Abstract
Factors controlling the anaerobic oxidation of ammonium with nitrate and nitrite were explored in a marine sediment from the Skagerrak in the Baltic-North Sea transition. In anoxic incubations with the addition of nitrite, approximately 65% of the nitrogen gas formation was due to anaerobic ammonium oxidation with nitrite, with the remainder being produced by denitrification. Anaerobic ammonium oxidation with nitrite exhibited a biological temperature response, with a rate optimum at 15°C and a maximum temperature of 37°C. The biological nature of the process and a 1:1 stoichiometry for the reaction between nitrite and ammonium indicated that the transformations might be attributed to the anammox process. Attempts to find other anaerobic ammonium-oxidizing processes in this sediment failed. The apparent Km of nitrite consumption was less than 3 μM, and the relative importance of ammonium oxidation with nitrite and denitrification for the production of nitrogen gas was independent of nitrite concentration. Thus, the quantitative importance of ammonium oxidation with nitrite in the jar incubations at elevated nitrite concentrations probably represents the in situ situation. With the addition of nitrate, the production of nitrite from nitrate was four times faster than its consumption and therefore did not limit the rate of ammonium oxidation. Accordingly, the rate of this process was the same whether nitrate or nitrite was added as electron acceptor. The addition of organic matter did not stimulate denitrification, possibly because it was outcompeted by manganese reduction or because transport limitation was removed due to homogenization of the sediment.
Nitrogen in the biosphere is distributed between a pool of N2, which is by far the larger pool and can be utilized only by prokaryotes that are able to fix N2, and a much smaller pool of fixed nitrogen that can be utilized by almost all living organisms. The availability of fixed nitrogen is a major factor regulating the primary production of the sea (10, 30). Once nitrogen has been transferred into the fixed pool by biological and industrial nitrogen fixation (12), it can be recycled many times between organic and inorganic forms before returning to the N2 pool. Until recently, denitrification was recognized as the only important process removing nitrogen from the fixed pool in natural environments (see, for example, reference 18). Recently, however, it was discovered that ammonium is oxidized anaerobically in sediments in the presence of nitrate and that this alternative pathway contributes significantly to benthic N2 production (25). In continental shelf sediments, up to 67% of the N2 formation was due to anaerobic ammonium oxidation with nitrate (or possibly nitrite) and only 33% of the N2 formation was due to denitrification (25). The contribution of anaerobic ammonium oxidation to N2 formation was observed to be greatest in sediment with low organic loading at a water depth of 695 m. Closer to shore, at a depth of 380 m, where the organic loading of the sediment was higher, anaerobic ammonium oxidation was responsible for 24% of the N2 production, and in a eutrophic coastal bay, the contribution of this process to the total N2 production was much lower (2%). It was therefore concluded that anaerobic ammonium oxidation was quantitatively most important in sediments with moderate organic loading, as is found on continental shelves. It has been suggested that nitrite accumulating through nitrate reduction is the actual oxidant for ammonium and that the reaction proceeds through the anammox pathway, which was originally described in a wastewater purification system (16). During anammox, NO2− and NH4+ are combined into N2 under anoxic conditions (16, 28) by microorganisms capable of autotrophic growth (27). The microorganisms responsible have not yet been isolated but are affiliated with the Planctomycetales order of bacteria (20, 21). The process has now also been described in other wastewater treatment systems (6, 9).
Here we report the results of further investigations of the factors controlling anaerobic ammonium oxidation in marine sediments and its relation to the anammox process. We explored the involvement of nitrite in anaerobic ammonium oxidation, quantified the kinetics of nitrite consumption, assessed the biological nature of the process, and further investigated its competitive relationship to denitrification.
MATERIALS AND METHODS
Study site and sampling.
Sediment was sampled by using a multiple corer (temperature and kinetics experiment) or a box corer (organic-matter experiment) at a water depth of 695 m in the Skagerrak in the North Sea between Denmark and Norway (station S9 [2, 3]) where anaerobic NH4+ oxidation has previously been demonstrated (25). All handling, storage, and incubation of sediment was done at bottom-water temperature (6°C) except for the incubations in the temperature experiment. Sediment from 10-cm (inside diameter) multiple-corer tubes was sectioned in a glove bag under an atmosphere of N2, and sediment from 13 to 38 mm below the surface, i.e., including the oxic-anoxic interface and the upper part of the anoxic sediment where NO3− and NO2− are present (2, 3, 24), was pooled from several cores to obtain a sufficient volume for the experiments and was stirred to homogeneity. The sediment was then processed as described below without being removed from the glove bag. Sediment from box cores was obtained by first removing the upper portion of sediment (approximately 15 mm) by using a thin Plexiglas plate and then transferring the next portion of sediment (approximately 25 mm) into a plastic box where it was stored under 2 cm of bottom water until the experiment was started. The time periods between the sampling and the start of the experiments were 20 h, 2 days, and 8 days for the organic-matter, kinetics, and temperature experiments, respectively.
