Abstract
The effectiveness of combined high pressure and heat treatment for reducing spore levels of Alicyclobacillus acidoterrestris, a thermoacidophilic spore-forming bacterium, in commercial pasteurized apple juice was investigated. Spores suspended in apple juice were successfully destroyed by combining high pressure with a mild or high temperature (45, 71, or 90°C).
In the 1980s, an acidophilic Bacillus species was isolated from apple juice and identified as a new type of spoilage bacterium (5). Originally named Bacillus acidoterrestris (7), this organism was later reclassified in a new genus, Alicyclobacillus (23), as ω-alicyclic fatty acid was the major membrane fatty acid component of its cells. Alicyclobacillus acidoterrestris is a motile, spore-forming, rod-shaped organism with a central, subterminal, or terminal oval spore and grows at pH values ranging from 2.5 to 6.0 at temperatures of 25 to 60°C (24). Because its spores have been shown to resist high temperatures and acidic environments, it has become an important potential spoilage concern for hot-fill fruit and vegetable juices.
The fruit juices and fruit juice-containing drinks that are most susceptible to this bacterium are either fresh (not heat treated) or pasteurized (but not ultrahigh temperature treated) (8, 16). The organism causes a flat sour type of spoilage and produces an offensive-smelling compound, guaiacol, and other taint chemicals (8, 16). Recently, A. acidoterrestris has been implicated in fruit juice spoilage incidents in the United Kingdom, Germany, and the United States (8, 16, 19). A survey was performed to determine the significance of acid-tolerant sporeformers in the food industry; 57 companies were surveyed, and 34 responses (60%) were obtained. Of the companies that responded, 12 (35%) had experienced spoilage that appeared to be caused by A. acidoterrestris (22).
Cerny et al. (5) and Splittstoesser et al. (19) reported that D values at 90°C were 15 and 16 to 23 min, respectively. Splittstoesser et al. (19) also reported D values of 2.4 to 2.8 min at 95°C. These data suggest that spores survive the typical juice pasteurization process that consists of holding at 86 to 96°C for 2 min. Given its ability to grow at pH values of <3.8 and to survive the typical juice pasteurization process, A. acidoterrestris has caused great concern in the fruit juice industry (1, 2, 13, 19, 20).
Orr et al. (16) reported the efficacy of disinfectants for killing spores of A. acidoterrestris. Their results showed reductions of about 2.2, 0.4, and 0.1 log in the number of viable A. acidoterrestris spores in a five-strain mixture when spores were suspended in 200-ppm chlorine, 500-ppm acidified sodium chlorite, and 0.2% H2O2 solutions, respectively, for 10 min at 23°C. When preparations were treated with either 1,000 ppm of chlorine or 4% H2O2, the number of viable spores was reduced by more than 5 log (17). However, consumers of processed foods dictate that the manufacturing industry should reduce the use of chemical additives and at the same time provide foods which are perceived to have received minimal physical processing.
Thus, this study was undertaken to investigate the effectiveness of combined pressure and heat treatment without use of chemicals for killing spores of A. acidoterrestris in apple juice.
Two strains of A. acidoterrestris, ATCC 49025 and NFPA1013 (apple juice isolate; National Food Processors Association, Dublin, Calif.), were used in this experiment. Cultures were grown for 2 days at 43°C on orange serum agar (OSA) (Becton Dickinson, Cockeysville, Md.) adjusted to pH 3.7 with filter-sterilized 10% tartaric acid and then stored at 4°C as stock cultures. To induce sporulation, cells grown at 43°C for 2 days on OSA were spread onto potato dextrose agar (pH 5.6; Becton Dickinson) in petri plates and incubated at 43°C for 7days until at least 80% of cells sporulated, as determined by microscopic examination. Spores of each strain were individually harvested by depositing approximately 5-ml portions of sterile water onto the surfaces of potato dextrose agar culture plates and were dislodged by gentle rubbing with a sterile swab. Pooled suspensions from 15 plates containing each strain were centrifuged at 4,000 × g for 20 min (4°C), and this was followed by resuspension in sterile water and centrifugation at 4,000 × g for 10 min (4°C). This procedure was repeated four times. The final pellets were resuspended in sterile phosphate buffer (pH 7.0) at concentrations of approximately 107 to 108 spores ml−1, combined, and stored in 1.8-ml cryogenic tubes (Fisher Scientific, Pittsburgh, Pa.) at −20°C until they were used. Each combined spore suspension was diluted with Alicyclobacillus-free commercial pasteurized apple juice (pH 3.7) to obtain a preparation containing approximately 106 spores ml−1. Two-milliliter portions of the of diluted spore suspension were put into sterile film bags for high-pressure treatment consisting of 207, 414, or 621 MPa of pressure for 1, 5, or 10 min. Each treatment was performed at an inner fluid temperature of 22, 45, 71, and 90°C (Engineered Pressure System; EPS Inc., Lebanon, Ohio). The film bags were cooled in ice water immediately following treatment. The surviving population of A. acidoterrestris was enumerated after duplicate spread plating of appropriate 10-fold serial dilutions in sterile phosphate buffer onto OSA (pH 3.7) and incubation at 43°C for 2 days.
