Abstract
This study presents the successful immobilization of a methotrexate-palladium complex onto the surface of magnetic CoFe2O4 nanoparticles through a simple and cost-efficient process. The environmentally friendly heterogeneous catalyst was comprehensively characterized using thermogravimetric analysis (TGA), energy dispersive spectroscopy (EDS), scanning electron microscopy (SEM), X-ray diffraction (XRD), vibrating sample magnetometry (VSM), inductively coupled plasma (ICP) atomic absorption spectroscopy, and Fourier transform infrared (FT-IR) spectroscopy. The catalyst demonstrated outstanding catalytic efficiency, achieving a 98% yield in the reduction of nitroarenes and facilitating the synthesis of diaryl sulfide derivatives under green conditions. Offering a practical and economical approach for immobilizing palladium-methotrexate complexes, this method serves as a promising alternative to conventional techniques in diaryl sulfide synthesis. Its advantages include easy recovery and reusable performance over four successive cycles. This catalyst holds significant potential for applications in both pharmaceutical and chemical industries.
Keywords: Sulfides, Cross‑coupling, Methotrexate, CoFe2O4
Subject terms: Chemistry, Catalysis, Inorganic chemistry
Introduction
The palladium-catalyzed coupling reaction between phenyl boronic acid and aryl halides offers an exceptionally efficient synthetic route for producing symmetric biaryls. This method has found extensive application in the synthesis of polymers, ligands, and pharmaceutical compounds1,2. Transition metal-catalyzed cross-coupling reactions are now widely recognized as a pivotal approach for forming both carbon–sulfur and carbon–carbon bonds. Known for its reliability and prominence in the field, this technique is a cornerstone of modern organic synthesis3,4. Among nanocatalysts, palladium is particularly notable for its versatility in facilitating C–C bond formation, especially in Sonogashira, Stille, and Suzuki coupling reactions, making it the preferred choice in these processes5–9. The creation of carbon–sulfur and carbon–carbon bonds remains an essential and adaptable strategy for generating a diverse array of compounds utilized in agriculture, pharmaceuticals, and electronic materials across the chemical industry10–13.
In recent years, various magnetic nanoparticles with efficient recycling and separation capabilities have been developed and utilized as catalysts for synthesizing a wide range of organic compounds. These nanoparticles are particularly notable for their ability to be easily separated from reaction mixtures using a simple bar magnet14–17. Among the different heterogeneous catalysts, CoFe2O4 nanoparticles have attracted considerable attention due to their simple synthesis methods and the versatility of their surface modification18,19.
In the present study, the use of methotrexate-palladium-supported CoFe2O4@SiO2 nanoparticles as an effective catalyst for the reduction of nitroarenes and the synthesis of diaryl sulfide derivatives has been investigated. Due to the inherent large surface area and magnetic properties of CoFe2O4 nanoparticles, this catalyst can significantly facilitate chemical reactions. The results of the study have shown that the aforementioned nanoparticles with palladium catalyst have high efficiency in the conversion of nitroarenes to aryl amines and help accelerate the reactions. Also, in this study, the synthesis of diaryl sulfides using nanoparticle catalytic methods has been effectively investigated. The findings indicate that this catalytic system with the ability to recycle and reuse can be a sustainable and cost-effective option for industrial and pharmaceutical applications. The quality of the final products has also been significantly improved using this catalyst. Overall, this study shows that methotrexate-palladium-supported CoFe2O4@SiO2 nanoparticles can be used as an efficient catalyst in organic chemistry and industrial processes.
Experimental
Preparation of CoFe2O4@SiO2-Pr-Mth-Pd
To synthesize CoFe2O4 nanoparticles (NPs), 10 mmol of Co(NO3)2·4H2O and 20 mmol of FeCl2·4H2O were prepared and mixed, followed by heating the mixture in a 70 °C water bath for 30 min. Subsequently, 5 g of NaOH were added, and the solution was stirred. The resulting particles were collected, washed several times with water, and dried at 60 °C, as depicted in Scheme 1. In the subsequent step, 2.0 g of the prepared CoFe2O4 were dispersed in 50 mL of ethanol (EtOH). To this solution, 4 g of polyethylene glycol (PEG) and 3 mL of tetraethyl orthosilicate (TEOS) were introduced, followed by stirring at room temperature for 24 h. The CoFe2O4@SiO2 product formed was separated using an external magnet, washed three times with ethanol and deionized water, and dried at 25 °C. For the preparation of CoFe2O4@SiO2-Pr, 1 g of CoFe2O4@SiO2 was dispersed in 30 mL of toluene via sonication for 30 min. Then, 3 mmol of 3-chloropropyl trimethoxy silane (Pr) was added, and the reaction mixture was refluxed with continuous stirring for 24 h. After completion, the product was isolated magnetically, thoroughly washed with ethyl acetate and ethanol, and dried in an oven at 50 °C for 24 h. To synthesize the CoFe2O4@SiO2-Pr-Mth complex, 3 g of CoFe2O4@SiO2-Pr were dispersed in 60 mL of toluene by sonicating for 35 min. Afterward, 3 mmol of methotrexate (Mth) was added to the reaction and stirred under reflux for 24 h. The final product was isolated using a bar magnet, washed thoroughly with ethyl acetate and water, and dried at 60 °C for 10 h. Finally, for the preparation of CoFe2O4@SiO2-Pr-Mth-Pd, 2 g of CoFe2O4@SiO2-Pr-Mth were combined with 3 mmol of palladium acetate (Pd(OAc)2) and 30 mL of ethanol in a flask. The reaction mixture was stirred at 60 °C for 48 h, after which 1.5 mmol of sodium borohydride (NaBH4) was added. The reaction proceeded for an additional 5 h. The final product, CoFe2O4@SiO2-Pr-Mth-Pd, was separated using a magnet, washed with ethanol and water, and dried under vacuum at 50 °C.
