Abstract
Background
Atrial fibrillation (AF) is a prevalent arrhythmia, the ineffective contraction of the atria leads to a decrease in effective cardiac output. AF patients are prone to hypotension during anesthesia, especially in the early stages of general anesthesia. We explored whether the inferior vena cava collapsibility index (IVCCI) or the superior vena cava distensibility index (SVCDI) could predict the occurrence of post-induction hypotension (PIH) and early intraoperative hypotension (eIOH) in AF patients.
Methods
A total of 77 AF patients undergoing left atrial appendage occlusion under general anesthesia were included in this study. The inferior vena cava was measured before induction and the superior vena cava after induction. The main outcome was the ultrasound measurements of IVCCI and SVCDI in AF patients and their association with hypotension during general anesthesia. Hypotension was classified as the mean arterial pressure (MAP) below 60 mmHg or more than 20% below the baseline level. The correlation between IVCCI, SVCDI and the percentage decrease in MAP was assessed. Receiver operating characteristic (ROC) curves of IVCCI, SVCDI were separately generated to predict PIH and eIOH. Logistic regression was employed to validate the risk factors for PIH and eIOH in AF patients.
Results
AF patients who developed PIH had a significantly higher IVCCI (P < 0.001) and developed eIOH had a significantly higher SVCDI (P < 0.001). ROC curve analysis showed that IVCCI had an accuracy of 85% to predict PIH at a cut-off point more than 34.1% (P < 0.001). SVCDI had an accuracy of 86% to predict eIOH at a cut-off point more than 17.8% (P < 0.001). After adjusting for confounding variables, IVCCI was an independent predictor of PIH (P < 0.001), while SVCDI was an independent predictor of eIOH (P < 0.001).
Conclusion
Preoperative IVCCI > 34.1% indicates a non-invasive predictor of PIH in AF patients; SVCDI > 17.8% suggest a reliable predictor of eIOH in AF patients.
Trial registration
This trial was registered on June 27, 2023 at the China Clinical Trial Center (http://www.chictr.org.cn; No. ChiCTR2300072846).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-025-03295-5.
Keywords: IVC-CI, SVC-DI, Atrial fibrillation, Post-induction hypotension, Early intraoperative hypotension
Backgound
Atrial fibrillation (AF) is one of the most common arrhythmias in adults, with a reported incidence and prevalence of 1.6% in China [1]. AF elevates the risk of ischemic stroke and systemic arterial embolism. In recent years, various open and percutaneous left atrial appendage occlusion devices have been developed, and some have shown comparable effectiveness to anticoagulants in preventing strokes in patients with AF [2]. Percutaneous left atrial appendage closure, commonly performed under general anesthesia to optimize procedural conditions through patient immobilization and prolonged transesophageal echocardiography (TEE), poses a significant risk of hypotension. This complication is particularly pronounced in elderly patients with pre-existing cardiovascular comorbidities. A decrease in arterial pressure during anesthesia induction can result in perioperative complications, such as acute kidney injury, heart failure, cerebral ischemia, and myocardial infarction [3–6]. Factors predicting hypotension include age, a higher American Society of Anesthesiologists (ASA) physical status, use of propofol, dosage of fentanyl, and importantly, volume status [7, 8]. Patients with AF also face an increased risk of post-induction hypotension (PIH) or early intraoperative hypotension (eIOH) during general anesthesia. Recently recommended dynamic parameters for assessing volume status include ultrasound measurements of the inferior vena cava (IVC) diameter with respiration [9, 10]. These parameters, such as the maximum diameter of the IVC (IVCmax) at the end of expiration and the collapsibility index (CI), have been suggested as rapid, noninvasive methods to predict anesthesia-related hypotension [11, 12]. However, patients with AF lack atrial contraction support during left ventricular diastolic filling, which can lead to elevated left atrial pressure and necessitate greater volume management. Additionally, AF may compromise the utility of cardiopulmonary interaction-based volume status measurements, such as stroke volume variation [13]. The effectiveness of IVC variability as an indicator in patients with AF has yet to be explored.
This study aims to explore the clinical value of the inferior vena cava collapsibility index and the superior vena cava distensibility index in predicting the occurrence of PIH and eIOH events in atrial fibrillation patients.
