Abstract
Background
Plasticity in sensory perception and tolerance to xenobiotics contributes to insects’ adaptive capacity and evolutionary success, by enabling them to cope with potentially toxic molecules from the environment or internal milieu. Odorant-binding proteins (OBPs) and chemosensory proteins (CSPs) have traditionally been studied in the context of chemoreception. However, accumulating evidence over the past few years indicates that these protein families can also sequester insecticide molecules. In doing so, the insecticide cannot reach its target site and can be more easily eliminated through the feces, complexed with these proteins. Thus, xenobiotic sequestration by OBPs and CSPs may lead to insecticide tolerance or even resistance.
In the Southern Cone, the kissing bug Triatoma infestans is the main vector of Trypanosoma cruzi, the protozoan parasite that causes Chagas disease. Vectorial transmission of T. cruzi has not been interrupted in certain regions of Argentina, where several populations of T. infestans highly resistant to insecticides have been reported. Understanding the molecular mechanisms underlying resistance is crucial for designing effective vector control strategies. In this context, studying protein families involved in insecticide sequestration is essential.
Results
We manually corrected predicted gene models and identified new sequences of chemosensory and odorant-binding proteins in five Hemiptera species with different feeding habits. Using this information, we mined the raw genome sequence of T. infestans to identify and characterize their orthologs based on sequence conservation and phylogenetic relationships. In total, 26 chemosensory and 49 odorant-binding proteins were identified in the T. infestans genome. Phylogenetic analysis, tissue-specific expression, and molecular docking with major insecticides were performed to assess possible roles.
Conclusions
This work represents the first comprehensive genomic analysis of chemosensory and odorant-binding protein families across Hemiptera species, as well as the first characterization of these gene families in T. infestans using genomic data. It contributes to a better understanding of the molecular basis of chemoreception and insecticide resistance in T. infestans.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-025-11967-2.
Keywords: Insecticide, Xenobiotic sequestration, Chemoreception, Insects, Kissing bugs
Background
Plasticity in coping with xenobiotics and sensory perception contributes significantly to the adaptive capacity and evolutionary success of insects [1, 2]. The main protein superfamilies traditionally studied in the context of insect detoxification and insecticide resistance are cytochrome P450s (CYPs), carboxyl/cholinesterases (CCEs), and glutathione S-transferases (GSTs) [3, 4]. Sensory-related gene families are mainly classified into odorant receptors, ionotropic receptors, gustatory receptors, PPK receptors, odorant-binding proteins (OBPs), and chemosensory proteins (CSPs) [5]. Both detoxification and chemosensory systems are involved in processing or handling xenobiotics from the environment or internal milieu (odor molecules, toxic dietary components, insecticides, etc.).
The kissing bug Triatoma infestans (Hemiptera: Reduviidae) is the main vector of Trypanosoma cruzi, the protozoan parasite that causes Chagas disease, in the Southern Cone. The World Health Organization (WHO) has included Chagas among the neglected tropical diseases; it is endemic to Central and South America and affects around 7 million people worldwide [6]. Vectorial transmission of T. cruzi has not been interrupted in the Southern Cone, particularly in the Gran Chaco ecoregion of Argentina, where several populations of T. infestans highly resistant to pyrethroids have been reported [7–9].
Since the 1980s, chemical control of triatomines has relied on pyrethroid insecticides [10]. However, high rates of pyrethroid resistance have been detected in T. infestans populations over the past 20 years, challenging the success of control campaigns [7–9]. According to the World Health Organization (WHO), understanding the molecular mechanisms underlying vector resistance to insecticides is crucial for designing effective vector control interventions [11].
The best-studied mechanisms of insecticide resistance in pest insects include mutations in insecticide target sites, increased detoxification metabolism, and cuticular changes that reduce insecticide penetration [12, 13]. The advent of next-generation sequencing technologies, and the resulting surge in available genomes and transcriptomes, has led to the identification of a new insecticide-resistance mechanism, which is xenobiotic sequestration [14]. By this mechanism, the concentration of toxic molecules available to interact with the target site is diminished. Accumulating evidence over recent years indicates that CSPs and OBPs can mediate insecticide sequestration, contributing to resistance [14–18]. The expression of both CSPs and OBPs is modulated in resistant insect populations [19–21] and following insecticide exposure [18, 22–25]. Functional experiments using RNAi-mediated gene silencing have also demonstrated the involvement of CSP and OBP family members in insecticide sequestration and resistance across different species [18, 26].
CSPs and OBPs are small, globular, and soluble proteins characterized by specific domains composed of four or six α-helices, connected by two or three disulfide bonds [27, 28]. OBPs are categorized into subfamilies based on structural features. Classic OBPs are defined by a conserved six-cysteine motif arranged in a characteristic pattern; Plus-C OBPs include two additional conserved cysteines and a proline. Minus-C OBPs lack two of the conserved cysteines. Atypical OBPs are characterized by having 9–10 cysteines and an extended C-terminal region. Two-domain OBPs contain two separate domains, each homologous to the classic OBP domain with six cysteines [29]. While this classification was originally proposed for dipterans, it may not adequately classify the great variety of OBP sequences found across different insect orders [29–31].
In a previous study, we identified members of the CSP family in a T. infestans transcriptome [24]. We also showed that the expression of some of these transcripts is modulated in response to deltamethrin exposure [24]. However, a comprehensive description of these gene families in T. infestans was limited by the absence of an annotated genome. A genome assembly of T. infestans is now publicly available (GenBank accession: GCA_011037195.1), but it consists of raw genomic sequence without gene annotations.
Feeding habits in insects can modulate their molecular machinery for sequestering toxic xenobiotics from the diet. Whereas phytophagous species must cope with plant defense molecules, hematophagous insects must deal with an excess of proteins and heme groups present in blood. A comparative analysis among insects with different feeding habits could reveal molecular adaptations to either hematophagy or plant feeding. Common features in protein families related to xenobiotic sequestration among species with similar diets could shed light on the molecules involved in sequestering different xenobiotics from the food source. For these reasons, in the present work, we manually curated gene models and identified new sequences of CSPs and OBPs in five Heteroptera species with different feeding habits. Using this manually curated set of CSPs and OBPs from all species, we mined the raw genome sequence of T. infestans to identify and characterize orthologs, based on sequence conservation and phylogenetic relationships. To infer putative roles, we studied the expression of CSPs and OBPs in T. infestans tissue-specific transcriptomes. Finally, we performed a molecular docking analysis of the complete set of T. infestans CSPs and OBPs with three neurotoxic insecticides from different classes. To our knowledge, this is the first study to mine the T. infestans genome and the first phylogenetic and structural analysis of CSPs and OBPs across Heteroptera.
Results and discussion
CSP and OBP encoding genes in Heteroptera genomes
Gene predictions in non-model insect genomes are initially performed using automatic pipelines; therefore, further manual analysis and correction or confirmation of gene models are necessary. In the case of CSPs and OBPs, such manual curation has not been published to date for the Heteroptera species analyzed here, except for Rhodnius prolixus [5]. We conducted a detailed manual analysis to identify and correct CSP and OBP gene predictions in the genomes of five Heteroptera species with different feeding habits (Fig. 1): the hematophagous Cimex lectularius (Cimicidae) and R. prolixus (Reduviidae); two phytophagous pentatomids, Halyomorpha halys and Nezara viridula; and the predator Orius laevigatus. As an outgroup, we included the non-heteropteran hemipteran Nilaparvata lugens, a monophagous species specialized in rice plants. The amino acid sequences of the identified CSPs and OBPs are provided in Additional file 1, including sequences that were confirmed or corrected from automatic predictions, as well as novel sequences identified in these genomes for the first time in this study. The characteristic features of all identified sequences are presented in Additional file 2.
Fig. 1.
A) CSP and B) OBP sequences identified in the genomes of the species analyzed. Black bars indicate the number of complete sequences, and gray bars indicate the number of incomplete sequences. Abbreviations: Clec, C. lectularius; Hhal, H. halys; Nvir, Ne. viridula; Olae, O. laevigatus; Rpro, R. prolixus; Nlug, Ni. lugens; Tinf, T. infestans.
Of the 19 CSPs automatically annotated in the C. lectularius genome, ClecCSP17 was incomplete, and ClecCSP8 was manually corrected (Additional files 1 and 2).
Twenty-three CSP sequences were found in H. halys, considering both genome and transcriptome data (Fig. 1 and Additional file 2). Of these, 19 were annotated in the automatic prediction. Sixteen of these transcripts were previously identified in an antennal transcriptome [32]. We corrected the annotation of hhalcsp3 and hhalcsp11, that were incorrectly annotated as a single gene in the genome (Additional files 1 and 2). Furthermore, the antennal transcriptome contained HhalCSP4, despite its absence in the genome [32]. Additionally, our analysis allowed the identification of six previously unpredicted sequences in the genome (Fig. 1 and Additional file 2).
Considering transcriptome [33] and genome searches (present work), a total of 33 CSPs were identified in Ne. viridula (Fig. 1, Additional files 1 and 2). Of these, 8 sequences from the genome are described here for the first time; 13 CSPs from the transcriptome were not predicted in the genome.
