Abstract
Viable and nonviable Weissella confusa strain PL9001 inhibited the binding of Helicobacter pylori to human gastric-cell line MKN-45 cells by more than 90%. Spent culture supernatant of PL9001 rapidly decreased the viability of H. pylori, rupturing cell walls. The results suggest that PL9001 is a probiotic that can reduce the infectivity and persistence of H. pylori.
Helicobacter pylori is a gram-negative microorganism that causes gastritis and gastric carcinoma (11, 12, 13), infects through the intake of food, and attaches to the gastric and duodenal mucous membranes (14, 27). Once attached, it remains for several decades and is not eliminated naturally. The ease with which it reinfects is the major cause of chronic gastritis. To remove H. pylori, antibiotic drugs (e.g., amoxicillin and clarithromycin), restrainers of proton pumping (e.g., omeprazole), and gastric acid removers (e.g., magnesium) have been used. H. pylori develops resistance to antibiotics, however, and stomach acid removers do not solve the infection problem.
It has been well documented that probiotic lactic acid-producing bacteria (LAB) can develop antagonistic activity against various human pathogens (3, 24). Certain LAB synthesize antimicrobial compounds that are related to the bacteriocin family (16). Other such compounds synthesized by LAB are well-known metabolic end products of lactic acid fermentation, such as lactic and acetic acids (21) and hydrogen peroxide, or are as yet unidentified (4). Because LAB are known to exist in the intestines and exert antibacterial activity on intestinal pathogens and because the stomach is a very harsh environment, with low pH and the presence of pepsin, most researchers have assumed that LAB cannot inhibit gastric pathogens. Recently, however, Lactobacillus salivarius (1), Lactobacillus acidophilus (3), and Bacillus subtilis (23) were found to produce antibacterial materials other than lactic acid against H. pylori. In human volunteers, the spent culture supernatants (SCSs) of the human L. acidophilus strain LB and Lactobacillus gasseri OLL2716 (LG21) were active against H. pylori (10, 20, 25).
In an effort to find new LAB with activity against H. pylori, more than 100 LAB were isolated from infant feces; one isolate was selected based on its strong inhibitory activity on H. pylori growth and on the adherence of H. pylori to the glycolipid, the binding moiety in the stomach (Lewis antigen isolated from reticulocytes of human O-type blood [5, 14, 15, 18]), as has been described in a previous paper (18). This isolate was identified as Weissella confusa, and its 16S rRNA sequence was registered in GenBank under the accession no. AF477495. The genus Weissella was separated recently from the genus Lactobacillus due to developments in DNA technology (28). W. confusa (previously known as Lactobacillus confusus and originally known as Lactobacillus coprophilus) is present in the normal microflora of human intestines (22, 28, 29, 30) and has been isolated worldwide from foods such as Greek salami (26), Malaysian chili bo (19), and Mexican pozol (2). However, no strain in this genus has yet been developed as a probiotic. In this study, the anti-H. pylori functions of W. confusa strain PL9001 were characterized.
H. pylori ATCC 43504 was grown on brucella solid medium containing amphotericin B (2.5 μg/ml; Fungizone) and Skirrow's supplement (2.5 IU of polymyxin B/ml, 10 μg of vancomycin/ml, 5 μg of trimethoprim/ml) and supplemented with 10% horse serum in 5 to 10% CO2. The inhibitory activity of the SCS on the growth of H. pylori was assayed with a well test by adding 100 μl of the SCS of PL9001 to each well formed with a sterilized Pasteur pipette on a solid medium inoculated with H. pylori. The SCS was subjected to low pH (pH 2), pepsin (30 U/ml), proteinase K (0.1 μg/ml), or heat treatment. After colonies appeared, the diameter of the inhibited growth zone was measured.
The ability of PL9001 to bind to human gastric-cell line MKN-45 cells was observed by Gram staining and scanning electron microscopy (SEM) (model JSM-5200; Jeol, Tokyo, Japan). MKN-45 cells were cultured in RPMI 1640 medium (Gibco-BRL, New York, N.Y.) containing 2 g of sodium bicarbonate/liter, 10% heat-inactivated fetal bovine serum, and antibiotic-antimycotic (pH 7.2) in a 30-mm-diameter dish (Nunc, Inc., Naperville, Ill.) for Gram staining or in a Lab Tak chamber slide system (Nunc, Inc.) for SEM by inoculating 3 × 105 viable cells into 2 ml of culture medium. A 6-day confluent monolayer was washed twice with 2 ml of phosphate-buffered saline before the adhesion assay. One hundred microliters of PL9001 (107 CFU in 1 ml) was added to 2 ml of a complete MKN medium and incubated at 37°C in a 5% CO2 incubator. After incubation for 60 min, the monolayer was washed twice with sterile phosphate-buffered saline (pH 7.4) and fixed with methanol. Cells were examined under a light microscope after Gram staining or by SEM. To observe the effect of the SCS on H. pylori, 1 ml of the SCS was mixed with the same volume of H. pylori in brucella broth. After various incubation times, bacterial cells were observed by SEM. For the competition assay, a mixture of H. pylori and PL9001 (1:10 [CFU]) was added to the MKN-45 cells, followed by incubation for 60 min at room temperature. To detect H. pylori bound to MKN-45 cells, fluorescein isothiocyanate (FITC)-conjugated antibody was used and cells were observed under a model FDX-35 fluorescence microscope (excitation filter, 450 to 490 nm; dichronic mirror, 505 nm; barrier filter, 520 nm; Nikon, Tokyo, Japan).
