Abstract
The antimicrobial effect of l-lactate was much greater than that of d-lactate over a range of concentrations for Escherichia coli O157 and non-O157 strains. Despite this, the intracellular pHs and membrane potentials of l-lactate- and d-lactate-treated cells were similar, suggesting that these factors are not involved in the antimicrobial action of l-lactate.
Escherichia coli O157 is a major cause for concern in the field of public health. Infections caused by this bacterium, though infrequent, are associated with high levels of morbidity and mortality and may have led to reduced public confidence in food safety, particularly for red meat products. There is growing interest in both how E. coli O157 enters the food chain and practical measures of prevention.
Organic acids have been used traditionally in abattoirs and the animal feed, food, and pharmaceutical industries to control pathogens (4). Recent evidence has suggested that these acids may be effective in controlling the proliferation of E. coli O157 (7). One of the most effective acids against this organism in vitro is lactate, and its effectiveness in combination with its wide availability, low cost, and generally “recognized-as-safe” status makes lactate a promising candidate as a control measure for E. coli O157 in farm and slaughterhouse environments. Investigations into the antimicrobial effect of lactate have previously focused on either l-lactate or, more frequently, the commercially available dl-lactate mixture which contains the isomers in variable proportions. A preliminary study at the Rowett Research Institute (11) has suggested that E. coli strains from pigs may be more susceptible to l-lactate than to d-lactate. We decided to extend this study to compare the relative contributions of the stereoisomers to the antimicrobial effect of lactate on various E. coli O157 and non-O157 isolates.
The E. coli strains used in this study are shown in Table1. Stationary-phase cells were prepared as previously described (10) to give a population of approximately 109 CFU ml−1. Cultures were treated with l-lactate (Sigma, Poole, United Kingdom) or d-lactate (Sigma) to produce a final pH of 3.8, and the viability was then determined (10). Specific death rates (SDR) were calculated by plotting the results of viability studies over time semilogarithmically and determining the negative value of the slope. The reduction in viable count could then be expressed as log10 CFU per milliliter per hour. All viability studies were performed in duplicate, and the results were compared using analysis of variance.
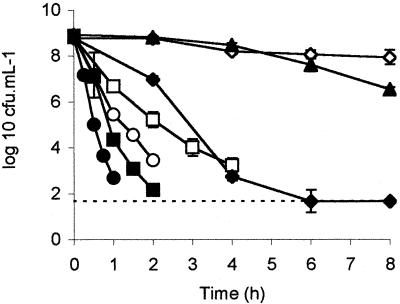
The effects of the individual stereoisomers of lactate on E. coli NCTC 12900 and F318 were examined. l-Lactate had a much greater antimicrobial effect than did d-lactate, reflecting previously published observations (11). The efficacies of both isomers were dose dependent, and a greater concentration of d-lactate than of l-lactate was required to obtain similar reductions in viability. The isomers and their concentrations in ascending order of efficacy (P < 0.05) were as follows: 100 mM d-lactate < 50 mM l-lactate < 150 mM d-lactate < 100 mM l-lactate (Fig. 1). The effects of treatments consisting of 200 mM d-lactate, 150 mM l-lactate, and 200 mM l-lactate did not differ from each other statistically but were greater (P < 0.05) than those of the other treatments.
FIG. 1.
Viability of NCTC 12900 in various concentrations of d- or l-lactate. d-Lactate was added to give final concentrations of 100 (◊), 150 (□), and 200 (○) mM. l-Lactate was added to give final concentrations of 50 (▴), 100 (⧫), 150 (▪), and 200 (•) mM. A dashed line represents the limit of detection (1.67 log10 CFU ml−1).
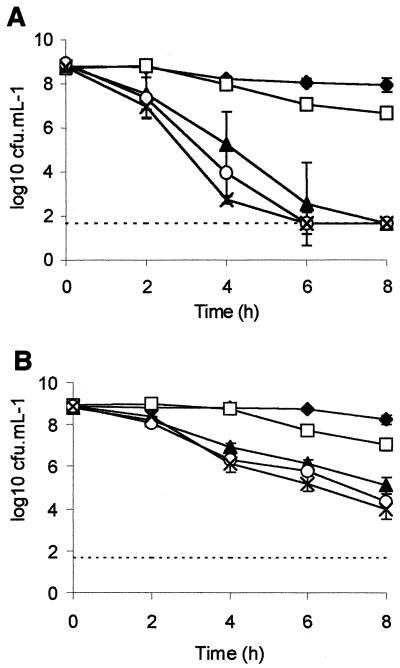
The viability of strain NCTC 12900 in various concentrations of d-lactate or l-lactate was determined. Stationary-phase cultures were challenged with various proportions of l-lactate and d-lactate such that the total concentration was 100 mM. l-Lactate at a concentration of 100 mM exerted a greater antimicrobial effect (P < 0.01) than 100 mM d-lactate for NCTC 12900 (Fig. 2A) and F318 (Fig. 2B). Increasing the proportion of the l isomer over that of the d isomer heightened the antimicrobial efficacy in a dose-dependent manner for both strains.
