, R. T. Stock, D. A. Stone, N. B. Taylor, G. M. Thorne, M. Tran, and K. J. Gruys. 1999. PHA production, from bacteria to plants. Int. J. Biol. Macromol. 25:303-306. [DOI] [PubMed] [Google Scholar]
- 64.Valentin, H. E., and D. Dennis. 1996. Metabolic pathway for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) formation in Nocardia corallina: inactivation of mutB by chromosomal integration of a kanamycin resistance gene. Appl. Environ. Microbiol. 62:372-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valentin, H. E., and D. Dennis. 1997. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J. Biotechnol. 58:33-38. [DOI] [PubMed] [Google Scholar]
- 66.Vemuri, G. N., M. A. Eiteman, and E. Altman. 2002. Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl. Environ. Microbiol. 68:1715-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams, D. R., A. Anderson, E. A. Dawes, and D. F. Ewing. 1994. Production of a co-polyester of 3-hydroxybutyric acid and 3-hydroxyvaleric acid from succinic acid by Rhodococcus ruber: biosynthetic considerations. Appl. Microbiol. Biotechnol. 40:717-723. [Google Scholar]
- 68.Wood, H. G., B. Jacobson, and B. L. Gerwin. 1969. Oxaloacetate transcarboxylase from Propionibacterium. Methods Enzymol. 13:215-230. [Google Scholar]