Nitrite dependence of ammonium oxidation.
Combinations of labeled and unlabeled nitrogen compounds (see below) were added to portions of sediment, and then the sediment was stirred for 1 min and transferred to 28-ml glass centrifuge tubes, which were capped with butyl rubber stoppers, leaving no headspace. Four parallel incubations were performed by adding nitrogen compounds from concentrated stock solutions to the following final concentrations: 100 μM 14NH4+ plus 50 μM 15NO3− for the first incubation, 100 μM 14NH4+ plus 50 μM 15NO2− for the second incubation, 25 μM 15NH4+ plus 100 μM 14NO3− for the third incubation, and 100 μM 15NH4+ for the fourth incubation.
Duplicate samples were taken at seven time points during the 48-h incubation by centrifugation of tubes from each treatment at 1,600 × g for 10 min. The stopper was then carefully removed, and a 2-ml sample was taken with a high-precision glass syringe with a hypodermic needle for analysis of the isotopic composition of N2. Approximately 0.1 ml of a 50% (wt/vol) ZnCl2 solution was drawn into the syringe after the sample had been drawn, and the content of the syringe was then slowly emptied into a 6.6-ml helium-flushed Exetainer vial (Labco, High Wycombe, United Kingdom), with care taken not to mix water and headspace. The overpressure was then removed before the needle was retracted. The remaining supernatant was filtered through a 0.45-μm-pore-size cellulose acetate filter into polypropylene vials and frozen for later analysis.
Temperature dependence.
Portions of sediment (9 ml each) were transferred to a series of 12.6-ml Exetainer glass vials (Labco). Each Exetainer vial was closed with a screw cap holding a 5-mm-thick butyl rubber septum and removed from the glove bag, and the headspace was immediately flushed with helium. The sediment was preincubated at the incubation temperatures of 6.5, 15.7, 25.6, 31.9, 35.7, 38.0, 41.5, 45.1, and 52.9°C for 12 h. The experiment was started by injection of 15NO3− and 14NH4+ from concentrated stock solutions into the vials, followed by vigorous shaking for 1 min until final concentrations of 50 and 100 μM, respectively, were reached.
For sampling, the Exetainer vials were shaken vigorously for 1 min and centrifuged at 1,600 × g for 10 min. To sample the headspace for the isotopic composition of N2, we first injected 2 ml of helium, flushed the syringe five times with the headspace, and finally retracted a 2-ml sample. The gas was transferred to an Exetainer vial (6.6 ml) prefilled with helium-gassed water. An open hypodermic needle allowed the excess water to escape as the gas was injected into the Exetainer vial. The pore-water supernatant was filtered through a 0.45-μm-pore-size cellulose acetate filter into polypropylene vials and frozen for later analysis. Sampling was performed in duplicate or triplicate three times during the incubation period at each incubation temperature. Incubation times varied from 4.3 h (35.7°C) to 14.2 h (6.7°C), which in all cases was too short for the depletion of the NO3−-plus-NO2− pool, and production of nitrogen gas could therefore be recorded throughout the incubation times at all temperatures. For determination of the initial isotopic composition of N2 for all incubations, samples were taken from three Exetainer vials preincubated at 6.5°C without added 15NO3− and 14NH4+.
Effects of organic matter.
Sediment was transferred from the plastic box into a glass beaker after first siphoning off the water on top of the sediment. The beaker was then placed in the anoxic glove bag, and the sediment was stirred until it was homogeneous and was then left for 2 h to allow the sediment oxygen consumption to remove any oxygen that might have entered while the sediment was handled outside the glove bag. The sediment was divided into two portions, one of which served as a control while the other received an aliquot of a highly concentrated pure culture of planktonic green algae (Tetraselmis sp.; Reed Mariculture, Inc., San Jose, Calif.) to a final concentration of 1.5 g (ash-free dry weight) per liter of sediment. After being stirred for 1 min, each of the two portions was split into two halves that received two different treatments. Each set consisted of a Tetraselmis-amended portion and an unamended portion, with one set receiving 100 μM 15NO3− and the other set receiving 100 μM 15NH4+ (final concentrations). The sediment was then transferred to glass centrifuge tubes and sampled as described above for the kinetics experiment.
Analysis.
