ABSTRACT
Regenerative endodontics has emerged as a promising and recognized approach for treating necrotic young permanent teeth. Based on advanced tissue engineering strategies, regenerative therapies, such as cell homing and cell-based transplantation, have been extensively investigated to achieve functional regeneration of the injured pulp-dentin complex. Injectable, thermo-responsive, and tailor-made 3D-printed scaffolds that carry antimicrobial, anti-inflammatory, and other signaling cues provide a powerful means of delivering drugs precisely within the narrow, branching anatomy of the root canal. By enhancing antimicrobial decontamination, an essential step in the regenerative process, these biomaterials establish a permissive microenvironment that promotes cellular migration, adhesion, and subsequent differentiation. Therefore, the current narrative review emphasizes emerging strategies and their optimization to enhance regenerative outcomes in immature teeth affected by pulp necrosis and apical periodontitis.
KEYWORDS: Growth differentiation factors, mesenchymal stem cell transplantation, regenerative endodontics, regenerative medicine, tissue engineering, tissue scaffolds
1. Introduction
Historically, a widely used approach among endodontists for treating immature teeth with pulp necrosis has been apexification. This is achieved either through the long-term application of calcium hydroxide to stimulate the formation of an apical barrier or by placing a mineral trioxide aggregate (MTA) apical plug, followed by conventional root canal obturation with gutta-percha [1,2]. However, these approaches are unable to restore the sensory and immune functions of the lost dental pulp, nor do they promote root thickening or lengthening [1,3,4]. As a result, thin and fragile radicular walls are maintained, which increases the risk of root fracture, particularly when calcium hydroxide is used over multiple visits [5].
Considering the drawbacks of conventional therapies, regenerative endodontic procedures (REPs) have emerged as a biologically driven alternative that facilitates the physiological restoration of compromised dental structures, including dentin, root tissues, and the cellular components of the pulp-dentin complex [6,7]. Early studies on REPs emphasized the role of evoked bleeding in promoting vascularization following prior disinfection, aiming to create an optimal environment for the formation of new tissue within the root canal (Figure 1). Notably, Ostby demonstrated the potential of this technique to facilitate these regenerative processes in vivo [8]. The earliest clinical evidence came from human case reports of what were then called “revascularization” procedures [9,10]. These studies showed marked root maturation and restored sensitivity in immature necrotic teeth treated with REPs. They also emphasized that a bacteria-tight coronal seal is indispensable for preventing microbial ingress, recommending a bonded resin restoration positioned apical to the cementoenamel junction (CEJ).
Figure 1.
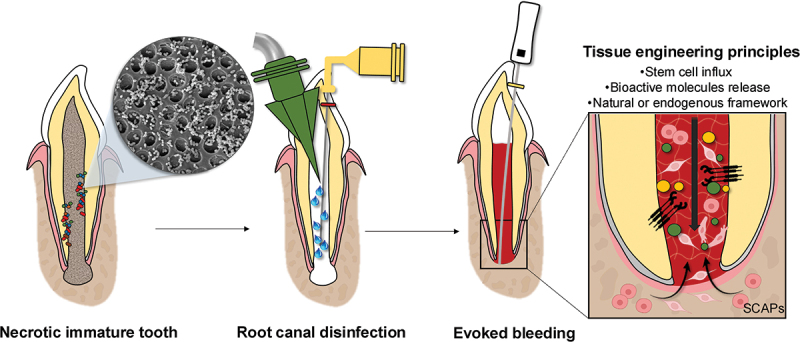
Schematic representation of the clinical sequence for regenerative endodontic procedures used to treat necrotic immature permanent teeth (elements created with BioRender.com).
The apical papilla in immature permanent teeth with necrotic pulps has already demonstrated viable potential to support regeneration, even in the presence of necrosis and apical periodontitis [11]. However, the literature reports non-uniform healing patterns in necrotic immature teeth undergoing REPs. While the “regeneration” process involves the restoration of damaged tissue by replacing it with tissue similar to the original, “repair” occurs when damaged tissue is replaced by a different type of tissue, often resulting in the loss of biological functions. Despite the possibility of pulp-like tissue regeneration after REPs, with nerves, capillaries, and specialized cells [12–14], most in vivo assessments have shown that the tissues formed in the canal space were mineralized tissues similar to cementum and bone, along with fibrous connective tissue resembling the periodontal ligament [15–21], exhibiting more characteristics of a reparative rather than a regenerative process. These different findings may be related to various variables, such as the experimental model and observation period used, which can influence the degree of inflammation and the residual bacterial concentration, as well as the evaluation of novel cell-based therapies for REPs.
