Abstract
The death kinetics of Aspergillus niger spores under high-pressure carbonation were investigated with respect to the concentration of dissolved CO2 (dCO2) and treatment temperature. All of the inactivation followed first-order death kinetics. The D value (decimal reduction time, or the time required for a 1-log-cycle reduction in the microbial population) in the saline carbonated at 10 MPa was 0.16 min at 52°C. The log D values were linearly related to the treatment temperature and the concentration of dCO2, but a significant interaction was observed between them.
Heat treatment in food processing is a broadly effective method for inactivating human pathogens and spoilage microorganisms. However, the high temperature involved can cause undesirable changes in nutritional and sensory properties.
The toxicity of CO2 has been demonstrated for a wide range of microorganisms (5, 6, 12, 17). Carbon dioxide, while exerting antimicrobial activity, causes little harm in foods, additives, etc.; therefore, it is a suitable agent for controlling food spoilage microorganisms (22, 23, 29). In recent years, the influence of high-pressure CO2 on the vegetative cells of various species has been demonstrated (1, 3, 4, 7, 8, 9, 10, 11, 13, 14, 18, 20, 28).
We succeeded in dissolving CO2 in an aqueous medium to a nearly saturated level by supplying high-pressure CO2 in microbubbles (high-pressure carbonation). As a consequence, the treatment time and temperature required for microbial inactivation could be substantially reduced (15, 16, 26). Recently, it was shown that the antimicrobial effect was not related to the pressure of CO2 but to the concentration of dissolved CO2 (dCO2) (27). The experiments were carried out with saturated dCO2 concentrations under various combinations of pressure and temperature conditions.
Most spoilages of fruit juice are caused by yeast and mold, because the germination of bacterial spores is inhibited at the pH of fruit juice (30). Shimoda et al. (27) have reported that the inactivation temperature of Saccharomyces cerevisiae was reduced by about 30°C under high-pressure carbonation. As mold spores have only a moderate thermal resistance, the heat treatment in fruit juice production is intended primarily to inactivate mold spores. The effects of pressurized CO2 on the inactivation of mold spores, however, have not been investigated. A mesophilic mold, Aspergillus niger, was used as a test strain because it is a common contaminant of foods and other products (24).
The purpose of the present paper was to investigate separately the effects of dCO2 concentration, treatment pressure, and temperature on the death kinetics of Aspergillus niger spores.
Fungal strain.
Spores of Aspergillus niger (ATCC 16888) were obtained from the Japan Collection of Microorganisms (Wako, Saitama, Japan).
Preparation of the spore suspension of A. niger.
The strain was cultured on potato dextrose agar plates (Eiken Chemical Co., Ltd., Tokyo, Japan) at 25°C over 10 days. Spores were collected by washing the surface of the culture. Finally, a spore suspension (about 108 to 109 CFU/ml) was prepared in physiological saline with 0.005% Tween 20.
High-pressure carbonation treatment.