The concentration of NO3− plus NO2− was determined by using the vanadium chloride reduction method (1) (NOx analyzer model 42c; Thermo Environmental Instruments, Inc., Franklin, Mass.). Nitrite was analyzed spectrophotometrically (7), and the concentration of NH4+ was determined by using the flow injection method with conductivity detection (8). For analysis of the isotopic composition of N2, the Exetainer vials were first shaken vigorously for 1 min in order to establish equilibrium between the N2 in the water and the N2 in the headspace (>96% of the N2 was in the headspace at equilibrium). The headspace was then injected into a gas chromatographic column coupled to a triple-collector isotopic ratio mass spectrometer (RoboPrep G+ in line with TracerMass; Europa Scientific, Crewe, United Kingdom), and the abundance and concentrations of 14N15N and 15N15N were analyzed. The isotopic composition of the NO3− pool was estimated from the concentrations of NO3− before and after the addition of 15NO3− (99.6% 15N), and the isotopic composition of the NH4+ pool was determined by using the combined microdiffusion-hypobromite method (19).
Calculations.
The rates of denitrification and NH4+ oxidation with NO2− in the homogenized sediment were calculated from the 15NO3− and 15NO2− addition experiments as previously described (25). The calculations are based on the production of 29N2 and 30N2 and assume a 1:1 pairing of N from NH4+ and NO3− or NO2− during anaerobic ammonium oxidation and random isotope pairing during denitrification. In the NO3− addition experiments, it is assumed that the 15N labeling rates of the NO3− and NO2− pools are identical.
Estimates of Km values for NO3− uptake in the sediment were obtained from progress curves of NO2− concentrations versus time (5). In the temperature experiment, the rates of NH4+ oxidation with NO2− and denitrification were determined from linear regressions of the amount of nitrogen gas produced by each of the processes versus time.
RESULTS
Nitrite dependence of ammonium oxidation.
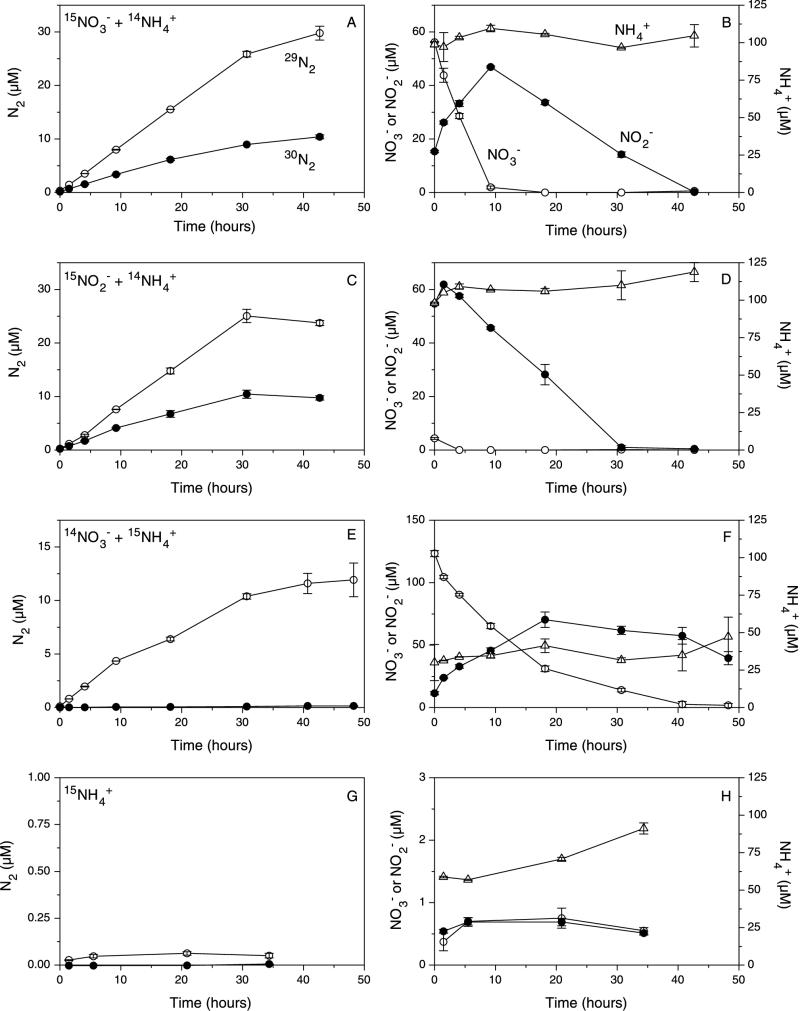
With no addition of NO3− or NO2−, the NH4+ concentration increased as a result of mineralization by 25 μM over 30 h of anoxic incubation (Fig. 1H), while in the experiments where either NO3− or NO2− was added, the NH4+ concentration remained stable during the consumption of NO3− and NO2− (Fig. 1B, D, and F). 15N labeling of the NH4+ pool showed that this difference was due to the consumption of NH4+ in the presence of NO3− and NO2−. Thus, the 15N content of the NH4+ pool was 53% at the beginning of the experiment but decreased to 16% at the end of the experiment due to dilution by unlabeled NH4+ produced by mineralization. In experiments with 15NH4+ and unlabeled NO3− and NO2−, the consumption of NH4+ was accompanied by the accumulation of 15N-labeled nitrogen gas, and all of the 15N converted to N2 was recovered as 29N2 (Fig. 1E). No N2 production occurred in the absence of NO3− and NO2− (Fig. 1G).
