Abstract
We describe the isolation and characterization of Rickettsia monacensis sp. nov. (type strain, IrR/MunichT) from an Ixodes ricinus tick collected in a city park, the English Garden in Munich, Germany. Rickettsiae were propagated in vitro with Ixodes scapularis cell line ISE6. BLAST analysis of the 16S rRNA, the citrate synthase, and the partial 190-kDa rickettsial outer membrane protein A (rOmpA) gene sequences demonstrated that the isolate was a spotted fever group (SFG) rickettsia closely related to several yet-to-be-cultivated rickettsiae associated with I. ricinus. Phylogenetic analysis of partial rompA sequences demonstrated that the isolate was genotypically different from other validated species of SFG rickettsiae. R. monacensis also replicated in cell lines derived from the ticks I. ricinus (IRE11) and Dermacentor andersoni (DAE100) and in the mammalian cell lines L-929 and Vero, causing cell lysis. Transmission electron microscopy of infected ISE6 and Vero cells showed rickettsiae within the cytoplasm, pseudopodia, nuclei, and vacuoles. Hamsters inoculated with R. monacensis had immunoglobulin G antibody titers as high as 1:16,384, as determined by indirect immunofluorescence assay. Western blot analyses demonstrated that the hamster sera cross-reacted with peptides from other phylogenetically distinct rickettsiae, including rOmpA. R. monacensis induced actin tails in both tick and mammalian cells similar to those reported for R. rickettsii. R. monacensis joins a growing list of SFG rickettsiae that colonize ticks but whose infectivity and pathogenicity for vertebrates are unknown.
Rickettsiae are arthropod-associated gram-negative prokaryotes that reside within the cytoplasm and sometimes nuclei of eukaryotic host cells (17). They are subdivided into the typhus or spotted fever group (SFG) on the basis of genotypic and phenotypic analyses (39). This is supported by sequence analyses of genes for 16S rRNA (42, 45, 49), the genus-common 17-kDa antigen (3, 58), citrate synthase (gltA) (45), and rickettsial outer membrane proteins A (rOmpA) (15, 57) and B (rOmpB) (43). rompA sequences have been found to be most useful for species differentiation within the SFG (15, 40, 41).
Several yet-to-be-cultivated SFG rickettsiae have been detected by the hemolymph test or PCR in Ixodes ricinus ticks from Switzerland (5), Spain (25), and Slovakia (45). For example, the Cadiz agent was detected in I. ricinus adults collected in southwestern Spain (25) and IRS3 and IRS4 were subsequently identified in I. ricinus ticks collected in two different regions of Slovakia (45). Sequence comparisons of 16S rRNA gene, gltA, and rompA sequences suggested that these rickettsiae represented new genotypes within the SFG (25, 45). These rickettsiae were distinct from Rickettsia helvetica, which was recently implicated in chronic perimyocarditis in humans (32), and Rickettsia slovaca, both of which are commonly associated with and isolated from I. ricinus (5, 8, 30, 31, 36, 46).
In this report, we describe the cultivation, actin-based motility, and partial molecular and immunologic characterization of an SFG rickettsia from a female I. ricinus tick collected in a European city park, the English Garden in Munich, Germany. The type strain organism, designated IrR/MunichT, was isolated by inoculation of tick tissues onto an Ixodes scapularis cell line, ISE6. We determined this rickettsia to be distinct from all other currently recognized rickettsial species and closely related to the yet-to-be-isolated Cadiz agent, IRS3, and IRS4. Thus, we propose that this novel rickettsia be formally named Rickettsia monacensis after its geographic origin.
MATERIALS AND METHODS
Tick collection.
Adult I. ricinus ticks were collected in May 1998 in the English Garden, a recreational park in Munich, Germany, by dragging a white flannel cloth along grassy areas in the northeastern section of the park. Ticks were maintained in glass vials within desiccator jars humidified with a saturated solution of Na2SO4 and in a photoperiod of 16 h of light (22°C) and 8 h of darkness (18°C).
Dissection and cultivation of I. ricinus tissues.
Internal tissues were dissected from 12 partially engorged females for the detection and isolation of microorganisms. Individual females were surface disinfected (23), and their salivary glands, Malpighian tubules, ovaries, and midgut tissues were aseptically removed. DNA was extracted from half of the salivary glands and midgut tissues for PCR and restriction fragment length polymorphism (RFLP) analyses (see below). The remaining organs were individually placed into wells of a 24-well plate (Becton Dickinson & Company, Franklin Lakes, N.J.) containing cells of the I. scapularis line ISE6 (for I. scapularis embryos from tick 6) (28). The medium was L15B300 with NaHCO3 (0.25%) and HEPES (25 mM) (27). Plates were incubated in a humidified candle jar at 34°C (29) for 5 days. The candle jars created an atmosphere containing lower oxygen and higher carbon dioxide levels, conditions conducive to the isolation of ehrlichiae from ticks (27, 29). Cultures from the same tick that remained uncontaminated were then pooled in 5 ml of fresh medium, transferred to 25-cm2 vented-cap flasks (Sarstedt, Newton, N.C.), and further incubated in a candle jar as described above. Culture medium was replaced weekly, and subsequent passages of infected cells and rickettsiae were made directly into 25-cm2 sealed-cap flasks (Sarstedt), removing the need for candle jars.
Rickettsial strains and animal cell lines.
R. monacensis IrR/MunichT multiplied in both tick and mammalian cell lines maintained in L15B300 (27). The type strain of R. monacensis, IrR/MunichT, has been deposited at the American Type Culture Collection (Manassas, Va.) and the WHO Collaborating Center for Tropical Diseases, Department of Pathology, University of Texas Medical Branch, Galveston. Cell lines from I. ricinus (IRE11; embryonic cell line) (U. G. Munderloh, unpublished data) and D. andersoni (DAE100; embryonic cell line) (48) ticks were also used. Line DAE100 was cleared of a chronic Rickettsia peacockii infection by incubation at 37°C for a month. We also used the mouse L-929 (ATCC CCL-1) and African green monkey kidney Vero (ATCC CCL-81) cell lines to grow R. monacensis. For transfer of R. monacensis between different cell lines, a host cell-free, semipurified rickettsial suspension was prepared from a confluent 25-cm2 culture. Cells were ruptured by repeated passage through a 27-gauge needle to release the rickettsiae. Large debris was removed by low-speed centrifugation (275 × g for 10 min), the supernatant was filtered through a 5.0-μm-pore-size syringe filter, and rickettsiae were inoculated into the heterologous target culture. R. helvetica C9P9 (6) was provided by Lorenza Beati (Department of Epidemiology and Public Health, Yale University School of Medicine) and maintained in IRE11 cells. R. peacockii DaE100R (48) was maintained in D. andersoni embryonic cell line DAE100, from which it originated. Rickettsia rickettsii Hlp#2 (35) (provided by Robert Heinzen, Rocky Mountain Laboratories, National Institutes of Health) and Rickettsia strain MOAa (26) were both maintained in I. scapularis cell line IDE2, which had previously been isolated from embryos of an “Ixodes dammini ” (= I. scapularis) female (28).
