Abstract
We have isolated a Lactobacillus plantarum strain (MiLAB 393) from grass silage that produces broad-spectrum antifungal compounds, active against food- and feed-borne filamentous fungi and yeasts in a dual-culture agar plate assay. Fusarium sporotrichioides and Aspergillus fumigatus were the most sensitive among the molds, and Kluyveromyces marxianus was the most sensitive yeast species. No inhibitory activity could be detected against the mold Penicillium roqueforti or the yeast Zygosaccharomyces bailii. An isolation procedure, employing a microtiter well spore germination bioassay, was devised to isolate active compounds from culture filtrate. Cell-free supernatant was fractionated on a C18 SPE column, and the 95% aqueous acetonitrile fraction was further separated on a preparative HPLC C18 column. Fractions active in the bioassay were then fractionated on a porous graphitic carbon column. The structures of the antifungal compounds cyclo(l-Phe-l-Pro), cyclo(l-Phe-trans-4-OH-l-Pro) and 3-phenyllactic acid (l/d isomer ratio, 9:1), were determined by nuclear magnetic resonance spectroscopy, mass spectrometry, and gas chromatography. MIC values against A. fumigatus and P. roqueforti were 20 mg ml−1 for cyclo(l-Phe-l-Pro) and 7.5 mg ml−1 for phenyllactic acid. Combinations of the antifungal compounds revealed weak synergistic effects. The production of the antifungal cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-Pro) by lactic acid bacteria is reported here for the first time.
Biopreservation, the use of microorganisms to preserve food and feed, has gained increasing interest during recent years due to consumers' demand for reduced use of chemical preservatives. The preserving ability of lactic acid bacteria (LAB) has been used since ancient times in food and animal feed, such as sauerkraut and silage. The antimicrobial effect of the LAB is mainly related to production of lactic and acetic acid, but for some strains, synthesis of bacteriocins is also of great importance (11, 18). Due to the “generally regarded as safe” status of LAB, the interest in using them for biopreservation has increased during recent years.
Molds and yeasts are important spoilage organisms in food and feed systems, where the potential mycotoxin production from molds is of particular concern (16). There are many reports on the production of antibacterial compounds by LAB (11, 17), but reports on inhibition of yeasts and molds are comparatively few. Magnusson and Schnürer (12) described production of a proteinaceous antifungal compound by a Lactobacillus coryniformis strain, while Lavermicocca et al. (10) reported isolation of the antifungal compounds phenyllactic acid and 4-hydroxyphenyllactic acid from Lactobacillus plantarum. Short-chain fatty acids, in particular caproic acid, produced by the sourdough bacterium Lactobacillus sanfrancisco also exhibit anti-mold activities (1). L. plantarum can produce fungus-inhibitory low-molecular-weight substances, such as benzoic acid, methylhydantoin, mevalonolactone, and cyclo(Gly-l-Leu) (14), while a fungistatic bacteriocin-like substance, pentocin TV35b, was isolated from a Lactobacillus pentosus strain (15).
We have isolated a Lactobacillus plantarum strain with antifungal properties from grass silage. Three antifungal substances produced by the strain have been isolated, and their structures have been determined. The substances have been identified as 3-phenyllactic acid, with a 9/1 ratio of l and d isomers and the two cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-Pro). This is the first report of production of these cyclic dipeptides by LAB. Furthermore, the antifungal properties of these dipeptides have not been clearly established previously.
MATERIALS AND METHODS
Cultures and media.
The strain MiLAB 393 was grown on MRS agar (Oxoid Ltd., Basingstoke, England) at 30°C in anaerobic jars under CO2-N2 atmosphere (GasPak System; BBL, Cockeysville, Md.). The culture was stored anaerobically on MRS agar plates at 5°C or for long-term storage at −70°C in a 15% glycerol salt solution (0.82 g of K2HPO4, 0.18 g of KH2PO4, 0.59 g of Na-citrate, and 0.25 g of MgSO4 · 7H2O per liter). Liquid cultures were grown in MRS broth (Oxoid).
