Abstract
Background
Aedes albopictus (Skuse, 1894) and Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae) are invasive species in the Hawaiian Islands as well as other islands of the Pacific and serve as the primary vectors of arboviruses like dengue virus. Despite its significance to public health, data on their insecticide resistance remains limited. Knowledge of the level of insecticide resistance is critical in developing effective mosquito control strategies, especially when an arboviral disease outbreak occurs.
Methods
This study investigated the phenotypic and genotypic resistance of Hawaiian Ae. albopictus and Ae. aegypti to permethrin, one of the commonly used pyrethroids. Genomic sequences of 93 Ae. albopictus samples from four Hawaiian Islands (Kaua’i, O’ahu, Maui, and the Island of Hawai’i) were screened for non-synonymous mutations in the voltage-gated sodium channel (VGSC) gene (chromosome 3: 345,142,499 − 345,663,668). Phenotypic resistance to permethrin was assessed using a modified Centers for Disease Control and Prevention (CDC) bottle bioassay on Ae. albopictus and Ae. aegypti collected from two socio-environmentally distinct sites.
Results
Among 4,101 single-nucleotide polymorphisms (SNPs) identified in the VGSC region of Ae. albopictus genomes from for Hawaiian Islands, 61 were classified as synonymous. No non-synonymous mutations were found, suggesting an absence of genotypic resistance to pyrethroids in these populations. In phenotypic assays, over 97% of Ae. albopictus and all Ae. aegypti individuals were knocked down within 10 minutes of permethrin exposure. These high knockdown rates indicate that both species remain phenotypically susceptible to permethrin.
Conclusions
This study is the first study reporting the phenotypic insecticide resistance profile of Hawaiian Aedes mosquitoes. Hawaiian populations of Ae. albopictus and Ae. aegypti remain susceptible to pyrethroids, as demonstrated by the absence of VGSC mutations and high knockdown rates in permethrin bioassays. While no genotypic and phenotypic resistance was detected in these two Aedes species, monitoring for resistance in other mosquito species and through alternative mechanisms is needed.
Keywords: Aedes, insecticide resistance, resistance mutation, Hawaii
Background
Among 119 Aedes species found in the Pacific Islands, Aedes albopictus and Aedes aegypti are recognized as the main vectors of arboviruses, including dengue, chikungunya, and Zika viruses [1]. They have spread across the Pacific Islands, and, notably, the Hawaiian Islands were one of the first documented regions in the world to report the invasion of these two Aedes mosquito species [2, 3]. These two Aedes mosquitoes invaded Hawaii before 1900, and since then, dengue outbreaks caused by both species have been reported [3].
The widespread distribution of these Aedes mosquitoes and their critical involvement in arbovirus transmission emphasize the necessity of evaluating their susceptibility to insecticides. Despite being major vectors in the Hawaiian Islands for a long time, there are no available publications on the insecticide resistance of Aedes mosquitoes from the region, except for limited references to a few individuals in global-scale research investigations [4]. The presence and development of insecticide resistance in mosquito populations can undermine the effectiveness of chemical products used for mosquito control [5].
Pyrethroids have constituted the majority in terms of quantity of usage among the four classes of insecticides approved by the World Health Organization for the control of disease vectors between 2000 and 2019 [6, 7]. Pyrethroid insecticides target voltage-gated sodium channels (VGSC), which are formed by four domains, each comprising six segments [8]. The binding of pyrethroids to VGSC results in the channels remaining open for an extended period of time, disrupting nerve function. Consequently, mutations in the VGSC gene interfere with the binding of pyrethroids, thereby conferring knockdown resistance (kdr) to pyrethroids to Aedes [9], Anopheles [10], and Culex [11] mosquitoes.
Non-synonymous mutations in the VGSC gene alter the amino acid sequence of the channel protein, thereby modifying its structure. The level of resistance conferred by such mutations can vary depending on their location on the VGSC gene [12]. Mutations occurring near the pyrethroid-binding site can hinder the attachment of the insecticide and confer resistance. The level of resistance may be further enhanced due to interactions between mutations when certain mutations occur in combination [9, 13]. The presence of such compound mutations has been frequently observed in field studies, which can be interpreted as a consequence of evolutionary selective pressure in environments where multiple insecticides are used. As resistance to pyrethroids increases, the effectiveness of existing insecticides decreases [9], highlighting the need for alternative chemicals or novel control strategies.
