Abstract
Aspergillus sojae belongs to the Aspergillus section Flavi but does not produce aflatoxins. The functionality of the A. sojae aflR gene (aflRs) was examined by transforming it into an ΔaflR strain of A. parasiticus, derived from a nitrate-nonutilizing, versicolorin A (VERA)-accumulating strain. The A. parasiticus aflR gene (aflRp) transformants produced VERA, but the aflRs transformants did not. Even when aflRs was placed under the control of the amylase gene (amyB) promoter of Aspergillus oryzae, the amy(p)::aflRs transformants did not produce VERA. A chimeric construct containing the aflRs promoter plus the aflRs N- and aflRp C-terminal coding regions could restore VERA production, but a construct containing the aflRp promoter plus the aflRp N- and aflRs C-terminal coding regions could not. These results show that the A. sojae aflR promoter is functional in A. parasiticus and that the HAHA motif does not affect the function of the resulting hybrid AflR. We conclude that the lack of aflatoxin production by A. sojae can be attributed, at least partially, to the premature termination defect in aflRs, which deletes the C-terminal transcription activation domain that is critical for the expression of aflatoxin biosynthetic genes.
Aspergillus sojae and A. oryzae are used in the production of enzymes and fermented foods and are taxonomically related to A. parasiticus and A. flavus. All four species belong to the Aspergillus section Flavi, which is commonly referred to as the A. flavus group. These species not only are morphologically similar but also share sufficient DNA sequence similarity that Kurtzman et al. (13) proposed that A. parasiticus, A. oryzae, and A. sojae be reduced to a varietal status. Geiser et al. (9) analyzed the genes involved in primary metabolism in strains of A. flavus and A. oryzae and concluded that A. oryzae is a species that evolved by domestication from one of the two A. flavus groups. Although the molecular evidence regarding the evolutionary origin of A. sojae is still inconclusive, it is generally agreed that A. sojae, which has never been isolated from the field, is a domesticated variant of A. parasiticus (29).
Neither A. sojae nor A. oryzae is known to produce aflatoxins (28), but homologues of several aflatoxin biosynthetic genes have been found in them (1, 12, 17). These genes apparently are not transcribed (16, 20, 27). The molecular mechanisms that prevent production of aflatoxins by A. oryzae isolates have been examined only recently. Kusumoto et al. (15) found that several strains of A. oryzae (groups 2 and 3) have partial deletions in the aflatoxin gene cluster. However, other A. oryzae strains (group 1) with intact aflatoxin gene clusters also were identified. Thus, the molecular mechanisms responsible for nontoxigenicity in A. oryzae appear to be diverse. Several studies have suggested that A. sojae isolates do not produce aflatoxins because of a defect in the aflatoxin pathway regulatory gene homologue, aflR (27). Most recently, Matsushima et al. (19) showed that A. sojae aflR (aflRs) does not elevate the production of aflatoxin precursors in a strain of A. parasiticus but that strains carrying an additional copy of A. parasiticus aflR (aflRp) do produce increased levels of these precursors.
The A. parasiticus aflR gene encodes AflR, a GAL4-type, zinc cluster transcriptional factor of 444 amino acids. AflR and its counterparts in A. flavus and A. nidulans are required for transactivation of the genes located in the aflatoxin and sterigmatocytin gene clusters (3, 4, 8, 21, 30, 32). The A. sojae aflR gene contains a 6-bp duplication and a substitution at position 1145 that results in a pretermination stop codon, TGA. The predicted A. sojae AflR thus has a distinct HAHA motif and is 62 amino acids shorter than the functional A. parasiticus AflR (19, 27).
In the present study, we constructed an A. parasiticus strain whose aflR gene was deleted. We used this strain to determine whether intact A. sojae aflR, the A. sojae aflR coding region fused to the strong inducible promoter of an amylase gene of A. oryzae, and A. parasiticus aflR containing the corresponding aflR carboxy-terminal coding region of A. sojae could complement the aflR deletion.
MATERIALS AND METHODS
Fungal strains and culture media.
A. parasiticus CS10-N2 (pyrG niaD ver-1 wh-1) was derived from A. parasiticus CS10 (pyrG ver-1 wh-1) (24), which is deficient in the versicolorin A (VERA) reductase gene, ver-1, that is in part responsible for converting VERA to demethylsterigmatocystin. This mutant strain accumulates VERA and cannot use nitrate. An isogenic aflRp deletion strain of CS10-N2, CS10-N2Δ aflR, was used to study aflRs functionality. Potato dextrose agar (PDA) and potato dextrose broth (PDB), which permit aflatoxin production, were purchased from Difco (Detroit, Mich.). PDB supplemented with maltose (1% [wt/vol]) was used for the induction of the A. oryzae amylase gene promoter in the aflR overexpression experiments.
Construction of the aflR disruption vector.
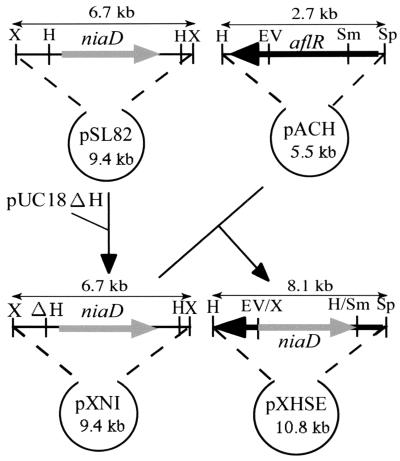
We constructed aflR disruption vector pXHSE (Fig. 1). The 6.7-kb niaD-containing HindIII-XbaI fragment of pXN1 was blunt ended with T4 DNA polymerase, ligated to pACH, pretreated with SmaI and EcoRV, blunt ended again, and dephosphorylated to produce pXHSE. The niaD-selectable marker in pXHSE replaced a large segment of the A. parasiticus aflR coding region corresponding to amino acids 7 to 444 of AflR.