The molds Aspergillus fumigatus J9, Aspergillus nidulans J283 (FSGC A4 wt), Fusarium sporotrichioides J304 (ITEM168), Penicillium roqueforti J268 (IBT 6754), Penicillium commune J238 (IBT 12400), and the yeasts Pichia anomala J121, Kluyveromyces marxianus J137 (CBS 6556), Rhodotorula mucilaginosa J350 (CFSQE 63), Debaromyces hansenii J187 (CBS 6958), Zygosaccharomyces bailii J109 (CBS 2852), Candida albicans J278 (CBS 562), and Saccharomyces cerevisiae J122 (ATCC 9793) are kept in the culture collection of the Department of Microbiology, Swedish University of Agricultural Sciences. The target fungi were chosen to represent potential spoilage fungi in, e.g., silage and dairy products (16). The exception was A. fumigatus, which was used in the bioassay, since it appears to be highly sensitive to LAB antifungal compounds (12) as well as being a serious pathogen of animals and humans (2). Molds were grown on malt extract agar slants (Oxoid) at 25°C for 5 to 7 days and stored at 4°C. Spore inocula were prepared by growing the molds on malt extract agar slants until sporulation and spores or conidia were collected by vigorously shaking slants with sterile peptone water (0.2% [wt/vol]). Yeast cell inocula were prepared from washed cultures grown in malt extract broth (2%; DIFCO Laboratories, Detroit Mich.) at 25 or 30°C for 12 h. Both mold (spores or conidia) and yeast cell concentrations were determined using a hemocytometer and adjusted to 105 spores (cells) per ml of sterile peptone water.
Isolation and identification of MiLAB 393.
L. plantarum MiLAB 393 was isolated from grass silage (Phleum pratense and Festuca pratensis with <20% red clover [Trifolium pratense]) in an experiment investigating the effect of addition of different biological agents on growth of the spoilage mold P. roqueforti in silage. The strain MiLAB 393 came from a control silo without chemical or biological additives that showed total growth inhibition of the inoculated P. roqueforti (unpublished data). From the silo, 10 g of silage was suspended in 90 ml of sterile peptone water (0.2% [wt/vol]) and mixed in a stomacher for 2 min. Dilutions were prepared using sterile peptone water, and 0.1-ml aliquots were surface spread on MRS agar plates. After anaerobic incubation at 30°C for 48 h, the bacterial cultures were transferred to fresh MRS plates for a second incubation.
MiLAB 393 was identified from both the fermentation pattern and the 16S rRNA gene sequence. The API 50 CHL test (bioMérieux, Marcy L'Etoile, France) was used for identification by fermentation pattern. For 16S ribosomal DNA sequence determination, chromosomal DNA was isolated using the DNeasy tissue kit (QIAGEN GmbH, Hilden, Germany). PCR amplification (94°C for 30 s, 54°C for 30 s, and 72°C for 80 s, 30 cycles) using primers 16S.S (5′-AGAGTTTGATCCTGGCTC-3′) and 16S.R (5′-CGGGAACGTATTCACCG-3′) amplified approximately 1,400 bp of the 16S ribosomal DNA. Sequencing of the amplified DNA was performed using the Thermo Sequenase dye terminator cycle sequencing kit (Amersham Biosciences, Uppsala, Sweden) and the automated sequence analyzer ABI PRISM 377XL (Perkin-Elmer). The culture is deposited as strain LMG-P21295 at the BCCM/LMG bacteria collection, Ghent, Belgium.
Determination of antifungal activity.
The inhibition spectrum of strain MiLAB 393 was determined by using the overlay method as described by Magnusson and Schnürer (12) but with some modifications of the scale used for gradation. Strain MiLAB 393 was inoculated in two 2-cm lines on MRS agar plates and incubated under anaerobic conditions at 30°C for 48 h. The plates were overlaid with malt extract soft agar containing 104 cells/spores ml−1 and incubated aerobically at 25 to 30°C for 24 to 48 h. Clear zones of inhibition were recorded and scored as follows: −, no visible inhibition; (+), visible inhibition only in the soft agar above the bacterial streak; +, inhibition area per bacterial streak of 0.1 to 3.0% of the petri dish; ++, inhibition area per bacterial streak of 3.0 to 8.0% of the petri dish; or +++, inhibition area per bacterial streak of >8.0% of the petri dish.
Determination of MIC values.
MICs were determined for the available commercial compounds, 3-phenyl-l-lactic acid (Sigma-Aldrich Chemie, Steinheim, Germany), 3-phenyl-d-lactic acid (Fluka Chemie AG, Buchs, Switzerland), and cyclo(l-Phe-l-Pro) (Bachem AG, Bubendorf, Switzerland). Both cyclo(l-Phe-l-Pro) and phenyllactic acid (both d and l form) were dissolved in water with 8% methanol, and the pH was adjusted to 4 with 2 M NaOH. A pH-adjusted 8% methanol-water solution without dissolved substances was used as a negative control. MIC determinations were performed in duplicates as serial twofold dilutions using the microtiter plate well method described by Magnusson and Schnürer (12) and with A. fumigatus and P. roqueforti as target organisms. In the microtiter plate well method, a 100-μl sample and 100 μl of MRS broth or malt extract broth (2%) containing 104 fungal spores ml−1 were added to each well. After 48 h incubation at 30°C, the inhibition was measured by optical density at 550 nm (Microplate Autoreader EL 309, Biotek Instruments, Winooski, Vt.). An inverted microscope and measurement using the naked eye were also used for estimation of fungal growth in the wells. The MIC was determined as the lowest concentration where total inhibition of spore germination was observed. Synergistic inhibitory effects against A. fumigatus and P. roqueforti were evaluated by testing cyclo(l-Phe-l-Pro) and 3-phenyl-l-lactic acid in combination and together with 100 mM lactic acid. A mixture of cyclo(l-Phe-l-Pro) at a concentration of 40 mg ml−1 and 3-phenyl-l-lactic acid at a concentration of 20 mg ml−1 was used in serial twofold dilutions. The pH of the mixtures was adjusted to 4 with 2 M NaOH. Lactic acid at 100 mM, representative of the concentration of a 48-h culture filtrate from L. plantarum MiLAB 393, had no effect on growth of A. fumigatus and P. roqueforti at pH 4.0.