Methods
Patients
We selected 77 patients with atrial fibrillation (aged 65–85) who underwent left atrial appendage occlusion under general anesthesia at Shanghai Zhoupu Hospital between June 2022 and January 2023, based on sample size calculations. The study protocol was reviewed and approved by the Institutional Ethics Committee of Shanghai Zhoupu Hospital (Registration number:2022-C-027-E0) and conformed to the ethical standards for medical research involving human subjects, as laid out in the 1964 Declaration of Helsinki and its later amendments. Participants provided written informed consent prior to taking part in the study The trial was registered at http://www.chictr.org.cn (registration number:ChiCTR2300072846) on 27 June 2023. Our reporting adhered to the STROBE guidelines. Participants in this study were at least 65 years old, with a body mass index (BMI) ranging from 18 to 32 kg m−2, an American Society of Anesthesiologists (ASA) physical status of 2 to 3, and scheduled for left atrial appendage occlusion. The exclusion criteria were as follows: emergency surgery, sudden life-threatening changes in vital signs, elevated intra-abdominal pressure, expected airway or mental dysfunction, diseases of the autonomic nervous system, current use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, presence of an implantable pacemaker or cardioverter, peripheral vascular disease, serious cardiovascular conditions, unstable angina, and an ejection fraction (EF) of less than 40%.
Materials and methods
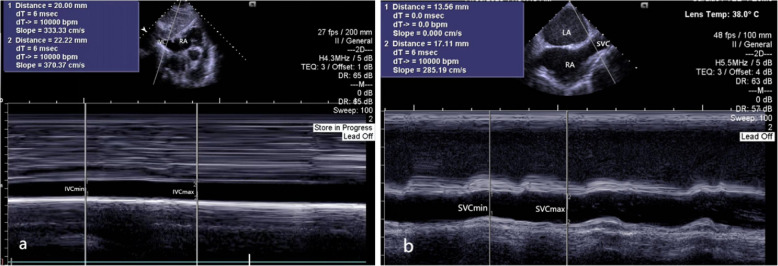
Upon entering the room, peripheral veins were opened, and lactated Ringer's solution was administered at a rate of 30 drops per minute, along with a pre-treatment of 0.05 mg kg−1 of midazolam. Hypertensive patients discontinued angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and diuretics prior to surgery. Patients were conscious, lying supine, and breathing spontaneously for at least five minutes before the examination. The maximum (IVCmax) and minimum (IVCmin) diameters of the inferior vena cava were measured using ultrasound during one spontaneous respiratory cycle in AF patients, as shown in Fig. 1a. The collapsibility index of inferior vena cava (IVCCI) was calculated using the following equation: collapsibility index = (IVCmax-IVCmin)/IVCmax*100 [12]. The ultrasound measurement of the inferior vena cava was performed as follows: the patient was positioned supine without a pillow, with hands relaxed at the sides, following the method described by the American Society of Echocardiography [14]: The subxiphoid approach was used, with a phased-array probe from a Siemens Healthineers ACUSON SC2000 machine placed subxiphoid to display the four-chamber view. The probe was then rotated downward towards the spine, with the orientation marker pointing towards the patient's head, to show the inferior vena cava entering the right atrium and the hepatic veins joining the inferior vena cava. The diameter of the inferior vena cava was measured 2 cm from the right atrial entrance, IVCmax and IVCmin were measured by freezing the ultrasound image at end-expiration and end-inspiration respectively. Each site was measured three times, and the average was taken; measurements differing by more than 2 mm were excluded.
Fig. 1.
M-mode assessment of IVCmax and IVCmin were measured during spontaneous breathing (a); TEE assessment of SVCmax and SVCmin were measured during mechanical ventilation (b)
After anesthesia induction, the maximum (SVCmax) and minimum (SVCmin) diameters of the superior vena cava were measured using a transesophageal ultrasound probe from a Siemens machine over one mechanical ventilation respiratory cycle in AF patients, as shown in Fig. 1b. The distensibility index of superior vena cava (SVCDI) was calculated using the following equation: distensibility index = (SVCmax-SVCmin)/SVCmin*100 [15]. The transesophageal ultrasound measurement of the superior vena cava was performed as follows: after the patient was under general anesthesia and mechanically ventilated, the transesophageal ultrasound was adjusted to a depth of 10 cm based on the mid-esophageal bicaval view. This view clearly displayed the left atrium, right atrium, interatrial septum, superior vena cava, inferior vena cava, partial pulmonary veins, and their connections to the right atrium, from which SVCmax and SVCmin were obtained. Each site was measured three times, and the average was taken; measurements differing by more than 2 mm were excluded. The measurement process did not exceed 3 min, and the entire induction period did not exceed 5 min. All ultrasound measurements were performed by an experienced anesthesiologist who was blinded to the study.