FIG. 1.
Concentrations of 29N2, 30N2, NO3−, NO2−, and NH4+ as functions of time in anoxic sediment incubations with addition of 15NO3− plus 14NH4+ (A and B), 15NO2− plus 14NH4+ (C and D), 15NH4+ plus 14NO3− (E and F), or 15NH4+ (G and H). In panels A and B, 15NO3− accounted for 98% of the NO3− pool; in panels C and D, 15NO2− accounted for 98% of the NO2− pool; in panels E and F, 15NH4+ initially accounted for 53% of the NH4+ pool, decreasing to 16% at the end of the experiment; and in panels G and H, the 15N content of the NH4+ pool decreased from 77 to 39% between the start and the end of the experiment. Error bars indicate standard errors.
In the experiments in which NO3− was added, there was a rapid decrease in the concentration of NO3− that was instantly accompanied by a transient appearance of NO2− (Fig. 1B and F). Production of 15N-labeled N2 was recorded immediately after addition of either 15NO3− or 15NO2− to the anoxic sediment (Fig. 1A and C), and the concentrations of both 29N2 and 30N2 increased linearly with time until all of the NO2− was consumed (Fig. 1B and D). Control experiments showed that less than 2% of the added 15NO3− was reduced to NH4+ in this sediment. Thus, dissimilatory NO3− reduction to NH4+ did not affect our results. The initial 15N content in the NO3− and NO2− pools of the two experiments was 98% and was assumed not to change with time. When NO2− was added, the very small amount of endogenous NO3− was rapidly consumed, after which the concentration of NO2− decreased linearly almost to zero.
The production rates of 29N2 and 30N2 differed only slightly between the experiments with the additions of 15NO3− (Fig. 1A) and 15NO2− (Fig. 1C). The production of 29N2 was 0.84 μM h−1 with NO3− and 0.82 μM h−1 with NO2−, and the production of 30N2 was 0.29 μM h−1 with NO3− and 0.34 μM h−1 with NO2−. Accordingly, the relative importance of anaerobic NH4+ oxidation in N2 production was almost the same whether NO3− or NO2− was added (74 and 71%, respectively). Calculations based on the changes between subsequent samplings during the incubations showed that there was no systematic variation in the relative importance of NH4+ oxidation and denitrification as a function of NO2− concentrations between 1 and 50 μM in either type of experiment (data not shown), which is in agreement with findings from previous experiments involving NO3− additions (25).
Kinetics of nitrite uptake.
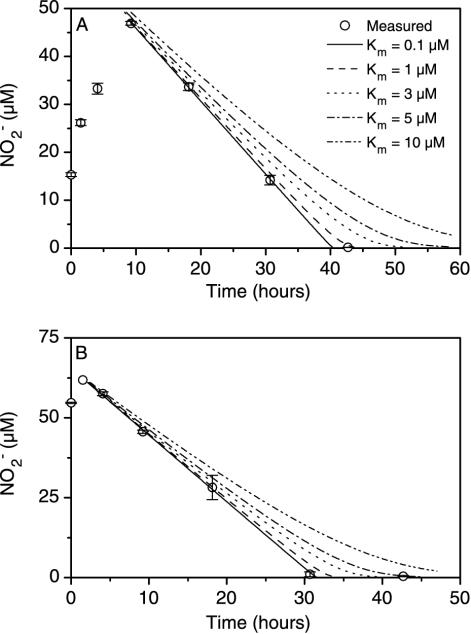
The theoretical time courses of NO2− concentration that were calculated assuming Michaelis-Menten kinetics with different Km values were compared with our measurements (Fig. 2). The parameters used for the 15NO3− addition experiment were as follows: maximum substrate consumption rate (Vmax) of −1.53 μM h−1 and initial substrate concentration (S0) of 61.1 μM. For the 15NO2− addition experiment, the parameters were as follows: Vmax of −2.09 μM h−1 and S0 of 65.4 μM. The best agreement between the theoretical and measured concentrations of NO2− was obtained for a Km value of 0.1 μM; however, acceptable agreement was found for Km values up to 3 μM. The Km value was hence estimated to be below 3 μM (Fig. 2A and B).
FIG. 2.
Concentrations of NO2− as functions of time in two experiments with either NO3− added (A) or NO2− added (B). The solid lines indicate the theoretical decrease in NO2− concentration assuming Michaelis-Menten kinetics and a Km value of 0.1 μM, a maximum NO2− reduction rate calculated as the slope of the linear portion of the curve, and an initial concentration calculated as the intersection of this linear regression with the y axis. The other lines depict the decrease in NO2− concentration assuming higher Km values. Error bars indicate standard errors.