The phenotypic resistance of Hawaiian Aedes mosquitoes to pyrethroids has never been reported [4, 14]. Available literature about genotypic resistance for the Hawaiian Ae. albopictus population is limited to a single study, which reported the absence of the resistant F1534 mutation in O’ahu, Hawaii [15]. Similarly, in the Hawaiian Ae. aegypti collected from the Islands of Hawai’i, no resistance that confers mutations has been identified [16]. On the other hand, the consistent detection of mutations in the VGSC gene of other insects, such as bed bugs, suggested the persistent use of pyrethroids in O’ahu, Hawaii [17], which could contribute to the development of pyrethroid resistance in Hawaiian mosquitoes if mosquitoes come indoors to bite people and come in contact with insecticide-treated surface. Given the limited data, further genetic surveillance and phenotypic characterization are needed to understand the insecticide resistance profiles of these populations. Such knowledge is crucial for devising mitigation strategies in genetic control trials and managing unforeseen circumstances that could compromise control effects [18].
In this study, we explored single-nucleotide polymorphisms (SNPs) in the coding DNA sequence (CDS) of VGSC to determine whether Hawaiian Ae. albopictus populations possess resistant mutations or not. We further conducted Centers for Disease Control and Prevention (CDC) bottle bioassays on Hawaiian Ae. albopictus and Ae. aegypti populations to characterize their phenotypic resistance levels to permethrin, a commonly used pyrethroid insecticide. Understanding the insecticide resistance profile will help in establishing effective vector management and control strategies.
Methods
We used 93 genomic sequences of the Hawaiian Ae. albopictus population (SRA Accession number: SAMN472862276-SAMN47286368) (Table S1), which were obtained from our previous study [19] to identify potential non-synonymous mutations in the VGSC region. These samples were collected from four Hawaiian Islands: Kaua’i, O’ahu, Maui, and the Island of Hawai’i. Variant calling was performed using Freebayes version 1.3.6 [20] based on the Ae. albopictus reference genome AalbF5 (GCF_035046485.1). The analysis focused on the VGSC region located on chromosome 3, spanning positions 345,142,499 − 345,663,668. Missing data was allowed up to 10%. To identify mutations within the CDS of the VGSC region, we examined SNPs in this region. Processing and filtering of SNPs were carried out using BCFtools version 1.13 [21] and VCFtools version 0.1.16 [22]. Indels were excluded, and only variants with a read depth between eight and 120 and a quality score of 30 or higher were retained. Visualization and confirmation of SNPs were conducted using the Integrative Genomics Viewer (IGV) version 2.18.4 [23], where identified variants were compared against the reference genome.
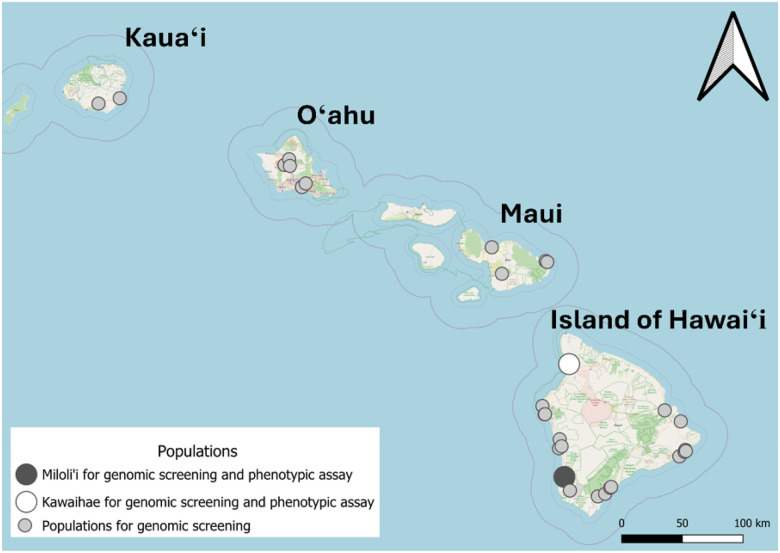
For phenotypic insecticide resistance assay, eggs of Ae. albopictus and Ae. aegypti were collected from Kawaihae and Miloli’i on the Islands of Hawai’i (Fig. 1). Kawaihae is a coastal town known for its harbor, functioning as a regional commercial port. Miloli’i is a coastal fishing village located over 3 km from the main road, where residents use rainwater catchment systems for their water supply. These two sites were selected to investigate variation in insecticide resistance between populations exposed to different socio-environmental settings. Collecting was conducted from February to March of 2023, using oviposition traps, filled with a seven-day-old grass infusion prepared by