Arterial pressure was measured invasively using an arterial line catheter, which was inserted before anesthesia induction. Hemodynamic data were collected both before (baseline) and after induction. Post-induction hypotension (PIH) was defined by a decrease in mean arterial pressure (MAP) of more than 20% from baseline or any recorded period of MAP below 60 mmHg [16]. MAP is continuously monitored and recorded within the first 15 min after the start of surgery. Early intraoperative hypotension (eIOH) was defined as a MAP below 60 mmHg or a reduction of more than 20% from the baseline level within the first 15 min after skin incision [8].
Anesthesia induction involved a regimen of sufentanil (0.35 µg kg−1) and propofol (1.5 mg kg−1), followed by tracheal intubation facilitated by cisatracurium (1.5 mg kg−1). Anesthesia was maintained with 1.5 vol% inhaled sevoflurane in oxygen-enriched air and remifentanil continuously infused at 6 µg kg−1 h−1. In cases of severe hypotension (MAP < 55 mmHg) or prolonged hypotension episodes (lasting ≥ 3 min) during the induction phase or early intraoperative period, intravenous ephedrine 6 mg or norepinephrine infusion at 200 µg h−1 [17]. However, patients who experience severe hypotension requiring pharmacological intervention during the induction phase or early intraoperative period are excluded.
Statistical analysis
Sample size
The sample size was calculated using PASS15.0 software, commonly utilized in clinical research. Based on a literature review, we used the following parameters: the sensitivity of the IVC to predict intraoperative hypotension is 80%, the specificity is 90%, α = 0.05. The calculation indicated that 70 cases are needed for this study. To account for a potential dropout rate of 10%, 77 AF patients will be included.
Data analysis
Data were compiled and analyzed using Microsoft Excel, for statistical analyses, we employed StataMP16.0 and PRISM10.0. Continuous variables are presented as means ± standard deviations if they are normally distributed, as confirmed by the Shapiro–Wilk W test. Non-normally distributed data are displayed as medians and interquartile ranges. For comparisons, we used Student's two-sample t-test and the Mann–Whitney U test. Categorical variables are shown as percentages and absolute case numbers. The χ2-test and Fisher's exact test were used for analyzing contingency tables as appropriate. Two-sided p-values are reported, with a significance threshold set at p < 0.05. The pearson correlation coefficient (r) was utilized to evaluate the association between measurements and the percentage decrease in MAP from baseline after the induction of general anesthesia.
ROC curve analysis was conducted to evaluate the predictive capability of the two ultrasound-derived parameters, IVCCI and SVCDI, for clinically significant of PIH and eIOH. The areas under the curves (AUCs) with 95% confidence intervals were computed. We compared the two ROC curves using the nonparametric method described by DeLong et al. [18]. Optimal cutoff values were determined by the values that maximize the Youden index (sensitivity + specificity-1). Logistic regression was employed to validate the risk factors for PIH and eIOH in AF patients.
Results
A total of 77 AF patients were initially included in this analysis, however, 7 were subsequently excluded due to inadequately visualized IVC in 3 cases and missing data in 4 cases, as shown in Fig. 2. Ultimately, 70 AF patients were analyzed. Thirty-four.
Fig. 2.
STROBE flow diagram. AF, atrial fibrillation; MAP, mean artery pressure; IVC, inferior vena cava; SVC, superior vena cava; PIH, post-induction hypotension; eIOH, early intraoperative hypotension
patients were male and 36 were female. Their mean age was 75.2 ± 6.3 years, mean BMI was 23.9 ± 3.2 kg m−2. Fifteen patients were ASA 2, while 55 were ASA 3. The demographic characteristics of AF patients are summarized in Table 1.
Table 1.