Since cyclic dipeptides also are reported to be antibacterial (5), the MIC for cyclo(l-Phe-l-Pro) against the producer strain MiLAB 393 was determined using the microtiter plate method described above.
Preparation of cell-free supernatant.
L. plantarum MiLAB 393 was inoculated to a concentration of 105 cells ml−1 in 2 liters of MRS broth or in the defined medium DM1 (13) as a still culture at 30°C for 48 h. DM1 contains no peptides or proteins; instead, free amino acids necessary for growth are added to the medium (13). Cell-free supernatant was prepared by centrifugation (7,500 × g for 15 min) and sterile filtration (0.45 μm-pore-size filter; Millipore). The cell-free culture filtrate was used for isolation of the antifungal compounds. Uninoculated MRS broth was used as a negative control, fractionated, and evaluated in the biotests using the same procedure as with the cell-free culture filtrate. All fractions from the purification process were evaluated for antifungal activity after concentration under vacuum and/or by freeze-drying or evaporation under compressed air. The microtiter plate bioassay described above was used with A. fumigatus as the indicator fungus.
SPE and high performance liquid chromatography (HPLC).
The supernatant was fractionated on a solid phase extraction (SPE) column (Isolute, C18 EC, 10 g; International Sorbent Technology Ltd., Hengoed, United Kingdom). The SPE column was activated with 20 ml of acetonitrile and equilibrated with 20 ml of water. Following sample loading, the column was washed with 5% aqueous acetonitrile and subsequently eluted with 95% aqueous acetonitrile. The 95% aqueous acetonitrile fraction was separated on a preparative HPLC system (piston pump 305 and 306; Gilson, Villiers-le-Bel, France) using a Discovery C18 column (100 by 21.2 mm, 5 μm; Supelco, Bellafonte, Pa.) and a linear gradient from 5% acetonitrile in water to 100% acetonitrile in 10 min followed by 5 min at 100% acetonitrile. The flow rate was 10 ml min−1, and the eluate was monitored using a UV detector (Gilson 118) at 210 nm. All fractions were collected in 96 2-ml deep-well plates using a fraction collector (Gilson Liquid Handler 215). Following bioassay, all active fractions were further fractionated on the same HPLC system using a Hypercarb porous graphitic carbon column (100 by 21.2 mm, 5 μm; ThermoQuest Runcorn, Cheshire, United Kingdom) and isocratic elution with 40% aqueous acetonitrile containing 0.06% trifluoroacetic acid. Fractions showing activity in the bioassay were dried in a vacuum centrifuge.
Structure determination of antifungal compounds.
The structures of the antifungal compounds were determined using nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), and gas chromatography (GC). NMR spectra were recorded on samples in CD3OD and/or dimethylsulphoxide-d6 and/or D2O on a Bruker DRX-600 NMR spectrometer (Bruker, Rheinstetten, Germany) equipped with a 2.5-mm microprobe. All spectra were recorded at 30°C, and one-dimensional 1H NMR experiments as well as two-dimensional 1H-1H correlation spectroscopy, 1H-13C heteronuclear multiple bond correlation, and 1H-13C heteronuclear multiple quantum coherence (HMQC) experiments were performed according to Bruker standard pulse sequences. Proton chemical shifts were determined from one-dimensional 1H NMR and from HMQC experiments, and 13C chemical shifts were determined from HMQC and 1H-13C heteronuclear multiple bond correlation experiments. Chemical shifts are reported relative to the solvent peaks (CD3OD: 1H δ 3.31 and 13C δ 49.15, dimethyl sulfoxide-d6 1H δ 2.50) except when D2O was used, where data are given relative to sodium 3-(trimethylsilyl)propionate (1H δ 0.00). MS was performed on an Esquire ion-trap MS (Bruker) with an electrospray ion source and on a JEOL JMS-SX/SX102A four-sector tandem MS (JEOL, Ltd., Tokyo, Japan) with a fast-atom-bombardment (FAB) ion source with glycerol as the matrix.