Scheme 1.
Synthesis of CoFe2O4@SiO2-Pr-Mth-Pd.
Aromatic sulfide formation catalyzed by CoFe2O4@SiO2-Pr-Mth-Pd
A mixture of aryl halide (2 mmol), sulfur (S8, 1 mmol), potassium hydroxide (KOH, 0.6 mmol), and CoFe2O4@SiO2-Pr-Mth-Pd nanocatalyst (0.02 g) was stirred in ethanol under reflux conditions. The reaction progress was monitored by thin-layer chromatography (TLC) (Hexzan: EtOAc, 8:2). Upon completion, the mixture was cooled to room temperature and diluted with water. The nanocatalyst was separated magnetically, rinsed with ethyl acetate, and removed. The aqueous layer was extracted with ethyl acetate, and the combined organic extracts were dried with sodium sulfate (Na2SO4, 1.5 g). The solvent was then evaporated to obtain pure ether derivatives, as illustrated in Scheme 2.
Scheme 2.
Synthesis of diaryl sulfide derivatives using CoFe2O4@SiO2-Pr-Mth-Pd.
Activity of CoFe2O4@SiO2-Pr-Mth-Pd catalyst in reduction of nitroarenes
In a typical reduction reaction, 1 mmol of nitroarene was treated with 0.02 g of the CoFe2O4@SiO2-Pr-Mth-Pd nanomagnetic catalyst in 3 ml of water. The reaction mixture was stirred at room temperature for 30 min. Next, 1.5 mol of NaBH4 were introduced, and the mixture was stirred vigorously under the same conditions until the reaction reached completion. The reaction’s progress was tracked using TLC (Hexzan: EtOAc, 7: 3). Upon completion, the CoFe2O4@SiO2-Pr-Mth-Pd nanocatalyst was separated effortlessly with a permanent magnet. To the resulting mixture, 10 ml of CH2Cl2 was added, and the organic layer was carefully separated from the aqueous phase using a separating funnel. The organic phase was dried over anhydrous magnesium sulfate. Afterward, the CH2Cl2 layer was concentrated under reduced pressure, and the crude product was subjected to purification by column chromatography on silica gel. A mixture of n-hexane and ethyl acetate served as the eluting solvents, enabling recovery of the final reduced product (Scheme 3).
Scheme 3.
Catalytic activity of CoFe2O4@SiO2-Pr-Mth-Pd catalyst in the reduction of nitrobenzene.
Selected NMR data
Diphenylsulfane (Table 2, Entry 1)1:H NMR (400 MHz, DMSO): δH = 7.47–7.59 (m, 10H) ppm. Bis(4-nitrophenyl)sulfane (Table 2, Entry 3):1H NMR (400 MHz, DMSO): δH = 7.61 (m, 3H), 7.77 (m, 4H) ppm. 4,4’-Thiodianiline (Table 2, Entry 7):1H NMR (400 MHz, DMSO): δH = 7.19 (m, 4H), 6.70 (m, 4H), 5.73 (s, 4H) ppm. 4,4’-Thiodiphenol (Table 2, Entry 9):1H NMR (400 MHz, DMSO): δH = 8.79 (s, 2H), 7.39 (m, 3H), 7.22 (m, 5H) ppm. Dibenzylsulfane (Table 2, Entry 14):1H NMR (400 MHz, DMSO): δH = 7.44 (s, 4H), 7.31 (m, 6H), 3.66 (m, 4H) ppm. bis(4-methoxyphenyl)sulfane (Table 2, Entry 6):1H NMR (400 MHz, DMSO): δH = 7.38–7.65 (m, 8H), 3.87 (m, 6H) ppm. 4,4’-thiodibenzaldehyde (Table 2, Entry 8):1H NMR (400 MHz, DMSO): δH = 8.79 (s, 1H), 7.49–7.59 (m, 8H) ppm. di-p-tolylsulfane (Table 21H NMR (400 MHz, DMSO): δH = 7.61 (d, J = 8 Hz, 3H), 7.44 (m, 4H), 7.33 (m, 1H), 1.96 (s, 6H) ppm.
Table 2.
