Abstract
Mundticin KS, a bacteriocin produced by Enterococcus mundtii NFRI 7393 isolated from grass silage in Thailand, is active against closely related lactic acid bacteria and the food-borne pathogen Listeria monocytogenes. In this study, biochemical and genetic characterization of mundticin KS was done. Mundticin KS was purified to homogeneity by ammonium sulfate precipitation, sequential ion-exchange chromatography, and solid-phase extraction. The gene cluster (mun locus) for mundticin KS production was cloned, and DNA sequencing revealed that the mun locus consists of three genes, designated munA, munB, and munC. The munA gene encodes a 58-amino-acid mundticin KS precursor, munB encodes a protein of 674 amino acids involved in translocation and processing of the bacteriocin, and munC encodes a mundticin KS immunity protein of 98 amino acids. Amino acid and nucleotide sequencing revealed the complete, unambiguous primary structure of mundticin KS; mundticin KS comprises a 43-amino-acid peptide with an amino acid sequence similar to that of mundticin ATO6 produced by E. mundtii ATO6. Mundticin KS and mundticin ATO6 are distinguished by the inversion of the last two amino acids at their respective C termini. These two mundticins were expressed in Escherichia coli as recombinant peptides and found to be different in activity against certain Lactobacillus strains, such as Lactobacillus plantarum and Lactobacillus curvatus. Mundticin KS was successfully expressed by transformation with the recombinant plasmid containing the mun locus in heterogeneous hosts such as E. faecium, L. curvatus, and Lactococcus lactis. Based on our results, the mun locus is located on a 50-kb plasmid, pML1, of E. mundtii NFRI 7393.
Among the various antimicrobial compounds produced by lactic acid bacteria (LAB), bacteriocins are ribosomally synthesized single polypeptides or posttranslationally modified polypeptides that are usually inhibitory only to closely related bacterial species (18, 22). In recent years, LAB bacteriocins have generated interest due to their potential as biopreservatives that could, at least partially, replace chemical preservatives (35). This interest is also due to the structural diversity of these bacteriocins and to the presence of many bacteriocin-producing LAB in a variety of naturally fermented products and feed products.
LAB bacteriocins are subdivided into four different classes based on biochemical and genetic characteristics (18, 21, 22). Class I and class II bacteriocins are by far the most extensively studied, because they are the most abundant and most prominent in industrial applications (27). Class I bacteriocins, named lantibiotics, contain two modified amino acid residues, lanthionine and methyllanthionine, which are formed posttranslationally (5). They are small (<5 kDa) membrane-active peptides. Nisin is the most extensively studied lantibiotic and is approved for use as a food preservative (31). Class II bacteriocins are small (<10 kDa), unmodified, heat-stable, membrane-active peptides and are usually characterized by a G-G-Xaa processing site (where Xaa is any amino acid) in the bacteriocin precursor. Several subgroups have been defined within class II, notably the class IIa bacteriocins, which contain a consensus YGNGV amino acid motif near the N terminus and are active against the food-borne pathogen Listeria monocytogenes (8, 22). Class IIa bacteriocins are widespread among LAB and include pediocins AcH and PA-1 produced by Pediococcus acidilactici (3, 24), piscicolin 126 produced by Carnobacterium piscicola (19), sakacin P and 674 produced by Lactobacillus sakei (16, 36), enterocin SE-K4 produced by Enterococcus faecalis (7), and leucosin A produced by Leuconostoc gelidum (13).
We previously did a survey of LAB from fermented tropical vegetables and silage in Thailand to search for bacteriocin producers (7) and successfully isolated several thermophilic enterococcal strains that produced bacteriocins against Enterococcus faecium. Enterocin SE-K4, belonging to class IIa bacteriocins, produced by one such thermophilic enterococcus, Enterococcus faecalis K-4, has previously been isolated and characterized (7).
In this report, we characterized an antilisterial peptide that belongs to class IIa bacteriocins and is produced by the enterococcal strain NFRI 7393, which was identified as a strain of Enterococcus mundtii. This peptide, mundticin KS, was described by N-terminal sequencing of the purified peptide and sequencing of the mundticin gene cluster. Comparison of the activities of recombinant mundticins expressed in Escherichia coli against indicator strains revealed that the C-terminal last two amino acid sequence of mundticin KS are important for target cell specificity. In heterogeneous hosts such as E. faecium, Lactobacillus curvatus, and Lactococcus lactis, mundticin KS was successfully expressed by transformation of the mun locus. The implications of our data for the individual gene function in the mun locus on the production of and immunity to the bacteriocin are discussed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and PCR primers.
Bacterial strains, plasmids and PCR primers used in this study are listed in Table 1, and the indicator strains used are listed in Table 2.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. mundtii NFRI 7393 | Producer of mundticin KS | Isolate from grass silage in Thailand |
| E. mundtii JCM 8731T | Type strain | Present study |
| E. faecium IFO13712 | Routine indicator for mundticin KS and cloning host for the mun locus | Present study |
| L. lactis IL-1403 | Host for transformation | 4 |
| L. curvatus JCM 1096 | Host for transformation | Present study |
| E. coli DH5α | F− φ80 lacZΔM15 endA1 recA1 hsdR17(rK− mK−) supE44 thi-1 ΔgyrA96 (ΔlacZYA-argF) U169 relA1 | GIBCO BRL |
| E. coli BL21-CodonPlus (DE3)-RIL | F−ompT hsdS (rB− mB−) dcm+ Tetrgal λ(DE3) endA Hte [argU ileY leuW Camr] | Stratagene |
| Plasmids | ||
| pBluescript SK(+) | E. coli cloning vector; bla | Stratagene |
| pIL253 | Broad-host-range vector in LAB derived from pAMβ1; multicopy, erm | 34 |
| pRH100 | E. coli-LAB shuttle vector; bla, erm | Present study |
| pRB1 | pRH100 with a 5.4-kb BamHI fragment containing munA, munB, and munC genes | Present study |
| pKB541 | pBluescript SK(+) with the same fragment as in pRB1 | Present study |
| pBH293 | pBluescript SK(+) with a 2.9-kb BamHI-HindIII fragment from the 5.4-kb insert in pKB541 | Present study |
| pKH181 | pBluescript SK(+) with a 1.8-kb HindIII fragment from the 5.4-kb insert in pKB541 | Present study |
| pBH072 | pBluescript SK(+) with a 0.7-kb BamHI-HindIII fragment from the 5.4-kb insert in pKB541 | Present study |
| pPROEX-HTb | E. coli expression and purification vector; bla | Gibco BRL |
| pRRO3B | pPROEX Hta derivative expressing a recombinant mature peptide of mundticin KS; bla | Present study |
| pET-3a | E. coli expression vector | Qiagen |
| pEM3B | pET-3a derivative overexpressing a recombinant mature peptide of mundticin KS; bla | Present study |
| pEM6A | pET-3a derivative overexpressing a recombinant mature peptide of mundticin ATO6; bla | Present study |
| pRK1 | pRH100 with a 3.4-kb NotI-EcoRI PCR fragment containing munA, munB, and munC genes | Present study |
| pRK45 | pRH100 with a 1.1-kb NotI-EcoRI PCR fragment containing only munC gene fused to the munA promoter | Present study |
| pRK55 | pRH100 with a 2.3-kb NotI-EcoRI PCR fragment containing munA and munB genes | Present study |
| pRK62 | pRH100 with a 3.1-kb NotI-EcoRI PCR fragment containing munA, munB, and munC genes without the munA promoter | Present study |
| pRK72 | pRH100 with a 2.6-kb NotI-EcoRI PCR fragment containing munA and munB genes | Present study |
| PCR primers | ||
| 7393-DN | AGGTTTTAAATACTACGGTAATGGAGTCT | |
| 7393-HC | CAAAGCTTAACCTATAAATAAGAATACTC | |
| 7393ETN | TATACATATGTCGTACTACCATCACCATCA | |
| 7393ETC1 | ATTGGATCCTTATTAACTTTTCCAACCAGC | |
| ET3aN | GGCCACGATGCGTCCGGCGTAGAGG | |
| ET3aC | TCTCATGTTTGACAGCTTATCATCG | |
| mSKN | GCAGCTGGTTGGAGTAAATAATAAGGATCC | |
| mSKC | GGATCCTTATTATTTACTCCAACCAGCTGC | |
| ORF1-F | TTTTGCGGCCGCTAAGACTATAGGATAGACAA | |
| ORF2-R | AAATGAATTCTTCATCAGAATGAATGGGAG | |
| ORF1-3R | TCCTCCTTGATTCCTTCATTTTTTGAACAA | |
| ORF3-F | TTGTTCAAAAAATGAAGGAATCAAGGAGGA | |
| BacN-F | TGAGGCGGCCGCAAGTTTTGAAGAAATTAACAGC | |
| ORF3-NR | TTACTCATGAATTCATCCTCCTTGATTCC |
TABLE 2.