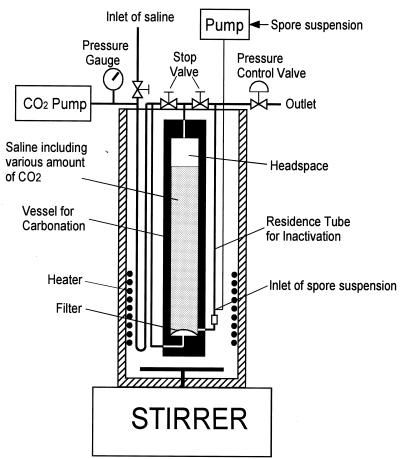
To simplify controlling the treatment temperature and dCO2 concentration, an original apparatus (Fig. 1) was constructed. At first, 200 ml of physiological saline was introduced into a vessel for carbonation. Microbubbles of pressurized CO2 were dispersed into the saline solution from a stainless steel filter (pore size, 10 μm) attached to the bottom of the carbonation vessel. After the carbonation, the solution was left to stand for about 10 min under pressure and then pressed out by introducing pressurized CO2 into the headspace. A suspension of A. niger spores (∼108 to ∼109 CFU/ml) was introduced into the flow of carbonated saline at a flow rate of 0.33 to 2.8 ml/min. The total flow rate of the carbonated saline and spore suspension ranged from 3.3 to 28 ml/min. After the carbonated saline containing the spores flowed through a residence tube (4-mm inside diameter, 56 cm in depth), it was decompressed via a pressure control valve, with the pressure being kept within ±0.1 MPa. It took only a few milliseconds to decompress the stream of carbonated spore suspension, since the entire stream flowed through the pressure-regulating port (about 1 mm2) under propulsion by a spring. The apparatus was placed in a water bath to keep the treatment temperature within ±0.2°C. The volume of gaseous CO2 generated from the carbonated spore suspension was measured in the outlet of the pressure control valve. Treatment conditions were as follows: pressure was at 5, 8, 10, 15, and 19 MPa; temperatures were at 44, 46, 48, 50, and 52°C; inactivation times were 0.25 to 2.1 min; and dCO2 concentrations wre 0 to 24.2 γ. The concentration of dCO2 was estimated as a Kuenen gas absorption coefficient (γ); e.g., a γ value of 24 means that there were 24 volumes of CO2 (in a normal state) in 1 volume of liquid.
FIG. 1.
Schematic diagram of the apparatus for microbial inactivation under high-pressure carbonation.
Enumeration of spore counts.
Samples were serially diluted with sterile physiological saline. Initial spore counts and counts of survivors were determined by plating 0.1-ml diluted or nondiluted samples on triplicate plates of potato dextrose agar (Eiken Chemical Co.). Colonies were counted after incubation at 30°C for 3 days.
Replication and statistical treatment.
All experiments were done in triplicate. The data presented are the means of results of three replicate experiments.
Kinetic parameters for inactivation of microbial cells under high-pressure carbonation.
The approach to describing changes in microbial populations as a function of time uses the survivor curve equation log [N/N0] = −t/D, where N is the microbial population at any time (t), N0 is the initial microbial population, and D is the decimal reduction time, or time required for a 1-log-cycle reduction in the microbial population.
The influence of temperature on the inactivation rates under high-pressure carbonation was expressed in terms of the thermal resistance constant [ZCO2(T)] with the model log [D/DT0] = −(T − T0)/ZCO2(T).
The reference decimal reduction time (DT0) is the magnitude at a reference temperature (T0) under a given concentration of dCO2. The thermal resistance constant 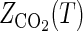 is the temperature increase needed to accomplish a 1-log-cycle reduction in the D value at a given dCO2 concentration.
is the temperature increase needed to accomplish a 1-log-cycle reduction in the D value at a given dCO2 concentration.
The influence of the dCO2 concentration on the inactivation rates was expressed in terms of the CO2 resistance constant [Ztemperature(γ)] using the following model: log [D/Dγ0] = −(γ − γ0)/Ztemperature(γ).
The reference decimal reduction time (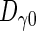 ) is the magnitude under a reference concentration (γ0) of dCO2 at a given temperature. The CO2 resistance constant
) is the magnitude under a reference concentration (γ0) of dCO2 at a given temperature. The CO2 resistance constant 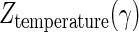 is the increase in the concentration of dCO2 needed to accomplish a 1-log-cycle reduction in the D value at a given temperature.
is the increase in the concentration of dCO2 needed to accomplish a 1-log-cycle reduction in the D value at a given temperature.
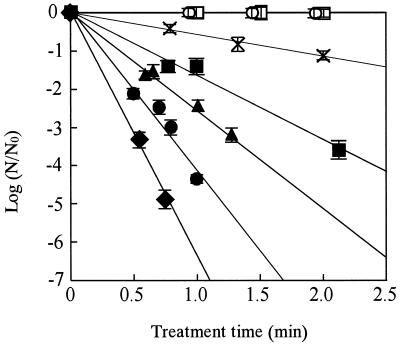
Figure 2 shows the survival curves of A. niger spores in the saline carbonated with microbubbles of CO2 at 10 MPa. The relationships between the values of 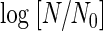 and treatment time strongly suggested first-order death kinetics. The concentration of dCO2 in the treated medium was 24.4 γ for 44°C, 24.2 γ for 46°C, 23.0 γ for 48°C, 21.7 γ for 50°C, and 22.9 γ for 52°C. These values ranged from 87 to 90% of the saturated levels estimated from the data of Seidell and Linke (25) and were reasonable values if we take into account a 10% dilution with spore suspension.
