Abstract
Background
Bleeding after tooth extraction is a significant challenge, particularly in patients taking anticoagulants such as aspirin and warfarin, as the use of these medications increases the risk of prolonged bleeding. This study aimed to evaluate the effectiveness of the hemostatic agents, Surgicel and Gelfoam, in controlling bleeding and improving healing after extraction in this patient group.
Methods
A clinical study was conducted on 40 patients, divided into two groups based on the type of anticoagulant used (aspirin or warfarin). Each patient received treatment with different hemostatic dressings: Gelfoam was applied to one side and Surgicel to the other. Bleeding was assessed using the VIBe scale, and the rate of gingival healing was measured using the Gingival Healing Index (GHI) on days three (D3) and seven (D7). Pain was also assessed using the Visual Analogue Scale (VAS), and late bleeding rates were analyzed 24 h after extraction.
Results
The results showed that Surgicel was more effective than Gelfoam in achieving hemostasis and reducing delayed bleeding (p < 0.05). Patients using Surgicel also reported faster improvement in tissue healing compared to patients using Gelfoam, particularly on day 7 after extraction. Additionally, pain scores were significantly lower in patients treated with Surgicel compared to Gelfoam, reflecting its role in improving patient comfort and accelerating healing.
Conclusions
This study supports the use of Surgicel as a more effective option for controlling bleeding and promoting tissue healing in patients taking anticoagulants, reducing the need for treatment adjustments that may increase the risk of thrombosis. The study recommends further studies to evaluate the long-term benefits of using different hemostatic agents in this patient population.
Trial registration
The trial was retrospectively registered at the ISRCTN registry (ISRCTN19155058) on 29 May 2025.
Keywords: Post-extraction bleeding, Anticoagulants, Aspirin, Warfarin, Hemostatic, Gelfoam, Surgicel, Bleeding control
Introduction
Tooth extraction is one of the most common procedures in dentistry; however, it is often associated with complications such as bleeding, pain, inflammation, and infection, which require effective management by dental professionals [1, 2]. Post-extraction bleeding presents a particular challenge in patients who are on anticoagulants, such as aspirin and warfarin, as the use of these medications increases the risk of prolonged bleeding, especially during the first few days following extraction [2–4]. Therefore, the identification of effective strategies to control bleeding and enhance the quality of life for patients during the post-extraction period is essential in clinical practice.
The blood clot at the site of tooth extraction is a crucial element in the wound healing process, as it stimulates the necessary immune response for physiological bone healing [5]. If this clot is displaced, it can result in delayed wound healing and increased pain, particularly in the hours immediately following the procedure [6]. Additionally, uncontrolled bleeding may require emergency medical interventions, such as blood transfusions, and heightens the risk of infection, exacerbating pain and inflammation, which could complicate the healing process [7].
Anticoagulants, such as aspirin and warfarin, are widely used for the prevention of blood clots and to reduce the risk of stroke and heart disease [8, 9]. Aspirin is typically used at low doses as an antiplatelet agent [10], while warfarin is an effective anticoagulant in conditions like atrial fibrillation and heart valve diseases [11]. Despite the therapeutic benefits of these medications, their use increases the risk of bleeding during surgical procedures, such as tooth extraction, necessitating the search for safe and effective treatment solutions to control bleeding without discontinuing therapy [12]. This is important to avoid the complications associated with abrupt cessation of these drugs. In this context, topical hemostatic agents play a significant role in achieving immediate hemostasis without affecting systemic blood clotting, allowing patients to continue their treatment without an increased risk of bleeding or thrombosis.
Hemostatic dressings are among the most effective means of controlling bleeding after tooth extraction, offering a safe mechanism for achieving local hemostasis without the need to adjust anticoagulant dosages [13]. Among these agents, Gelfoam and Surgicel are widely used hemostatic dressings in surgical procedures, including oral and maxillofacial surgeries [14]. Gelatin-based hemostatic materials were first developed in 1945, demonstrating their effectiveness in promoting blood clot formation [15]. Gelfoam is a highly absorbent gelatin sponge that acts as a mechanical barrier, gradually enhancing clot formation and being biologically resorbable over time [16, 17]. In contrast, Surgicel is composed of oxidized cellulose, which accelerates the clotting process through direct interaction with platelets. It also provides a protective barrier against infection, thereby reducing the risk of post-surgical infections [18–20].
The determination of the optimal choice between Gelfoam and Surgicel depends on several factors, including the speed of hemostasis, compatibility with oral tissues, and their effect on wound healing. While both Gelfoam and Surgicel have proven effective in reducing bleeding, their use in patients with bleeding disorders still requires further extensive clinical studies to determine which dressing provides better results with fewer complications [14].
This study aims to evaluate and compare the effectiveness of Gelfoam and Surgicel in controlling bleeding in patients on aspirin and warfarin therapy, ensuring the provision of safe and effective solutions for managing bleeding during tooth extraction procedures. Additionally, the study seeks to address the knowledge gap in the use of hemostatic agents in patients with bleeding disorders by providing clinical data that may contribute to improving treatment practices and the selection of the optimal dressing for each case, thereby enhancing the quality of care for patients at risk of bleeding.
Materials and methods
Study design and ethical considerations
This investigation was designed as a prospective, single-blinded, split-mouth, active-controlled clinical trial, adhering strictly to the ethical standards for biomedical research involving human subjects. The study protocol was developed and executed in complete alignment with the principles outlined in the Declaration of Helsinki [21] and followed the reporting standards provided by the CONSORT statement [22] to ensure transparency and methodological rigor. The study was conducted at the Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Damascus University, between November 2022 and March 2025. Ethical approval was obtained from the institutional Biomedical Research Ethics Committee (DN-150525-H27), and the study was retrospectively registered and approved in the ISRCTN registry (ISRCTN19155058) on 29/05/2025. The treatment protocol was standardized, and all procedures adhered to ethical guidelines. Participation was voluntary, with complete confidentiality ensured. Patients retained the right to withdraw at any point without impacting their future care.
