Abstract
The effect of addition of purified nisin Z in liposomes to cheese milk and of in situ production of nisin Z by Lactococcus lactis subsp. lactis biovar diacetylactis UL719 in the mixed starter on the inhibition of Listeria innocua in cheddar cheese was evaluated during 6 months of ripening. A cheese mixed starter culture containing Lactococcus lactis subsp. lactis biovar diacetylactis UL719 was selected for high-level nisin Z and acid production. Experimental cheddar cheeses were produced on a pilot scale, using the selected starter culture, from milk with added L. innocua (105 to 106 CFU/ml). Liposomes with purified nisin Z were prepared from proliposome H and added to cheese milk prior to renneting to give a final concentration of 300 IU/g of cheese. The nisin Z-producing strain and nisin Z-containing liposomes did not significantly affect cheese production and gross chemical composition of the cheeses. Immediately after cheese production, 3- and 1.5-log-unit reductions in viable counts of L. innocua were obtained in cheeses with encapsulated nisin and the nisinogenic starter, respectively. After 6 months, cheeses made with encapsulated nisin contained less than 10 CFU of L. innocua per g and 90% of the initial nisin activity, compared with 104 CFU/g and only 12% of initial activity in cheeses made with the nisinogenic starter. This study showed that encapsulation of nisin Z in liposomes can provide a powerful tool to improve nisin stability and inhibitory action in the cheese matrix while protecting the cheese starter from the detrimental action of nisin during cheese production.
Nisin is a cationic polypeptide of 34 amino acids produced by Lactococcus lactis strains (18, 47). Two natural variants of nisin (nisin A and nisin Z) are known and are equally distributed among nisin-producing strains (34). These variants differ by a single substitution, at position 27, with histidine (nisin A) and asparagine (nisin Z) (34). This structural modification gives nisin Z higher solubility and diffusion characteristics which are important for food applications (12).
Nisin has an inhibitory effect against a wide variety of gram-positive food-borne pathogens and spoilage microorganisms (40) and can also act on several gram-negative bacteria when the integrity of their outer membranes is disrupted (23, 43). The use of nisin as a food preservative dates back to 1956, when nisin was proposed to control growth and spore formation of Clostridium botulinum and Clostridium sporogenes in cheese (31). Nisin is the only bacteriocin that has been approved by the World Health Organization as a preservative in food (46), and Nisalpin, the commercial product containing 2.5% pure nisin A, is being legally used in more than 50 countries for specific food applications (10). However, the loss of nisin activity from the commercial form has been reported for several food products during storage (8, 9). Moreover, the use of nisin in its free form in cheese can be expensive and results in inhibitory effects against the suitable acidifying or aroma-producing starter cultures, decreasing growth and acidification (37). An alternative to the addition of free nisin to fermented food systems is the use of nisin-producing strains during fermentation processes (8, 29, 38). However, bacteriocin-producing organisms in cheese making can cause alterations in the cheese-making process, such as delayed acidification of the curd with a concomitant increase in residual lactose (17, 37).
To date, few attempts to use microencapsulated bacteriocin in foods have been reported in the literature. This strategy can improve nisin stability and distribution in the food matrix. In a meat model system, entrapment of pediocin AcH in liposomes made from phosphatidylcholine enhanced the antilisterial activity of pediocin compared with free pediocin (7). That study concluded that optimization of the encapsulation system and antimicrobial activity of the encapsulated bioactive agent in food systems still required investigation to obtain more efficient delivery of bacteriocin in foods. We recently demonstrated the efficacy of proliposome H (pro-lipo H), composed of higher-melting-point phospholipids, for encapsulating nisin Z (R. Laridi, E. E. Kheadr, R.-O. Benech, J. C. Vuillemard, C. Lacroix, and I. Fliss, submitted for publication). This system proved to withstand the cheddar cheese temperature cycle and did not appear to disturb cheese fermentation.
The present study aimed to (i) select a mixed starter culture containing the high-level nisin Z producer Lactococcus lactis subsp. lactis biovar diacetylactis UL719, (ii) compare the efficacies of liposome-encapsulated purified nisin Z and the selected mixed starter culture in inhibiting growth of Listeria innocua in cheddar cheese, and (iii) evaluate the activities and stabilities of both free and encapsulated nisin during cheddar cheese ripening.
MATERIALS AND METHODS
Bacteria and growth conditions.
Lactococcus lactis subsp. lactis biovar diacetylactis UL719, isolated from raw-milk cheese (13), was used as a nisin Z-producing strain (32). Pediococcus acidilactici UL5, from our collection, was used as indicator organism for the bacteriocin activity assay (4). L. innocua ATCC 33090 was obtained from the American Type Culture Collection (Manassas, Va.). The lactococcal strains tested for their acidification capacity during the selection of the cheese mixed culture were Lactococcus lactis subsp. cremoris ATCC 9596, Lactococcus lactis subsp. cremoris E8, and Lactococcus lactis subsp. cremoris KB from our collection. Lactococcus lactis subsp. lactis KBP, Lactococcus lactis subsp. lactis KB, and Lactococcus lactis subsp. lactis biovar diacetylactis KB were obtained from Ezal, Rhône Poulenc (Mississauga, Ontario, Canada). A commercial cheese mixed set culture (no. 911) from Chr. Hansen (Milwaukee, Wis.) was also tested for its compatibility with Lactococcus lactis subsp. lactis biovar diacetylactis UL719. All bacterial cultures were maintained in 20% glycerol stock at −80°C. Strains of Lactococcus spp. were grown in M17 broth medium (BDH-Merck, Darmstadt, Germany) supplemented with 0.5% (wt/vol) glucose and incubated overnight at 30°C (45). Lactococcus lactis subsp. lactis biovar diacetylactis UL719 was cultivated in MRS broth obtained from Rosell Institute Inc. (Montreal, Quebec, Canada) and incubated overnight at 30°C (11). L. innocua was reactivated in tryptic soy broth (Difco Laboratories, Detroit, Mich.) at 37°C. Before each experiment, each bacterial strain was subcultured at least three times (inoculation at 1%, vol/vol) at 24-h intervals.
Selection of a mixed starter culture.
