Abstract
Individual differences in neural circuits underlying emotional regulation, motivation, and decision-making are implicated in many psychiatric illnesses. Interindividual variability in these circuits may manifest, at least in part, as individual differences in impulsivity at both normative and clinically significant levels. Impulsivity reflects a tendency towards rapid, unplanned reactions to internal or external stimuli without considering potential negative consequences coupled with difficulty inhibiting responses.
Here, we use multivariate brain-based predictive models to explore the neural bases of impulsivity across multiple behavioral scales, neuroanatomical features (cortical thickness, surface area, and gray matter volume), and sexes (females and males) in a large sample of youth from the Adolescent Brain Cognitive Development (ABCD) Study at baseline (n = 9,099) and two-year follow-up (n = 6,432).
Impulsivity is significantly associated with neuroanatomical variability, and these associations vary across behavioral scales and neuroanatomical features. Impulsivity broadly maps onto cortical thickness in dispersed regions (e.g., inferior frontal, lateral occipital, superior frontal, entorhinal), as well as surface area and gray matter volume in specific medial (e.g., parahippocampal, cingulate) and polar (e.g., frontal and temporal) territories. Importantly, while many relationships are stable across sexes, others are sex-specific.
These results highlight the complexity of the relationships between neuroanatomy and impulsivity across scales, features, sexes, and time points in youth. These findings suggest that neuroanatomy, in combination with other biological and environmental factors, reflects a key driver of individual differences in impulsivity in youth. As such, neuroanatomical markers may help identify youth at increased risk for developing impulsivity-related illnesses. Furthermore, this work emphasizes the importance of adopting a multidimensional and sex-specific approach in neuroimaging and behavioral research.
Keywords: BIS/BAS, UPPS, neuroanatomy, machine learning, sex differences, development
INTRODUCTION
Impairments in emotional regulation, motivation, and decision-making are prevalent across a range of psychiatric illnesses1 and often emerge during early adolescence2,3. These impairments contribute to the heterogeneity observed within psychiatric illnesses and may initially appear as more fundamental alterations in processes and behaviors such as impulsivity4,5. Impulsivity is a multifaceted construct that reflects “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions to the impulsive individual or to others.6” Importantly, changes in impulsivity are a normal part of development. However, in some individuals, heightened levels of impulsivity may indicate increased risk for psychiatric illness7–9 (see Supplemental Materials for specific examples). A better understanding of the neurobiological underpinnings of impulsivity, especially in youth, is crucial for developing more effective prevention and intervention strategies for these at-risk individuals. Neuroimaging techniques offer a powerful tool for investigating these neural substrates and identifying potential biomarkers for interventions. Given that adolescence is a period of significant brain plasticity, interventions targeting these impulsivity-related neural circuits in youth may be most effective.
Although often treated as a single construct, the term impulsivity encompasses a variety of distinct but related functions that promote impulsive behavior10. These include an individual’s (in)ability to consider the consequences of a behavior (lack of premeditation), tendency to disengage from tasks due to boredom or difficulty before completion (lack of perseverance), responses to emotional states (positive and negative urgency), and motivation to experience rewarding sensations (sensation seeking)9. Impulsivity can also be considered a product of two systems that promote impaired self-regulation: the behavioral inhibition and approach systems (BIS/BAS)11. The BIS prevents actions that may lead to a negative outcome12, while the BAS encapsulates sensitivity to, and motivation for, reward/punishment, as well as escape from punishment, therefore encouraging incentive-motivated behavior12. These conceptualizations highlight the variability in how impulsivity is defined and measured, posing a challenge for research, and underscoring the need for greater conceptual clarity across different definitions of impusivity6,10. Consequently, a crucial consideration in exploring the neuroanatomical basis of impulsivity is the extent to which different, yet related, components of this construct may be subserved by shared versus distinct neurobiological substrates.
Corticolimbic and corticostriatal circuitry regulate impulsivity8,13–16. The corticolimbic system contributes to processing emotional salience and regulating emotional responses17, while the corticostriatal system is involved in motivated behavior, reward processing, learning, and habit formation18. These systems mature throughout development19 and this is accompanied by significant changes in synaptic connectivity and myelination20–22. Critically, this development is asynchronous. Limbic and striatal structures mature earlier than cortical structures, including the prefrontal cortex, resulting in heightened impulsivity during this period of developmental ‘mismatch’21,23,24. Behaviorally, emotional regulation, motivation, and impulse control evolve throughout development with rapid changes in early life followed by gradual changes during adolescence25–29. This change is paralleled by changes in their neural substrates beyond corticolimbic and corticostriatal circuits, such as the insula and cingulate cortex7,29,30. However, previous studies examining these relationships have often focused on establishing univariate, cross-sectional associations between specific brain circuits and individual behavioral measures in small sample sizes. In contrast, brain-based predictive models use machine learning to analyze whole-brain multivariate relationships between brain features and behavioral measures31, which account for the interconnected nature of the brain, unlike traditional analyses focused on single regions. These models along with the large sample sizes provided by big data initiatives can provide insights into the whole-brain neuroanatomical basis of impulsivity32. In addition, longitudinal data can be used to examine whether these relationships are consistent throughout development. Here, we leverage these approaches to garner insights into the neural substrates of impulsivity in youth.
Importantly, neurodevelopmental processes and behavioral expressions vary between males and females, raising questions about the extent to which sex-specific neuroanatomical patterns contribute to observed differences in impulsivity. There are significant sex differences in the developmental trajectories of corticolimbic and corticostriatal systems33,34, although findings have not always been consistent across studies. As an example, on average, females have greater relative volume in the prefrontal and orbitofrontal regions, while males have greater volume in ventral temporal and occipital regions35. Similarly, sex differences have been reported in impulsivity, but these results are also inconsistent36. Thus, it is plausible that sex differences exist across neuroanatomy, impulsivity, and their interrelationships, highlighting the importance of considering sex differences when studying impulsivity, particularly within a developmental framework. Furthermore, within the context of recently established large data initiatives, it remains to be determined whether sex differences in impulsivity are driven by unique neuroanatomical substrates. This can be addressed by establishing sex-specific relationships between neuroanatomy and impulsivity and examining the extent to which they overlap.
Cortical thickness (CT), surface area (SA), and gray matter volume (GMV) reflect different aspects of neuroanatomy. CT (i.e., distance between the brain’s outer surface and gray-white matter junction) reflects neuronal density and arrangement37,38. It increases rapidly during the prenatal period, continues growing after birth, peaks in early childhood (with regional variation), and then gradually thins39. Cortical SA (i.e., area of the pial surface) is linked to the organization and complexity of cortical columns37,38 as well as neuronal proliferation37,38 and gyrification40. SA expands prenatally and through childhood, peaking in late childhood/early adolescence, and then gradually declines39. GMV, encompassing thickness and SA, reflects the total amount of cells and synapses37,38, and generally follows the same developmental trajectory observed for SA39. Changes in these neuroanatomical features result from neurogenesis, synaptogenesis, synaptic pruning, cell death, and alterations in cell size and density, and are linked to various psychiatric conditions37,38. Importantly, these structural measures demonstrate high reliability and are considered more “trait-like” compared to functional measures, making them particularly valuable for investigation. Given these complexities, a multimodal analysis considering all three features of brain structure across all regions of the brain is warranter to reveal their unique and shared contributions to impulsivity, facilitating a more holistic understanding of these relationships.