FIG. 1.
Construction of the aflR disruption vector pHXSE. The aflR disruption vector pXHSE was constructed by ligating a 2.8-kb SacI-HindIII fragment of pHX1 containing A. parasiticus aflR (3) into the corresponding sites in pUC18 to generate pACH. A 6.7-kb XbaI fragment of pSL82 (10) was subcloned into the XbaI site of a pUC18 derivative from which the HindIII site had been removed. Then, the HindIII site of a 6.7-kb XbaI fragment in the niaD-niiA intergenic region (2) was eliminated by partial digestion with HindIII and self-ligation, yielding pXN1, which contained only one HindIII site. The 6.7-kb niaD-containing HindIII-XbaI fragment of pXN1 was blunt ended with T4 DNA polymerase, ligated to pACH, pretreated with SmaI and EcoRV, blunt ended, and dephosphorylated to yield pXHSE. EV, EcoRV; H, HindIII; S, SalI; Sm, SmaI; Sp, SphI; X, XbaI.
Generation of aflR disruption mutants.
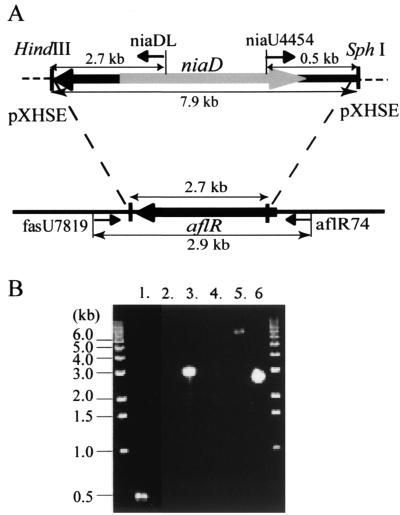
Plasmid pXHSE was linearized with HindIII and SphI before transformation to release the portion derived from pUC18 (Fig. 2A). Transformation of A. parasiticus CS10-N2 was carried out by a protoplast-polyethylene glycol method as previously described (10). Czapek Solution (CZ) agar (Difco) supplemented with Cove's trace-element solution (7), 20 mM uracil, and 0.6 M KCl was used for the regeneration of protoplasts after transformation. To confirm the occurrence of aflR gene disruption, we performed PCRs with the following primers: fasU7819, 5′-GTCGGCCACGATGAACCGATCCTAT-3′; aflR74, 5′-CTTGCCATACCGGGTGATAGATCAT-3′; niaU4454, 5′-GCCGCCGAGGACGCATATCCGAAGA-3′; and niaDL, 5′-CTTGCCATACCGGGTGATAGATCAT-3′ (Fig. 2). Genomic DNA of A. parasiticus transformants for PCR was isolated from mycelia grown in PDB for 72 h at 30°C with a DNeasy Plant Mini Kit (Qiagen, Chatsworth, Calif.).
FIG. 2.
Strategy and analysis of disruption of aflR of A. parasiticus. (A) Schematic representation of disruption of aflR in A. parasiticus. At the top is shown the replacement position of the aflR disruption plasmid pHXSE. The bottom of the figure shows the corresponding locus of aflR in A. parasiticus. Arrows show positions of oligonucleotide primers used for confirmation of aflR disruption. (B) Analysis of strains transformed with the aflR disruption plasmid. Insertion of niaD into aflR was confirmed by PCR with the primers fasU7819 and niaDL (lanes 1 and 2), aflR74 and niaU4454 (lanes 3 and 4), and fasU7819 and aflR74 (lane 5 and 6). Templates for PCR were prepared from CS10-N2 ΔaflR (lanes 1, 3, and 5) and CS10-N2 (lanes 2, 4, and 6).
RT-PCR.
Mycelia of A. parasiticus strains grown for 72 h at 30°C in PDB were harvested, blotted dry, and ground to a fine powder in liquid nitrogen with a mortar and pestle. Total RNA from 150 mg of mycelial powder per sample was prepared by using an RNeasy Total RNA Extraction Kit (Qiagen). The total RNA was treated with 1 to 2 U of RNase-free DNase at 37°C for 1 h to eliminate residual fungal DNA. The DNase-treated total RNA was purified with the RNeasy Total RNA Extraction Kit. Reverse transcription-PCR (RT-PCR) was carried out by using an Advantage RT-for-PCR Kit (Clontech, Palo Alto, Calif.) with an oligo(dT)18 primer. Primers flanking introns in aflatoxin genes were used to examine the presence of these gene transcripts in the total RNA samples. The aflR primers were 5′-CCGATTTCTTGGCTGAGT-3′ and 5′-TCCTCATCCACACAATCC-3′ (no intron). The aflJ primers were 5′-CTGGCTCCGTCAGCATCAGC-3′ and 5′-TATGCCATGATTATCGAGAC-3′ (intron 2). The nor1 primers were 5′-TGCAAACTGATATGGGCGAC-3′ and 5′-GAACTGATCGAGCGAAAGCC-3′ (intron 3). The omtA primers were 5′-GTCACCATGATGGAGCATGA-3′ and 5′-GCGCAAACGCAGTTGTTGAACG-3′ (intron 4). Two sets of primers derived from the A. parasiticus β-tubulin gene sequence (31) were used as the positive controls to confirm successful RT-PCR of the total RNA samples. One set was β-E26F (5′-TACCTTCAGACCGGCCAGTG-3′) and β-E26R (5′-GCAGCCCTCAGCCTCGCGAC-3′), which encompassed exons 2 to 6. Another set was β-I6F (5′-TACCTCACCTGCTCTGCCAT-3′) and β-I6R (5′-GTGAACTCCATCTCGTCCAT-3′) (intron 6).