and treatment time strongly suggested first-order death kinetics. The concentration of dCO2 in the treated medium was 24.4 γ for 44°C, 24.2 γ for 46°C, 23.0 γ for 48°C, 21.7 γ for 50°C, and 22.9 γ for 52°C. These values ranged from 87 to 90% of the saturated levels estimated from the data of Seidell and Linke (25) and were reasonable values if we take into account a 10% dilution with spore suspension.
FIG. 2.
Inactivation behaviors of A. niger spores in carbonated saline. The saline was carbonated at 10 MPa, and the concentrations of dCO2 were ∼21.7 to ∼24.2 γ. Symbols indicate results of experiments in carbonated saline at 44°C (×), 46°C (▪), 48°C (▴), 50°C (•), and 52°C (⧫); in physiological saline at 52°C (○); and in McIlvaine buffer (pH 3.0) at 52°C (□)
The D values of A. niger spores in carbonated saline at 10 MPa were estimated from the slopes of straight lines in Fig. 2. The values were 0.16 min for 52°C, 0.24 min for 50°C, and 0.39 min for 48°C (Table 1). On the other hand, the spores in physiological saline and McIlvaine buffer (pH 3.0) without CO2 were not killed at 52°C in the limited times.
TABLE 1.
Decimal reduction time, D value, in the inactivation of A. niger spores under high-pressure carbonation
| Temp (°C) | dCO2 concn (γ) | D (min) |
|---|---|---|
| 44 | 24.4 | 1.74 |
| 46 | 24.2 | 0.60 |
| 48 | 23.0 | 0.39 |
| 50 | 21.7 | 0.24 |
| 52 | 22.9 | 0.16 |
| 52a | 0 | |
| 52b | 0 |
Physiological saline without CO2.
McIlvaine buffer (pH 3.0) without CO2.
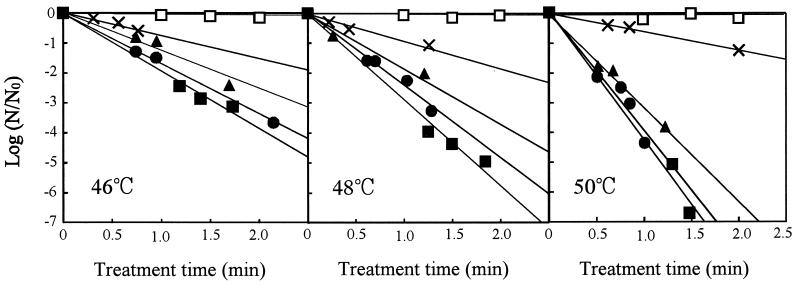
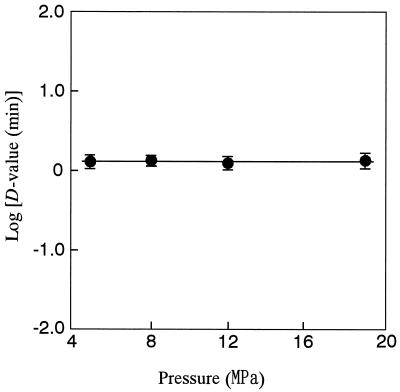
The effect of treatment pressure on the inactivation of A. niger spores is shown in Fig. 3. The spore suspension was introduced into saline carbonated at 5, 8, 10, and 19 MPa. In every treatment, the plots of the values of 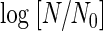 against treatment time showed a linear relationship. The slopes of three survival curves under carbonation at 5 MPa gave almost the same value independently of the treatment temperature, but the slopes became steep as the treatment pressure and temperature increased. On the other hand, D values in Fig. 4 show no significant difference at a fixed dCO2 concentration (γ = 15.5), independently of the treatment pressure. This result indicated that the antimicrobial activity of CO2 was dependent on the concentration of dCO2 and not on the treatment pressure. Thus, the increase in the dCO2 concentration was found to cause the increase in the inactivation rates at a given temperature, as shown in Fig. 3.