There has been a rapid expansion of research in regenerative endodontics, particularly over the past few years [22]. For instance, A PubMed search using the keywords “pulp regeneration,” “pulp revascularization,” “regenerative endodontics,” “regenerative endodontic procedures,” “pulp revitalization,” and “regenerative endodontic therapy” currently retrieves over 1,600 articles. This result reflects a growing emphasis on exploring novel biomaterials and scaffolds developed through advanced tissue engineering strategies [22], particularly aimed at regenerating a viable and functional pulp-dentin complex, rather than merely stimulating wound healing responses.
Clinically, undesirable outcomes have been reported after REPs, including persistent pain, tooth discoloration, and intracanal calcification. These factors may be related to prognostic indicators, material selection, and particularly the challenge of achieving proper root canal disinfection, which can impact the healing process following the procedure. Over the past few decades, advances in tissue engineering have transformed clinical practice and propelled regenerative medicine forward. Studies on stem-cell biology, growth factors, and three-dimensional scaffolds have clarified how each element promotes tissue repair and functional recovery. Rigorous infection-control protocols and the judicious use of biomaterials further create a microenvironment that supports healing. Collectively, these strategies encourage the regeneration of both soft and hard tissues, thereby enhancing the overall success of regenerative endodontic therapy [21].
This review provides a comprehensive analysis of the current challenges faced by researchers and clinicians in REPs, highlighting emerging strategies and their optimization to improve regenerative outcomes in both immature and mature teeth affected by pulp necrosis and apical periodontitis. It also looks ahead, exploring how to replicate the pulp’s complex microenvironment and facilitate seamless integration with host tissues, which are key steps toward more predictable and functional regeneration.
2. New approaches for debridement and infection control
Cleaning and disinfecting immature teeth present significant challenges due to their unique anatomical features and the ability of bacteria to penetrate deeply into the dentinal tubules and inaccessible areas [23]. Inflammatory responses and postoperative infections can then shape how the tissue heals after regenerative endodontic procedures [21,23,24]. If the infection remains, it can compromise the survival, differentiation, and maturation of stem cells from the apical papilla, thereby undermining regeneration.
While effective to a certain extent, traditional disinfection methods often fail to completely eliminate bacteria from root canal systems, as areas of reduced flow and irregularities may harbor pathogens and compromise the integrity of the tooth structure. Furthermore, the minimal mechanical preparation of immature teeth may limit biofilm disruption during REPs, making infection control reliant on the efficacy of chemical agents. Traditionally, triple or double antibiotic pastes and calcium hydroxide-based agents have been recommended as root canal disinfectants, following initial irrigation with chemical solutions such as sodium hypochlorite (NaOCl) and ethylenediaminetetraacetic acid (EDTA) [7]. Despite their antibacterial properties, studies have reported detrimental effects associated with commonly used antibiotics and substances incorporated into these medications, including dose-dependent toxicity to stem cells and impaired vascularization [25–27].
Antimicrobial approaches in regenerative endodontics have evolved from antibiotic pastes to electrospun fibers, then to three-dimensional (3D) constructs, and most recently to injectable hydrogels. Paired with advanced irrigation systems, these technologies enhance diffusion and enable the controlled delivery of antibacterial agents while maintaining a cell-friendly environment, thereby increasing the likelihood of successful regenerative outcomes (Figure 2).
Figure 2.

Antimicrobial biomaterials for infection control in regenerative endodontics. (A) scanning electron micrographs illustrate non-crosslinked and photo-crosslinked SilkMA scaffolds loaded with 10 wt% clindamycin/tinidazole (BiMix). (B) antibacterial activity is quantified by counting colony-forming units. (C) SEM (5000×) images show 7-day E. faecalis biofilms on dentin discs: untreated control, double-antibiotic paste (50 mg/mL metronidazole/ciprofloxacin), and SilkMA scaffolds with or without BiMix. (D) BiMix spectrum of activity against representative endodontic pathogens (Reprinted/adapted with permission from Narayanam et al. [28]. ©2024, elsevier). (ea) photograph of a tubular triple-antibiotic-eluting 3-D construct (TA-3DC) inserted into a dentin slice; (eb) schematic of the confocal-laser-scanning-microscopy (CLSM) workflow used to evaluate Actinomyces naeslundii biofilm. (F) CLSM micrographs of A. naeslundii biofilm before and after seven days of TA-3DC exposure. (G) SEM images of dentin surfaces after TA-3DC treatment (Reprinted/adapted with permission from Bottino et al. [133]. ©2019, John Wiley & sons). (H) SEM images of injectable chitosan hydrogels with and without azithromycin. (I) direct antibacterial assay comparing pure and azithromycin-laden chitosan hydrogels with chlorhexidine as a positive control. (J) SEM of a 7-day E. faecalis biofilm on dentin; (L) SEM after seven-day exposure to azithromycin-laden chitosan hydrogel. (M) Brown & Brenn staining of rat molar apices 28 days after regenerative procedures reveals abundant bacteria in the necrotic control (red arrows), but effective disinfection in groups treated with antibiotic paste or azithromycin hydrogel (Reprinted/adapted with permission from Reis-Prado et al. [21]. ©the authors, 2025, elsevier).