To determine the absolute configuration of the isolated 3-phenyllactic acid, a modified version of a published method (6) was used. An aliquot (<1 mg) of dry sample was dissolved in 1 ml of acetyl chloride-methanol (1:9, vol/vol) and heated for 10 min at 100°C. After cooling to room temperature, 1 ml of 10% disodium hydrogen phosphate solution was added and the sample was extracted twice with 0.9 ml of hexane. The organic layer was dried over anhydrous sodium sulfate, evaporated to approximately 50 μl, and analyzed on a GC-MS instrument (HP5890-HP5970; Hewlett-Packard, Palo-Alto, Calif.). GC-MS was performed on a chiral fused-silica (30 m by 0.25 mm) β-Dex 120, coated with 0.25 μm of 20% permethylated β-cyclodextrin in 80% poly-(35% diphenyl-65% dimethyl)-siloxane (Supelco) using a temperature gradient (140°C for 5 min; 140 to 180°C at 3°C min−1). The GC injector was kept at 200°C, and the GC-MS interface was kept at 200°C. Samples (1 to 5 μl) were injected in split mode (1:50) three times, and helium was used as the carrier gas (1 ml min−1). Commercial 3-phenyl-l-lactic acid and 3-phenyl-d-lactic acid were used as references.
To determine the absolute configuration of the cyclic dipeptides, a small amount (less than 0.1 mg) of each dipeptide was hydrolyzed in 6 M aqueous HCl (1 ml) at 110°C for 24 h. Each hydrolysate was dried under reduced pressure, redissolved in 200 μl of H2O, transferred to a Reacti-vial (Pierce, Rockford, Ill.), and freeze-dried. To each dried sample, 50 μl of 2-(S)-butanol and acetyl chloride (10:1) was added, and the solution was heated at 100°C for 40 min followed by evaporation under a stream of compressed air. Subsequently, perfluoropropanoic anhydride (50 μl) was added, and each vial was heated at 100°C for 40 min to give the corresponding amide. The sample was dried under a stream of compressed air. Reference samples were prepared by derivatization of commercial l-proline, dl-proline, l-phenylalanine, dl-phenylalanine, cis-4-hydroxy-l-proline, cis-4-hydroxy-d-proline, and trans-4-hydroxy-l-proline as outlined above. trans-4-hydroxy-d-proline was not available as a reference substance. Instead, trans-4-hydroxy-l-proline was esterified with 2-(R)-butanol, yielding an ester, which is enantiomeric to trans-4-hydroxy-d-proline esterified with 2-(S)-butanol. Since enantiomers behave identically on a nonchiral GC column, this substance was used as the missing reference substance, after amide formation as described above.
Prior to GC-MS analysis each sample was dissolved in toluene. GC-MS was performed on a fused-silica capillary column (BP5; 0.25 μm, 30 m × 0.25 mm; SGE Ltd., Ringwood, Austalia) using a temperature gradient (120°C for 5 min, 120 to 160°C at 2°C min−1). The GC injector was kept at 240°C, and the GC-MS interface was kept at 260°C. Samples (1 μl) were injected in split mode (1:50), and helium (1 ml min−1) was used as the carrier gas.
RESULTS
Identification of strain MiLAB 393.
The partial 16S rDNA sequence, 450 to 550 bp, encoding variable regions V1 and V2 from the isolate was used for searching in GenBank and showed 100% homology to the corresponding sequence of L. plantarum. The fermentation pattern from the API 50 CHL test identified the strain as an L. plantarum strain with a probability of 99.9%.
Spectrum of antifungal activity.
L. plantarum MiLAB 393 had activity against several mold and yeast species in an agar overlay method (Table 1). F. sporotrichioides and A. fumigatus were the most sensitive among the molds, and K. marxianus was the most sensitive yeast species. No activity could be detected against the food and feed spoilage mold P. roqueforti or the preservative-resistant yeast Z. bailii.
TABLE 1.
Antifungal inhibition spectrum of strain MiLAB 393 in a dual-culture agar overlay systema
| Mold or yeast strain | Inhibitionb |
|---|---|
| Molds | |
| A. fumigatus | +++ |
| A. nidulans | ++ |
| F. sporotrichioides | +++ |
| P. commune | ++ |
| P. roqueforti | − |
| Yeasts | |
| C. albicans | ++ |
| D. hansenii | ++ |
| K. marxianus | +++ |
| P. anomala | (+) |
| R. mucilaginosa | ++ |
| S. cerevisiae | (+) |
| Z. bailii | − |
The grading of the inhibition is based on the area of the inhibition zone surrounding the bacterial inocula.
