Abstract
Lactobacillus reuteri strain 121 produces a unique, highly branched, soluble glucan in which the majority of the linkages are of the α-(1→4) glucosidic type. The glucan also contains α-(1→6)-linked glucosyl units and 4,6-disubstituted α-glucosyl units at the branching points. Using degenerate primers, based on the amino acid sequences of conserved regions from known glucosyltransferase (gtf) genes from lactic acid bacteria, the L. reuteri strain 121 glucosyltransferase gene (gtfA) was isolated. The gtfA open reading frame (ORF) was 5,343 bp, and it encodes a protein of 1,781 amino acids with a deduced Mr of 198,637. The deduced amino acid sequence of GTFA revealed clear similarities with other glucosyltransferases. GTFA has a relatively large variable N-terminal domain (702 amino acids) with five unique repeats and a relatively short C-terminal domain (267 amino acids). The gtfA gene was expressed in Escherichia coli, yielding an active GTFA enzyme. With respect to binding type and size distribution, the recombinant GTFA enzyme and the L. reuteri strain 121 culture supernatants synthesized identical glucan polymers. Furthermore, the deduced amino acid sequence of the gtfA ORF and the N-terminal amino acid sequence of the glucosyltransferase isolated from culture supernatants of L. reuteri strain 121 were the same. GTFA is thus responsible for the synthesis of the unique glucan polymer in L. reuteri strain 121. This is the first report on the molecular characterization of a glucosyltransferase from a Lactobacillus strain.
Many lactic acid bacteria employ large extracellular enzymes, glucosyltransferases (GTFs) (EC 2.4.1.5; common name, glucansucrases), for the synthesis of high-molecular-weight α-glucans from sucrose. Moreover, low-molecular-weight oligosaccharides are produced in the presence of suitable acceptor molecules.
The GTF enzymes of oral streptococci and the dextransucrases (DSRs) and alternansucrases (ASRs) from Leuconostoc mesenteroides strains have been studied in the most detail. All GTFs from lactic acid bacteria share a common structure and are composed of four distinct domains: their N-terminal end starts with (i) a signal peptide of 32 to 34 amino acids, which is followed by (ii) a highly variable stretch of 123 to 129 amino acids, (iii) a highly conserved catalytic or sucrose binding domain of about 1,000 amino acids, and (iv) a C-terminal glucan binding domain of about 500 amino acids, which is composed of a series of tandem repeats (30).
Amino acid sequence comparisons revealed that GTFs possess a (β/α)8 barrel structure similar to that of glycoside hydrolases of family 13. This family includes, for instance, α-amylase and cyclodextrin glycosyltransferase (CGTase) (43). The core of the proteins belonging to this family comprises eight β-sheets alternated with eight α-helices. In GTFs, however, this (β/α)8 barrel structure is circularly permuted (12, 27). Therefore, GTFs are classified in family 70 of glycoside hydrolases (http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html). Some GTF enzymes, such as dextransucrase from L. mesenteroides strain B-512F, catalyze the formation of linear glucans containing mostly α-(1→6) linkages (dextrans). Other types synthesize dextrans with α-(1→2), α-(1→3), or α-(1→4) branches (16, 30). ASR from L. mesenteroides strain NRRL B-1355 synthesizes a glucan with alternating α-(1→6) and α-(1→3) glucosidic bonds (3). Recently, a GTF of L. mesenteroides strain NRLL B-1355 that synthesizes a glucan containing α-(1→2) glucosidic linkages has been characterized (39). There are few reports, however, about glucan synthesis in lactobacilli (13, 21, 37, 44, 45). A biochemical and molecular characterization of the enzyme(s) responsible for glucan synthesis in lactobacilli has not been reported.
In previous studies we have isolated a strain of Lactobacillus reuteri that is capable of producing both a fructan and a glucan. Depending on the carbon source in the culture medium, the GTF responsible for the synthesis of this polysaccharide material is completely cell associated or partly released into the culture medium (44, 45). This work describes the first molecular characterization of a Lactobacillus GTF gene (gtfA), expression of gtfA in Escherichia coli, and the characterization of its glucan product. The gtfA gene encodes a novel type of GTF, synthesizing a highly branched glucan with a unique structure containing α-(1→4) and α-(1→6) linkages.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
L. reuteri strains 121 and 35-5 (both obtained from the culture collection of TNO Nutrition and Food Research, Zeist, The Netherlands) were cultivated anaerobically at 37°C in MRS medium (Difco, Franklin Lakes, N.J.) (9) or in MRS-s medium (i.e., MRS medium with sucrose [100 g liter−1] instead of glucose [20 g liter−1]). E. coli DH5α (Phabagen, Utrecht, The Netherlands) (22), E. coli JM 109 (Promega, Madison, Wis.) (22), and E. coli TOP 10 (Invitrogen, Carlsbad, Calif.) were used as hosts for cloning purposes. Plasmids pCR2.1-TOPO (Invitrogen), pCR-XL-TOPO (Invitrogen), and pEMBL8 (11) were used for cloning of the GTF gene for sequencing purposes. Plasmid pBluescript II SK(+) (Stratagene, La Jolla, Calif.) was used for cloning of the complete GTF gene. Plasmid pET15b (Novagen, Madison, Wis.) was used for expression of the gtf gene in E. coli BL21 Star(DE3) (Invitrogen). E. coli strains were grown aerobically at 37°C in LB medium (4). E. coli strains containing recombinant plasmids were cultivated in LB medium with the appropriate antibiotic (ampicillin [100 μg ml−1] or kanamycin [50 μg ml−1]). Agar plates were made by adding 1.5% agar to the LB medium; when appropriate, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (20 μg ml−1) was added.
Isolation of DNA.