Characteristics of AF patients
| Variables | |
|---|---|
| Age (years) | 75.2 ± 6.3 |
| Sex | |
| Male | 34 (48.6%) |
| Female | 36 (51.4%) |
| BMI | 23.9 ± 3.2 |
| 18 ~ 23.99 kg m−2 | 36 (51.4%) |
| 24 ~ 27.99 kg m−2 | 24 (34.3%) |
| 28 ~ 32 kg m−2 | 10 (14.3%) |
| ASA physical status | |
| II | 15 (21.4%) |
| III | 55 (78.6%) |
| Hypertension | 62 (88.6%) |
| Diabetes mellitus | 27(38.6%) |
| Coronary artery disease | 57(81.4%) |
| MAP (mmHg) | 105.3 ± 12.7 |
| HR (per/min) | 76.8 ± 16.8 |
Values are mean ± SD, number of patients or number (%)
BMI body mass index, ASA American Society of Anesthesiologists, MAP mean artery pressure, HR heart rate
Thirty-two patients developed PIH after induction, 21 patients developed eIOH after skin incision. When comparing AF patients who developed PIH and eIOH with those who did not, we found that there were no significant differences between the two groups as regards sex, BMI, ASA status, baseline MAP or HR. Significant differences were observed in the IVCmax and IVCCI, where AF patients with PIH had a significantly higher IVCCI (P < 0.001) and a lower IVCmax (P < 0.005) compared to those who did not. Similarly, patients with AF who developed eIOH, both IVCmax and SVCmax were significantly lower than in the normal blood pressure group, whereas IVCCI and SVCDI were significantly higher (P < 0.001), as shown in Table 2.
Table 2.
Comparison of characteristics of AF patients with post-induction hypotension and early intraoperative hypotension
| Variables | Developed PIH | Developed eIOH | ||||
|---|---|---|---|---|---|---|
| Yes (n = 32) | No (n = 38) | P | Yes (n = 21) | Yes (n = 21) | P | |
| Age(years) | 74.7 ± 5.5 | 75.6 ± 7.0 | 0.57 | 75.7 ± 5.7 | 75.0 ± 6.6 | 0.68 |
| Sex | 0.49 | 0.35 | ||||
| Male | 17 (24%) | 17 (24%) | 12 (17%) | 22 (31%) | ||
| Female | 15 (22%) | 21 (30%) | 9 (13%) | 27 (39%) | ||
| BMI (%) | 0.45 | 0.04 | ||||
| 18 ~ 23.99 kg m−2 | 19 (27%) | 17 (24%) | 15 (21%) | 21 (30%) | ||
| 24 ~ 27.99 kg m−2 | 8 (12%) | 16 (23%) | 5 (8%) | 19 (27%) | ||
| 28 ~ 32 kg m−2 | 5 (7%) | 5 (7%) | 1 (1%) | 9 (13%) | ||
| ASA physical status | 0.93 | 0.35 | ||||
| II | 7 (10%) | 8 (11%) | 6(9%) | 9 (13%) | ||
| III | 25 (36%) | 30 (43%) | 15(21%) | 15 (21%) | ||
| MAP (mmHg) | 107.1 ± 13.8 | 103.0 ± 11.5 | 0.28 | 110.3 ± 15.0 | 103.6 ± 11.3 | 0.05 |
| HR (per/min) | 80.0 ± 21.1 | 74.1 ± 11.8 | 0.14 | 77.5 ± 14.4 | 76.5 ± 17.9 | 0.83 |
| IVCmax (mm) | 18.4 ± 3.3 | 21.2 ± 2.6 | < 0.001 | 17.5 ± 3.1 | 21.0 ± 2.7 | < 0.001 |
| IVCCI (%) | 40.9 ± 12.6 | 23.8 ± 8.9 | < 0.001 | 43.0 ± 10.1 | 26.8 ± 12.1 | < 0.001 |
| SVCmax (mm) | - | - | 15.3 ± 2.6 | 18.6 ± 2.4 | < 0.001 | |
| SVCDI (%) | - | - | 26.6 ± 7.1 | 16.0 ± 7.4 | < 0.001 | |
Values are mean ± SD, number of patients or number (%)
BMI body mass index, ASA American Society of Anesthesiologists, MAP mean artery pressure, HR heart rate, IVCmax maximum inferior vena cava diameter, IVCCI collapsibility index of inferior vena cava, SVCmax maximum superior vena cava diameter, SVCDI distensibility index of superior vena cava, PIH post-induction hypotension; eIOH, early intraoperative hypotension
After anesthesia induction, the IVCmax showed a low-degree negative correlation with the percentage decrease in MAP (r = − 0.42, P < 0.001). On the other hand, the IVCCI exhibited a significant positive correlation with the decrease in MAP (r = 0.55, P < 0.001) (Fig. 3a). During the early intraoperative period, IVCmax showed a low-degree negative correlation with the percentage decrease in MAP (r = − 0.48, P < 0.001), while IVCCI exhibited a low-degree positive correlation (r = 0.45, P < 0.001) (Fig. 3b). SVCmax demonstrated a significant negative correlation with the percentage decrease in MAP (r = − 0.54, P < 0.001), and SVCDI showed a significant positive correlation with the decrease in MAP (r = − 0.54, P < 0.001) (Fig. 3c).