An overnight M17 culture of lactococcal strain was standardized to an optical density of 0.5 at a wavelength of 600 nm, using a LKB model spectrophotometer (Biochrom, Cambridge, England). The standardized culture was used to inoculate 100 ml of sterilized reconstituted skim milk (11% [wt/vol] total solids) in 250-ml Erlenmeyer flasks, and incubation was carried out at 30°C in a water bath with mixing at 150 rpm until a pH of 5.2 ± 0.1 was obtained. The pH electrodes (Fisher Scientific, Montreal, Quebec, Canada) were sterilized by soaking in sodium hypochloride (6%) for 1 min followed by thorough rinsing with sterile deionized water (5). The pH electrodes were attached to an electronic switchbox (model 607; Orion, Boston, Mass.) connected to both a pH meter and a personal microcomputer for automatic measurements of pH at 30-s intervals. Samples were taken at 3-h intervals for bacterial counting, and nisin activity was determined at the end of incubation. In order to select a cheddar cheese starter with acidification activity suitable for cheddar cheese production, the available lactococcal strains were first tested in pure culture with an inoculation rate of 2% (vol/vol). Strains showing high acidification characteristics were further tested at a constant inoculation rate of 1% (vol/vol) in pure culture or in combination with 1% (vol/vol) Lactococcus lactis subsp. lactis biovar diacetylactis UL719. Experiments were done in triplicate with duplicate analysis.
Nisin Z production and purification.
An overnight MRS culture of Lactococcus lactis subsp. lactis biovar diacetylactis UL719 was centrifuged at 7,000 × g for 20 min at 4°C using a Biofuge 22R centrifuge (D-37520; Heraeus Instruments, Osterode, Germany). The supernatant was adjusted to pH 6.0 with 5 M NaOH, filter sterilized with 0.45-μm-pore-size filters (Nalge Co., Rochester, N.Y.), and purified by immunoaffinity chromatography using anti-nisin Z monoclonal antibodies (6). A glass column (50 cm high and 2.5 cm in diameter) was packed with 35 ml of Sepharose beads (CNBr-activated Sepharose 4B; Amersham Pharmacia Biotech, Quebec, Quebec, Canada). Anti-nisin Z monoclonal antibodies were coupled with NH2 groups to the Sepharose matrix according to the procedures of the manufacturer. The column was regenerated with 200 ml of 0.01 M phosphate-buffered saline at pH 6 at a flow rate of 10 ml/min, using a peristaltic pump (model EP1; Bio-Rad, Mississauga, Ontario, Canada). The filter-sterilized supernatant was injected into the column (10 ml/min). The column was then washed with 200 ml of phosphate-buffered saline (10 ml/min), and nisin Z was eluted with 80 ml of urea (6 M) at a flow rate of 5 ml/min. The eluted fractions were dialyzed for 24 h against acetic acid (0.05 M) in a 1,000-molecular-weight-cutoff cellulose ester dialysis bag (Spectra/ProΕ CE membrane; Spectrum Laboratories Inc., Houston, Tex.). The resulting dialysate was concentrated using a Speed Vac concentrator (SVC 100H/200H; Savant Instruments Inc., Holbrook, N.Y.). Protein contents of concentrates were determined as previously described (28). Nisin Z activity, determined by agar diffusion and competitive enzyme immunoassay (cEIA), and purity of nisin Z, tested with high-pressure liquid chromatography, were evaluated by the methods described previously by Daoudi et al. (6)
Liposome preparation.
Pro-lipo H, made up of hydrogenated phosphatidylcholine, was obtained from Lucas Meyer (Chelles, France). Liposomes were prepared from pro-lipo H by a procedure described previously (Laridi et al., submitted). Briefly, 5 g of pro-lipo H was mixed with 5 ml of an aqueous solution containing 5 mg of purified nisin Z per ml. The mixture was stirred (50 to 60 rpm) for 15 min at 65°C with a magnetic stirrer (PC-420, VWR; Corning, Mississauga, Ontario, Canada), diluted with 20 ml of deionized water, and stirred again for 15 min. Liposomes were separated from unencapsulated nisin Z by ultracentrifugation (model L8-70 M ultracentrifuge; Beckman, Palo Alto, Calif.) at 85,000 × g for 1 h at 20°C, washed twice with deionized water, and recentrifuged. The resultant supernatants were combined and used to determine the amount of unencapsulated nisin. Vesicles separated by centrifugation were suspended in deionized water, and the final volume was adjusted to 5 ml. One milliliter of vesicle suspension was mixed with 1 ml of 2% (vol/vol) Triton X-100 (Sigma Chemical Co., St. Louis, Mo.) to disrupt the membranes and release the encapsulated nisin, and then 20 μl of HCl (0.5 M) was added and the whole mixture was heated at 98°C for 5 min. The amounts of encapsulated and unencapsulated nisin were determined using the agar diffusion method, and the entrapment efficiency (EE) was calculated using the following equation: EE (%) = {encapsulated nisin (IU)/[encapsulated nisin (IU) + unencapsulated nisin (IU)]} × 100.
Cheese-making procedure.
A cheese starter mixture composed of Lactococcus lactis subsp. lactis (KB) and Lactococcus lactis subsp. cremoris (KB) at a 1:1 (vol/vol) ratio was selected from the acidification tests. The nisinogenic mixed starter used for cheese production was composed of the selected starter mixture and Lactococcus lactis subsp. lactis biovar diacetylactis UL719 at a ratio of 3:1 (vol/vol), permitting both high acid production and high nisin Z production. For cheese production, the strains of Lactococcus were grown separately overnight in sterilized reconstituted skim milk at 30°C. An overnight culture of L. innocua was used to inoculate cheese milk to a final concentration of 105 to 106 CFU/ml. L. innocua was chosen for this work instead of Listeria monocytogenes for safety reasons, since this work was done in a pilot laboratory and it was considered a suitable nonpathogenic replacement for L. monocytogenes (15).
Prior to cheese making, raw milk obtained from farm SMA (Quebec, Quebec, Canada) was standardized to a constant fat content of 3.4%, pasteurized at 72°C for 16 s, and cooled to 4°C. The milk was warmed to 32°C and inoculated with one of the starter cultures at 2% (vol/vol). Cheddar cheese was made by a standard procedure (24), using computer-controlled cheese equipment (INRA, Poligny, France) equipped with four 10-liter vats. Milk was ripened until the pH reached 6.5 (approximately 60 min), and liposomes containing nisin Z were added 5 min before rennet addition. Calcium chloride was added at 0.02% (wt/vol). Milk was coagulated at 32°C in 30 min using rennet Chy-Max extra from Chr. Hansen. The coagulum was cut, and the temperature was slowly raised to 39°C to induce syneresis and to increase curd firmness. Whey was drained, and samples were taken to determine nisin Z concentration and activity by using a cEIA (6) and the agar diffusion test (44), respectively. The curd was subjected to the cheddaring process for 120 min at 36°C until the pH reached 5.3 ± 0.1, milled, dry salted (2.0% [wt/wt] salt), transferred in a round mold, and pressed overnight. Following pressing, the cheese was cut and a representative sample of approximately 200 g was taken for proximate and microbiological analyses. Cheese blocks were then vacuum packed in plastic bags (4-ml thickness; Winpax Co., Winnipeg, Manitoba, Canada), coded, and ripened at 7°C for 6 months. The experimental cheeses and their codes are given in Table 1.