All experiments were duplicated and repeated three times. Data from three samples subjected to each treatment in each of three independent replicate experiments were analyzed. Data were subjected to the Statistical Analysis System (SAS Institute, Cary, N.C.) for analysis of variance, and Duncan's multiple range test was used to determine whether significant differences (P < 0.05) existed between mean values of treatments.
To obtain scanning electron microscopy (SEM) photographs, the treated spores were collected by centrifugation at 4,000 × g for 10 min, rinsed three times with 0.1 M phosphate-buffered saline (PBS) (pH 7.3), and fixed with 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M PBS overnight at 4°C. After washing with PBS, spores were postfixed in 1% osmium tetroxide in 0.1 M PBS and dehydrated by using a graded ethanol series (30, 50, 70, 95%, and absolute ethanol). The dehydrated samples were subjected to critical point drying with liquid CO2 by using standard procedures. Subsequently, the samples were mounted on aluminum specimen stubs by using electrically conducting carbon (TED Pella, Inc., Reading, Calif.) and sputter coated with approximately 30 nm of gold by using argon gas as the ionizing plasma. Images were obtained with an S-570 SEM (Hitachi, Tokyo, Japan) with secondary electrons at an acceleration voltage of 20 kV at room temperature.
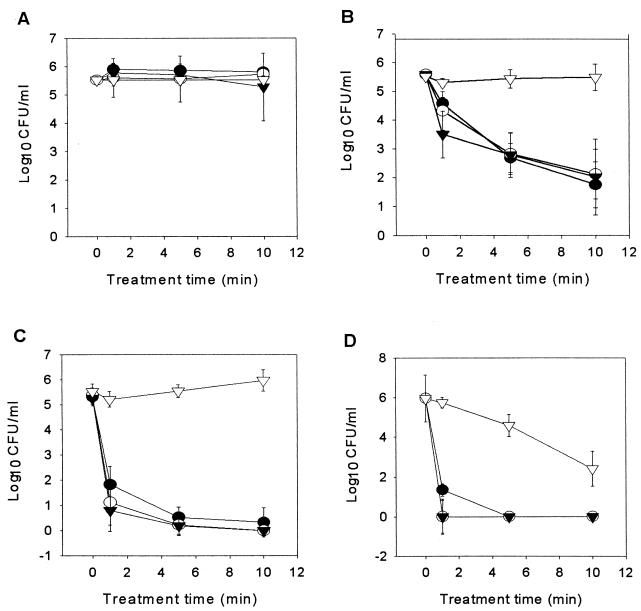
Figure 1 shows data for populations of A. acidoterrestris spores treated with high pressure and heat. Spore viability was not appreciably reduced by high pressure alone at room temperature (Fig. 1A). However, the viability of spores treated with high pressure and heat (45, 71, or 90°C) was significantly reduced (Fig. 1A, B, and C).
FIG. 1.
Survival curves for A. acidoterrestris spores in apple juice treated with 0 MPa (▿), 207 MPa (•), 414 MPa (○), and 621 MPa (▾) at room temperature (22°C) (A), 45°C (B), 71°C (C), and 90°C (D).
Treatment of spores at 45 or 71°C with atmospheric pressure for 10 min did not reduce spore viability, whereas the viability of spores treated with high pressures at 45 or 71°C was significantly reduced (Fig. 1B and C). Treatment with 207 MPa at 45°C for 10 min or at 71°C for 1 min resulted in more than a 3.5-log reduction in the number of viable spores (Fig. 1B and C); treatment with 414 or 621 MPa at 71°C for 1 min resulted in reduction of the number of viable spores by more than 4 logs (Fig. 1C); and treatment with 414 or 621 MPa at 71°C for 10 min reduced the number of viable spores to undetectable levels (<1 CFU ml−1), a >5.5-log reduction (Fig. 1C). The reductions in viability increased as treatment time increased at 45 or 71°C; however, there was not a significant difference among the effects of 207, 414, and 621 MPa on spore viability (P < 0.05) (Fig. 1B and C). There was not a significant reduction in viability when spores were heated at 90°C without high pressure for 1 min (Fig. 1D). However, heat treatment at 90°C combined with 414 or 621 MPa for 1 min reduced the number of viable spores by >5.5 logs to undetectable levels (<1 CFU ml−1) (Fig. 1D). Treatment with 207 MPa for 5 min at 90°C reduced the number of viable spores to undetectable levels (<1 CFU/ml) (Fig. 1D).