Here, we investigated the sex-specific neuroanatomical basis of impulsivity, across different neuroanatomical features and impulsivity measures, in a large sample of youth from the Adolescent Brain Cognitive Development (ABCD) Study at baseline and two-year follow-up. Using a data-driven, brain-based predictive modeling framework, we show that neuroanatomical features are associated with impulsivity and there are notable sex differences in these relationships. We also demonstrate that different domains of impulsivity are linked to shared and distinct neuroanatomical features. Some features vary across facets of impulsivity, others across sexes, and others across time points. These findings highlight substantial individual variability in the neural basis of impulsivity in youth. Understanding these distinct markers of impulsivity is crucial for establishing normative developmental patterns and paves the way for development of more effective early interventions grounded in neurobiological mechanism to prevent psychiatric illness.
METHODS
An overview of the methods is provided below. Details are in the Supplemental Materials.
Dataset
The ABCD Study is following a large community-based sample of children and adolescents throughout the course of development41. Participants are assessed on a comprehensive set of neuroimaging, behavioral, developmental, and psychiatric batteries. In this study, we used imaging and impulsivity data from 9,099 participants at baseline (ages 9–10 years) and 6,432 participants at the two-year follow-up (see Table S1 for demographic data). Details on data inclusion procedures (e.g., exclusion criteria for neuroimaging data) are provided in the Supplemental Materials (see Figures S1-S2 for our participant inclusion pipeline). The research protocol for the dataset was reviewed and approved by a central Institutional Review Board (IRB) at the University of California, San Diego, and, in some cases, by individual site IRBs. Parents or guardians provided written informed consent, and children assented before participation.
Neuroimaging
The neuroimaging protocol and specific parameters for T1-weighted scans are detailed in previous publications41,42. We used measures of CT (mean), SA (total), and GMV (total) for 68 cortical regions (34 per hemisphere) from the Desikan-Killiany parcellation as provided on the NIMH Data Archive. Regional SA and GMV measures, but not CT, were proportionally corrected for individual differences in intracranial volume by dividing the raw values by intracranial volume, as recommended by prior work43 (see Figure S3 for average measures and Figure S4 for sex differences in the measures).
Impulsivity
Impulsivity-related measures were derived from the Behavioral Inhibition/Activation System (BIS/BAS) and the Modified Urgency, (lack of) Planning (or Premeditation), (lack of) Perseverance, Sensation-Seeking, and Positive Urgency (UPPS-P44) Short Version scales, both of which have been validated in children12,45. We examined these measures separately, rather than combining them into summary or composite scores, as they assess distinct facets of impulsivity with potentially unique neural underpinnings, developmental trajectories, and sex differences.
Differences in Impulsivity Across Development and Across Sexes
We used non-parametric Mann-Whitney U rank tests to evaluate differences in the behavioral measures (4 BIS/BAS, 5 UPPS-P) between baseline and two-year follow-up as the scales were not normally distributed. We also used non-parametric Mann-Whitney U rank tests to evaluate sex differences in the behavioral measures at each time point. We corrected p-values for multiple comparisons within each behavioral scale using the Benjamini-Hochberg False Discovery Rate (q = 0.05) procedure46. We also computed sex-independent and sex-specific full correlations between the measures at each time point to evaluate their co-expression in youth.
Predictive Modeling
We used a cross-validated brain-based predictive modeling framework31 which we have leveraged in prior work43,47–51. This framework avoids data leakage and minimizes overfitting to capture robust, reliable, and interpretable associations between imaging-derived measures and phenotypic data. For each pair of neuroanatomical features and behavioral measure, we developed separate sets of sex-independent (i.e., including the entire sample) and sex-specific (i.e., in either females or males) linear ridge regression models at each of the two time points to predict impulsivity based on neuroanatomy. We trained each model on neuroanatomical features (either CT, SA, or GMV) from 68 regions to predict a single impulsivity measure. We quantified model performance using prediction accuracy51–54 and assessed significance in comparison to null distributions. We report test accuracies in our results to provide an unbiased estimate of model generalizability, as training accuracy can be inflated due to overfitting. We corrected p-values for multiple comparisons within each behavioral scale using the Benjamini-Hochberg procedure46.
Feature Weights
For models yielding reliable brain-behavior relationships in both sexes (as compared to null distributions), we conducted a series of analyses to determine feature importance and to guide mechanistic understanding. This conservative approach ensures that comparisons are only made where meaningful brain-behavior relationships are present in both sexes. We transformed the feature weights obtained from the models using the Haufe transformation55 (to increase their interpretability and reliability53,56,57) and then calculated a mean feature importance for each set of models. We computed cosine similarities between the mean feature importance values to evaluate overlap in the regional features associated with different impulsivity measures.
RESULTS
Expressions of impulsivity vary across youth.
Analyses examining the distributions of the impulsivity measures and the correlations between them at baseline and two-year follow-up are presented in the Supplemental Materials (see Figure S5 for results across all participants and S6 for sex-specific results). These distributions are consistent with prior work examining impulsivity in the ABCD Study58,59, indicating that our sample is representative of the cohort. Broadly, these analyses indicated modest within-scale correlations of measures suggesting that they capture partially overlapping aspects of behavior, and significant but weak between-scale correlations indicating that the UPPS-P and BIS/BAS, while potentially related, measure somewhat independent constructs60.
Neuroanatomy predicts impulsivity
Brain-based predictive models were used to quantify associations between neuroanatomy and impulsivity (Fig. 1, Table S2).
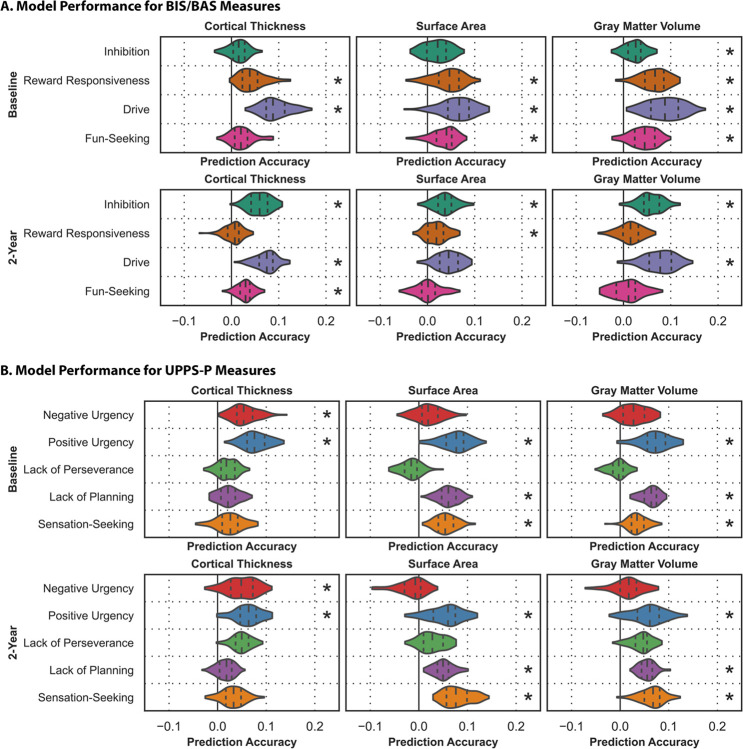
Figure 1. Neuroanatomy reflects individual differences in impulsivity.
Prediction accuracies (correlation between observed and predicted values) for models trained to predict behavioral measures from the BIS/BAS (A) and UPPS-P (B) scales. Results for models based on CT (left), SA (center), and GMV (right) at baseline (top) and two-year follow-up (bottom) are shown. The shape of the violins indicates the distribution of values, the dashed lines indicate the median, and the dotted lines indicate the interquartile range. Asterisks indicate the model captured significant associations.