Microscopy.
Cultures were observed weekly by phase-contrast microscopy to assess culture confluency, cytopathic effect, and the presence and relative abundance of rickettsiae. Cultures were periodically sampled, and cells were stained with Giemsa stain to determine percent infection.
R. monacensis bacteria passaged 4 and 16 times in ISE6 and Vero cells, respectively, were prepared for transmission electron microscopy (26) to assess the ultrastructure and intracellular location of microorganisms.
DNA extraction and preparation.
DNA was extracted from infected cell cultures and the salivary glands and guts of each tick with the Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.). Following alcohol precipitation, DNA was dissolved in 50 μl of water and stored at −70°C. DNA was extracted from IrR/MunichT at passages 1, 2, 8, and 36 in ISE6 cells and from R. helvetica at passage 2 in IRE11 cells (21, 57).
PCR amplification.
Rickettsia-specific primer sets (Table 1) were used for amplification and preliminary identification of rickettsial DNA in tick tissues and cell cultures. The PCR amplification conditions were as specified in the literature (Table 1), with a RoboCycler thermocycler (Stratagene, La Jolla, Calif.) and 50-μl reaction mixtures with 5 μl of template DNA or water (negative control). Amplification products were visualized by electrophoresis through 1.5% agarose gels stained with ethidium bromide.
TABLE 1.
PCR primer sets used in this study
| Primer set | Gene | Nucleotide sequence (5′-3′) | Size (bp) | Reference(s) |
|---|---|---|---|---|
| fD1-Rc16S.452n | 16SrRNA | AGAGTTTGATCCTGGCTCAG | 416 | 25, 56 |
| AACGTCATTATCTTCCTTGC | ||||
| RpCS.877p-RpCS.1258n | gltA | GGGGGCCTGCTCACGGCGG | 381 | 40 |
| ATTGCAAAAAGTACAGTGAACA | ||||
| Rr17 | 17-kDa antigen | GCTCTTGCAACTTCTATGTT | 434 | 58 |
| CATTGTTCGTCAGGTTGGCG | ||||
| Rr190.70p-Rr190.602n | rompA | ATGGCGAATATTTCTCCAAAA | 532 | 40 |
| AGTGCAGCATTCGCTCCCCCT |
DNA sequencing.
R. monacensis 16S rRNA, citrate synthase (gltA) and rompA gene PCR products, corresponding to the fD1-Rc16S.452n, RpCS.877p-RpCS.1258n, and Rr190.70p-Rr190.602n primer pairs, respectively, were sequenced (48). Template DNA was prepared with passage 2 of IrR/MunichT in ISE6 cells. Three clones of each partial gene PCR product were sequenced in both directions with an ABI 377 automated sequencer (Advanced Genetic Analysis Center, University of Minnesota). Sequences were aligned with the CLUSTAL W multiple-sequence alignment program (52) to find the consensus nucleotide sequence, and similarity to other rickettsial organisms was determined by running BLAST analyses (1).
RFLP.
We used RFLP analyses to characterize the 16S rRNA and gltA gene amplification products (Table 1) obtained from cultured IrR/MunichT (passages 1, 2, 8, and 36) to assess the homogeneity of the culture isolate. We compared these to products amplified from I. ricinus tick no. 5 (salivary glands and gut tissues) and R. helvetica. RsaI (Life Technologies) and BstXI (Takara Biotechnology, Shiga, Japan) were used to digest the 16S rRNA gene PCR products, while AluI and Sau3AI (Promega, Madison, Wis.) were used to digest the gltA PCR products. Diagnostic cutting sites for these enzymes were selected with the aid of the restriction mapping software Webcutter 2.0 (M. Heiman; http://www.firstmarket.com/firstmarket/cutter/cut2.html). PCR products (10-μl aliquots) were digested with 10 U of endonuclease for 4 h at 37°C for RsaI, AluI, and Sau3AI and 45°C for BstXI. Digested DNA was resolved by electrophoresis through an 8% polyacrylamide gel, stained with ethidium bromide, and visualized by UV illumination.
Phylogenetic analysis.
Partial rompA sequences of validated SFG rickettsial species (11; http://www.bacterio.cict.fr/) in the GenBank database were used to phylogenetically place R. monacensis (IrR/MunichT) among these species. Sequences corresponding to R. rickettsii rompA positions 92 to 581 (40) were manually aligned with conserved nucleotides as reference points. A dendrogram was constructed by the neighbor-joining method (44); PAUP∗, version 4.0b8 (PPC) (50); and Kimura's two-parameter option (22) to determine distances. Bootstrap analysis was used with 1,000 replicates to test the relative support for the branches produced by the neighbor-joining analysis (12). Trees were rooted with Rickettsia australis PHS on the basis of phylogenetic studies of the 16S rRNA (42, 45, 49), citrate synthase (45), rompB (43), and 23S rRNA genes, as well as sequences of the fmt-to-rrl spacer region (4).
Production of R. monacensis antisera.
Male Syrian hamsters (Mesocricetus auratus; 2 months old) were injected intraperitoneally with 106 IRE11 (three hamsters, passage 25) or Vero (three hamsters, passage 23) cells infected with R. monacensis. The cells were previously cultured at 34°C, and >95% were infected. Serum from an age-matched, uninfected hamster served as a control. Blood was collected 6 weeks later by cardiac puncture from hamsters killed by exposure to CO2. Animals were maintained and handled in accordance with all of the guidelines established by the National Institutes of Health and the University of Minnesota Animal Care and Use Committee. Titers of immunoglobulin G to different SFG rickettsial species were determined by indirect immunofluorescence assay (IFA) with 18-ring antigen slides (Erie Scientific, Portsmouth, N.H.). ISE6 cells infected with R. monacensis, R. helvetica, R. peacockii, R. rickettsii, or Rickettsia strain MOAa were suspended in culture medium, and aliquots were pipetted into individual rings. Slides were ambient air dried overnight, and cells were fixed and permeabilized in methanol for 4 min. Serial twofold dilutions of test and control hamster sera were applied to duplicate wells of IFA slides containing rickettsial antigen as described above and incubated for 1 h at 37°C. Bound antibodies were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-hamster IgG (heavy and light chains; Pierce, Rockford, Ill.) for 1 h at 37°C. Slides were counterstained for 4 min in 0.005% Evans blue, mounted in Vectashield antifade solution (Vector Laboratories, Burlingame, Calif.), and examined with a Nikon E400 Eclipse microscope fitted for epifluorescence illumination.