Seventeen CSPs were identified in the O. laevigatus genome, two of which were partial (Fig. 1, Additional files 1 and 2). Searches in R. prolixus databases did not yield additional CSPs beyond those reported previously in a comprehensive analysis [5] (Fig. 1, Additional files 1 and 2). The search in Ni. lugens yielded 18 positive hits, seven of which are reported here for the first time (NlugCSP12 to NlugCSP18). The sequence XP_03927276004.1 corresponded to two juxtaposed CSPs, which were split here into NlugCSP2 and NlugCSP3 (Additional file 2). The sequences NlugCSP17 (XP_039277645.1), NlugCSP13 (XP_022188430.2), NlugCSP5 (XP_022200187.2), NlugCSP6 (XP_022201642.1), and NlugCSP8 (XP_022188604.2) were manually corrected due to evident annotation artifacts, such as gene fusions or incorrectly assigned start or stop codons.
In C. lectularius, 31 OBPs were found, three of which were partial (XP_024084571.1, XP_014246950.1, and XP_024086027.1) (Additional file 2). Sixty-eight OBPs were identified in the H. halys genome; of these, 24 were identified here for the first time, and others were corrected by manual analysis (Additional file 2).
In Ne. viridula, 67 OBP genes were identified (Fig. 1 and Additional file 2), five of which were partial, and five automatic predictions were corrected (Fig. 1 and Additional file 2).
The search for OBPs in O. laevigatus resulted in 34 sequences; two of them were partial (OlaeOBP33 and OlaeOBP34), and two were corrected from automatic predictions (OlaeOBP11 and OlaeOBP23) (Fig. 1 and Additional file 2).
Our searches in R. prolixus revealed two previously unreported OBP sequences RproOBP28 (RPRC003602) and RproOBP29 (RPRC017720). The sequence RproOBP15 was manually corrected. The final set of OBPs in R. prolixus consists of 31 sequences, five of which lack one or more structural characteristics (Fig. 1 and Additional file 2).
Nineteen OBP genes were found in Ni. lugens; 9 of them are reported here for the first time, and 10 were previously reported in an antennal transcriptome [34].
Identification, annotation, and characterization of CSP and OBP encoding genes in T. infestans genomic database
An initial search in the assembled T. infestans genome revealed 21 CSP genes distributed across 14 scaffolds. After iterative searches and searches in transcriptomic databases [5, 24, 35], a total of 26 CSP-encoding transcripts were identified, 24 of which are likely complete, as inferred by the presence of all conserved family characteristics (Additional file 3 and Fig. 2A). A general feature format (GFF) file was generated with the reconstructed CSP genes present in the genome (Additional file 4); amino acid and nucleotide sequences are provided in Additional file 5, and the structural analysis of each sequence is presented in Additional file 3.
Fig. 2.
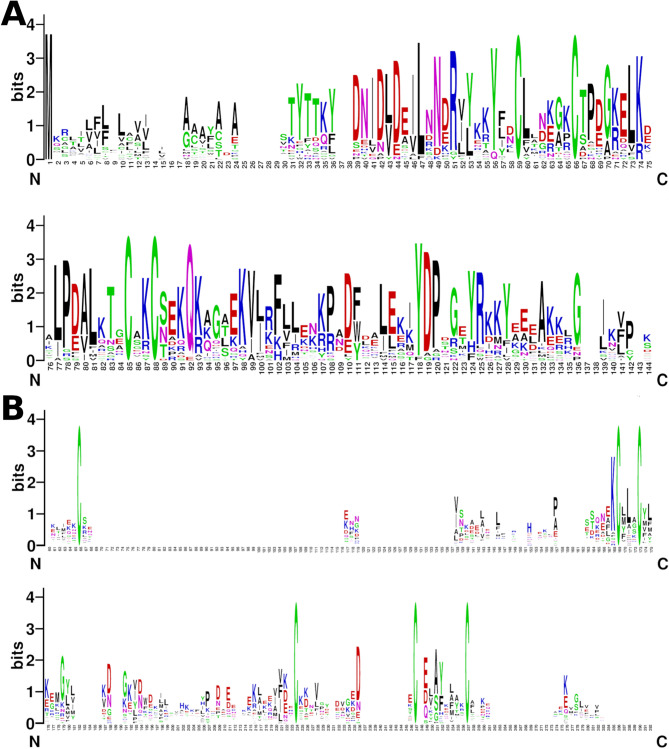
SeqLogo of T. infestans CSP (A) and OBP (B) sequences. A) The cysteines at positions 59, 66, 85, and 88 in the alignment of CSP8 are conserved in the 24 complete CSP sequences identified in T. infestans. Partial sequences CSP22 and CSP26 were not included. B) The cysteines at positions 67, 169, 173, 224, 247, and 257 in the alignment of OBP1 are conserved in the 31 complete OBP sequences identified in T. infestans. Neither partial OBP sequences nor any minus-C OBP sequences were included.
Fourteen predicted CSPs were identical in the genome and one or more transcriptomes (TinfCSP1, TinfCSP3, TinfCSP5, TinfCSP6, TinfCSP7, TinfCSP8, TinfCSP12, TinfCSP14, TinfCSP15, TinfCSP16, TinfCSP17, TinfCSP19, TinfCSP21, and TinfCSP24); three sequences were complete in the genome but partial in the transcriptomes (TinfCSP4, TinfCSP9, and TinfCSP22); three were absent in the transcriptomes and detected in the genome for the first time (TinfCSP23, TinfCSP25, and TinfCSP26), which could suggest low expression levels. Finally, six sequences were identified in transcriptomes but not in the genome (TinfCSP2, TinfCSP10, TinfCSP11, TinfCSP13, TinfCSP18, and TinfCSP20), reflecting the incompleteness of the genomic database. Figure 2A shows a sequence logo of the complete T. infestans CSP proteins predicted here. The larger number of CSPs observed in T. infestans compared to R. prolixus (26 and 19, respectively) could suggest a recent expansion of CSPs in the species or genus.
A total of 49 OBP-encoding transcripts were identified in T. infestans, 31 of which have all the conserved characteristics of the family, suggesting they are complete sequences (Fig. 2B, Additional files 3 and 5). Of these, 34 sequences were identified in the genome (distributed across 21 scaffolds) and confirmed in at least one transcriptome (TinfOBP1 to TinfOBP10, TinfOBP12 to TinfOBP20, TinfOBP22 to TinfOBP25, TinfOBP28 to TinfOBP34, TinfOBP39, TinfOBP43, TinfOBP44, and TinfOBP46), and five were found only in the genome (TinfOBP35, TinfOBP42, TinfOBP45, TinfOBP48, and TinfOBP49), which could indicate low expression levels. The 39 sequences in the genome were annotated in the GFF file presented in Additional file 4. Finally, 10 sequences were found in transcriptomes but were absent from the genome (TinfOBP11, TinfOBP21, TinfOBP26, TinfOBP27, TinfOBP35, TinfOBP36, TinfOBP37, TinfOBP40, TinfOBP41, and TinfOBP47). The lengths of T. infestans OBP sequences tend to be larger than those of CSPs, with an average length of 150.65 ± 3.05 vs. 127.25 ± 1.25 amino acids, respectively (Additional file 3). Regarding sequence conservation, OBPs are highly divergent, with an average per-site amino acid divergence of d = 1.70 and an overall sequence identity of 19.49%. In contrast, CSPs exhibit lower divergence (d = 1.05) and higher overall identity (38.44%) (Figs. 2A and B). The higher conservation of CSPs compared to the OBP family is a common observation in arthropods [31].
Phylogenetic analysis
To infer orthologies and generate function-related hypotheses, we conducted a phylogenetic analysis including CSP sequences from T. infestans and six other hemipterans (C. lectularius, H. halys, Ne. viridula, O. laevigatus, R. prolixus, and Ni. lugens) (Fig. 3). We also included Anopheles gambiae and Drosophila melanogaster, given the availability of high-quality genomes and functional information on CSP genes from these species [21, 36–38]. A CSP from Bemisia tabaci (AFJ342498.1) was used as an outgroup.
Fig. 3.
Phylogeny of the CSP family. See Additional files 1 and 5 for sequence IDs. A CSP from B. tabaci (AFJ342498.1) was used as an outgroup. Branch support values greater than 0.8 are indicated by gray circles
The phylogenetic analysis revealed two large clades composed exclusively of sequences from triatomines, suggesting a role in adaptation to the ecological niche of these species. In both of them, there is an expansion in T. infestans compared to R. prolixus. One of these clades contained 8 members: the closely related orthologs TinfCSP2 and RproCSP2 form a clade with TinfCSP3, RproCSP3, and RproCSP4. They are also closely associated with TinfCSP4, TinfCSP21, and TinfCSP25. TinfCSP21 was differentially expressed after treatment with a pyrethroid [24], suggesting a role in insecticide sequestration that could be shared by other members of this conserved clade.
A second clade composed exclusively of triatomine sequences had 5 members: four from T. infestans (TinfCSP12, TinfCSP15, TinfCSP14, and TinfCSP23) and 1 from R. prolixus (RproCSP12), which is a close ortholog of TinfCSP12. TinfCSP5 and TinfCSP6 were close orthologs of RproCSP5 and RproCSP6, respectively, with an extra sequence of T. infestans in each clade (TinfCSP24 and TinfCSP20, respectively). In both cases, the expansion observed in T. infestans could be due to recent duplication events during the evolution of the genus or species.