L. reuteri strain 121 total DNA was isolated according to the method in reference 32. Plasmid DNA of L. reuteri strain 121 was isolated using a modification of the methods described previously (2, 7). Prewarmed (37°C) fresh MRS broth (10 ml) was inoculated with 200 μl of an overnight culture and incubated for 2.5 h at 37°C. Cells were harvested by centrifugation and washed with 2 ml of sterile STE buffer (0.1 M NaCl, 10 mM Tris-HCl, 1 mM EDTA [pH 8]). After centrifugation, the pellet was resuspended in 380 μl of solution I (0.5 M sucrose, 50 mM Tris-HCl, 1 mM EDTA [pH 8]) containing lysozyme (2 mg/ml; Sigma, St. Louis, Mo.) and 6.6 U of mutanolysin (Sigma). After 1.5 h of incubation at 37°C, 50 μl of solution II (0.25 M EDTA, 50 mM Tris-HCl [pH 8]) and 30 μl of solution III (20% sodium dodecyl sulfate, 50 mM Tris-HCl, 20 mM EDTA [pH 8]) were added and the suspension was mixed. NaOH (30 μl of 3 M solution) was added, which was followed by 50 μl of 2 M Tris-HCl and 72 μl of 5 N NaCl. After extraction with equal volumes of phenol and chloroform, the DNA was precipitated with ethanol as described previously (35).
Plasmid DNA of E. coli was isolated using the alkaline lysis method (6) or with a Wizard Plus SV plasmid extraction kit (Promega).
Molecular techniques.
General procedures for cloning, E. coli transformations, DNA manipulations, and agarose gel electrophoresis were carried out as described previously (35). Restriction endonuclease digestions and ligations with T4 DNA ligase were performed as recommended by the enzyme suppliers (New England Biolabs, Beverly, Mass.; Roche Biochemicals, Basel, Switzerland). Primers were obtained from Eurogentec, Seraing, Belgium. Sequencing was performed according to the method in reference 36. DNA was amplified by PCR on a DNA Thermal Cycler 480 (Perkin-Elmer, Boston, Mass.) using ampliTAQ DNA polymerase (Perkin-Elmer) or Pwo DNA polymerase (Roche Biochemicals). For inverse PCR the Expand High Fidelity PCR system (Roche Biochemicals) was used as described by the supplier. Fragments were isolated from agarose gels using a Qiagen (Hilden, Germany) gel extraction kit following the instructions of the supplier.
For Southern hybridization, DNA was restricted with endonucleases, separated by agarose gel electrophoresis, and transferred to a Hybond nylon membrane (Amersham Pharmacia Biotech, Piscataway, N.J.) following the manufacturer's instructions. The probes, which were labeled with digoxigenin-dUTP, were prepared, and patterns of hybridization with the probes were examined with a digoxigenin DNA labeling and detection kit (Roche Biochemicals), following the manufacturer's instructions. Stringent probe hybridizations were performed at 65°C, and nonstringent hybridizations were performed at 45°C.
Identification and nucleotide sequence analysis of the GTF gene.
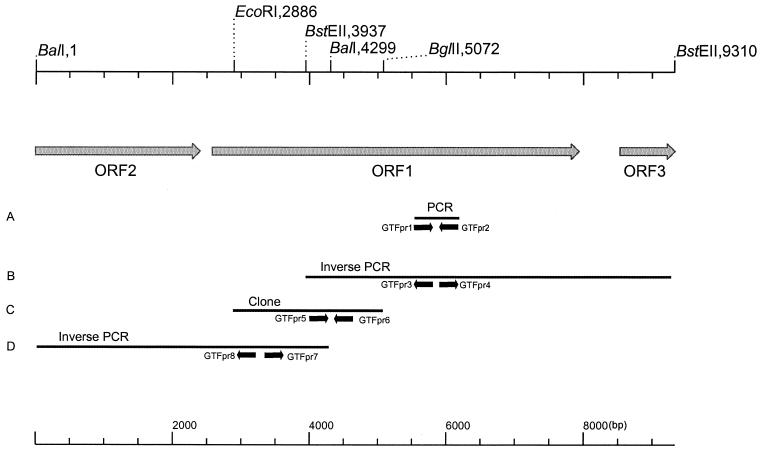
An overview of the isolation strategy of the gtf gene is given in Fig. 1. The first part of the GTF gene was isolated by amplification of chromosomal DNA of L. reuteri strain 121 with PCR using degenerate primers (GTFpr1, 5′-GAYAAKWSNAAKSYNRTNGTNSARGC-3′, and GTFpr2, 5′-ANRTCNCCRTARTANACNCKNG-3′ [Y = T or C; K = G or T; W = A or T; S = C or G; R = A or G; N = A, C, G, or T]) based on conserved amino acid sequences present in the catalytic core, as deduced from the GTF genes of Streptococcus downei (gtfS), Streptococcus mutans (gtfC), S. downei (gtfI), Streptococcus salivarius (gtfK and gtfM), and dsrA of L. mesenteroides (14, 18, 20, 31, 38, 40). An amplification product with the expected size of about 660 bp was obtained and cloned in E. coli JM 109 using the pCR2.1 vector. Analysis of its nucleotide sequence (659 bp [Fig. 1A]) confirmed its GTF identity. The 659-bp amplified fragment was used to design the primers GTFpr3 (5′-GGGCTTTCAGATGCAACTAATCGTTGGGG-3′) and GTFpr4 (5′-GCTTATTTGAGACAGCATCTGAGTCCATACC-3′) for inverse PCR. L. reuteri strain 121 chromosomal DNA was digested with BstEII and ligated, yielding circular DNA molecules. PCR with diverging primers GTFpr3 and GTFpr4 with the circular ligation products as template yielded an amplicon of 5,229 bp (Fig. 1B), which was cloned into pCR-XL-TOPO (Invitrogen). Based on the 5,229-bp nucleotide sequence, a probe encompassing GTFpr5 (5′-GGTAATGATAAGCGCCATACAG-3′) and GTFpr6 (5′-GATATACTGCTGCTACATCTGGAC-3′) was designed to screen a partial gene library of L. reuteri strain 121. This library was constructed by ligating EcoRI/BglII fragments of genomic DNA into the EcoRI/BamHI sites of pEMBL8. A positive clone was selected by colony blot hybridization (8) using Hybond N-filters (Amersham Pharmacia Biotech), and its nucleotide sequence was determined (2,186 bp [Fig. 1C]). This information was used to design the diverging primers GTFpr7 (5′-CGGGATTCAATTGAAATTGTTAGTCGATACAG-3′) and GTFpr8 (5′-GAGATGCGTTCGTTGCATTCCATCCTGAAAC-3′) for an inverse PCR step with BalI-digested and ligated chromosomal DNA. The inverse PCR fragment obtained was cloned into pCR-XL-TOPO (Invitrogen) and sequenced (4,299 bp [Fig. 1D]).
