Abstract
Grass carp reovirus type II (GCRV-II) has inflicted substantial economic damage to aquaculture industry due to highly contagious. To combat epidemic GCRV-II, we rational designed and constructed a multi-epitope nanoparticle vaccine (Pep-Fn) that consisted with cell penetrating peptide (CPP), epitope peptides, cell and grass carp-derived ferritin. Firstly, an anti-GCRV-II phage antibody library was constructed to screen antibodies for outer capsid proteins VP4 and VP35. Ab-1 and Ab-3 were successfully screened and demonstrated high affinity with GCRV-II particles. We further identified five potential epitopes (Pep1-Pep5) on the outer capsid protein recognized by Ab-1 and Ab-3 through protein-protein docking and alanine scanning mutagenesis. Then, a self-assembled nanoparticle displaying the Pep1-Pep5 and CPP on the surface was constructed for Pep-Fn preparation. Benefit from the nano-sized particle structure, Pep-Fn could overcome the body surface barrier and accumulate in the immune organs. Experiments demonstrated that Pep-Fn could effectively stimulate grass carp to produce anti-GCRV-II antibodies via immersion immunization and also provided protective effect against GCRV-II challenge. Collectively, our research provides a new vaccine design strategy for combating GCRV-II, and demonstrates the great potential of protein-based nanoparticle as a platform for GCRV-II vaccine development.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-025-02411-9.
Keywords: Grass carp reovirus type II, Phage antibody library, Antigen epitope, Nanoparticle vaccine
Introduction
Reoviridae, a family of double-stranded RNA virus, can be transmitted among multiple species, including teleost, plants, poultry and mammals, posing a significant threat to global public health and agricultural production [1–4]. As a typical reovirus, the pandemic of grass carp reovirus type II (GCRV-II) has caused great losses to aquaculture industry due to high fatality [5]. Vaccination is considered as a successful initiative to prevent viral infection in the world [6]. The design of an effective vaccine involves three critical steps, including the selection of antigens, adjuvant and delivery vector [7, 8]. The development and application of GCRV-II vaccine face two bottleneck problems: insufficient immune response activation leads to inadequate protective efficacy; alongside the lack of large-scale vaccination strategy leads to increased costs. The screening of key epitopes and advanced delivery system represent feasible approach to deal with above challenges [9].
Antigenic epitopes are the fundamental units within antigens capable of eliciting immune response, can be classified into B or T cell epitopes [10]. Traditional approach for epitope identification is mainly based on specific recognition of antigen-antibody, including truncated method, peptide scanning, and alanine scanning mutagenesis [11–13]. With the development of epitope analysis technology, such as high-throughput screening based on library and epitope prediction based on protein-protein docking, the efficiency of epitope identification was further enhanced. By combining phage display library technology screening and computer-aided prediction, our team reported the core neutralizing epitopes of two aquatic animal pathogens [14, 15]. Especially, with the progress of Cyro-EM, researchers can accurately locate epitopes through high resolution antibody-antigen complex structure, and the neutralizing epitopes of a series of pathogens such as, Influenza virus, Hepatitis C virus, SARS-CoV-2 and Nipah virus has been identified [16–19]. Each method has inherent strengths and weaknesses, while the integration of different approach enables rapid and precise epitope identification. Structural optimization of epitopes is another key step in the design of vaccine antigen. For example, epitope stability can be further enhanced through alteration of antigen amino acid sequences and have been widely used in respiratory syncytial virus (RSV) vaccine design [20, 21]. In addition, it has been reported that the preparation of vaccines by concatenating multiple epitopes can further enhance the protective immune response [22, 23].
Self-assemble nanoparticle demonstrate significant potential in vaccine development, owing to structural similarity to natural viruses, good biosafety and biocompatibility [24]. It has also been indicated that high-density epitope peptides displaying on nanoparticles can enhance immunogenicity, thereby activating immune response in host [25]. Various natural self-assembled proteins, such as virus-like particle, lumazine synthase and ferritin, have been reported to be developed vaccine design platforms [26–28]. Furthermore, researchers have artificially designed some self-assembled proteins, such as I53-50 A/B and mi3, which have enabled the development of SARS-CoV-2, RSV and influenza vaccines that effectively induce the production of neutralizing antibody in mouse models [29–31]. The small size of nanoparticle vaccine enables easy penetration through mucosal cells and recognized by APCs, not only diversifying vaccine immunization routes but also inducing robust mucosal immune response [32, 33]. However, the current understanding of the specific mechanism of nanoparticle-enhanced immunity is still insufficient, which limits its subsequent development efficiency.
In this research, an anti-GCRV-II phage antibody was firstly constructed for high affinity antibody screening. And the epitopes recognized by the antibodies screened from the library were identified. A novel epitope-based nanoparticle vaccine Pep-Fn was subsequently constructed and evaluated through animal experiments. Our research aims to establish a new vaccine development and application strategy, so as to provide important support for GCRV-II combating.
Materials and methods
Grass carp, virus and cell lines
Healthy grass carp (4–6 cm, 1.0 ± 0.2 g) were purchased from YueZhiLan market in Liupanshui (Guizhou, China). The grass carp were maintained at 28℃ and fed twice daily with low-protein diet. GCRV-II was isolated by our laboratory and stored in the form of tissue virus suspension [34]. The GCO and CIK cell lines were stored in our lab and cultured at 28℃ with 5% CO2, in DMEM medium supplemented with 10% FBS.
GCRV-II infection
Grass carp were injected intraperitoneally with GCRV-II (20 µL/fish) to stimulate the production of anti-GCRV-II polyclonal antibodies. The blood and spleen samples were collected from five fish at 1, 2, 3, 4 weeks after GCRV-II infection. Above 200 µL blood sample was stored at 4℃ overnight and then separated the serum for evaluation of GCRV-II-binding antibody level by indirect ELISA. Briefly, tissue virus suspension was coated on the microplate at 100 µL/well, and then incubated with serum pre-diluted in PBS solution as the antigen (1: 100), 1: 1000 diluted mouse anti-grass carp IgM (Frdbio) and 1: 5000 diluted goat anti-mouse IgG-HRP (Sangon). The binding signal was represented by measuring the absorbance at 450 nm. RNAs of spleen tissues were extracted and then reversed to cDNA. The cDNA was stored at a concentration of 100 ng/µL for PCR amplification.
Construction of anti-GCRV II phage display antibody library
The nucleotide sequences of VH and VL of grass carp immunoglobulin were found in NCBI, and the conserved sequences were obtained by comparative analysis of the frame region, so as to design degenerate primers for amplifying VH and VL genes (Table 1). Among them, the primers in group A contained a flexible linker (A-VH-R1 and A-VL-F1) for splicing VH and VL genes into single-chain antibody genes, while the primers in group B were only used to amplify VH gens without splicing process. All the amplified products were purified for the following amplification reaction.
Table 1.