Hydrogels composed of antimicrobial agents, such as antibiotics or herbal extracts, have garnered significant attention due to their biocompatibility, biodegradability, self-assembly properties, and potent, selective antimicrobial action [29–32]. The polymeric structure of these hydrogels enables efficient bacterial disinfection through direct contact, particularly in small gaps within dental tissues. Additionally, they offer long-term antimicrobial protection, preventing microbial recolonization after implantation. In contrast to traditional intracanal medicaments composed of calcium hydroxide or antibiotic pastes, injectable hydrogels are well-suited for endodontic regeneration because they mimic the properties of soft tissues, creating a physiologically favorable environment for cell growth and differentiation. By replicating the characteristics of extracellular matrix (ECM), such as high water content, controllable porosity, and suitable mechanical and physicochemical properties, they support natural cellular functions. Their injectability is another significant advantage for endodontic use, allowing them to fill complex root canal systems and function as physical barriers to prevent bacterial colonization and penetration, thereby filling voids and inaccessible areas.
While NaOCl is an effective irrigating solution, showing solvent and antimicrobial properties, its use in young teeth requires careful consideration of dosage, application techniques, and precautions to minimize risks and ensure optimal outcomes. Although studies have evaluated its performance at different concentrations, current guidelines recommend low dosages for use in REPs due to its increased toxicity. To mitigate the harmful effects of NaOCl while ensuring sustained release and efficient antimicrobial delivery in REPs, our group demonstrated that a NaOCl-loaded nanotube-modified gelatin-methacryloyl (GelMA) hydrogel enables the safe administration of this substance, exhibiting reduced toxicity, biodegradability, and antibacterial efficacy against E. faecalis biofilm [33]. GelMA is a semi-synthetic hydrogel used as a controlled drug delivery system due to its positive interactions with host tissues, biodegradability, and mechanical stability [34]. Additionally, these nanotube-based constructs feature nanostructured surfaces with a high aspect ratio and a hollow core, serving as nanocarriers with a large surface area for drug adsorption [33].
Beyond NaOCl-loaded GelMA hydrogels, GelMA-based drug delivery systems have also been explored by incorporating various antimicrobial agents to enable controlled and sustained release of the drugs within the root canal system. Aloe vera, a natural compound with broad-spectrum antibacterial properties and immunomodulatory effects, has been incorporated into photocrosslinkable GelMA nanofibers via electrospinning [35]. Findings indicate that GelMA/Aloe vera nanofibers at a 70:30 ratio exhibit potent antimicrobial activity, effectively reducing E. faecalis biofilm while demonstrating biocompatibility, collagen formation, and immunomodulatory effects. Specifically, these nanofibers promoted a macrophage phenotype polarization from M1 to M2 in an animal model, supporting their anti-inflammatory response [35]. A similar modulation of the M2 macrophage response was observed when Aloe vera was incorporated into a chitosan hydrogel [36]. The incorporation of antibiotics in GelMA also showed effective action against monospecies bacteria and biofilms [31,37,38]. Furthermore, the incorporation of specific ions, such as silver nanoparticles, has been proposed due to their broad-spectrum antibacterial properties against both Gram-negative and Gram-positive bacteria, as well as their lower likelihood of inducing microbial resistance compared to some antibiotics.