Synthesis of symmetrical sulfides via the reaction of S8 and aryl/alkyl halides catalyzed by peptide nanofibers decorated with CoFe2O4@SiO2-Pr-Mth-Pd.
| Entry | Aryl halide | Product | Time (min) | Yield (%) a, b | TON c | TOF (min−1) d |
|---|---|---|---|---|---|---|
| 1 | 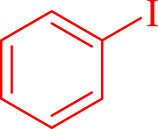 |
 |
60 | 98 | 272 | 272 |
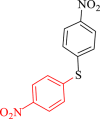
| 2 |  |
 |
25 | 92 | 255 | 621 |
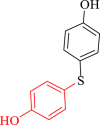
| 3 |  |
 |
60 | 93 | 258 | 258 |
| 4 |  |
 |
20 | 96 | 266 | 806 |
| 5 | 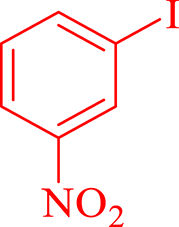 |
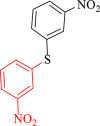 |
40 | 90 | 250 | 378 |
| 6 |  |
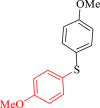 |
30 | 91 | 252 | 504 |
| 7 | 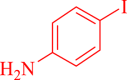 |
 |
30 | 94 | 261 | 522 |
| 8 | 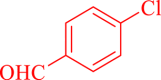 |
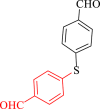 |
120 | 80 | 222 | 111 |
| 9 | 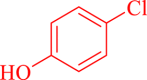 |
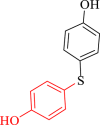 |
30 | 85 | 236 | 472 |
| 11 |  |
 |
60 | 92 | 255 | 255 |
| 12 |  |
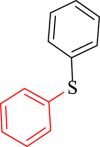 |
60 | 91 | 252 | 252 |
| 13 | 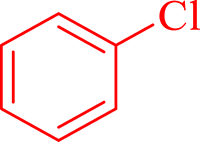 |
 |
40 | 96 | 266 | 403 |
| 14 |  |
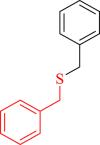 |
60 | 90 | 250 | 378 |
aIsolated yield.
bReaction conditions: aryl halide (2 mmol), sulfur (S8, 1 mmol), potassium hydroxide (KOH, 0.6 mmol), CoFe2O4@SiO2-Pr-Mth-Pd (20 mg), EtOH (3 mL).
cTON = Yield/Amount of catalyst.
dTOF = TON/Time.
Aniline(Table 4, Entry 1):1H NMR (400 MHz, DMSO): δH = 8.77 (d, 3H), 7.38 (m, 2H), 4.89 (s, 2H) ppm. 4-Chloroaniline(Table 4, Entry 2):1H NMR (400 MHz, DMSO): δH = 7.57 (s, 1H), 7.42 (m, 2H), 7.20 (s, 1H), 5.11 (s, 2H) ppm. 4-Aminophenol(Table 4, Entry 8):1H NMR (400 MHz, DMSO): δH = 8.75 (s, 1H), 7.35 (d, 4H), 4.69 (d, 2H) ppm.4-Aminobenzonitrile(Table 4, Entry 4):1H NMR (400 MHz, DMSO): δH = 7.33 (d, 2H), 6.73 (d, 1H), 5.75 (s, 2H) ppm. benzene-1,4-diamine(Table 4, Entry 10):1H NMR (400 MHz, DMSO): δH = 6.70 (s, 4H), 5.73 (s, 4H), ppm. 4-bromoaniline(Table 4, Entry 3):1H NMR (400 MHz, DMSO): δH = 7.19 (s, 2H), 6.74 (s, 2H), 5.74 (s, 2H), ppm. 3-aminobenzonitrile(Table 4, Entry 6):1H NMR (400 MHz, DMSO): δH = 7.62 (m, 3H), 7.26 (d, J = 24 Hz, 1H), 6.31 (s, 2H), ppm. 4-methoxyaniline (Table 4, Entry 5):1H NMR (400 MHz, DMSO): δH = 7.65 (m, 4H), 3.73 (s, 3H), ppm.
Table 4.
Nitroarene reduction catalyzed by CoFe2O4@SiO2-Pr-Mth-Pd magnetic catalyst.
| Entry | Aryl halide | Product | Time (min) | Yield (%)a,b | TON | TOF (min−1) |
|---|---|---|---|---|---|---|
| 1 |  |
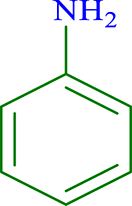 |
30 | 97 | 269 | 538 |
| 2 | 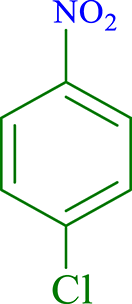 |
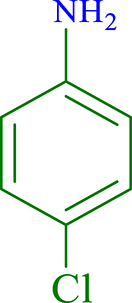 |
30 | 96 | 266 | 532 |
| 3 | 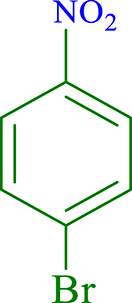 |
 |
60 | 91 | 252 | 252 |
| 4 |  |
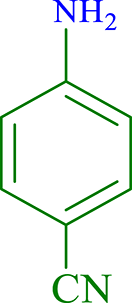 |
15 | 89 | 247 | 988 |
| 5 |  |
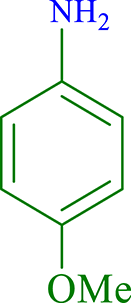 |
15 | 95 | 263 | 1052 |
| 6 | 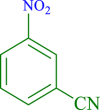 |
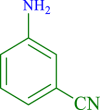 |
45 | 90 | 250 | 333 |
| 7 | 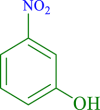 |
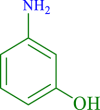 |
20 | 90 | 250 | 796 |
| 8 |  |
 |
15 | 97 | 269 | 1076 |
| 9 |  |
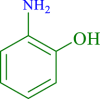 |
60 | 89 | 247 | 247 |
| 10 | 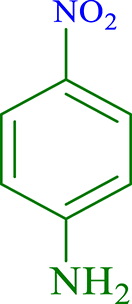 |
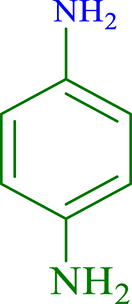 |
30 | 95 | 263 | 526 |
aIsolated yield.
bReaction conditions: Nitroarene (1 mmol), NaBH4 (1.5 mmol), CoFe2O4@SiO2-Pr-Mth-Pd (0.02 g), H2O (3 mL).