FIG. 1.
Strategy used for the isolation of the gtfA gene and surrounding regions from L. reuteri strain 121 chromosomal DNA. Primers are indicated with small, black arrows. (A) The fragment given is the 659-bp insert isolated with degenerated primers. (B and D) The fragments are regions amplified by inverse PCR (5,229 and 4,229 bp, respectively). (C) The fragment (2,186 bp) was obtained by colony blot hybridization of a partial gene library.
Construction of plasmids for expression of the GTF gene in E. coli.
Two separate PCRs with primers GTFpr11 to -14 were used to amplify the complete gtf gene (Fig. 2). The product of the first PCR, synthesized with primers GTFpr11 (5′-GATGCATGAGCTCCCATGGACCAACAAGTTCAGCAAGCTTCC-3′, containing SacI [in boldface type] and NcoI [underlined] sites) and GTFpr12 (5′-GTGCATTAAAGTACGTAACCAATCAGTATTTCCGG-3′), was digested with SacI/PstI. The product of the second PCR, primed with oligonucleotides GTFpr13 (5′-TTGATGGTATGGTGGCCTAATACTCTTACCC) and GTFpr14 (5′-ATATCGATGGGCCCCGGATCCTATTAGTGATGGTGATGGTGATGTAGTTTATTTTGATCAAGCATCTTACC-3′, containing ApaI [in boldface type] and BamHI [underlined] sites and a six-His tag [in italics]), was digested with PstI/ApaI. The resulting fragments (2,547 and 2,723 bp) were cloned in the corresponding sites of pBluescript II SK(+), yielding pBSP2500 and pBPA2700, respectively. Both plasmids were digested with ApaI/PstI, and the fragment from pBPA2700 containing the 3′ part of gtf was ligated into pBSP2500, yielding pBGTF1. Plasmid pBGTF1 was digested with NcoI/BamHI, and the resulting 5.3-kb fragment was ligated into the corresponding sites of the expression vector pET15b (Novagen) yielding p15gtf.
FIG. 2.
Overview of primers and restriction sites used for cloning of the gtfA gene in the expression vector pET15B (Novagen).
Dendrogram construction.
Amino acid sequences were aligned with Clustal W 1.74 (23), with a gap opening penalty of 30 and a gap extension penalty of 0.5. Amino acid sequences were obtained from GenBank (accession numbers are given in parentheses) and divided into the following three groups: (i) DSRB of L. mesenteroides NRRL B-1299 (AAB95453), DSRS of L. mesenteroides NRRL B-512F (AAA53749), DSRA of L. mesenteroides NRRL B-1299 (AAB40875), ASR of L. mesenteroides NRRL B-1355 (CAB65910), DSRT of L. mesenteroides NRRL B-512F (BAA90527), and GTFA of L. reuteri strain 121; (ii) GTFIa of Streptococcus sobrinus OMZ176 (BAA14241), GTFI of S. downei Mfe28 (BAA0296), GTFIs of S. sobrinus OMZ176 (BAA02976), GTFB of S. mutans GS5 (AAA88588), and GTFC of S. mutans GS5 (AAA88589); and (iii) GTFG of Streptococcus gordonii (AAC43483), GTFR of Streptococcus oralis ATCC 10557 (BAA95201), GTFS of S. downei Mfe28 (AAA26898), GTFM of S. salivarius ATCC 25975 (AAC41413), GTFL of S. salivarius ATCC 25975 (AAC41412), GTFN of S. salivarius ATCC 25975 (AAC05165), GTFD of S. mutans GS5 (AAA26895), GTFJ of S. salivarius ATCC 25975 (CAA77900), GTFT of S. sobrinus OMZ176 (D13928), and GTFK of S. salivarius ATCC 25975 (CAA77898). Amino acid sequences were aligned first within each group. The complete alignment was performed by aligning groups i to iii with each other. Tree construction was performed using TreeCon 1.3b (no correction for distance estimation, 10 bootstrap samples, and the neighbor-joining algorithm were used) (42).
Preparation of E. coli cell extracts.
Cells of E. coli BL21 Star(DE3) harboring p15gtf were harvested by centrifugation (10 min at 4°C at 10,000 × g) after 16 h of growth. The pellet was washed with 50 mM sodium acetate buffer, pH 5.5, containing 1 mM CaCl2 and 1% (vol/vol) Tween 80, and the suspension was centrifuged again (10 min at 4°C at 10,000 × g). Pelleted cells were resuspended in 50 mM sodium acetate buffer, pH 5.5, containing 1 mM CaCl2, 1% (vol/vol) Tween 80, and 5 mM β-mercaptoethanol. Cells were broken by sonication (seven 15-s pulses of 7 μ at 30-s intervals). Cell debris and intact cells were removed by centrifugation for 20 min at 4°C at 10,000 × g, and the resulting cell extract (supernatant) was used in the enzyme assays.
Enzyme assays.
Using E. coli cell extracts or L. reuteri strain 121 grown on MRS-s culture supernatant as the source of enzyme, GTF activity was measured by determining the release of fructose from sucrose at 37°C in 50 mM sodium acetate buffer, pH 5.5, containing 1 mM CaCl2 and 100 mM sucrose (44).
Amino acid sequence determination from the GTF from L. reuteri strain 121 using 2D-PAGE.
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) (i.e., isoelectric focusing followed by sodium dodecyl sulfate-PAGE) experiments were performed as described earlier (33), using the II xi Cell system according to the manufacturer's instructions (Bio-Rad, Hercules, Calif.). Chemicals and materials were from Bio-Rad, unless indicated otherwise.
(i) Sample preparation.