Fig. 3.

Scatter plots of IVCmax and IVCCI vs. post-induction (a) and early intraoperative hypotension decrease (b), and SVCmax with SVCDI (c) vs. early intraoperative hypotension decrease
The ROC curve analysis showed that AF patients with a AUROC of 95% confidence intervals was 0.77 (P < 0.001) for IVCCI and 0.86 (P < 0.001) for IVCmax to predict PIH. The optimal threshold for IVCmax was 19.4 mm (sensitivity 72%, specificity 79%), while for IVCCI it was 34.1% (sensitivity 84%, specificity 92%) (Fig. 4a). AUROC was 0.85 (P < 0.001) for predicting eIOH using IVCCI, the optimal threshold was 34.1% (sensitivity 91%, specificity 78%) (Fig. 4b); AUROC was 0.86 (P < 0.001) for predicting eIOH using SVCDI, the optimal threshold was 17.8% (sensitivity 95%, specificity 71%) (Fig. 4c).
Fig. 4.

Receiver operating characteristics (ROC) curve of IVCmax and IVCCI for predicting post-induction (a) and early intraoperative (b) hypotension; SVCmax with SVCDI (c) for early intraoperative hypotension
Table 3 shows that the IVCCI (OR = 1.20, 95% CI 1.09–1.31, P < 0.001) was associated with the occurrence of PIH in patients with AF, as did the IVCmax (OR = 0.55, 95% CI 0.37–0.82, P < 0.001). Other parameters were not associated with the occurrence of PIH.
Table 3.
Multivariate logistic regression for prediction post-induction hypotension
| OR | 95% CI | P | |
|---|---|---|---|
| Age (years) | 0.88 | 0.75 ~ 1.02 | 0.08 |
| BMI | 0.99 | 0.78 ~ 1.26 | 0.95 |
| HR (per/min) | 1.01 | 0.95 ~ 1.08 | 0.73 |
| MAP (mmHg) | 1.03 | 0.94 ~ 1.13 | 0.69 |
| IVCmax (mm) | 0.55 | 0.37 ~ 0.82 | < 0.05 |
| IVCCI (%) | 1.20 | 1.09 ~ 1.31 | < 0.001 |
BMI body mass index, HR heart rate, MAP mean artery pressure, IVCmax maximum inferior vena cava diameter, IVCCI collapsibility index of inferior vena cava, OR odds ratio, 95%CI 95% confidence interval
Table 4 shows the results of multivariate linear regression analysis factors predicting eIOH in patients with AF. After adjusting for age, BMI, and baseHR, baseMAP, it was found that IVCCI (OR = 1.16, 1.06–1.25, P < 0.05) and SVCDI (OR = 1.14, 1.02–1.27, P < 0.05) were independent predictors of eIOH.
Table 4.