Antimicrobial activity of mundticin KS
| Organism | Straina | Sensitivityb to mundticin KS |
|---|---|---|
| Bacillus subtilis | ATCC 6633 | − |
| 168 | − | |
| Bacillus cereus | IFO15305 | − |
| Enterococcus faecium | IFO13712 | +++ |
| Enterococcus faecalis | IFO12964 | +++ |
| Enterococcus mundtii | NFRI 7393c | − |
| JCM 8731T | +++ | |
| Lactobacillus plantarum | ATCC 8041 | + |
| Weissella viridescens | JCM 1174 | − |
| Lactobacillus lactis | NFRI 7307 | + |
| Lactobacillus brevis | JCM 1059 | − |
| Lactobacillus bavaricus | JCM 1129 | − |
| Lactobacillus curvatus | JCM 1096 | + |
| Lactobacillus casei subsp. casei | JCM 1134 | − |
| Salmonella enterica serovar Enteritidis | IFO3313 | − |
| Listeria monocytogenes | ATCC 15313 | ++ |
| ATCC 49594 | ++ | |
| Escherichia coli | HB101 | − |
| ATCC 10798 | − |
ATCC, American Type Culture Collection, Manassas, Va.; IFO, Institute for Fermentation, Osaka, Japan; NFRI, National Food Research Institute, Tsukuba, Japan; JCM, Japan Collection of Microorganisms, Wako, Japan.
Culture filtrate containing mundticin 7393 (6,400 AU/ml), which was preheated at 75°C for 1 h, was used for these sensitivity tests. Symbols: +, sensitive to mundticin KS (+, ++, and +++ reflect the degree of sensitivity); −, resistant to mundticin KS.
Mundticin KS producer strain.
Media, reagents, assays, and standard protein and DNA techniques.
All LAB were grown in Lactobacilli MRS medium (Difco, Detroit, Mich.), unless otherwise stated. Escherichia coli strains were grown in Luria-Bertani medium. For solid media, 1.5% agar was added to liquid media. Listeria, Salmonella, and Bacillus strains were cultured on TS agar medium (Nissui, Tokyo, Japan). Antibiotics were used at the following concentrations: chloramphenicol, 50 μg/ml; erythromycin, 5 μg/ml; and ampicillin, 100 μg/ml. Standard DNA manipulations were performed as described previously (32). Plasmid DNA isolation from Enterococcus strains was done by using the procedure of Anderson and McKay (1). Restriction and modifying enzymes were used according to the protocol recommended by the manufacturer. The PCR program, using an Ex Taq DNA polymerase (Takara Shuzo), comprised 4 min at 96°C followed by 30 cycles of 30 s at 95°C, 45 s at 55°C, and 2 min at 72°C, and then a final incubation for 7 min at 72°C using a GeneAmp 9700 thermal cycler (Applied Biosystems). An ABI PRISM 310 genetic analyzer and a BigDye terminator cycle sequencing FS ready reaction kit (Applied Biosystems) were used for DNA sequencing. DNASIS software (Hitachi Software Engineering) was used for sequence analysis. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tricine-SDS-PAGE) and Western blotting were done as described previously (20, 33). rTEV protease was purchased from GIBCO BRL (Gaithersburg, Md.). Protein concentrations were determined with a BCA protein assay reagent (Pierce) using crystalline bovine serum albumin as the standard. Amino acid sequencing was done using automated Edman degradation and an HPG1005A protein sequencing system (Hewlett-Packard).
Taxonomic characterization of strain NFRI 7393.
The fermentation potential of various sugars was measured using API Rapid CH fermentation strips (BioMerieux). The entire 16S rDNA of strain NFRI 7393 was amplified by PCR and directly sequenced using the method of Mori et al. (25). The 16S rDNA sequences of related microorganisms were obtained from DDJB, and the sequences were aligned using the DNASIS program. The 16S rDNA sequence of strain NFRI 7393 was deposited in the DDBJ under accession number AB066266. A DNA-DNA hybridization assay was carried out as described previously (9).
Bacteriocin activity and sensitivity to heat, pH, and degradative enzymes.
The spot-on-lawn method as described by van Reenen et al. (37) was used to determine the antimicrobial activity of mundticin KS using E. faecium IFO13712 as an indicator strain, unless otherwise stated. A clear inhibition zone of at least 2 mm in diameter was recorded as positive. One arbitrary unit (AU) of mundticin KS activity was defined as the reciprocal of the highest dilution that produced an inhibition zone of at least 2 mm in diameter. Aliquots of a crude extract containing mundticin KS (3,200 AU/ml) were exposed to heat treatments of 40, 60, 70, 80, 90, and 100°C for 60 min. The samples were then tested for activity against E. faecium IFO13712 by the spot-on-lawn method. In a separate experiment, samples of the mundticin KS were adjusted to pH values ranging from 3 to 12, incubated at 37°C for 30 min, neutralized to pH 6.5, and then tested for antimicrobial activity. Resistance of the purified mundticin KS to degradative enzymes was determined by incubating samples in the presence of proteinase K (mundticin, 5 U/mg), trypsin (mundticin, 30 U/mg), RNase A (mundticin, 2 U/mg), and lipase (mundticin, 3 U/mg) at 37°C for 1 h. All enzymes were obtained from Sigma Chemical Co. (St. Louis, Mo.). After incubation, the enzymes were heat inactivated for 15 min at 90°C and then the mundticin KS was tested for antimicrobial activity.
Isolation and purification of mundticin KS. (i) Concentration.
The mundticin KS was purified from 1-liter cultures of E. mundtii NFRI 7393 grown in MRS broth to the early stationary phase (A600 = ∼1.5) at 37°C. The cells were removed by centrifugation at 4,000 × g for 15 min at 4°C, after which the supernatant was adjusted to pH 6.5 and filtered through a 0.45-μm-pore-size HVLP membrane (Millipore SA). The mundticin KS was precipitated from the filtrate by using ammonium sulfate (final concentration of 60% [wt/vol]). The precipitate was collected by centrifugation at 14,300 × g and resuspended in 50 ml of 50 mM 2-morpholineethanesulfonic acid (MES) buffer (pH 5.0).
(ii) Cation-exchange chromatography.