TABLE 1.
Cheese treatments made in the studya
| Cheese (code) | Additionsb
|
||
|---|---|---|---|
| Lactococcus lactis subsp. lactis biovar diacetylactis UL719c | L. innocua | Encap- sulated nisin Zd | |
| Nisin-free cheese (NF) | − | − | − |
| In situ nisin-containing cheese (ISN) | + | − | − |
| L. innocua-containing nisin-free cheese (LNF) | − | + | − |
| L. innocua- and in situ nisin-containing cheese (LISN) | + | + | − |
| L. innocua- and liposome nisin-containing cheese (LLN) | − | + | + |
All cheeses were made using a 1:1 (vol/vol) mixed starter culture of Lactococcus lactis subsp. lactis (KB) and Lactococcus lactis subsp. cremoris (KB).
−, absent; +, present.
Nisin Z-producing strain.
Liposomes with encapsulated nisin were produced by diluting 5 g of pro-lipo H with 5 ml of a nisin Z aqueous solution (5 mg/ml). Following separation of liposomes, the resulting vesicles were added to 10 liters of cheese milk for a final concentration of 300 IU/g of cheese.
Cheese and whey composition.
The moisture and total nitrogen contents of cheese samples and the total nitrogen and trichloroacetic acid-soluble nitrogen contents in whey samples were determined using official methods of analysis (2). The fat content of cheese was determined by the Babcock fat test as previously described (3). Cheese pH was measured with a Spear Tip combination electrode (VWR Scientific, Ville Mont-Royal, Quebec, Canada).
Microbiological analyses.
Milk samples (10 ml) from the acidification test were vortexed for 2 min with 90 ml of sterile peptone water (0.15%, wt/vol). Serial dilutions were then made with peptone water, and appropriate dilutions were plated in triplicate on KMK agar for counting lactococci after incubation at 30°C for 48 h (21).
Cheese samples (10 g) taken in triplicate were homogenized with 90 ml of sterile 2% sodium citrate solution for 3 min in a Stomacher (Lab Blender 80; Seward Medical, London, England), as described previously (19). Cheese samples were then serially diluted 10-fold in 2% sodium citrate. Appropriate dilutions were plated in triplicate on Listeria selective agar base (Oxoid) with a selective supplement, SR140E (200 mg of cycloheximide, 10 mg of colistin sulfate, 5 mg of fosfomycin, 2.5 mg of acriflavine, and 1 mg of cefotan per 500 ml of medium). Plates were incubated at 37°C for 48 h. Appropriate dilutions were also plated on KMK agar to enumerate lactococci in cheeses.
Nisin Z extraction of milk, whey permeate, and cheese.
Milk and whey samples (2.5 ml) were extracted as previously described (16), with 10 ml of 0.2% (wt/vol) Tween 80 in 0.02 N HCl (Tween 80-HCl). The pH was adjusted to 2.5 ± 0.1 with 5 N HCl solution, and the samples were heated at 100°C for 5 min. Following cooling to 20°C, extracts were centrifuged at 4,000 × g for 20 min. The supernatants were held at 4°C for 30 min and filtered through a 0.22-μm-pore-size Ministart sterile filter (Sartorius AG, Göttingen, Germany). Milk and whey samples with known nisin Z concentrations were also extracted and used in activity test.
Freeze-dried cheese samples (4 g) were rehydrated with 8 ml of Tween 80-HCl, and the final pH was adjusted at 2 with 5 M HCl. The mixture was homogenized for 15 min at 60°C in a water bath. The homogenate was incubated at 60°C for 15 min, heated at 100°C for 3 min, vortexed for 2 min, and reheated at 100°C for 2 min. Following cooling to 4°C, the cheese homogenate was centrifuged at 10,000 × g for 30 min, and the resulting supernatant was recentrifuged at 10,000 × g for 20 min. The resulting supernatant was then filtered with a 0.2-μm-pore-size filter and concentrated with a Speed Vac concentrator. The concentrate was resuspended in 200 μl of Tween 80-HCl and tested for nisin activity with the agar diffusion test.
Agar diffusion assay.
The activity of nisin Z was determined by an agar diffusion test using P. acidilactici UL5 as the indicator organism (4). For quantification, standard solutions of purified nisin Z with concentrations from 0.625 to 10 μg/ml were prepared in Tween 80-HCl and spotted on the agar. After incubation at 30°C for 18 h, the diameters of zones of inhibition were recorded and compared with those obtained with standard solutions.
Transmission electron microscopy (TEM).
Cheese cubes (0.8 to 1.0 mm) were fixed overnight at 4°C in 0.05% (wt/vol) glutaraldehyde and 2.5% (wt/vol) paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). They were washed four times (10 min each) with sodium cacodylate buffer and postfixed for 2 h at 4°C in 1% (wt/vol) osmium tetroxide. After being washed four times with sodium cacodylate buffer, the samples were dehydrated in graded ethanol series, embedded in Quetol (Marivac, Halifax, Nova Scotia, Canada), and polymerized at 60°C for 24 h (1). Ultrathin sections (0.1 μm) of samples were cut with an ultramicrotome (Reichert-Jung, Vienna, Austria) and collected on Formvar-coated nickel grids (JBEM, Dorval, Quebec, Canada). The grids were dried, stained with uranyl acetate and lead citrate, and examined with a 1200 EX instrument (JEOL, Peabogy, Mass.) at 80 kV.
Statistical analysis.
All experimental cheese-making treatments were performed in triplicate with the same lot of milk, and all analyses were done in duplicate. Statistical analyses were performed with Stat View SE + Graphics (Abacus Concepts, Inc., Berkeley, Calif.). Significant differences between treatments were tested by analysis of variance. Treatment comparisons were performed using Fisher's protected least-significant differences (PLSD) test, with a P value of <0.05 considered significant.
RESULTS
Selection of a mixed starter culture.