Primers used to construct anti-GCRV-II phage antibody library
| Primer name | Nucleotide sequence |
|---|---|
| A-VH-F1 | TCAGGCCCAGCCGGCCTCTGAGTCAGCGGTCATTAAA |
| A-VH-F2 | TCAGGCCCAGCCGGCCAGTCTCAAGCTGTGGTAAAA |
| A-VH-F3 | TCAGGCCCAGCCGGCCATGGTAGTAAAACCAGGA |
| A-VH-R1 | GCTGCCACCTCCGCCTGAACCGCCTCCACC GGTGACTTTGGTTCCTTT |
| A-VL-F1 | GGCGGAGGTGGCTCTGGCGGTGGCGGATCG CCAGGACAAGAAGTMAGA |
| A-VL-R1 | ATAAGAATGCGGCCGCCACMAGTTTAGTTCCTCCACC |
| B-VH-F1 | TCAGGCCCAGCCGGCCTCTGAGTCAGCGGTCATTAAA |
| B-VH-F2 | TCAGGCCCAGCCGGCCAGTCTCAAGCTGTGGTAAAA |
| B-VH-F3 | TCAGGCCCAGCCGGCCATGGTAGTAAAACCAGGA |
| B-VH-R1 | ATAAGAATGCGGCCGCGGTGACTTTGGTTCCTTT |
The underlined part is the restriction enzyme site (Not I and Sfi I), the coarsened part is the frame region sequence, and the italic part is the inserted flexible linker (Gly4Ser)3. VH = heavy chain variable, VL = light chain variable
The group A primers were used to amplify VH and VL genes in the following reaction system: 2 µL cDNA, 1 µL upstream primer (A-VH-F1, A-VH-F2, A-VH-F3 and A -VL-F1), 1 µL downstream primer (A-VH-R1 and A-VL-F1), 21 µL ddH2O and 25 µL 2 × Rapid Taq Master Mix (Vazyme). The reaction procedure was carried out at 95 °C for 5 min; subsequently, 30 cycles of 95 °C for 60 s, 55 °C for 30 s and 72 °C for 60 s; last 72 °C for 10 min. According to the upstream and downstream primer combinations, these amplified products were correspondingly named as A-VH-1/2/3 and A-VL-1. Then A-VH-1/2/3 and A-VL-1 sequences were spliced a complete scFv by SOE-PCR as follow system: 2 µL template cDNA mixtures (A-VH-1/2/3 and A-VL-1 were 1 µL, respectively), 1 µL upstream primer (A-VH-F1/2/3), 1 µL downstream primer (A-VL-R1), 21 µL ddH2O and 25 µL 2 × Rapid Taq Master Mix. The spliced products were correspondingly named as scFv-1/2/3. The procedure and system for amplification of VH genes with group B primers were consistent with those of group A primers and the products were named as VH-1/2/3. The purified scFv-1/2/3 and VH -1/2/3 fragment were inserted into pCANTAB5E and then transformed in to the TG1 chemical competent cells. The 100 µL transformation products were spread on the 2×YT-Amp plate. After overnight culture, single clone was randomly selected from the plate for DNA sequencing.
The phage antibody library amplification and purification were carried out according to the previous method [35]. Firstly, 500 µL transformation mixture was added into 100 mL 2×YT-Amp medium which incubated at 37 °C and 180 rpm until the OD600 reached 0.45–0.6, then adding the helper phage M13KO7 (1 × 1011 pfu/mL, New England Biolabs) into the bacterial solution and culturing at 37 °C. cells After helper phage infection, bacterial cells were collected by centrifugation and resuspended with 100 mL 2×YT-AK medium, and then cultured at 37 °C and 180 rpm for 12 h. The supernatant of the culture was collected and then mixed with 24 mL PEG/NaCl. After the mixture was placed in ice bath for 12 h, the precipitated phage was collected and then resuspended in 1.5 mL PBS. This is the constructed anti-GCRV-II phage display antibody library, which can be used for subsequent screening.
Biopanning of phage display antibody library
The phage antibody library was subjected to four rounds of biopanning with coat antigen (GCRV-II virus suspension) for anti-GCRV-II phage antibody clone isolation and enrichment. And biopanning can be simply divided into three steps: binding, elution and amplification. Firstly, 96-well microplates were coated with virus suspension (100 µL/ well). The wells were washed sterile PBST for three times and then the blocking solution (BSA) was added. The phage antibody library solution (100 µL, 1010 pfu/mL) was added to the wells. After being washed 10 times with PBST (300 µL for 1 min, the first round was 10 times, and the remaining three rounds were 15, 20, 25 times, respectively), 100 µL Glycine-HCL solution (0.2 M, pH = 2.2) were added to each well to elute the bound phages. The elute was transferred to EP tube and an equal amount of Tris-HCL buffer (pH = 7.4) was added. The mixture was the first round of enrichment antibody library, and the next round of enrichment was performed after amplification. Each round of input library phage particles was quantified to 1010 pfu/mL and the output phage particles were quantified after each round biopanning. The total enrichment multiplier was calculated after four rounds biopanning.
Screening and DNA sequencing of positive phage colony
Phage-ELISA was carried out to screen positive phage clone from enriched phage antibody library. Briefly, the last round of phage antibody library was infected with TG-1 and then spread on the 2×YT-Amp plate. Subsequently, 31 individual anti-GCRV-II phage clones were randomly selected from plate and then cultured with 10 mL 2×YT-Amp medium at 37 °C for 6 h. According to the above method, those clones were prepared into phage supernatant was quantified into the same concentration for following phage-ELISA experiment. Tissue virus suspension was used as the antigen, phage supernatant as primary antibody, and mouse anti-M13 Major Coat Protein (HRP) as secondary antibody (1:1000, Santa Cruz Biotechnology). The OD450 of each well was measure after the color reaction. Anti-GCRV-II phage clone with the P/N ratio more than 2.1 was identified as positive. “P” was bound with anti-GCRV-II phage particles, and “N” was helper phage. After infection and expansion, the positive phage clones were selected for DNA sequencing.
Detection of the affinity between recombinant antibody protein and GCRV-II
The prokaryotic expression vector pET-32a-Ab-1 and pET-32a-Ab-3 were constructed by Azenta Life Science and then transformed into the Rosetta complement cell (TransGen Biotech). The expression strain was cultured in LB-Amp medium till OD600 of 0.6 and further induced with 0.5 mM IPTG at 37 °C for 8 h. The bacteria culture was centrifuged, and the precipitated cells was collected and lysed by sonication (3 s/2 s, work/stop, total 20 min). After centrifugation of lysate, the recombinant protein in supernatant and precipitate was analyzed by SDS-PAGE and then purified using Ni-NTA 6FF (Sangon). The purified of recombinant Ab-1 and Ab-3 protein analyzed by Western blot (primary antibody: anti 6× His tag mouse mAb, Sangon; secondary antibody: goat anti-mouse IgG-HRP, Sangon). The recombinant protein was stored at -80 °C after the concentration was determined by using BCA protein quantification kit (Vazyme). The affinity between the recombinant antibody protein and GCRV-II particles was determined by indirect ELISA. We firstly prepared differently gradient molar concentrations of recombinant Ab-1 and Ab-3 protein solution. Then, the microplate was coated with GCRV-II (100 µL/ well) and bound with the recombinant protein solution after blocking and washing. The antibody selection was same as the above WB experiment, and following detection steps were consistent with the phage-ELISA section.
Identification of key antigen-antibody interaction
The structures of GCRV-II capsid proteins VP4, VP35 and high affinity antibody Ab-1, Ab-3 were predicted by AlphaFold 2 [36]. The antigen-antibody docking results were predicted by using ClusPro [37], including four models, Ab-1-VP4, Ab-1-VP35, Ab-3-VP4, Ab-3-VP35. All the protein structures and docking models were visualized by PyMOL software. The binding energies and two-dimensional interaction regions of antigen-antibody docking model were analyzed by using PDBePISA and LigPlus, respectively. In order to further analyze the key amino acid residues and regions of antigen-antibody interaction, alanine was used substitute VP4K7, VP4T9, VP4Y11, VP4N16, VP4K68, VP4E73, VP4I74, VP4T75, VP4D160, VP4T162, VP4Y169, VP4V170, VP4E172 of VP4 and VP35H72, VP35G73, VP35C74, VP35I75, VP35P76, VP35E79, VP35H80, VP35W83, VP35Y87, VP35D91, VP35M93 of VP35, respectively. This series of antigen mutants were re-docked with Ab-1 and Ab-3. The potential antigenic epitopes were identified according to the change of binding energies. The corresponding five peptides Pep1-Pep5 were synthesized by SynthBio (purity of > 80%). The reaction between peptides and GCRV-II positive serum (collected in 2.2) was analyzed by indirect-ELISA.