Inhibition was graded as follows: −, no visible inhibition; (+), visible inhibition only in the soft agar above the bacterial streak; +, inhibition area per bacterial streak of 0.1 to 3.0% of the petri dish; ++, inhibition area per bacterial streak of 3.0 to 8.0% of the petri dish; +++, inhibition area per bacterial streak of >8.0% of the petri dish.
Purification and characterization of antifungal compounds.
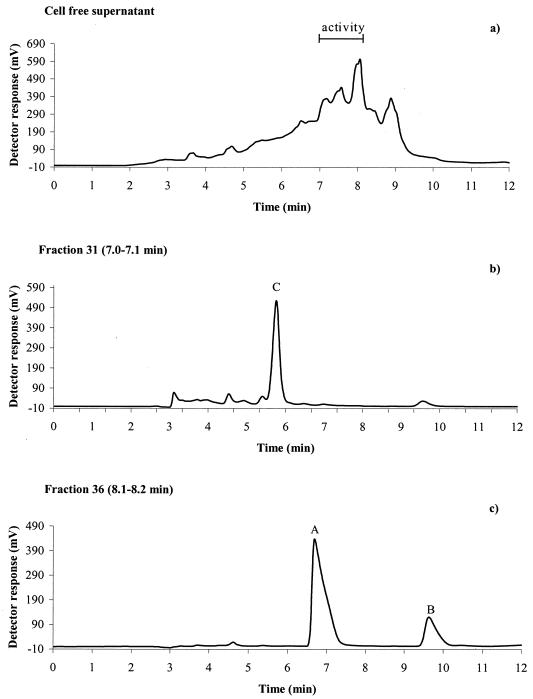
Antifungal activity of the cell-free supernatant resulting from growth of strain MiLAB 393 in MRS broth was recovered in the 95% acetonitrile phase after SPE on a C18 column, indicating a hydrophobic nature of the antifungal compound or compounds. Further separation of the filtrate (95% acetonitrile phase from SPE) by HPLC using a C18 column and activity assay in microtiter wells against A. fumigatus delimited the activity to seven (fractions 31 to 37) of a total of 64 fractions collected (Fig. 1a). After further separation by HPLC using a porous graphitic carbon column, three active compounds, A, B, and C were isolated (Fig. 1b and c). No activity was observed from the corresponding fractions isolated from noninoculated MRS broth. Data from the experiments elucidating the chemical structures of compounds A, B, and C are summarized below.
FIG. 1.
Chromatograms from isolation of antifungal compounds from culture broth of L. plantarum MiLAB 393. (a) Separation of the 95% acetonitrile phase (SPE) from cell supernatant on an HPLC C18 column. Activity against A. fumigatus was detected in fractions 31 to 37 (retention time, 7.0 to 8.2 min). Chromatograms from a second preparative HPLC on a porous graphitic carbon column of fraction 31 with activity at peak C (b) and fraction 36 with activity at peaks A and B (c) are shown; see the text for chromatographic conditions.
For compound A, solid; 1H NMR (600 MHz, D2O); δ 7.38 (H-5 and H-9), 7.31 (H-6 to H-8), 4.27 (H-2), 3.11 (H-3a), 2.89 (H-3b); GC retention time (tR) (min); d-phenyllactic acid 16.81 (standard 16.82) and l-phenyllactic acid 17.05 (standard 17.07); ESI-MS (m/z): 165 (M+H)+; HPLC tR (min): 6.7. For compound B, solid; 1H NMR (600 MHz, CD3OD); δ 7.95 (NH, dimethylsulphoxide-d6 used as solvent), 7.27 (H-13 and H-15), 7.24 (H-12 and H-16), 7.22 (H-14), 4.44 (H-3), 4.07 (H-9), 3.55 (H-6a), 3.37 (H-6b), 3.17 (2H, H-10), 2.10 (H-8a), 1.81 (2H, H-7), 1.25 (H-8b); 13C NMR (150 MHz, CD3OD); δ 170.86 (C-1), 166.86 (C-4), 137.37 (C-11), 131.11 (C-16 and C-12), 129.62 (C-13 and C-15), 128.19 (C-14), 60.03 (C-9), 57.71 (C-3), 45.94 (C-6), 38.20 (C-10), 29.34 (C-8), 22.68 (C-7); FAB-MS (m/z): 245 (M+H)+; HPLC tR (min): 9.6. For compound C, viscous oil; 1H NMR (600 MHz, CD3OD); δ 7.95 (NH, dimethyl sulfoxide-d6 used as solvent), 7.27 (H-13 and H-15), 7.24 (H-12 and H-16), 7.22 (H-14), 4.48 (H-3), 4.37 (H-9), 4.28 (H-7), 3.71 (H-6a), 3.29 (H-6b), 3.17 (2H, H-10), 2.07 (H-8a), 1.39 (H-8b); 13C NMR (150 MHz, CD3OD); δ 171.29 (C-1), 167.19 (C-4), 137.51 (C-11), 131.14 (C-12 and C-16), 129.63 (C-13 and C-15), 128.23 (C-14), 68.65 (C-7), 58.35 (C-9), 57.65 (C-3), 55.19 (C-6), 38.76 (C-8), 37.95 (C-10); FAB-MS (m/z): 261 (M+H)+; HPLC tR (min): 5.8.