Temperature dependence.
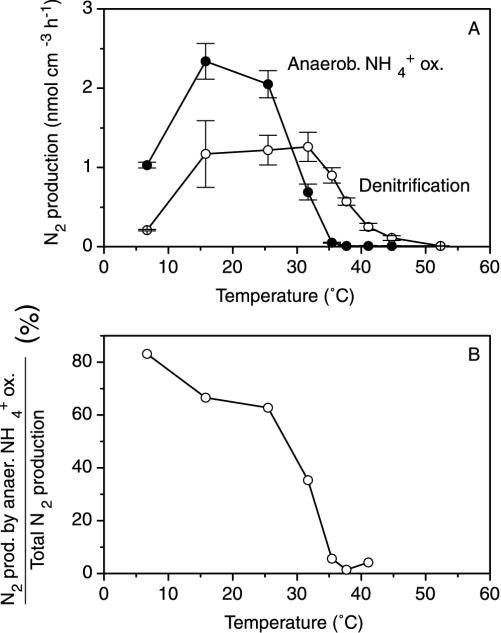
Anaerobic NH4+ oxidation and denitrification responded differently to changes in temperature. The rate of anaerobic NH4+ oxidation was highest at 15°C and decreased sharply above 25°C, reaching zero around 37°C (Fig. 3A). Denitrification had a wider optimum range (from 15 to 32°C), decreased less abruptly towards higher temperatures, and was detectable at temperatures up to 45°C. The relative importance of NH4+ oxidation with NO2− for N2 production decreased with increasing temperature (Fig. 3B). Ammonium oxidation with NO2− was responsible for approximately 80% of the total nitrogen gas production at the in situ temperature of 6°C, whereas more than 98% of the nitrogen gas was produced by denitrification at 37°C. The activation energy was 61 kJ mol−1.
FIG. 3.
Rates of dinitrogen production by anaerobic oxidation of NH4+ with NO2− and by denitrification as a function of temperature (A) and the production of dinitrogen by the oxidation of NH4+ with NO2− relative to the total dinitrogen production in the sediment (B). Error bars indicate standard errors.
Effects of organic matter on anaerobic ammonium oxidation and denitrification.
The addition of organic matter resulted in a statistically significant (P < 0.05) stimulation of the rate of mineralization, as indicated by a 102% increase in the rate of NH4+ production (from 1.5 to 3.1 μM N h−1). Denitrification was only slightly stimulated (from 0.58 to 0.62 μM N h−1 [8% increase]; not statistically significant), and NH4+ oxidation with NO2− was only slightly reduced (from 0.57 to 0.53 μM N h−1 [8% decrease]; not statistically significant) by the addition of organic matter. This resulted in a decrease in the relative importance of NH4+ oxidation with NO2− for the total N2 production (from 49.7% in the unamended experiment to 45.9% in the amended experiment). As in the other experiments, NO2− accumulated transiently. With the addition of 15NH4+ alone, no transfer of 15N from NH4+ to N2 could be detected in either the amended or the unamended experiment (data not shown).
DISCUSSION
Nitrite dependence of ammonium oxidation.
Previous experiments that demonstrated anaerobic NH4+ oxidation in sediments were all performed with the addition of NO3−, and although NO2− was suggested to be the oxidant for NH4+ based on patterns of isotope pairing, a direct involvement of NO3− could not be excluded (25). The results presented here directly demonstrate that NO2− alone may serve as an oxidant for NH4+ and that NH4+ oxidation is not directly dependent on NO3−. Thus, anaerobic NH4+ oxidation proceeded in the absence of NO3− both after the depletion of added NO3− and when NO2− was added (Fig. 1). Furthermore, the process proceeded at similar rates both in the presence of NO3− and NO2− and in the presence of NO2− alone, which indicates that NO3− was not involved in pathways of NH4+ oxidation that bypass NO2−. As in the previous sediment studies and studies of anammox in wastewater reactors, the patterns of isotope pairing were also consistent with N2 formation through a 1:1 pairing of nitrogen from NO2− and NH4+. Thus, if NO3− were the direct oxidant of NH4+, as was originally suggested for the wastewater systems (16), then the oxidation states of the nitrogen atoms in NO3− and NH4+ would require that 5 NH4+ react with 3 NO3− to form 4 N2. Consequently, at least some 30N2 should be formed in the experiments with the addition of 15NH4+ and 14NO3−, but that was clearly not the case (Fig. 1E). By contrast, the simpler stoichiometry of NH4+ oxidation with NO2− is consistent with a 1:1 pairing of their nitrogen atoms: NO2− + NH4+ → N2 + 2H2O. We therefore infer that the anaerobic NH4+ oxidation in the sediment occurred as a 1:1 reaction between NO2− and NH4+.