Quantitation of aflR gene expression by real-time PCR.
The real-time PCR was performed in a two-step RT-PCR procedure with oligo(dT) as the extension primer. The first-strand cDNA was generated by RT-PCR with the SYBR Green RT-PCR kit (PE Biosystems [P/N 4306225], Foster City, Calif.). The thermal cycling parameters for the RT reactions are incubation at 25°C for 10 min, reverse transcription at 48°C for 30 min, and reverse transcriptase inactivation at 95°C for 5 min. Sample cDNAs were used in three serial dilutions: 1 μl straight, followed by 10e and 100-fold dilutions. The primer pair of A. parasiticus β-actin gene was used as a positive control for normalization. No template control (NTC) was used as the negative control. The aflR primer pair used was the forward primer 5′-TCCGCCATCTTTTCTCACCA-3′ and the reverse primer 5′-CCGAATTCCGAATCGACTGTTA-3′. The β-actin primers were as follows: forward, 5′-CCGACCGTATGCAGAAGAA-3′; reverse, 5′-ACTGCCCTTGCTCCCTCTTCCATGAA-3′. The aflR primers used in the real-time PCR experiment were designed to not amplify the transcript of second copy aflR. Real-time PCR was performed by using the GeneAmp 5700 Sequence Detection System (PE Biosystems). The thermal cycling parameters consisted of an initial heating at 95°C for 10 min, denaturing at 95°C for 15 s, and annealing and extension at 60°C for 1 min, with an amplification of 40 cycles. The relative quantitation values of aflR transcripts were obtained by using the comparative Ct method (ΔΔCt) as described elsewhere (Relative Quantitation of Gene Expression [User Bulletin No. 2]; PE Biosystems) (22).
Northern blot analysis.
A total of 5 μg of total RNA was fractionated in a 0.4 M formaldehyde-1.2% agarose gel and transferred to a GeneScreen Plus membrane (DuPont NEN Research Products, Boston, Mass.). The membrane was probed with aflatoxin gene DNA probes prepared with a DIG High Prime DNA Labeling and Detection Kit (Roche, Indianapolis, Ind.).
Vectors for aflR genetic complementation.
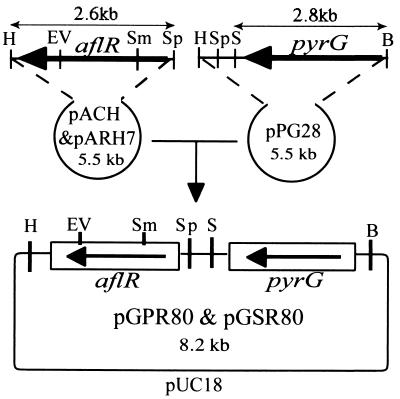
Vectors containing the A. parasiticus pyrG-selectable marker and the complete aflR genes of A. parasiticus and A. sojae were constructed for genetic complementation experiments. A 2.8-kb BamHI-SalI fragment from pBZ5, which contains the A. parasiticus pyrG gene (23), was cloned into pUC18 to give pPG28. The 2.6-kb HindIII-SphI fragment of pACH containing A. parasiticus aflR and the corresponding 2.6-kb HindIII-SphI fragment of pARH7 containing A. sojae aflR (19) were ligated to the HindIII-SphI-digested pPG28; the resulting constructs were designated pGPR80 and pGSR80, respectively (Fig. 3).
FIG. 3.
Vectors for introducing aflR into A. parasiticus. pGPR80 and pGSR80 contain an aflR fragment from A. parasiticus and A. sojae, respectively. B, BamHI; EV, EcoRV; H, HindIII; S, SalI; Sm, SmaI; Sp, SphI.
Fusion vectors for forced expression of the aflRs and aflRp coding regions.
Vectors containing the aflR coding regions of A. parasiticus and A. sojae placed under the control of the A. oryzae TaKa-amylase A gene (amyB) promoter (11) were constructed for the aflR overexpression experiments. An SphI site was introduced at the initiation codon of aflR in pGPR80 and pGSR80 by using a QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, Calif.). A 600-bp fragment containing the amyB gene promoter was amplified from the genomic DNA of A. oryzae RIB40 by using the following PCR primers: AoAmy5′-SP (5′-GAACTACGTGGAATGCATGCTGTTTTGATC-3′) and AoAmy3′-SP (5′-ACCACGCGACCAGCATGCATGCCTTATGTG-3′) (with the SphI site underlined). The PCR fragment was digested with SphI and inserted into the SphI site of pGPR80 and pGSR80; the resulting constructs were designated pGAmP and pGAmS, respectively. Sequences of the promoter and C-terminal region of aflR in each vector were confirmed.