CT: Models based on CT accurately predicted reward-responsiveness (prediction accuracy, r = 0.044, pFDR<0.001), drive (r = 0.093, pFDR<0.001), negative urgency (r = 0.055, pFDR=0.020), and positive urgency (r = 0.078, pFDR=0.015) at baseline; and inhibition (r = 0.059, pFDR=0.022), drive (r = 0.073, pFDR=0.004), fun-seeking (r = 0.029, pFDR=0.028), negative urgency (r = 0.049, pFDR=0.008), and positive urgency (r = 0.062, pFDR<0.001) at two-year follow-up.
SA: Models based on SA accurately predicted reward-responsiveness (r = 0.043, pFDR<0.001), drive (r = 0.064, pFDR=0.004), fun-seeking (r = 0.035, pFDR = < 0.001), positive urgency (r = 0.074, pFDR<0.001), lack of planning (r = 0.061, pFDR<0.001), and sensation-seeking (r = 0.055, pFDR=0.003) at baseline; and inhibition (r = 0.037, pFDR=0.044), reward-responsiveness (r = 0.019, pFDR=0.044), positive urgency (r = 0.055, pFDR=0.040), lack of planning (r = 0.050, pFDR=0.040), and sensation-seeking (r = 0.080, pFDR=0.025) at two-year follow-up.
GMV: Models based on GMV accurately predicted inhibition (r = 0.024, pFDR=0.023), reward-responsiveness (r = 0.065, pFDR=0.002), drive (r = 0.088, pFDR=0.004), fun-seeking (r = 0.043, pFDR=0.002), positive urgency (r = 0.071 pFDR<0.001), lack of planning (r = 0.062, pFDR<0.001), and sensation-seeking (r = 0.036, pFDR=0.033) at baseline; and inhibition (r = 0.058, pFDR=0.010), drive (r = 0.077, pFDR<0.001), positive urgency (r = 0.059, pFDR<0.001), lack of planning (r = 0.056, pFDR=0.030), and sensation-seeking (r = 0.065, pFDR=0.005) at two-year follow-up.
We next used the same framework to examine sex-specific associations (Fig. 2, Tables S3-S4).
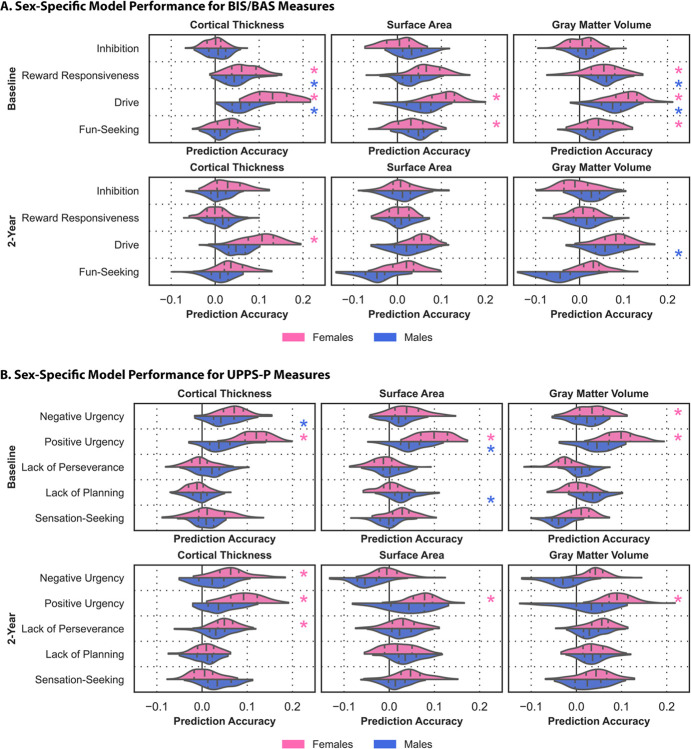
Figure 2. Sex influences associations between neuroanatomy and impulsivity.
Prediction accuracies (correlation between observed and prediction values) for sex-specific models trained to predict behavioral measures from the BIS/BAS (A) and UPPS-P (B) scales. Results for female-specific (pink) and male-specific (blue) models based on CT (left), SA (center), and GMV (right) at baseline (top) and two-year follow-up (bottom) are shown. The shape of the violins indicates the distribution of values, the dashed lines indicate the median, and the dotted lines indicate the interquartile range. Asterisks indicate the model captured significant associations.
CT: Sex-specific models based on CT accurately predicted reward-responsiveness (rfemale=0.065, pfemale=0.002; rmale=0.046, pmale=0.008) and drive (rfemale=0.133, pfemale<0.001; rmale=0.060, pmale=0.004) in both sexes, negative urgency (r = 0.048, p = 0.015) in males, and positive urgency (r = 0.119, p < 0.001) in females at baseline; and drive (r = 104, p = 0.028), negative urgency (r = 0.065, p = 0.005), positive urgency (r = 0.093, p < 0.001), and lack of perseverance (r = 0.051, p = 0.038) in females at two-year follow-up.
SA: Sex-specific models based on SA accurately predicted positive urgency (rfemale=0.099, pfemale<0.001; rmale=0.046, pmale<0.005) in both sexes, drive (r = 0.106, p < 0.001) and fun-seeking (r = 0.045 p = 0.006) in females, and lack of planning (r = 0.031, p = 0.008) in males at baseline; and positive urgency in females (r = 0.075, p = 0.005) at two-year follow-up.
GMV: Sex-specific models based on GMV accurately predicted reward-responsiveness (rfemale=0.052, pfemale=0.028; rmale=0.059, pmale=0.020) and drive (rfemale=0.105 pfemale<0.001; rmale=0.072, pmale<0.001) in both sexes, fun-seeking (r = 0.045, p = 0.006), and negative urgency (r = 0.034, p = 0.040) in females at baseline; and drive (r = 0.060, p < 0.001) in males and positive urgency in females (r = 0.094, p = 0.005) at two-year follow-up.
Changes in impulsivity are part of normative development and, at their extremes, are linked to the development of mental illnesses later in life61,62. We show that individual variations in neuroanatomy can predict impulsivity in youth, and these relationships are generally stable across development. While certain measures can be predicted in both sexes, others yield significant results only in females, which may be due to the reliability of measures across sexes, reporting biases, and data quality, among other factors.
Impulsivity maps onto shared and distinct brain regions
We derived the feature importance maps from the models and computed cosine similarities to evaluate overlap, focusing on models that captured significant associations. These results, presented in their entirety in Fig. 3A, indicated that models predicting measures from the same scale captured largely overlapping associations. As an example, CT features associated with positive and negative urgency were highly similar at both time points (cosine similarity, sbaseline=0.69, stwo−year=0.77). Further, while some models predicting measures from different scales were similar, others were orthogonal or opposite. For example, baseline CT features associated with reward-responsiveness and drive were dissimilar from those associated with lack of planning and sensation seeking (−0.60 ≤ s≤−0.29), but similar to those associated with positive urgency (0.62 ≤ s ≤ 0.64). These observed patterns were generally consistent across time points for CT and SA, but less so for GMV.