Catalyst characterizations
FTIR spectroscopy with a KBr pellet was utilized to verify the successful functionalization of CoFe2O4@SiO2-Pr-Mth-Pd MNPs. The absorption bands associated with CoFe2O4 (Fig. 1a) and CoFe2O4@SiO2-Pr (Fig. 1b) corresponded closely to those reported in previous studies20–23. In the spectrum for CoFe2O4@SiO2-Pr-Mth (Fig. 1c), distinct absorption bands around 1597 cm−1 and 1655 cm−1 were detected, representing aliphatic C = N and C = O stretching vibrations, respectively. Furthermore, a broad absorption within the range of 2500–3400 cm−1 was identified, which was attributed to the hydroxycarboxylic acid group in the Mth structure. This evidence confirms the successful immobilization of Mth-Pd on the substrate.
Fig. 1.
Comparative study of FT-IR spectra of a) CoFe2O4, b) CoFe2O4@SiO2-Pr, and c) CoFe2O4@SiO2-Pr-Mth-Pd.
As illustrated in Fig. 2, X-ray diffraction (XRD) analysis was conducted on both the CoFe2O4@SiO2-Pr-Mth-Pd and CoFe2O4 samples. The results confirm that the crystal structure of CoFe2O4 within the material remains well-preserved and aligns with standard patterns. Notably, the analysis reveals that the attachment of the Pr-Mth-Pd complex to CoFe2O4 does not alter the phase morphology of the catalyst, ensuring its original structure remains intact. This indicates that the surface modification of CoFe2O4 using the Pr-Mth-Pd complex does not negatively impact the integrity of its crystal structure.
Fig. 2.
XRD spectrum of a) CoFe2O4, and b) CoFe2O4@SiO2-Pr-Mth-Pd.
Thermogravimetric analysis (TGA) is a reliable and efficient technique for assessing the thermal stability of magnetic nanoparticles. It plays an essential role in confirming and interpreting the bond formation between magnetite nanoparticles and organic complexes. In Fig. 3a, a slight weight loss is noted, which is attributed to the oxidation of hydroxyl groups on the CoFe2O4 surface. In Fig. 3b, the initial 10% weight loss below 200 °C is associated with the removal of physically adsorbed solvents. This is followed by a further weight loss of 28% between 200 °C and 1000 °C, corresponding to the degradation of organic layers on the CoFe2O4 surface. These observations validate the successful grafting of organic groups onto the CoFe2O4 magnetic nanoparticles.
Fig. 3.
TGA curve of a) CoFe2O4, b) CoFe2O4@SiO2-Pr-Mth-Pd.
The elemental composition of CoFe2O4@SiO2-Pr-Mth-Pd was examined through EDX analysis. As shown in Fig. 4, the results confirmed the presence of Pd, C, Si, O, N, Fe, and Co within the catalyst, aligning with the reference data from the EDX pattern database.
Fig. 4.
EDS analysis of CoFe2O4@SiO2-Pr-Mth-Pd.
Scanning electron microscope (SEM) images of CoFe2O4@SiO2-Pr-Mth-Pd nanoparticles, as illustrated in Fig. 5, reveal their distinct morphology and grain-like structure. The observed features indicate the presence of extremely small, discrete particles with nanometer-scale dimensions. Such a structure significantly influences the material’s physical characteristics, particularly its potential magnetic properties. The nanoscale structure enhances the surface-to-volume ratio, which, in turn, increases the specific surface area. This crucial attribute plays a pivotal role in shaping the behavior and overall performance of the CoFe2O4@SiO2-Pr-Mth-Pd nanoparticles.
Fig. 5.

SEM images of CoFe2O4@SiO2-Pr-Mth-Pd.
The aggregation of nanoparticles within the synthesized catalyst was analyzed through transmission electron microscopy (TEM). This technique plays a vital role in providing in-depth insights into the structure and morphology of catalytic nanoparticles. High-resolution images captured via TEM allowed for a detailed examination of the degree and pattern of nanoparticle aggregation in the catalyst material. As depicted in the TEM images (Fig. 6), the nanoparticles appeared as spherical structures with nanoscale dimensions.
Fig. 6.

TEM images of CoFe2O4@SiO2-Pr-Mth-Pd.
The ICP technique was later utilized to measure the palladium content in the initial catalyst and assess the degree of palladium leaching after recycling. The results showed that the palladium concentrations in both the fresh and recycled catalysts were 1.8 × 10⁻4 and 1.7 × 10⁻4 mol·g⁻1, respectively, demonstrating negligible palladium leaching from the CoFe2O4@SiO2-Pr-Mth-Pd framework.