FIG. 2.
Viability of NCTC 12900 (A) and F318 (B) treated with various combinations of d- and l-lactate. Acids were added to give a final combined concentration of 100 mM and consisted of 100 mM d-lactate (⧫), 75 mM d-lactate plus 25 mM l-lactate (□), 50 mM d-lactate plus 50 mM l-lactate (▴), 25 mM d-lactate plus 75 mM l-lactate (O), and 100 mM l-lactate (×). A dashed line represents the limit of detection (1.67 log10 CFU ml−1).
A range of O157 and non-O157 E. coli isolates were assayed to determine whether the greater efficacy of l-lactate than of d-lactate is widespread in E. coli strains. Stationary-phase cells were incubated in 100 mM l-lactate or d-lactate for 3 h. The isolates were much less sensitive (P < 0.00001) to d-lactate than to l-lactate, suggesting that this effect is prevalent among E. coli strains (Table 1). These results may have implications for improving the efficacy of lactic acid decontaminant preparations and probiotic lactic acid bacteria. The abilities of the various strains to survive lactate challenge were highly variable. When the E. coli isolates were grouped as O157 or non-O157 strains, there was no statistical difference between the groups in their sensitivities to d-lactate. However, the non-O157 strains were more susceptible to l-lactate than were the O157 strains. Previous studies comparing the levels of acid tolerance of E. coli strains have suggested that either E. coli O157 strains are more acid tolerant than non-O157 E. coli strains, or there is no difference between the two groups (2).
TABLE 1.
Effect of lactate on the viability of various O157 and non-O157 E. coli strains
| Strain | Origin | Serotype | Decrease in viabilitya
|
|
|---|---|---|---|---|
| d-Lactate | l-Lactate | |||
| NCTC 12900 | Human | O157:H7 (VT−)b | −0.08 | 10.2 |
| NCTC 13126 | Human | O157:H7 (VT−) | 0.92 | 8.52 |
| NCTC 12079 | Human | O157:H7 | 7.6 | 6.40 |
| AUIO-5c | Cattle feces | O157:H7 | 0.42 | 4.66 |
| AUIO-7c | Raw milk | O157:H7 | 0.08 | 4.77 |
| AUIO-13c | Minced beef | O157:H7 | 3.53 | 6.00 |
| AUIO-309c | Cheese | O157:H7 | 0.17 | 4.76 |
| AUIO-NDc | Sheep feces | O157:H7 | 1.42 | 4.49 |
| F318 | Sheep rumen | O162 | 1.25 | 10.4 |
| F38 | Sheep rumen | O rough | 4.08 | 8.83 |
| EC17 | Pig | O106:NM | −0.25 | 10.6 |
| EC30 | Bison | O113:H21 | 6.83 | 16.1 |
| EC33 | Sheep | O7:H21 | 2.00 | 15.4 |
| EC45 | Pig | ON:HM | 1.58 | 13.2 |
| EC47 | Sheep | ON:H18 | −2.08 | 5.75 |
| EC67 | Goat | O4:H43 | 0.08 | 9.42 |
Decrease in viable cell numbers, expressed in CFU (108) ml−1, following 3 h of incubation in 100 mM lactate at pH 3.8. Negative values indicate growth. Results are the average of two repeat experiments, and the average standard deviation was 1.54 × 108 CFU ml−1.
VT−, verocytotoxin negative.
Kindly supplied by Ian Ogden, Department of Medical Microbiology, University of Aberdeen, Aberdeen Royal Hospitals Trust, Foresterhill, Aberdeen, United Kingdom.
Ethanol has been previously shown to enhance the killing of E. coli O157 strains by lactate (7). We examined the effect of ethanol on d- and l-lactate-mediated viability by using stationary-phase cultures treated with 5% (vol/vol) ethanol and a 100 mM concentration of the organic acid (Table 2). The SDR of NCTC 12900 treated with ethanol and d-lactate (1.36 log10 CFU ml−1 h−1) was much greater (P < 0.05) than that of NCTC 12900 treated with d-lactate alone (0.16 log10 CFU ml−1 h−1). This result concurs with previous data for dl-lactate (2). The SDR were similar for NCTC 12900 treated with l-lactate both with and without ethanol (2.00 and 2.12 log10 CFU ml−1 h−1, respectively). Ethanol increased the SDR of d-lactate and l-lactate-treated F318 cells. Perhaps the ability of ethanol to perturb membranes or the alteration in fatty acid composition associated with alcohols increased the uptake of the undissociated acid (8).