Demonstration of actin tails.
Polymerized (F-)actin associated with R. monacensis in tick and mammalian cells was visualized as previously described (20, 26). D. andersoni DAE100 and mouse L-929 cells were seeded onto glass coverslips in 24-well plates at a concentration of 5 × 105 cells/well in 1 ml of medium. R. monacensis bacteria were liberated from a 25-cm2 culture, partially purified as specified above, and diluted in medium, and 0.1-ml aliquots of 102-, 103-, and 104-fold dilutions were added to wells.
We used dual fluorescence staining to visualize rickettsiae and their associated F-actin structures. Rickettsiae were reacted with hamster anti-R. monacensis serum at a dilution of 1:4,096 in phosphate-buffered saline with 3% bovine serum albumin and labeled with goat anti-hamster IgG (heavy and light chains) conjugated with FITC (Pierce) for visualization of the labeled rickettsiae. Texas red phalloidin (Molecular Probes, Eugene, Oreg.) at a dilution of 1:20 in phosphate-buffered saline with 3% bovine serum albumin was added together with the secondary antibody to stain F-actin. Coverslips were mounted onto microscope slides with a drop of Vectashield and examined by fluorescence microscopy with a Nikon E800 microscope fitted with a CoolCam 2000 video camera (Imaging Center, University of Minnesota). Digital images of sequential horizontal focal planes of infected cells were captured and then merged with ImagePro Plus software (Media Cybernetics, Des Moines, Iowa).
Western blot analyses.
We compared antigens of R. monacensis to those from R. helvetica, Rickettsia strain MOAa, and R. rickettsii by using high-titer (1:16,382) anti-R. monacensis sera from two hamsters inoculated with R. monacensis grown in Vero cells at 34°C (see above). Semipurified rickettsiae were prepared from IRE11 or IDE2 cultures as already described. Rickettsiae were further purified by centrifugation (20,000 × g for 40 min at 4°C) through 30% diatrizoate (Hypaque 76; Nycomed Inc., Princeton, N.J.), washed in Hanks' balanced salt solution, and resuspended in 0.5 M Tris-HCl, pH 6.8. Approximate protein concentrations were determined by UV spectrophotometry. Proteins were denatured, diluted, separated (65 μg/well) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (24) through 8 to 16% gradient minigels (ISC BioExpress, Kaysville, Utah), and blotted onto polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, Mass.) (34). Duplicate gels stained with Coomassie blue and blots developed with polyclonal hamster anti-R. monacensis sera (diluted 1:300) were compared with blots reacted with murine monoclonal antibody (MAb) 13-5 (2) (diluted 1:500; provided by T. Hackstadt, Rocky Mountain Laboratories, National Institutes of Health) to identify rOmpA. Peroxidase-labeled goat anti-mouse IgG (diluted 1:1000; Kirkegaard & Perry Laboratories) was used to detect the MAb, and peroxidase-labeled goat anti-hamster IgG (heavy and light chains; diluted 1:1,000; Kirkegaard & Perry Laboratories) to visualize antigens on blots reacted with polyclonal sera. Blots were developed with the 4CN Membrane Peroxidase Substrate System (Kirkegaard & Perry Laboratories).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA, citrate synthase, and rompA gene PCR products of IrR/MunichT have been deposited in the GenBank database and assigned accession numbers AY048818, AY048817, and AF201329, respectively.
RESULTS
Isolation and in vitro maintenance of IrR/MunichT.
The midgut and Malpighian tubules from 1 of the 12 partially engorged I. ricinus ticks (no. 2) yielded a rickettsial isolate in ISE6 cultures that was subsequently designated IrR/MunichT. Electron microscopy and DNA sequence data (see below) confirmed the rickettsial identity of the bacteria. Intra- and extracellular coccobacillary microorganisms were first observed by phase-contrast microscopy 26 days after initiation of cultures and were also observed in Giemsa-stained cell spreads. We maintained the isolate for the first three transfers simply by passage of infected cultures (diluted 1:10). The rickettsiae became cytopathic for ISE6 cells after the third passage (5 months), causing cell lysis. Subsequent transfers of IrR/MunichT were accomplished by transferring 0.05 to 0.1 ml of an infected cell suspension to an uninfected, confluent (approximately 5 × 106 cells/ml), 25-cm2, 5-ml culture once every 7 to 10 days. Numerous extracellular rickettsiae were observed at the later stages (7 days and later) of infection, when more than 80% of the cells were infected and necrotic foci were apparent in the cell layer. Once stable in ISE6 cells, R. monacensis could be successfully transferred to other tick and mammalian cell lines.
Isolate IrR/MunichT replicated in both tick (ISE6, IRE11, and DAE100) and mammalian (L-929 and Vero) cell lines. Growth in L-929 and Vero cells was much slower than in tick cells, even though the incubation temperature for all was 34°C. In mammalian cells, passages were therefore made with 0.5 to 1 ml of an infected cell suspension. IrR/MunichT grew fastest in IRE11, and an inoculum of 0.5% caused complete host cell lysis in 1 week. Growth and cytopathogenicity of IrR/MunichT were retarded in cultures (DAE100 and Vero cells) maintained at 37°C, which required monthly, or longer, transfer intervals.
Ultrastructure of IrR/MunichT.