Unadjusted and adjusted odds ratios for predicting early intraoperative hypotension
| Unadjusted analysis | Adjusted analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 0.95 | 0.80 ~ 1.13 | 0.56 | 0.94 | 0.81 ~ 1.10 | 0.48 | 0.98 | 0.87 ~ 1.11 | 0.74 |
| BMI | 0.78 | 0.55 ~ 1.10 | 0.16 | 0.76 | 0.54 ~ 1.05 | 0.10 | 0.78 | 0.60 ~ 1.02 | 0.07 |
| HR (per/min) | 1.00 | 0.95 ~ 1.05 | 0.95 | 0.99 | 0.95 ~ 1.03 | 0.61 | 1.02 | 0.98 ~ 1.06 | 0.44 |
| MAP (mmHg) | 0.98 | 0.88 ~ 1.08 | 0.67 | 1.00 | 0.92 ~ 1.08 | 0.94 | 1.00 | 0.93 ~ 1.07 | 0.95 |
| IVCmax (mm) | 0.57 | 0.36 ~ 0.91 | < 0.05 | 0.53 | 0.34 ~ 0.81 | < 0.05 | − | − | − |
| IVC-CI (%) | 1.14 | 1.03 ~ 1.26 | < 0.05 | 1.16 | 1.06 ~ 1.27 | < 0.05 | − | − | − |
| SVCmax (mm) | 0.92 | 0.60 ~ 1.40 | 0.69 | − | − | − | 0.64 | 0.44 ~ 0.94 | < 0.05 |
| SVC-DI (%) | 1.12 | 1.00 ~ 1.25 | 0.06 | − | − | − | 1.14 | 1.02 ~ 1.27 | < 0.05 |
BMI body mass index, HR heart rate, MAP mean artery pressure, IVCmax maximum inferior vena cava diameter, IVCCI collapsibility index of inferior vena cava, SVCmax maximum superior vena cava diameter, SVCDI distensibility index of superior vena cava, OR odds ratio, 95%CI 95% confidence interval
Discussion
This observational study found that pre-operative ultrasound measurements of IVCCI was an independent predictor of PIH and eIOH (P < 0.001), while SVCDI was an independent predictor of eIOH (P < 0.001). Moreover, patients with AF who developed PIH and eIOH exhibited a higher preoperative IVCCI and SVCDI.
In this study, 46% of AF patients experienced PIH, 30% experienced eIOH. All included patients were aged 65 years or older, suffered from AF, and over half had hypertension. Most were classified as ASA grade II or higher. During anesthesia induction, intravenous administration of drugs such as propofol and sufentanil can induce hypotension [19, 20]. To minimize the impact of these drugs, induction dosages were calculated based on body weight. The pathophysiological characteristics of AF involve disrupted atrial electrical activity leading to fibrillatory motion instead of effective contraction, resulting in incomplete blood ejection from the atria and stasis, especially in the left atrium [21]. Furthermore, decreased vascular reactivity, increased sensitivity to anesthetics, and extended preoperative fasting and preparation times contribute to the inherent susceptibility of AF patients to cardiovascular instability and hypotension.
During atrial fibrillation, heart rate and blood pressure exhibit low sensitivity in assessing volume responsiveness, and the unstable ventricular rate also renders many monitoring techniques that are feasible in normal patients unreliable as reference indicators. Perrine Bortolotti et al. reported that both IVCmax and IVCCI can predict fluid responsiveness in patients with arrhythmias in the ICU, with an area under the ROC curve of 0.93 (0.86–1) for both parameters. IVCmax < 11 mm predicted fluid responsiveness with a sensitivity of 83% and a specificity of 88%; an IVC-CI ≥ 39% predicted it with a sensitivity of 93% and a specificity of 88% [22]. Our study found that IVCmax predicts PIH in AF patients with an area under the ROC curve of 0.77 (0.65–0.88), a threshold of 19.4 mm, a sensitivity of 79%, and a specificity of 72%; IVCCI predicts PIH with an area under the ROC curve of 0.86 (0.75–0.97), a threshold of 34.1%, a sensitivity of 92%, and a specificity of 84%. Even in the presence of arrhythmias, IVCCI remains a predictive indicator of PIH.