steeping 160 g of Guinea grass (Megathyrsus maximus) in 19 L of water [24]. Subsequently, eggs were transferred to the laboratory of the Hawaii Department of Health in Hilo, Hawaii, for rearing and storage, following procedures outlined by the PacMOSSI (Pacific Mosquito Surveillance Strengthening for Impact) consortium [25]. After conditioning and drying, eggs were kept in sealed plastic containers (30 cm [L] × 15 cm [W] × 11 cm [H]; Sterilite) until used for hatching. In April and May, the eggs were placed in a 25 cm [L] × 20 cm [W] × 4 cm [H] tray with tap water, which had been boiled and cooled to ambient temperature. The larvae fed ground fish food (TetraMin; Tetra, VA) hatched and developed into pupae in approximately seven days. Pupae were transferred into 12 cm-diameter, plastic cups (946 ml; Taral Plastics, CA) and then placed in mosquito-rearing cages (30.5 cm [L] × 30.5 cm [W] × 30.5 cm [H]; BioQuip 1450B; company is out of business and its location information no longer available.). Cotton balls wet with 10% sugar solution were provided on top of the rearing cages for the emerging adults. The temperature in the rearing environment was 25.0 ± 0.6 °C (M ± SD), and the humidity was 68.8 ± 4.0%. Both female and male adults that were 3–8 days old were used for the bottle bioassay.
Figure 1.
Locations of Aedes mosquito populations analyzed via genome screening and phenotypic assay across four Hawaiian Islands. Populations represented by dark gray (Miloli i) and white (Kawaihae) circles were included in both genomic screening and phenotypic assay. Light gray circles indicate populations included in genomic screening only. This map was created using the Free and Open Source QGIS.
A CDC bottle bioassay was conducted to test phenotypic resistance to permethrin in two Hawaiian populations of the two species. We followed the CDC protocol [26] with a minor modification in which only one individual was introduced into each test bottle to assess the resistance of individual mosquitoes, and 25–30 individuals were introduced into a control bottle. This allows correlation to be made between the phenotype data and genotype data for each individual in subsequent analysis. This approach has been used in Ae. aegypti research [9, 27] to uncover relationships between phenotype and genotypes. A stock solution of permethrin provided by the CDC was diluted in acetone to a concentration of the diagnostic dose (43 μl/ml). Thirty test bottles were coated with 1 ml of the diluted permethrin solution, while a control bottle was coated with 1 ml of acetone. Their knockdown was recorded at 5, 10, 15, 20, 30, 45, 60, 90, and 120 minutes. The diagnostic time was 10 minutes of exposure [26].
Statistical analyses were conducted to evaluate differences in survival rates across species and populations. Log-rank tests were applied to survival data recorded at 5 and 10 minutes to identify significant differences between species and populations. These time points were selected as 10 minutes of continuous exposure to permethrin, which is the diagnostic time for these two species [26]. Analyses were conducted using Python 3.10 with the lifeline library version 0.27.8 [28]. Bonferroni corrections were applied for multiple comparisons.
Results
In the VGSC region of the Ae. albopictus genome, a total of 4,101 SNPs were identified across the 93 genomic sequences from four Hawaiian Islands. Within the CDS region, 61 synonymous mutations were observed. Notably, no non-synonymous mutations were found, indicating an absence of amino acid changes in the VGSC region of the Hawaiian Ae. albopictus populations.