Because of the poor performance of nisin-producing strains in producing acid and their reduced proteolytic capacity and inhibitory effect on conventional starter cultures (27), a balanced mixed culture for cheddar cheese manufacture leading to appropriate production of acid and nisin Z was selected. Seven strains of lactococci and Lactococcus lactis subsp. lactis biovar diacetylactis UL719 were first tested in pure culture for their acidifying activity in milk with an inoculum ratio of 2% (vol/vol) (data not shown). Four Lactococcus strains (Lactococcus lactis subsp. cremoris E8, Lactococcus lactis subsp. cremoris KB, Lactococcus lactis subsp. lactis KBP, and Lactococcus lactis subsp. lactis KB) were selected for their ability to acidify milk at the highest rate; a pH of 5.2 ± 0.1 was obtained in <6 h. These strains were further evaluated using a 1% inoculum ratio. A commercial cheese starter culture from Chr. Hansen (coded COM) was also tested for its acidifying capacity. With a 1% inoculum, Lactococcus lactis subsp. cremoris E8 took the longest time to reach pH 5.2, which was double that obtained with a 2% inoculum. Strains Lactococcus lactis subsp. cremoris KB, Lactococcus lactis subsp. lactis KBP, and Lactococcus lactis subsp. lactis KB were selected for their ability to grow with Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (Table 2).
TABLE 2.
Acidifying activity and viable cell counts of different lactococci grown in milk in pure culture or in combination with Lactococcus lactis subsp. lactis biovar diacetylactis UL719
| Culture | Meana ± SD
|
||
|---|---|---|---|
| Time to reach pH 5.2 ± 0.1 (min) | Cell count (log CFU/ml) | Nisin Z activity (IU/ml) | |
| Lactococcus lactis subsp. cremoris E8 | 897 ± 15.5a | 8.02 ± 0.017f | |
| Lactococcus lactis subsp. cremoris KB | 430 ± 4.0g | 8.47 ± 0.036a,b,c | |
| Lactococcus lactis subsp. lactis KBP | 435 ± 6.5f,g | 8.44 ± 0.032b,c,d | |
| Lactococcus lactis subsp. lactis KB | 411 ± 8.5h | 8.50 ± 0.067a,b | |
| Lactococcus lactis subsp. lactis biovar diacetylactis UL719 | 828 ± 15.5b | 8.38 ± 0.081d | 450 ± 20.1a |
| COM | 521 ± 5.5d | 8.28 ± 0.019e | |
| Lactococcus lactis subsp. cremoris KB-Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (1:1) | 447 ± 7.5f,g | 8.39 ± 0.057c,d | 344 ± 9.0c,d |
| Lactococcus lactis subsp. lactis KBP-Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (1:1) | 523 ± 14.0d | 8.05 ± 0.078f | 243 ± 8.0f |
| Lactococcus lactis subsp. lactis KB-Lactococcus lactis subsp. lactis biovar diacetylactis UL719L (1:1) | 418 ± 13.5g,h | 8.50 ± 0.045a,b | 356 ± 10.5c |
| COM-Lactococcus lactis subsp. lactis Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (1:1) | 594 ± 11.0c | 7.87 ± 0.072g | 336 ± 2.0d |
| Lactococcus lactis subsp. cremoris KB-Lactococcus lactis subsp. lactis KB-Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (1:1:1) | 512 ± 5.0d | 8.39 ± 0.072c,d | 408 ± 6.0b |
| Lactococcus lactis subsp. cremoris KB-Lactococcus lactis subsp. lactis KB-Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (1.5:1.5:1) | 469 ± 12.0e | 8.55 ± 0.040a | 306 ± 2.0e |
Means within the same column without a common letter are significantly different (P < 0.05) by the PLSD test.
The three selected strains were individually evaluated for their tolerance to Lactococcus lactis subsp. lactis biovar diacetylactis UL719 at a ratio of 1:1 (vol/vol) (Table 2). The tested commercial culture (COM) and Lactococcus lactis subsp. lactis KBP appeared to be sensitive to nisin Z, as their combined culture with Lactococcus lactis subsp. lactis biovar diacetylactis UL719 showed a significantly slower acid production and lower viable cell count (P < 0.05) than those with pure cultures (Table 2). When Lactococcus lactis subsp. lactis KB and Lactococcus lactis subsp. cremoris KB cells were grown in the presence of Lactococcus lactis subsp. lactis biovar diacetylactis UL719, acid production and total viable counts in such mixed cultures did not significantly differ from those in pure cultures (P < 0.05). However, nisin Z production by Lactococcus lactis subsp. lactis biovar diacetylactis UL719 decreased significantly in the presence of the other lactococci, from 450 IU/ml to 344 and 356 IU/ml when grown in combination with Lactococcus lactis subsp. cremoris KB or Lactococcus lactis subsp. lactis KB, respectively.
In order to obtain the optimal mixed culture composition for cheddar cheese manufacture, mixed cultures containing Lactococcus lactis subsp. cremoris KB, Lactococcus lactis subsp. lactis KB, and Lactococcus lactis subsp. lactis biovar diacetylactis UL719 at a ratio of 1:1:1 or 1.5:1.5:1 (vol/vol/vol) were inoculated in milk at a level of 2% (vol/vol) and evaluated for acid and nisin Z production. The increase of the proportion of Lactococcus lactis subsp. lactis biovar diacetylactis UL719 in the 1:1:1 coculture inoculum resulted in a significant increase in the acidification time and nisin Z production compared with the 1.5:1.5:1 inoculum (Table 2). The 1.5:1.5:1.0 ratio was then selected for cheese production as a compromise for high acid production and acceptable nisin Z levels.
Cheese and whey compositions.
The use of a mixed culture containing Lactococcus lactis subsp. lactis biovar diacetylactis UL719 or liposome-encapsulated nisin Z did not significantly affect cheddar cheese manufacture. The time required to reach pH 5.3 ± 0.1 at the salting stage was approximately 20 to 30 min longer for Lactococcus lactis subsp. lactis biovar diacetylactis UL719- and liposome-containing cheeses than for nisin-free control cheeses. Addition of the nisin Z-producing strain or nisin Z-encapsulated liposomes did not influence significantly the composition of whey. The mean compositions of whey from control and experimental vats were 0.78% ± 0.028% total protein and 33.94% ± 1.53% trichloroacetic acid-soluble nitrogen/total nitrogen. On the other hand, nisin Z could not be detected in whey samples from experimental cheese either by cEIA or by the agar diffusion test.
Composition data for control and experimental cheeses after the pressing stage are given in Table 3. The chemical compositions of control and Lactococcus lactis subsp. lactis biovar diacetylactis UL719-containing cheeses appeared to be similar, while the cheeses with added encapsulated nisin Z contained 1.73% ± 0.46% higher moisture levels (P < 0.05) than control cheeses (Table 3).
TABLE 3.