Sample size calculation
The sample size was determined using G*Power V 3.1.9.4 software to ensure the accuracy of statistical analysis. The calculation was based on effect size (f = 0.47), significance level (α = 0.05), and (95%) statistical power (1 - β err prob), utilizing an ANOVA test. Based on these parameters, the minimum sample size was determined to be 40 patients, who were assigned to the two groups according to the inclusion criteria specified in the study. To refine the accuracy of the calculations, a pilot study was conducted on 10 patients to estimate the effect size.
Eligibility criteria
Inclusion and exclusion criteria were defined to ensure the selection of a homogeneous patient sample, which would allow for reliable results and minimize factors that could influence the accuracy of the statistical analysis.
Inclusion criteria
Patients were selected based on the following criteria:
Age between 40 and 75 years.
Patients use aspirin at a daily dose of 80 mg or oral warfarin.
Patients using warfarin must have a stable INR value within the therapeutic range (2-3.5) [23].
Obtaining written informed consent from all participants, with their commitment to maintaining the anticoagulant doses unchanged throughout the study period.
Requirement for simple tooth extractions of paired teeth, without the need for complex surgical intervention.
Exclusion criteria
Patients who could potentially affect the study results or who had conditions that precluded their participation were excluded based on the following criteria:
Presence of uncontrolled systemic diseases that contraindicate tooth extraction.
Participation in other clinical trials during the study period is necessary to avoid treatment overlap and influence on results.
Unstable INR, where patients with fluctuations in INR outside the required therapeutic range (2-3.5) before extraction or during follow-up were excluded [23].
Severe alcohol or tobacco dependence due to their negative effects on wound healing post-extraction.
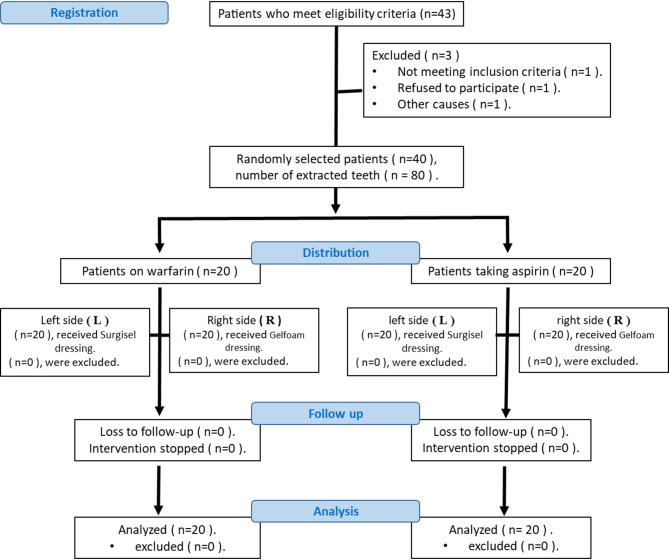
The CONSORT diagram is provided to illustrate the distribution of patients into different treatment groups according to the study protocol.
Figure 1 illustrates the CONSORT flow diagram. A total of 20 aspirin users and 20 warfarin users were evaluated in the Department of Oral and Maxillofacial Surgery.
Fig. 1.
CONSORT Flow Diagram
Patients will be divided into two groups:
Group A (Aspirin Users) (n = 20): Gelfoam (G) will be applied to the right side (R) and Surgicel (S) to the left side (L).
Group W (Warfarin Users) (n = 20): Gelfoam (G) will be applied to the right side (R) and Surgicel (S) to the left side (L).
Based on the split-mouth design, each patient has undergone treatment on both sides to ensure a fair comparison between the dressings [24]. Patients were assigned to the two groups based on the type of anticoagulant therapy used post-extraction. The dressings used include Gelfoam (Hemosponge) of size 10 × 10 × 10 mm from India and Surgicel (SURGICEL™) of size 0.5 × 2 cm from Switzerland.
Therapeutic procedure
Demographic data, along with medical and dental history, were recorded for all patients. Necessary clinical and radiographic examinations were performed. To ensure eligibility, consulting physicians were consulted to confirm the stability of the patient’s health, excluding those with unstable medical conditions. The INR value was measured before the extraction using the CoaguChek® XS device (Roche Diagnostics, Indiana, USA) to ensure it was within the therapeutic range (2-3.5) without requiring discontinuation of warfarin [23]. All extractions were performed by a skilled surgeon experienced in managing patients with bleeding disorders, ensuring standardized procedures, and minimizing clinical errors.
Local anesthesia was administered using 2% lidocaine with 1:80,000 epinephrine (Hons Ltd., Seongnam, South Korea) via a dental syringe (Dental Lab, Guangdong, China) and a 27 × ¾ inch needle (J. Morita, Connecticut, USA) to ensure effective pain and bleeding control during the procedure. The extraction was performed by an atraumatic extraction technique using periotomes, which was employed to preserve the socket structure and minimize trauma [1, 25].