Chimeric vectors for determining the region responsible for the defect in A. sojae aflR.
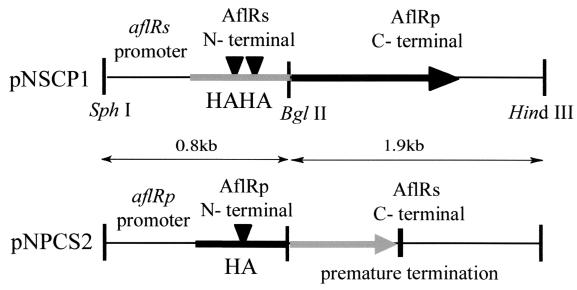
The 1.9-kb BglII-HindIII fragment containing the carboxyl-terminal coding region (amino acids 164 to 444) of aflRp in pGPR80 was swapped with the corresponding region of aflRs in pGSR80 to give two chimeric constructs, pNSCP1 and pNPCS2, respectively (Fig. 4). Regions around the BglII site in each vector were sequenced and confirmed.
FIG. 4.
Construction of chimera aflR between A. sojae and A. parasiticus. aflRs and aflRp were swapped at the BglII site. pNSCP1 consisted of the aflRs promoter, AflRs N-terminal domain, and AflRp C-terminal domain. pNPCS2 consisted of the aflRp promoter, AflRp N-terminal domain, and AflRs C-terminal domain.
Analysis of VERA production in transformants.
Fungal transformants were grown in PDB at 30°C for 3 days. Aflatoxin intermediates extracted from mycelia with acetone and chloroform (4:1 [vol/vol]) were separated by silica gel thin-layer chromatography with an ether-methanol-water (96:3:1 [vol/vol/vol]) solvent system as previously described (6).
RESULTS
Generation of A. parasiticus aflR-disrupted strains.
We used a double-crossover strategy with a linearized vector that contained the A. parasiticus niaD gene as a selectable marker to disrupt the A. parasiticus aflR gene (Fig. 2A). Transformation of A. parasiticus CS10-N2 with pXHSE digested with HindIII and SphI produced more than 500 transformants on CZ regeneration plates in a single experiment. One hundred randomly selected transformants were screened for VERA production on PDA plates. About 10% of these transformants did not accumulate the bright yellow VERA on the PDA plates, which suggested that the aflR gene in these transformants had been disrupted. Disruption of aflR was confirmed by PCR with the niaD internal primers (niaU4454 and niaDL) and the aflR external primers (fasU7819 and aflR74) (Fig. 2A). When genomic DNA of two nonpigmented transformants was amplified, primer pair niaU4454-aflR74 gave a 0.5-kb PCR fragment, and primer pair niaDL-fasU7819 gave a 3.0-kb PCR fragment (Fig. 2B, lanes 1 and 3). In contrast, no PCR fragments were obtained from A. parasiticus CS10-N2 genomic DNA (Fig. 2B, lanes 2 and 4). Moreover, primer pair fasU7819-aflR74 yielded an 8.2-kb PCR fragment from the genomic DNA of the nonpigmented transformants and a 2.9-kb PCR fragment from the CS10-N2 genomic DNA (Fig. 2B, lanes 5 and 6). These results indicate that the niaD marker had been inserted into the aflR locus through a double-crossover event in the nonpigmented transformants. One of the transformants, CS10-N2 ΔaflR, was used in the subsequent genetic complementation experiments.
Complementation of A. parasiticus CS10 ΔaflR with intact aflR of A. sojae and A. parasiticus.
To determine whether A. sojae aflR (aflRs) could complement the genetic defect of A. parasiticus aflR (aflRp), we transformed pGSR80 or pGPR80 (Fig. 3) into A. parasiticus CS10-N2 ΔaflR. Of the 12 aflRs (pGSR80) transformants examined, none produced VERA on the PDA plates, whereas 8 of 15 aflRp (pGPR80) transformants produced VERA (Table 1).
TABLE 1.
Introduction of A. sojae aflR into an A. parasiticus aflR-disrupted strain
| Introduced plasmid containing aflRa | No. of transformants
|
|
|---|---|---|
| VERA production | Total | |
| Intacta | ||
| pGSR80 (A. sojae aflR)b | 0 | 12 |
| pGPR80 (A. parasiticus aflR) | 8 | 15 |
| Amylase gene promoter-aflR fusiona | ||
| pGAmS (A. sojae aflR)b | 0 | 20 |
| pGAmP (A. parasiticus aflR) | 10 | 20 |
| Chimera aflR between A. sojae and A. parasiticusa,c | ||
| pNSCP1 | 8 | 20 |
| pNPCS2 | 0 | 20 |
Differences between the data are significant according to the Fisher's exact test (P < 0.05).
pGSR80 and pGAmS are replicated twice, and no VERA production was seen.
pNSCP1 indicates a plasmid containing the aflRs promoter, the AflRs N-terminal moiety, and the AflRp C-terminal moiety. pNPCS2 indicates a plasmid containing the aflRp promoter, the AflRp N-terminal moiety, and the AflRs C-terminal moiety.
RT-PCR analyses of aflR gene expression in CS10 ΔaflR and its aflRs transformant.