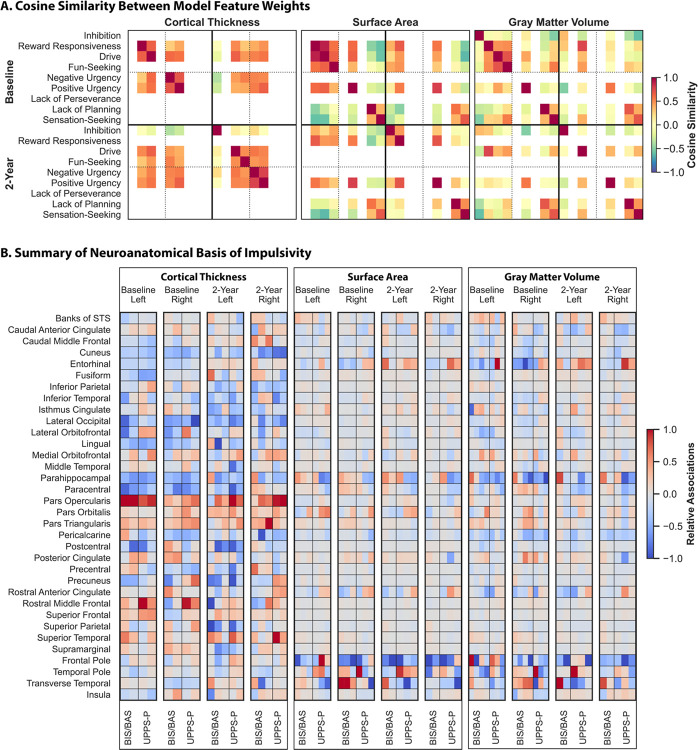
Figure 3. Shared and unique neuroanatomical features are linked to impulsivity.
Cosine similarities between the Haufe-transformed regional feature weights from models trained to predict impulsivity (A). Results for models based on CT (left), SA (center), and GMV (right) that captured significant associations are shown. Warmer colors indicate greater similarity, cooler colors indicate a greater dissimilarity. Rows and columns corresponding to models that did not capture significant associations are left blank.
Relative regional associations (Haufe-transformed feature weights) between neuroanatomy and impulsivity derived from the models based on CT (left), SA (center), and GMV (right) (B). Left and right hemisphere are shown as denoted by the top, x-axis labels. Warmer colors indicate a stronger positive association, cooler colors indicate a stronger negative association. To facilitate visualization, association values for each set of models were divided by the maximum value for that model. Results are only shown for models that captured significant associations, as outlined below (ordered left to right), and measures from the scales are separated by vertical lines.
CT, Baseline: Reward Responsiveness, Drive, Negative Urgency, Positive Urgency
CT, Two-Year: Inhibition, Drive, Fun-Seeking, Negative Urgency, Positive Urgency
SA, Baseline: Reward Responsiveness, Drive, Fun-Seeking, Positive Urgency, Lack of Planning, Sensation-Seeking
SA, Two--Year: Inhibition, Reward Responsiveness, Positive Urgency, Lack of Planning, Sensation-Seeking
GMV, Baseline: Inhibition, Reward Responsiveness, Drive, Fun-Seeking, Positive Urgency, Lack of Planning, Sensation-Seeking
GMV, Two-Year: Inhibition, Drive, Fun-Seeking, Positive Urgency, Lack of Planning, Sensation-Seeking
We also evaluated the similarities in the neuroanatomical features associated with impulsivity measures across the sexes, focusing on the five pairs of models that yielded significant results in both sexes at baseline. GMV features associated with reward-responsiveness at baseline were quite similar across the sexes (s = 0.53), along with SA features associated with positive urgency (s = 0.53). However, other associations were considerably different across the sexes. Models based on CT to predict reward-responsiveness and drive captured distinct associations in males and females (sreward−responsiveness=0.24, sdrive=0.37), as well as those based on GMV to predict drive (s = 0.36).
These analyses reveal that impulsivity maps onto shared and distinct neuroanatomical features. Across the entire sample, reward sensitivity and urgency share a common neuroanatomical basis that is distinct from the neural substrates of lack of planning and sensation-seeking. These findings suggest that the BIS/BAS and UPPS-P scales, though separable constructs, share some neuroanatomical features while also exhibiting distinct features. Importantly, some of these associations differ across the sexes, suggesting the presence of sex-specific neuroanatomical substrates.
Neuroanatomical basis of impulsivity varies across imaging modalities
CT features associated with impulsivity were widespread, while SA and GMV features overlapped and were more localized (full results shown in Fig. 3B).
CT: Impulsivity was broadly negatively associated with CT in bilateral cuneus and lateral occipital regions (i.e., youth who were more impulsive had less CT in these areas relative to those who were less impulsive) and was positively associated with CT in the bilateral inferior frontal gyrus, particularly in the pars opercularis, superior frontal, and superior temporal regions (Fig. 4A–D for 2 representative BIS/BAS and UPPS-P measures at each time point, Figure S7 for all other measures). Impulsivity was also negatively associated with CT in bilateral entorhinal, lingual, parahippocampal, and paracentral regions at baseline, but these associations were less consistent at two-year follow-up. Other regions exhibited measure- and hemisphere-specific relationships. For example, impulsivity was broadly negatively associated with CT in the left postcentral and precuneus regions, but BIS/BAS measures were positively associated with CT in the right postcentral region while UPPS-P measures were positively associated with CT in right precuneus at baseline.
Figure 4. Associations between CT and impulsivity are dispersed throughout the cortex, while those between SA (and gray matter) and impulsivity are localized.
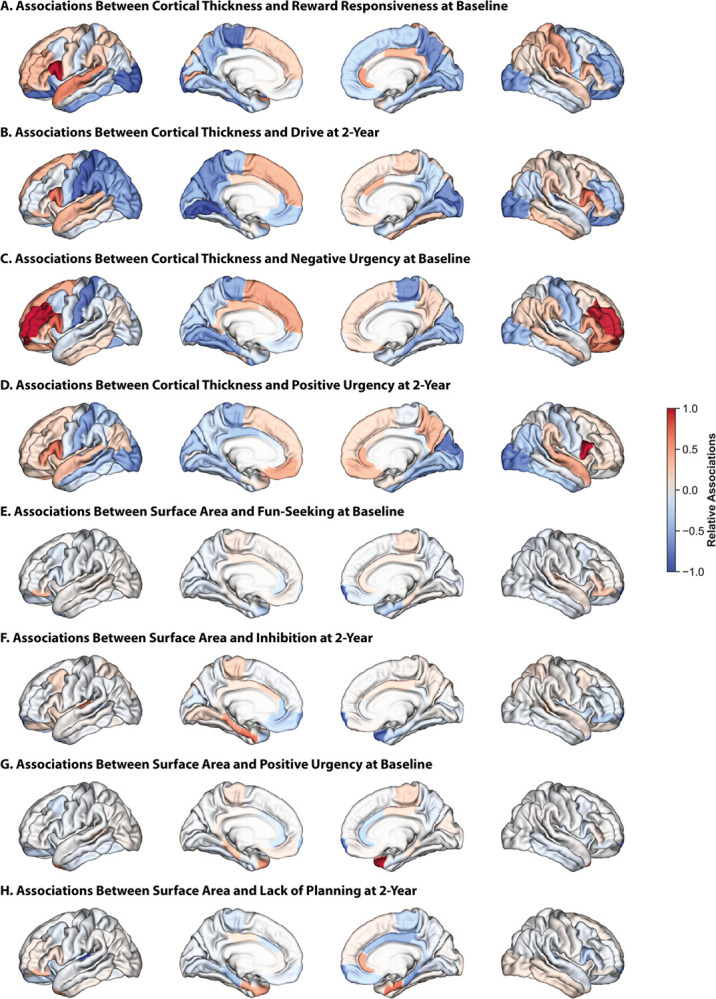
Relative regional associations (Haufe-transformed feature weights) from models trained on CT data to predict reward-responsiveness at baseline (A), drive at two-year follow-up (B), negative urgency at baseline (C), and positive urgency at two-year follow-up (D). Relative regional associations from models trained on SA data to predict fun-seeking at baseline (E), inhibition at two-year follow-up (F), positive urgency at baseline (G), lack of planning at two-year follow-up (H). Lateral (outer) and medial (inner) surfaces for left (left) and right (right) hemispheres are shown. Warmer colors indicate a stronger positive association, cooler colors indicate a stronger negative association. To facilitate visualization, association values for each set of models were divided by the maximum value for that model.