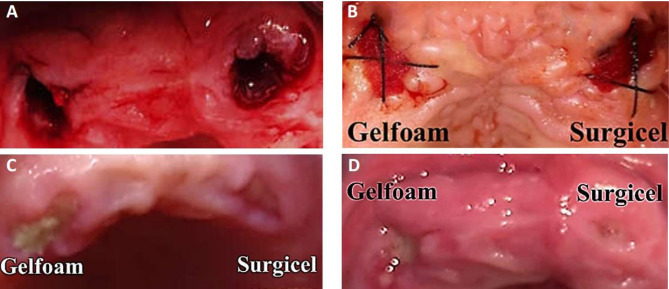
To ensure hemostasis, one of the hemostatic dressings (Gelfoam or Surgicel) was applied directly into the dental socket using a dry, sterile instrument and secured with a figure-of-8 suture using 3.0 silk sutures (TUDOR® DVR-4942, Champion Biotech & Pharma Corp, Manila, Philippines). For clinical documentation and monitoring of the healing process, clinical photographs of the site were taken immediately after the extraction (Fig. 2A) and dressing application (Fig. 2B), then on the third day (Fig. 2C), and finally on the seventh day (Fig. 2D) to evaluate wound healing during the first-week post-extraction.
Fig. 2.
A After extraction, B Bandage application, C After three days, D After seven days
Primary outcome measure - clinical variable study
Bleeding severity
Bleeding severity was assessed using the Validated Intraoperative Bleeding Scale (VIBe) at two different time points following tooth extraction. This scale is the first bleeding severity scale validated by the U.S. Food and Drug Administration, specifically developed to study the effects of hemostatic agents during surgical procedures. The scale classifies bleeding into several grades based on its severity and impact on surgical visibility, with the following classifications:
(0) No visible bleeding.
(1) Mild bleeding that does not affect visibility and does not require additional intervention.
(2) Mild bleeding that obscures visibility but does not necessitate halting the surgery.
(3) Moderate bleeding that affects visibility and may require repeated suction or temporary cessation of the surgery.
(4) Severe bleeding that obstructs visibility and requires intensive interventions.
(5) Acute, life-threatening bleeding that makes visibility impossible and demands emergency measures to stop the hemorrhage [26].
Bleeding severity was measured at two time points: T0, immediately after extraction, to evaluate the effectiveness of the hemostatic dressing in controlling primary bleeding, and T1, two hours after the extraction, to assess reactionary bleeding, which typically occurs two hours post-extraction and is more common in patients with bleeding disorders [27]. Bleeding was evaluated in both groups using the VIBe scale to confirm the formation of a blood clot.
Delayed bleeding
Delayed bleeding was monitored for up to 24 h following tooth extraction through clinical evaluation [28–30], as well as follow-up phone calls to ensure that no subsequent bleeding occurred.
Gingival healing index
The Gingival Healing Index, developed by Landry et al. [31], was utilized in this study to assess the healing process following tooth extraction on the third (D3) and seventh (D7) days. The index aims to evaluate the tissue response, initiate the healing process, track its progression, and identify the need for additional interventions to accelerate healing.
The index is based on five levels to classify the healing status:
Very Poor Healing: Significant delay in healing with symptoms such as inflammation or ulcers.
Poor Healing: Some improvement, but with ongoing bleeding, pain, or inflammation.
Good Healing: Noticeable healing progress with mild side effects such as redness or swelling.
Very Good Healing: Substantial improvement in healing with minimal signs of ongoing inflammation.
Excellent Healing: Ideal healing with no signs of inflammation or complications.
Visual analog scale (VAS)
The pain was subjectively assessed by patients using the Visual Analog Scale (VAS) at three time points following tooth extraction and hemostatic dressing application: Day 1 (D1) to assess initial postoperative pain, Day 3 (D3) to evaluate pain as healing commenced, and Day 7 (D7) to assess pain reduction over time. Patients rated their pain intensity on a Visual Analogue Scale (VAS) on designated days after receiving instruction on how to use the scale. They were clinically examined on days 3 and 7 [32]. If the pain score exceeded six on the VAS, patients were advised to take 500 mg paracetamol tablets three times daily (total of 1.5 g) for pain relief, with the recommendation to avoid analgesics within eight hours prior to the next assessment to prevent interference with the measurement [33]. The VAS scores were categorized as follows: 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, 7–9 = severe pain, and 10 = worst possible pain [34]. The inter-rater reliability coefficient (Kappa) for the examiner was > 0.8.
Statistical analysis
The data were analyzed using GraphPad Prism V9, where appropriate statistical tests were applied, including the independent t-test to compare groups and ANOVA to analyze differences between measurement times within each group. Descriptive analysis was used to determine means and standard deviations, in addition to Chi-square tests to assess relationships between categorical variables. A difference was considered statistically significant when the p-value was < 0.05.
Results
Based on the inclusion criteria, 40 patients were recruited (20 patients taking aspirin and 20 patients taking warfarin), with 80 corresponding teeth extracted per patient. Of the 80 teeth extracted, 48 were molars (60%), 20 premolars (25%), and 12 anterior teeth (15%). The distribution of tooth types was balanced across treatment sides, minimizing bias related to anatomical site. Participants were recruited between January 2022 and March 2025. Table 1 shows the basic demographic and clinical characteristics of the study participants. More than half of the participants were female (22; 55%), and male participants (18; 45%) had an average age of 58.1 years (standard deviation 6.89; range 40–75 years). The mean INR value was 2.58 (standard deviation 0.32; range 2.1–3.1).
Table 1.
Baseline demographic and clinical characteristics of study participants
| Characteristics | n = 40 |
|---|---|
| Sex | |
| Female n (%) | 22 (55%) |
| Male n (%) | 18 (45%) |
| Age (years) | |
| Mean ± SD | 58.1 ± 6.89 |
| Min – Max | 40–75 |
| INR value | |
| Mean ± SD | 2.58 ± 0.32 |
| Min – Max | 2.1–3.1 |
Analysis of bleeding severity using the vibe scale
The severity of bleeding was assessed using the VIBe Scale after tooth extraction at two distinct time points: T0 (immediately after extraction) to evaluate the efficacy of hemostatic dressings in controlling primary bleeding, and T1 (two hours after extraction) to monitor for reactionary bleeding following the subsidence of the vasoconstrictive effect.