against treatment time showed a linear relationship. The slopes of three survival curves under carbonation at 5 MPa gave almost the same value independently of the treatment temperature, but the slopes became steep as the treatment pressure and temperature increased. On the other hand, D values in Fig. 4 show no significant difference at a fixed dCO2 concentration (γ = 15.5), independently of the treatment pressure. This result indicated that the antimicrobial activity of CO2 was dependent on the concentration of dCO2 and not on the treatment pressure. Thus, the increase in the dCO2 concentration was found to cause the increase in the inactivation rates at a given temperature, as shown in Fig. 3.
FIG. 3.
Inactivation behaviors of A. niger spores in carbonated (closed symbols) and noncarbonated (□) saline. Carbonations were carried out to the saturated levels at 5 MPa (×), 8 MPa (▴), 10 MPa (•), and 19 MPa (▪).
FIG. 4.
Effect of the treatment pressure on the inactivation of A. niger spores at a fixed concentration (15.5 γ) of dCO2. The treatment temperature was 50°C.
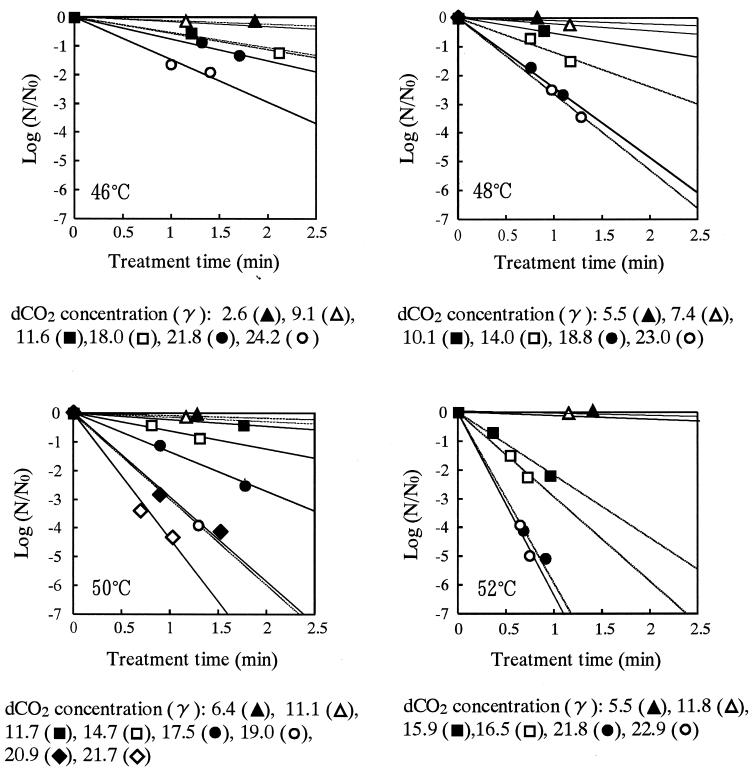
To elucidate the effects of the concentration of dCO2 and treatment temperature on the CO2 inactivation of A. niger spores, treatments were done with various combinations of dCO2 concentration and treatment temperature. The plots of the values of 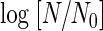 against treatment time suggested that they have a linear relationship (Fig. 5). Comparison of these four plots seemed to suggest the existence of a significant interaction between the effects of treatment temperature and dCO2 concentration on inactivation.
against treatment time suggested that they have a linear relationship (Fig. 5). Comparison of these four plots seemed to suggest the existence of a significant interaction between the effects of treatment temperature and dCO2 concentration on inactivation.
FIG. 5.
Effect of dCO2 concentration on the inactivation of A. niger spores under carbonation at 10 MPa.