Design, construction and characterization of multi-epitope nanoparticle vaccine
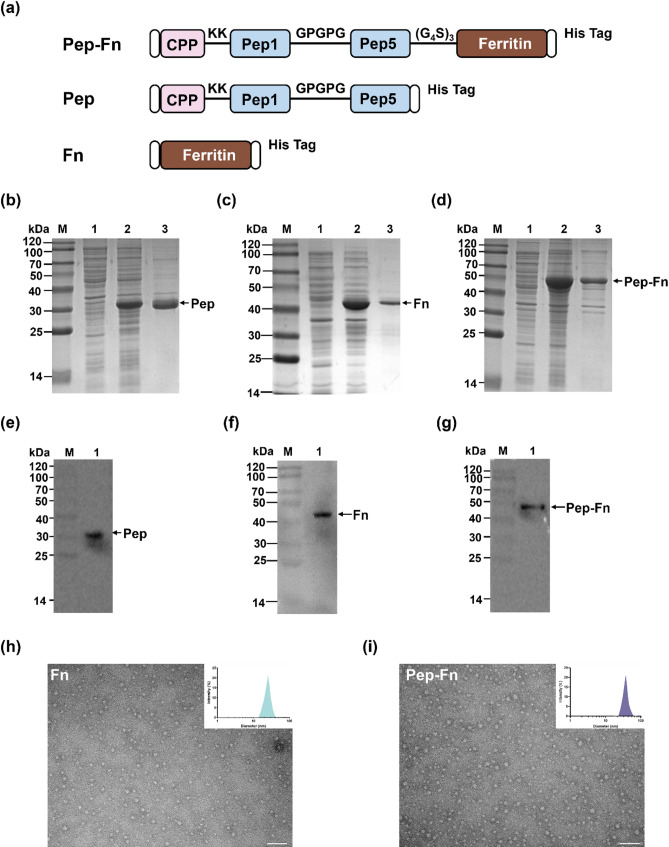
The design strategy of the multi-epitope nanoparticle vaccine Pep-Fn was shown in Fig. 4a. The expression strains (Rosetta) containing recombinant plasmids pET32a-Pep, pET32a-Fn and pET32a-Pep-Fn were constructed by Azenta Life Science. The expression and purification of recombinant Pep, Fn and Pep-Fn protein were analyzed by SDS-PAGE and WB, respectively. The antibody selection was same as the above WB experiment in 2.6. Dynamic light scattering (DLS) was employed to measure the hydrodynamic diameters and polydispersity index of Fn and Pep-Fn nanoparticles. The data were analyzed using the instrument’s software (Malvern Panalytical). The morphology of nanoparticles was detected by negative transmission electron microscopic (TEM) analysis and images was captured using HT7800 (HITACHI).
Fig. 4.
Design, construction, and characterization of nanoparticle vaccine. (a) Schematic diagram of components of recombinant Pep, Fn, and Pep-Fn proteins. (b)-(d) SDS-PAGE recombinant Pep, Fn, and Pep-Fn proteins. Lane M: protein marker; Lane 1: Rosetta transformed with pET-32a induced by IPTG; Lane 2: Rosetta transformed with pET-32a-Pep/pET-32a-Fn/pET-32a-Pep-Fn (supernatant) induced by IPTG; Lane 3: purified Pep/Fn/Pep-Fn protein. (e)-(g) WB analysis of recombinant Pep/Fn/Pep-Fn proteins. Lane M: protein marker; Lane 1: purified Pep/Fn/Pep-Fn protein. (h) and (i) Hydrodynamic diameters and transmission electron microscopy images of Fn and Pep-Fn nanoparticles, scale bar: 50 nm
Biosafety evaluation of Pep-Fn
The biosafety of Pep-Fn was evaluated in grass carp cell liner and grass carp individual. The cytotoxicity assay of Pep-Fn was detected by using CCK-8 cell counting kit (Vazyme). The GCO and CIK cells were inoculated in 96-well plates at a density of 1 × 105 and then incubated with different concentration Pep-Fn solution at 28 °C for 24 h. Subsequently, CCK-8 solution was added to each well (10 µL/ well) and incubated at 28 °C for 4 h. The OD450 of each well was measured and cell viability was calculated as per manufacturer’s instructions. For in vivo experiments, grass carp were divided into four groups (50 fish/ group), including control, Pep, Fn and Pep-Fn group. Grass carp in treatment group were immersed with 20 mg/L Pep, Fn and Pep-Fn for 24 h and then transferred to new tanks. Four grass carp from each group were selected to collect gill, liver, kidney and spleen tissues. Those tissues were used to prepare paraffin sections and observed under Ni-U microscope (Nikon) after H&E staining.
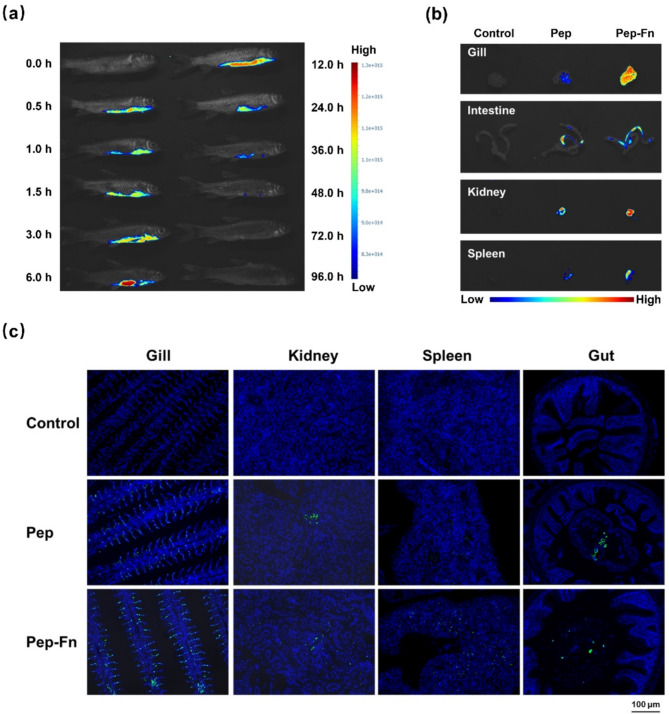
Detection the bio-distribution of Pep-Fn
The bio-distribution of Pep-Fn in grass carp after bath immunization was characterized using FITC-labeled protein (FITC-Pep and FITC-Pep-Fn). Briefly, grass carp were divided into control, Pep and Pep-Fn group (30 fish/ group). Grass carp in treatment group were immersion immunized with 20 mg/L FITC-Pep and FITC-Pep-Fn for 6 h and then transferred to new tanks. Fluorescence images of vaccinated fish were collected using VISQUE in vivo Smart-LF system (Vieworks) in different timepoints. Additionally, gill, gut, kidney and spleen tissues were collected from each group at 6 h after immunization and the fluorescence signals were detected. Those tissues were also prepared into frozen sections and stained with DAPI. A fluorescence microscope DM6 B (Leica) was used to observe and capture images of these sections.
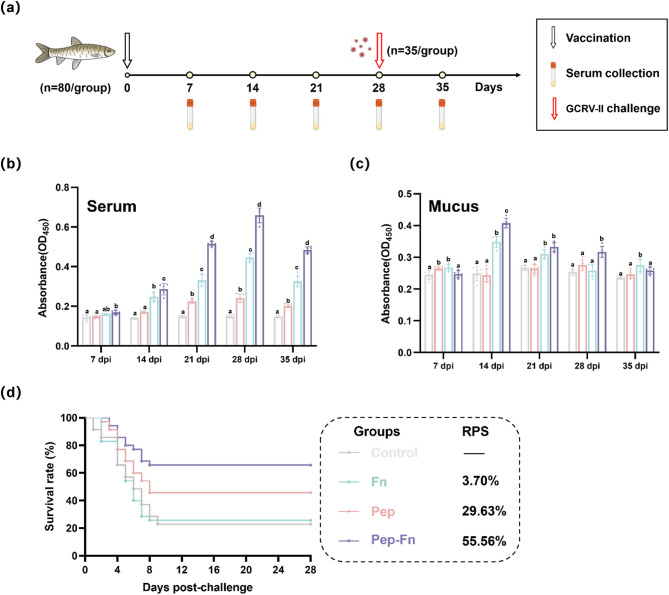
Immune effect evaluation of Pep-Fn
Grass carp were divided into four groups (80 fish/ group), including control, Pep, Fn and Pep-Fn group. For immunization, grass carp in treatment group were immersed with 20 mg/L Pep, Fn and Pep-Fn for 6 h and transferred to new tanks. For sampling, five fish from each group were selected to collect serum and mucus at different timepoints. Serum samples were obtained as above described. Mucus in skin and gill were rubbed with sterile cotton and then vortexed in EP tubes containing 0.2 mL PBS [38]. The cotton was squeezed to collect the absorbed liquid and then centrifuged to obtain the supernatant. The specific antibody level in serum and mucus was determined by indirect ELISA.