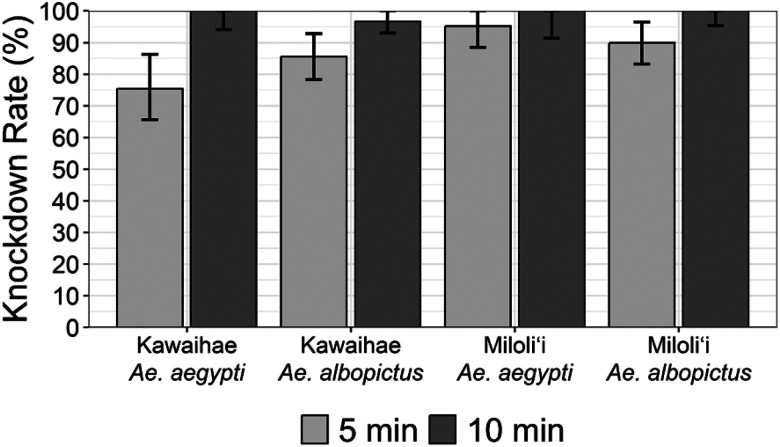
A total of 169 Ae. albopictus and 102 Ae. aegypti were tested using the modified CDC bottle bioassay, with 90 Ae. albopictus and 61 Ae. aegypti collected from Kawaihae, and 79 Ae. albopictus and 41 Ae. aegypti from Miloli’i. All Ae. aegypti from Kawaihae and Miloli’i were knocked down at 10 minutes, indicating the susceptible population according to the criteria provided in [26]. The mortality rate of Ae. albopictus from Kawaihae and Miloli’i were 96.7% and 100% at 10 minutes, respectively (Fig. 2). It indicates that the two Ae. albopictus populations are susceptible to permethrin. The mortality rate of the Kawaihae Ae. albopictus population reached 100% at 15 minutes of exposure. On the other hand, none of the control Aedes mosquitoes were dead at the diagnostic time.
Figure 2.
Knockdown rates of Ae. aegypti and Ae. albopictus mosquitoes collected from Kawaihae and Miloli’i in the Island of Hawai’i at 5- and 10-min exposure to permethrin (43 μl/bottle).
Statistical analyses revealed that the knockdown time of Kawaihae Ae. aegypti population significantly differed from that of the Miloli’i Ae. aegypti (adjusted P < 0.001). However, no significant differences were found between Kawaihae Ae. albopictus and Miloli’i Ae. albopictus (adjusted P = 0.24), between Kawaihae Ae. albopictus and Kawaihae Ae. aegypti (adjusted P = 0.56), and between Miloli’i Ae. albopictus and Miloli’i Ae. aegypti (adjusted P = 0.25).
Discussion
The screening of non-synonymous mutations in the VGSC gene of Hawaiian Ae. albopictus populations indicated that this species has not developed target site insensitivity mutations yet. The samples utilized in genetic screening were obtained from a broader range, including Kaua’i, O’ahu, Maui, and the Island of Hawai’i, than the samples used for the modified CDC bottle bioassay. This suggests that the susceptibility to pyrethroids is not confined to the Island of Hawai’i populations but could extend across Ae. albopictus populations throughout the Hawaiian Islands. The lack of standing genetic variations in the VGSC among Hawaiian populations could minimize the potential for insecticide resistance to rise via selection on standing variations, although other forms of mutations, such as de-novo mutations [29], are possible. Adaptive introgression [30, 31] is also possible but unlikely in Hawaii because no other Aedes species occur there that could hybridize and produce viable offspring.
This study is the first study reporting the phenotypic insecticide resistance profile of Hawaiian Aedes mosquitoes. All phenotypic insecticide resistance tests by populations and species showed that Hawaiian Ae. albopictus and Ae. aegypti populations are susceptible to permethrin. Statistical analyses showed Kawaihae Ae. aegypti are significantly different from the Miloli’i population. The significant difference appears to be due to the relatively lower mortality rate of Kawaihae Ae. aegypti population when exposed to permethrin for five minutes. However, it does not indicate that the population is resistant to permethrin, as their mortality rate eventually reached 100% at 10 minutes of exposure.
Since previous studies on insecticide resistance mutations in Hawaiian Aedes mosquitoes have only focused on a limited number of genes [15, 16], we cannot rule out the presence of other resistance-associated mutations. Pyrethroid resistance relies not only on VGSC mutations but also on metabolic resistance mechanisms, including detoxification by cytochrome P450 monooxygenases [32, 33]. Therefore, further studies should investigate pyrethroid resistance that may have developed through other mechanisms, as well as resistance to other classes of insecticides, such as organophosphates or carbamates, to provide a comprehensive potential resistance profile for Hawaiian mosquitoes.