The VIBe Scale was applied to quantify the bleeding severity for each treatment group (aspirin and warfarin) based on the effect of the hemostatic agents, Gelfoam and Surgicel. The scale categorizes bleeding severity from 0 (no visible bleeding) to 5 (severe and life-threatening bleeding requiring emergency measures), depending on its impact on the surgical field and the need for additional intervention.
The results revealed significant differences in bleeding severity between the treatment groups, as shown in Table 2. In the aspirin group, patients who received Surgicel exhibited lower bleeding values than those who received Gelfoam. The VIBe score was higher in patients who used Gelfoam than those who used Surgicel at T0, with this difference persisting at T1, indicating better control of bleeding with Surgicel.
Table 2.
Comparison of bleeding severity according to the vibe score at time points T0 and T1 for each treatment group
| Group | Distinct Time | R (Gelfoam) | L (Surgicel) |
|---|---|---|---|
| Average ± SD | Average ± SD | ||
| Aspirin | T0 | 2.0 ± 1.03 | 1.8 ± 0.95 |
| Aspirin | T1 | 2.2 ± 1.10 | 1.75 ± 0.79 |
| Warfarin | T0 | 2.45 ± 1.00 | 2.1 ± 1.02 |
| Warfarin | T1 | 2.65 ± 0.99 | 2.4 ± 1.05 |
In the warfarin group, bleeding severity was generally higher compared to the aspirin group. Patients who used Gelfoam recorded higher bleeding values at T0, which increased significantly at T1, whereas patients who used Surgicel exhibited less bleeding and greater stability two hours post-extraction, suggesting its superiority in controlling reactionary bleeding.
Statistical analyses using ANOVA for repeated measures demonstrated a significant temporal difference in bleeding severity across time points (p < 0.0001). Independent t-tests further confirmed that warfarin users experienced greater bleeding compared to aspirin users across all time points (p < 0.0001). Additionally, paired t-tests revealed that Surgicel was more effective than Gelfoam in reducing bleeding, with a statistically significant difference (p < 0.0001). These findings suggest that Surgicel represents a more effective option for controlling bleeding after tooth extraction, particularly in patients prone to excessive bleeding, such as those on anticoagulant therapy.
A subgroup analysis was conducted comparing bleeding scores between patients with INR values of 2.1–2.5 and those with INR values of 2.6–3.1. While a trend toward increased bleeding in the higher INR subgroup was observed, the difference was not statistically significant (p > 0.05).
Delayed bleeding results
Delayed bleeding was assessed within the first 24 h after tooth extraction through direct clinical follow-up and phone calls with the patients. The results showed in Table 3 that the rate of delayed bleeding was higher in patients taking warfarin than those on aspirin, which aligns with the known effect of anticoagulants in prolonging bleeding time. In the aspirin group, the incidence of delayed bleeding was 10% with Gelfoam and 5% with Surgicel, indicating better control of Surgicel. In the warfarin group, the incidence of delayed bleeding was 15% with Gelfoam and 10% with Surgicel, confirming that patients using Gelfoam were more prone to delayed bleeding compared to those using Surgicel.
Table 3.
Frequency and percentage distribution of delayed bleeding occurrence or Non-Occurrence in the groups
| Group | Bandage | Frequency Distribution | Percentile Distribution | ||||
|---|---|---|---|---|---|---|---|
| Bleeding | Non-Bleeding | P (n) | Bleeding | Non-Bleeding | P (%) | ||
| Aspirin | R (Gelfoam) | 2n= | 18n= | 20n= | 10% | 90% | 100% |
| L (Surgicel) | n = 1 | n = 19 | 20n= | 5% | 95% | 100% | |
| Warfarin | R (Gelfoam) | n = 3 | n = 17 | 20n= | 15% | 85% | 100% |
| L (Surgicel) | 2n= | n = 18 | 20n= | 10% | 90% | 100% | |
Statistical analysis using the Chi-square Test confirmed significant differences between Gelfoam and Surgicel in controlling delayed bleeding (p < 0.05), with Surgicel proving more effective in reducing delayed bleeding compared to Gelfoam. The analysis also showed that delayed bleeding was more common in the warfarin group compared to the aspirin group (p < 0.05). These results support the hypothesis that Surgicel provides more effective control of delayed bleeding after tooth extraction than Gelfoam due to its hemostatic properties that help stabilize the blood clot and prevent its breakdown after extraction.