SA: Impulsivity was broadly negatively associated with SA in bilateral frontal poles at both time points (Fig. 4E–H for 2 representative BIS/BAS and UPPS-P measures at each time point, Figure S8 for all other measures). Positive urgency was also positively associated with SA in bilateral temporal poles, but associations with temporal pole regions were more nuanced for other measures. Certain regions also exhibited opposite associations. For example, BIS/BAS measures were positively associated with SA in the transverse temporal and parahippocampal regions and negatively associated with SA in the rostral anterior cingulate and entorhinal regions, while the opposite was true for UPPS-P measures.
GMV: Associations between GMV and impulsivity largely paralleled those with SA, although they were less pronounced and included a few exceptions (Figure S9 for 2 representative BIS/BAS and UPPS-P measures at each time point, Figure S10 for all other measures). Impulsivity was broadly negatively associated with GMV in the pericalcarine region, and inhibition was positively associated with frontal pole volumes but negatively associated with isthmus cingulate volumes.
These findings highlight the presence of multivariate relationships between neuroanatomy and impulsivity. While some associations are similar across the behavioral scales, others are less consistent. These results suggest a distributed network of brain regions encode individual differences in impulsive behaviors, and these relationships are dynamic.
There are sex differences in the neuroanatomical basis of impulsivity
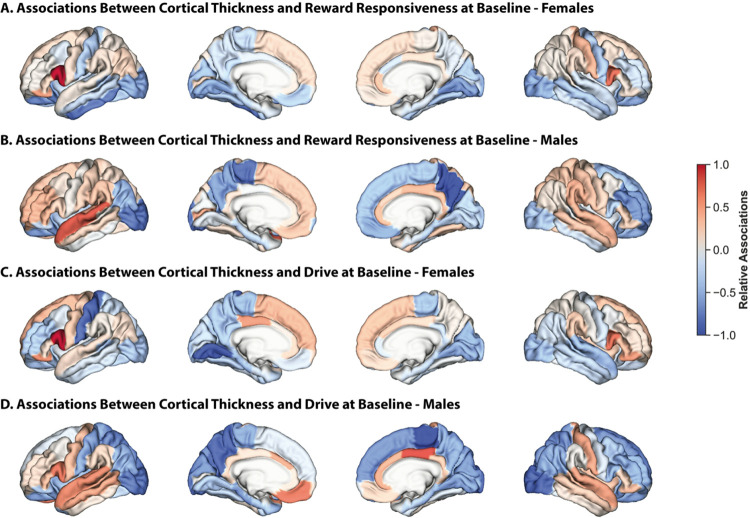
We assessed sex differences in the associations between neuroanatomy and impulsivity, focusing on the models that were significant in both sexes. Across both sexes, BAS measures were negatively associated with CT in the entorhinal, lateral occipital, lateral orbitofrontal, and parahippocampal regions (Fig. 5). In females, they were also positively associated with CT in bilateral inferior frontal gyrus and, to a lesser extent, in the superior frontal gyrus, while in males, these relationships were present in the left hemisphere, but the opposite relationships were present in the right hemisphere. Further, although these measures were negatively associated with CT in the lingual, paracentral, and precuneus regions in both sexes, these relationships were stronger in the lingual regions in females and in the paracentral and precuneus regions in males.
Figure 5. There are sex differences in the associations between CT and reward sensitivity.
Relative regional associations (Haufe-transformed feature weights) from models trained on CT data to predict reward-responsiveness at baseline in females (A) and males (B), and drive at baseline in females (C) and males (D). Lateral (outer) and medial (inner) surfaces for left (left) and right (right) hemispheres are shown. Warmer colors indicate a stronger positive association, cooler colors indicate a stronger negative association. To facilitate visualization, association values for each set of models were divided by the maximum value for that model.
Relationships between GMV and reward-responsiveness at baseline were largely shared across the sexes, but those between GMV and drive were considerably different, although the differences were predominantly in strength rather than directionality (Figure S11). As an example, GMV in the right posterior cingulate and right pars opercularis exhibited stronger relative positive associations with drive in females than in males. Results from all other sex-specific significant models generally resembled the results from the sex-independent models (Figures S12–14).
These findings suggest that while many of these relationships are shared across the sexes, there are also important differences in the neuroanatomical underpinnings of impulsivity. These sex-specific relationships may, in part, explain observed sex differences in impulsive behaviors and vulnerability to impulsivity-related psychiatric illness.
DISCUSSION
Examining multiple facets of impulsivity and neuroanatomical features, we demonstrate that individual variability in neuroanatomy in a large cohort of youth is associated with impulsivity across facets and features, and these relationships, to some extent, differ between sexes. We show that impulsivity measures map onto shared and distinct brain regions across neuroanatomical features, and these relationships are largely stable across development. Some relationships overlap across the impulsivity measures and across neuroanatomical features, and are consistent across the sexes, while others vary across facets, features, and sexes. These results shed light on how individual differences in neuroanatomy throughout development contribute to the diverse expressions of impulsivity in youth. These findings also suggest that impulsivity maps onto specific patterns of neuroanatomy that, alongside other risk factors, could help identify youth at risk for impulsivity-related disorders.
Impulsivity is, in part, driven by individual differences in corticolimbic, corticostriatal and motor-sensory circuits8,13–16,63. Recent advances in brain-based predictive modeling allow us to examine whole-brain multivariate relationships31, unlike traditional univariate analyses focused on individual regions. In recent years, a few studies have used this approach to investigate the neuroanatomical basis of impulsivity, although they have generally focused on specific neuroimaging features and impulsivity measures, ignored sex effects, and considered these relationships at a single time point8,53,64,65. Using a multivariate approach, we replicate univariate findings and demonstrate that impulsivity maps onto a dispersed set of cortical regions. While some relationships are shared across facets of impulsivity, others are distinct. One particularly stable relationship appears to be in the pars opercularis, where CT is positively linked to impulsivity measures from both scales at both time points. These results align with those from prior neuroanatomical, functional, and electrophysiological studies showing that the pars opercularis plays an important role in impulse control66 across motor67–69 and speech70,71 domains.
Here, we find that impulsivity is associated with reduced cortical thickness in the visual network and increased cortical thickness in heteromodal association networks (default, attention, and frontoparietal). Gray matter alterations in the visual network, responsible for the processing of visual stimuli, have been linked to impulsivity72. These associations may explain difficulties with visual attention and increased distractibility that are common in impulsive individuals72. In addition, functional network alterations in heteromodal association cortices are linked to broad psychopathology53,68,73–77. Structural alterations in these regions associated with impulsivity may, in part, explain the functional alterations that subsequently underlie various psychological disorders. Our analyses also revealed widespread associations between impulsivity and both surface area and gray matter volume within the limbic network, although the direction of these associations was dependent on the specific impulsivity measure and limbic region examined. The limbic network, which plays a crucial role in higher-order cognitive processes related to emotions, memory, and motivation, shows particularly interesting patterns in relation to impulsivity. Specifically, impulsivity is negatively linked to D2/3 receptor binding in limbic structures78, and the spatial density of these receptors has been linked to altered functional connectivity patterns in substance use disorder79. The structural alterations we observe here to be linked to impulsivity may, in part, be associated with these receptor density differences and related functional connectivity changes, potentially contributing to the vulnerability for impulsivity-related disorders. Collectively, these findings demonstrate that impulsivity is associated with a complex pattern of neurobiological alterations across multiple networks, providing important insights into the neurobiological mechanisms underlying impulsive behavior throughout development and its relationship to psychopathology.