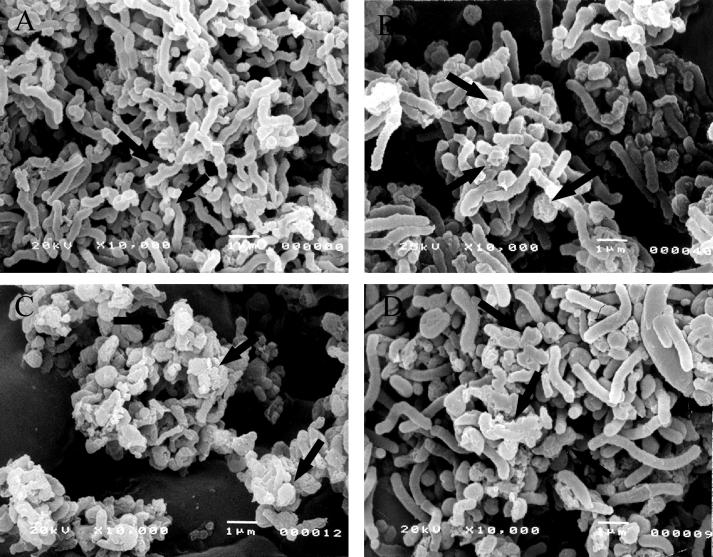
Among the 100 LAB isolated from infant feces, several isolates that showed good inhibitory activity on the adherence of H. pylori to glycolipid spotted on a thin-layer chromatography plate were selected. One isolate (PL9001), which showed the best inhibitory activity on the growth of H. pylori in a well test, was selected and characterized further. A growth inhibition zone was observed even after the SCS of PL9001 was subjected to pH 2, pH 4, pepsin, proteinase K, or heat treatment (data not shown). When H. pylori was treated with the SCS, the cells changed from helical form (Fig. 1A) to coccoid form and became necrotic (Fig. 1C), even after treatment with the neutralized SCS (Fig. 1D). A similar coccoid form was observed with H. pylori treated with amoxicillin (Fig. 1B); others have previously observed the same result after treatment with the SCS of L. acidophilus (10), and this form is known to result in a loss of infectivity (6, 17). Because the neutralized SCS had the same effect, there must be a factor(s) for necrosis in addition to lactic acid and low pH. The antibacterial activity was stable after low pH, pepsin, proteinase, or heat treatment; thus, the bacteriocin(s) produced by PL9001 seems to belong to the class II bacteriocins, nonproteinaceous materials that rupture the cell wall (16).
FIG. 1.
Scanning electron micrographs of H. pylori with no treatment (A) and after treatment with amoxicillin (0.01 μg/ml) (B), the SCS of W. confusa PL9001 for 5 min (C), or neutralized SCS (pH 7.0) for 5 min (D). Magnification, ×10,000.