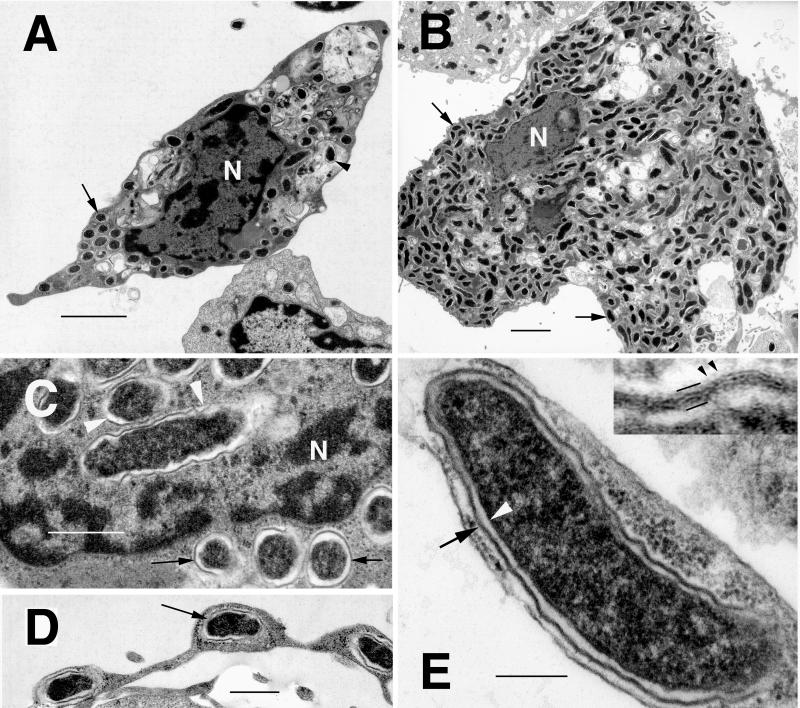
We examined the ultrastructure of IrR/MunichT in ISE6 (Fig. 1A) and Vero (Fig. 1B) cells. In both, the bacteria had the ultrastructural features of rickettsiae and were observed free in the cytoplasm (Fig. 1A and B, arrows) and pseudopodia (Fig. 1D, arrow) and occasionally within nuclei (Fig. 1C, arrowheads) or vacuoles (Fig. 1A, arrowhead). Extracellular rickettsiae were associated with coated pits at the cell surface. The coccobacillary rickettsiae were approximately 1.0 to 1.5 by 0.3 to 0.4 μm and were delineated by an inner periplasmic membrane (Fig. 1E, arrowhead), a periplasmic space, and a trilaminar cell wall (Fig. 1E, arrow) of varying thickness and a faint microcapsular layer (Fig. 1E, insert, arrowheads). An electron-translucent zone or slime layer surrounded the cell wall and separated the bacterium from the cytoplasm of the host cell, a feature of SFG rickettsiae (18, 20). However, the IrR/MunichT slime layer was thin (<30 nm) in comparison with that of R. rickettsii (30 to 60 nm) (47, 53). There was no difference in the overall ultrastructure of IrR/MunichT in tick or mammalian cells maintained at 34°C.
FIG. 1.
Transmission electron photomicrographs of R. monacensis IrR/MunichT within cultured tick and mammalian cells. (A) Infected I. scapularis (ISE6) cell with rickettsiae in the cytoplasm (arrow), as well as being digested in vacuoles (arrowhead). Bar = 2 μm. N, host cell nucleus. (B) Vero cell filled with cytoplasmic rickettsiae (arrows). N, host cell nucleus. Bar = 2 μm. (C) R. monacensis within the nucleus (N, arrowheads) and cytoplasm (arrow) of an ISE6 cell. Bar = 0.5 μm. (D) Rickettsia (arrow) within a pseudopodial extension of an ISE6 cell. Bar = 0.5 μm. (E) High magnification of R. monacensis showing the typical rickettsial morphology of the organism. Bar = 0.2 μm. Shown are the inner periplasmic membrane (white arrowhead), the electron-lucent periplasmic space, and the cell wall (arrow). The insert shows an enlargement of the outer membrane. Bars delineate the trilaminar cell wall; small arrowheads indicate the faint microcapsular layer.
Molecular analyses of IrR/MunichT (i) PCR and RFLP analysis.
Our results demonstrated that IrR/MunichT is an SFG rickettsia. Primer sets for the 16S rRNA (fD1-Rc16S.452n), citrate synthase (RpCS.877p-RpCS.1258n), and 17-kDa Rickettsia-common antigen (Rr17) genes gave PCR products of approximately 440, 380, and 435 bp, respectively, as expected for rickettsiae, and indistinguishable from corresponding PCR products for R. helvetica. These primers also yielded PCR products of the same sizes from another tick (no. 5) that was culture negative. Thus, 2 of the 12 I. ricinus ticks appeared to have been infected with an SFG rickettsia. The rompA primer set (Rr190.70p-Rr190.602n) gave PCR products of the expected size (approximately 532 bp) for cultured IrR/MunichT and the second tick but failed to amplify products from R. helvetica, as previously reported (6, 15, 36, 41).
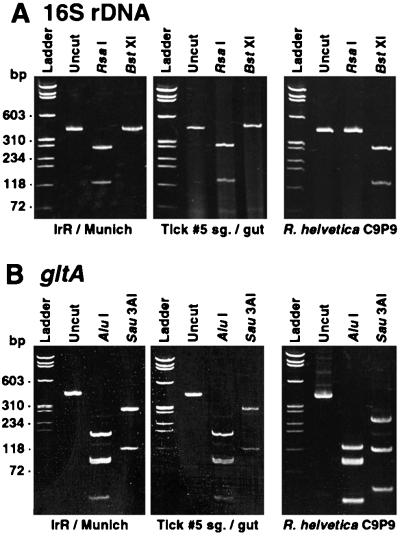
RFLP analysis of 16S rRNA and citrate synthase (gltA) gene PCR products demonstrated that the two ticks were infected with the same rickettsia, which had a genotype unlike that of R. helvetica (Fig. 2). The restriction patterns of the 16S rRNA gene PCR products of IrR/MunichT and tick no. 5 digested with RsaI (132 and 304 bp) and BstXI (uncut) were identical. These profiles were as expected from sequence data (see below) and distinct from those obtained with R. helvetica (RsaI uncut, and BstXI cut, 132 and 304 bp). The RFLP patterns obtained from citrate synthase DNA of IrR/MunichT digested with AluI (43, 81, 87, and 165 bp; the predicted 6-bp fragment was not observed) and Sau3AI (107 and 275 bp) were also as predicted and identical to those from tick no. 5. They differed from the R. helvetica patterns (AluI, 43, 43, 81, 87, and 122 bp [the predicted 6-bp fragment was not observed]; Sau3AI, 48, 107, and 227 bp). In addition, the restriction profiles obtained from IrR/MunichT subcultured 1, 2, 8, or 36 times did not differ, an indication of the purity of the isolate.
FIG. 2.