Gingival healing index
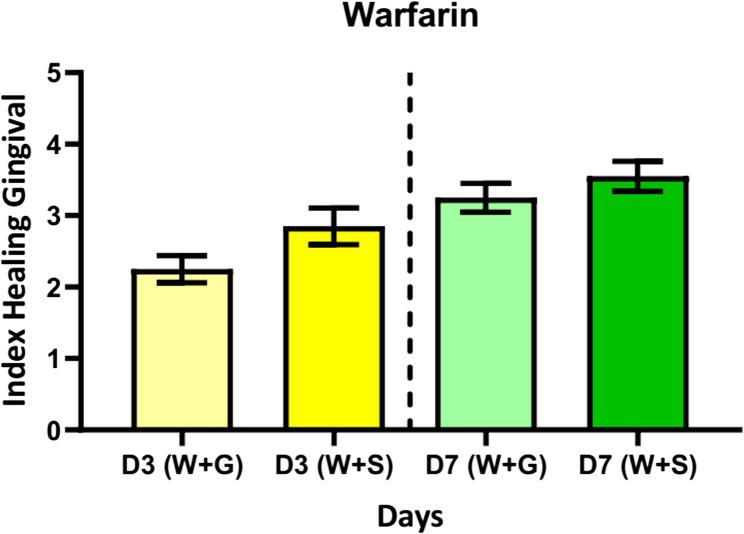
The gingival healing rates were assessed using the Gingival Healing Index on the third (D3) and seventh (D7) days after tooth extraction to compare the effects of Surgicel and Gelfoam in patients taking aspirin and warfarin. The results showed a gradual improvement in healing rates over time, with patients in the aspirin group showing higher healing rates than the warfarin group. As shown in Fig. 3, at D3, the average gingival healing rate for the Gelfoam group was (3 ± 1.07), improving to (3.6 ± 0.99) at D7, while for the Surgicel group, it was (3.35 ± 1.03) at D3 and (3.85 ± 0.93) at D7. These findings suggest that Surgicel is superior in promoting tissue healing. In the warfarin group, patients who used Gelfoam exhibited a lower healing rate, with an average score of (2.25 ± 0.85) at D3, which improved to (3.25 ± 0.91) at D7. On the other hand, patients who used Surgicel demonstrated a more noticeable improvement, with a score of (2.85 ± 1.13) at D3 and (3.55 ± 0.94) at D7, as shown in Fig. 4. Statistical analysis using the Paired T-test revealed a significant difference (p < 0.05) between Surgicel and Gelfoam within each group, indicating that Surgicel was more effective in promoting tissue healing. Furthermore, Independent T-tests showed that patients taking warfarin had lower healing rates compared to those taking aspirin, with a statistically significant difference (p < 0.05) on D7. These results confirm that Surgicel provides a faster healing response compared to Gelfoam, making it a more effective option, especially for patients on anticoagulants such as warfarin. Surgicel contributes to improved blood clot stability and reduces potential complications following tooth extraction.
Fig. 3.
Healing rate for aspirin patients over days
Fig. 4.
Healing rate for warfarin patients over days
Pain assessment results (VAS Scale)
The severity of pain was assessed using the Visual Analog Scale (VAS), which is an ordinal scale composed of 11 levels at three time points after tooth extraction: Day 1 (D1), Day 3 (D3), and Day 7 (D7). The results showed significant differences between the treatment groups across the different time periods, as shown in Table 4.
Table 4.
Descriptive statistics for mean VAS scores at different time points for the study groups
| Day | Group | Average | SD | Max | Min | |
|---|---|---|---|---|---|---|
|
Warfarin (n = 20) |
D1 | (W + G) | 8.25 | 0.79 | 9 | 7 |
| (W + S) | 6.90 | 0.97 | 9 | 6 | ||
| D3 | (W + G) | 5.60 | 0.60 | 7 | 5 | |
| (W + S) | 4.55 | 0.60 | 6 | 4 | ||
| D7 | (W + G) | 3.65 | 0.59 | 5 | 3 | |
| (W + S) | 2.15 | 0.67 | 3 | 1 | ||
|
Asprin (n = 20) |
D1 | (A + G) | 8.05 | 0.69 | 9 | 7 |
| (A + S) | 5.85 | 0.81 | 7 | 5 | ||
| D3 | (A + G) | 4.75 | 0.64 | 6 | 4 | |
| (A + S) | 3.50 | 0.61 | 5 | 3 | ||
| D7 | (A + G) | 2.65 | 0.49 | 3 | 2 | |
| (A + S) | 1.65 | 0.49 | 2 | 1 |
On Day 1 (D1), patients taking warfarin reported higher pain levels compared to those taking aspirin. The average VAS scores in the warfarin group were 8.25 with Gelfoam and 6.9 with Surgicel, while in the aspirin group, the average pain score was 8.05 with Gelfoam and 5.85 with Surgicel. As the healing process progressed, a marked decrease in pain levels was observed by Day 3 (D3). In the warfarin group, the average VAS score was 5.6 with Gelfoam and 4.55 with Surgicel, while in the aspirin group, the average scores were 4.75 with Gelfoam and 3.5 with Surgicel. On Day 7 (D7), the noticeable decline in pain continued, with the average VAS scores in the warfarin group being 3.65 with Gelfoam and 2.15 with Surgicel, and in the aspirin group, 2.65 with Gelfoam and 1.65 with Surgicel, indicating recovery and reduced pain over time.
Statistical analysis of these results using a repeated-measures ANOVA test confirmed a significant time-dependent reduction in pain levels in both groups (p < 0.0001). Independent t-tests showed that patients on warfarin experienced more pain compared to aspirin users across all time points (p < 0.0001). Additionally, paired t-tests confirmed that Surgicel was more effective than Gelfoam in reducing pain, with a statistically significant difference (p < 0.0001).
These results suggest that Surgicel may be a better option for pain management following tooth extraction compared to Gelfoam, likely due to its hemostatic and anti-inflammatory properties, which help reduce inflammation and promote tissue healing, thereby improving patient comfort post-surgery. These findings align with previous research demonstrating Surgicel’s efficacy in reducing inflammation and accelerating wound healing, making it a preferred choice for pain control after dental extractions. However, since both extractions were performed on the same day, patients may have experienced generalized or overlapping pain sensations, which limits the precision of pain localization to one side. Future studies may consider staging extractions to improve the accuracy of pain assessment.