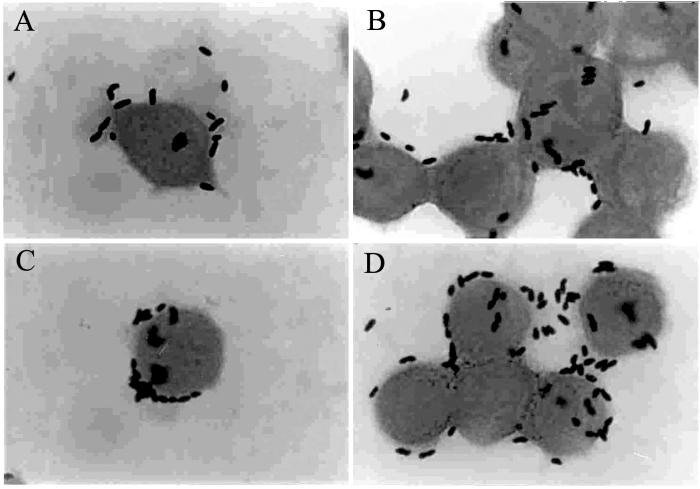
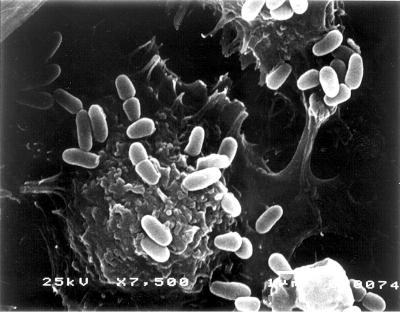
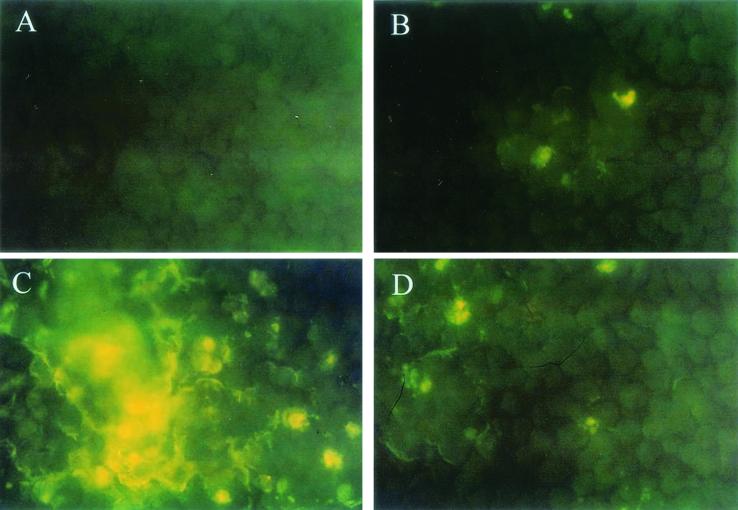
PL9001 can bind to gastric cells (Fig. 2A and B), and this binding ability remained in the nonviable cells heated at 75°C for 15 min (Fig. 2C and D). As observed by SEM, many cells with the characteristic club shape of W. confusa were found on the surface of MKN-45 cells (Fig. 3). The prevention of the adherence of H. pylori by PL9001 was confirmed with FITC-conjugated antibody. As shown in Fig. 4, both live and nonviable PL9001 inhibited the binding of H. pylori to MKN-45 cells. The binding activity of nonviable PL9001 suggests that PL9001 binds directly to the stomach via a mechanism similar to that involved in the binding of LAB to the intestinal epithelial cells (7, 8, 9) and that nonviable as well as viable PL9001 can prevent the adherence of H. pylori to the stomach. The results of this study show that strain PL9001 has a dual inhibitory activity on H. pylori—bactericidal activity and prevention of adherence to gastric mucous cells—and has the potential to be developed as a new probiotic for the stomach.
FIG. 2.
Gram staining of MKN-45 cells after incubation with W. confusa PL9001. After 60 min of incubation with live (A and B) or nonviable (C and D) PL9001, cells were washed and Gram stained. Magnification, ×1,000.
FIG. 3.
Scanning electron micrograph of MKN-45 cells treated with W. confusa PL9001. Magnification, ×7,500.
FIG. 4.
Competition between H. pylori and W. confusa PL9001 for binding to MKN-45 cells. H. pylori and PL9001 were added to MKN-45 cells, H. pylori was detected with FITC-conjugated antibody, and observations were made with a fluorescence microscope. Shown are MKN-45 cells with no treatment (control) (A), with H. pylori and live PL9001 (B), with H. pylori (C), and with H. pylori and nonviable PL9001 (D). Magnification, ×400.
Acknowledgments
This work was supported by PLBio, Ltd., of Korea.