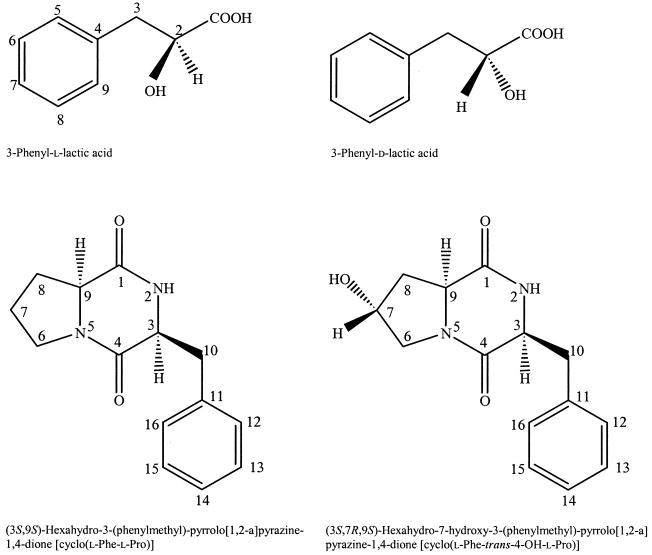
Compound A was identified as 3-phenyllactic acid by comparing data from NMR, ESI-MS, and HPLC with data from commercial 3-phenyllactic acid. The isolated 3-phenyllactic acid was determined to be in a 9/1 ratio of the L and D configuration by analysis of the corresponding methyl ester on a chiral GC column and using commercial d- and l-phenyllactic acid as standards. Compound B was identified as cyclo(Phe-Pro) by comparing data from NMR, FAB-MS, and HPLC with data from commercial cyclo(l-Phe-l-Pro). The NMR and FAB-MS data of compound C were in good agreement with previously published data of cyclo(l-Phe-trans-4-OH-l-Pro) (8). Absolute configuration determination of the amino acids constituting the isolated cyclic dipeptides [cyclo(Phe-Pro) and cyclo(Phe-4-OH-Pro)] was done by comparing the retention time of standards and samples from the GC analysis of the derivatized amino acids. The amino acids were identified as l-phenylalanine, l-proline, and trans-4-hydroxy-l-proline (Table 2). Thus, the antifungal dipeptides were cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-Pro) (Fig 2.).
TABLE 2.
GC analysis of amino acids constituting compounds B, cyclo(Phe-Pro), and C, cyclo(Phe-4-OH-Pro), isolated from L. plantarum MiLAB 393a
| Compound | Retention time (min)
|
||
|---|---|---|---|
| Reference | Compound B | Compound C | |
| d-Pro | 10.12 | ||
| l-Pro | 10.31 | 10.31 | |
| trans-4-OH-d-Prob | 11.91 | ||
| trans-4-OH-l-Pro | 12.11 | 12.15 | |
| cis-4-OH-d-Pro | 14.46 | ||
| cis-4-OH-l-Pro | 14.70 | ||
| d-Phe | 20.33 | ||
| l-Phe | 20.57 | 20.56 | 20.60 |
The cyclic dipeptides were hydrolyzed with 6M HCl and subsequently derivatized with 2-(S)-butanol and perfluoropropionic anhydride. The GC analysis was performed using a nonchiral GC column (see the text for details).
The amino acid trans-4-OH-d-Pro was not commercially available. To obtain a reference compound behaving identically on a nonchiral GC column, the enantiomeric compound trans-4-OH-l-Pro was derivatized with 2-(R)-butanol and perfluoropropionic anhydride.
FIG. 2.
Antifungal compounds isolated from supernatant of L. plantarum MiLAB 393 grown in MRS broth.
Production of compounds in defined medium.
Since two of the identified active compounds were cyclic dipeptides, the defined medium DM1, containing only free amino acids instead of peptides, was used to verify that the cyclic dipeptides were produced by the bacteria. Comparison of HPLC retention time and FAB-MS spectra with a reference compound confirmed that cyclo(l-Phe-l-Pro) was produced in the defined medium. The presence of cyclo(l-Phe-trans-4-OH-l-Pro) was confirmed by comparing the retention time from HPLC separation with previously isolated cyclo(l-Phe-trans-4-OH-l-Pro). Thus, the conclusion is that both cyclic dipeptides are produced by the bacteria in DM1. Isolation of the two dipeptides was done by using the same procedure as when MRS broth was used as the medium and with the same volume of supernatant. Comparison of the weight of the isolated dipeptides showed that approximately 10 times less material was produced when DM1 was used.