Supernatants of cultures of L. reuteri strain 121 grown on MRS-s (10 ml precipitated with 2 ml of 30% trichloroacetic acid) in the exponential phase of growth, harvested by centrifugation (10,000 × g at 4°C for 10 min), were used as a source of GTF protein.
(ii) GTF activity staining.
After separation in the second dimension, GTF activity was identified as described previously (44), using periodic acid-Schiff staining. The same gel was silver stained (Genotech, St. Louis, Mo.) to identify other proteins on the gel, thereby facilitating the identification of the GTF spot on the blot (see below).
(iii) Amino acid sequencing.
A second gel obtained after 2D-PAGE, identical to the GTF activity stained gel, was blotted according to the method in reference 26 onto a polyvinylidene difluoride membrane (Roche Biochemicals) with a semidry electroblotter (Ancos, Hoejby, Denmark). The polyvinylidene difluoride membrane was stained with Coomassie brilliant blue R-250. The N-terminal amino acid sequence of the protein band which corresponded with the activity band obtained by periodic acid-Schiff staining was determined on a Perkin-Elmer ABI 476A automated sequencer using Edman degradation (NAPS Protein Sequencing and Peptide Mapping Laboratory, Vancouver, Canada).
Characterization of the glucans produced.
Glucans were produced by incubating the enzyme preparations overnight, using the conditions described above under “Enzyme assays.” Glucans produced by L. reuteri strain 121 and strain 35-5 (producing only the wild-type glucan and not the fructan) and glucans produced with the GTF expressed in E. coli were isolated by precipitation with ethanol (44). Nuclear magnetic resonance (NMR) spectroscopy and methylation analysis were performed as described earlier (44). The molecular weights of the glucans were determined by high-performance size exclusion chromatography (HPSEC) coupled online with a multiangle laser light scattering (MALLS) and a differential refractive index detection (Schambeck SDF). The HPSEC system consisted of an isocratic pump, an injection valve, a guard column, and a set of two SEC columns in series (Shodex SB806MHQ column and TSK gel 6000PW). A Dawn-DSP-F (Wyatt Technology, St. Barbara, Calif.) laser photometer (HeNe [λ = 632.8 nm]) equipped with a K5 flow cell, thermostatted by a Peltier heating system, was used as a MALLS detector. Samples were filtered through a 0.45-μm-pore-size filter (MILLEX), and the injection volume was 220 μl. Na2SO4 (0.1 M) was used as eluent at a flow rate of 0.8 ml min−1. Pullulan and dextran samples with Mw ranging from 4 × 104 to 2 × 106 were used as standards. Determinations were performed in duplicate.
Nucleotide accession number.
The nucleotide sequence of gtfA has been assigned accession no. AX306822 by GenBank.
RESULTS
Isolation and nucleotide sequence analysis of the putative L. reuteri strain 121 GTF gene.
Based on sequence homology between conserved regions located in the catalytic core of different gtf genes of gram-positive bacteria, degenerate primers were designed and used for PCR with chromosomal DNA of L. reuteri strain 121 as the template. A single fragment of 659 bp (Fig. 1) was obtained, and sequence analysis confirmed its gtf identity. Southern hybridization of chromosomal DNA of L. reuteri strain 121 with this 659-bp amplified PCR fragment, followed by washing under nonstringent conditions, revealed one hybridizing fragment. Plasmid DNA of L. reuteri did not show hybridization.
In subsequent steps a total of 9,310 bp was obtained and sequenced (Fig. 1). One complete open reading frame (ORF) and two partial ORFs were located on this compiled sequence: ORF1 (5,343 bp [Fig. 1]), which encodes a putative GTF (GTFA); ORF2 (2,403 bp), upstream of ORF1; and ORF3 (786 bp), downstream of gtfA. The deduced amino acid sequence of ORF2 showed homology with GTFs, whereas the deduced amino acid sequence of ORF3 did not show significant homology to any protein present in databases. The gtfA gene encodes a putative protein of 1,781 amino acids, with a deduced molecular weight of 198,637 and a pI of 5.04. It was preceded by a putative ribosomal binding site (GAAGGAG), localized 6 bp upstream from the ATG start codon. According to the consensus promoter sequences described previously for lactobacilli (34), a potential promoter sequence in the sequence upstream of gtfA (42 bp from the start codon) could be identified, with a −35 sequence (TTGAAA) separated by 19 bp from a −10 sequence (TATAAT).
Two inverted repeats were located 61 bp downstream from the gtfA termination codon. These repeats could form a stem (20 bp)-loop (12 nucleotides) secondary structure with a ΔG value of −20.4 kcal mol−1, followed by a series of thymidine residues, suggesting a rho-independent transcription termination signal (5).
Amino acid sequence alignments of L. reuteri GTFA with other GTFs.
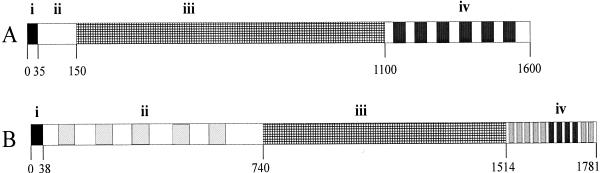
Alignment of the amino acid sequence of GTFA with other GTFs, performed with BLAST (1), revealed clear similarities. The highest similarity (46% identity and 59% similarity within 1,261 amino acids) at the amino acid level was found with ASR from L. mesenteroides NRRL B-1355. The putative protein structure of GTFA was similar to that of other GTFs containing (i) an N-terminal signal sequence of 38 amino acids, (ii) a relatively large variable N-terminal domain of 702 amino acids, (iii) a catalytic domain of 774 amino acids, and (iv) a C-terminal domain of 267 amino acids (Fig. 3).
FIG. 3.
Schematic representation of GTFs in general (A) and GTFA of L. reuteri strain 121 (B), showing the four different domains—(i) N-terminal signal sequence, (ii) variable region with five RDV repeats (light grey boxes), (iii) catalytic domain, and (iv) C-terminal (putative) glucan binding domain with four YG repeating units (dark grey boxes)—according to the definition of Giffard and Jacques (19) and seven less- conserved YG repeating units (light grey boxes).