Impulsivity follows a non-linear trend, increasing during childhood and adolescence and decreasing throughout adulthood80–82. This trajectory mirrors developmental changes observed in neuroanatomy. During adolescence and early adulthood, significant maturation occurs in the prefrontal cortex, a region critical for emotional regulation and impulse control20–22. This maturation involves synaptic pruning and increased white matter connectivity to refine neural circuits, leading to improved cognitive control and decreased impulsivity22. Our results show that the neuroanatomical basis of impulsivity is not static, potentially reflecting these broader developmental changes. While some regions show consistent relationships, others demonstrate changes from baseline to two-year follow-up, suggesting that changes in impulsivity may be driven by shifts in the underlying neuroanatomical associations. Further, deviations from typical developmental trajectories in neuroanatomy may underlie impulsivity-related deficits.
Research on sex differences in impulsivity has produced mixed findings36,83. The most consistent finding is that females exhibit greater inhibition and males exhibit greater sensation-seeking36. Activation-related impulsivity is comparable across the sexes36, though differences have been reported for specific rewards84. One study examining relationships between CT and a single global measure of impulsivity in the ABCD cohort reported significant associations in males but not the entire sample85. A separate study exploring the volumetric correlates of impulsivity in the same cohort found that lack of premeditation and sensation seeking were related to larger volumes in many cortical and subcortical regions, while positive urgency was related to smaller volumes in those same regions86. They also found that many of the relationships were stronger in females. These studies highlight the need for sex-specific investigations. Our analyses build on this work using a multivariate machine learning approach and show that while some brain-impulsivity associations are consistent across the sexes, others are not. In some cases, the same regions even exhibit opposite relationships across the sexes. Notably, we find that regions showing sex-specific associations with impulsivity are predominantly heteromodal association cortices. These same regions demonstrate established structural35 and functional87 sex differences, which are specifically coupled to regional expression of sex-chromosome genes and show enrichment for distinct cell-type signatures35,88,89. These findings suggest that sex differences in impulsivity may have a strong biological basis, rooted in sex-specific patterns of gene expression and cellular organization within key brain networks.
There are several limitations to this work. First, we used a single dataset. Although participants reflect different demographic groups, income levels, and living environments, our findings may be limited in generalizability90,91. To maximize the robustness of our work, we included all participants with complete imaging and impulsivity data and used a cross-validated predictive modeling framework known to yield reliable results51. Second, we only considered binary sex due to data availability and thus were not able to assess these relationships in intersex or other non-binary populations. We also did not consider the effects of gender, which influences neurobiology47 and behavior92. Third, the brain continues to develop throughout adolescence, with females and males reaching developmental milestones at different times93. Here, we used data from two time points but did not include older ages due to data availability. As a result, the associations we report may continue to shift throughout development. Our work serves as an empirical baseline from which theses trajectories may be tracked in later waves of the ABCD study. Fourth, we analyzed relationships between cortical structures and impulsivity, but did not consider subcortical or cerebellar regions. A more complete understanding of neural substrates of impulsivity will require future research incorporating these subcortical and cerebellar regions. Finally, we examined the neural basis of impulsivity and explored sex differences in these relationships. However, we did not consider the effects of other biological or environmental factors (e.g., genetics, pubertal maturation, urbanicity)94–97. Future analyses within global open-access datasets that consider the effects of additional biological and environmental factors can address these limitations and provide additional evidence to confirm (or refute) these findings.
Increases in impulsivity are a typical part of development, and, when significant, may be linked to risk for psychiatric illness. Understanding the neuroanatomical basis of impulsivity paves the way for development of more effective early interventions grounded in neurobiological mechanism to prevent psychiatric illness. These findings highlight how neuroanatomy underlies the diverse expressions of impulsivity throughout development and may represent potential markers of psychiatric risk. In addition, this work emphasizes the importance of conducting sex-disaggregated analyses when examining brain-behavior relationships.
Supplementary Material
Supplementary Files
This is a list of supplementary files associated with this preprint. Click to download.
Funding Sources:
This work was supported by the following sources: Brain and Behavior Research Foundation (Young Investigator Grant to ED), Northwell Health Advancing Women in Science and Medicine (Career Development Award to ED and Educational Achievement Award to ED), Feinstein Institutes for Medical Research (Emerging Scientist Award to ED), NIAAA (R01AA027553 to SWY), NIDA (R01DA053301 to SWY), NIMH (2R01MH120080 to AJH and BTTY), Stanford University Knight-Hennessy Scholars Program (JAR) and the National Academies of Sciences, Engineering, and Medicine’s Ford Foundation Predoctoral Fellowship (JAR). SWY is also supported by the Canadian Institute of Health Research (CIHR) and by Women’s Healthy Research at Yale (WHRY). BTTY is also supported by the NUS Yong Loo Lin School of Medicine (NUHSRO/2020/124/TMR/LOA), the Singapore National Medical Research Council (NMRC) LCG (OFLCG19May-0035), NMRC CTG-IIT (CTGIIT23jan-0001), NMRC OF-IRG (OFIRG24jan-0006), NMRC STaR (STaR20nov-0003), Singapore Ministry of Health (MOH) Centre Grant (CG21APR1009). Any opinions, findings, and conclusions or recommendations expressed here are those of the authors and do not necessarily reflect the views of the funders.
Funding Statement
This work was supported by the following sources: Brain and Behavior Research Foundation (Young Investigator Grant to ED), Northwell Health Advancing Women in Science and Medicine (Career Development Award to ED and Educational Achievement Award to ED), Feinstein Institutes for Medical Research (Emerging Scientist Award to ED), NIAAA (R01AA027553 to SWY), NIDA (R01DA053301 to SWY), NIMH (2R01MH120080 to AJH and BTTY), Stanford University Knight-Hennessy Scholars Program (JAR) and the National Academies of Sciences, Engineering, and Medicine’s Ford Foundation Predoctoral Fellowship (JAR). SWY is also supported by the Canadian Institute of Health Research (CIHR) and by Women’s Healthy Research at Yale (WHRY). BTTY is also supported by the NUS Yong Loo Lin School of Medicine (NUHSRO/2020/124/TMR/LOA), the Singapore National Medical Research Council (NMRC) LCG (OFLCG19May-0035), NMRC CTG-IIT (CTGIIT23jan-0001), NMRC OF-IRG (OFIRG24jan-0006), NMRC STaR (STaR20nov-0003), Singapore Ministry of Health (MOH) Centre Grant (CG21APR1009). Any opinions, findings, and conclusions or recommendations expressed here are those of the authors and do not necessarily reflect the views of the funders.
Footnotes
Disclosures: The authors have nothing to disclose.
Additional Declarations: The authors have declared there is NO conflict of interest to disclose
Contributor Information
Elvisha Dhamala, Feinstein Institutes for Medical Research.
Erynn Christensen, Feinstein Institutes for Medical Research.
Jocelyn Ricard, Stanford University.
Noelle Arcaro, Feinstein Institutes for Medical Research.
Simran Bhola, Feinstein Institutes for Medical Research.
Lisa Wiersch, Feinstein Institutes for Medical Research.
Katharina Brosch, Feinstein Institutes for Medical Research.
B.T. Thomas Yeo, National University of Singapore.
Avram Holmes, Rutgers University.
Sarah Yip, Yale School of Medicine.
Data Availability Statement:
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Code Availability Statement:
Code used for the analyses are on GitHub: https://github.com/elvisha/neuroanat_impulsivity.