A clade composed only of sequences from phytophagous species included 9 sequences from H. halys (HhalCSP1 to HhalCSP3, HhalCSP6, HhalCSP7, HhalCSP15, HhalCSP18, HhalCSP22, and HhalCSP23), 8 from Ne. viridula (NvirCSP1 to NvirCSP5, NvirCSP26, NvirCSP27 and NvirCSP32), and 1 sequence from Ni. lugens (NlugCSP3). With only 1 extra sequence from H. halys, there is a 1:1 orthology among the pentatomid CSPs (Fig. 3). The conservation observed among phytophagous species suggests that members of this clade could be involved in coping with plant xenobiotics.
The phylogenetic tree showed a clade composed only of CSPs from non-phytophagous Hemiptera, i.e., species with hematophagous or predatory feeding habits. The close orthologs TinfCSP1 and RproCSP1 grouped with an expansion in C. lectularius (ClecCSP3, ClecCSP4, ClecCSP8, and ClecCSP16) and two sequences from O. laevigatus (OlaeCSP3 and OlaeCSP8).
Other clades formed in the phylogenetic tree tend to be composed of sequences from several hemipteran species with different feeding habits. TinfCSP8 was present in a 1:1 orthology with CSP sequences from the other Hemiptera species analyzed, except for O. laevigatus, suggesting its conservation throughout the group and a possible loss during the evolution of the predator (Fig. 3). TinfCSP9 grouped with sequences from the two dipterans, H. halys, Ne. viridula, R. prolixus, and Ni. lugens, in a clade closely related to a group containing TinfCSP7, its close ortholog RproCSP7, 2 conserved sequences from pentatomids (NvirCSP12 and HhalCSP10), and sequences from C. lectularius (ClecCSP7), O. laevigatus (OlaeCSP7), Ni. lugens (NlugCSP7), and An. gambiae (AgamCSP8) (Fig. 3). The sequences TinfCSP18 and RproCSP18 are close orthologs but are not conserved in the other species analyzed. TinfCSP19, ClecCSP1, OlaeCSP1, and RproCSP19 are close orthologs in a clade that included a sequence from H. halys (HhalCSP21). TinfCSP10 and RproCSP10, as well as TinfCSP11 and RproCSP11, are close orthologs in a clade formed by other hemipteran CSPs (ClecCSP10, HhalCSP4, HhalCSP16, NvirCSP6, OlaeCSP10, OlaeCSP14, OlaeCSP15, and NlugCSP10) (Fig. 3).
The close orthologues TinfCSP16 and RproCSP16 were grouped in a clade with HhalCSP9, ClecCSP6, NvirCSP8, and OlaeCSP6. This clade was closely related to another one that contained ClecCSP5, NvirCSP16, and OlaeCSP5. These two clades were further grouped with NvirCSP14 and NlugCSP5. The close orthologues TinfCSP17 and RproCSP17 were in a 1:1 relationship with CSPs from pentatomids (HhalCSP8 and NvirCSP7), O. laevigatus (OlaeCSP4), and Ni. lugens (NlugCSP4). NlugCSP4 has recently been shown to play a crucial role in resistance to the neonicotinoid imidacloprid by binding and sequestering the insecticide [17]. Related roles for its close orthologues in other hemipteran species could be explored. NlugCSP2, another gene reported to be involved in imidacloprid resistance [17], formed a separate clade with three additional Ni. lugens sequences (NlugCSP1, NlugCSP8, and NlugCSP14). AgamCSP2, which has been shown to play a role in pyrethroid resistance [18], clustered in a separate clade within the dipteran group, along with AgamCSP1, AgamCSP3, and DmelCSP2. Phylogenetic analysis indicated close orthology between TinfCSP13, NvirCSP15, and RproCSP13.
A similar phylogenetic analysis to that conducted for CSPs was performed for OBPs, using an OBP from B. tabaci (XP_018896211.1) as an outgroup. The classification of OBPs was based on sequence conservation relative to previously characterized proteins in Diptera, Lepidoptera, and other Hemiptera species, as well as phylogenetic relationships [31, 39–45] (Fig. 4). Most sequences were classified as Classic or Plus-C OBPs with good support, and representatives from all the studied species were found for these classes (Fig. 4). Minus-C OBPs were detected only in D. melanogaster and the triatomines; the sequence-level differences between these groups suggest independent origins (Fig. 4). Our analysis revealed 3 Minus-C OBPs in R. prolixus (RproOBP12, RproOBP26, RproOBP27) and 9 in T. infestans (TinfOBP12, TinfOBP26, TinfOBP27, TinfOBP35, TinfOBP39, TinfOBP40, TinfOBP41, TinfOBP45, TinfOBP46) (Fig. 4). Given that Minus-C OBPs are generally absent or under-represented in Hemiptera genomes (present results; [31, 46, 47]), the presence of multiple sequences encoding this subgroup in triatomines is a remarkable finding. RproOBP27 has been implicated in the reception of sexual pheromones [48]; sequence conservation suggests a similar role for related Minus-C OBPs in triatomines.
Fig. 4.
Phylogeny of the OBP family. See Additional files 1 and 5 for sequence IDs. An OBP from B. tabaci (XP_018896211.1) was used as an outgroup. Branch support values greater than 0.8 are indicated by gray circles
Two-domain OBPs likely originated from the fusion of two Classic OBP genes [31]. In an exhaustive survey across 20 arthropod genomes—including Diptera (12 Drosophila species and An. gambiae), Lepidoptera (Bombyx mori), Coleoptera (Tribolium castaneum), Hymenoptera (Apis mellifera), Phthiraptera (Pediculus humanus), Hemiptera (Acyrthosiphon pisum), Crustacea (Daphnia pulex), and Chelicerata (Ixodes scapularis)—Vieira and Rozas [31] found two-domain OBPs only in Diptera genomes. Interestingly, we found that this OBP class, although poorly represented, is present in Hemiptera, with one sequence each in Ne. viridula, O. laevigatus, and Ni. lugens (NvirOBP73, OlaeOBP26, and NlugOBP16) (Fig. 4). Sequence-level differences suggest independent origins of two-domain OBPs in Hemiptera.
Two clades were composed exclusively of Classic OBP sequences from triatomines. In both cases, expansions in T. infestans compared to R. prolixus were observed, suggesting recent duplication events: 7 sequences vs. 3 (TinfOBP9, TinfOBP10, TinfOBP11, TinfOBP31, TinfOBP32, TinfOBP36, and TinfOBP38 vs. RproOBP9, RproOBP10, and RproOBP11), and 9 sequences vs. 4 (TinfOBP5, TinfOBP13, TinfOBP14, TinfOBP22, TinfOBP23, TinfOBP30, TinfOBP37, TinfOBP42, and TinfOBP43 vs. RproOBP13, RproOBP14, RproOBP22, and RproOBP23). As previously mentioned, the triatomine-exclusive clades may be associated with adaptation to a specific ecological niche.
Three large clades were composed exclusively of pentatomid OBPs, all classified as classic, except for NvirOBP73, which is a two-domain OBP (Fig. 4). One of these clades contains 7 sequences from H. halys (HhalOBP24, HhalOBP26, HhalOBP44, HhalOBP49, HhalOBP50, HhalOBP58, HhalOBP69) and 7 from Ne. viridula (NvirOBP13, NvirOBP24, NvirOBP74 to NvirOBP77, NvirOBP79). Another pentatomid clade included 5 sequences from H. halys (HhalOBP12, HhalOBP17, HhalOBP20, HhalOBP27, HhalOBP35) and 6 from Ne. viridula (NvirOBP1, NvirOBP6, NvirOBP10, NvirOBP12, NvirOBP19, NvirOBP67). A sequence from T. infestans (TinfOBP15) was classified as related to these pentatomid clades. Another large clade composed of pentatomid classic OBPs included 14 sequences from H. halys (HhalOBP36, HhalOBP40 to HhalOBP43, HhalOBP45, HhalOBP53, HhalOBP56, HhalOBP57, HhalOBP59, HhalOBP60, HhalOBP64) and 14 from Ne. viridula (NvirOBP15, NvirOBP16, NvirOBP30, NvirOBP32, NvirOBP35, NvirOBP41, NvirOBP42, NvirOBP69, NvirOBP70, NvirOBP72, NvirOBP73, NvirOBP84 to NvirOBP86). Finally, a large clade composed solely of pentatomid Plus-C OBPs comprised 9 sequences from H. halys (HhalOBP3, HhalOBP9, HhalOBP10, HhalOBP13, HhalOBP28, HhalOBP33, HhalOBP51, HhalOBP55, HhalOBP68) and 7 from Ne. viridula (NvirOBP8, NvirOBP23, NvirOBP24, NvirOBP27, NvirOBP53, NvirOBP62, NvirOBP64).
Similar to CSPs, some clades were composed of sequences from multiple hemipteran species with different feeding habits. The sequence TinfOBP2 showed 1:1 orthology with ClecOBP7, three identical sequences from O. laevigatus (OlaeOBP7, OlaeOBP10, and OlaeOBP12), and NlugOBP7. Another clade was composed of 3 sequences from T. infestans (TinfOBP18, TinfOBP19, and TinfOBP20), each with close orthologs in R. prolixus (RproOBP18, RproOBP19, and RproOBP20, respectively). This clade also included sequences from C. lectularius (ClecOBP17, ClecOBP22, and ClecOBP12), the pentatomids (HhalOBP14, HhalOBP16, HhalOBP25, NvirOBP3, NvirOBP7, NvirOBP11), O. laevigatus (OlaeOBP17 and OlaeOBP22), and D. melanogaster (DmelOBP57a and DmelOBP57b) (Fig. 4). Within this clade, HhalOBP14, HhalOBP16, and HhalOBP25 encode proteins previously reported to bind the alarm pheromone and certain plant volatiles in H. halys, along with HhalOBP15 and HhalOBP20 [49]. A highly conserved clade containing sequences from all Heteroptera species analyzed (ClecOBP14, HhalOBP20, NvirOBP1, RproOBP24, OlaeOBP13, OlaeOBP14, and TinfOBP24) suggests a conserved role across this group.