The crude extract was applied onto a 7-ml TSK gel SP-TOYOPEARL 550(c) column that had been equilibrated with 50 mM MES buffer (pH 5.0). The column was washed with 50 ml of MES buffer, and proteins were eluted with an NaCl linear gradient from 0 to 1.0 M at a flow rate of 5 ml/min. Fractions (5 ml each) were collected and tested for activity against E. faecium IFO13712. Acetone (final concentration of 75% [vol/vol]) was added to the active fractions (0.6 to 0.9 M NaCl elute) and incubated overnight at −20°C. The precipitate was collected by centrifugation at 28,000 × g and dried under vacuum.
(iii) Solid-phase extraction.
The dry residue was dissolved in 50 mM MES at pH 6.0 and then centrifuged at 10,000 × g for 5 min. The supernatant was subsequently used for purification by solid-phase extraction. The supernatant was loaded onto a Sep-Pak Plus C18 cartridge (Waters, Division of Millipore Corp.) equilibrated with 50 mM MES at pH 6.0. After the Sep-Pak cartridge was washed with 10 ml of 10% CH3CN containing 0.1% trifluoroacetic acid, the mundticin KS was eluted successively with 4 ml each of 30, 40, and 50% CH3CN containing 0.1% trifluoroacetic acid. Fractions (1 ml each) were collected, and a 2-μl portion of each fraction was tested for antimicrobial activity. Active fractions (the first 3-ml fraction; 40% CH3CN elute) containing the purified mundticin KS were combined, dried under vacuum, and stored at −20°C.
The purified mundticin KS was subjected to Tricine-SDS-PAGE with protein markers ranging from 2.35 to 46 kDa (Rainbow Marker; Amersham Pharmacia Biotech). One-half of the gel was stained with Coomassie brilliant blue R250. The position of the active peptide was determined by overlaying the other half of the gel, which had been washed three times for 10 min each with 100 ml of distilled H2O, with cells of E. faecium IFO13712 (approximately 106 cells/ml) embedded in MRS agar (0.8% agar [wt/vol]).
Transformation of LAB by electroporation.
The cells of LAB for electroporation were prepared according to the procedure of Dunny et al. (6), frozen in liquid nitrogen, and stored at −80°C. Electroporation was done with a Gene Pulser apparatus (Bio-Rad Laboratories) at a capacitance of 25 μF in all experiments. Plasmid DNA (1 μg in a volume of less than 2 μl) was added to 0.1 ml of the thawed cells. This mixture was placed in sterile 0.2-cm-wide electroporation cuvettes and held on ice for 5 min. Immediately following the application of an electric pulse at the field strength of 1.25 kV/cm (resistance was set to 200 Ω), 1 ml of prechilled MRS broth containing 0.5 M sucrose was added to the DNA-cell mixture and incubated for 1.5 h at 30°C (L. curvatus and L. lactis) or 37°C (E. faecium). Then, 0.1-ml aliquots of the culture were plated onto selective media, with or without prior dilutions. Using this protocol, transformation efficiencies of 104 to 105 transformants per microgram of plasmid DNA were routinely obtained.
Cloning of the mundticin KS gene.
An E. mundtii NFRI 7393 gene library was constructed in E. coli DH5α by ligating an Sau3AI partial digest (4- to 7-kb fragments) of its total DNA into the BamHI site of the E. coli-LAB shuttle vector pRH100 (see the next section). The library consisted of 10 sublibraries, each containing approximately 2,000 transformants. The plasmid DNAs from the sublibraries were electroporated into E. faecium IFO13712 cells. The cells were then plated without dilution onto MRS agar supplemented with 5% (vol/vol) filter-sterilized culture supernatant containing mundticin KS (12,800 AU/ml) and erythromycin (MRCE medium) for screening of the mundticin KS gene, and with dilution onto MRS agar containing only erythromycin for determination of transformation efficiencies. Transformant colonies developed on MRCE plates were checked for mundticin KS production and screened for plasmid content. In this way, the clone harboring pRB1 was eventually obtained. pRB1 contained a 5.4-kb insert (Table 1).
Construction of recombinant plasmids.
pRH 100 was constructed by inserting the 4.9-kb EcoRI-XhoI fragment of pIL253 (34) into the corresponding sites in the polylinker region of pBluescript SK(+). The plasmid pRH100 is an E. coli-LAB shuttle vector that possesses the ampicillin resistance gene as a selectable marker in E. coli, and the erythromycin-resistance gene as a selectable marker in LAB strains. pRH100 retained similar unique restriction sites for gene cloning to pBluescript SK(+) (XhoI, SacI, SacII, NotI, SpeI, BamHI, SmaI, PstI, and EcoRI). pKB541 was constructed by inserting the 5.4-kb BamHI fragment from pRB1 into the corresponding site in the polylinker region of pBluescript SK(+). Similarly, each of the fragments—2.9-kb BamHI-HindIII fragment, 1.8-kb HindIII fragment, and 0.7-kb BamHI-HindIII fragment—from the 5.4-kb BamHI fragment of pRB1 was subcloned into pBluescript SK(+) to yield pBH293, pKH181, and pBH072, respectively. The presence of the correct insert was confirmed in these constructs by restriction analysis.
pEM3B expressing the recombinant mundticin KS peptide was constructed as follows: the region encoding the mature peptide was amplified from the munA gene using pKB541 as a template, with 7393-DN and 7393-HC as primers. The primers included DraI and HindIII cleavage sites for ligation with the E. coli vector, pPROEX-HTb. The resulting 229-bp PCR product was digested with DraI and HindIII and ligated with EheI/HindIII-digested pPROEX-HTb to yield pRRO3B. Correct structure of the plasmid was confirmed by DNA sequencing. The region encoding the recombinant peptide was PCR amplified using pRRO3B as a template with 7393ETN (including an NdeI site) and 7393ETC1 (including a BamHI site) as primers. The PCR products were digested with NdeI and BamHI and ligated with NdeI/BamHI-digested pET-3a to yield pEM3B. DNA sequencing confirmed the correct structure of this plasmid. pEM6A expressing the recombinant mundticin ATO6 peptide was constructed by PCR in vitro mutagenesis in the following manner. First, two regions of the munA gene in pEM3B were PCR amplified with ET3aN and mSKC as primers and with ET3aC and mSKN as primers. The mSKC and mSKN are the primers for introduction of mutations in the munA gene to change the last two amino acids at its C termini from lysine-serine to serine-lysine, identical to the mundticin ATO6 sequence. Second, using the resulting PCR products of 342 and 540 bp as templates, PCR amplification with ET3aN and ET3aC as primers was done to create the mutated munA gene. The 850-bp PCR product was digested with BglII and HindIII and ligated with similarly digested pET-3a to yield pEM6A. DNA sequencing confirmed the correct structure of this plasmid. pEM3B and pEM6A are expected to direct the recombinant peptides of mundticin KS and mundticin ATO6, respectively, both of which possess the same N-terminal extension with a His tag sequence to facilitate their purification by affinity chromatography (15), followed by a specific rTEV protease cleavage site.
pRK1 and pRK62 containing the entire mun locus (consisting of munA, munB, and munC genes) with and without a putative munA promoter, respectively, were constructed as follows. The mun locus with the munA promoter was PCR amplified using pKB541 as a template, with open reading frame 1-F (ORF1-F) and ORF2-R as primers. The mun locus lacking the munA promoter was PCR amplified with BacN-F and ORF2-R as primers, which included NotI and EcoRI cleavage sites, respectively. The resulting PCR products of 3.4 and 3.1 kb were digested with NotI and EcoRI and ligated with NotI/EcoRI-digested pRH100 to yield pRK1 and pRK62, respectively. In a similar manner, the NotI/EcoRI-digested fragments of 2.4 and 2.7 kb, which were PCR amplified with BacN-F or with ORF1-F and ORF3-NR as primers, were inserted into the pRH100 NotI/EcoRI cloning site to yield pRK55 (containing munA and munB without the munA promoter) and pRK72 (containing munA and munB with the munA promoter), respectively. pRK45, containing only the munC gene fused to the munA promoter, was constructed in the following manner. First, PCR amplification of the munA promoter (with ORF1-F and ORF1-3R as primers) and the munC gene (with ORF3-F and ORF2-R as primers) was done using pKB541 as a template. Second, using the resulting PCR products of 270 and 800 bp as templates, PCR amplification with ORF1-F and ORF2-R as primers was done to create the munC gene fused to the munA promoter. The 1.1-kb PCR product was digested with NotI and EcoRI and ligated with NotI/EcoRI-digested pRH100 to yield pRK45. DNA sequencing confirmed the correct structures of these plasmids.