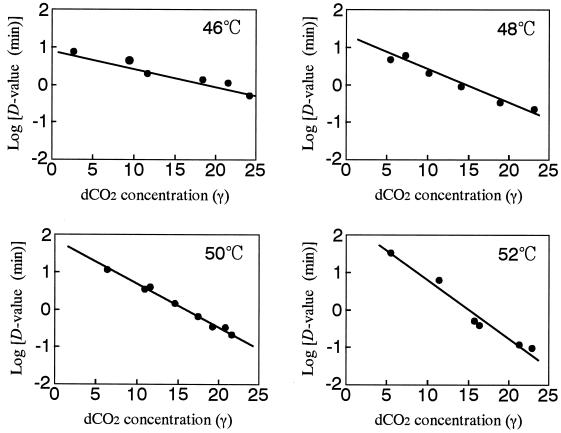
Figure 6 shows the plots of log D values against the concentration of dCO2 at 46, 48, 50, and 52°C. These plots gave linear relationships, and the slopes increased as the treatment temperature increased. These results indicated that the CO2 sensitivity of A. niger spores was consistent in the wide range of the dCO2 concentration at a given temperature but that the sensitivity increased as the treatment temperature increased. The CO2 resistance constants, 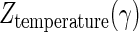 , estimated from the slopes of straight lines in Fig. 6 are listed in Table 2. The values were significantly influenced by the treatment temperature and decreased from 21.8 γ at 46°C to 6.4 γ at 52°C. This demonstrated that the CO2 sensitivity increased as the treatment temperature increased.
, estimated from the slopes of straight lines in Fig. 6 are listed in Table 2. The values were significantly influenced by the treatment temperature and decreased from 21.8 γ at 46°C to 6.4 γ at 52°C. This demonstrated that the CO2 sensitivity increased as the treatment temperature increased.
FIG. 6.
Relationship between the log D value and dCO2 concentration.
TABLE 2.
CO2 resistance constant, Ztemperature(γ), in the inactivation of A. niger spores under high-pressure carbonation
| Temp (°C) | Ztemperature(γ) |
|---|---|
| 46 | 21.8 |
| 48 | 11.5 |
| 50 | 8.4 |
| 52 | 6.4 |
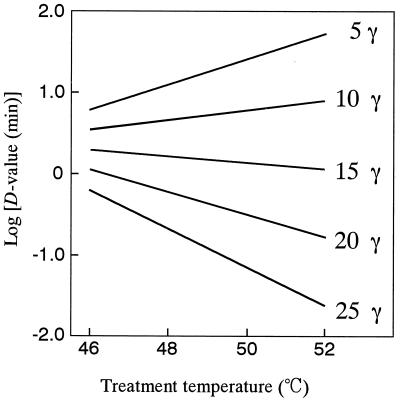
The CO2 resistance constant [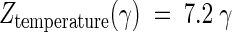 ] for the inactivation of S. cerevisiae under high-pressure carbonation was independent of treatment temperature (27). On the other hand, the CO2 resistance constant for A. niger spores was significantly dependent on the treatment temperature (Table 2). This might be attributed to structural and physiological differences between S. cerevisiae cells, which are living cells, and A. niger spores, which are resting cells. To make clear the interaction between CO2 and thermal resistance constants, the log D values were plotted against the treatment temperature, with the concentration of dCO2 being taken as a parameter. Figure 7 was constituted from the values on the straight lines in Fig. 6 and shows a very interesting profile of the inactivation of A. niger spores under high-pressure carbonation. That is, in the low dCO2 concentration (γ < 10), the log D values linearly increased with the increase in the treatment temperature. This phenomenon is contradictory to what is commonly known about pasteurization. The treatments under the conditions of lower pressure and lower temperature might have some advantages with respect to food quality as well as equipment cost, although the antimicrobial activity was quite limited and thus a prolonged treatment time was needed. At the medium concentration of dCO2 (γ value of 10 to 20), the D values were hardly influenced by the treatment temperature. At the high concentration (γ > 20), the log D values linearly decreased with the increase in the treatment temperature. In the carbonated saline at 52°C and 25 γ, the D value of A. niger spores was reduced by about four cycles in a log scale compared with the value in noncarbonated medium. The values of the thermal resistance constant (
] for the inactivation of S. cerevisiae under high-pressure carbonation was independent of treatment temperature (27). On the other hand, the CO2 resistance constant for A. niger spores was significantly dependent on the treatment temperature (Table 2). This might be attributed to structural and physiological differences between S. cerevisiae cells, which are living cells, and A. niger spores, which are resting cells. To make clear the interaction between CO2 and thermal resistance constants, the log D values were plotted against the treatment temperature, with the concentration of dCO2 being taken as a parameter. Figure 7 was constituted from the values on