TinfOBP1 and TinfOBP29 were grouped with 3 sequences from C. lectularius (ClecOBP1, ClecOBP2, ClecOBP16), 3 from H. halys (HhalOBP2, HhalOBP7, HhalOBP19), 2 from Ne. viridula (NvirOBP5, NvirOBP9), 2 from O. laevigatus (OlaeOBP1, OlaeOBP16), 2 from R. prolixus (RproOBP1, RproOBP29), and 3 from Ni. lugens (NlugOBP1, NlugOBP5, NlugOBP16). Within this clade, NlugOBP5 has been shown to bind and sequester the organophosphate chlorpyrifos, suggesting a role in insecticide tolerance and/or resistance [50]. Additionally, NlugOBP1 is highly expressed in the salivary glands and inhibits plant-defense molecules when the planthopper feeds on rice [51]. Based on these findings, a role in xenobiotic sequestration can be proposed for OBPs within this clade.
NlugOBP3, which sequesters nitenpyram and sulfoxaflor [52], was phylogenetically associated with other hemipteran sequences (ClecOBP3, HhalOBP37, NvirOBP34, and OlaeOBP3). TinfOBP8 and RproOBP8 are related to ClecOBP11, HhalOBP11, NvirOBP25, OlaeOBP11, and NlugOBP11 in a conserved clade present in all Hemiptera species analyzed. The close orthologues TinfOBP21 and RproOBP21 were associated with ClecOBP20, ClecOBP21, HhalOBP48, NvirOBP39, OlaeOBP20, and TinfOBP25 (Fig. 4).
TinfOBP6 was grouped with sequences from all species under analysis: ClecOBP15, HhalOBP8, NvirOBP14, RproOBP6, OlaeOBP15, NlugOBP15, AgamOBP4, AgamOBP5, and DmelOBP76a. The latter, also known as LUSH, is required for the activity of pheromone-sensitive neurons [53]; orthologues of this gene could be candidates for studying this function in Hemiptera. The close orthologues TinfOBP17 and RproOBP17 were grouped with 2 sequences from C. lectularius (ClecOBP8 and ClecOBP23), 1 from H. halys (HhalOBP30), 1 from Ne. viridula (NvirOBP4), and 3 from O. laevigatus (OlaeOBP8, OlaeOBP23, and OlaeOBP24). This clade was phylogenetically related to 1 sequence from Ni. lugens (NlugOBP8), 5 transcripts from An. gambiae (AgamOBP1, AgamOBP2, AgamOBP3, AgamOBP7, and AgamOBP15/16), and three from D. melanogaster (DmelOBP83a, DmelOBP83b, and DmelOBP69a). AgamOBP1 has been shown to bind indole, an essential metabolite in positive plant–insect interactions [54].
TinfOBP16 was associated with ClecOBP13, HhalOBP22, NvirOBP36, and NlugOBP13. DmelOBP59a is an atypical OBP with clear orthologues in all insect species except Hymenoptera [29]. We found clear orthologues of this sequence in the Hemiptera analyzed here, with the exception of Ne. viridula and R. prolixus (ClecOBP18, HhalOBP5, OlaeOBP18, NlugOBP18, and TinfOBP28). Likewise, DmelOBP73a is a classic OBP with close orthologues in all insect species except Hymenoptera [29]. We identified 1:1 orthology in all the species analyzed here except O. laevigatus (ClecOBP6, HhalOBP1, NvirOBP48, RproOBP4-NTE, NlugOBP6, and TinfOBP4). The conservation of a sequence across distant species suggests an evolutionarily conserved role; the absence of a highly conserved sequence in one or a few genomes could be explained by incomplete databases.
DmelOBP56a is the closest orthologue of PregOBP56a from the carnivorous blowfly Phormia regina. The latter OBP is highly expressed in mouthparts and has been involved in the solubilization of fatty acids from the food source [55]. Our phylogenetic analysis indicates that these OBPs have close orthologues in triatomines and pentatomids (HhalOBP38, NvirOBP29, RproOBP7, TinfOBP7), pointing to a potential role of these molecules in the solubilization of food components.
Besides classic OBPs, the Plus-C OBP group was the only one with representatives from all the species analyzed, with a predominance in dipterans and pentatomids (H. halys: 14, Ne. viridula: 12, An. gambiae: 19 and D. melanogaster: 13). The Plus-C triatomine OBP sequences are RproOBP3, TinfOBP3 and TinfOBP34. C. lectularius had 1 Plus-C OBP (ClecOBP9), and O. laevigatus and Ni. lugens had 3 sequences each (OlaeOBP4, OlaeOBP6, OlaeOBP9, NlugOBP4, NlugOBP9 and NlugOBP10) (Fig. 3). This group was divided into different clades: some composed of sequences from several species, suggesting a conserved role across evolution, whereas species-specific clades were also observed (Fig. 4).
CSPs and OBPs expression in T. infestans transcriptomes from salivary glands and antennae
As stated above, both CSP and OBP families have been associated with chemoreception and xenobiotic sequestration. It remains unclear whether the molecules involved in coping with environmental toxins also participate in odor transport, or if distinct proteins perform specialized roles. We hypothesized that expression pattern analysis could provide clues to investigate whether a particular CSP or OBP is associated with xenobiotic sequestration, chemoreception, or both. Hence, we analyzed public transcriptomes from T. infestans antennae [5] and salivary glands [56] to infer the potential roles of the different CSPs and OBPs.
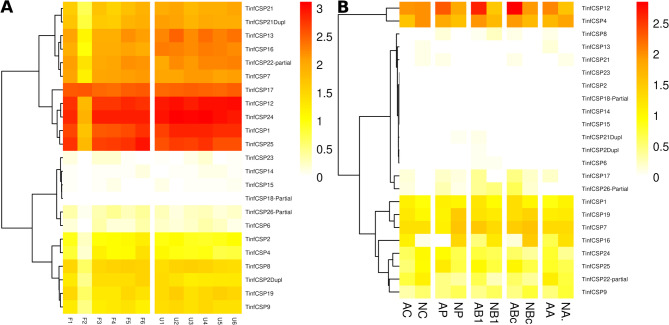
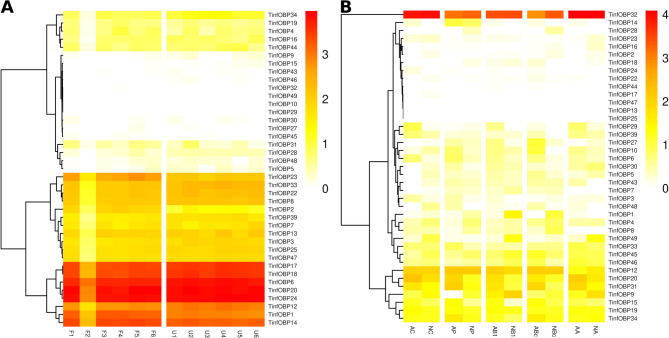
During feeding, both hematophagous and phytophagous hemipterans inject saliva into the bite site. This saliva contains a diverse array of molecules that counteract the host’s defense mechanisms, including OBPs and CSPs that may sequester toxic compounds from the food source [51, 57]. Indeed, the role of a salivary Ni. lugens OBP in inhibiting plant defense molecules has been demonstrated [51]. Beyond hemipterans, the carnivorous blowfly P. regina contains a salivary OBP required for fatty acid solubilization during feeding [55]. We hypothesized that transcripts highly expressed in antennae are likely related to olfaction, whereas those highly expressed in salivary glands could be involved in feeding and sequestering compounds from the food source. Additionally, transcripts with high expression in both tissues may have a constitutive role. Furthermore, gene expression under different feeding conditions and/or developmental stages could suggest physiological functions. The public bioprojects used included antennal samples from fed and unfed fifth instar nymphs [5], and salivary glands from adults or nymphs from different populations [56]. We found that the expression of each CSP or OBP gene did not vary depending on the condition analyzed in each transcriptome (Figs. 5 and 6), indicating constitutive expression across different physiological states. However, comparisons between antennae and salivary glands revealed significant differences in both CSP and OBP expression.
Fig. 5.
CSP expression levels represented as log10 (FPKM+1) in T. infestans. Each column represents a different library from the dataset. A) antennal and B) salivary gland transcriptomes. F: Fed, U: Unfed; A: Adult, N: Nymphal; C: Chile, P: Peru, B1: Bolivian F1, Bc: Bolivian colony, A: Argentina. Cluster analysis with dendrograms grouped proteins by similar expression patterns
Fig. 6.
OBP expression levels represented as log10 (FPKM + 1) in T. infestans. Each column represents a different library from the dataset. A) antennal and B) salivary gland transcriptomes. F: Fed, U: Unfed; A: Adult, N: Nymphal; C: Chile, P: Perú, B1: Bolivian F1, Bc: Bolivian colony, A: Argentina. Cluster analysis with dendrograms grouped the proteins by similar expression patterns
Those CSP highly expressed in salivary glands were TinfCSP4 and TinfCSP12, which were also expressed in antennae at medium or high levels (Fig. 5), suggesting a constitutive role. Interestingly, TinfCSP4 is phylogenetically related to TinfCSP21, which was regulated after intoxication with deltamethrin [24]; besides, TinfCSP12 had a high affinity with insecticides in docking experiments (see below). The high expression in salivary glands reinforce the hypothesis of a role of these CSPs in xenobiotic sequestration.