With NO2− as the direct oxidant of NH4+, the reduction of NO3− to NO2− is a prerequisite for NH4+ oxidation with NO2− to gain access to the pool of NO3−, which is generally much larger than the pool of NO2− in the marine environment (12). In the S9 sediment, however, the reduction rate of NO3− during the first 10 h of the experiment was four times faster than the reduction rate of NO2− during the last 30 h of the experiment (Fig. 1B), and thus the reduction of NO3− to NO2− did not limit NH4+ oxidation with NO2−. This is further supported by the fact that the production rates of 29N2 were the same in the experiments with added 15NO3− and 15NO2−. The reduction of NO3− to NO2− can be carried out by a number of different microorganisms, including denitrifying and other anaerobic-respiring bacteria as well as fermenting bacteria, and there is generally a high capacity for this process in sediments (4, 13). It is thus generally expected that the NH4+ oxidation with NO2− in marine sediments is not limited by the reduction of NO3− to NO2−. In intact sediment, NO2− may also be produced in the oxic surface layer by nitrification and then be transported into the anoxic zone, where it may support an anaerobic NH4+ oxidation.
It has been suggested that oxidation of NH4+ with MnO2 could convert nitrogen from NH4+ to N2 under anoxic conditions (11, 15). However, in agreement with our previous findings (24), NH4+ was not oxidized to N2 in the absence of NO3− or NO2− (Fig. 1G).
Temperature dependence.
The typical biological temperature spectrum provides good evidence that anaerobic NH4+ oxidation in the sediment is microbially catalyzed (Fig. 3A). The temperature optimum for NH4+ oxidation with NO2− of approximately 15°C found in this sediment is much lower than the optimum for the anammox process of 37°C found in wastewater treatment systems (14, 22). The activation energies of the anammox process in wastewater reactors (22) and the NH4+ oxidation with NO2− in marine sediment were similar (70 and 61 kJ mol−1, respectively). The bottom-water temperature at station S9 is between 4 and 6°C throughout the year (23), and a special adaptation to this low temperature can be expected. Therefore, even though the data shown in Fig. 3 indicate that denitrification would be favored over NH4+ oxidation with NO2− at higher temperatures, this does not necessarily imply that NH4+ oxidation with NO2− would be of lesser importance in warmer environments. Instead, the two rather different temperature optima found in these two environments may indicate that the NH4+ oxidation with NO2− is rather flexible with respect to temperature, and different temperature characteristics may be found in other environments. Similar relatively narrow temperature ranges have also been reported for nitrification in sediments, with the optimum temperature varying as a function of the temperatures in situ (26).
Kinetics.
The relative quantitative importance of N2 production by NH4+ oxidation with NO2− and denitrification was found to be independent of the NO2− concentration. We therefore argue that even though the NO2− and NO3− concentrations applied in these experiments were higher than those found in situ (typically ranging from 0 to 30 μM for NO3− and from 0 to 5 μM for NO2− [B. Thamdrup and T. Dalsgaard, unpublished data]), the balance between NH4+ oxidation with NO2− and denitrification found in the anoxic jar experiments represents the balance under in situ conditions. The importance of NH4+ oxidation with NO2− for nitrogen cycling in this sediment was underlined by the fact that the consumption of NH4+ by the process was able to keep pace with the concomitant NH4+ production by mineralization (Fig. 1B and D).
In these experiments, the other substrate for NH4+ oxidation with NO2−, NH4+, did not reach a level where it limited the rate of the process. For example, in the experiments with addition of organic matter, the average NH4+ concentrations were 52 μM without and 250 μM with addition of the algal culture (data not shown). This fivefold increase in NH4+ concentration did not stimulate NH4+ oxidation with NO2−, and we conclude that the Km value for NH4+ uptake by this process must be well below 50 μM.
The Km value for NO2− uptake in the sediment was estimated by use of the progress curve technique (5) to be below 3 μM (Fig. 2). Since the balance between N2 production through NH4+ oxidation with NO2− and through denitrification was independent of NO2− concentration, we assumed that the balance between the NO2− uptakes by these two processes was also independent of NO2− concentration and that the two processes had a similar affinity for NO2− within the range of concentrations examined here. For anammox sludge, the Km value was found to be less than 7 μM (22), and the Km for NO3− uptake in denitrifying bacterial communities was between 1.8 and 13.7 μM (17). The Km for anaerobic ammonium oxidation in marine sediments thus seems to be in the same range as that of other nitrate- or nitrite-reducing bacteria.