The deduced N-terminal amino acid sequence of GTFA contained a putative secretion peptide with a predicted signal peptidase cleavage site between amino acids 38 and 39 (http://www.cbs.dtu.dk/services/SignalP/). To confirm this cleavage site, the L. reuteri strain 121 enzyme was purified by 2D-PAGE and subjected to N-terminal sequence analysis. The first 13 amino acids were identified as DQQVQQASTLQDQ; except for the 10th residue the sequence was identical to that of the deduced amino acid sequence following the predicted cleavage site. 2D-PAGE experiments also confirmed the predicted Mr and pI of GTFA. Within the deduced N-terminal variable region of GTFA, a series of five repeating units was found. These repeating units, designated RDV, were on average 41 amino acids long, separated on average by 71 amino acids (Fig. 4). These repeats have never been seen in other GTF enzymes and showed no significant homology to any protein motifs present in the databases.
FIG. 4.
Alignment of the five unique repeat elements (RDV1 to RDV5) from the N-terminal region of GTFA of L. reuteri strain 121. Conserved (.), highly conserved (:), and identical (∗) residues are indicated. The RDV motif is indicated in boldface type [R(N/P)DV]. Two other conserved motifs, SGF and R(F/Y)S, are indicated by boxes.
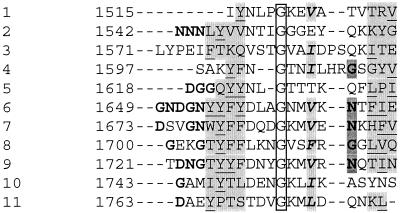
The putative catalytic domain of GTFA showed high similarity (about 45% identity and 60% similarity) to other known streptococcal and Leuconostoc GTF enzymes. However, not all of the conserved amino acids found in the other GTFs were found in the deduced amino acid sequence of GTFA. Particularly in the region downstream of Asp1024, 3 out of 10 conserved amino acids were not found in GTFA (Fig. 5). One of the conserved amino acid substitutions in this region of GTFA, Ile1029, was also found in amylosucrase, a GTF from Neisseria polysaccharea synthesizing an α-(1→4) glucan (10).
FIG. 5.
Alignment of parts of the catalytic cores of DSRS of L. mesenteroides NRRL B-512F (46), GTFD of S. mutans GS5 (15), ASR of L. mesenteroides NRRL B-1355 (3), GTFA of L. reuteri strain 121, and amylosucrase (AS) of N. polysaccharea (10). Amino acids which are conserved in all GTFs but not in GTFA are shown in boldface type (also underlined). Symbols: ∗, identical residue; :, highly conserved residue; ., conserved residue; ⇓, putative catalytic residue; ▿, residue possibly playing a role in binding of acceptor molecules and in the transfer of the glucosyl residue; ⋄, putative chloride binding site. The alignment shown is based on an alignment of glucansucrases made by Monchois et al. (30).
The relatively short C-terminal domain of GTFA contains four YG-repeating units according to the definition of Giffard and Jacques (19) and seven YG-repeating units which are less conserved (Fig. 6).
FIG. 6.
Alignment of repeats in the C-terminal region of GTFA of L. reuteri strain 121. YG repeats consist of, from left to right, (i) four to six residues that usually include glycine and/or aspartate and/or asparagine residues (indicated in boldface type), (ii) a cluster of one to four residues (lightly shaded) that nearly always includes tyrosine (underlined), (iii) three to four poorly conserved residues, (iv) a glycine residue (boxed), (v) two poorly conserved residues, (vi) a residue that is usually hydrophobic (indicated in boldface italic type and lightly shaded), (vii) one poorly conserved residue, (viii) a neutral polar residue (darkly shaded) that is usually glycine or asparagine (indicated in bold type), (ix) one poorly conserved residue, and (x) three residues (darkly shaded) that include one to three hydrophobic residues (underlined) (19). Four repeats (rows 6, 7, 8, and 9) match exactly the consensus sequence defined by Giffard and Jacques (19). The other seven show lower homology with the YG repeats.
Dendrogram.
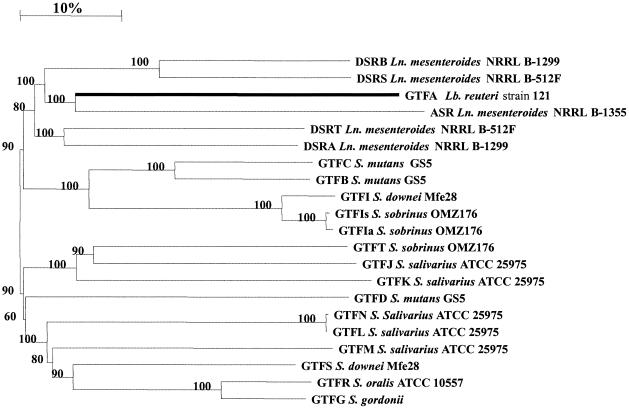
Construction of a dendrogram (Fig. 7), based on the complete amino acid sequences of different GTFs of lactic acid bacteria, revealed that GTFA of L. reuteri strain 121 is distinct from other GTFs known. Again, GTFA is most closely related to the ASR of L. mesenteroides NRRL B-1355 (3).
FIG. 7.
Dendrogram of GTFs of lactic acid bacteria. The horizontal distances are a measure for the differences at the amino acid level. The length of the upper bar indicates a 10% difference. Bootstrap values are given at the root of each branch (as a percentage). The GTFA of L. reuteri strain 121 is indicated with a thick black line.
Analysis of the glucans produced by L. reuteri and E. coli containing p15gtf.
Extracts of E. coli(p15gtf) cells and supernatants of sucrose-grown cultures of L. reuteri incubated with sucrose both produced high-molecular-weight glucans. Using HPSEC-MALLS, the average molecular weight of the glucan produced by L. reuteri strain 35-5 was determined to be 4 × 107 (±5% SEM), whereas that of the glucan produced by E. coli harboring p15gtf was 8 × 107 (±5% SEM).