Purification of recombinant mundticins and preparation of an antiserum against mundticin KS.
For overexpression of the recombinant mature peptides of mundticin KS and mundticin ATO6, E. coli BL21-CodonPlus(DE3)-RIL cells harboring pEM3Bor pEM6A were grown in Luria-Bertani broth containing ampicillin and chloramphenicol to mid-logarithmic phase (A600 = ∼0.5) at 37°C. Isopropyl-β-d-thiogalactopyranoside was then added to the cultures to a final concentration of 1 mM, and the incubation was continued for 2 h. Using crude extracts prepared from the induced cells, the recombinant mature peptides were purified by affinity chromatography with Ni-Sepharose Fast Flow (Pharmacia Biotech), followed by solid-phase extraction with a Sep-Pak Plus C18 cartridge (see the preceding section). Typically, about 10 mg of the purified peptides was obtained from 1 liter of the induced culture. Rabbit polyclonal antiserum to mundticin KS was generated against the purified recombinant mundticin KS. The polyclonal anti-mundticin KS antiserum was used as the primary antibody in Western analysis at a dilution of 1:3,000.
rTEVprotease digestion was done using a reaction volume of 100 μl containing 20 μg of the purified peptide and 20 U of the protease. After incubation at 15°C overnight, the reactions were stopped by adding Ni-Sepharose resin to adsorb the protease. After centrifugation, the peptides in the supernatant were heated at 70oC for 30 min and then tested for bacteriocin activity to determine their specific activities.
RESULTS
Identification of bacteriocin producer strain NFRI 7393.
The fermentation potential of various sugars of strain NFRI 7393 (data not shown) agreed well with that of E. mundtii. Sequencing of the entire 16S rDNA of strain NFRI 7393 revealed a perfect match to that of E. mundtii JCM 8731T. Furthermore, strain NFRI 7393 was found to have 75% sequence similarity with E. mundtii JCM 8731T by the DNA-DNA hybridization experiment (data not shown). These characteristics clearly identify strain NFRI 7393 as a strain of E. mundtii.
Antimicrobial activity.
Mundticin KS was found to be active against all strains of Enterococcus spp. tested, against three strains of Lactobacillus spp., and against two strains of L. monocytogenes used in this study (Table 2). However, other gram-positive bacteria, including four LAB strains and two species of Bacillus, and gram-negative bacteria were resistant to mundticin KS (Table 2).
Sensitivity to heat, pH, and proteolytic or other enzymes.
Mundticin KS was resistant to heat treatments of up to 90°C for 60 min (data not shown). Approximately 50% of the antimicrobial activity was retained after 60 min at 100°C. Mundticin KS was not markedly affected by incubation at pH ranging from 3 to 12 but was sensitive to trypsin and proteinase K and resistant to lipase and RNase A.
Production of mundticin KS.
E. mundtii NFRI 7393 produced 6,400 to 12,800 AU of mundticin KS per ml of culture medium at the early stationary phase at growth temperatures ranging from 15 to 40°C (data not shown). Mundticin KS production markedly decreased at 42°C (<500 AU/ml) and completely disappeared at 45°C, whereas the producer strain grew at 42 and 45°C as well as it did at 37°C. Mundticin KS production occurred during the exponential phase of growth at 37°C (data not shown).
Purification of mundticin KS and determination of its N-terminal amino acid sequence.
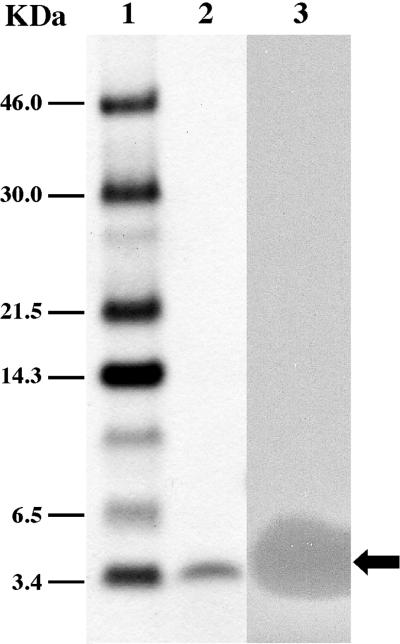
Mundticin KS was purified to homogeneity from the supernatant of an early stationary phase culture grown at 37°C in MRS broth. Results are summarized in Table 3. Ammonium sulfate precipitation was successfully used to concentrate the activity from the growth medium. Approximately a 50-fold increase in the specific activity and an 80% recovery of mundticin KS were obtained (Table 3). Purification of the crude extract of mundticin KS by chromatography on a TSK gel SP-TOYOPEARL 550(c) column yielded a 504-fold increase in specific activity with a 67.5% recovery (Table 3). The largest increase in specific activity, 1,930-fold with a 25.6% recovery, was obtained after the final solid-phase extraction step. The final specific activity of the purified mundticin KS was about 3.3 × 106 AU/mg. Separation on Tricine-SDS-PAGE yielded only one active peptide band (Fig. 1). The purified peptide was analyzed by a protein sequencer, which determined the N-terminal amino acid sequence of KYYGNGVSXNKKGXSV.
TABLE 3.
Mundticin KS purification
| Step | Volume (ml) | Total protein (mg) | Total activity (AU, 103) | Sp act (AU/mg) | Yield (%) | Purifi- cation (fold) |
|---|---|---|---|---|---|---|
| Culture supernatant | 1,000 | 5,870 | 1,000 | 170 | 100 | 1 |
| (NH4)2SO4 ppt | 50 | 99 | 800 | 8,080 | 80 | 47 |
| SP-TOYOPEARL | 75 | 7.9 | 675 | 85,400 | 67.5 | 504 |
| Sep-Pak C18 | 3 | 0.78 | 256 | 328,600 | 25.6 | 1,930 |
FIG. 1.
Separation of purified mundticin KS by Tricine-SDS-PAGE. Lane 1, Rainbow protein size markers; lane 2, mundticin KS stained with Coomassie brilliant blue R250; lane 3, mundticin KS overlaid with cells of E. faecium IFO13712 embedded in MRS agar (0.8% [wt/vol] agar). The arrow indicates the active peptide band.
Cloning and nucleotide sequence analysis of the bacteriocin locus.