Four T. infestans CSPs (TinfCSP13, TinfCSP17, TinfCSP21 and TinfCSP21dupl) showed high expression in antenna and low or no expression in salivary glands (Fig. 5), suggesting a probable role in olfaction. TinfCSP14, TinfCSP15, and TinfCSP23 are low expressed in both tissues in the conditions analyzed. TinfCSP13 transcription was downregulated in antennae after feeding in T. infestans [5]; this protein was not detected in the salivary glands transcriptome (Fig. 5). This expression pattern could be in agreement with a role in host-seeking by olfaction in starved animals.
TinfOBP32 was highly expressed in salivary glands, while it was not expressed in antennae. TinfOBP1, TinfOBP6, TinfOBP14, TinfOBP17, TinfOBP18, and TinfOBP24 were highly expressed in the antennae but showed little or no expression in salivary glands, pointing to a role in chemoreception (Fig. 6). Similarly, TinfOBP8, TinfOBP22, TinfOBP23, and TinfOBP33 were expressed at medium to high levels in antennae, whereas their expression in salivary glands was low or absent. On the other hand, TinfOBP9, TinfOBP15, TinfOBP45, TinfOBP46, and TinfOBP49 showed medium expression in salivary glands, with low or no expression in antennae (Fig. 6), suggesting a role in the sequestration of dietary xenobiotics.
Modelling of T. infestans CSPs and OBPs interaction with insecticides
Given that CSP and/or OBP family members could have a role in insecticide sequestration, we modeled the docking of all the predicted proteins from T. infestans with three classes of insecticides intensively used worldwide for vector control: a pyrethroid (deltamethrin), a phenylpyrazole (fipronil), and an organophosphate (malathion) (Additional file 6). Docking affinities are semi-quantitative because absolute values may differ between systems and scoring functions. As a rule of thumb, for molecules like those evaluated in this work, energies higher than−5 kcal mol⁻¹ represent weak or no binding,−5.1 to−7 kcal mol⁻¹ moderate affinity, and lower than−7 kcal mol⁻¹ probably indicate high-affinity binders [58, 59].
The results point to the capacity of T. infestans CSPs to efficiently bind, and probably sequester, different insecticides. On average, a higher favorable energy for docking was found with deltamethrin (−8.57 ± 0.27 kcal mol⁻¹) followed by fipronil (−8.16 ± 0.14 kcal mol⁻¹); the lowest favorable average energy was found for malathion (−5.38 ± 0.09 kcal mol⁻¹) (Additional file 6). TinfCSP19 had the highest favorable energy for binding both deltamethrin and malathion (best affinity−11.00 and−6.30 kcal mol⁻¹ respectively), whereas the most favorable docking with fipronil was found for TinfCSP10 (best affinity−10.10 kcal mol⁻¹) (Fig. 7).
Fig. 7.

Docking of A) deltamethrin and B) malathion inside TinfCSP19. C Docking of fipronil inside TinfCSP10 (ribbon representation)
In the case of OBPs, deltamethrin and fipronil showed similar average docking energies (−6.11 ± 0.25 and−5.91 ± 0.28 kcal mol⁻¹, respectively), followed by malathion (−4.83 ± 0.14 kcal mol⁻¹). TinfOBP45 had the most favorable interaction with both deltamethrin (best affinity−10.22 kcal mol⁻¹) and malathion (best affinity−6.82 kcal mol⁻¹). Interestingly, the expression of this OBP in salivary glands—but not in antennae (Fig. 6)—suggests its role in sequestering toxic xenobiotics. For fipronil, the most favorable energy was calculated for TinfOBP16 (best affinity−9.83 kcal mol⁻¹) (Fig. 8 and Additional file 6).
Fig. 8.
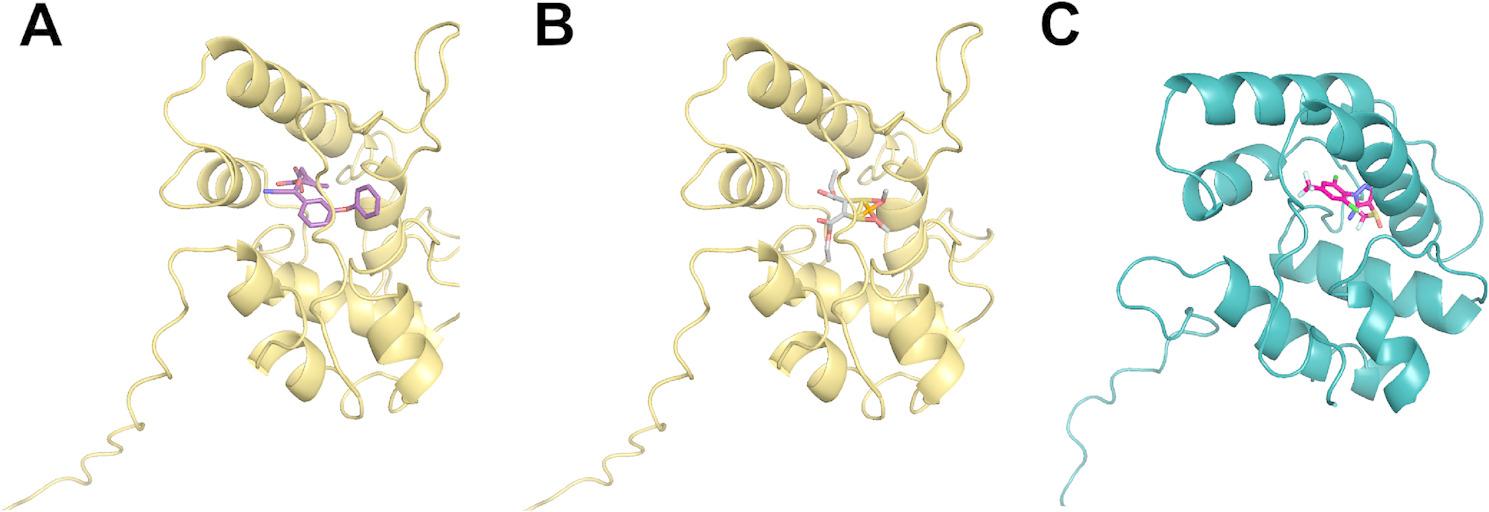
Docking of A) deltamethrin and B) malathion inside TinfOBP45. C) Docking of fipronil inside TinfOBP16 (ribbon representation)
Among the CSPs and OBPs that exhibited the most favorable insecticide binding energies, TinfCSP19 and TinfOBP16 are located within hemiptera-specific clades containing genes from most of the analyzed species, suggesting a possible conserved sequestering function. Additionally, TinfOBP18 and TinfOBP20 are not only associated with hemipteran genes but also with DmelOBP57a and DmelOBP57b. In contrast, TinfCSP2, TinfCSP10, TinfCSP12, and TinfCSP15, as well as TinfOBP35, TinfOBP41, and TinfOBP45, are restricted to triatomine-specific clades.
Concluding remarks
This work represents the first comprehensive genomic analysis of CSP and OBP protein families across Hemiptera species, as well as the first characterization of these gene families in T. infestans using genomic data. A common observation in our analysis was the inaccuracy of several automatic gene predictions; also, many sequences were detected in transcriptomes but absent in genomes. These facts reflect the incompleteness of genomic assemblies and/or predicted protein sets, and highlight the need for manual correction and careful revision of automatic annotations in non-model insects. To our knowledge, we report here for the first time the existence of two-domain OBPs in non-dipteran species. Since these genes likely result from the fusion of two different OBPs, this event could have occurred independently multiple times during evolution. Alternatively, two-domain OBPs in different species could share a common ancestral origin and have been lost during evolution in most non-dipteran genomes. Given the lack of sequence conservation among two-domain OBPs from different species, the hypothesis of independent gene fusion events seems more likely.
Regarding the CSP and OBP complement size, our analysis did not reveal common features that could be associated with feeding habits among the species included. However, some CSP and OBP members are phylogenetically grouped in clades that correlate with ecological niches or feeding habitats, suggesting a possible role in sequestering harmful dietary components. We identified significant expansions and species-specific duplications of these protein families in T. infestans through integrated phylogenetic, structural, and docking analyses. The detection of high expression levels in both antennal and salivary gland transcriptomes reinforces the evidence of the potential roles of CSPs and OBPs in coping with xenobiotics from odor sources and/or potentially toxic compounds from the diet.
Overall, the tissue expression and docking results suggest a role for several CSPs and OBPs in insecticide sequestration. Docking results indicate that the potential for sequestering deltamethrin and fipronil could be more efficient than that for malathion in T. infestans. However, the concentrations of malathion used in the field to control triatomines are more than 40 times higher than those of deltamethrin, in terms of grams per square meter [60]. This suggests that sequestration might not be the key factor affecting the efficacy of these insecticides; instead, differences could be related to their distinct target sites, cuticle penetration capacity, or metabolic detoxification. While pyrethroids are the insecticides of choice for T. infestans control, malathion was used in the past but discontinued in many regions due to toxicological concerns [61]. Both classes demonstrated high efficacy in controlling T. infestans, except in populations highly resistant to pyrethroids, which were associated with point mutations at their target sites [62–64]. However, populations with similar mutation frequencies displayed varying resistance profiles to pyrethroids [64], suggesting the existence of additional resistance mechanisms yet to be identified. Moreover, fipronil has not been used in field control programs for T. infestans, but experimental studies have shown high variability in susceptibility across different populations [65]. It would be interesting to determine the contribution of insecticide sequestration to the high resistance observed in field populations of T. infestans and to their tolerance to fipronil.