Discussion
The formation of a stable blood clot after tooth extraction is a crucial factor in ensuring proper tissue healing and minimizing the risk of bleeding and postoperative complications [5]. A stable blood clot is essential for sealing the extraction site and promoting tissue and bone healing. However, patients taking anticoagulants such as aspirin and warfarin are at a higher risk of prolonged bleeding due to the effects of these medications on coagulation mechanisms [8, 9]. This may lead to recurrent bleeding, delayed wound healing, and an increased likelihood of complications such as dry sockets [35]. Therefore, using effective topical hemostatic agents has become essential to mitigate these risks and improve clinical outcomes following tooth extraction. Hemostatic agents such as Gelfoam and Surgicel are effective in achieving hemostasis after tooth extraction [14, 36]. Gelfoam, an absorbable gelatin sponge, acts as a physical barrier that absorbs blood and provides a surface for the activation of natural coagulation mechanisms, aiding in the formation of a stable blood clot [6, 15, 17]. In contrast, Surgicel, an oxidized cellulose material, offers a dual action by enhancing coagulation through its interaction with platelets while also possessing antimicrobial properties that reduce the risk of infection and wound inflammation [19, 20, 37]. The importance of using these hemostatic agents in patients on anticoagulant therapy lies in their ability to control bleeding without requiring the discontinuation of medication, thereby maintaining the balance between hemostasis and anticoagulation and reducing the risk of thrombotic events associated with drug withdrawal [13]. This finding is consistent with previous reports by Soliman et al. (2023), who found Surgicel superior to Gelfoam in achieving intraoperative hemostasis in vascular surgery patients [38]. Similarly, a clinical study by Richardson et al. (2022) in oral surgery reported that Surgicel provided faster and more stable hemostasis compared to gelatin-based agents [14].
The study included 40 patients who underwent a total of 80 tooth extractions. They were divided into two groups based on the type of anticoagulant used: the warfarin group (W) and the aspirin group (A), with 20 patients in each group. Gelfoam was applied to the right side (R) and Surgicel to the left side (L) in both groups, allowing for a direct comparison of the hemostatic effectiveness and healing outcomes of the two dressings. In terms of demographic distribution, females constituted 55% of the study population, while males accounted for 45%, with a relatively even distribution across both groups. The patients’ ages ranged from 45 to 75 years, with a mean age of 58.10 ± 6.89 years, reflecting a focus on an age group more susceptible to anticoagulant-related risks [39]. Although the distribution of extracted tooth types was not the primary variable of interest, we ensured that the types (molars, premolars, and anterior teeth) were relatively evenly distributed between the treatment sites. This balance reduces the likelihood that differences in healing outcomes were due to the anatomical variability of the extraction site.
Strict exclusion criteria were applied to ensure the accuracy of results and the homogeneity of the sample. Patients with uncontrolled systemic diseases that could affect the surgical procedure or impair wound healing were excluded [40]. Additionally, participants involved in other research studies during the same period were excluded to prevent treatment interference and its potential impact on outcomes [41]. Moreover, heavy smokers and alcohol-dependent individuals were excluded due to their negative impact on wound healing and the increased likelihood of post-extraction complications [40]. Patients with unstable INR values (outside the therapeutic range of 2–3.5) were also excluded due to the increased risk of bleeding and its potential effect on statistical accuracy [42]. While all patients included in the study had INR values within the therapeutic range (2.1–3.1), this range still represents a spectrum of coagulation status that may influence bleeding risk. A post hoc analysis showed a trend toward increased bleeding in patients with INR values > 2.5, although the difference was not statistically significant. Larger studies may be needed to determine whether narrower INR stratification reveals clinically meaningful differences.
This study aimed to evaluate the effectiveness of Surgicel and Gelfoam in controlling bleeding, reducing pain, and promoting tissue healing after tooth extraction in patients taking aspirin and warfarin. The findings demonstrated the superiority of Surgicel in achieving hemostasis and reducing bleeding compared to Gelfoam. Patients treated with Surgicel recorded lower VIBe Scores at T0 and T1, indicating a more efficient hemostatic response. Additionally, patients on warfarin experienced higher bleeding levels than those on aspirin, consistent with previous studies on the increased surgical bleeding risk associated with anticoagulants [43, 44]. The results showed a gradual decrease in pain intensity over D1, D3, and D7, with Surgicel proving more effective pain reduction than Gelfoam [38]. Patients treated with Surgicel reported significantly lower VAS Scores, particularly on Day 3 and Day 7, suggesting its potential role in minimizing inflammation and enhancing post-extraction comfort. These findings align with previous evidence by Mp (2016), indicating that oxidized cellulose dressings, such as Surgicel, may aid in reducing local inflammation and improving tissue healing.
Regarding gingival healing, our findings indicate that Surgicel-treated sites exhibited higher Gingival Healing Index scores by Day 7. These results corroborate studies by Al-Attar et al. (2023) [45] and Genyk et al. (2016) [46]. This suggests that Surgicel stabilizes the blood clot, accelerating healing and reducing potential complications [47]. Furthermore, patients on warfarin experienced more bleeding and slower healing compared to those on aspirin, consistent with existing literature highlighting the greater anticoagulant potency of warfarin by Yoshikawa et al., and Lee et al. [3, 12].
Late bleeding was assessed 24 h post-extraction [38], and the study found that patients treated with Surgicel were less likely to experience late bleeding than those treated with Gelfoam; this difference was statistically significant (p < 0.05).
These findings underscore the clinical value of Surgicel as a preferred hemostatic agent in patients undergoing dental extraction while on anticoagulant therapy. Its application was associated with reduced risk of clot disintegration and a lower incidence of late bleeding events. Based on the outcomes of this study, Surgicel may offer superior efficacy in controlling post-extraction bleeding, promoting tissue healing, and alleviating postoperative pain, particularly in patients treated with aspirin or warfarin. It is important to note, however, that postoperative bleeding following dental extractions is relatively rare, even among anticoagulated patients. Although this study focused on a high-risk population, the overall incidence of bleeding events remained low, which may limit the generalizability of the findings. Further long-term, multicenter studies with larger sample sizes are recommended to assess the extended clinical benefits of hemostatic agents and to compare their impact on healing and patient comfort across broader patient populations. Moreover, future research should consider the inclusion of patients treated with novel oral anticoagulants such as direct oral anticoagulants (DOACs) to evaluate their bleeding profiles and healing responses in comparison with traditional agents like aspirin and warfarin. This study also has several limitations. Notably, the absence of a suture-only control group prevented us from assessing baseline healing and bleeding outcomes without hemostatic intervention. While the split-mouth design provided robust intra-individual comparisons, future studies should consider including a no-hemostat control arm to more clearly define the added benefit of hemostatic dressings in anticoagulated patients. Another limitation involves the assessment of postoperative pain. As both surgical sites were treated in a single session, patients may have experienced difficulty in localizing discomfort to a specific side. Although the split-mouth approach reduces intersubject variability, it may compromise the accuracy of pain differentiation. Future studies could improve on this by staging extractions and allowing sufficient healing time between procedures.