The potential use of other polymers as drug delivery systems has been explored in endodontics due to their improved mechanical properties and biocompatibility. Moreover, the application of broad-spectrum intracanal medications in REPs is essential for effective infection control during the procedure. For instance, BiMix-laden SilkMA scaffolds incorporating multiple antibiotics have demonstrated cytocompatibility while significantly reducing the viability of endodontic bacteria commonly found in young teeth and markedly inhibiting biofilm formation [28,39]. The antimicrobial features exhibited by these systems are crucial for promoting tissue healing by suppressing inflammation and supporting cell differentiation, collagen matrix deposition, and the formation of loose connective or hard tissue.
Clinicians face challenges in effectively debriding wide infected root canals with fragile dentinal walls. REPs require a careful balance between infection control and principles of bioengineering. Traditional disinfection protocols have been increasingly questioned, particularly due to their detrimental effects on crucial growth factors, as well as stem cell survival and differentiation. The limitations of these conventional methods in promoting optimal tissue regeneration have driven the investigation of alternative antimicrobial approaches and modern scaffold-based strategies [27]. Scaffolds support stem cells and bioactive molecules, control the release of growth factors, and enhance stem cell differentiation, boosting tissue regeneration. Hence, integrating effective disinfection protocols with advanced scaffold-based approaches represents a promising step forward in regenerative endodontics to achieve more predictable and functional tissue regeneration.
3. Bioengineered scaffolds and cellular strategies for pulp-dentin regeneration
3.1. Cell homing versus cell-based therapies
3.1.1. Evoked bleeding and platelet-rich concentrates
Cell-homing strategies couple the body’s own stem or progenitor cells, guiding them to sites of injury through a coordinated action of signaling molecules, extracellular matrix cues, and microenvironmental factors [40]. Between 1961 and 1971, Ostby emerged as a pioneer in REPs, investigating the potential for tissue repair by deliberately inducing apical bleeding through over-instrumentation before performing a partial root filling [8,41]. This technique, commonly referred to as “revascularization,” was first performed on a necrotic and immature human premolar by Iwaya et al. in 2001 [9]. The procedure led to continued root development and thickening of the root canal walls with mineralized tissue and has since been widely adopted in clinical practice. Periapical bleeding is intentionally induced to form an intracanal blood clot, typically by extending an endodontic file beyond the root apex. However, excessive instrumentation may damage residual pulp tissue and disrupt odontoblast differentiation.
The blood clot formed following evoked bleeding serves as a three-dimensional scaffold, facilitating the migration and attachment of endogenous progenitor cells while also providing a reservoir of endogenous growth factors, thereby functioning as a cell-homing strategy. The granulation tissue generated after apical bleeding acts as a precursor for tissue healing; however, the composition and concentration of cells within the fibrin clot remain unpredictable. Moreover, erythrocytes within the blood clot inevitably undergo necrosis, which may alter their biological characteristics [42]. Therefore, platelet-rich concentrates have been introduced as autologous scaffolds for application within the root canal.
Platelet-rich plasma (PRP) is an autologous source of growth factors obtained by centrifuging whole blood to achieve elevated concentrations that exceed physiological levels [43]. However, its use in tissue regeneration may be limited by the presence of anticoagulants, which can interfere with the healing process [43]. To overcome this limitation, platelet-rich fibrin (PRF) was developed as a second-generation platelet concentrate, featuring a dense fibrin matrix enriched with platelets, leukocytes, and cytokines, which may also enhance infection control [40]. PRF not only preserves growth factors but also ensures their gradual release over time, enhancing its regenerative capacity. These platelet-derived biomaterials facilitate cell migration and differentiation, stimulate angiogenesis, produce anti-inflammatory mediators, and contribute to wound healing [44,45]. The regenerative efficacy of autologous platelet concentrates is influenced by factors such as platelet concentration and the quantity and type of leukocytes incorporated within the fibrin network. The injectable form of PRF (i-PRF) is highly adaptable, as it penetrates complex root canal anatomy and fills voids and narrow spaces. In vitro, it also enhances the behavior of stem cells from the apical papilla (SCAPs) [45]. Clinically, i-PRF and other autologous platelet concentrates have shown comparable benefits, supporting root maturation, periapical healing, and recovery of vitality in necrotic teeth [2,46]. This liquid, injectable form of PRF, prepared by centrifugation at 700 rpm for 3 minutes, also release higher concentrations of various growth factors, promoting greater fibroblast migration, mineralization, angiogenesis, and expression of platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and collagen type I compared to conventional PRF membrane [45,47]. Furthermore, the development of a loose and porous fibrin network of i-PRF may support stem cell infiltration into the scaffold [48]. Although combining evoked bleeding with i-PRF might further enhance regenerative outcomes and facilitate clinical application while reducing procedure time by allowing injection into the complex root canal anatomy, this strategy has yet to be systematically investigated in vivo.