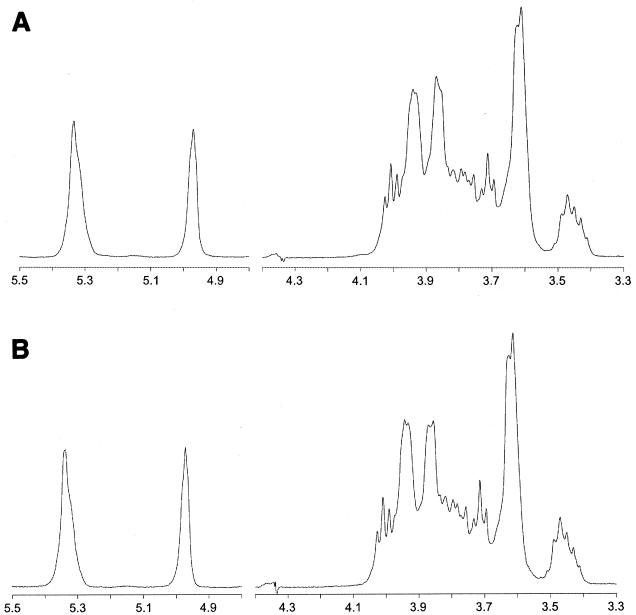
The 1H NMR spectra of the glucans produced by the recombinant GTF present in E. coli and the GTF enzyme in L. reuteri strain 35-5, producing only the glucan, were virtually identical (Fig. 8). Comparison of both 1H NMR spectra with that of potato starch (17) showed that both glucans consist of α-(1→4)- and α-(1→6)-linked glucopyranosyl units. The identical nature of the glucans was confirmed by methylation analysis (Table 1).
FIG. 8.
500-MHz 1H NMR spectra of the glucan produced by L. reuteri strain 35-5 GTFA present in culture supernatant (A) and by E. coli GTFA cell extracts (B), recorded in D2O at 80°C. Chemical shifts are given in parts per million relative to the signal of internal acetone (δ = 2.225).
TABLE 1.
Methylation analysis of the glucans produced by L. reuteri strains and E. coli GTFA
| Type of glucosyl unit | % Methylation
|
||
|---|---|---|---|
| L. reuteri strain 121 | L. reuteri strain 35-5 | E. coli (pBGTF1) | |
| Glcp-(1→ | 24 | 25 | 21 |
| →4)-Glcp-(1→ | 42 | 43 | 44 |
| →6)-Glcp-(1→ | 22 | 21 | 24 |
| →4,6)-Glcp-(1→ | 12 | 11 | 11 |
DISCUSSION
This work reports the molecular characterization of the first Lactobacillus gene (gtfA) encoding a GTF enzyme (GTFA). A detailed analysis showed that GTFA produces a unique soluble glucan in which the majority of the linkages are of the α-(1→4) glucosidic type. The glucan also contains α-(1→6)-linked glucosyl units and 4,6-disubstituted α-glucosyl units at the branching points. Expression of gtfA in E. coli yielded an active GTF synthesizing the same highly branched soluble glucan.
GTFA showed high similarity with streptococcal and Leuconostoc GTFs. Similar to other GTFs, GTFA contains an N-terminal signal sequence, a variable N-terminal domain, a catalytic core, and a C-terminal domain. Striking features of GTFA are its relatively large variable domain (702 amino acids), which contains five distinct unique repeats (RDV repeats) (Fig. 4), and its relatively short C-terminal domain (267 amino acids). ASR of L. mesenteroides NRRL B-1355 also possesses three N-terminal repeats (24), but these do not show homology to the N-terminal repeats found in GTFA. The exact function of the variable domain is unknown. The variable domain of GTFI from S. downei MFe28 contains no repeats and is five times smaller than the GTFA domain. Deletion of the GTFI variable domain yielded a mutant enzyme which retained function (28).
Based on alignments with other GTFs from lactic acid bacteria (30), the putative catalytic residues in L. reuteri strain 121 GTFA are Asp1024, Glu1061, and Asp1133. The putative calcium-binding site is Asp986, and the putative chloride binding site is Arg1022. Addition of Ca2+ increases enzyme activity and stability (data not shown). Five residues may play a role in the binding of acceptor molecules and the transfer of the glucosyl residue. These are GTFA residues Asp1027, Asn1028, Asp1062, and Trp1063 (Fig. 5). The fifth amino acid, possibly playing a role in acceptor binding or transfer of the glucosyl residue, a Ser in other GTFs (except for ASR of L. mesenteroides and DSRA of L. mesenteroides), was replaced by Asn1064 in GTFA.
The C-terminal domain of GTFA, which consists of 267 amino acids, is shorter than corresponding domains in other GTFs (∼500 amino acids). The C-terminal domain of streptococcal and Leuconostoc GTFs consist of a series of different tandem repeats, which have been divided into four classes: A, B, C, and D repeats. These repeats exhibit high similarity to the repeats found in the glucan binding protein from S. mutans as well as the ligand binding domains in Clostridium difficile toxin A and the lysins from Streptococcus pneumoniae (18, 47). DSRS from L. mesenteroides NRRL B-512F also contains, in addition to A and C repeats, N repeats, which have not been identified in streptococcal GTFs. ASR from L. mesenteroides NRRL B-1355 contains a single A repeat and distinct short repeats DG(X)4APY (24). Within the A, B, C, and D repeats, a repeating unit designated YG can be distinguished (19). The A, B, C, and D repeats present in distinct patterns in the C-terminal domain of other GTFs were not found in GTFA. Instead, four YG repeating units and seven less-conserved YG repeats could be identified (Fig. 6).