Determination of MICs.
Commercial reference samples of 3-phenyllactic acid, both the l and d forms, and cyclo(l-Phe-l-Pro) were used for determination of fungal inhibitory concentrations with A. fumigatus and P. roqueforti as indicator organisms. For both fungi the MIC for total growth inhibition was 7.5 mg ml−1 for 3-phenyllactic acid and 20 mg ml−1 for cyclo(l-Phe-l-Pro). When 3-phenyl-l-lactic acid and cyclo(l-Phe-l-Pro) were combined, the mixture containing 5 mg of 3-phenyl-l-lactic ml−1 and 10 mg of cyclo(l-Phe-l-Pro) ml−1 gave total inhibition, revealing weak synergistic effects. Addition of 100 mM lactic acid (pH 4) in the mixtures gave no further inhibitory effects. The MIC for the cyclic dipeptide cyclo(l-Phe-l-Pro) against the producer strain MiLAB 393 was 30 mg ml−1.
DISCUSSION
LAB with antifungal activities could have potential as biopreservatives, preventing growth of spoilage molds and yeasts in food and feed systems. In this study we report the antifungal properties and identification of fungal inhibitory substances from the L. plantarum strain MiLAB 393. Three antifungal substances were isolated, and the chemical structures were determined as 3-phenyllactic acid (both l and d forms in a 9/1 ratio), cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-Pro). Phenyllactic acid from L. plantarum has previously been reported in fungal inhibition (10), and the D-enantiomer of 3-phenyllactic acid from the yeastlike fungus Geotrichum candidum has been found to exhibit antibacterial properties (3). We found a lower concentration for inhibition of fungal growth than Lavermicocca et al. (10), who found total inhibition concentrations ranging from 50 to 166 mg ml−1 with various fungi. This is probably due to the use of target fungi of different species and possibly a more sensitive test system. We used a microtiter plate well method where the fungal spores are more directly exposed to the substance than in the disk agar diffusion assay used by Lavermicocca et al. (10).
Cyclic dipeptides have previously been reported to be both antibacterial and antifungal (5). Niku-Paavola et al. (14) also reported production of an antifungal cyclic dipeptide, cyclo(Gly-l-Leu), from an L. plantarum strain. The cyclic dipeptides identified in this work have to our knowledge never before been shown to be produced from LAB, nor has the antifungal effect of cyclo(l-Phe-trans-4-OH-l-Pro) been reported earlier. The results further show that combining the peptide cyclo(l-Phe-l-Pro) and 3-phenyllactic acid leads to weak synergistic fungal inhibitory effects.
Cyclo(l-Phe-l-Pro) has also been isolated from Pseudomonas fluorescens and Pseudomonas alcaligenes cell-free culture supernatants and reported to be involved in quorum sensing mechanisms (7), which synchronize bacterial physiological responses in a cell density-dependent manner. For gram-positive bacteria, peptide pheromones are the most common signal molecules, whereas gram-negative bacteria use lactone-based pheromones (9). Quorum sensing in LAB is exemplified by larger signal peptides regulating the production of certain bacteriocins (9). The observation by Holden et al. (7) that gram-negative bacteria could produce the cyclic dipeptide cyclo(l-Phe-l-Pro), which activated quorum sensing mechanisms, and our finding that cyclo(l-Phe-l-Pro) is produced by L. plantarum could suggest a possibility of cross talk among signaling systems of gram-positive and gram-negative bacteria.
In the agar test system used for determination of antifungal spectrum in this study, strain MiLAB 393 showed a broad inhibitory spectrum with effect against both filamentous fungi and yeasts. This dual-culture system is based on diffusion of the inhibitory substances into the agar, and consequently lactic acid will also contribute to the inhibition. Both P. roqueforti and Z. bailii are tolerant of low pH and regarded as resistant to weak acid preservatives (16), which might explain the lack of inhibition of these fungi in this test system. However, total inhibition of spore germination of P. roqueforti with 3-phenyllactic acid and cyclo(l-Phe-l-Pro), at the same concentration as with A. fumigatus, was observed in the more sensitive microtiter well method.
The fungal inhibitory concentrations of the antifungal compounds isolated in this work are high compared to antifungal drugs, such as amphotericin B and nikkomycin Z, that inhibit fungal growth at concentrations in the microgram-per-milliliter range (4). The antifungal activity of the isolated cyclic dipeptides is most probably a secondary effect; the primary reason for production might instead be related to quorum sensing or other unknown mechanisms. Nevertheless, the antifungal activity of these substances most likely is of considerable importance for the fungal inhibitory activity of the bacterial strain MiLAB 393.