Conclusions
This study highlights the importance of selecting appropriate hemostatic agents to improve the outcomes of tooth extractions in patients taking anticoagulants such as aspirin and warfarin. The primary challenge lies in effectively controlling bleeding without compromising anticoagulant efficacy or increasing the risk of complications. The results demonstrate that Surgicel provides superior hemostatic control compared to Gelfoam, as it helps reduce delayed bleeding, accelerates gingival tissue healing, and minimizes pain, making it the preferred treatment option for this patient group.
Acknowledgements
The authors thank the University of Damascus for providing support throughout this research project.
Abbreviations
- CONSORT
Consolidated Standards of Reporting Trials
- ISRCTN
International Standard Randomized Controlled Trial Number
- INR
International Normalized Ratio
- VIBe
Validated Intraoperative Bleeding
- GHI
Gingival Healing Index
- VAS
Visual Analogue Scale
- D1, D3, D7
Day 1, Day 3, Day 7 (postoperative time points)
- T0
Immediately After Extraction
- T1
Two Hours After Extraction
- RCT
Randomized Clinical Trial
- DOACs
Direct Oral Anticoagulants
Authors’ contributions
W.I. experimented and drafted the manuscript. W.S. wrote the manuscript and performed the statistical analysis. W.I. planned the experiments, supervised the project, and critically reviewed the manuscript. All authors have read and approved the manuscript.
Funding
Not applicable.
Data availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was provided by the Biomedical Research Ethics Committee of the Faculty of Dentistry, Damascus University (Approval ID: DN-150525-H27). The trial was retrospectively registered at the ISRCTN registry (ISRCTN19155058) on 29/05/2025. All participants signed written informed consent before enrollment. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that there is no conflict of interest related to the publication of this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gadhia A, Pepper T. Oral surgery, [internet]xtraction of [internet]eeth, in StatPearls [Internet]. StatPearls Publishing; 2023.
- 2.Goswami A, et al. A general overview of post extraction complications-prevention, management and importance of post extraction advices. Fortune J Health Sci. 2020;3(3):135–47. [Google Scholar]
- 3.Yoshikawa H, et al. Safety of tooth extraction in patients receiving direct oral anticoagulant treatment versus warfarin: a prospective observation study. Int J Oral Maxillofac Surg. 2019;48(8):1102–8. [DOI] [PubMed] [Google Scholar]
- 4.Ono S, et al. Risk of post-extraction bleeding with direct oral anticoagulant compared with warfarin: retrospective cohort study using large scale claims data in Japan. Thromb Res. 2023;222:24–30. [DOI] [PubMed] [Google Scholar]
- 5.de Gomes S. Molecular and cellular aspects of socket healing in the absence and presence of graft materials and autologous platelet concentrates: a focused review. J Oral Maxillofacial Res. 2019;10(3):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajkin BV, et al. Dental extractions and risk of bleeding in patients taking single and dual antiplatelet treatment. Br J Oral Maxillofac Surg. 2015;53(1):39–43. [DOI] [PubMed] [Google Scholar]
- 7.Banasiewicz T et al. Principles of minimize bleeding and the transfusion of blood and its components in operated patients-surgical aspects. Polski Przegląd Chirurgiczny. 2023;95(5). https://pubmed.ncbi.nlm.nih.gov/38084044. [DOI] [PubMed]
- 8.Steinhubl SR, et al. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150(6):379–86. [DOI] [PubMed] [Google Scholar]
- 9.Framework MRCsGPR. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351(9098):233–41. [PubMed] [Google Scholar]
- 10.Ren Z, et al. Comparing efficacy and safety of low-dose versus standard-dose antiplatelet therapy in stroke patients: a meta-analysis. Front Pharmacol. 2025;15:1484130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawwas GK, Lewis JD, Cuker A. Direct oral anticoagulants compared with warfarin in patients with atrial fibrillation and valvular heart disease without mechanical valves. J Am Heart Association. 2025;14(4):e035478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J-I, et al. Analysis of postoperative bleeding after oral surgery in patients receiving anticoagulants: A retrospective study. Medicina. 2025;61(3):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kämmerer PW, et al. Oral surgery during therapy with anticoagulants—a systematic review. Clin Oral Invest. 2015;19(2):171–80. [DOI] [PubMed] [Google Scholar]
- 14.Richardson S, Khandeparkar R, Banerjee P. The role of gelfoam and surgicel in cases of Le fort I advancement in achieving effective haemostatais. Adv Oral Maxillofacial Surg. 2022;8:100325. [Google Scholar]
- 15.Irfan NI, et al. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent J. 2022;34(8):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Campos N, Furlaneto F, Buischi YDP. Bleeding in dental surgery. 2019.