Compositions of control and experimental cheeses after manufacture
| Cheesea | Chemical parameters (mean ± SD)b
|
||||
|---|---|---|---|---|---|
| Moisture content (%) | Total proteins (%) | Fat content (%) | Acidity (%) | pH | |
| NF | 37.72 ± 0.23b | 23.72 ± 0.52a | 32.25 ± 0.35a | 0.882 ± 0.031a | 5.2 ± 0.1a |
| LNF | 37.11 ± 0.43c | 23.48 ± 0.11a | 32.25 ± 0.35a | 0.882 ± 0.018a | 5.2 ± 0.1a |
| ISN | 37.72 ± 0.27b | 23.50 ± 0.05a | 31.75 ± 0.35a,b | 0.816 ± 0.010b,c | 5.2 ± 0.1a |
| LISN | 37.46 ± 0.31b,c | 23.47 ± 0.33a | 31.50 ± 0.10b | 0.812 ± 0.010c | 5.2 ± 0.1a |
| LLN | 39.19 ± 0.15a | 23.73 ± 0.37a | 32.40 ± 0.56a | 0.846 ± 0.014a,b | 5.2 ± 0.1a |
See Table 1.
Means within the same column without a common letter are significantly different (P < 0.05) by the PLSD test.
TEM observation of liposomes.
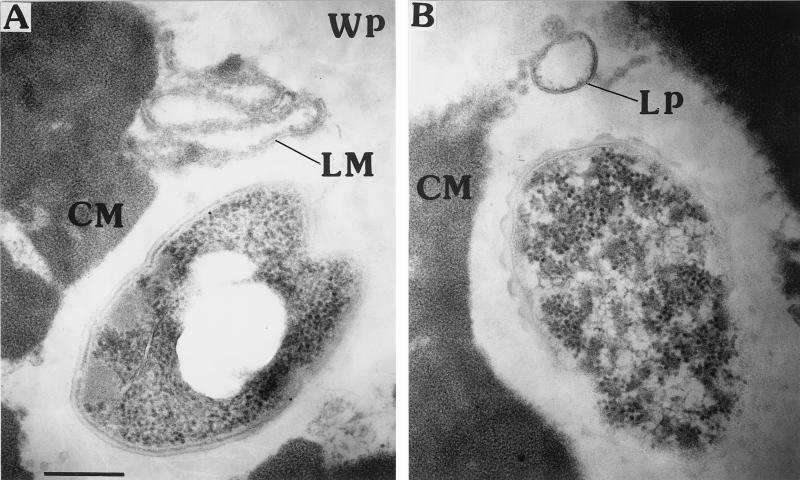
The mean entrapment efficiency for nisin Z in liposomes prepared from pro-lipo H for three trials was 47% ± 2.7%. The majority of the intact vesicles were small unilamellar vesicles with a diameter range of 80 to 120 nm and could be observed during the first month of ripening (Fig. 1B). However, large unilamellar vesicles were apparently less stable, and many of them appeared in linear or unlamellar structures even in cheese samples taken immediately after production (Fig. 1A). In addition, linear liposome membranes were observed throughout the ripening period. Electron microscopy observations of liposomes in cheese showed that in 0-day-old cheeses (Fig. 1A) as well as during cheese aging (Fig. 1B), liposome vesicles and membranes appeared to be located together either in the whey pocket or at the fat-casein interface.
FIG. 1.
Liposome stability in cheddar cheese matrix. (A) Fresh cheese; (B) 1-month-old cheese. CM, casein matrix; WP, whey pocket; LP, liposome vesicle; LM, liposome membrane. Bar, 200 nm.
Nisin Z stability in cheddar cheese.
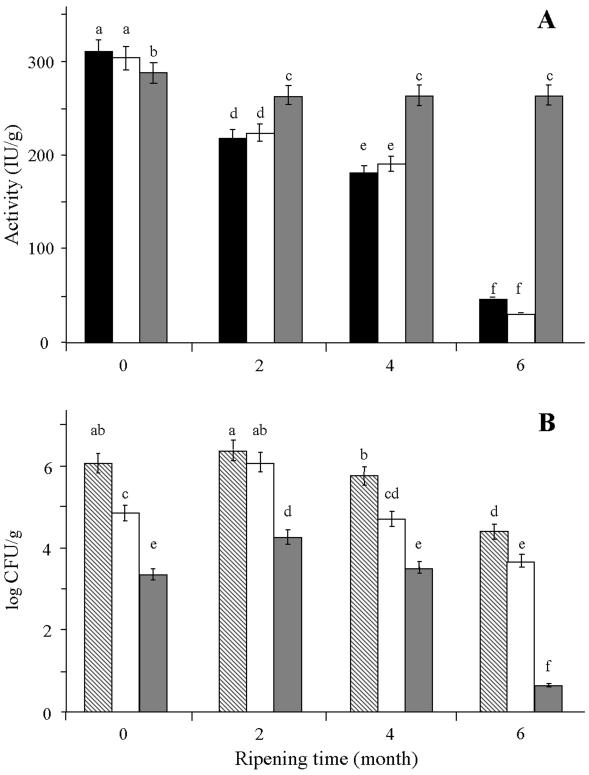
At the beginning of ripening, nisin Z activities in experimental cheeses with added Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (ISN and LISN cheeses [Table 1]) or encapsulated nisin (LLN cheese) were 315 ± 12 and 288 ± 11 IU/g, respectively (Fig. 2A). During aging, a progressive decline in nisin Z activity was observed in cheeses with added Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (ISN and LISN); a nisin activity of 27 ± 2 IU/g was detected after 6 months of ripening, representing 12% of the initial activity determined in 0-day-old cheeses. No significant differences in nisin Z activity could be detected between cheeses with added Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (ISN and LISN) throughout ripening. However, the encapsulation of nisin in liposomes largely improved its stability in the cheese matrix; nisin activity decreased only slightly to 263 ± 10 IU/g after 2 months, representing 90% of the initial activity, and remained stable until the end of the ripening period.
FIG. 2.
Changes in nisin Z activity (A) and L. innocua viable counts (B) during ripening of cheddar cheeses made with a nisin Z-producing culture (dark bars, treatment ISN; clear bars, treatment LISN) or encapsulated nisin Z (gray bars, treatment LLN) and of nisin-free cheddar cheese (hatched bars, treatment LNF). Means within the same group of bars without a common letter are significantly different (P < 0.05) by the PLSD test.
Lactococcal and L. innocua populations during cheddar cheese storage.