RFLP analyses of R. monacensis IrR/MunichT, I. ricinus tick no. 5 salivary glands (sg.) and gut tissues, and R. helvetica C9P9 PCR products. (A) 16S rRNA gene PCR products from the fD1-Rc16S.452n primer set uncut or digested with restriction endonuclease RsaI or BstXI. (B) Citrate synthase gene (gltA) sequences corresponding to the RpCS.877p-RpCS.1258n primer set, uncut or digested with restriction endonuclease AluI or Sau3AI. The molecular sizes indicated on the left correspond to φX174 replicative-form DNA digested with HaeIII (Life Technologies).
(ii) BLAST and phylogenetic analyses.
To identify the SFG rickettsia infecting the I. ricinus females, we cloned and sequenced PCR products generated from the 16S rRNA, citrate synthase, and rompA genes. BLAST analyses demonstrated a high level of sequence similarity of IrR/MunichT to three other rickettsiae detected in I. ricinus ticks, the Cadiz agent, IRS3, and IRS4. The 16S rRNA gene sequences of IrR/MunichT, the Cadiz agent, IRS3, and IRS4 were identical (GenBank accession numbers Y08783, AF141907, and AF141908, respectively). Partial gltA sequences from IrR/MunichT and IRS4 were the same but differed from that of IRS3 by 1 nucleotide and from that of the Cadiz agent by 13 nucleotides (GenBank accession numbers AF141906, AF140706, and Y08784, respectively). The partial rompA sequence was more similar to that of IRS4, with 2 nucleotide differences, than to that of either IRS3 or the Cadiz agent, from which it had 6 and 10 nucleotide differences, respectively (GenBank accession numbers AF141911, AF141909, and Y08785, respectively).
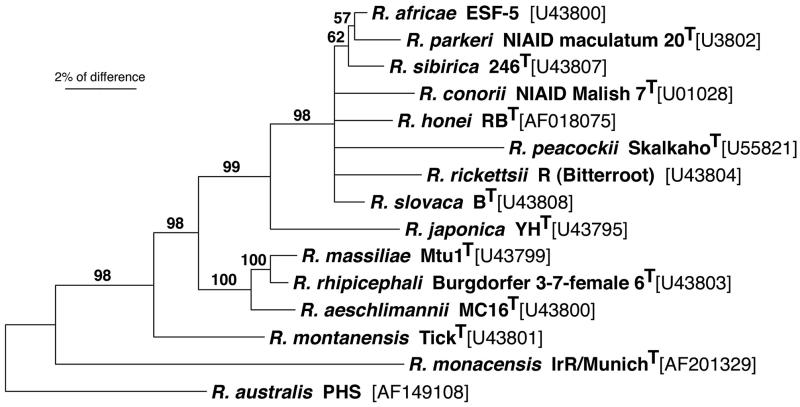
A neighbor-joining analysis based on partial rompA sequences demonstrated the unique genotype of IrR/MunichT among those of the validated species of the SFG (Fig. 3). Along with R. australis, IrR/MunichT was placed most basal with respect to the other rickettsiae included in this analysis (98% bootstrap support).
FIG. 3.
Neighbor-joining phylogram based on partial rompA sequences showing the phylogenetic placement of R. monacensis sp. nov. IrR/MunichT among the validated SFG rickettsial species. Bootstrap support (>50%) for phylogenetic groupings and the scale of percent difference between taxa are indicated. R. australis PHS was used as the outgroup (see Materials and Methods).
Antibody responses of hamsters inoculated with IrR/MunichT.
Five of six hamsters inoculated with IrR/MunichT seroconverted but appeared healthy throughout the course of the study. The IgG titer endpoints of four of the sera tested by IFA against other SFG rickettsiae are shown in Table 2. The highest titer was obtained with IrR/MunichT as the antigen, and titers ranging from 1:64 to 1:512 were obtained with the other rickettsiae. Thus, IrR/MunichT induced IgG antibodies that cross-reacted, albeit at lower titers, with phylogenetically distinct SFG rickettsiae.
TABLE 2.
Comparative titers of IgG to selected SFG rickettsiae in sera of hamsters inoculated with R. monacensis IrR/MunichT-infected host cellsa
| Antigen | Tick cells (IRE11)b | Mammalian cells (Vero)c | Control serumd |
|---|---|---|---|
| Rickettsia monacensis IrR/MunichT | 1:16,384/1:16,384 | 1:16,384/1:16,384 | 1:4 |
| Rickettsia peacockii DaE100R | 1:256/1:512 | 1:256/1:512 | 1:1 |
| Rickettsia strain MOAa | 1:256/1:512 | 1:128/1:512 | 1:1 |
| Rickettsia rickettsii Hlp#2 | 1:128/1:256 | 1:128/1:256 | 1:4 |
| Rickettsia helvetica C9P9 | 1:128/1:128 | 1:64/1:512 | 1:1 |
Intraperitoneal inoculation of 1 × 106 infected cells per hamster.
Sera from two hamsters, 67 days postinoculation.
Sera from two hamsters, 50 days postinoculation.
Negative control hamster serum.
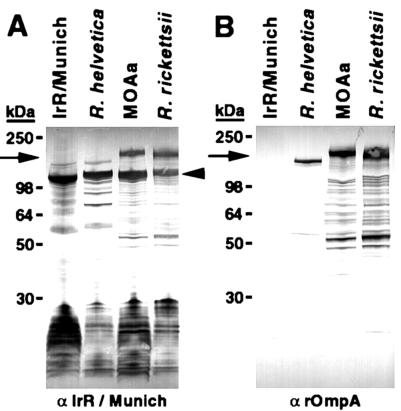
Western blot assays with polyclonal hamster sera or MAb 13-5 to rOmpA of R. rickettsii identified further differences between IrR/MunichT and other SFG rickettsiae. The pooled anti-IrR/MunichT sera from seroconverted hamsters infected with Vero cell-grown IrR/MunichT recognized a number of peptides from IrR/MunichT (Fig. 4A) and other SFG rickettsiae, apparently including rOmpA. The sera bound a 145-kDa protein in both IrR/MunichT and R. helvetica and a 190-kDa antigen (arrow) in Rickettsia strain MOAa and R. rickettsii. In all of the rickettsiae, the anti-IrR/MunichT antisera also recognized bands of approximately 120 kDa (arrowhead) and a series of low-molecular-mass (<30-kDa) bands. MAb 13-5 did not react with any proteins of IrR/MunichT (Fig. 4B) but recognized a series of bands in R. helvetica, Rickettsia strain MOAa, and R. rickettsii. The most prominent bands (arrow) were approximately 145 kDa for R. helvetica and 190 kDa for R. rickettsii and Rickettsia strain MOAa.