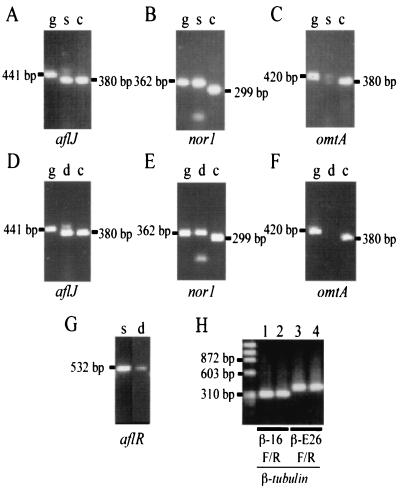
RT-PCR analysis performed with the first-strand cDNA prepared from DNase-treated, 72-h total RNA resulted in the amplification of aflR, aflJ, nor1, and omtA (Fig. 5A to G) from CS10-N2 ΔaflR, its aflRs transformant S8, and CS10-N2, which has a functional aflRp. This process was successful because only processed size transcripts of the A. parasiticus β-tubulin gene (Fig. 5H) were amplified from the first-strand cDNA. If β-tubulin gene transcripts were processed, primers βI6F/R and βΕ26F/R yielded 346- and 382-bp fragments, respectively, but if left unprocessed they yielded 462- and 691-bp fragments. Moreover, no PCR fragment was obtained from the DNase-treated total RNA. Thus, the template RNA was not contaminated by carryover genomic DNA. The processed sizes of the aflJ transcripts were amplified from cDNA of CS10-N2, CS10-N2ΔaflR, and CS10-N2aflRs-S8 (Fig. 5A and D). On the other hand, only the unprocessed size of the nor1 transcript was recovered from CS10-N2ΔaflR and CS10-N2aflRs-S8 (Fig. 5B and E). The omtA transcripts were detected at low levels in CS10-N2 aflRs-S8 (Fig. 5C) but not at all in CS10-N2ΔaflR (Fig. 5F). RT-PCR analysis detected aflR gene transcripts in both CS10-N2ΔaflR and CS10-N2 aflRs-S8 that had aflRs integrated at the pyrG locus (Fig. 5G). However, the levels of aflatoxin gene transcripts expressed in CS10-N2 ΔaflR and the afiRs transformant were not detectable by conventional Northern blot analysis (data not shown).
FIG. 5.
Profile of aflatoxin gene transcripts of CS10ΔaflR and its aflRs transformant detected by RT-PCR. (A to F) Lane g, genomic DNA of CS10-N2 (control); lane c, cDNA of CS10-N2 (control); lane s, cDNA of CS10-N2 aflRs-S8 (pGSR80); lane d, cDNA of CS10-N2 ΔaflR. (A and D) aflJ unprocessed (expected size of fragment, 441 bp), aflJ processed (380 bp). (B and E) nor1 unprocessed (362 bp) and nor1 processed (299 bp). (C and F) omtA unprocessed (42 0bp) and omtA processed (380 bp). (G) aflR (532 bp). (H) β-Tubulin gene (control). The processed sizes of the transcripts by primers β-I6F/R and β-E26F/R are 346 and 382 bp, respectively. Lane 1, β-I6F/R, CS10-N2 ΔaflR; lane 2, β-I6F/R, CS10-N2 aflRs-S8; lane 3, β-E26F/R, CS10-N2 ΔaflR; lane 4, β-E26F/R, CS10-N2 aflRs-S8.
Quantitation of aflR gene expression by real-time PCR.
To quantify the level of aflR expression, a real-time PCR experiment was carried out. The parental strain of CS10-N2 ΔaflR contains a partially duplicated aflatoxin gene cluster (26). To avoid the detection of transcript from the second copy aflR, we used PCR primers that did not amplify the transcript from the second copy aflR. The data from each sample treatment were normalized against that for the β-actin positive control. The experimental data are accurate with minimum errors, as demonstrated both by the positive control (β-actin) and the NTC and by the dissociation curve. Due to the disruption of the aflR gene in the CS10N2 ΔaflR mutant, no aflR transcription was detected. The reaction threshold of the ΔaflR strain was at ca. 33 cycles, i.e., approximately the same as for the NTC. The level of transcription of aflR in the nondisrupted strain (CS10N2) is about two-thirds (100 versus 147%) the level of that for the A. parasiticus aflR-complemented strain (CS10N2 aflR p-P8) (Table 2). The A. sojae aflR in the A. parasiticus background is expressed well enough to more than half of the level of the nondisrupted strain.
TABLE 2.
Relative expression of Aspergillus sojae aflR to A. parasiticus aflR
| Ratio type | Relative expression (mean ± SE)a in:
|
|||
|---|---|---|---|---|
| CS10N2 | CS10N2 ΔaflR | CS10N2 aflRp-P8 | CS10N2 aflRs-S8 | |
| Ratio to CS10N2 aflRs-S8 | 1.82 ± 1.11 | 0.00 | 2.51 ± 1.28 | 1.00 |
| Ratio to CS10N2 | 1.00 | 0.00 | 1.47 ± 0.53 | 0.68 ± 0.32 |
The values are based on three determinations. Steady-state mRNA levels were determined by real-time RT-PCR from total RNA prepared from 48-h-old mycelia grown in PDB medium supplemented with uracil. CS10N2 is the parent of CS10N2 ΔaflR. CS10N2 ΔaflR is an aflR-disrupted strain. CS10N2 aflRp-P8 is CS10N2 ΔaflR complemented with pGPR80 (A. parasiticus aflR). CS10N2 afls-S8 is CS10N2 ΔaflR complemented with pGSR80 (A. sojae aflR).