We adopted a modified CDC bottle bioassay to perform insecticide resistance testing at an individual level. This method allows us to analyze the relationship between phenotypic insecticide resistance and genotypic insecticide resistance [9, 27]. However, since no individuals with phenotypic resistance were identified in this study, we did not investigate potential mutations associated with genotypic insecticide resistance from the sampled we used for the CDC bottle bioassay.
While this study highlights the susceptibility of Hawaiian Ae. albopictus and Ae. aegypti populations to permethrin, additional research is needed to monitor insecticide resistance in other Hawaiian mosquito species, such as Ae. japonicus and Wyeomyia mitchellii. There are indications that Hawaiian populations may have a chance to develop insecticide resistance. Mutations in the VGSC gene have been observed in several Ae. aegypti populations in the PICTs, such as Vanuatu, Kiribati, New Caledonia, and Fiji [4] Given the high invasiveness of Ae. albopictus and Ae. aegypti [1, 4], the insecticide-resistant population could be introduced and established if early detection and adequate response are not implemented in these islands. In addition, in the O’ahu region, VGSC mutations in bed bugs were consistently detected in 2009 and 2019, alongside the emergence of new mutations in 2019 [17]. It indicated that the persistent use of pyrethroid insecticide in Hawaii could exert selection pressure for insects to develop resistance under increased insecticide exposure. Even if this insecticide is not specifically targeted at mosquitoes, it may still contribute to the development of insecticide resistance in mosquito populations [34]. On the other hand, given the high volume of tourism in Hawaii, it is also possible that bed bugs with insecticide resistance were introduced from outside Hawaii rather than developing their resistance locally. Moreover, mosquito biting could take place predominantly outdoors in Hawaii, reducing the frequence of direct contact with insecticide-treated surface indoors for bedbug treatment. It may explain why insecticide resistance has not been observed in Aedes mosquitoes in this study.
Conclusion
This study is the first study reporting the phenotypic insecticide resistance profile of Hawaiian Aedes mosquitoes. In this study, we found no evidence of pyrethroid resistance in Hawaiian populations of Ae. albopictus and Ae. aegypti, demonstrating their susceptibility to permethrin based on both genotypic and phenotypic analyses. Therefore, the use of permethrin remains viable option for Aedes control in Hawaii. The absence of any mutations in the VGSC gene and the lack of phenotypic resistance observed through modified CDC bottle bioassay suggest that these mosquito species and populations have not yet developed resistance to pyrethroids. Given that the genetic screening included samples from multiple Hawaiian Islands, this susceptibility may be widespread across the Hawaiian Islands. These findings underscore the effectiveness of permethrin in controlling Hawaiian Aedes mosquitoes. Expanding resistance monitoring to include other mosquito species and mechanisms will be crucial to sustaining effective vector control strategies in Hawaii.
Supplementary Material
This is a list of supplementary files associated with this preprint. Click to download.
Acknowledgement
We would like to thank Dr. Ana L. Romero-Weaver for her help with the experimental setup and the staff of the Hawaii Department of Health who assisted with mosquito egg collections.
Funding
This research has been supported by a grant from the U.S. Environmental Protection Agency’s Science to Achieve Results (STAR) program (Agreement No. 84020401), the USDA National Institute of Food and Agriculture multi-state Hatch Project (1025565 and 7007941), the Southern IPM Center working group grant as part of National Institute of Food and Agriculture (NIFA) Crop Protection and Pest Management Regional Coordination Program (Agreement No. 2022-70006-38002), and National Institute of Health (R35GM156217). It has not been formally reviewed by the EPA, USDA, or NIH. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. The EPA, USDA, and NIH do not endorse any products or commercial services mentioned in this publication.
Funding Statement
This research has been supported by a grant from the U.S. Environmental Protection Agency’s Science to Achieve Results (STAR) program (Agreement No. 84020401), the USDA National Institute of Food and Agriculture multi-state Hatch Project (1025565 and 7007941), the Southern IPM Center working group grant as part of National Institute of Food and Agriculture (NIFA) Crop Protection and Pest Management Regional Coordination Program (Agreement No. 2022-70006-38002), and National Institute of Health (R35GM156217). It has not been formally reviewed by the EPA, USDA, or NIH. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. The EPA, USDA, and NIH do not endorse any products or commercial services mentioned in this publication.