- 17.Clements W, et al. Let Us settle the controversy—gelfoam is a safe intravascular embolic agent. Br J Radiol. 2024;97(1157):933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mp SK. Local hemostatic agents in the management of bleeding in oral surgery. Asian J Pharm Clin Res. 2016;9(3):35–41. [Google Scholar]
- 19.Schreiber MA, Neveleff DJ. Achieving hemostasis with topical hemostats: making clinically and economically appropriate decisions in the surgical and trauma settings. AORN J. 2011;94(5):S1–20. [DOI] [PubMed] [Google Scholar]
- 20.Masoudi M, Wiseman J, Wiseman SM. A contemporary systematic review of the complications associated with SURGICEL. Expert Rev Med Dev. 2023;20(9):741–52. [DOI] [PubMed] [Google Scholar]
- 21.Goodyear MD, Krleza-Jeric K, Lemmens T. The declaration of Helsinki. 2007, Br Med J Publishing Group. pp. 624–5. [DOI] [PMC free article] [PubMed]
- 22.Cuschieri S. The CONSORT statement. Saudi J Anaesth 2019; 13: S27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu S-Y, Lin L-H, Hsue S-S. Management of dental extractions in patients on warfarin and antiplatelet therapy. J Formos Med Assoc. 2018;117(11):979–86. [DOI] [PubMed] [Google Scholar]
- 24.Zanatta RF, et al. Guidelines for conducting split-mouth clinical studies in restorative dentistry. Brazilian Dent Sci. 2017;20(2):29–37. [Google Scholar]
- 25.Dave M, et al. An evaluation of sepsis in dentistry. BDJ Team. 2021;8(7):32–9. [DOI] [PubMed] [Google Scholar]
- 26.Tibi PR, et al. Global observational survey verifying surgeon utilization of the validated intraoperative bleeding (VIBe) scale for use in clinical practice. Surg Pract Sci. 2023;12:100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cocero N, Basso M, Grosso S, Carossa S. Direct oral anticoagulants and medical comorbidities in patients needing dental extractions: management of the risk of bleeding. J Oral Maxillofac Surg. 2019;77(3):463–70. [DOI] [PubMed] [Google Scholar]
- 28.Czembirek C, et al. Causes and timing of delayed bleeding after oral surgery. Clin Oral Invest. 2014;18:1655–61. [DOI] [PubMed] [Google Scholar]
- 29.Brennan MT, Wynn RL, Miller CS. Aspirin and bleeding in dentistry: an update and recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 2007;104(3):316–23. [DOI] [PubMed] [Google Scholar]
- 30.Lockhart P, Gibson J, Pond S, Leitch J. Dental management considerations for the patient with an acquired coagulopathy. Part 1: coagulopathies from systemic disease. Br Dent J. 2003;195(8):439–45. [DOI] [PubMed] [Google Scholar]
- 31.Hamzani Y, Chaushu G. Evaluation of early wound healing scales/indexes in oral surgery: A literature review. Clin Implant Dent Relat Res. 2018;20(6):1030–5. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoudi A, et al. Efficacy of a new hemostatic dental sponge in controlling bleeding, pain, and dry socket following mandibular posterior teeth extraction—a split-mouth randomized double-blind clinical trial. J Clin Med. 2023;12(14):4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahé I, et al. Interaction between Paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica. 2006;91(12):1621–7. [PubMed] [Google Scholar]
- 34.Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: myths, truths and clinical relevance. Scandinavian J Pain. 2016;13(1):67–75. [DOI] [PubMed] [Google Scholar]
- 35.Pouplin A, Kormann DP, Dolivet G, Phulpin B. Hemorrhagic shock after dental extractions in a patient with anticoagulant and antiplatelet therapy: a case report. Int J Surg Case Rep. 2025:111375. 10.1016/j.ijscr.2025.111375. [DOI] [PMC free article] [PubMed]
- 36.Kademani D. Dental, Oral, and Maxillofacial Emergencies. Oral Medicine and Medically Complex Patients: Oral Medicine and Medically Complex Patients, 2025: pp. 187–243.
- 37.Cassano R, et al. Chitosan hemostatic dressings: properties and surgical applications. Polymers. 2024;16(13):1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soliman M, et al. The efficacy of bloodstop iX, surgicel, and gelfoam in vascular operations: First-in-Human Head-to-Head study. Innovations. 2023;18(3):276–81. [DOI] [PubMed] [Google Scholar]
- 39.Driver JA et al. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 2008:337. 10.1136/bmj.a2467. [DOI] [PMC free article] [PubMed]
- 40.Li Y, Hecht SS. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem Toxicol. 2022;165:113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornu C, et al. Experimental designs for small randomised clinical trials: an algorithm for choice. Orphanet J Rare Dis. 2013;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witt DM, et al. Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood J Am Soc Hematol. 2009;114(5):952–6. [DOI] [PubMed] [Google Scholar]
- 43.Menger MM, et al. Vascularization strategies in the prevention of nonunion formation. Tissue Eng Part B: Reviews. 2021;27(2):107–32. [DOI] [PubMed] [Google Scholar]
- 44.Ravanshad S, et. al Comparative risk of Gastrointestinal major bleeding with Rivaroxaban and warfarin. J Iranian Med Council. 2024. 10.18502/jimc.v8i2.17702.
- 45.Al-Attar N, et al. Safety and hemostatic effectiveness of SURGICEL® powder in mild and moderate intraoperative bleeding. Clin Appl Thromb Hemost. 2023;29:10760296231190376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genyk Y, et al. Fibrin sealant patch (TachoSil) vs oxidized regenerated cellulose patch (Surgicel Original) for the secondary treatment of local bleeding in patients undergoing hepatic resection: a randomized controlled trial. J Am Coll Surg. 2016;222(3):261–8. [DOI] [PubMed] [Google Scholar]
- 47.Bhavsar AK. A comparative clinical and optical evaluation of Self-filling osmotic tissue expander in augmenting keratinized tissue around dentulous region. Rajiv Gandhi University of Health Sciences (India); 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.