The highest overall homology of GTFA at the amino acid level was found with ASR from L. mesenteroides NRRL B-1355 (3), which is responsible for the synthesis of an alternan with 50% α-(1→6) and 50% α-(1→3) linkages, and with DSRS from L. mesenteroides NRRL B-512F (29, 46), which synthesizes a dextran with 95% α-(1→6) and 5% α-(1→3) linkages. Homology of GTFA with other GTFs was highest in the highly conserved putative catalytic domain, which had roughly the same size and structure as the corresponding domains of other GTFs. However, not all the conserved residues were found in the L. reuteri strain 121 GTFA. Relatively many differences with amino acids conserved in other GTFs were found directly downstream of the putative catalytic Asp1024 (Fig. 5). This region constitutes the α/β-barrel 4 of the enzymes of family 13 of glycoside hydrolases (27). The domain directly downstream of the catalytic Asp1024 contains the conserved amino acids Asp-Ala-Val-Asp-Asn in other GTFs. In CGTase these residues constitute part of the acceptor binding site (residues Asp229-Ala-Val-Lys-His233 in Bacillus circulans 251 CGTase), responsible for the stereospecific positioning of the molecule accepting the glucosyl unit (25). The structure of this acceptor site determines the type of glucosidic bond formed (41). In the corresponding region of GTFA, Pro1026 is found in a position where a conserved Val is found in other GTFs (Fig. 5). Compared with Val, the presence of Pro causes a more rigid protein structure, which may have a direct effect on the type of glucosidic bonds formed in the glucan synthesized by the enzyme. The presence of the Pro1026 residue could therefore be part of the explanation for the unique structure of the glucan with α-(1→4) and α-(1→6) bonds produced by GTFA. The conserved Val is present in amylosucrase (Fig. 5), a GTF synthesizing α-(1→4) bonds. However, immediately downstream of this Val, the conserved Asp-Asn residues are replaced by Ala-Phe (10). The following amino acid in amylosucrase is Ile, which is also present at that position in GTFA, whereas in other GTFs a conserved Val is found (Fig. 5). This also suggests that the above-mentioned region downstream of the catalytic Asp1024 may be of influence on the type of bonds being formed. Therefore, Pro1026 and Ile1029 of GTFA are likely targets for site-directed mutagenesis experiments.
The partial ORF upstream of gtfA (ORF2 [Fig. 1]) may encode a second GTF enzyme in L. reuteri strain 121. However, the N-terminal amino acid sequence of GTF purified from culture supernatants of L. reuteri strain 121 was the same as the deduced N-terminal amino acid sequence of the gtfA gene, and the Mr and pI of the purified enzyme were the same as those predicted from the nucleotide sequence of the gtfA gene. Furthermore, the 1H NMR spectra of the glucans produced by the L. reuteri GTFA present in culture supernatant and by the E. coli GTFA in cell extracts were virtually identical (Fig. 8). This, combined with the results of the methylation (Table 1) and the molecular weight determinations of the glucans, shows that the E. coli GTFA and the L. reuteri enzyme present in culture supernatants synthesize the same glucan with a unique structure: a highly branched glucan containing α-(1→4) and α-(1→6) bonds. Therefore, it is concluded that the gtfA gene encodes the active GTF of L. reuteri strain 121.
In the future, ORF2 upstream of gtfA will be characterized in further detail and GTFA will be characterized molecularly and biochemically.
Acknowledgments
S.K. and G.H.V.G.-S. contributed equally to this work.
We thank Jos van der Vossen, Egbert Smit, Dick van den Berg, and Peter Terpstra for help with the isolation of gtfA; Isabel Capron for the HPSEC-MALLS analyses; Joost Uitdehaag, Bart van der Veen, and Hans Leemhuis for discussions concerning the structure-function relationships of family 13 glycoside hydrolases; and Sacha van Hijum for his contribution in the construction of the dendrogram.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmids DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arguello-Morales, M. A., M. Remaud-Simeon, S. Pizzut, P. Sarcabal, and P. Monsan. 2000. Sequence analysis of the gene encoding alternansucrase, a sucrose glucosyltransferase from Leuconostoc mesenteroides NRRL B-1355. FEMS Microbiol. Lett. 182:81-85. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. E. Brent, D. D. Kingston, J. G. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Bektesh, S. L., and J. P. Richardson. 1980. A rho-recognition site on phage lambda cro-gene mRNA. Nature 283:102-104. [DOI] [PubMed] [Google Scholar]
- 6.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger, J. H., and L. M. T. Dicks. 1994. Technique for isolating plasmids from exopolysaccharides-producing Lactobacillus spp. Biotechnol. Technol. 8:769-772. [Google Scholar]
- 8.Datta, A. R., and A. M. MacQuillan. 1987. Salt tolerance of lactose-grown Vibrio parahaemolyticus carrying Escherichia coli lac genes. Appl. Environ. Microbiol. 53:466-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 10.De Montalk, G. P., M. Remaud-Simeon, R. M. Willemot, V. Planchot, and P. Monsan. 1999. Sequence analysis of the gene encoding amylosucrase from Neisseria polysaccharea and characterization of the recombinant enzyme. J. Bacteriol. 181:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dente, L., G. Cesareni, and R. Cortese. 1983. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 11:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devulapalle, K. S., S. D. Goodman, Q. Gao, A. Hemsley, and G. Mooser. 1997. Knowledge-based model of a glucosyltransferase from the oral bacterial group of mutans streptococci. Protein Sci. 6:2489-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunican, L. K., and H. W. Seeley, Jr. 1963. Temperature-sensitive dextransucrase production by a lactobacillus. J. Bacteriol. 86:1079-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferretti, J. J., M. L. Gilpin, and R. R. Russell. 1987. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J. Bacteriol. 169:4271-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara, T., Y. Terao, T. Hoshino, S. Kawabata, T. Ooshima, S. Sobue, S. Kimura, and S. Hamada. 1998. Molecular analyses of glucosyltransferase genes among strains of Streptococcus mutans. FEMS Microbiol. Lett. 161:331-336. [DOI] [PubMed] [Google Scholar]