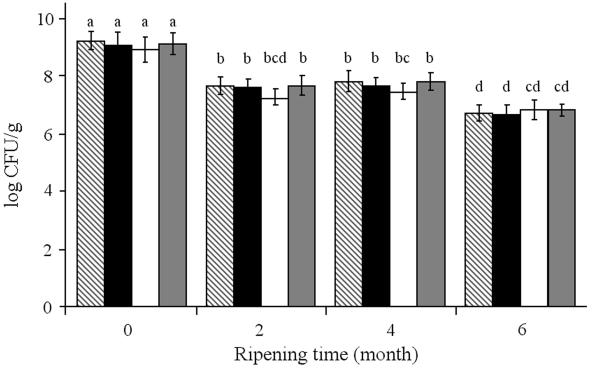
Figure 3 shows the viable cell counts for lactococci in control and experimental cheeses during 6 months of ripening. The initial numbers of lactococci did not differ significantly (P < 0.05) among cheeses. During ripening there was a gradual decrease in viable lactococci which was similar for control and experimental cheeses (P < 0.05).
FIG. 3.
Changes in lactococcal viable cell counts during ripening of nisin-free cheddar cheese (hatched bars, treatment LNF) and of cheddar cheeses with an added nisin Z-producing strain (dark bars, treatment ISN; open bars, treatment LISN) or with encapsulated nisin Z (gray bars, treatment LLN). Means within the same group of bars without a common letter are significantly different (P < 0.05) by the PLSD test.
The survival of L. innocua in cheeses manufactured with Lactococcus lactis subsp. lactis biovar diacetylactis UL719 (LISN) or encapsulated nisin Z (LLN) was monitored for 6 months of ripening and compared with that in nisin-free L. innocua-containing cheese (LNF), as shown in Fig. 2B. Following production, 1.5- and 3.0-log-unit reductions in L. innocua viable counts were observed in cheeses made with Lactococcus lactis subsp. lactis biovar diacetylactis UL719 and encapsulated nisin, respectively, compared with nisin-free L. innocua-containing cheeses. During ripening, the number of viable L. innocua organisms decreased gradually in all cheeses. Moreover, cheeses subjected to the LISN and LLN treatments showed significantly lower L. innocua viable counts than LNF cheese, but the viable L. innocua counts were always at least 1.2 log units lower for liposome-containing cheeses (LLN) than for Lactococcus lactis subsp. lactis biovar diacetylactis UL719-containing cheeses. After 6 months, the viable counts of L. innocua in liposome-containing cheeses were below the detection limit of the method (10 CFU/g), whereas they were 3.7 × 104 and 4.3 × 104 CFU/g, respectively, in LISN and LNF cheeses.
DISCUSSION
In this study, a mixed starter culture containing the nisin Z-producing strain Lactococcus lactis subsp. lactis biovar diacetylactis UL719 was developed and used for cheddar cheese production at a total inoculation level of 2% (vol/vol). This starter mixture was composed of three lactococci, Lactococcus lactis subsp. cremoris KB, Lactococcus lactis subsp. lactis KB, and Lactococcus lactis subsp. lactis biovar diacetylactis UL719, at a ratio of 1.5:1.5:1 (vol/vol/vol) for high nisin and acid production, as previously suggested (5, 20, 30, 42). The level of nisin Z produced by this mixed culture (306 IU/ml) remained within the recommended range of from 250 to 300 IU/g for controlling spoilage organisms in various cheeses (39).
The composition data for control and experimental cheeses on the day of manufacture indicated that the nisin Z-producing strain did not significantly affect cheese composition. However, liposome-treated cheeses (LLN cheeses) contained slightly higher moisture levels (P < 0.05) than control cheeses. An increased moisture content of liposome-supplemented cheeses has been previously reported for cheddar cheese (22, 26) and has been attributed to water binding at the surface of the liposome membrane.
The encapsulation of nisin in phospholipid vesicles protected the starter culture from the inhibiting effects of nisin, as indicated by the times needed to reach pH 5.2 ± 0.1. These times were similar for liposome-containing and control cheeses, even though the milk used for manufacturing liposome-containing cheese (LLN) contained a high nisin Z concentration of 300 IU/ml at the inoculation step. To date, few attempts to encapsulate bacteriocins in liposome vesicles have been made. Pediocin AcH has been encapsulated in multilamellar vesicles made of phosphatidylcholine with an entrapment efficiency of 18% (7). Those authors suggested that the use of higher-melting-point lipids or varying capsule polarities might provide a powerful tool for more efficient delivery of bioactive agents in food systems. Based on this study, we recently developed and optimized the encapsulation of nisin Z in liposomes prepared from pro-lipo H, which were made from hydrogenated phosphatidylcholine (Laridi et al., submitted). The increase of nisin Z concentration from 0.1 to 5 mg/ml correlated with an increased entrapment efficiency of from 34 to 47%. This phenomenon was attributed to the insertion and/or immobilization of nisin Z in liposome membranes. The insertion of nisin molecules in lipid membranes has been found to disturb the amphiphilic balance of the lipid membrane by increasing the hydrophobic contribution; as a consequence, this would favor the nonlamellar inverted phase (14). This might explain the formation of unlamellar structures observed at the beginning of ripening.
The nisin Z activity and stability data during ripening of experimental cheddar cheeses produced with a nisinogenic mixed starter reported in our study are in accordance with those reported previously for Gouda cheese (5). The loss of nisin activity during storage has been observed in several food systems and seems to be correlated with nisin degradation (8, 9). The mechanism of nisin degradation is complex and has not been characterized yet. In a cheese matrix, nisin stability is highly influenced by cheese components and storage conditions (41). The decline in nisin activity produced by Lactococcus lactis subsp. lactis biovar diacetylactis UL719 during ripening may be due to the action of proteolytic enzymes or nisinase from lactic acid bacteria, which may act on nisin peptides and result in the degradation of the active peptide (27). Furthermore, the diffusion of nisin molecules to the fat phase of the cheese matrix may also partly contribute to the observed decline in nisin activity in cheese. This is because the nisin in fat may not be recovered during the preparation of cheese samples for nisin activity assay (5). On the other hand, the higher stability of encapsulated nisin may be attributed to its maintenance at a high concentration and purity inside liposomal vesicles or immobilized on liposomal membranes (Laridi et al., submitted). By using TEM and monoclonal anti-nisin Z antibodies, nisin was shown to be encapsulated in the internal aqueous phase of liposome vesicles and immobilized along the inner hydrophobic liposome membrane. It appears that both forms, encapsulated and immobilized, can protect the nisin peptide by reducing its accessibility to inhibitory substances and other detrimental conditions present in the surrounding medium. For example, this system can be used to deliver nisin for the control of spoilage and pathogenic bacteria in cheeses with a high pH, where nisin activity is highly reduced.