the straight lines in Fig. 6 and shows a very interesting profile of the inactivation of A. niger spores under high-pressure carbonation. That is, in the low dCO2 concentration (γ < 10), the log D values linearly increased with the increase in the treatment temperature. This phenomenon is contradictory to what is commonly known about pasteurization. The treatments under the conditions of lower pressure and lower temperature might have some advantages with respect to food quality as well as equipment cost, although the antimicrobial activity was quite limited and thus a prolonged treatment time was needed. At the medium concentration of dCO2 (γ value of 10 to 20), the D values were hardly influenced by the treatment temperature. At the high concentration (γ > 20), the log D values linearly decreased with the increase in the treatment temperature. In the carbonated saline at 52°C and 25 γ, the D value of A. niger spores was reduced by about four cycles in a log scale compared with the value in noncarbonated medium. The values of the thermal resistance constant (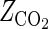 ) estimated from the slopes in Fig. 7 are listed in Table 3. These values indicated that the thermal sensitivity became larger as the concentration of dCO2 increased. As shown in Fig. 7, there was a significant interaction between the treatment temperature and dCO2 concentration. To explain the interaction is very difficult, but it might be caused by the difference in the levels of temperature dependence on the distribution of CO2 between the medium and spores and the thermal sensitivity of the spores under high-pressure carbonation.
) estimated from the slopes in Fig. 7 are listed in Table 3. These values indicated that the thermal sensitivity became larger as the concentration of dCO2 increased. As shown in Fig. 7, there was a significant interaction between the treatment temperature and dCO2 concentration. To explain the interaction is very difficult, but it might be caused by the difference in the levels of temperature dependence on the distribution of CO2 between the medium and spores and the thermal sensitivity of the spores under high-pressure carbonation.
FIG. 7.
Effect of the interaction between the treatment temperature and dCO2 concentration on the log D values of A. niger spores under carbonation.
TABLE 3.
Thermal resistance constant, ZCO2(T), in the inactivation of A. niger spores under high-pressure carbonation
| dCO2 concn (γ) | ZCO2(T) |
|---|---|
| 5 | −6.1 |
| 10 | −14.7 |
| 15 | 31.0 |
| 20 | 7.2 |
| 25 | 4.2 |
The antimicrobial effects of compressed CO2 have been extensively studied to elucidate the mechanism. In such studies, the death of target cells has been variously explained as follows: the acidification by dCO2 may cause the inactivation of key enzymes related to the essential metabolic process (1, 5, 6, 7, 13, 18), the extraction of intracellular substances such as hydrophobic compounds in the cell wall and cytoplasmic membrane may result in microbial death (18), cell rupture due to the expansion of CO2 within the cells may induce a loss of viability (3, 26), the damage to the cell membrane due to swelling with compressed CO2 may kill the cells, or an “anesthesia effect” may induce the inhibition of metabolic systems (13, 14, 27). Authors have demonstrated that the inactivation power of CO2 was much greater in a continuous treatment (sudden decompression) under high-pressure carbonation than in a batch treatment (slow decompression) (26). In the continuous treatments, the enhancement of the inactivation power could be attributed to cell bursting due to sudden expansion of compressed CO2 in the cells. Debs-Louka et al. (3) observed no significant difference in viability after a subcritical CO2 treatment with three different decompression times, but they described how rapid decompression under supercritical conditions could provoke cell rupture.