A change in the controlling factors for denitrification was expected to affect the balance between denitrification and NH4+ oxidation with NO2−. One such factor was expected to be the availability of organic matter, which should favor denitrification. However, the addition of organic matter to the sediment did not have a significant effect on NH4+ oxidation with NO2− or on denitrification. The addition of the algal debris clearly stimulated the mineralization in the sediment, as indicated by the doubling of the NH4+ production. The insignificant stimulation of denitrification by the addition of organic matter indicates either that the denitrifying bacteria were already saturated with electron donors or that they were outcompeted by other pathways of carbon oxidation. The first scenario could be a result of the homogenization of the sediment, which destroys the gradients that exist in the intact sediment, allowing the bacteria to utilize electron donors from sediment strata from which they were spatially separated in the intact sediment. The incubation may have been too short for the denitrifying community to respond to the increased substrate availability through growth. Alternatively, a major fraction of the added organic matter could be oxidized through dissimilatory Mn oxide reduction. A dominance of Mn-reducing bacteria over denitrifiers in the competition for organic matter is supported by the relatively low rate of denitrification relative to NH4+ accumulation in the sediment without added organic matter. Assuming a typical ratio of carbon oxidation to NH4+ accumulation (the molar CO2 production to NH4+ accumulation ratios reported for Skagerrak sediments range from 5 to 12 [2, 3]) and a ratio of 5:4 for CO2 production and NO3− consumption during organotrophic denitrification, we estimate that denitrification supported 2 to 9% of the carbon oxidation in the incubations. So, we attribute the lack of an effect of carbon addition on the rate of denitrification to either the short incubation time or the unusually high Mn oxide content in sediment at station S9. For more typical marine sediments, where Mn reduction is insignificant in anaerobic carbon oxidation, we expect that the addition of reactive organic matter, given a sufficient response time, would significantly stimulate denitrification at the expense of NH4+ oxidation with NO2−.
Together with our previous findings (25), our demonstration here of the NO2− dependence of ammonium oxidation, the biological catalysis of the process, and the 1:1 stoichiometry between NO2− and NH4+ provides a strong indication that anaerobic NH4+ oxidation with NO2− in sediments occurs through the anammox pathway. Further verification studies should include the detection and possibly the cultivation of the organisms that carry out the process and an investigation of the intermediates in the reaction. The anammox process has two characteristic intermediates, hydroxylamine and hydrazine (29), and identification of these in the marine process would further verify that the biochemistry of the anammox process is also responsible for the anaerobic oxidation of NH4+ in marine sediments.
Acknowledgments
We thank the masters, crews, and scientific parties of the cruises onboard R/V Dana and R/V Arne Tiselius for their assistance during the fieldwork. Egon B. Frandsen, Tanja Quottrup, Kitte Gerlich Lauridsen, Marlene Skjærbæk, and Anna Haxen are gratefully acknowledged for excellent technical assistance. We thank Stefan Hulth for the opportunity to join the R/V Arne Tiselius cruise and the Danish National Research Council for sponsoring the R/V Dana cruise.
B.T. was supported by the Danish National Research Foundation through the Danish Center for Earth System Science.
REFERENCES
- 1.Braman, R. S., and S. A. Hendrix. 1989. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Anal. Chem. 61:2715-2718. [DOI] [PubMed] [Google Scholar]
- 2.Canfield, D. E., B. B. Jørgensen, H. Fossing, R. N. Glud, J. K. Gundersen, N. B. Ramsing, B. Thamdrup, J. W. Hansen, L. P. Nielsen, and P. O. J. Hall. 1993. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 113:27-40. [DOI] [PubMed] [Google Scholar]
- 3.Canfield, D. E., B. Thamdrup, and J. W. Hansen. 1993. The anaerobic degradation of organic matter in Danish coastal sediments: iron reduction, manganese reduction, and sulfate reduction. Geochim. Cosmochim. Acta 57:3867-3883. [DOI] [PubMed] [Google Scholar]
- 4.Cole, J. A. 1987. Assimilatory and dissimilatory reduction of nitrate to ammonia, p. 281-329. In J. A. Cole and J. Ferguson (ed.), The nitrogen and sulphur cycles. Society for General Microbiology Symposium 42. Cambridge University Press, Cambridge, England.
- 5.Dalsgaard, T., and F. Bak. 1994. Nitrate reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil: sulfide inhibition, kinetics, and regulation. Appl. Environ. Microbiol. 60:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egli, K., U. Fanger, P. J. J. Alvarez, H. Siegrist, J. R. van der Meer, and A. J. B. Zehnder. 2001. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 175:198-207. [DOI] [PubMed] [Google Scholar]
- 7.Grasshoff, K., M. Ehrhardt, and K. Kremling. 1983. Methods of seawater analysis, 2nd ed. Verlag Chemie GmbH, Weinheim, Germany.