It appears that counts of previously selected lactococci were not related to the nisin Z content of cheeses. Others have also shown that starter culture cell populations are at a maximum during or shortly after manufacture and decline thereafter (33). Although the initial concentrations of nisin Z in Lactococcus lactis subsp. lactis biovar diacetylactis UL719- and liposome-containing cheeses were very similar (300 ± 12 IU/g of cheese), encapsulated nisin was much more effective at inhibiting L. innocua than UL719. Several studies have reported the use of nisin-producing starters to inhibit pathogens in cheese (35, 36). This strategy showed variable effects, ranging from complete to no inhibition for the same pathogen. For example, the use of nisin-producing cultures provided only partial inhibition of L. monocytogenes in feta cheese and had no effect on the same organism in Camembert cheese (35). In the present study, the use of Lactococcus lactis subsp. lactis biovar diacetylactis UL719 in a mixed starter culture did not appear to be the best strategy to inhibit L. innocua in cheddar cheese. However, a mixed culture containing a nisin Z-producing strain might be used for controlling postcontaminant organisms, as they are usually present in low numbers. The effectiveness of encapsulated nisin Z in reducing the viability of L. innocua, compared with nisin produced by Lactococcus lactis subsp. lactis biovar diacetylactis UL719, could be due to its maintenance at a higher concentration and purity. The presence of liposome vesicles or membranes at the fat-casein interface and whey pockets (Fig. 1), where bacteria are concentrated (25), may result in a higher accessibility of nisin to bacterial cells.
In conclusion, the entrapment of nisin in phospholipid vesicles could be a promising alternative for addition of nisin in cheese compared with direct addition of free nisin in milk or the utilization of a mixed starter culture with a nisinogenic strain. Selection of mixed cultures with high acid and nisin production and aromatic properties can be difficult and time-consuming, and the extensive use of this strategy for cheese preservation may lead to the development of nisin-resistant strains. This study demonstrated that cheese production using a nisin-containing starter culture could be successfully performed if the composition of the mixed starter is optimized. The effectiveness of liposome-encapsulated purified nisin Z in inhibiting L. innocua in a cheddar cheese matrix proved to be useful for improving nisin stability and controlling contaminating bacteria in cheddar cheese. Further research will be required to determine the parameters responsible for long-term stability and availability of encapsulated nisin Z.
Acknowledgments
This research was carried out within the program of the Canadian Research Network on Lactic Acid Bacteria, supported by the National Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada, Novalait Inc., The Dairy Farmers of Canada, Rosell-Lallemand Inc., and the Fond pour les Chercheurs et l'Avancement de la Recherche from the Province of Quebec.
REFERENCES
- 1.Abad, A., K. R. Cease, and R. A. Blanchette. 1987. A rapid technique using epoxy resin Quetol 651 to prepare woody plant tissue for ultrastructural study. Can. J. Bot. 66:677-682. [Google Scholar]
- 2.AOAC. 1990. Official methods of analysis, 15th ed., p. 840-850. AOAC International, Arlington, Va.
- 3.Bartels, H. J., M. E. Johnson, and N. F. Olson. 1987. Accelerated ripening of Gouda cheese. II. Effect of freeze-shocked Lactobacillus helveticus on proteolysis and flavor development. Milchwissenschaft 42:139-144. [Google Scholar]
- 4.Bouksaim, M., I. Fliss, J. Meghrous, and R. E. Simard. 1998. Immunodot detection of nisin Z in milk and whey using enhanced chemoluminescence. J. Appl. Microbiol. 84:176-184. [DOI] [PubMed] [Google Scholar]
- 5.Bouksaim, M., C. Lacroix, P. Audet, and R. E. Simard. 2000. Effect of mixed starter composition on nisin Z production by Lactococcus lactis subsp. lactis biovar. diacetylactis UL719 during production and ripening of Gouda cheese. Int. J. Food Microbiol. 59:141-156. [DOI] [PubMed] [Google Scholar]
- 6.Daoudi, L., C. Turcotte, C. Lacroix, and I. Fliss. 2001. Production and characterization of anti-nisin Z monoclonal antibodies: suitability for distinguishing active form inactive forms through a competitive enzyme immunoassay. Appl. Microbiol. Biotechnol. 56:114-119. [DOI] [PubMed] [Google Scholar]
- 7.Degnan, A. J., and J. B. Luchansky. 1992. Influence of beef tallow and muscle on the antilisterial activity of pediocin AcH and liposome-encapsulated pediocin AcH. J. Food Prot. 55:552-554. [DOI] [PubMed] [Google Scholar]
- 8.Delves-Broughton, J. 1990. Nisin and its application as a food preservative. J. Soc. Dairy Technol. 43:73-76. [Google Scholar]
- 9.Delves-Broughton, J. 1990. Nisin and its uses as food preservative. Food Technol. 44:100-117. [Google Scholar]
- 10.Delves-Broughton, J., and M. Friis. 1998. Nisin preparation—production, specification and assay procedure, in the use of nisin in cheese making, p. 18-19. International Dairy Federation document no. 329. International Dairy Federation, Brussels, Belgium.