For cloning of the mundticin gene cluster (mun locus) responsible for mundticin KS production, we first constructed a gene library of E. mundtii NFRI 7393 in the E. coli-LAB shuttle vector, pRH100. In an attempt to express the mun locus in E. faecium, we transformed plasmid DNAs from the gene library into E. faecium IFO13712 by electroporation. Positive selection for resistance to mundticin KS, which used MRCE medium containing the bacteriocin in addition to erythromycin, was used for the screening. This strategy was based on evidence of gene coding for a bacteriocin and its immunity gene found clustered in the genetic organization of most bacteriocins characterized to date (18, 30). If this gene clustering was also true for mundticin KS, the transformants harboring the recombinant plasmid with the mun locus should be highly enriched among those appearing on MRCE plates. Each MRCE plate was expected to contain about 2,000 transformed cells, as judged by plating the same transformation mixture on MRS agar containing erythromycin only. A total of 78 transformants were obtained by screening about 2 × 105 transformants and were tested for mundticin KS production. Of these 78 transformants, 32 were found to produce mundticin KS, and all routinely exhibited two- to threefold-higher production of the bacteriocin compared with that of E. mundtii NFRI 7393. Such higher production is possibly due to a gene dosage effect. Restriction analysis of the recombinant plasmids from five such transformants showed that the plasmids contained the same 5.4-kb BamHI fragment insert. One of the recombinant plasmids was designated pRB1 and used in this study. The N-terminal amino acid sequence of mundticin KS from the pRB1 transformant of E. faecium was identical to that from the original producer strain. The insert DNA in pRB1 and its restriction fragments were subcloned into the E. coli vector pBluescript SK(+) for DNA sequencing.
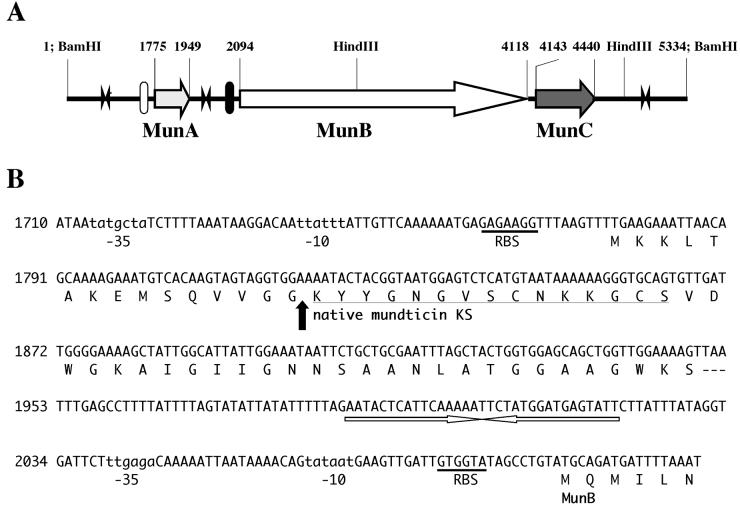
Sequences of the 2.9-kb BamHI-HindIII (in pBH293), 1.8-kb HindIII (in pKH181), and 0.7-kb HindIII-BamHI (in pBH072) fragments were determined and assembled, yielding a 5,334-bp sequence (Fig. 2A). When translated in all possible reading frames, this sequence revealed three ORFs. The first ORF (munA) encodes a 58-amino-acid prepeptide of mundticin KS with a putative promoter region and a stable stem and loop structure (ΔG = −14.4 kcal) possibly acting as a rho-independent transcriptional terminator (Fig. 2B). The possible start codon for munA is TTG, and the prepeptide contains a leader peptide of 15 amino acids with a consensus G-G-Xaa-processing site (18). Thus, amino acid and nucleotide sequencing determined the complete, unambiguous primary structure of mundticin KS, which comprises 43 amino acids with an estimated molecular mass of 4.290 kDa. The second ORF (munB) encodes a protein of 674 amino acids. The MunB protein exhibits significant end-to-end homology with the ABC transporter proteins found in other bacteriocin gene clusters such as EntT (717 amino acids, 42.7% identity) (28), CbnT (716 amino acids, 42.5% identity) (29), SppT (718 amino acids, 39% identity) (17), and PapD (724 amino acids, 36% identity) (26) proteins, which are involved in the excretion and maturation of enterocin A, carnobacteriocin B2, sakacin P, and pediocin AcH, respectively. The third ORF (munC) starts only 25 bp downstream from the termination codon of munB and encodes a protein of 98 amino acids, which exhibits homology with the piscicolin 126 immunity protein (PisI) (68% identity) (19) and sakacin P immunity protein (SpiA) (50% identity) (17). The two genes, munB and munC, have a putative common promoter upstream of munB, a common rho-independent terminator (ΔG = −27.6 kcal) downstream of munC, and independent ribosome-binding sites (Fig. 2A).
FIG. 2.
(A) Gene organization of the mun locus. Horizontal arrows that designate the direction of transcription represent the ORFs. Filled and open bars indicate putative promoters. Two arrowheads in opposite directions indicate terminators. Relevant nucleotide numbers and restriction sites are also shown. (B) Nucleotide sequence of the region encoding mundticin KS and the deduced amino acid sequence. Putative promoter regions are indicated in lowercase letters, and ribosome binding sites (RBS) are underlined. The N-terminal amino acid sequence of purified mundticin KS determined in this study is underlined. The arrow indicates the processing site of the peptide. The nucleotide sequence with arrows in opposite directions represents a transcriptional terminator with a stem and loop structure. The nucleotide sequence of the mun locus has been deposited in DDJB under accession number AB066267.
The BLASTX protein database homology search on the deduced mature peptide, mundticin KS, revealed a high homology with class IIa bacteriocins such as mundticin ATO6 (94% identity) (2), pisciolin 126 (84% identity) (19), sakacin P (65% identity) (36), and pediocin AcH (60% identity) (3). Mundticin KS has the two cysteine residues (C-9 and C-14) forming the disulfide bridge, which are well conserved in all class IIa bacteriocins. The only difference between mundticin KS and mundticin ATO6 is the last two amino acid positions at the C terminus, which are Lys-Ser (in mundticin KS) and Ser-Lys (in mundticin ATO6). No DNA homology was found (using the BLASTN DNA database) with any DNA sequences reported for bacteriocins.
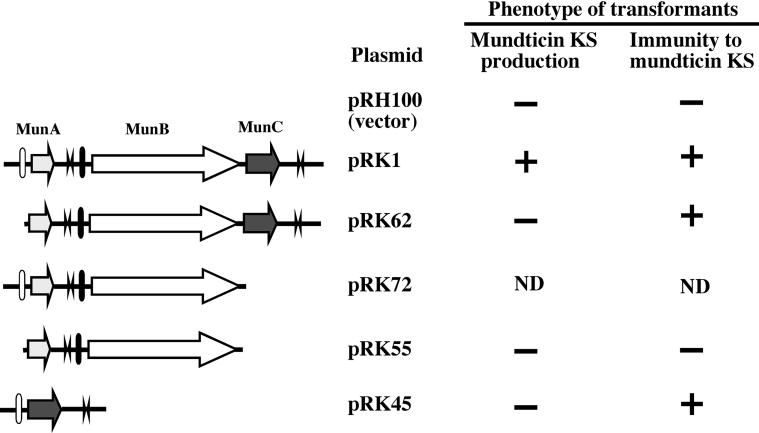
Deletion analysis of the mun locus.
Five deletion derivatives of the mun locus were constructed by PCR amplification in the shuttle vector pRH100 and transformed into E. faecium IFO13712 cells. The transformants were then tested for production of and sensitivity to mundticin KS. The results are summarized in Fig. 3. pRK72, with a 2.7-kb insert deleting only munC, was found to cause a severe growth defect in recipient cells because the transformation induced only small colonies (diameter, <1 mm) on the selective medium after 3 days at 37°C, whereas transformation with other deletion mutants and the vector alone resulted in normal-size colonies (diameter, 2 to 3 mm) after overnight culture at 37°C. The transformant harboring pRK1 (which contained a 3.4-kb insert with an intact mun locus) produced mundticin KS and exhibited immunity to it. Neither mundticin KS production nor immunity was observed in the transformant harboring pRK55 containing munA and munB without the munA promoter or the vector-only transformant. Transformants of either pRK45 containing a 1.1-kb insert that only has munC fused to the munA promoter or pRK62 containing a 3.1-kb insert lacking only munA promoter did not produce mundticin KS, but both exhibited immunity to it. These results clearly indicate that munC encodes the immunity protein to mundticin KS.