As alternative explanations for the antimicrobial effects of CO2, it will be worthwhile to consider the decrease of intracellular pH which is induced by ready penetration of CO2 into the cells and by its dissociation within the cells, as well as the anesthesia effect due to the accumulation of CO2 in the cytoplasmic membrane. These inactivation mechanisms could be attributed to the specific effects of CO2 compared to the effects of other organic acids used as an acidulants (2, 9, 19, 21). They may also affect microbial inactivation during high-pressure carbonation.
To elucidate the inactivation mechanism, it will be important to differentiate the inactivation caused by sudden decompression from that which occurs during high-pressure carbonation.
In conclusion, the inactivation of A. niger spores under high-pressure carbonation followed first-order death kinetics. The separate and combined effects of temperature and dCO2 concentration on inactivation were elucidated. These results permit an estimation of the D value for the inactivation of A. niger spores at any temperature and any dCO2 concentration. The high-pressure carbonation treatment could be realized in fruit juice processing, because there is no problem in incorporating such a treatment into a commercial process.
REFERENCES
- 1.Ballestra, P., A. A. Da Silva, and J. L. Cuq. 1996. Inactivation of Escherichia coli by carbon dioxide under pressure. J. Food Sci. 61:829-836. [Google Scholar]
- 2.Becker, Z. E. 1933. A comparison between the action of carbonic acid and other acids upon the living cell. Protoplasma 25:161-175. [Google Scholar]
- 3.Debs-Louka, E., N. Louka, G. Abraham, V. Chabot, and K. Allaf. 1999. Effect of compressed carbon dioxide on microbial cell viability. Appl. Environ. Microbiol. 65:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillow, A. K., F. Dehghani, J. S. Hrkach, N. R. Foster, and R. Langer. 1999. Bacterial inactivation by using near- and supercritical carbon dioxide. Proc. Natl. Acad. Sci. USA 96:10344-10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon, N. M., and D. B. Kell. 1989. The inhibition by CO2 of the growth and metabolism of micro-organisms. J. Appl. Bacteriol. 67:109-136. [DOI] [PubMed] [Google Scholar]
- 6.Donald, J. R., C. L. Jones, and A. R. M. MacLean. 1924. The effect of carbonation on bacteria in beverages. Am. J. Public Health 14:122-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enomoto, A., K. Nakamura, K. Nagai, T. Hashimoto, and M. Hakoda. 1997. Inactivation of food microorganisms by high-pressure carbon dioxide treatment with or without explosive decompression. Biosci. Biotechnol. Biochem. 61:1133-1137. [DOI] [PubMed] [Google Scholar]
- 8.Enomoto, A., K. Nakamura, M. Hakoda, and N. Amaya. 1997. Lethal effect of high-pressure carbon dioxide on a bacterial spores. J. Ferment. Bioeng. 83:305-307. [Google Scholar]
- 9.Erkmen, O. 2000. Antimicrobial effects of pressurised carbon dioxide on Brochothrix thermosphacta in broth and foods. J. Sci. Food Agric. 80:1365-1370. [Google Scholar]
- 10.Erkmen, O. 2000. Antimicrobial effect of pressurised carbon dioxide on Enterococcus faecalis in physiological saline and foods. J. Sci. Food Agric. 80:465-470. [Google Scholar]
- 11.Erkmen, O. 2001. Kinetic analysis of Listeria monocytogenes inactivation by high pressure carbon dioxide. J. Food Eng. 47:7-10. [Google Scholar]
- 12.Haas, G. J., J. R. Prescott, E. Dudley, R. Dik, C. Hintlian, and L. Keane. 1989. Inactivation of microorganisms by carbon dioxide under pressure. J. Food Safety 9:253-265. [Google Scholar]