This work contributes to a better understanding of molecules likely involved in chemosensory functions and xenobiotic sequestration in T. infestans. Further functional studies using RNAi-mediated gene silencing or related techniques will be necessary to test the role of candidate CSPs and OBPs in coping with xenobiotics. Identifying proteins involved in insecticide sequestration has potential implications for vector control strategies. Inhibiting these proteins—either through chemical agents or RNAi-mediated gene silencing—could increase the susceptibility of vectors to insecticides, enabling the use of lower doses and/or restoring sensitivity in resistant populations.
Methods
Gene identification and sequence analysis
BLASTp searches were performed using PFAM domains PF03392 (CSP) and PF01395 (OBP) as queries, with an expected threshold of < 0.0001. The following predicted protein databases were used (NCBI accession numbers): C. lectularius (GCF_000648675.2), H. halys (GCF_000696795.3), Ne. viridula (GCA_928085145.1), O. laevigatus (GCA_018703685.1), R. prolixus (GCA_000181055.3), and Ni. lugens (GCF_014356525.2). PFAM HMM profiles for each family (CSP: PF03392.18; OBP: PF01395.27) were used to perform searches with hmmscan (HMMER v3.4 – http://hmmer.org) on the aforementioned protein databases, with a threshold value set at 1e−10. Positive hits from both BLASTp and hmmscan analyses were used as queries for iterative BLASTp searches to detect additional sequences missed by the initial domain-based search. False positives were discarded by a final BLASTp search against the NCBI non-redundant database restricted to insects. Bibliographic searches were conducted to compare the obtained CSP and OBP datasets with previously published information [5, 32, 33, 61, 66]. All positive matches were carefully analyzed using homologous sequences as references to correct errors and create gene models if necessary.
Given the absence of predicted gene models in the T. infestans genomic sequence (NCBI accession number: GCA_011037195.1), a different strategy was used for sequence identification. The CSP and OBP sequences obtained in the previous step (Additional file 1), along with proteins reported in Ac. pisum, Aedes aegypti, Triatoma brasiliensis, Triatoma dimidiata, and Triatoma pallidipennis [23, 35, 67–70], were used as queries for tBLASTn searches against the T. infestans genome. Gene models were reconstructed using the Fgenesh + tool from Softberry, employing similar proteins from related insects [71]. Public transcriptomes of T. infestans [5, 24, 35] were used to correct the predicted gene models. Artemis software [72] was used to generate the general feature format (.gff) file (Additional file 4) of the predicted CSP and OBP gene models.
All identified CSP and OBP sequences were analyzed to assess their characteristic features: SignalP software [73] was used to predict the presence of signal peptides; Clustal Ω was used to align the sequences to confirm the presence of conserved cysteines [74]. InterProScan v85.0 [75] was used to identify PFAM domains. PSIPRED [76] was used for secondary structure analysis. WebLogo was used to generate sequence logos of the complete T. infestans CSPs and OBPs to provide an accurate description of the conserved features of each family [77]. T. infestans CSP and OBP names were assigned according to the phylogenetic tree (see below) and orthology relationships with CSPs and OBPs from R. prolixus, whose names were originally assigned by Mesquita et al., 2015 [78].
Phylogenetic analysis
Amino acid alignments (excluding the signal peptide region) were performed using MAFFT [79] with the G-INS-i option and the following configuration: “leave gappy regions” activated; unaligned level = 0.1; offset value = 0.12; maxiterate = 1000. Alignments were trimmed with trimAl v1.2 [80] using default parameters except for the gap threshold, which was fixed at 0.3. Trimmed alignments were used to build phylogenetic trees for each family with IQ-TREE v1.6.12 [81] with the following parameters: -B 1000, -alrt 1000. Branch support was estimated with the approximate Likelihood Ratio Test based on the Shimodaira-Hasegawa-like procedure (aLRT-SH) [82, 83]. The best-fit amino acid substitution model estimated by ModelFinder (integrated in IQ-TREE) for each protein family and chosen according to the Bayesian Information Criterion (BIC) was Q.yeast + I + G4 for the CSP family and LG + R8 for OBPs. Phylogenetic trees were rooted using a CSP or OBP sequence from B. tabaci, a non-heteropteran hemipteran insect of the suborder Sternorrhyncha [84], and edited with iTOL (https://itol.embl.de). Branch support values between 80 and 100 were marked with a circle scaled accordingly.
The sequences were named according to previous annotations available for H. halys [32], Ne. viridula [33], R. prolixus [5], Ni. lugens [66], An. gambiae [43], and D. melanogaster [31, 39]. These annotations, along with the phylogenetic relationships observed in the trees, were used to name the sequences reported here for the first time.
For the classification of the OBP subfamilies, the sequences were aligned with reference proteins from D. melanogaster and An. gambiae and analyzed for the presence of conserved regions and specific residues. These results were complemented with the phylogenetic trees obtained and sequence identity analyses performed using CD-HIT (with an identity threshold of 30% to confirm the proposed annotation). Given that reports of Minus-C OBPs in Hemiptera are scarce [46, 85–87], additional alignments including species from different insect orders with well-characterized OBP repertoires (Ap. mellifera, Cydia pomonella, B. mori, and Aphis glycine) [40–42, 88] were performed to assign the final classification.
For the phylogenetic trees, sequences longer than 100 amino acids and containing all the conserved characteristics of the family were used. Shorter and/or incomplete sequences were kept for analysis but not for tree generation.
Tissue-specific transcriptomes
T. infestans RNA libraries from antennal and salivary gland transcriptomes were used [5, 56]. Quality control of the FASTQ files and removal of low-quality sequences and adapters was performed with the FastQC tool (available at www.bioinformatics.babraham.ac.uk/projects/fastqc), followed by Trimmomatic v0.39 [89]. The trimmed sequences were mapped to the T. infestans reference genome (GCA_011037195.1) using STAR software v2.7.10a [90] with --outFilterMultimapNmax set to 1. Previously, the genome was indexed with the GenomeGenerate mode from STAR software (options: --genomeSAsparseD 5 --genomeSAindexNbases 13 --genomeChrBinNbits 16). Gene-level read counts were extracted with the option --quantMode GeneCounts, and Fragments Per Kilobase Million (FPKM) values were calculated for normalization. Gene expression data (log10(FPKM+1)) was used to create heatmaps using the 1.0.12 version package in RStudio; dendrograms were plotted using the default hierarchical clustering based on Euclidean distance between genes.
Molecular modeling and docking
The CSP and OBP sequences predicted from the T. infestans genome were used to generate structural models using Modeller 9.25 [91], treating each structure as a receptor in independent runs. For each protein family, multiple structures available in the RCSB PDB [92] were used as templates: specifically, 1K19, 1KX8, 1N8U, 1N8V, 2GVS, and 2JNT for CSPs, and 3S0D, 5BXU, and 2QDI for OBPs. The modeling process followed the advanced procedures outlined in the software manual. For each sequence, 100 models were built, selecting the one with the best (lowest) Discrete Optimized Protein Energy (DOPE) score (best affinity). We also present the average affinity from all the runs (Additional file 6). Insecticide Ligand structures were obtained from DrugBank 5.0 [93]. Before docking runs, the receptor and ligand were prepared using the MGLTools suite (prepare_ligand.py and prepare_receptor.py scripts) [94]. Docking runs were performed using AutoDock Vina 1.1.2 [95], with a box fully covering the receptor, under default parameters, producing between 7 and 10 modes per complex. For each protein, the lowest (best) binding energy is reported.
Supplementary Information
Additional file 1. Curated amino acid sequences of CSPs and OBPs from six hemipteran species. This file contains CSP and OBP amino acid sequences from C. lectularius, H. halys, Ne. viridula, O. laevigatus, R. prolixus, and Ni. lugens, including manually confirmed or corrected annotations and novel sequences identified in this study.
Additional file 2. Structural features of A) CSPs and B) OBPs in selected hemipteran species. Includes gene and protein identifiers, sequence lengths, subfamily classification, and key structural hallmarks (start and stop codons, predicted signal peptides, and conserved cysteine patterns).
Additional file 3. Structural characteristics of T. infestans A) CSP and B) OBP sequences. Sequence features and annotations of chemosensory and odorant-binding proteins identified in T. infestans.
Additional file 4. Gene models of T. infestans CSPs and OBPs. General feature format (GFF) files corresponding to CSP and OBP genes identified in T. infestans genome.
Additional file 5. Amino acid and nucleotide sequences of T. infestans CSPs and OBPs. FASTA-format sequences of all CSP and OBP genes from T. infestans, including both coding and protein sequences.
Additional file 6. Molecular docking results for T. infestans A) CSPs and B) OBPs with selected insecticides. Binding energy values from docking simulations assessing potential interactions between CSP/OBP proteins and various insecticide compounds (malathion, fipronil and deltamethrin).
Acknowledgements
Not applicable.