- 8.Hall, P. O. J., and R. C. Aller. 1992. Rapid, small-volume, flow injection analysis for CO2 and NH4+ in marine and freshwaters. Limnol. Oceanogr. 37:1113-1119. [Google Scholar]
- 9.Helmer, C., S. Kunst, S. Juretschenko, M. C. Schmid, K. H. Schleifer, and M. Wagner. 1999. Nitrogen loss in a nitrifying biofilm system. Water Sci. Technol. 39:13-21. [Google Scholar]
- 10.Howarth, R. W. 1988. Nutrient limitation of net primary production in marine ecosystems. Annu. Rev. Ecol. 19:89-110. [Google Scholar]
- 11.Hulth, S., R. C. Aller, and F. Gilbert. 1999. Coupled anoxic nitrification/manganese reduction in marine sediments. Geochim. Cosmochim. Acta 63:49-66. [Google Scholar]
- 12.Jaffe, D. A. 2000. The nitrogen cycle, p. 322-342. In M. C. Jacobson, R. J. Charlson, H. Rohde, and G. H. Orians (ed.), Earth system science. Academic Press, San Diego, Calif.
- 13.Knowles, R. 1982. Denitrification. Microbiol. Rev. 46:43-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuenen, J. G., and M. S. M. Jetten. 2001. Extraordinary anaerobic ammonium-oxidizing bacteria. ASM News 67:456-462. [Google Scholar]
- 15.Luther, G. W. I., B. Sundby, P. J. Lewis, and N. Silverburg. 1997. Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim. Cosmochim. Acta 61:4043-4052. [Google Scholar]
- 16.Mulder, A., A. A. van de Graaf, L. A. Robertson, and J. G. Kuenen. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 16:177-184. [Google Scholar]
- 17.Murray, R. E., L. L. Parsons, and M. S. Smith. 1989. Kinetics of nitrate utilization by mixed populations of denitrifying bacteria. Appl. Environ. Microbiol. 55:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon, S. W., J. W. Ammerman, L. P. Atkinson, V. M. Berounsky, G. Billen, W. C. Boicourt, W. R. Boynton, T. M. Church, D. M. Ditoro, R. Elmgren, J. H. Garber, A. E. Giblin, R. A. Jahnke, N. J. P. Owens, M. E. Q. Pilson, and S. P. Sitzinger. 1996. The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35:141-180. [Google Scholar]
- 19.Risgaard-Petersen, N., S. Rysgaard, and N. P. Revsbech. 1995. Combined microdiffusion-hypobromite oxidation method for determining nitrogen-15 isotope in ammonium. Soil Sci. Soc. Am. J. 59:1077-1080. [Google Scholar]
- 20.Schmid, M., U. Twachtmann, M. Klein, M. Strous, S. Juretschko, M. Jetten, J. W. Metzger, K. H. Schleifer, and M. Wagner. 2000. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 23:93-106. [DOI] [PubMed] [Google Scholar]
- 21.Strous, M., J. A. Fuerst, E. H. M. Kramer, S. Logemann, G. Muyzer, K. T. van de Pas-Schoonen, R. Webb, J. G. Kuenen, and M. S. M. Jetten. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446-449. [DOI] [PubMed] [Google Scholar]
- 22.Strous, M., J. G. Kuenen, and M. S. M. Jetten. 1999. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 65:3248-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svansson, A. 1975. Physical and chemical oceanography of the Skagerrak and the Kattegat. I. Open sea conditions. Fishery Board of Sweden, Institute of Marine Research, report no. 1. Bohusläningens AB, Uddevalla, Sweden.
- 24.Thamdrup, B., and T. Dalsgaard. 2000. The fate of ammonium in anoxic manganese oxide-rich marine sediment. Geochim. Cosmochim. Acta 64:4157-4164. [Google Scholar]
- 25.Thamdrup, B., and T. Dalsgaard. 2002. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68:1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thamdrup, B., and S. Fleischer. 1998. Temperature dependence of oxygen respiration, nitrogen mineralization, and nitrification in arctic sediments. Aquat. Microb. Ecol. 15:191-199. [Google Scholar]
- 27.van de Graaf, A., A. Mulder, P. de Brujin, M. S. M. Jetten, L. A. Robertson, and J. G. Kuenen. 1995. Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 61:1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Graaf, A., A. Mulder, H. Slijkhuis, L. A. Robertson, and J. G. Kuenen. 1990. Anoxic ammonium oxidation, p. 388-391. In C. Christiansen, L. Munck, and J. Villadsen (ed.), Proceedings of the Fifth European Congress on Biotechnology, vol. 1. Munksgaard, Copenhagen, Denmark. [Google Scholar]
- 29.van de Graaf, A. A., P. de Brujin, L. A. Robertson, M. S. M. Jetten, and J. G. Kuenen. 1997. Metabolic pathway of anaerobic ammonium oxidation on the basis of 15N studies in the fluidized bed reactor. Microbiology 143:2415-2421. [DOI] [PubMed] [Google Scholar]
- 30.Vitousek, P. M., and R. W. Howarth. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87-115. [Google Scholar]