During general anesthesia with mechanical ventilation, transesophageal ultrasound assessment of the superior vena cava is relatively straightforward [23]. Compared to conventional ultrasound, transesophageal echocardiography (TEE) performs vascular measurements directly behind the heart through the esophagus. This approach avoids interference from chest structures and pulmonary gas, thereby obtaining clearer images and more challenging views to acquire than standard echocardiograms. Additionally, TEE employs higher-frequency probes, which provide superior resolution in vascular measurements and yield more accurate results [24]. The study found that during mechanical ventilation, the area under the ROC curve for SVCDI in predicting eIOH in AF patients was 0.86 (0.76–0.95). The cutoff was 17.8%, with a sensitivity of 95% and specificity of 71%, indicating a significant predictive relationship between SVCDI and eIOH. Patients who developed eIOH had higher SVCDI compared to those with early intraoperative normotension among atrial fibrillation patients. In a large cohort study, Vignon P et al. compared several dynamic echocardiographic parameters for predicting fluid responsiveness and reported that the area under the ROC curve of SVC-DI (0.75) was significantly greater than that of IVCmax (0.63) and PPV (0.67) in mechanically ventilated patients [25]. The optimal cutoff for SVC-DI was 21%, with a sensitivity of 61% and specificity of 84%. It has been demonstrated that venous collapsibility in the great veins results from systemic filling pressure-driven venous return, rather than aortic pressure transmission [26–28]. Charbonneau et al. found that in mechanically ventilated septic patients, the SVC demonstrated higher accuracy than the IVC in predicting fluid responsiveness. The threshold for SVC to predict fluid responsiveness was 29%, with a sensitivity of 54% and a specificity of 89%, while the threshold for IVC was 21%, with a sensitivity of 38% and a specificity of 61% [29]. These results are largely consistent with some of our findings.Consequently, assessing the vena cava collapsibility/distensibility index is clinically valuable for predicting hypotension. Even in the presence of AF, IVC-CI and SVC-DI can still serve as predictive indicators for hypotension during general anesthesia.
There are some limitations of our study. A relatively small number of patients were included, although the estimated power of the study was sufficient to detect significant reliable results. Since SVCDI was measured using TEE, which is only used in certain types of surgery like left atrial appendage occlusion, the results may not apply to other procedures where TEE is not used. The study participants were elderly patients with relatively unstable hemodynamics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and diuretics were withdrawn prior to surgery. The relationship between preoperative oral administration of different medications and the magnitude of blood pressure reduction following induction and during the early intraoperative period requires more diverse clinical settings to validate and further develop the findings of this study.
Conclusion
In AF patients, IVCCI and SVCDI can predict the occurrence of PIH and eIOH, preoperative IVCCI > 34.1% indicates a non-invasive predictor of PIH; SVCDI > 17.8% suggest reliable predictors of eIOH.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- PIH
Post-induction hypotension
- eIOH
Early intraoperative hypotension
- IVCmax
Maximum inferior vena cava diameter
- IVCmin
Minimum inferior vena cava diameter
- SVCmax
Maximum superior vena cava diameter
- SVCmin
Minimum superior vena cava diameter
- IVC-CI
Collapsibility index of inferior vena cava
- SVC-DI
Distensibility index of superior vena cava
- ROC
Receiver Operating Characteristics
- MAP
Mean artery pressure
Authors’ contributions
XML: design the project; GSY, SWH, CHZ, collected the data; GSY, JZ analyzed and interpreted the data; XML wrote the manuscript; HJL and JZ critical revised the manuscript; All authors read and approved the final manuscript.
Funding
The study and publication of the manuscript was supported by the Shanghai Medical Innovation & Development Foundation (SMIDF2024-128–4).
Medical Application Foundation of Suzhou (SKY2021042).
Pudong New District Health Commission Key Discipline Cluster for Cardiovascular Diseases Development Program (PWZxq2022-11).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author in response to reasonable requests.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of Shanghai Zhoupu Hospital (Ethical number: 2022-C-027-E0) and registered in Chinese Clinical Trial Register (registration number: ChiCTR2300072846). The study was also conformed to the ethical standards for medical research involving human subjects, as laid out in the 1964 Declaration of Helsinki and its later amendments.Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haijian Liu, Email: liuhaijian1978@163.com.
Jiang Zhu, Email: zhujiangsz@126.com.