The N2 adsorption/desorption isotherm of the synthesized CoFe2O4 nanoparticles is presented in Fig. 7, derived from the BET analysis. The observed mesoporous structure is advantageous for increasing the material’s specific surface area. Based on BJH method calculations, the CoFe2O4 nanoparticles exhibit a specific surface area of 61.93 m2/g, a pore volume of 0.173 cm3/g, and an average pore diameter of 8.036 nm.
Fig. 7.

(a) N2 adsorption/desorption curve of the CoFe2O4.
The magnetic properties of CoFe₂O₄ and CoFe₂O₄@SiO₂-Pr-Mth-Pd nanostructures were evaluated at room temperature using a VSM, as illustrated in Fig. 8. The magnetization curves revealed saturation magnetization (Ms) values of 61 emu/g for CoFe₂O₄ and 42 emu/g for CoFe₂O₄@SiO₂-Pr-Mth-Pd. These Ms values confirm the ferrimagnetic nature of both materials. The decrease in saturation magnetization from 61 emu/g for CoFe₂O₄ to 42 emu/g for CoFe₂O₄@SiO₂-Pr-Mth-Pd reflects the impact of the SiO₂ coating and subsequent surface modification. As silica is a non-magnetic material, its presence as a coating increases the total weight of the material without contributing to its magnetization. Since Ms is determined based on the overall mass of the sample, this results in a lower Ms value. Additionally, the coating and surface modifications may alter the surface structure of the CoFe₂O₄ nanoparticles, potentially affecting the arrangement of surface spins and, in turn, reducing the overall magnetization.
Fig. 8.
VSM curves of (a) CoFe2O4 (b) CoFe2O4@SiO2-Pr-Mth-Pd.
Catalytic study
Checking catalytic activity of CoFe2O4@SiO2-Pr-Mth-Pd for the C–S coupling reaction
We examined the performance of the magnetic CoFe2O4@SiO2-Pr-Mth-Pd complex as a catalyst in the one-pot reaction involving iodobenzene and S8, which serves as a model reaction for producing the desired diaryl sulfide (Table 1). Initially, the reaction was tested under reflux conditions using various green solvents, including acetonitrile, ethanol, water, ethyl acetate (EtOAc), PEG-400, as well as under solvent-free conditions. Notably, ethanol emerged as the most effective solvent, delivering the highest yield. Subsequent investigations focused on catalyst loading, where it was determined that 20 mg of the catalyst was sufficient to achieve optimal transformation. Increasing the catalyst amount or prolonging the reaction time offered no additional improvement in efficiency. Furthermore, temperature was identified as a critical parameter; a significant drop in reaction efficiency was observed when the temperature was reduced to room temperature. In the final stage of optimization, comparisons were made between catalyst-free conditions and reactions involving catalyst intermediates to assess the effectiveness of the Pd complex. The best results were obtained when CoFe2O4@SiO2-Pr-Mth-Pd served as the catalyst. The optimized reaction conditions, detailed in Table 1, include using 0.02 g of the catalyst and 0.6 mmol of KOH (Table 1, entries 1–22) as the base, with the reaction conducted in ethanol under reflux conditions.
Table 1.
Optimization of the reaction conditions for the C–S coupling using iodobenzene and S8 as sulfur transfer agent.
| Entry | Cat. (mg) | Solvent | Base | T. (˚C) | T. (min) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | - | EtOH | KOH | Reflux | 2 days | N. R |
| 2 | 5 | EtOH | KOH | Reflux | 60 | 44 |
| 3 | 10 | EtOH | KOH | Reflux | 60 | 75 |
| 4 | 15 | EtOH | KOH | Reflux | 60 | 90 |
| 5 | 20 | EtOH | KOH | Reflux | 60 | 98 |
| 6 | 25 | EtOH | KOH | Reflux | 60 | 98 |
| 7 | 20 | EtOH | KOH | Reflux | 120 | 98 |
| 8 | 20 | EtOH | KOH | Reflux | 30 | 56 |
| 9 | 20 | Acetonitrile | KOH | Reflux | 60 | 40 |
| 10 | 20 | EtOAc | KOH | Reflux | 60 | 48 |
| 11 | 20 | H2O | KOH | Reflux | 60 | 73 |
| 12 | 20 | PEG-400 | KOH | 120 | 60 | 86 |
| 13 | 20 | Solvent-free | KOH | 120 | 60 | 94 |
| 14 | 20 | EtOH | K2CO3 | Reflux | 60 | 91 |
| 15 | 20 | EtOH | Cs2CO3 | Reflux | 60 | 81 |
| 16 | 20 | EtOH | Na2CO3 | Reflux | 60 | 89 |
| 17 | 20 | EtOH | NaOH | Reflux | 60 | 82 |
| 18 | 20 | EtOH | KOH | r.t | 60 | N. R |
| 19 | 20 | EtOH | KOH | 50 | 60 | 46 |
| 20 | 20 | EtOH | KOH | 70 | 60 | 89 |
| 21 | 20 | EtOH | KOH | Reflux | 60 | –a |
| 22 | 20 | EtOH | KOH | Reflux | 60 | –b |
| 23 | 20 | EtOH | KOH c | Reflux | 60 | 83 |
aReaction in the presence of CoFe2O4.
bReaction in the presence of CoFe2O4@SiO2-Pr.
cReaction in the presence of 0.4 mmol of KOH.