- 11.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 12.De Vos, W. M., J. W. Mulders, R. J. Siezen, J. Hugenholtz, and O. P. Kuipers. 1993. Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl. Environ. Microbiol. 59:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djae, A., C. Lacroix, D. Thuault, C. M. Bourgeois, and R. E. Simard. 1995. Characterisation of diacetin B, a bacteriocin from Lactococcus lactis subsp. lactis UL 720. Can. J. Microbiol. 41:832-841. [DOI] [PubMed] [Google Scholar]
- 14.El Jastimi, R., and M. Lafleur. 1999. Nisin promotes the formation of non-lamellar inverted phases in unsaturated phosphatidylethanolamines. Biochim. Biophys. Acta 1418:97-105. [DOI] [PubMed] [Google Scholar]
- 15.Fairchild, T. M., and P. M. Foegeding. 1993. A proposed nonpathogenic biological indicator for thermal inactivation of Listeria monocytogenes. Appl. Environ. Microbiol. 59:1247-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler, G. G., B. Jarvis, and J. Tramer. 1975. The assay of nisin in foods. Soc. Appl. Bacteriol. Tech. Ser. 8:91-105. [Google Scholar]
- 17.Fox, P. F., J. M. Wallace, S. Morgan, C. M. Lynch, E. J. Niland, and J. Tobin. 1996. Acceleration of cheese ripening. Antonie Leeuwenhoek 70:271-297. [DOI] [PubMed] [Google Scholar]
- 18.Gross, E., and J. L. Morell. 1971. The structure of nisin. J. Am. Chem. Soc. 93:4634-4635. [DOI] [PubMed] [Google Scholar]
- 19.Guerzoni, M. E., L. Vannini, C. Chaves Lopez, R. Lanciotti, G. Suzzi, and A. Gianotti. 1999. Effect of high pressure homogenization on microbial and chemico-physical characteristics of goat cheeses. J. Dairy Sci. 82:851-862. [DOI] [PubMed] [Google Scholar]
- 20.Hurst, A. 1981. Nisin. Adv. Appl. Microbiol. 27:85-123. [Google Scholar]
- 21.Kempler, G. M., and L. L. McKay. 1980. Improved medium for detection of citrate fermenting Streptococcus lactis subsp. diacetylactis. Appl. Environ. Microbiol. 4:926-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheadr, E. E., J. C. Vuillemard, and S. A. El Deeb. 2000. Accelerated Cheddar cheese ripening with encapsulated proteinases. Int. J. Food Sci. Technol. 35:483-495. [Google Scholar]
- 23.Kordel, M., and H. G. Sahl. 1986. Susceptibility of bacterial eucaryotic and artificial membranes to the disruptive action of the cationic peptides Pep5 and nisin. FEMS Microbiol. Lett. 34:139-144. [Google Scholar]
- 24.Kosikowski, F. V. 1982. Cheddar cheese, p. 228-260. In F. V. Kosikowski (ed.), Cheese and fermented milk foods, 3rd ed. F. V. Kosikowski and Associates, Brookdontale, N.Y.
- 25.Laloy, E., J. C. Vuillemard, M. El-Soda, and R. E. Simard. 1995. Influence of the fat content of Cheddar cheese on retention and localization of starter. Int. Dairy J. 6:729-740. [Google Scholar]
- 26.Laloy, E., J. C. Vuillemard, and R. E. Simard. 1998. Characterization of liposomes and their effect on the properties of Cheddar cheese during ripening. Lait 78:401-412. [Google Scholar]
- 27.Lipinska, E. 1977. Nisin and its application, p. 103-130. In M. Woodbine (ed.), Antimicrobials and antibiotics in agriculture. Butterworths, London, United Kingdom.
- 28.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:256-275. [PubMed] [Google Scholar]
- 29.Maisner-Patin, S., N. Deschamps, S. R. Tatini, and J. Richard. 1992. Inhibition of Listeria monocytogenes in Camembert cheese made with nisin-producing starter. Lait 72:249-263. [Google Scholar]
- 30.Martìnez-Cuesta, M. C., J. K. Kok, E. Herranz, C. Pealez, T. Requena, and G. Buist. 2000. Requirement of autolytic activity for bacteriocin-induced lysis. Appl. Environ. Microbiol. 66:3174-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattick, A. T., and A. Hirsch. 1956. Manufacture and preservation of cheese. U.S. patent 2,744,827.
- 32.Meghrous, J., C. Lacroix, M. Bouksaim, G. Lapointe, and R. E. Simard. 1997. Genetic and biochemical characterization of nisin Z produced by Lactococcus lactis ssp. lactis biovar. diacetylactis UL719. J. Appl. Microbiol. 83:133-138. [DOI] [PubMed] [Google Scholar]
- 33.Midje, L. D., E. D. Bastian, H. A. Morris, F. B. Martin, T. Bridgeman, and Z. M. Vickers. 2000. Flavor enhancement of reduced fat cheddar cheese using an integrated culturing system. J. Agric. Food Chem. 48:1630-1636. [DOI] [PubMed] [Google Scholar]
- 34.Mulders, J. W., I. J. Boerrigter, H. S. Rollema, R. J. Siezen, and W. M. De Vos. 1991. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant, Eur. J. Biochem. 201:581-584. [DOI] [PubMed] [Google Scholar]
- 35.Ramsaran, H., J. Chen, B. Brunke, A. Hill, and M. W. Griffiths. 1998. Survival of bioluminescent Listeria monocytogenes and Escherichia coli O157:H7 in soft cheeses. J. Dairy Sci. 81:1810-1817. [DOI] [PubMed] [Google Scholar]
- 36.Richard, J. A., T. F. Bozoglu, and B. Ray. 1996. Use of bacteriocin producing starters advantageously in dairy industry, p. 137-154. In T. M. Bozoglu and B. Ray (ed.), Lactic acid bacteria: current advances in metabolism, genetics and applications. Springer-Verlag, Berlin, Germany.
- 37.Roberts, R. F. 1991. Development of a nisin-producing starter culture for use during Cheddar cheese manufacture to inhibit spoilage in high-moisture pasteurized process cheese spreads. Ph.D. thesis. University of Minnesota, Minneapolis.
- 38.Roberts, R. F., E. A. Zottola, and L. L. McKay. 1992. Use of nisin-producing starter culture suitable for Cheddar cheese manufacture. J. Dairy Sci. 75:2353-2363. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, R. F., and E. A. Zottola. 1993. Shelf life of pasteurized process cheese spreads made from Cheddar cheese manufactured with a nisin-producing starter culture. J. Dairy Sci. 76:1829-1836. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez, J. M. 1996. Antimicrobial spectrum, structure, properties and mode of action of nisin, a bacteriocin produced by Lactococcus lactis. Int. J. Food Sci. Technol. 2:61-68. [Google Scholar]
- 41.Rollema, H. S., O. P. Kuipers, P. Both, W. M. De Vos, and R. J. Siezen. 1995. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol. 61:2873-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan, M. P., M. C. Rea, C. Hill, and R. P. Ross. 1996. An application in Cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, Lacticin 3147. Appl. Environ. Microbiol. 62:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens, K. A., B. W. Sheldon, N. A. Klapes, and T. R. Klaenhammer. 1991. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl. Environ. Microbiol. 57:3613-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suàrez, A. M., J. J. Azcona, J. M. Rodriguez, B. Sanz, and P. E. Hernandez. 1997. One step purification of nisin A by immunoaffinity chromatography. Appl. Environ. Microbiol. 63:4990-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Environ. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenbergh, P. A. 1993. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol. Rev. 12:221-238. [Google Scholar]
- 47.Verheul, A., N. J. Russell, R. V. Hof, F. M. Rombouts, and T. Abee. 1997. Modifications of membrane phospholipid composition in nisin-resistant Listeria monocytogenes Scott A. Appl. Environ. Microbiol. 63:3451-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]