TABLE 2.
SDR following various treatments with 100 mM lactate
| Treatmentb | SDR (log10 CFU ml−1 h−1)a
|
|
|---|---|---|
| NTCC 12900 | F318 | |
| d-Lactate | 0.16ABC | 0.24A |
| l-Lactate | 2.12B | 0.71 |
| d-Lactate + ethanol | 1.36C | 0.79D |
| l-Lactate + ethanol | 2.00 | 2.62D |
| d-Lactate, 20°C | 0.04 | 0.04 |
| l-Lactate, 20°C | 0.57 | 0.07 |
SDR were calculated as the reduction in the viable count per hour and are the average of two repeat experiments. SDR followed by the same letters differ statistically.
The final pH of the cultures was 3.8, and the concentration of ethanol was 5%. Unless otherwise stated, the temperature was 37°C.
The effect of temperature on the antimicrobial effect of lactate was investigated. Stationary-phase cultures of NCTC 12900 and F318 were incubated at 20°C in the presence of 100 mM d-lactate or l-lactate. The SDR of cells treated at 20°C were lower than those of the lactate-treated isolates at 37°C (Table 2). This finding concurs with previously reported data showing that O157 strains became more susceptible to 1.5% lactic acid with increasing temperature (14). Acid dissociation constants are inversely affected by temperature (4). The steady-state intracellular lactate anion concentration that accumulated at 37°C would therefore be greater than that at 20°C, assuming that lactate is able to freely permeate the cytoplasmic membrane. This may explain the reduced susceptibility of E. coli strains to lactate at 20°C. Another possible explanation is that alterations in the fatty acid content of E. coli membranes occur at lower temperatures (8).
The antimicrobial mode of action of organic acids has not been satisfactorily explained (4). Traditionally, only undissociated acid was thought to freely permeate the membrane, where it released toxic acid anions and protons intracellularly according to the intracellular pH (pHi), the protons causing acidification of the cytoplasm and dissipation of the transmembrane proton potential (6). However, this rationale has been dismissed as too simplistic (4), and other mechanisms have been proposed. Cherrington et al. (5) showed that bacteriostatic concentrations of propionic and formic acids interfered with E. coli macromolecular synthesis and it has been proposed that sorbic acid acts as a membrane-active compound for yeasts (13). Jordan et al. (7) showed that lactate caused a reduction in the pHi and suggested that the proton gradient (ΔpH) had collapsed. We decided to investigate the components of the proton motive force with respect to d-lactate and l-lactate.
The pHi, the ΔpH, and the membrane potential (ΔΨ) were determined by a centrifugation method described previously (9). Stationary-phase cells were incubated in 100 mM d- or l-lactate for 10 min. Measurements were performed three to five times. As shown in Table 3, the ΔpH values of cells treated with d-lactate or l-lactate were similar (1.02 and 1.14, respectively), as were the ΔΨ values (−37.54 and −42.92 mV, respectively). Similar trends were observed for F318 cells (Table 3). Although the ΔΨ measurements were relatively low, they were comparable to previous results for nongrowing E. coli in anaerobic environments (1). d-Lactate, which has little antimicrobial effect at 100 mM, caused a reduction in pHi similar to that of the bactericidal agent l-lactate. This suggests that the antimicrobial action of l-lactate is not due to the collapse of the pHi, as previously suggested (7). The reduction in pHi is probably due to a tolerance mechanism that prevents the accumulation of large amounts of acid anions, which was suggested previously (12). As neither the ΔpH nor the ΔΨ has been abolished, it is unlikely that the toxicity of lactate is due to uncoupling. Given that as d-lactate is the major fermentative product of E. coli at low pH under anaerobic conditions (3), it is possible that this organism has developed methods of effectively dealing with high intracellular concentrations of d-lactate, such as efflux mechanisms or conversion to nonacidic end products.
TABLE 3.
Effect of lactate on the proton motive force
| Strain | Acid | Temp (°C) | pHoa | pHib | ΔpHc | ZΔpHd | ΔΨe | Δpf |
|---|---|---|---|---|---|---|---|---|
| NCTC 12900 | l-Lactate | 37 | 3.76 | 4.90 | 1.14 | 70.68 | −42.92 | −113.60 |
| d-Lactate | 37 | 3.84 | 4.86 | 1.02 | 63.05 | −37.54 | −100.63 | |
| F318 | l-Lactate | 37 | 3.81 | 4.34 | 0.53 | 32.65 | −20.35 | −53.00 |
| d-Lactate | 37 | 3.79 | 4.30 | 0.51 | 31.62 | −25.01 | −56.63 |
pHo, external pH in pH units.
pHi values (pH units) are the average of three to five repeat experiments.
pH gradient (pHi − pHo) expressed in pH units.
pH gradient expressed in millivolts, where Z is 62 at 37°C and 58 at 20°C.