Following the optimization of reaction conditions, the catalytic efficiency of CoFe2O4@SiO2-Pr-Mth-Pd was evaluated using a range of Ar-X substrates, as detailed in Table 2. Aryl halides featuring both electron-donating and electron-withdrawing groups, including nitro, methyl, bromo, methoxy, and chloro, were synthesized successfully in short reaction times with outstanding yields. Additional details can be found in Table 2.
Scheme 4 outlines the catalytic cycle underlying the production of sulfides facilitated by CoFe2O4@SiO2-Pr-Mth-Pd. The process begins with the reaction of S8 and KOH, yielding potassium disulfide. This intermediate then interacts with CoFe2O4@SiO2-Pr-Mth-Pd, resulting in the formation of palladium disulfide. Palladium disulfide proceeds to undergo oxidative addition with an aryl halide, forming intermediate 1. Following this, reductive elimination leads to the generation of intermediate 2. The cycle continues as intermediate 2 undergoes another oxidative addition reaction with a second aryl halide, yielding compound 3. The final step involves reductive elimination, producing diphenyl sulfide, and regenerating the palladium catalyst, as demonstrated in Scheme 423.
Scheme 4.
Possible mechanism for the synthesis of sulfides.
To determine the optimal conditions, the catalytic performance of CoFe2O4@SiO2-Pr-Mth-Pd was assessed through a model reaction involving nitrobenzene and sodium borohydride. The study explored the effects of various factors, such as catalyst concentration, solvent type, and reaction temperature, on the overall outcome. Reaction progress was monitored via TLC, and upon completion, the product was isolated using ethyl acetate as the extraction solvent. The best results were obtained in water (H2O) at 90 °C, using 1.5 mmol of NaBH4 and 0.02 g of CoFe2O4@SiO2-Pr-Mth-Pd, as detailed in Table 3.
Table 3.
Optimization of conditions for reduction reaction of nitrobenzene with sodium borohydride in the presence of CoFe2O4@SiO2-Pr-Mth-Pd magnetic catalyst.
| Entry | Cat. (g) | Solvent |
NaBH4 (mmol) |
T. (˚C) | T. (min) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | - | H2O | 1.5 | 90 | 1 day | N.R |
| 2 | 0.005 | H2O | 1.5 | 90 | 15 | 55 |
| 3 | 0.007 | H2O | 1.5 | 90 | 15 | 80 |
| 4 | 0.01 | H2O | 1.5 | 90 | 15 | 93 |
| 5 | 0.02 | H2O | 1.5 | 90 | 15 | 98 |
| 6 | 0.025 | H2O | 1.5 | 90 | 15 | 98 |
| 7 | 0.02 | PEG-400 | 1.5 | 90 | 15 | 36 |
| 8 | 0.02 | hexane | 1.5 | 90 | 15 | 49 |
| 9 | 0.02 | EtOAc | 1.5 | 90 | 15 | 74 |
| 10 | 0.02 | Acetonitrile | 1.5 | 90 | 15 | 89 |
| 11 | 0.02 | EtOH | 1.5 | 90 | 15 | 93 |
| 12 | 0.02 | H2O | 1 | 90 | 15 | 83 |
| 13 | 0.02 | H2O | 0.9 | 90 | 15 | 78 |
| 14 | 0.02 | H2O | 0.7 | 90 | 15 | 41 |
| 15 | 0.02 | H2O | 0.3 | 90 | 15 | 35 |
| 16 | 0.02 | H2O | 1.5 | 70 | 15 | 73 |
| 17 | 0.02 | H2O | 1.5 | 50 | 15 | 59 |
| 18 | 0.02 | H2O | 1.5 | r.t | 15 | 41 |
| 19 | 0.02 | H2O | 1.5 | 90 | 15 | –a |
| 20 | 0.02 | H2O | 1.5 | 90 | 15 | –b |
a Reaction in the presence of CoFe2O4.
b Reaction in the presence of CoFe2O4@SiO2-Pr.
After optimizing the reaction conditions, we explored the effectiveness of the CoFe2O4@SiO2-Pr-Mth-Pd catalyst in reducing nitroarenes. As outlined in Table 4, the findings revealed outstanding yields and remarkable TOFs for the tested aryl bromides and iodides.
Building on previous articles and the supporting evidence provided, Scheme 5 depicts the catalytic cycle for the reduction of nitroarenes mediated by CoFe2O4@SiO2-Pr-Mth-Pd. It further proposes a mechanism for this reduction process, which employs NaBH4 as the reducing agent and is facilitated by the CoFe2O4@SiO2-Pr-Mth-Pd nanocatalyst. The mechanism outlines possible intermediates (I–IV), which are visually represented within Scheme 524.
Scheme 5.
A Proposed mechanism for reduction of nitroarene.
A hot filtration experiment was conducted to evaluate the heterogeneity of CoFe2O4@SiO2-Pr-Mth-Pd during the coupling reaction between iodobenzene and S8. The findings indicated that 59% of the product was formed at the midpoint of the reaction. To further investigate, the experiment was repeated with the catalyst removed at the halfway mark, allowing the reaction to proceed for an additional 30 min in the absence of the catalyst. The final yield reached 61%. These outcomes confirm that no copper leaching occurred.