At 28 days after immunization (dpi), 35 fish were selected from each group and maintained in new tanks for GCRV-II challenge experiment. The chosen grass carp were injected intraperitoneally with GCRV-II (40 µL/fish). The mortality of grass carp in each group was recorded daily for 28 days. The relative percentage survival (RPS) was calculated at the end of the experiment.
Statistical analysis
All the data were analyzed using GraphPad Prism 10. One-way ANOVA was used for multiple component comparisons. Significant differences are marked with asterisks or letters. * p < 0.05 was considered statistically significant.
Results
Ab-1 and Ab-3 were successfully screened from the anti-GCRV-II phage display antibody library
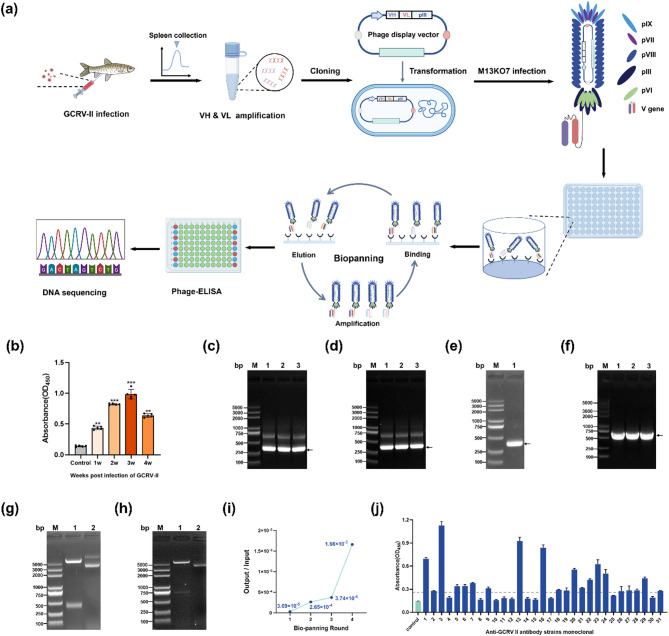
Construction, biopanning and screening process of anti-GCRV-II phage antibody library were shown in Fig. 1a. In order to obtain the anti-GCRV-II antibody genes from spleen B cells for library construction, grass carp were infected with GCRV-II through artificial injection. The serum antibody level gradually increased and reached the peak at 21 d after infection (Fig. 1b). This result suggested that spleen collected on 21 d after infection could be used for total RNA extraction to the subsequent amplification of antibody genes. Total RNA was reversed to cDNA, and the V region genes were then amplified using specific primers (Fig. 1c and e). Then VH and VL genes were used as template to splice into the scFv fragments (Fig. 1f). The sdAb and scFv fragments were cloned into pCANTAB5E vector, followed by the transformation of host bacteria, resulting in antibody library of 1.27 × 105 cfu / mL. The results of Not I and Sfi I digestion also showed the recombinant vector pCANTAB5E-sdFv and pCANTAB5E-scFv were successfully constructed (Fig. 1g and h).
Fig. 1.
Construction, biopanning and screening of anti-GCRV-II phage antibody library. (a) Schematic illustration of the utilization of the phage display antibody library to obtain anti-GCRV-II antibody. (b) Serum antibody level of grass carp after GCRV-II infection. Three biological and technical replicates were set in ELISA. (c) Grass carp VH genes amplified by Group A primers. Lane M: DNA marker; Lane 1–3: amplification products. (d) Grass carp VH genes amplified by Group B primers. Lane M: DNA marker; Lane 1–3: amplification products. (e) Grass carp VL genes amplified by Group A primers. Lane M: DNA marker; Lane 1: amplification products. (e) Grass carp VH genes amplified by Group A primers. Lane M: DNA marker; Lane 1–3: amplification products. (f) VH and VL spliced to scFv by SOE-PCR, Lane M: DNA marker; Lane 1–3: amplification products. (g) and (h) Double digestion identification (Not I + Sfi I) of recombinant plasmid pCANTAB5E-VH and pCANTAB5E-scFv. Lane M: DNA marker; Lane 1: pCANTAB5E-VH / pCANTAB5E-scFv, Lan2: digestion of pCANTAB5E-VH / pCANTAB5E-scFv. (i) Input of biopanning for anti-GCRV-II phage display antibody library. (j) Antibody affinity detection by phage-ELISA. Three technical replicates were manipulated for testing
The phage display antibody library was constructed through M13KO7 infection. After four bounds of biopanning, phage binding to GCRV-II particle were obviously enriched, with total enrichment multiplier of 54 times (Fig. 1i). A total of 21 positive clones were identified through phage-ELISA, with the ratio of P/N > 2 (Fig. 1j). The antibody gene of top 10 positive clones were sequenced. The results shown that A1, A7, A20, A22, A23 and A24 were the same with only heavy chain, named Ab-1; A3, A13, A16 and A29 were the same with complete scFv sequence, named Ab-3. The amino acid sequences of Ab-1 and Ab-3 were shown in Fig S1.
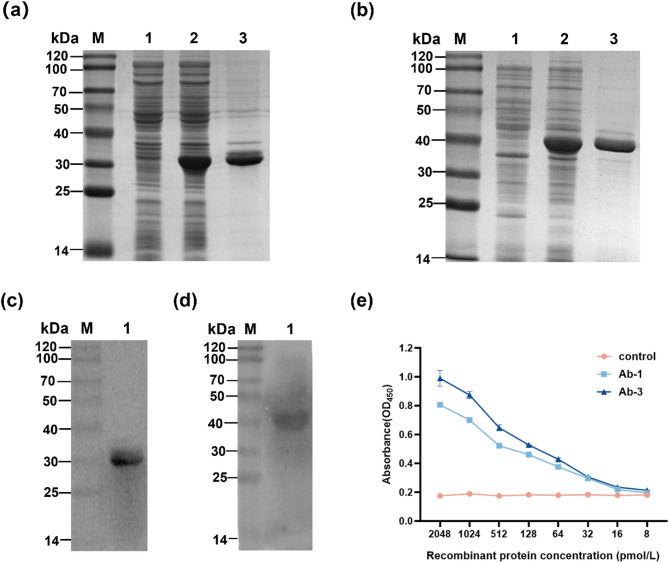
Fig. 2.
Expression, purification and affinity detection of recombinant antibody protein. (a) and (b) SDS PAGE analysis of recombinant protein Ab-1/Ab-3. Lane M: protein marker; Lane 1: Rosetta transformed with pET-32a induced by IPTG; Lane 2: Rosetta transformed with pET-32a-Ab-1/ pET-32a-Ab-3 (supernatant) induced by IPTG; Lane 3: purified Ab-1/Ab-3 protein. (c) and (d) WB analysis of recombinant protein Ab-1/Ab-3. Lane M: protein marker; Lane 1: purified Ab-1/Ab-3 protein. (e) The GCRV-II affinity of the recombinant antibody protein Ab-1 and Ab-3
Ab-1 and Ab-3 have high affinity with GCRV-II
Recombinant Ab-1 and Ab-3 protein were prepared and purified. The results showed that the sizes of purified Ab-1 and Ab-3 protein was approximately 30 and 40 kDa, respectively (Fig. 2a-d). Subsequently, we detected the affinity of purified proteins with GCRV-II through indirect ELISA. The results indicated that at Ab-3 demonstrated higher intensity than Ab-1 the same concentration (Fig. 2e). At the highest concentration, the OD450 of Ab-1 and Ab-3 were 0.807 and 0.991, respectively.