References
- 1.Sloan E. et al. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clinical psychology review 57, 141–163 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Cáceda R., Nemeroff C. B. & Harvey P. D. Toward an understanding of decision making in severe mental illness. The Journal of neuropsychiatry and clinical neurosciences 26, 196–213 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Lincoln T. M., Schulze L. & Renneberg B. The role of emotion regulation in the characterization, development and treatment of psychopathology. Nature Reviews Psychology 1, 272–286 (2022). [Google Scholar]
- 4.Sonmez A. I., Garcia J. Q., Thitiseranee L., Blacker C. J. & Lewis C. P. Scoping review: Transdiagnostic measurement of impulsivity domains in youth using the UPPS impulsive behavior scales. Journal of the American Academy of Child & Adolescent Psychiatry (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struijs S. Y. et al. Approach and avoidance tendencies in depression and anxiety disorders. Psychiatry research 256, 475–481 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Moeller F. G., Barratt E. S., Dougherty D. M., Schmitz J. M. & Swann A. C. Psychiatric aspects of impulsivity. American journal of psychiatry 158, 1783–1793 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Chambers R. A., Taylor J. R. & Potenza M. N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American journal of psychiatry 160, 1041–1052 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohler R. et al. Identification of a composite latent dimension of reward and impulsivity across clinical, behavioral, and neurobiological domains among youth. Biological psychiatry: cognitive neuroscience and neuroimaging 9, 407–416 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swann A. C., Pazzaglia P., Nicholls A., Dougherty D. M. & Moeller F. G. Impulsivity and phase of illness in bipolar disorder. Journal of affective disorders 73, 105–111 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Strickland J. C. & Johnson M. W. Rejecting impulsivity as a psychological construct: A theoretical, empirical, and sociocultural argument. Psychological review 128, 336 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braddock K. H. et al. Impulsivity partially mediates the relationship between BIS/BAS and risky health behaviors. Journal of personality 79, 793–810 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Pagliaccio D. et al. Revising the BIS/BAS Scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychological assessment 28, 429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean-Richard-Dit-Bressel P., Killcross S. & McNally G. P. Behavioral and neurobiological mechanisms of punishment: implications for psychiatric disorders. Neuropsychopharmacology 43, 1639–1650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardinal R. N., Winstanley C. A., Robbins T. W. & Everitt B. J. Limbic corticostriatal systems and delayed reinforcement. Annals of the New York Academy of Sciences 1021, 33–50 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Scheres A., Milham M. P., Knutson B. & Castellanos F. X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiat 61, 720–724 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Piantadosi P. T., Halladay L. R., Radke A. K. & Holmes A. Advances in understanding meso-corticolimbic-striatal systems mediating risky reward seeking. Journal of neurochemistry 157, 1547–1571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovner R., Oler J. A. & Kalin N. H. Cortico-limbic interactions mediate adaptive and maladaptive responses relevant to psychopathology. American Journal of Psychiatry 176, 987–999 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Hare J., Calakos N. & Yin H. H. Recent insights into corticostriatal circuit mechanisms underlying habits. Current opinion in behavioral sciences 20, 40–46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhlhaas P. J. et al. Towards a youth mental health paradigm: a perspective and roadmap. Molecular Psychiatry 28, 3171–3181 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey B. J., Getz S. & Galvan A. The adolescent brain. Developmental review 28, 62–77 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey B. J., Jones R. M. & Hare T. A. The adolescent brain. Ann N Y Acad Sci 1124, 111–126, doi: 10.1196/annals.1440.010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luna B. The relevance of immaturities in the juvenile brain to culpability and rehabilitation. The Hastings law journal 63, 1469 (2012). [PMC free article] [PubMed] [Google Scholar]
- 23.Somerville L. H., Jones R. M. & Casey B. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cognition 72, 124–133 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blakemore S. J. The social brain in adolescence. Nat Rev Neurosci 9, 267–277, doi: 10.1038/nrn2353 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Schweizer S., Gotlib I. H. & Blakemore S.-J. The role of affective control in emotion regulation during adolescence. Emotion 20, 80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young K. S., Sandman C. F. & Craske M. G. Positive and negative emotion regulation in adolescence: links to anxiety and depression. Brain sciences 9, 76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvers J. A. Adolescence as a pivotal period for emotion regulation development. Current opinion in psychology 44, 258–263 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Ripke S. et al. Reward processing and intertemporal decision making in adults and adolescents: The role of impulsivity and decision consistency. Brain research 1478, 36–47 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology 52, 263–276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey B., Heller A. S., Gee D. G. & Cohen A. O. Development of the emotional brain. Neuroscience letters 693, 29–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhamala E., Yeo B. T. & Holmes A. J. Methodological Considerations for Brain-Based Predictive Modelling in Psychiatry. Biol Psychiat (2022). [DOI] [PubMed] [Google Scholar]
- 32.Bzdok D. & Yeo B. T. Inference in the age of big data: Future perspectives on neuroscience. Neuroimage 155, 549–564 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Matte Bon G., Kraft D., Comasco E., Derntl B. & Kaufmann T. Modeling brain sex in the limbic system as phenotype for female-prevalent mental disorders. Biology of Sex Differences 15, 1–15 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laakso A. et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiat 52, 759–763 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Liu S., Seidlitz J., Blumenthal J. D., Clasen L. S. & Raznahan A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proceedings of the National Academy of Sciences 117, 18788–18798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross C. P., Copping L. T. & Campbell A. Sex differences in impulsivity: a meta-analysis. Psychological bulletin 137, 97 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature Reviews Neuroscience 10, 724–735 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakic P., Ayoub A. E., Breunig J. J. & Dominguez M. H. Decision by division: making cortical maps. Trends in neurosciences 32, 291–301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bethlehem R. A. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White T., Su S., Schmidt M., Kao C.-Y. & Sapiro G. The development of gyrification in childhood and adolescence. Brain Cognition 72, 36–45 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casey B. J. et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental cognitive neuroscience 32, 43–54, doi: 10.1016/j.dcn.2018.03.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagler D. J. Jr. et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 202, 116091, doi: 10.1016/j.neuroimage.2019.116091 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhamala E. et al. Proportional intracranial volume correction differentially biases behavioral predictions across neuroanatomical features and populations. NeuroImage, doi: 10.1016/j.neuroimage.2022.119485 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desikan R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980, doi: 10.1016/j.neuroimage.2006.01.021 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Geurten M., Catale C., Gay P., Deplus S. & Billieux J. Measuring impulsivity in children: adaptation and validation of a short version of the UPPS-P impulsive behaviors scale in children and investigation of its links with ADHD. Journal of attention disorders 25, 105–114 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B 57, 289–300 (1995). [Google Scholar]
- 47.Dhamala E., Bassett D. S., Yeo B. T. & Holmes A. J. Functional brain networks are associated with both sex and gender in children. Science Advances 10, eadn4202 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhamala E. et al. Sex differences in the functional network underpinnings of psychotic-like experiences in children. bioRxiv, 2024.2004. 2022.590660 (2024). [Google Scholar]