Membrane potentials, expressed in millivolts, are the average of three to five repeat experiments.
Proton motive force expressed in millivolts (ΔΨ − ZΔpH).
It has been suggested that direct comparisons of different organic acids with respect to their antimicrobial activities are difficult because of the variation in physical characteristics (4). Studies comparing the antimicrobial effects of the isomers of lactate may provide a useful tool in elucidating the mechanism of action of this acid. As the isomers of lactate have the same pKas and similar structures, it is likely that they will share a similarity in nonspecific interactions but will differ with respect to specific interactions such as enzymatic reactions.
In conclusion, l-lactate has a much greater antimicrobial effect than d-lactate for a wide range of E. coli O157 and non-O157 isolates. This finding may have implications for the use of lactate as an antimicrobial agent and the use of lactic acid bacteria as probiotics. There was no difference between d-lactate- and l-lactate-treated cells with respect to the transmembrane pH gradient, suggesting that the antimicrobial mode of action of l-lactate does not involve abolition of the pHi. This study also highlights the potential use of the isomers of lactate as a tool for elucidating the mechanism of action of lactate.
Acknowledgments
We thank Harry J. Flint, Rowett Research Institute, and Ian Booth, University of Aberdeen, for helpful discussions; Ian Ogden, Department of Medical Microbiology, University of Aberdeen, for providing us with E. coli O157 strains; and Anthony J. Richardson and Kenneth W. Young, Rowett Research Institute, for technical assistance.
We also thank the Department of the Environment, Farming and Rural Affairs (grant OZ0702) and Scottish Enterprise for financial support. The Rowett Research Institute is funded by the Scottish Executive Environment and Rural Affairs Department.
REFERENCES
- 1.Axe, D. D., and J. E. Bailey. 1995. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8-19. [DOI] [PubMed] [Google Scholar]
- 2.Booth, I. R., F. M. Thomson-Carter, P. Carter, S. Jordan, S. Park, L. Malcolm, and J. Glover. 1999. Acid tolerance of Escherichia coli—the sting in the tail?, p. 27-38. In C. S. Stewart and H. J. Flint (ed.), Escherichia coli O157 in farm animals. CAB International, Wallingford, United Kingdom.
- 3.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The idhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology (Reading) 143:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Cherrington, C. A., M. Hinton, G. S. Mead, and I. Chopra. 1991. Organic acids: chemistry, antibacterial activity and practical applications. Adv. Microb. Physiol. 32:87-108. [DOI] [PubMed] [Google Scholar]
- 5.Cherrington, C. A., M. Hinton, and I. Chopra. 1990. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J. Appl. Bacteriol. 68:69-74. [DOI] [PubMed] [Google Scholar]
- 6.Eklund, T. 1983. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol. 69:814-821. [DOI] [PubMed] [Google Scholar]
- 7.Jordan, S. L., J. Glover, L. Malcolm, F. M. Thomson-Carter, I. R. Booth, and S. F. Park. 1999. Augmentation of killing of Escherichia coli O157 by combinations of lactate, ethanol, and low-pH conditions. Appl. Environ. Microbiol. 65:1308-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadner, R. J. 1996. Cytoplasmic membrane, p 58-87. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed.; vol. 1. American Society for Microbiology, Washington, D.C.
- 9.Kroll, R. G., and I. R. Booth. 1981. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem. J. 198:691-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McWilliam Leitch, E. C., and C. S. Stewart. Susceptibility of Escherichia coli O157 and non-O157 isolates to lactate. Lett. Appl. Microbiol., in press. [DOI] [PubMed]
- 11.Probiotic Consortium. 1993. Efficacy and mode of action of probiotics, vol. 1. Non-ruminants, p. 93-95. Rowett Research Institute, Aberdeen, United Kingdom.
- 12.Russell, J. B. 1992. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J. Appl. Bacteriol. 73:363-370. [Google Scholar]
- 13.Stratford, M., and P. A. Anslow. 1998. Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative.' Lett. Appl. Microbiol. 27:203-206. [DOI] [PubMed] [Google Scholar]
- 14.Venkitanaryanan, K. S., T. Zhao, and M. P. Doyle. 1999. Inactivation of Escherichia coli O157:H7 by combinations of GRAS chemicals and temperature. Food Microbiol. 16:75-82. [Google Scholar]