Computer-aided detection of five potential epitopes Pep1-Pep-5 in outer capsid protein
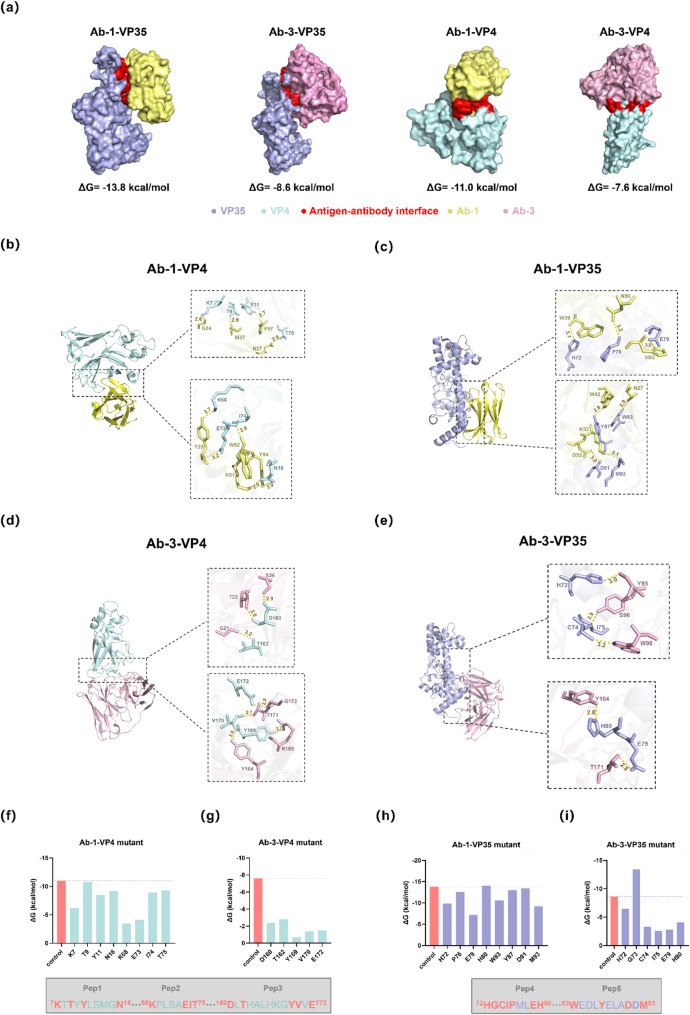
Four docking model (Ab-1-VP35, Ab-3-VP35, Ab-1-VP4 and Ab-3-VP4) were generated by ClusPro and visualized by PyMOL (Fig. 3a). The docking results showed that the binding energies of above four docking model was − 13.8, -8.6, -11.0 and − 7.6 kcal/mol, respectively, which are all spontaneous exothermic reactions. Additionally, we found that the interaction site were concentrated in the VP47–16, VP468–75, VP4160–172, VP3572–80 and VP3583–93 through analysis of the two-dimensional interaction interface (Fig. 3b-e). The hydrogen bonds of antigen-antibody interaction are mainly concentrated in these regions (e.g., Ab-1K91, Ab-1K91 with VP4N16; Ab-3S26, Ab-3T23 with VP4D160). Alanine was further used to substitute the binding amino acid residues in the above five regions to generate antigen mutants and then re-docking with antibody. The results showed that only the binding energies of Ab-3-VP35G73A and Ab-1-VP35H80A decreased after alanine substitution (Fig. 3f-i). According to the change of binding energy, five potential epitopes were identified and then named Pep1-Pep5.
Fig. 3.
Identification of the key interaction site between antibody and antigen. (a) VP4 and VP35 was docked with Ab-1 and Ab-3, respectively. The interaction region between antigen and antibody is marked in red. (b)-(e) Illustration of key amino acid residues in antigen-antibody docking model. (f)-(i) The change of antigen mutant-antibody docking binding energy
To determine the sequence conservation of Pep1-Pep5, the existing VP4 and VP35 sequences of different GCRV-II strains on NCBI were downloaded for multi-sequence alignment. The results demonstrated that the sequence of Pep1-Pep5 were highly conserved among GCRV-II strains, and the amino acid identify of Pep1 was 100% (Fig.S2). In order to further analyze the immunogenicity of Pep1-Pep5, the corresponding peptides were synthesized and the reactive ability with GCRV-II positive serum was detected. The results indicated that these five peptides were reactive with positive serum and showed a dose-dependent effect (Fig.S3).
Construction and characterization of multi-epitope nanoparticle vaccine
To further enhance immunogenicity and delivery efficiency, a novel nanoparticle vaccine Pep-Fn was constructed in this research. Pep-Fn consists of three components: cell penetrating peptide, five antigen epitope peptides and grass carp-derived ferritin (Fn). The results indicated that purified Pep, Fn, and Pep-Fn proteins with an expected size of 30, 40 and 50 kDa, respectively (Fig. 4b-g). The Fn and Pep-Fn nanoparticles were further characterized by DLS and TEM. The results showed that numerous uniformly shaped particles were observed in the TEM images of Fn and Pep-Fn (Fig. 4h). Additionally, Pep-Fn (35 nm) exhibits a larger average size than Fn (25 nm). Combined with the above results, we confirm that the nanoparticle vaccine displaying antigen on the surface were successfully constructed.
Pep-Fn can penetrate the surface barrier to reach the immune organs
The biosafety of Pep-Fn was evaluated before the formal immunization experiments. Firstly, the cytotoxicity of Pep-Fn to CIK and GCO cells was determined. The results showed that Pep-Fn did not affect the cell viability at a high treatment concentration (Fig. 5a-b). Furthermore, the absence of histopathological lesions in grass carp tissues was confirmed after immunization (Fig. 5c). The above results indicated that Pep-Fn had good biosafety.
Fig. 5.
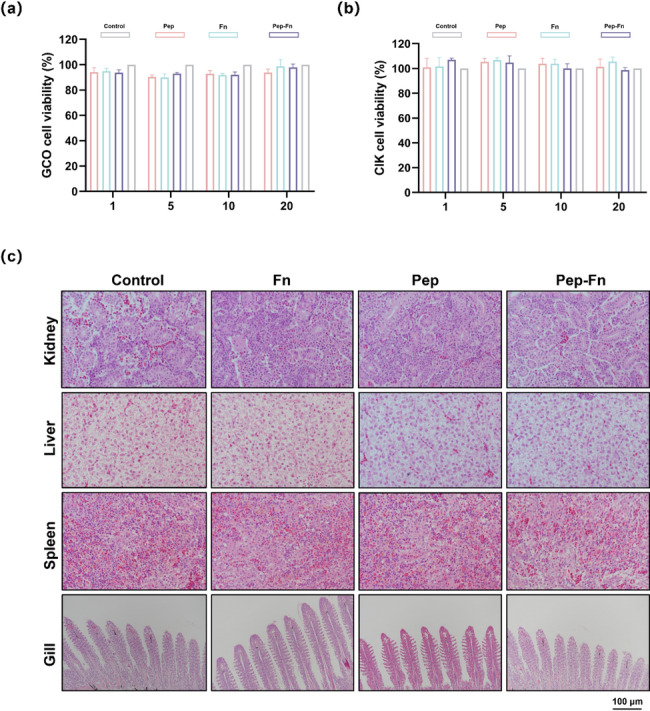
Biosafety evaluation of multi-epitope nanoparticle vaccine in vitro and in vivo. (a) Cell viability of GCO and CIK cells after incubation with different concentrations of Pep, Fn and Pep-Fn for 24 h. (b) Hematoxylin-eosin staining of tissues of control and treated fish
The bio-distribution of Pep-Fn was validated through in vivo imaging. The fluorescence intensity in grass carp were significantly increased at 6 h after immunization (Fig. 6a) and strong fluorescence signals could be detected in gill, intestine, spleen and kidney tissues at the same time (Fig. 6b-c). With the prolongation of time, the fluorescence intensity gradually decreased, and almost no fluorescence signal was detected at 48 h after immunization. These results indicated that Pep-Fn could effectively overcome the surface barrier and then present to the immune organs, thereby ensuring the induction of immune responses in grass carp.