- 49.Dhamala E., Jamison K. W., Jaywant A., Dennis S. & Kuceyeski A. Distinct functional and structural connections predict crystallised and fluid cognition in healthy adults. Human Brain Mapping 42, 3102–3118, doi: 10.1002/hbm.25420 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhamala E., Jamison K. W., Jaywant A. & Kuceyeski A. Shared functional connections within and between cortical networks predict cognitive abilities in adult males and females. Human Brain Mapping, doi: 10.1002/hbm.25709 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhamala E. et al. Brain-based predictions of psychiatric illness-linked behaviors across the sexes. Biol Psychiat (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J. et al. Relationship between prediction accuracy and feature importance reliability: An empirical and theoretical study. NeuroImage 274, 120115 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J. et al. Shared and unique brain network features predict cognitive, personality, and mental health scores in the ABCD study. Nature communications 13, 2217, doi: 10.1038/s41467-022-29766-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J. et al. Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage 196, 126–141, doi: 10.1016/j.neuroimage.2019.04.016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haufe S. et al. On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage 87, 96–110 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Tian Y. & Zalesky A. Machine learning prediction of cognition from functional connectivity: Are feature weights reliable? bioRxiv (2021). [DOI] [PubMed] [Google Scholar]
- 57.Chen J. et al. There is no fundamental trade-off between prediction accuracy and feature importance reliability. bioRxiv (2022). [Google Scholar]
- 58.Owens M. M. et al. Neuroanatomical correlates of impulsive traits in children aged 9 to 10. Journal of abnormal psychology 129, 831 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shao I. Y. et al. From individual motivation to substance use initiation: A longitudinal cohort study assessing the associations between reward sensitivity and subsequent risk of substance use initiation among US adolescents. Addictive Behaviors 160, 108162 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akoglu H. User’s guide to correlation coefficients. Turkish journal of emergency medicine 18, 91–93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardoso Melo R. D., Groen R. N. & Hartman C. A. Reward sensitivity at age 13 predicts the future course of psychopathology symptoms. Frontiers in Psychiatry 13, 818047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Read R. W. et al. A study of impulsivity and adverse childhood experiences in a population health setting. Frontiers in Public Health 12, 1447008 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kebets V. et al. Somatosensory-Motor Dysconnectivity Spans Multiple Transdiagnostic Dimensions of Psychopathology. Biol Psychiatry 86, 779–791, doi: 10.1016/j.biopsych.2019.06.013 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Ooi L. Q. R. et al. Comparison of individualized behavioral predictions across anatomical, diffusion and functional connectivity MRI. NeuroImage, 119636 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Ide J. S. et al. Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: An exploratory voxel-based morphometry study of the ABCD project data. Neuroimage 220, 117085 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forstmann B. U., van den Wildenberg W. P. & Ridderinkhof K. R. Neural mechanisms, temporal dynamics, and individual differences in interference control. Journal of Cognitive Neuroscience 20, 1854–1865 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Rizzolatti G., Fogassi L. & Gallese V. Motor and cognitive functions of the ventral premotor cortex. Current opinion in neurobiology 12, 149–154 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Holmes A. J., Hollinshead M. O., Roffman J. L., Smoller J. W. & Buckner R. L. Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. Journal of neuroscience 36, 4038–4049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy B. J. & Wagner A. D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York academy of sciences 1224, 40–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loh K. K. et al. Cognitive control of orofacial motor and vocal responses in the ventrolateral and dorsomedial human frontal cortex. Proceedings of the National Academy of Sciences 117, 4994–5005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao L., Silva A. B., Kurteff G. L. & Chang E. F. Inhibitory control of speech production in the human premotor frontal cortex. Nature Human Behaviour, 1–16 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ide J. S., Tung H. C., Yang C.-T., Tseng Y.-C. & Li C.-S. R. Barratt impulsivity in healthy adults is associated with higher gray matter concentration in the parietal occipital cortex that represents peripheral visual field. Frontiers in Human Neuroscience 11, 222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker J. T. et al. Functional connectomics of affective and psychotic pathology. Proceedings of the National Academy of Sciences 116, 9050–9059 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J. et al. Intrinsic Connectivity Patterns of Task-Defined Brain Networks Allow Individual Prediction of Cognitive Symptom Dimension of Schizophrenia and Are Linked to Molecular Architecture. Biol Psychiatry 89, 308–319, doi: 10.1016/j.biopsych.2020.09.024 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong R. et al. Spatial Topography of Individual-Specific Cortical Networks Predicts Human Cognition, Personality, and Emotion. Cerebral Cortex 29, 2533–2551, doi: 10.1093/cercor/bhy123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cole M. W., Repovš G. & Anticevic A. The frontoparietal control system: a central role in mental health. The Neuroscientist 20, 652–664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia C. H. et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun 9, 3003, doi: 10.1038/s41467-018-05317-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barlow R. L. et al. Ventral striatal D2/3 receptor availability is associated with impulsive choice behavior as well as limbic corticostriatal connectivity. International Journal of Neuropsychopharmacology 21, 705–715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricard J. A. et al. A shared spatial topography links the functional connectome correlates of cocaine use disorder and dopamine D2/3 receptor densities. Communications Biology 7, 1178 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Betts M. J. et al. Learning in anticipation of reward and punishment: perspectives across the human lifespan. Neurobiology of aging 96, 49–57 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Melo R. D. C., Schreuder M. J., Groen R. N., Sarsembayeva D. & Hartman C. A. Reward sensitivity across the lifespan in males and females and its associations with psychopathology. Personality and Individual Differences 204, 112041 (2023). [Google Scholar]
- 82.Hammond C. J., Potenza M. N. & Mayes L. C. Development of impulse control, inhibition, and self-regulatory behaviors in normative populations across the lifespan. Vol. 232 (Oxford University Press; New York, NY, 2012). [Google Scholar]
- 83.Weafer J. & de Wit H. Sex differences in impulsive action and impulsive choice. Addictive behaviors 39, 1573–1579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barendse M. et al. Sex and pubertal variation in reward-related behavior and neural activation in early adolescents. Developmental Cognitive Neuroscience 66, 101358 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Assari S. Sex Differences in the Association between Cortical Thickness and Children’s Behavioral Inhibition. Journal of psychology & behavior research 2, 49–64 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Chen Y. et al. Gray matter volumetric correlates of dimensional impulsivity traits in children: Sex differences and heritability. Human brain mapping 43, 2634–2652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shanmugan S. et al. Sex differences in the functional topography of association networks in youth. Proceedings of the National Academy of Sciences 119, e2110416119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DeCasien A. R., Guma E., Liu S. & Raznahan A. Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biology of sex differences 13, 43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X.-H. et al. The cell-type underpinnings of the human functional cortical connectome. Nature Neuroscience 28, 150–160 (2025). [DOI] [PubMed] [Google Scholar]
- 90.Ricard J. et al. Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nature Neuroscience, 1–8 (2022). [DOI] [PubMed] [Google Scholar]
- 91.Dhamala E. et al. Considering the interconnected nature of social identities in neuroimaging research. Nature Neuroscience, 1–12 (2024). [DOI] [PubMed] [Google Scholar]
- 92.Wierenga L. M. et al. Recommendations for a better understanding of sex and gender in neuroscience of mental health. Biological Psychiatry Global Open Science, 100283 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marek S., Tervo-Clemmens B., Calabro F., Nichols T. & Dosenbach N. Reproducible brain-wide association studies require thousands of individuals. Nature (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bevilacqua L. & Goldman D. Genetics of impulsive behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20120380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pauli R. et al. Action initiation and punishment learning differ from childhood to adolescence while reward learning remains stable. Nature communications 14, 5689 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bezdjian S., Baker L. A. & Tuvblad C. Genetic and environmental influences on impulsivity: A meta-analysis of twin, family and adoption studies. Clinical psychology review 31, 1209–1223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meidenbauer K. L. et al. Evidence for environmental influences on impulsivity and aggression. Urban Forestry & Urban Greening 103, 128594 (2025). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Code used for the analyses are on GitHub: https://github.com/elvisha/neuroanat_impulsivity.