As described above, high pressure alone did not reduce the levels of spores in apple juice, but high pressure was more effective as the temperature was increased (Fig. 1). Apparently, spores of A. acidoterrestris are relatively resistant to pressure (21). The intrinsic resistance of spores has been explained by the lack of solvation-derived effects in these relatively dry structures (18). High-pressure inactivation has been attributed to pressure-induced germination and the subsequent destruction of vegetative cells (18). The dipicolinic acid content of bacterial spores is thought to be closely associated with heat resistance properties. Leakage of dipicolinic acid from spores treated with pressure has been reported by Sale et al. (18), and the data provide good evidence of the efficacy of combined pressure and temperature treatment for spore destruction. Furthermore, there is good evidence that moderate increases in temperature enhance the germination effect of pressure (3, 4, 8, 9, 11, 12, 14, 18). Pressure germination kinetic studies show that a likely mechanism for pressure-induced germination is protoplast hydration (9, 15).
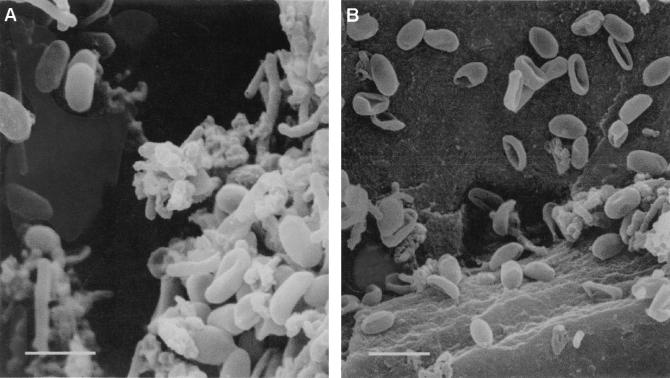
SEM photographs of untreated and treated spores showed that crushed and destroyed spores resulted from the combined effects of high pressure and temperature (Fig. 2). After treatment with 207 Mpa for 5 min at 45°C, some spores were crushed and other spores were shattered (Fig. 2B).
FIG. 2.
SEM photographs of A. acidoterrestris spores. (A) Untreated normal spores (short rods). (B) Spores crushed and destroyed by combined treatment with high pressure and temperature. The spores were treated with 207 MPa for 5 min at 45°C. Bars = 3.0 μm.
Pressure-germinated cells could also be inactivated by a range of other chemical and physical agents (8). Furthermore, elucidation of the pressure-mediated germination effect could open increased opportunities for commercial exploitation, particularly if chemical treatment is undesirable. Thus, there is an opportunity for using combined pressure and temperature processes for sterilization of low-acid foods in pressure ranges that are commercially feasible (8).
In 1884 Certes described the effects of high pressure on microbiological spoilage processes (6, 8). In 1899 Hite (10) reported the results of his successful application of high pressure for the purpose of food preservation. Despite the early beginnings of this technology, there have been relatively few commercial applications of high pressure for microbial inactivation in foods. This has been mainly due to the economic constraints of high-pressure vessel manufacture and operation and the lack of any significant advantage of the process over established methods of preservation. In recent years, the situation has changed, and high-pressure technology is available on a commercial scale at reasonable cost (14). Consumer demand for minimally processed foods provides a strong motivation for commercial development.
In this study, spores of A. acidoterrestris suspended in commercial apple juice were successfully destroyed by combining high pressure with mild to high temperatures. Thus, the results can be applied to the acidic food industry, especially the manufacture of fruit juice and fruit juice-containing drinks.
Acknowledgments
We acknowledge the financial support provided by the International Marketing Program for Agricultural Commodities and Trade.