Further evaluation of LAB for antifungal properties could lead to useful biopreservation systems, preventing fungal spoilage and mycotoxin formation in both food and animal feed.
Acknowledgments
The financial support of the Foundation for Strategic Environmental Research (MISTRA) and the Swedish Farmers Foundation for Agricultural Research (SLF) is gratefully acknowledged.
Lennart Kenne, Karin Jacobsson, Hans Jonsson, and Stefan Roos gave helpful comments on the manuscript.
REFERENCES
- 1.Corsetti, A., M. Gobbetti, J. Rossi, and P. Damiani. 1998. Antimould activity of sourdough lactic acid bacteria: identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1 Appl. Microbiol. Biotechnol. 50:253-256. [DOI] [PubMed] [Google Scholar]
- 2.deHoog, G. S. 1996. Risk assessment of fungi reported from humans and animals. Mycoses 39:407-417. [DOI] [PubMed] [Google Scholar]
- 3.Dieuleveux, V., S. Lemarinier, and M. Gueguen. 1998. Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int. J. Food Microbiol. 40:177-183. [DOI] [PubMed] [Google Scholar]
- 4.Frändberg, E., C. Petersson, L. N. Lundgren, and J. Schnürer. 2000. Streptomyces halstedii K122 produces the antifungal compounds bafilomycin B1 and C1. Can. J. Microbiol. 46:753-758. [PubMed] [Google Scholar]
- 5.Graz, M., A. Hunt, H. Jamie, G. Grant, and P. Milne. 1999. Antimicrobial activity of selected cyclic dipeptides. Pharmazie 54:772-775. [PubMed] [Google Scholar]
- 6.Heil, M., F. Podebrad, T. Beck, A. Mosandl, A. C. Sewell, and H. Bohles. 1998. Enantioselective multidimensional gas chromatography mass spectrometry in the analysis of urinary organic acids. J. Chromatogr. B 714:119-126. [DOI] [PubMed] [Google Scholar]
- 7.Holden, M. T. G., S. R. Chhabra, R. de Nys, P. Stead, N. J. Bainton, P. J. Hill, M. Manefield, N. Kumar, M. Labatte, D. England, S. Rice, M. Givskov, G. P. C. Salmond, G. Stewart, B. W. Bycroft, S. A. Kjelleberg, and P. Williams. 1999. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol. Microbiol. 33:1254-1266. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, Z., K. G. Boyd, A. Mearns-Spragg, D. R. Adams, P. C. Wright, and J. G. Burgess. 2000. Two diketopiperazines and one halogenated phenol from cultures of the marine bacterium Pseudoalteromonas luteoviolacea. Nat. Prod. Lett. 14:435-440. [Google Scholar]
- 9.Kleerebezem, M., and L. E. Quadri. 2001. Peptide pheromone-dependent regulation of antimicrobial peptide production in Gram-positive bacteria: a case of multicellular behavior. Peptides 22:1579-1596. [DOI] [PubMed] [Google Scholar]
- 10.Lavermicocca, P., F. Valerio, A. Evidente, S. Lazzaroni, A. Corsetti, and M. Gobbetti. 2000. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 66:4084-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindgren, S. E., and W. J. Dobrogosz. 1990. Antagonistic activities of lactic-acid bacteria in food and feed fermentations. FEMS Microbiol. Rev. 87:149-163. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson, J., and J. Schnürer. 2001. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moretro, T., B. F. Hagen, and L. Axelsson. 1998. A new, completely defined medium for meat lactobacilli. J. Appl. Microbiol. 85:715-722. [Google Scholar]
- 14.Niku-Paavola, M. L., A. Laitila, T. Mattila-Sandholm, and A. Haikara. 1999. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 86:29-35. [DOI] [PubMed] [Google Scholar]
- 15.Okkers, D. J., L. M. Dicks, M. Silvester, J. J. Joubert, and H. J. Odendaal. 1999. Characterization of pentocin TV35b, a bacteriocin-like peptide isolated from Lactobacillus pentosus with a fungistatic effect on Candida albicans. J. Appl. Microbiol. 87:726-734. [DOI] [PubMed] [Google Scholar]
- 16.Pitt, J. J., and A. D. Hocking. 1999. Fungi and food spoilage, 2nd ed. Aspen Publications, New York, N.Y.
- 17.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 18.Vandenbergh, P. A. 1993. Lactic-acid bacteria, their metabolic products and interference with microbial-growth. FEMS Microbiol. Rev. 12:221-238. [Google Scholar]