Nevertheless, cell homing approaches may present additional limitations, including the risk of pulp canal calcification or obliteration, as well as tooth staining. The latter complication occurs due to the interaction between blood-derived products (introduced during evoked bleeding) and dentinal tubules, or from the chemical reactions of certain biomaterials used in the procedure, potentially compromising long-term clinical success [49]. Furthermore, traditional cell-free strategies have generally failed to fully restore the native architecture and function of the pulp tissue. In this context, the concept of tissue engineering, which gained prominence in the late 1990s, introduced cell-based therapies as a promising alternative aimed at achieving true regeneration of the pulp-dentin complex [50].
3.1.2. Stem cell transplantation
Mooney et al. were pioneers in demonstrating the formation of tissue resembling native dental pulp by using constructs composed of cultured fibroblasts seeded onto synthetic extracellular matrices made of polyglycolic acid (PGA) [50]. This strategy involves transplanting exogenous stem cells, either autologous or allogeneic, isolated from various sources such as bone marrow, skin, blood vessels, adipose tissue, and dental tissues, into the host, in combination with scaffold systems and/or bioactive growth factors to support tissue regeneration. Both clinical and preclinical studies have demonstrated that stem cells seeded onto scaffolds, such as autologous constructs containing dental pulp stem cells (DPSCs), can effectively promote the formation of dense, pulp-like tissue with a rich capillary network, as well as support the healing of periapical lesions, even in fully developed teeth with pulp necrosis [13,51–56].
Other stem cell populations, such as bone marrow mesenchymal stem cells (BMMSCs) and SCAPs, have shown greater mineralization and differentiation potential compared to DPSCs. These cells have been incorporated into bioceramic materials, yielding promising results, particularly in promoting biomineralization [57,58]. These findings support a potential future incorporation of stem cells into materials used as cervical barriers following blood clot formation in REPs. Another promising alternative is the scaffold-free approach, which relies on the ability of cells to self-organize into three-dimensional tissues without the use of external scaffolding. This method was developed to achieve a higher initial cell density than traditional scaffold-based systems, allowing for direct cell-cell interactions without interference from exogenous materials. Scaffold-free strategies utilize cell sheets [59], spheroids [60], or three-dimensional constructs [61]. The success of this approach depends on the intrinsic capacity of these cellular units to merge and form larger, functional tissue constructs.
Compared to cell-based transplantation, cell homing strategies may offer a simpler and more cost-effective alternative, particularly in clinical settings where clinicians may lack specialized training. Nonetheless, cell-based therapies still face several challenges, including difficulties in standardizing protocols for stem cell isolation and expansion, identifying optimal combinations of growth factors and scaffold materials, risks of immune rejection, ethical concerns, and the translational gap in applying dental pulp stem/progenitor cell research to human clinical models [62].
An essential concept in dental pulp regeneration is that an ideal construct should replicate the structural and functional characteristics of the native extracellular matrix. In addition to supporting vascularization, it integrates effectively with host tissue and allows for gradual remodeling over time. Certain physiomechanical properties, such as porosity and interconnectivity, are crucial for facilitating the diffusion of growth factors, directing cellular responses, and promoting tissue repair. Additionally, there is a growing attention to cell-free regenerative strategies, which may overcome some of the limitations associated with cell-based methods. Hence, the following sections explore a range of scaffolds functionalized with bioactive agents that demonstrate significant regenerative potential and favorable biological performance for applications in tissue engineering.
3.2. Polymers
The use of polymer-based scaffolds is gaining increasing attention in biomedical research due to their ability to support tissue regeneration and enhance patient outcomes by promoting faster and more effective healing [63]. Polymers are macromolecules consisting of repeating monomer units connected through covalent bonds. These biomaterials typically degrade through hydrolysis, producing intermediate natural metabolites and allowing for controlled degradation rates.
A thorough understanding of their synthesis and fabrication processes is crucial for advancing biomedical technologies [64]. Both natural and synthetic polymers are commonly utilized in scaffold design. In regenerative medicine, researchers have investigated the use of polymer blends, which combine natural and/or synthetic polymers, during scaffold fabrication to address various mechanical, thermal, and biological limitations associated with individual polymer types and the characteristics of damaged tissue [65–67]. Table 1 summarizes the main characteristics and regenerative outcomes of natural versus synthetic polymers used in regenerative dentistry.