FIG. 3.
Deletion mutant analysis of the mun locus. Deletion mutants of the mun locus constructed in the vector plasmid, pRH100, by PCR are depicted on the left. Filled and open bars represent putative promoters. Two arrowheads in opposite directions indicate terminators. E. faecium IFO13712 transformants harboring the indicated deletion mutant plasmids were tested for production of and immunity against mundticin KS, as described in Materials and Methods. Symbols: +, production of or resistance to mundticin KS; −, no production of or sensitivity to mundticin KS. ND, not determined.
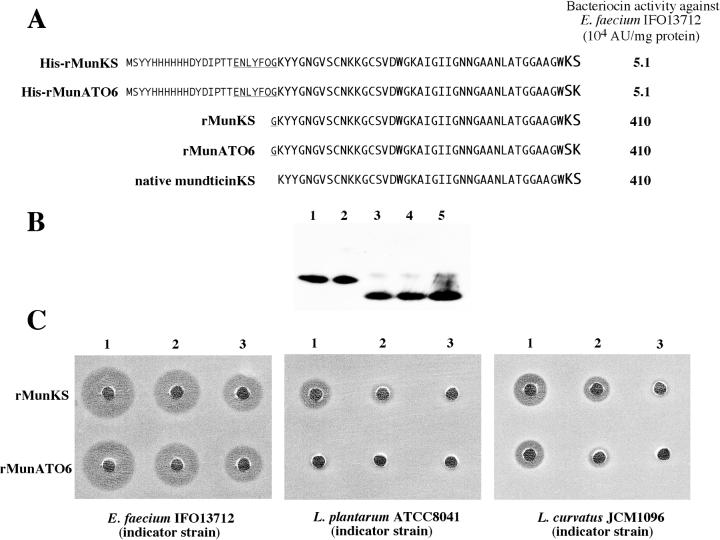
Expression of recombinant peptides of mundticin KS and mundticin ATO6 in E. coli.
The recombinant mature peptides (His-rMunKS and His-rMunATO6), both of which have the same N-terminal extension sequence with a His tag followed by a specific rTEV protease cleavage site, were overexpressed in E. coli. Both His-rMunKS and His-rMunATO6 exhibited low bacteriocin activity, compared with that of the native mundticin KS (Fig. 4A). Upon digestion with the rTEV protease for the removal of N-terminal extended sequence, the recombinant peptides were both converted to peptides (rMunKS and rMunATO6) of 44 amino acids with an extra glycine residue attached to the N terminus of the native peptide sequence. The rMunKS and rMunATO6 are distinguished by the inversion of the last two amino acids at their respective C termini, similar to the inversion of the corresponding native forms. These peptides comigrated with native mundticin KS on a Tricine-SDS-PAGE gel (Fig. 4B) and exhibited the same bacteriocin activity as the native mundticin KS against E. faecium IFO13712. However, rMunATO6 was found to have lower specific activities than rMunKS against L. curvatus JCM 1096 (about 50% of the activity) and L. plantarum ATCC 8041 (about 10% of the activity) (Fig. 4C).
FIG. 4.
Bacteriocin activity of the recombinant peptides of mundticin KS and mundticin ATO6 expressed in E. coli. (A) Specific activities of the peptides against E. faecium IFO13712. Amino acid sequences of the recombinant peptides with and without rTEV digestion, along with that of native mundticin KS, are shown on the left. The His tag sequence is written in small capital letters, and the rTEV protease cleavage site is underlined. (B) Western analysis of the peptides. Each lane contained 0.1 μg of the peptides. After Tricine-SDS-PAGE, Western blotting and detection were done using the anti-mundticin KS serum as described in Materials and Methods. Lane 1, His-rMunKS peptide; lane 2, His-rMunATO6 peptide; lane 3, rMunKS peptide; lane 4, rMunATO6 peptide; lane 5, native mundticin KS. (C) Bacteriocin activity of rMunKS and rMunATO6 peptides to LAB strains. In 20 μl of solution, 200, 67, and 40 ng of the peptides were added to wells 1, 2, and 3, respectively, of each MRS agar plate seeded with the indicated LAB strain cells. The plates were then incubated overnight at 30°C.
Mundticin KS expression in heterogeneous LAB.
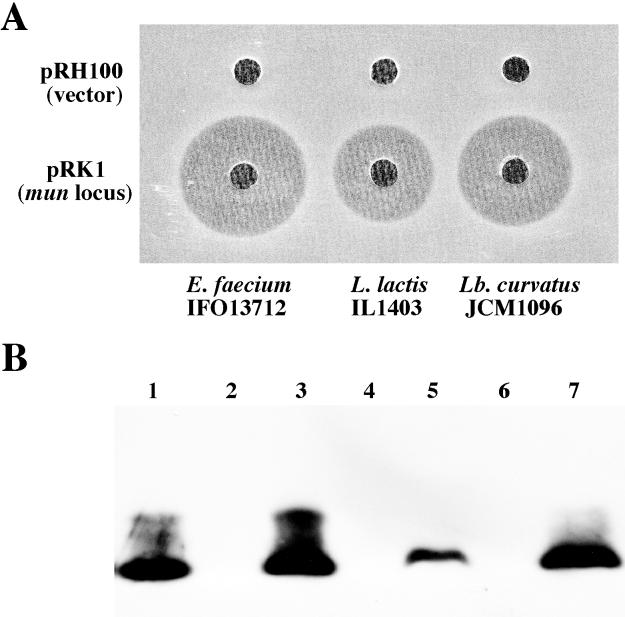
We tried to express mundticin KS in heterogeneous LAB other than E. faecium; L. curvatus JCM 1096 and L. lactis IL-1403 were transformed with the vector pRH100 and pRK1 containing the entire mun locus. The resulting transformants were then tested for production of and sensitivity to mundticin KS. The results are summarized in Fig. 5. pRK1 transformants of both L. curvatus and L. lactis produced mundticin KS as evidenced by bacteriocin activity (Fig. 5A) and Western analysis (Fig. 5B), and exhibited immunity to it. Neither mundticin KS production nor immunity was observed in the vector-only transformants. The results indicate that the mun locus consisting of munA, munB, and munC is sufficient for the mundticin KS production.
FIG. 5.
Mundticin KS production in heterogeneous hosts by transformation with pRK1 containing mun locus. (A) Bacteriocin activity of culture supernatants from transformants. pRH100 (vector) or pRK1 transformants of the indicated LAB strains were grown in MRS broth containing erythromycin (1 μg/ml) at 30°C overnight. Each well of the MRS agar plate seeded with E. faecium IFO13712 cells contained 20 μl of the culture supernatants. (B) Western analysis of culture supernatants from transformants. Western blotting and detection were done using the anti-mundticin KS serum as in Fig. 4. Each lane contained 20 μl of the culture supernatants except that lane 1 contained 0.1 μg of purified mundticin KS. Lane 2, pRH100 transformant of E. faecium; lane 3, pRK1 transformant of E. faecium; lane 4, pRH100 transformant of L. lactis; lane 5, pRK1 transformant of L. lactis; lane 6, pRH100 transformant of L. curvatus; lane 7, pRK1 transformant of L. curvatus.