FIG. 4.
Western blot analyses of R. monacensis IrR/MunichT, R. helvetica C9P9, Rickettsia strain MOAa, and R. rickettsii Hlp#2 sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated antigens reacted with pooled polyclonal sera from two hamsters inoculated with IrR/MunichT (A) and MAb 13-5 to rOmpA (B). Arrows indicate the approximate positions of rOmpA, as determined by MAb 13-5. The arrowhead indicates the approximate position of rOmpB.
Actin tail formation of IrR/MunichT in vitro.
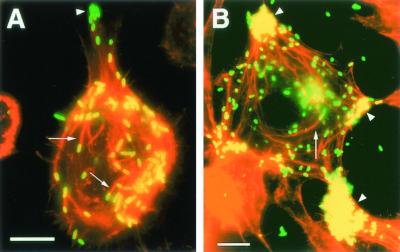
Simultaneous labeling of rickettsiae with FITC and of F-actin with Texas red showed that IrR/MunichT induced actin tails in both tick (DAE100) (Fig. 5A, arrows) and mammalian (L-929) (Fig. 5B, arrows) cells. In both cell types, they were approximately 20 μm long. Rickettsiae migrating centrifugally through thin pseudopodia appeared as if tethered to cells by actin tails, and rickettsial clusters found at the poles of host cells were sometimes associated with actin bundles (Fig. 5B, arrowheads).
FIG. 5.
Immunofluorescence photomicrographs of R. monacensis IrR/MunichT-infected eukaryotic cells as visualized by simultaneous excitation of the appropriate wavelengths for the Texas red and FITC fluorochromes. Rickettsiae were labeled with a hamster anti-IrR/MunichT polyclonal serum reacted with a secondary anti-hamster IgG antibody conjugated to FITC, and host cell actin was labeled with Texas red phalloidin. The arrows indicate actin tail structures associated with rickettsiae. The arrowheads indicate clustering of rickettsiae at the poles of cells. Panels: A, tick (D. andersoni) DAE100 cells; B, mouse (Mus musculus) L-929 cells. Bars = 10 μm.
DISCUSSION
A wide geographic distribution throughout Europe (55) and a broad host range that encompasses mammals, birds, and reptiles expose I. ricinus to diverse blood-borne pathogens (37, 38). Several different SFG rickettsiae have been detected in I. ricinus. R. helvetica and R. slovaca can be readily isolated from I. ricinus and propagated in mammalian cell cultures (5, 46). In addition, other yet-to-be-cultured rickettsiae have been detected by the hemolymph test and PCR in I. ricinus ticks from Switzerland (5), Spain (25), and Slovakia (45). We have isolated an SFG rickettsia from I. ricinus collected in Munich, Germany, by tick cell culture and partially characterized it with respect to growth in tick and mammalian cells and antigen expression and by PCR and nucleotide sequence analysis of selected diagnostic genes. Our partial gltA and rompA sequences and serological findings demonstrated this isolate to be distinct from R. helvetica, R. slovaca, and other previously validated species of SFG rickettsiae. Accordingly, we propose to name it R. monacensis sp. nov. and designate IrR/MunichT the type strain in recognition of its geographic origin.
Our analysis of three different genes confirmed the genotypic difference between R. monacensis and either R. helvetica or R. slovaca. Most informative was our analysis of R. monacensis rompA with the primer set Rr190.70p-Rr190.602n. This primer set does not detect the widely distributed species R. helvetica (6, 8, 30-32, 36), indicating that the gene is missing or modified. R. helvetica is one of the few SFG species in which this rOmpA primer set does not mediate amplification of a PCR product (6), underscoring the need to apply multiple methods and primers for detection and isolation of pathogens from arthropods. Furthermore, the failure to detect any of the predicted restriction products for R. helvetica and R. slovaca confirmed their absence in our culture or in the ticks. Our phylogenetic analysis of partial rompA sequences placed R. monacensis basal with respect to the validated SFG rickettsial species included in the analysis, with the exception of R. australis. BLAST analyses of the 16S rRNA, gltA, and rompA gene sequences demonstrated a close relationship of R. monacensis with the Cadiz agent, IRS3, and IRS4. The PCR-amplified 16S sequence of IrR/MunichT showed 100% similarity to those of the Cadiz agent, ISR3, and ISR4, and the PCR-amplified citrate synthase gene sequence of IrR/MunichT showed 100% similarity to that of IRS4 but less to those of IRS3 (99.77%) and the Cadiz agent (97.01%). The partial rompA gene sequence also indicated that IrR/MunichT is closer to IRS4 (99.62% similar) than to IRS3 (98.88% similar) and the Cadiz agent (98.12% similar). Nevertheless, further studies and more sequence data are needed to resolve the phylogenetic relationship of R. monacensis to the Cadiz agent, IRS3, and IRS4 and to SFG rickettsiae detected in other species of Ixodes ticks (7, 33). The isolation of these organisms in tick cell culture systems could facilitate such studies.
Our serologic data provide further evidence that R. monacensis is a novel SFG rickettsia. MAb 13-5 against rOmpA of R. rickettsii cross-reacts with most other species of SFG rickettsiae (2), and we have demonstrated its reactivity with R. helvetica, Rickettsia strain MOAa, and R. rickettsii. By contrast, R. monacensis, similar to R. australis, failed to react with MAb 13-5, which is further evidence of a closer relationship between these two species. Hamsters inoculated with IrR/MunichT seroconverted with a specific IgG titer of 1:16,384, demonstrating its ability to elicit an adaptive immune response. The high antibody titer suggested an established infection, as seen with the U strains of R. rickettsii (9). This is reflected in the numerous antigens detected in Western blots. Experimental tick transmission of R. monacensis to mammalian hosts will be important to the evaluation of its possible role as a tick-borne pathogen. The availability of an in vitro culture system for this organism will allow further assessment of its epidemiologic potential with serosurveys to identify populations at risk, as has been done for R. helvetica and Anaplasma phagocytophila (10, 13, 14). Assessments of antibody titers in vertebrates resident in the English Garden would also be instructive.
The location of R. monacensis within nuclei and pseudopodia is characteristic of rickettsiae that induce actin polymerization for mobility (20, 26). The actin tails of R. monacensis were similar in length and shape to those formed by other SFG rickettsiae (e.g., R. rickettsii and R. conorii) (16, 54). Moreover, R. monacensis tended to cluster at the poles of host cells, where they were associated with bundles of actin, as observed with R. rickettsii (19). The ability to polymerize eukaryotic host cell actin is thought to facilitate intracellular, as well as intercellular, movement of rickettsiae (20, 51). The long actin tails induced by R. monacensis are characteristic of SFG rickettsiae, such as R. rickettsii, that spread rapidly within hosts.