Authors' contributions
MV: Conceptualization, investigation, data curation, formal analysis, visualization, writing the original draft. LT: Conceptualization, investigation, data curation, formal analysis, writing review and editing. IS: formal analysis. ADN: formal analysis, data acquisition, AA: formal analysis, data acquisition. SO: Conceptualization, investigation, formal analysis, writing the original draft, project administration.
Funding
S.O., A.D.N., and A.A. are researchers of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). M.V. is a recipient of a research fellowship from CONICET. Research fellowships from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), awarded to L.T. and I.S., also provided funding for this work.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mariano Volonté and Lucila Traverso contributed equally to this work.
Change history
9/21/2025
The original publication was updated to re-order the additional files to correspond to their captions.
References
- 1.Anton S, Rössler W. Plasticity and modulation of olfactory circuits in insects. Cell Tissue Res. 2021;383(1):149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadenne C, Barrozo RB, Anton S. Plasticity in Insect Olfaction: To Smell or Not to Smell? Annu. Rev. of Entomol., vol. 61. Annual Reviews Inc., pp. 317–333, 11-Mar-2016. [DOI] [PubMed]
- 3.Traverso L, et al. Comparative and functional triatomine genomics reveals reductions and expansions in insecticide resistance-related gene families. PLoS Negl Trop Dis. 2017;11(2): e0005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volonté M, Traverso L, Estivalis JML, Almeida FC, Ons S. Comparative analysis of detoxification-related gene superfamilies across five hemipteran species. BMC Genomics. 2022;23(1):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latorre Estivalis JM, Traverso L, Pontes G, Lorenzo MG. The antennal transcriptome of Triatoma infestans reveals substantial expression changes triggered by a blood meal. BMC Genomics. 2022;23(1): 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cucunubá ZM, et al. The epidemiology of Chagas disease in the Americas. The Lancet Regional Health. 2024. 10.1016/j.lana.2024.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fronza G, Toloza AC, Picollo MI, Spillmann C, Mougabure-Cueto GA. Geographical variation of deltamethrin susceptibility of Triatoma infestans (Hemiptera: Reduviidae) in Argentina with emphasis on a resistant focus in the Gran Chaco. J Med Entomol. 2016;53(4):880–7. [DOI] [PubMed] [Google Scholar]
- 8.Germano MD, Acevedo GR, Cueto GAM, Toloza AC, Vassena CV, Picollo MI. New findings of insecticide resistance in Triatoma infestans (Heteroptera: Reduviidae) from the Gran Chaco. J Med Entomol. 2010;47(6):1077–81. [DOI] [PubMed] [Google Scholar]
- 9.Sierra I, Capriotti N, Fronza G, Mougabure-Cueto G, Ons S. Kdr mutations in Triatoma infestans from the Gran Chaco are distributed in two differentiated foci: implications for pyrethroid resistance management. Acta Trop. 2016;158:208–13. [DOI] [PubMed] [Google Scholar]
- 10.Mougabure-Cueto G, Picollo MI. Insecticide resistance in vector Chagas disease: evolution, mechanisms and management. Acta Trop. 2015;149:70–85. [DOI] [PubMed] [Google Scholar]
- 11.W. H. O. Manual for monitoring insecticide resistance in mosquito vectors and selecting appropriate interventions. Geneva: World Health Organization; 2022.
- 12.Feyereisen R. Molecular biology of insecticide resistance. Toxicol Lett. 1995;82–83:83–90. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Schuler M, Berenbaum M. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–53. [DOI] [PubMed] [Google Scholar]
- 14.Ingham V, Grigoraki L, Ranson H. Pyrethroid resistance mechanisms in the major malaria vector species complex. Entomol Gen. 2023. 10.1127/entomologia/2023/1880. [Google Scholar]
- 15.Abendroth J, Moural T, Wei H, Zhu F. Roles of insect odorant binding proteins in communication and xenobiotic adaptation. Front Insect Sci. 2023. 10.3389/finsc.2023.1274197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correy G, et al. Overcoming insecticide resistance through computational inhibitor design. Proc Natl Acad Sci U S A. 2019;116(42):21012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng M, et al. Chemosensory proteins protect Nilaparvata lugens from imidacloprid by sequestering the insecticide and facilitating metabolic detoxification. J Agric Food Chem. 2025;73(7):3951–66. [DOI] [PubMed] [Google Scholar]
- 18.Ingham VA, et al. A sensory appendage protein protects malaria vectors from pyrethroids. Nature. 2020;577(7790):376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kefi M, et al. Transcriptomic analysis of resistance and short-term induction response to pyrethroids, in Anopheles coluzzii legs. BMC Genomics. 2021;22(1):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, et al. Overexpression of two odorant binding proteins confers chlorpyrifos resistance in the green peach aphid Myzus persicae. J Agric Food Chem. 2024;72(36):20101–13. [DOI] [PubMed] [Google Scholar]
- 21.Ingham VA, Wagstaff S, Ranson H. Transcriptomic meta-signatures identified in Anopheles Gambiae populations reveal previously undetected insecticide resistance mechanisms. Nat Commun. 2018;9(1): 5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X, Jiang Y, Zhang L, Cai Y. Effects of insecticides chlorpyrifos, Emamectin benzoate and fipronil on Spodoptera Litura might be mediated by OBPs and CSPs. Bull Entomol Res. 2018;108(5):658–66. [DOI] [PubMed] [Google Scholar]
- 23.Sierra I, et al. Transcriptomic analysis and molecular docking reveal genes involved in the response of Aedes aegypti larvae to an essential oil extracted from eucalyptus. PLoS Negl Trop Dis. 2021. 10.1371/journal.pntd.0009587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traverso L, et al. Transcriptomic modulation in response to an intoxication with deltamethrin in a population of Triatoma infestans with low resistance to pyrethroids. PLoS Negl Trop Dis. 2022;16(6): e0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Q, Liang Z, Chen B. Evidence for the participation of chemosensory proteins in response to insecticide challenge in Conopomorpha sinensis. J Agric Food Chem. Jan.2023;71(3):1360–8. [DOI] [PubMed] [Google Scholar]
- 26.Qiu Y-L, et al. A sublethal dose of neonicotinoid imidacloprid precisely sensed and detoxified by a C-minus odorant-binding protein 17 highly expressed in the legs of Apis cerana. Sci Total Environ. 2023;885: 163762. [DOI] [PubMed] [Google Scholar]
- 27.Leal WS, Nikonova L, Peng G. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 1999;464(1–2):85–90. [DOI] [PubMed] [Google Scholar]
- 28.Tegoni M, Campanacci V, Cambillau C. Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem Sci. 2004;29(5):257–64. [DOI] [PubMed] [Google Scholar]
- 29.Rihani K, Ferveur J-F, Briand L. The 40-year mystery of insect odorant-binding proteins. Biomolecules. 2021. 10.3390/biom11040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rondón J, Pisarenco V, Rozas J, Hurtado J, Hasson E. Evolution of the odorant-binding protein gene family in Drosophila. Front Ecol Evol. 2022. 10.3389/fevo.2022.957247. [Google Scholar]
- 31.Vieira FG, Rozas J. Comparative genomics of the odorant-binding and chemosensory protein gene families across the arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol. 2011;3(1):476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun D, et al. Identification of candidate olfactory genes in the antennal transcriptome of the stink bug Halyomorpha halys. Front Physiol. 2020. 10.3389/fphys.2020.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z-Z, et al. Candidate genes coding for odorant binding proteins and chemosensory proteins identified from dissected antennae and mouthparts of the Southern green stink bug Nezara viridula. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2019;31: 100594. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S-S, Sun Z, Ma W, Chen W, Wang M-Q. De novo analysis of the Nilaparvata lugens (Stål) antenna transcriptome and expression patterns of olfactory genes. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2014;9:31–9. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Barnetche J, et al. Adaptations in energy metabolism and gene family expansions revealed by comparative transcriptomics of three Chagas disease triatomine vectors. BMC Genomics. 2018;19(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habtewold T, et al. A chromosomal reference genome sequence for the malaria mosquito, Anopheles gambiae, Giles, 1902, Ifakara strain. Wellcome Open Res. 2023;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoskins R, et al. The release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 2015. 10.1101/gr.185579.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swarup S, Williams TI, Anholt RRH. Functional dissection of odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav. 2011;10(6):648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hekmat-Scafe DS, Scafe CR, McKinney AJ, Tanouye MA. Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res. 2002;12(9):1357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forêt S, Maleszka R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 2006;16:1404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Y, Tang H, Bohne C, Plettner E. Binding conformation and kinetics of two pheromone-binding proteins from the gypsy moth Lymantria dispar with biological and nonbiological ligands. Biochemistry. 2009;49:793–801. [DOI] [PubMed] [Google Scholar]
- 42.Huang C, et al. Comparative genomics provide insights into function and evolution of odorant binding proteins in Cydia pomonella. Front Physiol. 2021;12: 690185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manoharan M, Ng Fuk Chong M, Vaïtinadapoulé A, Frumence E, Sowdhamini R, Offmann B. Comparative genomics of odorant binding proteins in Anopheles gambiae, Aedes aegypti, and Culex quinquefasciatus. Genome Biol Evol. 2013;5(1):163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S-N, et al. Identification of odorant-binding proteins and functional analysis of antenna-specific AplaOBP1 in the emerald ash borer, Agrilus planipennis. J Pest Sci. 2020. 10.1007/s10340-019-01188-4. [Google Scholar]
- 45.Zhou J-J, et al. Revisiting the odorant-binding protein LUSH of Drosophila melanogaster: evidence for odour recognition and discrimination. FEBS Lett. 2004;558:1–3. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, et al. Identification and expression profiles analysis of odorant-binding proteins in soybean aphid, Aphis glycines (Hemiptera: Aphididae). Insect Sci. 2020;27(5):1019–30. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Zhao N, Cai L, Liu N, Zhu J, Yang B. High-quality chromosome-level scaffolds of the plant bug Pachypeltis Micranthus provide insights into the availability of Mikania Micrantha control. BMC Genomics. 2023;24(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira D, Brito N, Franco T, Moreira M, Leal W, Melo A. Functional characterization of odorant binding protein 27 (RproOBP27) from Rhodnius prolixus antennae. Front Physiol. 2018. 10.3389/fphys.2018.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, et al. Expressional and functional comparisons of five clustered odorant binding proteins in the brown marmorated stink bug Halyomorpha halys. Int J Biol Macromol. 2022;206:759–67. [DOI] [PubMed] [Google Scholar]
- 50.Deng M, et al. Mechanistic exploration of odorant binding protein-mediated Chlorpyrifos resistance in Nilaparvata lugens: insights from insecticide sequestration and transcriptional regulation. Int J Biol Macromol. 2025;284: 138108. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, et al. A salivary odorant-binding protein mediates Nilaparvata lugens feeding and host plant phytohormone suppression. Int J Mol Sci. 2021;22: 4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, et al. Odorant binding protein 3 is associated with nitenpyram and sulfoxaflor resistance in Nilaparvata lugens. Int J Biol Macromol. 2022;209:1352–8. [DOI] [PubMed] [Google Scholar]
- 53.Xu P, Atkinson R, Jones DNM, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45(2):193–200. [DOI] [PubMed] [Google Scholar]
- 54.Biessmann H, et al. The Anopheles gambiae odorant binding protein 1 (AgamOBP1) mediates indole recognition in the antennae of female mosquitoes. PLoS One. 2010;5(3): e9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishida Y, Ishibashi J, Leal WS. Fatty acid solubilizer from the oral disk of the blowfly. PLoS One. 2013;8(1): e51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz A, et al. An updated insight into the sialotranscriptome of Triatoma infestans: developmental stage and geographic variations. PLoS Negl Trop Dis. 2014;8(12): e3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santiago PB, et al. The pharmacopea within Triatomine salivary glands. Trends Parasitol. 2020;36(3):250–65. [DOI] [PubMed] [Google Scholar]
- 58.Vieira TF et al. In Silico Identification of Protein Targets Associated to the Insecticide Activity of Eugenol Derivatives, Chemistry Proceedings, vol. 3, no. 1. 2021.