Overexpression of aflRs and aflRp in A. parasiticus CS10-N2 ΔaflR.
To examine whether overexpression of aflRs could complement the aflR mutation in CS10-N2 ΔaflR, we constructed pGAmP and pGAmS, in which the aflRp and aflRs coding regions, respectively, were placed under the control of the A. oryzae amylase gene promoter. We chose 20 colonies each of pGAmP and pGAmS transformants at random and transferred them onto PDA plates with or without 1% maltose, which is required for the induction of the amylase gene promoter. Half of the pGAmP (aflRp) transformants activated aflatoxin biosynthetic genes, as judged by VERA production on PDA. VERA production by the transformants was increased by the addition of maltose to the PDA. None of the pGAmS (aflRs) transformants produced VERA on either medium. Promoter function of pGAmS was confirmed by using a chimera vector, exchanging the 1.9-kb BglII-HindIII fragment containing the carboxyl-terminal region of aflRs in pGAmS for that of aflRs in pGAmP. About half of the transformant with the chimera vector produced VERA on PDA (data not shown).
Effect of the aflRs promoter, the HAHA motif, and truncation of the carboxy-terminal 62 amino acids on the function of A. sojae AflR.
There are three differences in the aflR genes of A. sojae and A. parasiticus. One difference is a nucleotide substitution of G for A at position −132 in the aflR promoter (1). There also are two differences between the predicted AflR proteins of A. sojae and A. parasiticus: a duplication of His-Ala and a nonsense mutation give change in the coding region of aflRs, resulting in an AflR protein in A. sojae that is 62 amino acids shorter than the protein from A. parasiticus (27). To examine the effect of these differences on the functions of aflRs and AflRs, we constructed two chimeras (Fig. 4). pNSCP1 consisted of the aflRs promoter and encoded the AflRs N-terminal moiety plus the AflRp C-terminal moiety. pNPCS2 consisted of the aflRp promoter and encoded the AflRp N-terminal moiety and the AflRs C-terminal moiety. Forty percent of the CS10-N2 ΔaflR transformants transformed with pNSCP1 showed transactivation of the aflatoxin biosynthetic genes, based on VERA production. However, pNPCS2 could not complement the aflR mutation of CS10-N2 ΔaflR.
DISCUSSION
In A. parasiticus and A. flavus, AflR is the only known transcriptional regulator of aflatoxin-related genes that result in aflatoxin production. aflR homologues are found in non-aflatoxin-producing A. sojae, which is used industrially in food fermentation, but transcription of these homolgues is not detectable (20, 27). Determining the cause of transcriptional loss of aflR in A. sojae is important because this fungus is used to produce products that are routinely used for human consumption.
The C-terminal domain of active AflR from A. parasiticus or A. flavus is the transcription-activating domain of this protein (5). The AflR homologue from A. sojae (AflRs), if expressed, lacks this domain (27) and has little or no transcriptional activation activity (19). Introduction of an extra copy of aflR in an A. parasiticus wild-type strain increased aflatoxin production (3), but the introduction of aflRs had no discernible effect on the level of aflatoxin production.
It is difficult to separate weak activity attributable to AflRs from background expression from the original copy of aflRp. Moreover, the complete functional loss of AflRp in the host strain while keeping other aflR-related signal transduction intact is important for detecting weak transcriptional activity and for sensitive monitoring of AflR activity. In addition, aflR-deficient strains obtained by UV mutagenesis were not suitable for this experiment because this form of mutagenesis could cause other lesions that could adversely affect the outcome. To avoid background activity from wild-type aflRp, we created aflR-disrupted strains by homologous recombination and examined AflRs functionality in an A. parasiticus ΔaflR strain that allowed us to monitor AflR activity.
We constructed an aflR-disrupted strain from an A. parasiticus VERA-accumulating mutant (24) to assess the function of aflRs in vivo. VERA is a precursor of aflatoxins; the mutant does not produce any aflatoxin (33). The parent strain, CS10-N2, accumulated VERA, and colonies turned bright yellow on PDA plates. Inactivation of genes in the aflatoxin biosynthetic pathway by the aflR disruption prevented the creation of VERA, and colonies of the ΔaflR strains remained white. Thus, the ΔaflR strains were suitable for aflR complementation tests because of their distinguishable phenotype.
A. parasiticus strains have two copies of aflR (18, 26, 27). One is located outside the aflatoxin gene cluster and is inactivated by functional loss resulting from amino acid substitution or insufficient transcription (J. W. Cary, K. C. Ehrlich, M. S. Wright, P.-K. Chang, and D. Bhatnagar, unpublished data). Disruption of the active copy, which is located in the aflatoxin gene cluster, eliminates detectable AflR function from cells. Using PCR, we confirmed that the aflR disruption mutation occurred at the active aflR locus in the aflatoxin gene cluster by double-crossover recombination (Fig. 2A). Real-time PCR did not detect transcript from the first copy aflR of CS10-N2 ΔaflR (Table 2). So the transcript detected by the RT-PCR experiment was derived from the second copy of aflR in CS10-N2 ΔaflR (Fig. 5G). aflJ, nor1, and omtA were also detected by RT-PCR in both CS10-N2 ΔaflR and aflRs transformants, but these strains did not produce VERA, and conventional Northern blot analysis could not detect the transcription of nor1, ver1, or omtA (data not shown). These results suggest that RT-PCR detected the basal-level transcription (25) of genes not activated by functional AflR.