Description of Rickettsia monacensis sp. nov.
R. monacensis (mo.na.cen′sis. M.L. n. Monacum, Munich, a German city; M. L. adj. monacensis, from/of Munich) sp. nov., for a novel SFG rickettsial species isolated from a female I. ricinus tick collected in the English Garden in Munich, Germany. The type strain (IrR/MunichT) maintained in tick and mammalian cell cultures was characterized. The type strain was used to generate partial 16S rRNA gene, rompA, and citrate synthase gene (gltA) sequences. The PCR and RFLP profiles obtained were the same as those obtained from another tick collected at the same time in the same area.
A prokaryote found in the castor bean tick (I. ricinus) that grows intracellularly in cultures of mammalian (mouse L-929 and African green monkey Vero) and tick (I. ricinus IRE11, I. scapularis ISE6, and D. andersoni DAE100) cells. The organism has an ultrastructure that conforms to that of rickettsiae, with a size range of 1 to 1.5 by 0.3 to 0.4 μm. Organisms are found free in the cytoplasm and occasionally within the nuclei of host cells. Not enclosed in host-provided membranes except when within digestive vacuoles of tick cells. The periplasmic membrane delineates an evenly mottled body, separated from the cell wall by a thin (20-nm) periplasmic space. The inner and outer leaflets of the trilaminar cell wall are equal in thickness, and the outer leaflet is lined with a faint microcapsular layer. The slime layer surrounding the microbes is thin (<30 nm). The rickettsiae are capable of inducing polymerization of host actin in both tick and mammalian cells, with tails averaging 20 μm in length. Polyclonal antibodies from laboratory hamsters cross-react significantly in IFAs with other SFG rickettsiae, but the titers of polyclonal antibodies against the homologous antigen are the highest and those against R. helvetica are the lowest. The most prominent antigens recognized on Western blots are approximately 120 to 145 kDa in size. IrR/MunichT does not react with MAb 13-5 against rOmpA of R. rickettsii. DNA primers complementary to regions of rickettsial genes for citrate synthase, the 17-kDa antigen, and rompA mediate amplification of specific products. Restriction of the 16S rRNA gene PCR product with RsaI yields two fragments, of 132 and 304 bp, but its restriction with BstXI yielded none. Restriction of the citrate synthase gene PCR product with AluI yielded fragments of 6, 43, 87, and 165 bp, and its restriction with Sau3AI yielded fragments of 107 and 275 bp.
Acknowledgments
This research was supported by a University of Minnesota Graduate School Doctoral Dissertation Fellowship award to Jason A. Simser. Research was also supported by state funds from the Minnesota Agriculture Experiment Station and Public Health Service grants (AR37909 and AI49424) from the National Institutes of Health.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Anacker, R. L., R. E. Mann, and C. Gonzales. 1987. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J. Clin. Microbiol. 25:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, B. E., and T. Tzianabos. 1989. Comparative sequence analysis of a genus-common rickettsial antigen gene. J. Bacteriol. 171:5199-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, S. G. E., D. R. Stothard, P. Fuerst, and C. G. Kurland. 1999. Molecular phylogeny and rearrangement of rRNA genes in Rickettsia species. Mol. Biol. Evol. 16:987-995. [DOI] [PubMed] [Google Scholar]
- 5.Beati, L., P.-F. Humair, A. Aeschlimann, and D. Raoult. 1994. Identification of spotted fever group rickettsiae isolated from Dermacentor marginatus and Ixodes ricinus ticks collected in Switzerland. Am. J. Trop. Med. Hyg. 51:138-148. [DOI] [PubMed] [Google Scholar]
- 6.Beati, L., O. Peter, W. Burgdorfer, A. Aeschlimann, and D. Raoult. 1993. Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group rickettsiae. Int. J. Syst. Bacteriol. 43:521-526. [DOI] [PubMed] [Google Scholar]
- 7.Billings, A. N., G. J. Teltow, S. C. Weaver, and D. H. Walker. 1998. Molecular characterization of a novel Rickettsia species from Ixodes scapularis in Texas. Emerg. Infect. Dis. 4:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgdorfer, W., A. Aeschlimann, O. Peter, S. F. Hayes, and R. N. Philip. 1979. Ixodes ricinus: vector of a hitherto undescribed spotted fever group agent in Switzerland. Acta Trop. 36:357-367. [PubMed] [Google Scholar]
- 9.Dasch, G. A., and E. Weiss. 1992. The genera Rickettsia, Rochalimaea, Ehrlichia, Cowdria, and Neorickettsia, p. 2407-2470. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, vol. 3. Springer Verlag, New York, N.Y.
- 10.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designations of Ehrlichia equi and ′HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. E vol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 11.Euzéby, J. P. 1997. List of bacterial names with standing in nomenclature: a folder available on the internet. Int. J. Syst. Bacteriol. 47:590-592. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 13.Fingerle, V., J. L. Goodman, R. C. Johnson, T. J. Kurtti, U. G. Munderloh, and B. Wilske. 1999. Epidemiological aspects of human granulocytic ehrlichiosis in southern Germany. Wien. Klin. Wochenschr. 111:1000-1004. [PubMed] [Google Scholar]
- 14.Fournier, P.-E., F. Grunnenberger, B. Jaulhac, G. Gastinger, and D. Raoult. 2000. Evidence of Rickettsia helvetica infection in humans, eastern France. Emerg. Infect. Dis. 6:389-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier, P.-E., V. Roux, and D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 48:839-849. [DOI] [PubMed] [Google Scholar]
- 16.Gouin, E., H. Gantelet, I. L. Egile, H. Ohayon, V. Villiers, P. Gounon, and P. J. Sansonetti. 1999. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J. Cell Sci. 112:1697-1708. [DOI] [PubMed] [Google Scholar]
- 17.Hackstadt, T. 1996. The biology of rickettsiae. Infect. Agents Dis. 5:127-143. [PubMed] [Google Scholar]
- 18.Hayes, S. F., and W. Burgdorfer. 1982. Reactivation of Rickettsia rickettsii in Dermacentor andersoni ticks: an ultrastructural analysis. Infect. Immun. 37:779-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzen, R. A., S. S. Grieshaber, L. S. Van Kirk, and C. J. Devin. 1999. Dynamics of actin-based movement by Rickettsia rickettsii in Vero cells. Infect. Immun. 67:4201-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuchi, R. 1989. Simple and rapid preparation of samples for PCR, p. 31-43. In H. A. Ehrlich (ed.), PCR technology, principles and applications for DNA amplification. Stockton Press, New York, N.Y.