References
- 1.Shi S, Tang Y, Zhao Q, et al. Prevalence and risk of atrial fibrillation in China: a national cross-sectional epidemiological study. Lancet Regional Health-Western Pacific. 2022:100439. 10.1016/j.lanwpc.2022.100439. [DOI] [PMC free article] [PubMed]
- 2.Masoudi FA, Calkins H, Kavinsky CJ, et al. 2015 ACC/HRS/SCAI left atrial appendage occlusion device societal overview. J Am Coll Cardiol. 2015;66:1497–513. [DOI] [PubMed] [Google Scholar]
- 3.van Waes JA, van Klei WA, Wijeysundera DN, et al. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124:35–44. [DOI] [PubMed] [Google Scholar]
- 4.Bijker JB, van Klei WA, Vergouwe Y, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–26. [DOI] [PubMed] [Google Scholar]
- 5.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. [DOI] [PubMed] [Google Scholar]
- 6.Lienhart A, Auroy Y, Pequignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105:1087–97. [DOI] [PubMed] [Google Scholar]
- 7.Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–6. [DOI] [PubMed] [Google Scholar]
- 8.Südfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57–64. [DOI] [PubMed] [Google Scholar]
- 9.Kalantari K, Chang JN, Ronco C, et al. Assessment of intravascular volume status and volume responsiveness in critically ill patients. Kidney Int. 2013;83:1017–28. [DOI] [PubMed] [Google Scholar]
- 10.Peacock WF, Soto KM. Current techniques of fluid status assessment. Contrib Nephrol. 2010;164:128–42. [DOI] [PubMed] [Google Scholar]
- 11.Çelebi Yamanoğlu NG, Yamanoğlu A, Parlak İ, et al. The role of inferior vena cava diameter in volume status monitoring; the best sonographic measurement method. Am J Emerg Med. 2015;33(3):433–8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Critchley LA. Inferior vena cava ultrasnography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124(3):580–9. [DOI] [PubMed] [Google Scholar]
- 13.Eysenck W, Ammar A, Kanthasamy V, et al. A trial of three non-invasive blood pressure monitors compared with invasive blood pressure assessment in atrial fibrillation and sinus rhythm. Int J Clin Pract. 2019:e13410. 10.1111/ijcp.13410. [DOI] [PubMed]
- 14.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. Am Soc Echocardiogr. 2019;32:1–64. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani MM, Fantoni DT, Gimenes AM, et al. Clinical monitoring of cardiac output assessed by transoesophageal echocardiography in anesthetised dogs: a comparison with the thermodilution technique. Biomed Chromatogr. 2017;13(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turan A, Chang C, Cohen B, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 2019;130(4):550–9. [DOI] [PubMed] [Google Scholar]
- 18.DeLong eR, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 19.Möller Petrun A, Kamenik M. Bispectral index-guided induction of general anesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013;110(3):388–96. [DOI] [PubMed] [Google Scholar]
- 20.Green RS, Butler MB. Post-intubation hypotension in general anesthesia: a retrospective analysis. J Intensive Care Med. 2016;31(10):667–75. [DOI] [PubMed] [Google Scholar]
- 21.Eichenlaub M, Mueller-Edenborn B, Minners J, et al. Left atrial hypertension, electrical conduction slowing, and mechanical dysfunction: the pathophysiological triad in atrial fibrillation-associated atrial cardiomyopathy. Front Physiol. 2021;12:670527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bortolotti P, Colling D, Colas V, et al. Respiratory changes of the inferior vena cava diameter predict fluid responsiveness in spontaneously breathing patients with cardiac arrhythmias. Ann Intensive Care. 2018;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowie BS, Kluger R, Rex S, et al. The relationship between superior vena cava diameter and collapsibility and central venous pressure. Anesth Intensive Care. 2015;43(3):357–60. [DOI] [PubMed] [Google Scholar]
- 24.Cioccari L, Baur HR, Berger D, et al. Hemodynamic assessment of critically ill patients using a miniaturized transesophageal echocardiography probe. Crit Care. 2013;17(3):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignon P, Repessé X, Bégot E, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017;195:1022–32. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Tomida M, Ando T, et al. Cardiac variation of inferior vena cava: new concept in the evaluation of intravascular blood volume. J Med Ultrasonics. 2013;40:205–9. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Qian K, Ando T, et al. Cardiac variation of internal jugular vein for the evaluation of hemodynamics. Ultrasound Med Biol. 2016;42:1764–70. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Dong J, Zhou J, et al. Dynamic assessment of the central vein throughout the cardiac cycle in adults with no right heart disease by cardiac CT. Clin Imaging. 2021;69:120–5. [DOI] [PubMed] [Google Scholar]
- 29.Charbonneau H, Riu B, Faron M, et al. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cava diameters. Crit Care. 2014;18(5):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author in response to reasonable requests.