- 13.Hong, S.-I., and Y.-R. Pyun. 1999. Inactivation kinetics of Lactobacillus plantarum by high pressure carbon dioxide. J. Food Sci. 64:728-733. [Google Scholar]
- 14.Isenschmid, A., I. W. Marison, and U. Stocker. 1995. The influence of pressure and temperature of compressed CO2 on the survival of yeast cells. J. Biotechnol. 39:229-237. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa, H., M. Shimoda, H. Shiratsuchi, and Y. Osajima. 1995. Sterilization of microorganisms by the supercritical carbon dioxide micro-bubble method. Biosci. Biotechnol. Biochem. 59:1949-1950. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, H., M. Shimoda, K. Tamaya, A. Yonekura, T. Kawano, and Y. Osajima. 1997. Inactivation of Bacillus spores by the supercritical carbon dioxide micro-bubble method. Biosci. Biotechnol. Biochem. 61:1022-1023. [DOI] [PubMed] [Google Scholar]
- 17.Jones, R. P., and P. F. Greenfield. 1982. Effects of carbon dioxide on yeast growth and fermentation. Enzyme Microb. Technol. 4:210-223. [Google Scholar]
- 18.Kamihira, M., M. Taniguchi, and T. Kobayashi. 1987. Sterilization of microorganisms with supercritical carbon dioxide. Agric. Biol. Chem. 51:407-412. [Google Scholar]
- 19.King, J. S., and L. A. Mabitt. 1982. Preservation of raw milk by the addition of carbon dioxide. J. Dairy Res. 49:439-447. [Google Scholar]
- 20.Kumagai, H., C. Hata, and K. Nakamura. 1997. CO2 sorption by microbial cells and sterilization by high-pressure CO2. Biosci. Biotechnol. Biochem. 61:931-935. [Google Scholar]
- 21.Lin, H.-M., Z. Yang, and L.-F. Chen. 1993. Inactivation of Leuconostoc dextranicum with carbon dioxide under pressure. Chem. Eng. J. 52:B29-B34. [Google Scholar]
- 22.Molin, G. 1983. The resistance to carbon dioxide of some food related bacteria. Eur. J. Appl. Microbiol. Biotechnol. 18:214-217. [Google Scholar]
- 23.Molin, G., I.-M. Stenstrom, and A. Termstrom. 1983. The microbiological flora of herring fillets after storage in carbon dioxide, nitrogen or air at 2°C. J. Appl. Bacteriol. 55:49-56. [DOI] [PubMed] [Google Scholar]
- 24.Samson, R. A., E. S. Hoekstra, J. C. Frisvad, and O. Filtenborg. 1995. Introduction to food-borne fungi, p. 64-65. Centraalbureau voor Schimmelculture, Baarn, The Netherlands.
- 25.Seidell, A., and W. F. Linke. 1958. Solubilities of inorganic and metal organic compounds, 4th ed., vol. I and II. D. Van Nostrand Company, Princeton, N.J.
- 26.Shimoda, M., Y. Yamamoto, J. Cocunubo-Castellanos, H. Tonoike, T. Kawano, H. Ishikawa, and Y. Osajima. 1998. Antimicrobial effects of pressured carbon dioxide in a continuous flow system. J. Food Sci. 63:709-712. [Google Scholar]
- 27.Shimoda, M., J. Cocunubo-Castellanos, H. Kago, M. Miyake, Y. Osajima, and I. Hayakawa. 2001. The influence of dissolved CO2 concentration on the death kinetics of Saccharomyces cerevisiae. J. Appl. Microbiol. 91:306-311. [DOI] [PubMed] [Google Scholar]
- 28.Wei, C. I., M. O. Balaban, S. Y. Fernando, and A. J. Peplow. 1991. Bacterial effect of high pressure CO2 treatment on foods spiked with Listeria or Salmonella. J. Food Prot. 54:189-193. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe, S. K. 1980. Use of CO- and CO2-enriched atmospheres for meats, fish, and produce. Food Technol. 34:55-58. [Google Scholar]
- 30.Zook, C. D., M. E. Parish, R. J. Braddock, and M. O. Balaban. 1999. High pressure inactivation kinetics of Saccharomyces cerevisiae ascospores in orange and apple juices. J. Food Sci. 64:533-535. [Google Scholar]