Catalyst recyclability
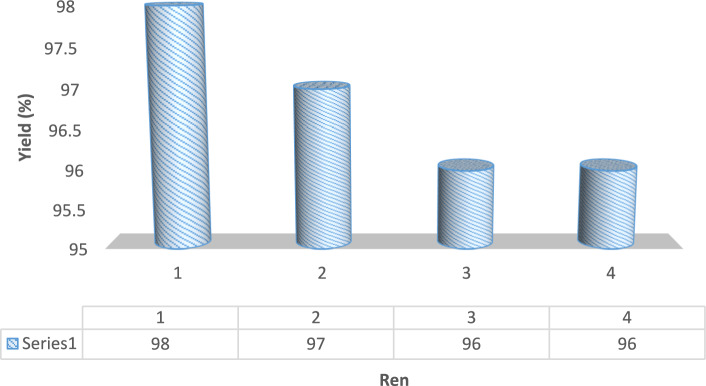
To assess the stability of the catalytic activity, the reaction between S8 and Ar-I was selected as a model system and conducted in ethanol using 0.02 g of CoFe2O4@SiO2-Pr-Mth-Pd. The catalyst was conveniently retrieved with the aid of a magnet, with recovery amounts measured at 0.0196, 0.0192, and 0.0188 g for each cycle, respectively, as detailed in the experimental section. Impressively, CoFe2O4@SiO2-Pr-Mth-Pd exhibited consistent performance and proved reusable across four successive cycles without any observable decline in catalytic efficiency, as illustrated in Fig. 9.
Fig. 9.
Recyclability of CoFe2O4@SiO2-Pr-Mth-Pd.
The FT-IR spectrum analysis of the recycled nanocatalyst shows no significant changes following the recovery process. This uniformity in the spectral data effectively demonstrates the structural stability and integrity of the CoFe2O4@SiO2-Pr-Mth-Pd composite material, as illustrated in Fig. 10.
Fig. 10.
Comparative study of FT-IR spectra of the recovered catalyst.
Conclusion
In this project, we successfully developed CoFe2O4@SiO2-Pr-Mth-Pd, a highly efficient, eco-friendly, and recoverable catalyst. Comprehensive characterization of the catalyst was performed using FTIR, XRD, EDS, TEM, TGA, VSM, SEM, and ICP analyses. It showcased broad applicability in promoting the reduction of nitroarenes and facilitating C–S coupling reactions. A noteworthy aspect of CoFe2O4@SiO2-Pr-Mth-Pd is its straightforward synthesis from commonly available commercial materials. Furthermore, the nanocatalyst offers significant benefits, including excellent catalytic performance, ease of separation, and reusability.
Supplementary Information
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia for supporting this work through project number TU-DSPP-2024-19.
Author contributions
Anjan Kumar. Mohammed Al-Bahrani. Magda H. Abdellattif. Vicky Jain. Suhas Ballal. Munthar Kadhim Abosaoda. Abhayveer Singh. T. Krithiga. Subhashree Ray. and Ojas Prakashbhai Doshi.Funding acquisition, Supervision, Conceptualization, Resources, Writing-review & editing.
Funding
The research was funded by Taif University Saudi Arabia project number TU-DSPP-2024-19.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. If anyone would like to request data from this study, they should contact Magda H. Abdellattif. Chemistry Department, College of Sciences, University College of Taraba, Taif University, Saudi Arabia, Email: ahmedmnaglah@gmail.com.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-12436-2.
References
- 1.Ashraf, M. A., Liu, Z., Li, C. & Zhang, D. Fe3O4@HcdMeen-Pd(0) Organic-Inorganic Hybrid: As a novel heterogeneous nanocatalyst for chemo and homoselective heck C-C cross-coupling synthesis of butyl cinnamates. Catal. Lett.10.1007/s10562-020-03509-0 (2021). [Google Scholar]
- 2.Veisi, H. et al. Recent advances in the application of magnetic nanocatalysts in multicomponent reactions. RSC Adv.13, 20530–20556 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taheri Kal Koshvandi, A., Heravi, M. M. & Momeni, T. Current applications of suzuki-miyaura coupling reaction in the total synthesis of natural products: An update. Appl. Org. Chem.32, e4210 (2018). [Google Scholar]
- 4.Chen, Z. et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol.13, 702–707 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Ashraf, M. A., Liu, Z., Zhang, D. & Alimoradi, A. L-lysine-Pd Complex Supported on Fe 3 O 4 MNPs: A novel recoverable magnetic nanocatalyst for Suzuki C-C Cross-Coupling reaction. Appl. Organomet. Chem.34, e5668 (2020). [Google Scholar]
- 6.Kandathil, V. et al. NHC-Pd complex heterogenized on graphene oxide for cross-coupling reactions and supercapacitor applications. Appl. Org. Chem.34, e5924 (2020). [Google Scholar]
- 7.Kanchana, U. S., Diana, E. J., Mathew, T. V. & Anilkumar, G. Palladium-catalyzed cross-coupling reactions of coumarin derivatives: An overview. Appl. Org. Chem.34, 5983 (2020). [Google Scholar]
- 8.Lakshmidevi, J., Naidu, B. R. & Venkateswarlu, K. CuI in biorenewable basic medium: Three novel and low E-factor Suzuki-Miyaura cross-coupling reactions. Mol. Catalysis522, 112237 (2022). [Google Scholar]