REFERENCES
- 1.Aiba, Y., N. Suzuki, A. M. Kabir, A. Takagi, and Y. Koga. 1998. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am. J. Gastroenterol. 93:2097-2101. [DOI] [PubMed] [Google Scholar]
- 2.Ampe, F., N. ben Omar, C. Moizan, C. Wacher, and J. P. Guyot. 1999. Polyphasic study of the spatial distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation-independent methods to investigate traditional fermentations. Appl. Environ. Microbiol. 65:5464-5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1994. Lactobacillus acidophilus LA1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernet-Camard, M.-F., V. Liévin, D. Brassart, J.-R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boren, T., P. Falk, K. A. Roth, G. Larson, and S. Normark. 1993. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262:1892-1895. [DOI] [PubMed] [Google Scholar]
- 6.Caternich, C. E., and K. M. Makin. 1991. Characterization of the morphological conversion of Helicobacter pylori from bacillary to coccoid forms. Scand. J. Gastroenterol. 26:58-64. [PubMed] [Google Scholar]
- 7.Chauvière, G., M. H. Coconnier, S. Kernéis, A. Darfeuille-Michaud, B. Joly, and A. L. Servin. 1992. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from enterocyte-like Caco-2 cells in culture. FEMS Microbiol. Lett. 91:213-218. [DOI] [PubMed] [Google Scholar]
- 8.Chauvière, G., M. H. Coconnier, S. Kernéis, J. Fourniat, and A. L. Servin. 1992. Adherence of human Lactobacillus acidophilus onto human enterocyte-like cells, Caco-2 and HT-29 in culture. J. Gen. Microbiol. 138:1689-1696. [DOI] [PubMed] [Google Scholar]
- 9.Coconnier, M. H., V. Liévin, M.-F. Bernet-Camard, S. Hudault, and A. L. Servin. 1997. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 41:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coconnier, M. H., V. Liévin, E. Hemery, and A. L. Servin. 1998. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 64:4573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover, T. L., and M. J. Blaser. 1995. Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News 61:21-26. [Google Scholar]
- 12.Dubois, A. 1995. Spiral bacteria in the human stomach: the gastric helicobacters. Emerg. Infect. Dis. 1:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enno, A., J. L. O'Rourke, C. R. Howlett, A. Jack, M. F. Dixon, and A. Lee. 1995. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis: a mouse model of Helicobacter pylori-induced gastric lymphoma. Am. J. Pathol. 147:217-222. [PMC free article] [PubMed] [Google Scholar]
- 14.Hemalatha, S. G., B. Drumm, and P. Sherman. 1991. Adherence of Helicobacter pylori to human gastric epithelial cells in vitro. J. Med. Microbiol. 35:197-202. [DOI] [PubMed] [Google Scholar]
- 15.Heneghan, M. A., A. P. Moran, K. M. Feeley, E. L. Egan, J. Goulding, C. E. Connolly, and C. F. McCarthy. 1998. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonization density and the consequent inflammatory response. FEMS Immunol. Med. Microbiol. 20:257-266. [DOI] [PubMed] [Google Scholar]
- 16.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusters, J. G., M. M. Gerrits, J. A. G. van Strijp, and C. M. J. E. Vandenbrouke-Grauls. 1997. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect. Immun. 65:3672-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, Y., E. Shin, J. Lee, and J. H. Park. 1999. Lactobacillus acidophilus inhibits the Helicobacter pylori adherence. J. Microb. Biotechnol. 9:794-797. [Google Scholar]
- 19.Leisner, J. J., B. Pot, H. Christensen, G. Rusul, J. E. Olsen, W. Wee, K. Muhamad, and H. M. Ghazali. 1999. Identification of lactic acid bacteria from chili bo, a Malaysian food ingredient. Appl. Environ. Microbiol. 65:599-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michetti, P., G. Dorta, D. Brassart, D. Vouillamoz, W. Schwitzer, C. Felley, A. L. Blum, N. Porta, M. Rouvet, and I. Corthesy-Theulaz. 1995. L. acidophilus supernatant as an adjuvant in the therapy of H. pylori in humans. Gastroenterology 108:A166. [Google Scholar]
- 21.Midolo, P. D., J. R. Lambert, R. Hull, F. Luo, and M. L. Grayson. 1995. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 79:475-479. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuoka, T. 1992. The human gastrointestinal tract, p. 69-114. In B. J. B. Wood (ed.), The lactic acid bacteria, vol. 1. The lactic acid bacteria in health and disease. Elsevier Applied Science, London, United Kingdom.
- 23.Pinchuk, I. V., P. Bressollier, B. Verneuil, B. Fenet, I. B. Sorokulova, F. Megraud, and M. C. Urdaci. 2001. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 45:3156-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid, G. 1999. The scientific basis for probiotic strains of Lactobacillus. Appl. Environ. Microbiol. 65:3763-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto, I., M. Igarashi, K. Kimura, A. Takagi, T. Miwa, and Y. Koga. 2001. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J. Antimicrob. Chemother. 47:709-710. [DOI] [PubMed] [Google Scholar]
- 26.Samelis, J., F. Maurogenakis, and J. Metaxopoulos. 1994. Characterisation of lactic acid bacteria isolated from naturally fermented Greek dry salami. Int. J. Food Microbiol. 23:179-196. [DOI] [PubMed] [Google Scholar]
- 27.Slomiany, B. L., and A. Slomiany. 1992. Mechanism of Helicobacter pylori pathogenesis: focus on mucus. J. Clin. Gastroenterol. 14(Suppl.):S114-S121. [PubMed] [Google Scholar]
- 28.Stiles, M. E., and W. H. Holzapfel. 1997. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 36:1-29. [DOI] [PubMed] [Google Scholar]
- 29.Tannock, J. W., A. Tilsala-Timisjarvi, S. Rodtong, J. Ng, K. Munro, and T. Alatossava. 1999. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl. Environ. Microbiol. 65:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]