Footnotes
Competing Interests
OSA is a founder of Agragene, Inc. and Synvect, Inc. with equity interest. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. All other authors declare no competing interests.
Contributor Information
Sangwoo Seok, University of Florida.
Miles T. McCollum, University of Florida.
Christopher M. Jacobsen, Hawaii State Department of Health.
Omar S. Akbari, University of California.
Derrick K. Mathias, University of Florida.
Yoosook Lee, University of Florida.
Data Availability
Data is provided within the manuscript as a supplementary information file.
References
- 1.Russell TL, Burkot TR. A guide to mosquitoes in the Pacific. Pacific Community; 2023. [Google Scholar]
- 2.Perkins RCL. Fauna hawaiiensis Volume 1. Part VI. London: Cambridge University Press; 1913. [Google Scholar]
- 3.Winchester JC, Kapan DD. History of Aedes mosquitoes in Hawaii. J. Am. Mosq. Control. Assoc. 2013;29:154–163; doi: 10.2987/12-6292R.1 [DOI] [PubMed] [Google Scholar]
- 4.Seok S, Raz CD, Miller JH, Malcolm AN, Eason MD, Romero-Weaver AL, et al. Arboviral disease outbreaks, Aedes mosquitoes, and vector control efforts in the Pacific. Front. Trop. Dis. 2023;4:1035273; doi: 10.3389/fitd.2023.1035273 [DOI] [Google Scholar]
- 5.Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007;63:628–633; doi: 10.1002/ps.1406 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg H, Zaim M, Yadav RS, Soares A, Ameneshewa B, Mnzava A, et al. Global trends in the use of insecticides to control vector-borne diseases. Environ. Health Perspect. 2012;120:577–582; doi: 10.1289/ehp.1104340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg H, da Silva Bezerra HS, Al-Eryani S, Chanda E, Nagpal BN, Knox TB, et al. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021;11:23867; doi: 10.1038/s41598-021-03367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014;50:1–17; doi: 10.1016/j.ibmb.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mack LK, Kelly ET, Lee Y, Brisco KK, Shen KV, Zahid A, et al. Frequency of sodium channel genotypes and association with pyrethrum knockdown time in population of Californian Aedes aegypti. Parasites Vectors 2021;14:141; doi: 10.1186/s13071-021-04627-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reimer L, Fondjo E, Patchoké S, Diallo B, Lee Y, Ng A, et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J. Med. Entomol. 2008;45:260–266; doi: 10.1093/jmedent/45.2.260 [DOI] [PubMed] [Google Scholar]
- 11.Pichler V, Itokawa K, Caputo B, Marco CMD, Serini P, Bellini R, et al. Unbiased sequence analysis of vgsc gene reveals circulation of novel and known knock-down resistance mutations in Culex pipiens, challenging vector control measures. J. Pest Sci. 2024;98:869–880; doi: 10.1007/s10340-024-01818-6 [DOI] [Google Scholar]
- 12.Uemura N, Itokawa K, Komagata O, Kasai S. Recent advances in the study of knockdown resistance mutations in Aedes mosquitoes with a focus on several remarkable mutations. Curr. Opin. Insect Sci. 2024;63:101178; doi: 10.1016/j.cois.2024.101178 [DOI] [PubMed] [Google Scholar]
- 13.Kasai S, Itokawa K, Uemura N, Takaoka A, Furutani S, Maekawa Y, et al. Discovery of super-insecticide-resistance dengue mosquitoes in Asia: Threats of concomitant knockdown resistance mutations. Sci. Adv. 2022;8:eabq7345; doi: 10.1126/sciadv.abq7345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11:e0005625; doi: 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Bonizzoni M, Zhong D, Zhou G, Cai S, Li Y, et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus. PLoS Negl. Trop. Dis. 2016;10:e0004696; doi: 10.1371/journal.pntd.0004696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosme LV, Gloria-Soria A, Caccone A, Powell JR, Martins AJ. Evolution of kdr haplotypes in worldwide populations of Aedes aegypti: Independent origins of the F1534C kdr mutation. PLoS Negl. Trop. Dis. 2020;14:e0008219; doi: 10.1371/journal.pntd.0008219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CD, Levine BA, Vargo EL, Schal C, Booth W. Recent detection of multiple populations of the tropical bed bug (Hemiptera: Cimicidae) exhibiting kdr-associated mutations in Hawaii. J. Med. Entomol. 2020;57:1077–1081; doi: 10.1093/jme/tjaa022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Anne. Rev. Entomol. 2015;60:537–559; doi: 10.1146/annurev-ento-010814-020828 [DOI] [PubMed] [Google Scholar]