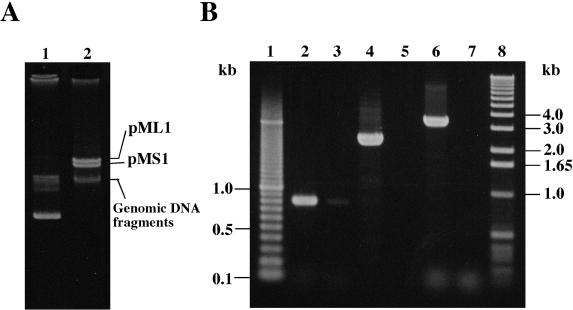
Localization of the mun locus on a large plasmid.
Plasmid analysis revealed that E. mundtii NFRI 7393 harbored two large plasmids of similar size, ∼50 kb, designated pMS1 and pML1 (Fig. 6A). Preliminary Southern hybridization analysis, using the 3.4-kb fragment containing the entire mun locus from pRK1 and the PCR-amplified 16S rDNA fragment as probes, demonstrated that the mun probe specifically hybridized to the plasmid positions (data not shown). Further PCR analysis using each gel-purified plasmid DNA as a template clearly confirmed the localization of the mun locus on the plasmid, pML1 (Fig. 6B).
FIG. 6.
Localization of the mun locus in a large plasmid, pML1, of E. mundtii NFRI 7393. (A) Plasmid profiles of E. mundtii NFRI 7393. The plasmids were separated by 0.6% agarose gel electrophoresis. Lane 1, a marker plasmid, pV1, of 28 kb (20); lane 2, plasmids of E. mundtii NFRI 7393. (B) Localization of the mun locus by PCR analysis. pML1 and pMS1 plasmid DNAs were carefully gel purified. Using the purified pML1 plasmid DNA (lanes 2, 4, and 6) or pMS1 plasmid DNA (lanes 3, 5, and 7) as the template, PCR amplification for the mun locus was done with three sets of primer combinations. Combination 1, primers ORF3-F and ORF2-R (lanes 2 and 3); combination 2, primers BacN-F and ORF3-NR (lanes 4 and 5); combination 3, primers ORF1-F and ORF2-R (lanes 6 and 7). The mun locus was expected to generate PCR products of 0.8, 2.7, or 3.4 kb in length when primer combination 1, 2, or 3 was used, respectively. Lanes 1 and 8 contain 100-bp and 1-kb DNA ladder markers, respectively.
DISCUSSION
In this report, we have described the purification and genetic characterization of a plasmid-encoded bacteriocin produced by E. mundtii NFRI 7393, which was isolated from grass silage in Thailand. Production of mundticin KS is restricted to conditions below 42°C in this thermophilic strain that is capable of growing in temperatures of up to 50°C, whereas a similar thermophile, E. faecalis K-4, produces enterocin SE-K4 maximally at 43 to 45°C, as we previously described (7).
Mundticin KS is a positively charged, hydrophobic, 43-amino-acid peptide that contains the highly conserved YGNGV motif found in the N-terminal part of many class IIa bacteriocins in the classification described by Klaenhammer (22). Mundticin KS is strongly inhibitory against L. monocytogenes as well as Enterococcus spp. (Table 2). Gene sequence analysis clearly reveals that mundticin KS is translated as a prepeptide that contains glycine residues in the −1 and −2 positions relative to the processing site, as found in most class IIa bacteriocin precursors (18). We assigned a possible TTG start codon in the mundticin KS structural gene, munA, for the following two reasons, although this codon is unusual for a bacteriocin structural gene (but not notably for other genes related to bacteriocin production). The first reason is that a ribosome binding site (GAGAAGG) was found 8 bases upstream, which is a spacing observed in many bacterial genes (Fig. 2B). Havarstein et al. (14) determined an extended consensus sequence for double-glycine type leaders, namely, LSXXELXXIXGG. Other signatures of the leader, according to Havarstein et al., were that positions −4, −7, −12, and −15 were hydrophobic residues and that positions −8, −9, and −11 were hydrophilic residues. The second reason is that a 15-amino-acid leader of the munA sequence (see Fig. 2B) fits well with these signatures proposed by Havarstein et al. Based on the primary structure, inhibitory spectrum and heat stability, mundticin KS should belong to the group of heat-stable, Listeria-active class IIa bacteriocins.
Mundticin KS exhibits high homology to the bacteriocins in the pediocin family, notably the bacteriocin produced by E. mundtii ATO6 (2), which was isolated from fresh chicory endive. The full primary amino acid sequence of mundticin ATO6 has been determined by peptide sequencing analysis (2) and is distinguished from that of mundticin KS only by the inversion of the last two amino acids at the C terminus. We succeeded in obtaining the recombinant mundticins, rMunKS and rMunATO6, by expressing in E. coli as the peptides containing a His tag sequence and digesting them with rTEV protease to remove the extra sequence. The rMunKS and rMunATO6, although they both have an extra glycine residue at the N terminus of the natural forms, were found to be biologically active and to have specific activities comparable to that of the native mundticin KS against E. faecium IFO13712. Nevertheless, rMunATO6 exhibited lower specific activities than rMun KS against L. curvatus JCM 1096 and L. plantarum ATCC 8041 (Fig. 5), possibly due to the difference between the last two amino acid positions at their C termini as found in the natural forms. Some studies suggest that the somewhat more diverse C-terminal half of pediocin-like bacteriocins is an important determinant of target cell specificity (10, 11, 12) as opposed to the relatively well conserved N-terminal half, and our results pinpoint a specific extreme C-terminal sequence element that plays a role in determining this specificity. Note that the bacteriocin activity difference between rMunKS and rMunATO6 found in this study is strain-specific and that this does not reflect general increases or decreases in potency.
Our positive selection procedure was successful for cloning the mun locus from an E. mundtii NFRI 7393 gene library. This procedure was simple and based on heat stability of mundticin KS (for preparation of selective agar plates) and on evidence that most of the bacteriocin gene clusters characterized to date also include their immunity genes. Our selection procedure might be applicable for cloning of gene clusters for other heat-stable class I and class IIa bacteriocins.
The mun locus consists of three genes, munA, munB, and munC (Fig. 2). The structural gene munA encodes a 58-amino-acid mundticin precursor. The munB gene encodes a 674-amino-acid polypeptide which shows significant similarity to the ABC transporter proteins such as EntT (27) and CbnT (28), implicated in translocation and/or processing functions of other bacteriocins. The munC gene encodes a mundticin KS immunity protein, as evidenced by our deletion analysis (Fig. 3) and by the close similarity to the other bacteriocin immunity proteins. In this study, we were able to express mundticin KS in heterogeneous hosts such as E. faecium, L. curvatus, and L. lactis by transformation with the recombinant plasmid pRK1 containing the entire mun locus, clearly indicating that the mun locus is solely responsible for mundticin KS production. We cannot exclude the possibility that in the case of mundticin KS expression in L. lactis IL-1403 the bacteriocin might be secreted by the products of an ABC transporter and accessory gene located on the chromosome of this strain (38). This L. lactis strain has been used previously in heterologous expression studies for class IIa bacteriocin structural and immunity genes in the absence of transport genes (23). Nevertheless, we did show that they can get the mundticin KS expressed in two other heterologous hosts, which does point to the fact that this bacteriocin does not need an accessory gene for transport, unlike that of other bacteriocins (8). When transformed into E. faecium, the plasmid pRK72 in which the mun locus is lacking only munC was found to induce small colonies (diameter, <1 mm), due to the production of active bacteriocin secreted into the medium by the expression of an intact MunA and MunB. Without any expression of MunC (immunity), accumulation of bacteriocin will eventually effectively stop further growth and therefore yield small colonies on a plate.