Fig. 6.
Detection the bio-distribution of multi-epitope nanoparticle vaccine. (a) Fluorescence images in vaccinated fish at different times. (b) Ex vivo fluorescence images of isolated fish tissues 6 h after bath immunization. (c) The immunofluorescence images of vaccinated fish tissues 6 h after bath immunization
Pep-Fn provides a robust immunoprotective effect against GCRV-II in grass carp
The specific antibody levels in serum and mucus were detected by indirect ELISA. As shown in Fig. 7b-c, the serum and mucus specific antibody increased rapidly on the 14 dpi and reached the peak on the 28 and 21dpi, respectively. Meanwhile, the serum specific antibody level in Pep-Fn group was significantly higher than that in Pep group at the same time point. At 28 dpi, GCRV-II challenge test was carried out to further assess the immunoprotection of Pep-Fn. The Pep-Fn showed the highest RPS of 55.56%. The above results show that Pep-Fn can induce specific immune response in grass carp via immersion immunization, thus providing anti-GCRV-II immune protection.
Fig. 7.
Evaluation of the protective immune responses in vaccinated fish. (a) Schematic illustration showing the immunization, challenge, and sampling program. (b) Specific antibody levels in serum and mucus at different time points. (c) Survival rate and relative percentage survival of vaccinated fish after the GCRV-II challenge. Significant differences are marked with different letters
Discussion
Due to the absence of commercial vaccines, GCRV-II poses a serious threat to the grass carp aquaculture industry. An effective and economically vaccine is urgently needed to control the outbreak of GCRV-II. For GCRV-II vaccines, in addition to providing a strong immune protection effect, large-scale immunization route should also be available. In recent years, nanocarriers have provided a new platform for vaccine design due to their unique size and delivery capabilities, and have brought new opportunities for the development of fish vaccines [39]. Based on this, our research can be divided into two parts. The first is to combine phage display technology with bioinformatics analysis to identify the key antigenic epitopes of GCRV-II. The second is to construct an epitope-based nanoparticle vaccine that can induce an immune response in grass carp through immersion immunization. Our lab has previously reported a ferritin-based vaccine that can provides a strong protection to common carp after SVCV challenge [9].
Phage display library technology is an efficient in vitro antibody screening technology that can quickly obtain antibodies against target antigens through integrating the antibody phenotype and genotype on individual phage particles [40–42]. For example, researchers rapidly identified multiple high-neutralizing antibodies from the phage antibody library within just 6 days in the early outbreak of COVID-19 [43]. Library size and diversity are crucial for rapid isolation of high-affinity antibodies [44]. In this research, we specifically constructed a combinatorial antibody library in scFv or VH format using spleen B cells from grass carp infected with GCRV-II. The capacity of anti-GCRV-II antibody library was determined to be 1.27 × 105 cfu/mL, which can be used for subsequent separation of antibodies. Biopanning is another important factor to obtain the target antibody from the library. In general case, phage clones binding the target antigen will be effectively enriched through 3–5 rounds of panning. It was also observed that the output of the phages increased rapidly in the third round of panning. Combined phage-ELISA and DNA sequencing, the sequence of two high-affinAb-1 and Ab-3 were obtained from 31 individuals. According to the sequence information, recombinant Ab-1 and Ab-3 protein were prepared to further verify the affinity between those two antibodies and GCRV-II particles. Both Ab-1 and Ab-3 demonstrated strong binding affinity to GCRV-II in ELISA experiments.
Antibody is an important tool in virological research. The preparation of antiviral antibodies and the analysis of epitopes recognized by antibodies are the basis for the development of therapeutic antibody drugs and epitope-based vaccines [45]. Combined protein structure simulations and protein-protein docking, five binding sites VP47–16, VP468–75, VP4160–172, VP3572–80 and VP3583–93 on the outer capsid protein that target by Ab-1 and Ab-3 were identified. To further reveal the key amino acid residues in outer capsid protein required for Ab-1 and Ab-3 binding, a series of point mutants were generated in these five sites via alanine substitution. The results indicated that only mutant VP35H80A and VP35G73A demonstrated smaller binding energy to Ab-1 and Ab-3, respectively. Based on this result, we speculated five potential dominant epitopes Pep1-Pep5, and corresponding synthesized peptides demonstrated a certain reactivity with GCRV-II-positive serum. This result suggested that Pep1-Pep5 can be designed as vaccine antigen.
In the development process of immersion vaccine, it is very important to ensure that the antigen epitope can effectively pass through the fish mucosal barrier and transmit to APC [46]. To achieve the above purpose, we designed a novel nanoparticle vaccine Pep-Fn, which combines with cell-penetrating peptide, epitope peptides and self-assembled protein. Briefly, CPP and five epitopes were linked by two lysine, and then displayed on the surface of nanoparticle platform. CPP could effectively deliver protein inside cells, and the nano size of Pep-Fn is more easily uptaken by APCs [47, 48]. Meanwhile, the grass carp-derived ferritin was employed to further improve the biosafety and biocompatibility of Pep-Fn. After immersed with FITC-labeled vaccine, strong fluorescence signals were detected in spleen and kidney tissues. This result demonstrated the ability of Pep-Fn to penetrate the mucus barrier. Antibody is one of the most important effector molecules in humoral immune response [49]. The specific antibody level in serum and mucus were up-regulated in different degrees after vaccination. Consistent with this result, grass carp vaccinated with Pep-Fn showed higher survival rate than those vaccinated with Pep after GCRV-II challenge. These results indicated that Pep-Fn successfully induced the immune response in grass carp via immersion vaccination, thereby providing protective effect against GCRV-II. The above-mentioned results prove the effectiveness of the vaccine design strategy, that is, the combination of CPP and self-assembled protein are ideal vaccine delivery platform.
Undoubtedly, there are still certain limitations in this research. Specifically, the low capacity of the anti-GCRV-II antibody library limits the discovery of more high-affinity antibodies. This may require us to further enhance the efficiency of PCR amplification, antibody gene and phagemid ligation, and recombinant vector transformation [50]. In addition, due to the lack of sensitive cell lines for in vitro culture of GCRV-II, we did not verify whether Ab-1 and Ab-3 are neutralizing antibodies, and whether Pep1-Pep5 are neutralizing epitopes. Since the absence of neutralizing assays makes it difficult to assess the true protective potential of Pep-Fn. In order to further improve the possibility of Pep-Fn application, we also need to optimize the parameters of immersion immunization procedure, including different immunization time, vaccine concentration and fish destiny [51].
In conclusion, we successfully screened two high-affinity antibody Ab-1 and Ab-3 from anti-GCRV-II phage antibody library. Five potential epitopes Pep1-Pep5 on the outer capsid protein were identified through bioinformatics analysis. Moreover, we designed and constructed a multi-epitope nanoparticle vaccine that could provide a robust immune protection to grass carp through immersion immunization. This research provides new references for the design of nanoparticle vaccine, paving the way for its accessible and broader commercial application in aquaculture.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 1 Figure S1 The amino acid sequences of Ab-1(a) and Ab-3 (b).
Supplementary Material 2 Figure S2 The conservative analysis of Pep1-Pep5 in different strains of GCRV-II.
Supplementary Material 3 Figure S3 The change curve of positive serum antibody activity with peptide concentration. Serum from uninfected grass carp was used as control.
Acknowledgements
This work was supported by National Key Research and Development Program (2023YFD2402400).
Author contributions
Fei-Fan Xu: Data curation; Formal Analysis; Writing and reviewing-original draft. Zhao Zhao: Formal Analysis; Software; Writing-original draft. Zhu-Yang Deng: Data curation; Visualization. Jia-Lun Tang: Formal Analysis; Validation. Bin Zhu: Funding acquisition; Methodology; Project administration; Supervision.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
All the experiments were reviewed and approved by the Animal Experiment Committee, Northwest A&F University (DK2023159).