- 22.Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 23.Kurtti, T. J., U. G. Munderloh, C. A. N. Hughes, S. M. Engstrom, and R. C. Johnson. 1996. Resistance to tick-borne spirochete challenge induced by Borrelia burgdorferi strains that differ in expression of outer surface proteins. Infect. Immun. 64:4148-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Marquez, F. J., M. A. Muniain, R. C. Soriguer, G. Izquierdo, J. Rodriguez-Bano, and M. V. Borobio. 1998. Genotypic identification of an undescribed spotted fever group Rickettsia in Ixodes ricinus from southwestern Spain. Am. J. Trop. Med. Hyg. 58:570-577. [DOI] [PubMed] [Google Scholar]
- 26.Munderloh, U. G., S. F. Hayes, J. Cummings, and T. J. Kurtti. 1998. Microscopy of spotted fever rickettsia movement through tick cells. Microsc. Microanal. 4:115-121. [Google Scholar]
- 27.Munderloh, U. G., S. D. Jauron, V. Fingerle, L. Leitritz, S. F. Hayes, J. M. Hautman, C. M. Nelson, B. W. Huberty, T. J. Kurtti, G. G. Ahlstrand, B. Greig, M. A. Mellencamp, and J. L. Goodman. 1999. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 37:2518-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munderloh, U. G., Y. Liu, M. Wang, C. Chen, and T. J. Kurtti. 1994. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80:533-543. [PubMed] [Google Scholar]
- 29.Munderloh, U. G., J. E. Madigan, J. S. Dumler, J. L. Goodman, S. F. Hayes, J. E. Barlough, C. M. Nelson, and T. J. Kurtti. 1996. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J. Clin. Microbiol. 34:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson, K., T. G. T. Jaenson, I. Uhnoo, O. Lindquist, B. Pettersson, M. Uhlen, G. Friman, and C. Pahlson. 1997. Characterization of a spotted fever group rickettsia from Ixodes ricinus ticks in Sweden. J. Clin. Microbiol. 35:243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson, K., O. Lindquist, A. J. Liu, T. G. T. Jaenson, G. Friman, and C. Pahlson. 1999. Rickettsia helvetica in Ixodes ricinus ticks in Sweden. J. Clin. Microbiol. 37:400-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson, K., O. Lindquist, and C. Pahlson. 1999. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet 354:1169-1173. [DOI] [PubMed] [Google Scholar]
- 33.Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obonyo, M., U. G. Munderloh, V. Fingerle, B. Wilske, and T. J. Kurtti. 1999. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker, R. R., E. G. Pickens, D. B. Lackman, E. J. Bell, and F. B. Thraikill. 1951. Isolation and characterization of Rocky Mountain spotted fever rickettsiae from the rabbit tick Heamaphysalis leporis-palustris Packard. Public Health Rep. 66:455-463. [PMC free article] [PubMed] [Google Scholar]
- 36.Parola, P., L. Beati, M. Cambon, and D. Raoult. 1998. First isolation of Rickettsia helvetica from Ixodes ricinus ticks in France. Eur. J. Clin. Microbiol. Infect. Dis. 17:95-100. [DOI] [PubMed] [Google Scholar]
- 37.Parola, P., and D. Raoult. 2001. Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 7:80-83. [DOI] [PubMed] [Google Scholar]
- 38.Randolph, S. E. 2001. The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 356:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roux, V., P.-E. Fournier, and D. Raoult. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roux, V., and D. Raoult. 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146:385-396. [DOI] [PubMed] [Google Scholar]
- 43.Roux, V., and D. Raoult. 2000. Phylogenetic analysis of the members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. E vol. Microbiol. 50:1449-1455. [DOI] [PubMed] [Google Scholar]
- 44.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 45.Sekeyova, Z., P.-E. Fournier, J. Rehacek, and D. Raoult. 2000. Characterization of a new spotted fever group rickettsia detected in Ixodes ricinus (Acari:Ixodidae) collected in Slovakia. J. Med. Entomol. 37:707-713. [DOI] [PubMed] [Google Scholar]
- 46.Sekeyova, Z., V. Roux, W. Xu, J. Rehacek, and D. Raoult. 1998. Rickettsia slovaca sp. nov., a member of the spotted fever group rickettsiae. Int. J. Syst. Bacteriol. 48:1455-1462. [DOI] [PubMed] [Google Scholar]
- 47.Silverman, D. J., and C. L. Wisseman. 1979. In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect. Immun. 26:714-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stothard, D. R., and P. A. Fuerst. 1995. Evolutionary analysis of the spotted fever and typhus groups of Rickettsia using 16S rRNA gene sequences. Syst. Appl. Microbiol. 18:52-61. [Google Scholar]
- 50.Swofford, D. L. 2001. PAUP∗: phylogenetic analysis using parsimony (∗and other methods), 4.0b8 (PPC) ed. Sinauer Associates, Sunderland, Mass.
- 51.Teysseire, N., C. Chiche-Portiche, and D. Raoult. 1992. Intracellular movements of Rickettsia conorii and R. typhi based on actin polymerization. Res. Microbiol. 143:821-829. [DOI] [PubMed] [Google Scholar]
- 52.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Todd, W. J., W. Burgdorfer, and G. P. Wray. 1983. Detection of fibrils associated with Rickettsia rickettsii. Infect. Immun. 41:1252-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Kirk, L. S., S. F. Hayes, and R. A. Heinzen. 2000. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect. Immun. 68:4706-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varma, M. G. R. 1989. Tick-borne diseases, p. 55-70. Geographical distribution of arthropod-borne diseases and their principal vectors. World Health Organization, Geneva, Switzerland.
- 56.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weller, S. J., G. D. Baldridge, U. G. Munderloh, H. Noda, J. Simser, and T. J. Kurtti. 1998. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J. Clin. Microbiol. 36:1305-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, S. G., J. B. Sacci, M. E. Schriefer, E. M. Anderson, K. K. Fujioka, F. J. Sorvillo, A. R. Barr, and A. F. Azad. 1992. Typhus and typhuslike rickettsiae associated with opossums and their fleas in Los Angeles county, California. J. Clin. Microbiol. 30:1758-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]