Our genetic results (Fig. 3) further predict the genetic control mechanism of the mun locus. Functionality of the munA promoter is deduced by the phenotypes of transformants with pRK1, pRK55, and pRK62. Functionality of the munB promoter is also supported by the phenotype of the pRK62 transformant. Therefore, munA and munBC are probably under independent transcriptional control in the mun locus. However, detailed transcriptional studies are needed to clarify the transcriptional control mechanism of the mun locus.
Our expression system of the recombinant peptides in E. coli, which rapidly produces ca. 10 mg of highly purified and biologically active peptide from 1 liter of cultured cells and high-specificity polyclonal anti-mundticin KS serum, should be an effective tool for clarifying the biochemical character of MunB and MunC functions with respect to production of and immunity against mundticin KS. Such experiments are in progress in our laboratory.
Acknowledgments
This work was supported by a grant-in-aid (Pioneer program) from the Ministry of Agriculture, Forestry and Fisheries of Japan.
We are grateful to Miho Tone and Hiroko Fukuda for technical assistance and to Chise Suzuki for helpful discussions and critical comments on the manuscript. We thank A. Chopin for the generous gift of L. lactis IL-1403 and the plasmid pIL 253.
REFERENCES
- 1.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennik, M. H., B. Vanloo, R. Brasseur, L. G. Gorris, and E. J. Smid. 1998. A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii: full characterization and interaction with target organisms. Biochim. Biophys. Acta 1373:47-58. [DOI] [PubMed] [Google Scholar]
- 3.Bukhtiyarova, M., R. Yang, and B. Ray. 1994. Analysis of the pediocin AcH gene cluster from plasmid pSMB74 and its expression in a pediocin-negative Pediococcus acidilactici strain. Appl. Environ. Microbiol. 60:3405-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopin, M. C., A. Chopin, A. Rouault, and D. Simon. 1986. Cloning in Streptococcus lactis of plasmid-mediated UV resistance and effect on prophage stability. Appl. Environ. Microbiol. 51:233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos, W. M., O. P. Kuipers, J. R. van der Meer, and R. J. Siezen. 1995. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by gram-positive bacteria. Mol. Microbiol. 17:427-437. [DOI] [PubMed] [Google Scholar]
- 6.Dunny, G. M., L. N. Lee, and D. J. LeBlanc. 1991. Improved electroporation and cloning vector system for gram-positive bacteria. Appl. Environ. Microbiol. 57:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguchi, T., K. Kaminaka, J. Shima, S. Kawamoto, K. Mori, S. H. Choi, K. Doi, S. Ohmomo, and S. Ogata. 2001. Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci. Enterococcus faecalis K-4. Biosci. Biotechnol. Biochem. 65:247-253. [DOI] [PubMed] [Google Scholar]
- 8.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 9.Ezaki, T., Y. Hashimoto, N. Takeuchi, H. Yamamoto, S. L. Liu, H. Miura, K. Matsui, and E. Yabuuchi. 1988. Simple genetic method to identify viridans group streptococci by colorimetric dot hybridization and fluorometric hybridization in microdilution wells. J. Clin. Microbiol. 26:1708-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fimland, G., R. Jack, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1998. The bactericidal activity of pediocin PA-1 is specifically inhibited by a 15-mer fragment that spans the bacteriocin from the center toward the C terminus. Appl. Environ. Microbiol. 64:5057-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fregeau Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 13.Hastings, J. W., M. Sailer, K. Johnson, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 173:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havarstein, L. S., H. Holo, and I. F. Nes. 1994. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by gram-positive bacteria. Microbiology 140:2383-2389. [DOI] [PubMed] [Google Scholar]
- 15.Hochuli, E. 1988. Large-scale chromatography of recombinant proteins. J. Chromatogr. 444:293-302. [DOI] [PubMed] [Google Scholar]
- 16.Holck, A. L., L. Axelsson, K. Huhne, and L. Krockel. 1994. Purification and cloning of sakacin 674, a bacteriocin from Lactobacillus sake Lb674. FEMS Microbiol. Lett. 115:143-149. [DOI] [PubMed] [Google Scholar]
- 17.Huhne, K., L. Axelsson, A. Holck, and L. Krockel. 1996. Analysis of the sakacin P gene cluster from Lactobacillus sake Lb674 and its expression in sakacin-negative Lb. sake strains. Microbiology 142:1437-1448. [DOI] [PubMed] [Google Scholar]
- 18.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamoto, S., H. Watanabe, A. Hesketh, J. C. Ensign, and K. Ochi. 1997. Expression analysis of the ssgA gene product, associated with sporulation and cell division in Streptomyces griseus. Microbiology 143:1077-1086. [DOI] [PubMed] [Google Scholar]
- 21.Klaenhammer, T. R. 1988. Bacteriocins of lactic acid bacteria. Biochimie 70:337-349. [DOI] [PubMed] [Google Scholar]
- 22.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 23.Martinez, J. M., J. Kok, J. W. Sanders, and P. E. Hernandez. 2000. Heterologous coproduction of enterocin A and Pediocin PA-1 by Lactococcus lactis: detection by specific peptide-directed antibodies. Appl. Environ. Microbiol. 66:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marugg, J. D., C. F. Gonzalez, B. S. Kunka, A. M. Ledeboer, M. J. Pucci, M. Y. Toonen, S. A. Walker, L. C. Zoetmulder, and P. A. Vandenbergh. 1992. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, and bacteriocin from Pediococcus acidilactici PAC1.0. Appl. Environ. Microbiol. 58:2360-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori, K., K. Yamazaki, T. Ishiyama, M. Katsumata, K. Kobayashi, Y. Kawai, N. Inoue, and H. Shinano. 1997. Comparative sequence analyses of the genes coding for 16S rRNA of Lactobacillus casei-related taxa. Int. J. Syst. Bacteriol. 47:54-57. [DOI] [PubMed] [Google Scholar]
- 26.Motlagh, A., M. Bukhtiyarova, and B. Ray. 1994. Complete nucleotide sequence of pSMB 74, a plasmid encoding the production of pediocin AcH in Pediococcus acidilactici. Lett. Appl. Microbiol. 18:305-312. [DOI] [PubMed] [Google Scholar]
- 27.Nes, I. F., D. B. Diep, L. S. Havarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 28.O'Keeffe, T., C. Hill, and R. P. Ross. 1999. Characterization and heterologous expression of the genes encoding enterocin a production, immunity, and regulation in Enterococcus faecium DPC1146. Appl. Environ. Microbiol. 65:1506-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quadri, L. E., M. Kleerebezem, O. P. Kuipers, W. M. de Vos, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1997. Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J. Bacteriol. 179:6163-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley, M. A. 1998. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 31.Sahl, H. G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 34.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 35.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 36.Tichaczek, P. S., R. F. Vogel, and W. P. Hammes. 1994. Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH 673. Microbiology 140:361-367. [DOI] [PubMed] [Google Scholar]
- 37.van Reenen, C. A., L. M. Dicks, and M. L. Chikindas. 1998. Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J. Appl. Microbiol. 84:1131-1137. [DOI] [PubMed] [Google Scholar]
- 38.Venema, K., M. H. R. Dost, P. A. H. Beun, A. J. Haandrikman, G. Venema, and J. Kok. 1996. The genes for secretion and maturation of lactococcins are located of the chromosome of Lactococcus lactis IL1403. Appl. Environ. Microbiol. 62:1689-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]