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei-Fan Xu and Zhao Zhao contributed equally to this work.
References
- 1.Wang Z, Xu C, Zhang Y, Huo X, Su J. Dietary supplementation with nanoparticle CMCS-20a enhances the resistance to GCRV infection in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2022;127:572–84. [DOI] [PubMed] [Google Scholar]
- 2.Zhou G, Xu D, Xu D, Zhang M. Southern rice black-streaked dwarf virus: a white-backed planthopper-transmitted fijivirus threatening rice production in Asia. Front Microbiol. 2013;4:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Wang X, Gao Y, Qi X. The over-40-years-epidemic of infectious bursal disease virus in China. Viruses. 2022;14(10):2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Cui K, Wang H, Liu F, Huang K, Duan Z, Wang F, Shi D, Liu Q. A milk-based self-assemble rotavirus VP6-ferritin nanoparticle vaccine elicited protection against the viral infection. J Nanobiotechnol. 2019;17(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su H, Su J. Cyprinid viral diseases and vaccine development. Fish Shellfish Immunol. 2018;83:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang G, Zhong L, Qian M, Wang M, Cui R. Filamentous bacteriophages, natural nanoparticles, for viral vaccine strategies. Nanoscale. 2022;14(16):5942–59. [DOI] [PubMed] [Google Scholar]
- 7.Gause KT, Wheatley AK, Cui J, Yan Y, Kent SJ, Caruso F. Immunological principles guiding the rational design of particles for vaccine delivery. ACS Nano. 2017;11(1):54–68. [DOI] [PubMed] [Google Scholar]
- 8.Mohsen MO, Gomes AC, Cabral-Miranda G, Krueger CC, Leoratti FM, Stein JV, Bachmann MF. Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J Control Release. 2017;251:92–100. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Zhao Z, Jia YJ, Zhang PQ, Sun Y, Zhou YC, Wang GX, Zhu B. Rationally designed self-assembling nanovaccines elicit robust mucosal and systemic immunity against rhabdovirus. ACS Appl Mater Interfaces. 2024;16(1):228–44. [DOI] [PubMed] [Google Scholar]
- 10.Skwarczynski M, Toth I. 2016. Peptide-based synthetic vaccines. Chem Sci. 2016 7(2): 842–854. [DOI] [PMC free article] [PubMed]
- 11.Chen R, Wen Y, Yu E, Yang J, Liang Y, Song D, Wen Y, Wu R, Zhao Q, Du S, Yan Q, Han X, Cao S, Huang X. Identification of an immunodominant neutralizing epitope of porcine Deltacoronavirus spike protein. Int J Biol Macromol. 2023;242(Pt 4):125190. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Sheng Y, Ji P, Deng Y, Sun Y, Chen Y, Nan Y, Hiscox JA, Zhou EM, Liu B, Zhao Q. A broad-specificity neutralizing nanobody against hepatitis E virus capsid protein. J Immunol. 2024;213(4):442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Yang X, Shi H, Zhang J, Feng T, Liu D, Zhang X, Chen J, Shi D, Feng L. Identification of two novel B-cell epitopes located on the spike protein of swine acute diarrhea syndrome coronavirus. Int J Biol Macromol. 2024;278(Pt 4):135049. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YY, Zhao L, Wei XF, Sun TZ, Xu FF, Wang GX, Zhu B. Vaccine molecule design based on phage display and computational modeling against rhabdovirus. J Immunol. 2024;212(4):551–62. [DOI] [PubMed] [Google Scholar]
- 15.Gong YM, Wei XF, Zheng YY, Li Y, Yu Q, Li PF, Zhu B. Combining phage display technology with in silico-designed epitope vaccine to elicit robust antibody responses against emerging pathogen tilapia lake virus. J Virol. 2023;97(4):e0005023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma H, Guo Y, Tang H, Tseng CK, Wang L, Zong H, Wang Z, He Y, Chang Y, Wang S, Huang H, Ke Y, Yuan Y, Wu M, Zhang Y, Drelich A, Kempaiah KR, Peng BH, Wang A, Yang K, Yin H, Liu J, Yue Y, Xu W, Zhu S, Ji T, Zhang X, Wang Z, Li G, Liu G, Song J, Mu L, Xiang Z, Song Z, Chen H, Bian Y, Zhang B, Chen H, Zhang J, Liao Y, Zhang L, Yang L, Chen Y, Gilly J, Xiao X, Han L, Jiang H, Xie Y, Zhou Q, Zhu J. Broad ultra-potent neutralization of SARS-CoV-2 variants by monoclonal antibodies specific to the tip of RBD. Cell Discov. 2022;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederhofer J, Tsybovsky Y, Nguyen L, Raab JE, Creanga A, Stephens T, Gillespie RA, Syeda HZ, FisherBE, Skertic M, Yap C, Schaub AJ, Rawi R, Kwong PD, Graham BS, McDermott AB, Andrews SF, King NP, Kanekiyo M. Protective human monoclonal antibodies target conserved sites of vulnerability on the underside of influenza virus neuraminidase. Immunity. 2024;57(3):574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frumento N, Sinnis-Bourozikas A, Paul HT, Stavrakis G, Zahid MN, Wang S, Ray SC, Flyak AI, Shaw GM, Cox AL, Bailey JR. Neutralizing antibodies evolve to exploit vulnerable sites in the HCV envelope glycoprotein E2 and mediate spontaneous clearance of infection. Immunity. 2024;57(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Sun Y, Shen Z, Wang C, Qian J, Mao Q, Wang Y, Song W, Kong Y, Zhan C, Chen Z, Dimitrov DS, Yang Z, Jiang S, Wu F, Lu L, Ying T, Sun L, Wu Y. Fully human single-domain antibody targeting a highly conserved cryptic epitope on the Nipah virus G protein. Nat Commun. 2024;15(1):6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, Holman LA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, Gaudinski MR, Kumar A, Chang LA, Moin SM, Hill JP, DiPiazza AT, Schwartz RM, Kueltzo L, Cooper JW, Chen P, Stein JA, Carlton K, Gall JG, Nason MC, Kwong PD, Chen GL, Mascola JR, McLellan JS, Ledgerwood JE, Graham BS, VRC 317 Study Team. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365(6452):505–9. [DOI] [PubMed] [Google Scholar]
- 21.Lin M, Yin Y, Zhao X, Wang C, Zhu X, Zhan L, Chen L, Wang S, Lin X, Zhang J, Xia N, Zheng Z. A truncated pre-F protein mRNA vaccine elicits an enhanced immune response and protection against respiratory syncytial virus. Nat Commun. 2025;16(1):1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren M, Abdullah SW, Pei C, Guo H, Sun S. Use of virus-like particles and nanoparticle-based vaccines for combating picornavirus infections. Vet Res. 2024;55(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ols S, Lenart K, Arcoverde Cerveira R, Miranda MC, Brunette N, Kochmann J, Corcoran M, Skotheim R, Philomin A, Cagigi A, Fiala B, Wrenn S, Marcandalli J, Hellgren F, Thompson EA, Lin A, Gegenfurtner F, Kumar A, Chen M, Phad GE, Graham BS, Perez L, Borst AJ, Karlsson Hedestam GB, Ruckwardt TJ, King NP, Loré K. Multivalent antigen display on nanoparticle immunogens increases B cell clonotype diversity and neutralization breadth to pneumoviruses. Immunity. 2023;56(10):2425–2441.e14. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Zhu J, Wang S, Li M, Sun X, Liu S, Wang Y, Li R, Zhang G. Modular nano-antigen display platform for pigs induces potent immune responses. ACS Nano. 2024;18(42):29152–77. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Gao Y, Su T, Zhang L, Zhou H, Zhang J, Sun H, Bai J, Jiang P. Nanoparticle vaccine triggers interferon-gamma production and confers protective immunity against porcine reproductive and respiratory syndrome virus. ACS Nano. 2025;19(1):852–70. [DOI] [PubMed] [Google Scholar]