The aflR disruption could be complemented, and these strains could produce VERA after integration of aflRp (pGPR80) at the pyrG locus (data not shown) but not by the integration of aflRs (pGSR80) in the same locus. The transcription level of aflRs in strain CS10N2 aflRs-S8, which has aflRs in its pyrG site, is more than half of that of CS10N2 (Table 2). These results suggest that the failure of aflRs transformants to produce VERA was not caused by gene silencing mediated by a positional effect.
DNA sequence information suggests that aflRs will produce a truncated protein (AflRs) compared to AflRp. Using our chimeric constructs we showed that the aflRs promoter is functional, that the HAHA motif in the AflRs N-terminal domain is probably not involved in the functional loss of AflRs activity, and that the loss of the 62-amino-acid sequence from the C terminus of AflRs is probably responsible for the observed loss of activity.
Matsushima et al. (20) reported that without the expression of aflRs there was no production of aflatoxin in A. sojae in spite of the presence of the toxin pathway genes in this fungus. However, we detected transcription of aflRs, i.e., that the level is equivalent to that of aflRp (CS10-N2), in an A. parasiticus background. The transcription of aflR and other aflatoxin-related genes (aflJ, nor1, and omtA) was also detected by RT-PCR in the A. parasiticus ΔaflR strain. Thus, the lack of a functional AflRs is insufficient to explain the complete repression of aflRs and other aflatoxin-related genes in A. sojae (20, 27). Kusumoto et al. (14, 16) reported that they did not detect several aflatoxin gene transcripts from A. oryzae, whose aflR does not have nonsense mutations as does aflRs (16, 27). Therefore, we conclude that the nonsense mutation in AflR is a reason for the nonproduction of aflatoxin in A. sojae but that other factors might also be involved. Factors involved in the complete repression of aflRs in A. sojae are still unknown.
It is generally accepted that A. sojae and A. oryzae never produce aflatoxins under any culture conditions (28). We conclude that the lack of aflatoxin production by A. sojae can be attributed, at least in part, to the premature termination defect in aflRs, which deletes the C-terminal transcription activation domain that is critical for the expression of aflatoxin biosynthetic genes. The lack of A. sojae aflR expression in the A. sojae genetic background coupled with its relatively high expression in an A. parasiticus genetic background indicates that an additional defect(s), in addition to the premature termination of the aflR transcript, may occur in the industrial strain A. sojae and reduce even further the potential ability of these strains to synthesize aflatoxins.
Acknowledgments
We thank Kenneth C. Ehrlich for helpful discussions during the real-time PCR experiments and Michelle Faust and Karen Gillespie for technical assistance.
REFERENCES
- 1.Chang, P.-K., D. Bhatnagar, T. E. Cleveland, and J. W. Bennett. 1995. Sequence variability in homologs of the aflatoxin pathway gene aflR distinguishes species in Aspergillus section flavi. Appl. Environ. Microbiol. 61:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, P.-K., K. Ehrlich, J. E. Linz, D. Bhatnagar, T. E. Cleveland, and J. W. Bennett. 1996. Characterization of the Aspergillus parasiticus niaD and niiA gene cluster. Curr. Genet. 30:68-75. [DOI] [PubMed] [Google Scholar]
- 3.Chang, P.-K., K. Ehrlich, J. Yu, D. Bhatnagar, and T. E. Cleveland. 1995. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 61:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, P.-K., J. W. Cary, D. Bhatnagar, T. E. Cleveland, J. W. Bennett, J. E. Linz, C. P. Woloshuk, and G. A. Payne. 1993. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl. Environ. Microbiol. 59:3273-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, P.-K., J. Yu, D. Bhatnagar, and T. E. Cleveland. 1999. The carboxy-terminal portion of the aflatoxin pathway regulatory protein AFLR of Aspergillus parasiticus activates GAL::lacZ gene expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 65:2508-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleveland, T. E., D. Bhatnagar, and R. L. Brown. 1991. Aflatoxin production via cross-feeding of pathway intermediates during co-fermentation of aflatoxin pathway-blocked Aspergillus parasiticus mutants. Appl. Environ. Microbiol. 57:2907-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51-56. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich, K. C., B. G. Montalbano, D. Bhatnagar, and T. E. Cleveland. 1998. Alteration of different domains in AFLR affects aflatoxin pathway metabolism in Aspergillus parasiticus transformants. Fungal Genet. Biol. 23:279-287. [DOI] [PubMed] [Google Scholar]
- 9.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horng, J. S., P.-K. Chang, J. J. Pestka, and J. E. Linz. 1990. Development of a homologous transformation system for Aspergillus parasiticus with the gene encoding nitrite reductase. Mol. Gen. Genet. 224:294-296. [DOI] [PubMed] [Google Scholar]