REFERENCES
- 1.April, J. P., J. E. Rushing, and P. M. Foegeding. 1998. Heat resistance of Alicyclobacillus acidoterrestris spores as affected by various pH values and organic acids. J. Food Prot. 61:41-46. [DOI] [PubMed] [Google Scholar]
- 2.Brown, K. L. 2000. Control of bacterial spores. Br. Med. Bull. 56:158-171. [DOI] [PubMed] [Google Scholar]
- 3.Butz, P., and H. Ludwig. 1989. Pressure inactivation of microorganisms at moderate temperatures. Physica 139:875-877. [Google Scholar]
- 4.Butz, P., J. Ricem, U. Trangott, H. Weber, and H. Ludwig. 1990. Hoch-druckinakinakivjerung von bakterien ind bakteriensporen. Pharm. Ind. 52:487-491. [Google Scholar]
- 5.Cerny, G., W. Hennlich, and K. Poralla. 1984. Spoilage of fruit juice by bacilli: isolation and characterization of the spoiling microorganism. Z. Lebensm. Unters. Forsch. 179:224-227. [DOI] [PubMed] [Google Scholar]
- 6.Certes, J. C. 1884. De l'action des hautes pressions sur les phenomenes de la purefaction et sur la vitalite des microorganisms d'eau donce et d'eau de mer. C. R. Acad. Sci. 99:385-388. [Google Scholar]
- 7.Deinhard, G., P. Blanz, K. Poralla, and E. Altan. 1987. Bacillus acidoterrestris sp. nov., a new thermotolerant acidophile isolated from different soils. Syst. Appl. Microbiol. 10:47-53. [Google Scholar]
- 8.Evangelia, K., S. B. Ioannis, E. D. Alison, D. B. Joss, and R. A. Martin. 1999. Alicyclobacillus acidoterrestris in fruit juices and its control by nisin. Int. J. Food Sci. Technol. 34:81-85. [Google Scholar]
- 9.Gould, G. W., and A. J. H. Sale. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:335-346. [DOI] [PubMed] [Google Scholar]
- 10.Hite, B. H. 1899. The effects of pressure in the preservation of milk. W. Va. Agric. Exp. Stn. Bull. 58:15-35. [Google Scholar]
- 11.Hoover, D. G. 1993. Pressure effect on biological systems. Food Technol. 47:150-155. [Google Scholar]
- 12.Mallidis, C. G., and D. Drizou. 1991. Effect of simultaneous application of heat and pressure on the survival of bacterial spores. J. Appl. Bacteriol. 71:285-288. [DOI] [PubMed] [Google Scholar]
- 13.McIntyte, S., J. Y. Ikawa, N. Parkinson, J. Haglund, and J. Lee. 1995. Characteristics of an acidophilic Bacillus strain isolated from shelf-stable juices. J. Food Prot. 58:319-321. [DOI] [PubMed] [Google Scholar]
- 14.Morgan, S. M., R. P. Ross, T. Beresford, and C. Hill. 2000. Combination of hydrostatic pressure and lacticin 3147 causes increased killing of Staphylococcus and Listeria. J. Appl. Microbiol. 88:414-420. [DOI] [PubMed] [Google Scholar]
- 15.Murrell, W. G., and Wills. 1977. Inactivation of Bacillus spore germination by hydrostatic pressure: effect of temperature. J. Bacteriol. 129:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr, R. V., L. Robert, C. J. H. Shewfelt, T. Sebhat, and L. Y. R. Beuchat. 2000. Detection of guaiacol produced by Alicyclobacillus acidoterrestris in apple juice by sensory and chromatographic analyses, and comparison with spore and vegetative cell populations. J. Food Prot. 63:1517-1522. [DOI] [PubMed] [Google Scholar]
- 17.Pettipher, G. L., M. E. Osmundson, and J. M. Murphy. 1997. Methods for the detection and enumeration of Alicyclobacillus acidoterrestris and investigation of growth and taint in fruit juice and fruit-containing drinks. Lett. Appl. Microbiol. 24:185-189. [DOI] [PubMed] [Google Scholar]
- 18.Sale, A. J. H., G. W. Gould, and W. A. Hamilton. 1970. Inactivation of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:323-334. [DOI] [PubMed] [Google Scholar]
- 19.Splittstoesser, D. F., C. Y. Lee, and J. J. Churry. 1998. Control of Alicyclobacillus in the juice industry. Dairy Food Environ. Sanit. 18:585-587. [Google Scholar]
- 20.Spring, M. P. 1995. Detectives on a ‘juicey’ case. Food Qual. 1:32. [Google Scholar]
- 21.Timson, W. J., and A. J. Short. 1965. Resistance of microorganisms to hydrostatic pressure. Biotechnol. Bioeng. 7:139-159. [Google Scholar]
- 22.Walls, I., and R. Chuyate. 2000. Spoilage of fruit juices by Alicyclobacillus acidoterrestris. Food Aust. 52:286-288. [PubMed] [Google Scholar]
- 23.Wisotzkey, J. D., P. Jurtshuk, G. E. Fox, G. Deinhard, and K. Poralla. 1992. Comparative sequence analysis of the 16S rRNA DNA of Bacillus acidocaldarius, Bacillus acidoterrestris, and Bacillus cycloheptancius and proposal for creation of new genus, Alicyclobacillus. Int. J. Syst. Bacteriol. 42:263-269. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki, K., H. Teduka, and H. Shinano. 1996. Isolation and identification of Alicyclobacillus acidoterrestris from acidic beverages. Biosci. Biotechnol. Biochem. 60:543-545. [DOI] [PubMed] [Google Scholar]