- 26.Qian C, Liu X, Xu Q, Wang Z, Chen J, Li T, Zheng Q, Yu H, Gu Y, Li S, Xia N. Recent progress on the versatility of virus-like particles. Vaccines. 2020;8(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladenstein R, Fischer M, Bacher A. The lumazine synthase/riboflavin synthase complex: shapes and functions of a highly variable enzyme system. FEBS J. 2013;280(11):2537–63. [DOI] [PubMed] [Google Scholar]
- 28.Georgiev IS, Joyce MG, Chen RE, Leung K, McKee K, Druz A, Van Galen JG, Kanekiyo M, Tsybovsky Y, Yang ES, Yang Y, Acharya P, Pancera M, Thomas PV, Wanninger T, Yassine HM, Baxa U, Doria-Rose NA, Cheng C, Graham BS, Mascola JR, Kwong PD. Two-component ferritin nanoparticles for multimerization of diverse trimeric antigens. ACS Infect Dis. 2018;4(5):788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walls AC, Fiala B, Schäfer A, Wrenn S, Pham MN, Murphy M, Tse LV, Shehata L, O’Connor MA, Chen C, Navarro MJ, Miranda MC, Pettie D, Ravichandran R, Kraft JC, Ogohara C, Palser A, Chalk S, Lee EC, Guerriero K, Kepl E, Chow CM, Sydeman C, Hodge EA, Brown B, Fuller JT, Dinnon KH 3rd, Gralinski LE, Leist SR, Gully KL, Lewis TB, Guttman M, Chu HY, Lee KK, Fuller DH, Baric RS, Kellam P, Carter L, Pepper M, Sheahan TP, Veesler D, King NP. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell. 2020;183(5):1367–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, Hodge E, Benhaim M, Ravichandran R, Carter L, Sheffler W, Brunner L, Lawrenz M, Dubois P, Lanzavecchia A, Sallusto F, Lee KK, Veesler D, Correnti CE, Stewart LJ, Baker D, Loré K, Perez L, King NP. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019;176(6):1420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyoglu-Barnum S, Ellis D, Gillespie RA, Hutchinson GB, Park YJ, Moin SM, Acton OJ, Ravichandran R, Murphy M, Pettie D, Matheson N, Carter L, Creanga A, Watson MJ, Kephart S, Ataca S, Vaile JR, Ueda G, Crank MC, Stewart L, Lee KK, Guttman M, Baker D, Mascola JR, Veesler D, Graham BS, King NP, Kanekiyo M. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021;592(7855):623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin L, Zhang H, Zhou Y, Umeshappa CS, Gao H. Nanovaccine-based strategies to overcome challenges in the whole vaccination cascade for tumor immunotherapy. Small. 2021;17(28):e2006000. [DOI] [PubMed] [Google Scholar]
- 33.Suberi A, Grun MK, Mao T, Israelow B, Reschke M, Grundler J, Akhtar L, Lee T, Shin K, Piotrowski-Daspit AS, Homer RJ, Iwasaki A, Suh HW, Saltzman WM. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci Transl Med. 2023;15(709):eabq0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu FF, Deng ZY, Li JK, Chen LY, Liu YQ, Jiang HF, Zhu B. Self-assembled nanoparticle vaccine based on the grass carp ferritin remarkably enhance the protective immune responses against GCRV type II infection. Aquaculture. 2025;602:742349. [Google Scholar]
- 35.Dong S, Gao M, Bo Z, Guan L, Hu X, Zhang H, Liu B, Li P, He K, Liu X, Zhang C. Production and characterization of a single-chain variable fragment antibody from a site-saturation mutagenesis library derived from the anti-Cry1A monoclonal antibody. Int J Biol Macromol. 2020;149:60–9. [DOI] [PubMed] [Google Scholar]
- 36.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with alphafold. Nature. 2021;596(7873):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Beglov D, Vajda S. The ClusPro web server for protein-protein docking. Nat Protoc. 2017;12(2):255–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C, Zhao Z, Zhang PQ, Guo S, Zhu B. TLR2-mediated mucosal immune priming boosts anti-rhabdoviral immunity in early vertebrates. Antiviral Res. 2022;203:105346. [DOI] [PubMed] [Google Scholar]
- 39.Facciolà A, Visalli G, Laganà P, La Fauci V, Squeri R, Pellicanò GF, Nunnari G, Trovato M, Di Pietro A. The new era of vaccines: the nanovaccinology. Eur Rev Med Pharmacol Sci. 2019;23(16):7163–82. [DOI] [PubMed] [Google Scholar]
- 40.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–7. [DOI] [PubMed] [Google Scholar]
- 41.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348(6301):552–4. [DOI] [PubMed] [Google Scholar]
- 42.Nagano K, Tsutsumi Y. Phage display technology as a powerful platform for antibody drug discovery. Viruses. 2021;13(2):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, Chen C, Drelich A, Martinez DR, Gralinski LE, Sun Z, Schäfer A, Kulkarni SS, Liu X, Leist SR, Zhelev DV, Zhang L, Kim YJ, Peterson EC, Conard A, Mellors JW, Tseng CK, Falzarano D, Baric RS, Dimitrov DS. Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection. Proc Natl Acad Sci USA. 2020;117(47):29832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C, Zhang C, Zhong J, Hu H, Luo S, Liu X, Zhang X, Liu Y, Liu X. Construction of an immunized rabbit phage display library for selecting high activity against Bacillus thuringiensis Cry1F toxin single-chain antibodies. J Agric Food Chem. 2017;65(29):6016–22. [DOI] [PubMed] [Google Scholar]
- 45.Goydel RS, Rader C. Antibody-based cancer therapy. Oncogene. 2021;40(21):3655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papadopoulou A, Monaghan SJ, Bagwell N, Alves MT, Verner-Jeffreys D, Wallis T, Davie A, Adams A, Migaud H. Efficacy testing of an immersion vaccine against Aeromonas salmonicida and immunocompetence in ballan wrasse (Labrus bergylta, Ascanius). Fish Shellfish Immunol. 2022;121:505–15. [DOI] [PubMed] [Google Scholar]
- 47.Arabzadeh S, Amiri Tehranizadeh Z, Moalemzadeh Haghighi H, Charbgoo F, Ramezani M, Soltani F. Design, synthesis, and in vitro evaluation of low molecular weight protamine (LMWP)-based amphiphilic conjugates as gene delivery carriers. AAPS PharmSciTech. 2019;20:111. [DOI] [PubMed] [Google Scholar]
- 48.Song J, Wang M, Zhou L, Tian P, Sun Z, Sun J, Wang X, Zhuang G, Jiang D, Wu Y, Zhang G. A candidate nanoparticle vaccine comprised of multiple epitopes of the African swine fever virus elicits a robust immune response. J Nanobiotechnol. 2023;21(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura R, Sawada SI, Mukai SA, Sasaki Y, Akiyoshi K. Antigen delivery to antigen-presenting cells for adaptive immune response by self-assembled anionic polysaccharide nanogel vaccines. Biomacromolecules. 2020;21(2):621–9. [DOI] [PubMed] [Google Scholar]
- 50.Tohidkia MR, Barar J, Asadi F, Omidi Y. Molecular considerations for development of phage antibody libraries. J Drug Target. 2012;20(3):195–208. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Z, Xiong Y, Zhang C, Jia YJ, Qiu DK, Wang GX, Zhu B. Optimization of the efficacy of a SWCNTs-based subunit vaccine against infectious spleen and kidney necrosis virus in mandarin fish. Fish Shellfish Immunol. 2020;106:190–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1 Figure S1 The amino acid sequences of Ab-1(a) and Ab-3 (b).
Supplementary Material 2 Figure S2 The conservative analysis of Pep1-Pep5 in different strains of GCRV-II.
Supplementary Material 3 Figure S3 The change curve of positive serum antibody activity with peptide concentration. Serum from uninfected grass carp was used as control.
Data Availability Statement
No datasets were generated or analysed during the current study.