- 19.Seok S, Vorsino AE, Collier TC, Hapairai L, Jacobsen CM, et al. Population genomics of Aedes albopictus across remote Pacific Islands for genetic biocontrol considerations. PLoS Negl. Trop. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv. 2012;07.3907; doi: 10.48550/arXiv.1207.3907 [DOI] [Google Scholar]
- 21.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of SAMtools and BCFtools. Giascience 2012;10:giab008; doi: 10.1093/gigascience/giab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics 2011;27:2156–2158; doi: 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson JT, Thorvaldsdóttir H, Wenger AM, Zehir A, Mesirov JP. Variant review with the integrative genomics viewer. Cancer Res. 2017;77:e31–e34; doi: 10.1158/0008-5472.CAN-17-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brisco KK, Jacobsen CM, Seok S, Wang X, Lee Y, Akbari OS, et al. Field evaluation of In2Care mosquito traps to control Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Hawai’i Island. J. Med. Entomol. 2023;60:364–372; doi: 10.1093/jme/tjad005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PacMOSSI consortium. Standard operating procedure for maintaining Aedes aegypti and Aedes albopictus colony mosquitoes. PacMOSSI; 2022. [Google Scholar]
- 26.McAllister JC, Scott M. CONUS manual for evaluating insecticide resistance in mosquitoes using the CDC bottle bioassay kit. Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/mosquitoes/pdfs/CONUS-508.pdf. Accessed 01 May 2023. [Google Scholar]
- 27.Seok S, Blore K, Smoleroff S, Lee Y. Investigation of insecticide resistance profile of St. Johns County populations of Aedes aegypti and Aedes albopictus to permethrin. J. F. Mosq. Control Assoc. 2025;72:89–91; doi: 10.32473/jfmca.72.1.139364 [DOI] [Google Scholar]
- 28.Davidson-Pilon C. Lifelines: Survival analysis in Python. J. Open Source Softw. 2019;4:1317; doi: 10.21105/joss.01317 [DOI] [Google Scholar]
- 29.Endersby-Harshman NM, Schmidt TL, Chung J, van Rooyen A, Weeks AR, Hoffmann AA. Heterogeneous genetic invasions of three insecticide resistance mutations in Indo-Pacific populations of Aedes aegypti (L.). Mol. Ecol. 2020;29:1628–1641; doi: 10.1111/mec.15430 [DOI] [PubMed] [Google Scholar]
- 30.Norris LC, Main BJ, Lee Y, et al. Adaptative introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc. Natl. Acad. Sci. USA 2015;112:815–820; doi: 10.1073/pnas.1418892112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Main BJ, Lee Y, Collier TC, Fofana A, Cornel AJ, Lanzaro GC. Complex genome evolution in Anopheles coluzzii associated with increased insecticide usage in Mali. Mol. Ecol. 2015;24:5145–5157; doi: 10.1111/mec.13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dusfour I, Zorrilla P, Guidez A, Issaly J, Girod R, Guillaumot L, et al. Deltamethrin resistance mechanisms in Aedes aegypti populations from three French overseas territories worldwide. PLoS Negl. Trop. Dis. 2015;9:e0004226; doi: 10.1371/journal.pntd.0004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman RU, Souza B, Uddin I, Carrara L, Brito LP, Costa MM, et al. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci. Rep. 2021;11:4555; doi: 10.1038/s41598-021-83465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weston DP, Poynton HC, Wellborn GA, Lydy MJ, Blalock BJ, Sepulveda MS, et al. Multiple origins of pyrethroid insecticide resistance across the species complex of a nontarget aquatic crustacean, Hyalella Azteca. Proc. Natl. Acad. Sci. USA 2013;110:16532–16537; doi: 10.1073/pnas.1302023110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript as a supplementary information file.