- 16.Funane, K., T. Ishii, M. Matsushita, K. Hori, K. Mizuno, H. Takahara, Y. Kitamura, and M. Kobayashi. 2001. Water-soluble and water-insoluble glucans produced by Escherichia coli recombinant dextransucrases from Leuconostoc mesenteroides NRRL B-512F. Carbohydr. Res. 334:19-25. [DOI] [PubMed] [Google Scholar]
- 17.Gidley, M. J. 1985. Quantification of the structural features of starch polysaccharides by NMR spectroscopy. Carbohydr. Res. 139:85-93. [Google Scholar]
- 18.Giffard, P. M., D. M. Allen, C. P. Milward, C. L. Simpson, and N. A. Jacques. 1993. Sequence of the gtfK gene of Streptococcus salivarius ATCC 25975 and evolution of the gtf genes of oral streptococci. J. Gen. Microbiol. 139:1511-1522. [DOI] [PubMed] [Google Scholar]
- 19.Giffard, P. M., and N. A. Jacques. 1994. Definition of a fundamental repeating unit in streptococcal glucosyltransferase glucan-binding regions and related sequences. J. Dent. Res. 73:1133-1141. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore, K. S., R. R. Russell, and J. J. Ferretti. 1990. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect. Immun. 58:2452-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond, B. F. 1969. Dextran production by a human oral strain of Lactobacillus casei. Arch. Oral Biol. 14:879-890. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 23.Higgins, D. G., and P. M. Sharp. 1988. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237-244. [DOI] [PubMed] [Google Scholar]
- 24.Janecek, S., B. Svensson, and R. R. Russell. 2000. Location of repeat elements in glucansucrases of Leuconostoc and Streptococcus species. FEMS Microbiol. Lett. 192:53-57. [DOI] [PubMed] [Google Scholar]
- 25.Knegtel, R. M., B. Strokopytov, D. Penninga, O. G. Faber, H. J. Rozeboom, K. H. Kalk, L. Dijkhuizen, and B. W. Dijkstra. 1995. Crystallographic studies of the interaction of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 with natural substrates and products. J. Biol. Chem. 270:29256-29264. [DOI] [PubMed] [Google Scholar]
- 26.Kyhse-Andersen, J. 1984. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 10:203-209. [DOI] [PubMed] [Google Scholar]
- 27.MacGregor, E. A., H. M. Jespersen, and B. Svensson. 1996. A circularly permuted alpha-amylase-type alpha/beta-barrel structure in glucan-synthesizing glucosyltransferases. FEBS Lett. 378:263-266. [DOI] [PubMed] [Google Scholar]
- 28.Monchois, V., M. A. Arguello-Morales, and R. R. Russell. 1999. Isolation of an active catalytic core of Streptococcus downei MFe28 GTF-I glucosyltransferase. J. Bacteriol. 181:2290-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monchois, V., M. Remaud-Simeon, R. R. Russell, P. Monsan, and R. M. Willemot. 1997. Characterization of Leuconostoc mesenteroides NRRL B-512F dextransucrase (DSRS) and identification of amino-acid residues playing a key role in enzyme activity. Appl. Microbiol. Biotechnol. 48:465-472. [DOI] [PubMed] [Google Scholar]
- 30.Monchois, V., R. M. Willemot, and P. Monsan. 1999. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol. Rev. 23:131-151. [DOI] [PubMed] [Google Scholar]
- 31.Monchois, V., R. M. Willemot, M. Remaud-Simeon, C. Croux, and P. Monsan. 1996. Cloning and sequencing of a gene coding for a novel dextransucrase from Leuconostoc mesenteroides NRRL B-1299 synthesizing only α(1-6) and α(1-3) linkages. Gene 182:23-32. [DOI] [PubMed] [Google Scholar]
- 32.Nagy, I., G. Schoofs, F. Compernolle, P. Proost, J. Vanderleyden, and R. de Mot. 1995. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J. Bacteriol. 177:676-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 34.Pouwels, P. H., and R. J. Leer. 1993. Genetics of lactobacilli: plasmids and gene expression. Antonie Leeuwenhoek 64:85-107. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidebotham, R. L. 1974. Dextrans. Adv. Carbohydr. Chem. Biochem. 30:371-444. [DOI] [PubMed] [Google Scholar]
- 38.Simpson, C. L., P. M. Giffard, and N. A. Jacques. 1995. Streptococcus salivarius ATCC 25975 possesses at least two genes coding for primer-independent glucosyltransferases. Infect. Immun. 63:609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, M. R., R. Y. Wong, R. E. Lundin, and J. A. Ahlgren. 1998. A mutant strain of Leuconostoc mesenteroides B-1355 producing a glucosyltransferase synthesizing 32 α(1→2) glucosidic linkages. J. Ind. Microbiol. Biotechnol. 21:37-45. [Google Scholar]
- 40.Ueda, S., T. Shiroza, and H. K. Kuramitsu. 1988. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene 69:101-109. [DOI] [PubMed] [Google Scholar]
- 41.Uitdehaag, J. C., R. Mosi, K. H. Kalk, B. A. Van der Veen, L. Dijkhuizen, S. G. Withers, and B. W. Dijkstra. 1999. X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the alpha-amylase family. Nat. Struct. Biol. 6:432-436. [DOI] [PubMed] [Google Scholar]
- 42.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 43.Van der Veen, B. A., J. C. Uitdehaag, B. W. Dijkstra, and L. Dijkhuizen. 2000. Engineering of cyclodextrin glycosyltransferase reaction and product specificity. Biochim. Biophys. Acta 1543:336-360. [DOI] [PubMed] [Google Scholar]
- 44.Van Geel-Schutten, G. H., E. J. Faber, E. Smit, K. Bonting, M. R. Smith, B. Ten Brink, J. P. Kamerling, J. F. Vliegenthart, and L. Dijkhuizen. 1999. Biochemical and structural characterization of the glucan and fructan exopolysaccharides synthesized by the Lactobacillus reuteri wild-type strain and by mutant strains. Appl. Environ. Microbiol. 65:3008-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Geel-Schutten, G. H., F. Flesch, B. Ten Brink, M. R. Smith, and L. Dijkhuizen. 1998. Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides. Appl. Microbiol. Biotechnol. 50:697-703. [Google Scholar]
- 46.Wilke-Douglas, M., Perchorowicz, J. T., Houck, C. M., and Thomas, B. R. 1989. Methods and compositions for altering physical characteristics of fruit and fruit products. PTC WO patent 89/12386.
- 47.Wren, B. W. 1991. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5:797-803. [DOI] [PubMed] [Google Scholar]