- 11.Kanemori, Y., K. Gomi, K. Kitamoto, C. Kumagai, and G. Tamura. 1999. Insertion analysis of putative functional elements in the promoter region of a heterologous Aspergillus oryzae TaKa-amylase A gene (amyB) by using a heterologous Aspergillus nidulans amdS-lacZ fusion gene system. Biosci. Biotechnol. Biochem. 63:180-183. [DOI] [PubMed] [Google Scholar]
- 12.Klich, M. A., P.-K. Chang, E. J. Mullaney, D. Bhatnagar, and T. E. Cleveland. 1995. Hybridization of genes involved in aflatoxin biosynthesis to DNA of aflatoxigenic and non-aflatoxigenic aspergilli. Appl. Microbiol. Biotechnol. 44:439-443. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzman, C. P., B. W. Horn, and C. W. Hesseltine. 1987. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Leeuwenhoek 53:147-158. [DOI] [PubMed] [Google Scholar]
- 14.Kusumoto, K., K. Yabe, Y. Nogata, and H. Ohta. 1998. Aspergillus oryzae with or without aflatoxin biosynthetic gene ver-1. Appl. Microbiol. Biotechnol. 50:98-104. [DOI] [PubMed] [Google Scholar]
- 15.Kusumoto, K., Y. Narata, and H. Ohta. 2000. Directed deletions in the aflatoxin biosynthesis gene homolog cluster of Aspergillus oryzae. Curr. Genet. 37:104-111. [DOI] [PubMed] [Google Scholar]
- 16.Kusumoto, K., K. Yabe, Y. Nagata, and H. Ohta. 1998. Transcript of a homolog of aflR, a regulatory gene for aflatoxin synthesis in Aspergillus parasiticus, was not detected in Aspergillus oryzae strains. FEMS Microbiol. Lett. 169:303-307. [DOI] [PubMed] [Google Scholar]
- 17.Kusumoto, K., K. Mori, Y. Nogata, H. Ohta, and M. Manabe. 1996. Homologs of an aflatoxin biosynthetic gene ver-1 in the strains of Aspergillus oryzae and its related species. J. Ferment. Bioeng. 82:161-164. [Google Scholar]
- 18.Liang, S.-H., C. D. Skory, and J. F. Linz. 1996. Characterization of the function of the ver-1A and ver-1B genes, involved in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 62:4568-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushima, K., P.-K. Chang, J. Yu, D. Bhatnagar, and T. E. Cleveland. 2001. Pre-termination in aflR of Aspergillus sojae inhibits aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 55:585-589. [DOI] [PubMed] [Google Scholar]
- 20.Matsushima, K., K. Yashiro, Y. Hanya, K. Abe, K. Yabe, and T. Hamasaki. 2001. Absence of aflatoxin biosynthesis in koji mold (Aspergillus sojae). Appl. Microbiol. Biotechnol. 55:771-776. [DOI] [PubMed] [Google Scholar]
- 21.Payne, G. A., G. J. Nystrom, D. Bhatnagar, T. E. Cleveland, and C. P. Woloshuk. 1993. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl. Environ. Microbiol. 59:156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittgen, T. D., B. A. Zakrajsek, A. G. Mills, V. Gorn, M. J. Singer, and M. W. Reed. 2000. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285:194-204. [DOI] [PubMed] [Google Scholar]
- 23.Skory, C. D., J. S. Horng, J. J. Pestka, and J. E. Linz. 1990. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl. Environ. Microbiol. 56:3315-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skory, C. D., P.-K. Chang, J. Cary, and J. E. Linz. 1992. Isolation and characterization of gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 58:3527-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tjian, R., and T. Maniatis. 1994. Transcription activation: a complex puzzle with few easy pieces. Cell 77:5-8. [DOI] [PubMed] [Google Scholar]
- 26.Trial, F., N. Mahanti, M. Rarick, R. Mehigh, S. H. Liang, R. Zhou, and J. E. Linz. 1995. Physical and transcriptional map of an aflatoxin gene cluster in Aspergillus parasiticus and functional disruption of a gene involved early in the aflatoxin pathway. Appl. Environ. Microbiol. 61:2665-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson, A. J., L. J. Fuller, D. J. Jeens, D. B. Archer. 1999. Homologs of aflatoxin biosynthesis genes and sequence of aflR in Aspergillus oryzae and Aspergillus sojae. Appl. Environ. Microbiol. 65:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, D. L., and S. C. Jong. 1986. Production of aflatoxins by strains of the Aspergillus flavus group maintained in ATCC. Mycopathologia 93:19-24. [DOI] [PubMed] [Google Scholar]
- 29.Wicklow, D. T. 1984. Adaptation in wild and domesticated yellow-green aspergilli, p. 78-86. In H. Kurata and Y. Ueno (ed.), Toxigenic fungi: their toxins and health hazards. Elsevier, Amsterdam, The Netherlands.
- 30.Woloshuk, C. P., K. R. Foutz, J. F. Brewer, D. Bhatnagar, T. E. Cleveland, and G. A. Payne. 1994. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 60:2408-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, T. S., and J. E. Linz. 1993. Recombinational inactivation of the gene encoding nitrate reductase in Aspergillus parasiticus. Appl. Environ. Microbiol. 59:2998-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, J.-H., R. A. E. Butchko, M. Fernandes, N. P. Keller, T. J. Leonard, and T. H. Adams. 1996. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29:549-555. [DOI] [PubMed] [Google Scholar]
- 33.Yu, J., P.-K. Chang, J. W. Cary, M. Wright, D. Bhatnagar, T. E. Cleveland, G. A. Payne, and J. E. Linz. 1995. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus. Appl. Environ. Microbiol. 61:2365-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]