- 9.Bagade, K. S., Jadhav, D. D. & Kumbhar, A. S. Solid-state Suzuki-Miyaura cross-coupling: A sustainable approach by DDIL-stabilized magnetic separable and recyclable Pd nanoparticles with total solvent bypass. Appl. Organomet. Chem.38, e7503 (2024). [Google Scholar]
- 10.Gorji, S. & Ghorbani-Vaghei, R. Ag nanoparticles stabilized on basalt fibers as a novel, stable, and reusable catalyst for Suzuki-Miyaura coupling reactions. Appl. Org. Chem.35, e6018 (2021). [Google Scholar]
- 11.Azizollahi, H., Eshghi, H. & García-López, J.-A. Fe3O4-SAHPG-Pd0 nanoparticles: A ligand-free and low Pd loading quasiheterogeneous catalyst active for mild Suzuki-Miyaura coupling and C-H activation of pyrimidine cores. Appl. Org. Chem.35, e6020 (2021). [Google Scholar]
- 12.Aabaka, S. R. et al. Nanocellulose Supported PdNPs as in situ Formed Nano Catalyst for the Suzuki Coupling Reaction in Aqueous Media: A Green Approach and Waste to Wealth. J. Organomet. Chem.937, 121719 (2021). [Google Scholar]
- 13.Han, C. et al. Enhanced support effects in single-atom copper-incorporated carbon nitride for photocatalytic suzuki cross-coupling reactions. Appl. Catal. B320, 121954 (2023). [Google Scholar]
- 14.Chen, M.-N., Mo, L.-P., Cui, Z.-S. & Zhang, Z.-H. Magnetic nanocatalysts: Synthesis and application in multicomponent reactions. Current Opinion in Green and Sustainable Chemistry15, 27–37 (2019). [Google Scholar]
- 15.Mhaldar, P. M., Patil, M. V., Rashinkar, G. S. & Pore, D. M. Magnetically Recoverable Palladium Nanocatalyst [Pd(II)-Benz-Am-Fe3O4@SiO2] for Ullmann Type Homocoupling of Aryl halides with N2H4 as an Efficient Reductant. J. Inorganic Org. Polymers Mater.32, 3053–3066 (2022). [Google Scholar]
- 16.Bagade, K. S. & Kumbhar, A. S. APTES immobilized copper-doped nitrogen quantum dots (CuNPs@N-GQDs@APTES): An efficient heterogeneous nanocatalyst for multicomponent synthesis of 5-substituted-1H-tetrazoles. J. Organomet. Chem.1030, 4624–4645 (2025). [Google Scholar]
- 17.Shinde, G. & Thakur, J. Magnetically Recyclable Ag@Fe2O3 Core-shell Nanostructured Catalyst for One-pot Synthesis of 2-Aryl Benzimidazole and Benzothiazole. Current Organocatalysis09, 237–251 (2022). [Google Scholar]
- 18.Muniyappan, N. & Sabiah, S. Synthesis, structure, and characterization of picolyl- and benzyl-linked biphenyl palladium N-heterocyclic carbene complexes and their catalytic activity in acylative cross-coupling reactions. Appl. Org. Chem.34, e5421 (2020). [Google Scholar]
- 19.Alamgholiloo, H., Rostamnia, S. & Noroozi, P. N. Extended architectures constructed of thiourea-modified SBA-15 nanoreactor: A versatile new support for the fabrication of palladium pre-catalyst. Appl. Organomet. Chem.34, e5452 (2020). [Google Scholar]
- 20.Mohammadi, S. & Naeimi, H. Functionalized CoFe2O4/lamellar mesopore silica anchored to melamine nanocomposite as a novel catalyst for synthesis of 4H-chromenes under mild conditions. Appl. Org. Chem.34, e5630 (2020). [Google Scholar]
- 21.Zeng, Y., Xie, J., Xiao, X., Chen, L. & Zhu, X. Synthesis of CoFe2O4@SiO2–NH2 and its application in adsorption of trace lead. RSC Adv.14, 589–601 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmasry, M., Elshahat, M., Ramadan, R. & Abdelhameed, R. Selective photocatalytic reduction of nitroarenes into amines based on cobalt/copper ferrite and cobalt-doped copper ferrite nano-photocatalyst. J. Mater. Sci.: Mater. Electron.32, 1–17 (2021). [Google Scholar]
- 23.Xu, Z., Zihan, Z., Rui, W., Fei, L. & Jiang, H. Potassium salts catalyzed oxidative coupling of alkanethiols with sulfur: the effect of solid-liquid phase transfer of crown ether. Phosphorus, Sulfur Silicon Relat. Elem.199, 90–102 (2024). [Google Scholar]
- 24.Manjunatha, K. et al. Magnetic nanoparticle-tethered Schiff base–palladium(II): Highly active and reusable heterogeneous catalyst for Suzuki-Miyaura cross-coupling and reduction of nitroarenes in aqueous medium at room temperature. Appl. Organomet. Chem.32, e4266 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. If anyone would like to request data from this study, they should contact Magda H. Abdellattif. Chemistry Department, College of Sciences, University College of Taraba, Taif University, Saudi Arabia, Email: ahmedmnaglah@gmail.com.