- 59.Dong J, Wang K, Sun Y-L, Tian C-H, Wang S. Antennal transcriptome analysis of odorant-binding proteins and characterization of GOBP2 in the variegated cutworm Peridroma saucia. Front Physiol. 2023;14: 1241324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gürtler RE, Cecere MC. In: Guarneri A, Lorenzo M, editors. Chagas disease vector control BT - Triatominae - The biology of Chagas disease vectors. Cham: Springer International Publishing; 2021. pp. 491–535. [Google Scholar]
- 61.Benoit JB, et al. Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nat Commun. 2016;7(1):10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fabro J, et al. Identification of a point mutation associated with pyrethroid resistance in the para-type sodium channel of Triatoma infestans, a vector of chagas’ disease. Infect Genet Evol. 2012;12(2):487–91. [DOI] [PubMed] [Google Scholar]
- 63.Capriotti N, Mougabure-Cueto G, Rivera-Pomar R, Ons S. L925I mutation in the para-type sodium channel is associated with pyrethroid resistance in Triatoma infestans from the Gran Chaco region. PLoS Negl Trop Dis. 2014;8(1): e2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sierra I, Capriotti N, Fronza G, Mougabure-Cueto G, Ons S. Kdr mutations in Triatoma infestans from the Gran Chaco are distributed in two differentiated foci: implications for pyrethroid resistance management. Acta Trop. 2016. 10.1016/j.actatropica.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Mougabure-Cueto G, Picollo MI. In: Guarneri A, Lorenzo M, editors. Insecticide resistance in triatomines BT - Triatominae - The biology of Chagas disease vectors. Cham: Springer International Publishing; 2021. pp. 537–55. [Google Scholar]
- 66.Xue J, et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014;15(12):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marchant A, Mougel F, Jacquin-Joly E, Costa J, Almeida CE, Harry M. Under-expression of chemosensory genes in domiciliary bugs of the Chagas disease vector Triatoma brasiliensis. PLoS Negl Trop Dis. 2016;10(10): e0005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richards S et al. Feb., Genome sequence of the pea aphid Acyrthosiphon pisum, PLoS Biol., 2010; 8, e1000313 [DOI] [PMC free article] [PubMed]
- 69.Zhou J-J, He X-L, Pickett JA, Field LM. Identification of odorant-binding proteins of the yellow fever mosquito Aedes aegypti: genome annotation and comparative analyses. Insect Mol Biol. 2008;17(2):147–63. [DOI] [PubMed] [Google Scholar]
- 70.Zhou J-J et al. Mar., Genome annotation and comparative analyses of the odorant-binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum, Insect Mol. Biol., vol. 19, no. s2, pp. 113–122, 2010. [DOI] [PubMed]
- 71.Solovyev V, Kosarev P, Seledsov I, Vorobyev D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006;7(1): S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rutherford K, et al. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16(10):944–5. [DOI] [PubMed] [Google Scholar]
- 73.Almagro Armenteros JJ, et al. Signalp 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37(4):420–3. [DOI] [PubMed] [Google Scholar]
- 74.Sievers F, Higgins DG. Clustal omega, accurate alignment of very large numbers of sequences. In: Russell DJ, editor. in Multiple sequence alignment methods. Totowa, NJ: Humana; 2014. pp. 105–16. [DOI] [PubMed] [Google Scholar]
- 75.Blum M, et al. The interpro protein families and domains database: 20 years on. Nucleic Acids Res. 2021;49(D1):D344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buchan DWA, Jones DT. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Res. 2019;47(W1):W402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crooks G, Hon G, Chandonia J-M, Brenner S. Weblogo: a sequence logo generator. Genome Res. 2004;14:1188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mesquita RD, et al. Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc Natl Acad Sci U S A. 2015;112(48):14936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33(2):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. Trimal: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-tree: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate Maximum-Likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–21. [DOI] [PubMed] [Google Scholar]
- 83.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. Modelfinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14(6):587–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gäde G, Marco H. The adipokinetic peptides of Hemiptera: structure, function, and evolutionary trends. Front Insect Sci. 2022;2: 891615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farias LR, et al. Transcriptome-based identification of highly similar odorant-binding proteins among Neotropical stink bugs and their egg parasitoid. PLoS One. 2015;10(7): e0132286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang R, et al. Identification and expression profile analysis of odorant binding protein and chemosensory protein genes in Bemisia tabaci MED by head transcriptome. PLoS One. 2017;12(2): e0171739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Z, et al. The chromosome-scale reference genome of Mirid Bugs (Adelphocoris suturalis) genome provides insights into omnivory, insecticide resistance, and survival adaptation. BMC Biol. 2023;21(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L, Yin H, Zhu Z, Yang S, Fan J. A detailed spatial expression analysis of wing phenotypes reveals novel patterns of odorant binding proteins in the soybean aphid, Aphis Glycines. Front Physiol. 2021;12: 702973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics. 2016;54(1):5.6.1-5.6.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berman HM. The Protein Data Bank. Nucleic Acids Res. 2000;28(1):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wishart DS, et al. DrugBank 5.0: a major update to the drugbank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris GM, Huey R, Olson AJ. Using autodock for ligand-receptor docking. Curr Protoc Bioinformatics. 2008;24(1):8.14.1-8.14.40. [DOI] [PubMed] [Google Scholar]
- 95.Trott O, Olson AJ. Autodock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Curated amino acid sequences of CSPs and OBPs from six hemipteran species. This file contains CSP and OBP amino acid sequences from C. lectularius, H. halys, Ne. viridula, O. laevigatus, R. prolixus, and Ni. lugens, including manually confirmed or corrected annotations and novel sequences identified in this study.
Additional file 2. Structural features of A) CSPs and B) OBPs in selected hemipteran species. Includes gene and protein identifiers, sequence lengths, subfamily classification, and key structural hallmarks (start and stop codons, predicted signal peptides, and conserved cysteine patterns).
Additional file 3. Structural characteristics of T. infestans A) CSP and B) OBP sequences. Sequence features and annotations of chemosensory and odorant-binding proteins identified in T. infestans.
Additional file 4. Gene models of T. infestans CSPs and OBPs. General feature format (GFF) files corresponding to CSP and OBP genes identified in T. infestans genome.
Additional file 5. Amino acid and nucleotide sequences of T. infestans CSPs and OBPs. FASTA-format sequences of all CSP and OBP genes from T. infestans, including both coding and protein sequences.
Additional file 6. Molecular docking results for T. infestans A) CSPs and B) OBPs with selected insecticides. Binding energy values from docking simulations assessing potential interactions between CSP/OBP proteins and various insecticide compounds (malathion, fipronil and deltamethrin).
Data Availability Statement
Data is provided within the manuscript or supplementary information files.