Table 1.
Main characteristics and regenerative outcomes of natural versus synthetic polymers widely used in tissue engineering.
| Characteristics | Polymers |
|
|---|---|---|
| Natural | Synthetic | |
| Behavior | Naturally controlled | Properties are engineered |
| Classes and structure | Polypeptide- and protein-based, polysaccharide-based, and polynucleotide-based materials with similar or nonidentical repeating units. | Thermoplastic polymers, thermoset polymers, and elastomers with identical repeating units. |
| Mechanical performance | Usually poor stability, low elastic modulus, and weak mechanical strength. | Usually good mechanical behavior. |
| Degradation profile | Usually biodegradable, with rapid in vivo degradation and increasing solubility over time. | Some are biodegradable, showing an adjustable degradation rate. |
| Cytocompatibility/Biocompatibility | Excellent biocompatibility, low toxicity, and good cell adhesion. | Biocompatible, low bioactivity, and limited cell adhesion. |
| Regenerative outcomes | Organized matrix formation in vivo similar to that of pulpal tissue, vascularization, and innervation [12,68,69]; growth factors release, induce stem cell differentiation and mineralization [70,71]; and exhibit antimicrobial activity, especially when loaded with bioactive agents [21,28,72], while reducing inflammation [72]. | Odontoblastic differentiation [73] and mineralization [74,75]; innervation [76,77]; antibacterial activity as drug delivery systems [78]; and pulp-like tissue formation when combined with stem cells [79]. |
In regenerative endodontics, blood clots serve as a natural scaffold with inherent biological properties. Despite advances in scaffolding matrices, challenges such as immune compatibility, cell loss during periapical laceration, uncontrolled scaffold degradation rates, difficulty in inducing intracanal bleeding, particularly in the presence of large periapical lesions, and the lack of standardized clinical protocols persist. From this perspective, various natural polymers, such as polysaccharides, glycosaminoglycans, and proteins, can be incorporated or derived, including chitosan, fibrin, alginate, hyaluronic acid, collagen, gelatin, silk fibroin, and albumin. These different materials have been extensively studied for REPs due to their biocompatibility, biomimetic nature, accessibility, cost-effectiveness, and ability to form hydrogels [80].
3.2.1. Synthetic materials
Synthetic materials are recognized for their high water absorption capacity, tunable mechanical properties, thermal stability, and enhanced durability, in addition to being relatively cost-effective [81]. These synthetic polymers also serve to deliver a variety of bioactive agents, including anti-inflammatory compounds, growth factors, and adhesive proteins [82], further expanding their applicability and versatility in the manufacturing of scaffolds for REPs. Moreover, scaffolds fabricated from synthetic polymers may offer superior handling characteristics and a more straightforward manufacturing process compared to those fabricated from natural biomaterials.
The characteristics of polymers can be influenced by their chemical composition, degree of crosslinking, and fabrication techniques [81]. Synthetic hydrogels are typically classified into two types: degradable types, such as polyglycolic acid (PGA) and polylactic acid (PLA), and non-degradable types, such as polyethylene glycol (PEG). Scaffolds fabricated from PGA and PLA have demonstrated the ability to support dental pulp cell adhesion and proliferation [82], promote stem cell differentiation, and facilitate the formation of vascularized pulp-like tissue. Additionally, they have shown potential to enhance mineralized tissue formation in vivo [83,84], and can serve as drug delivery systems to reduce bacterial load during tissue regeneration [85]. Regarding PEG, both in vitro and in vivo evaluations have demonstrated that it promotes cellular vascularization and osteogenic differentiation, resulting in the formation of vascularized soft connective tissue that resembles dental pulp in vivo [86].
However, unlike natural polymers, these biomaterials exhibit less similarity to the native extracellular environment of dental pulp. Furthermore, synthetic structures may induce inflammatory responses in vivo due to the production of acidic by-products during hydrolytic degradation, which can lead to a localized decrease in pH [63,82,87]. These limitations may encourage the exploration of more naturally derived materials for use in REPs.
3.2.2. Collagen and hydrogel-based scaffolds
In addition to their injectable nature, which facilitates delivery through the complex anatomy of the root canal system, natural hydrogel systems (e.g., chitosan, alginate, fibrin, collagen) offer several advantages for dental pulp regeneration. These include biocompatibility, viscoelastic properties comparable to those of soft connective tissues, such as dental pulp, rapid diffusion of nutrients and metabolites, and the efficient capacity for cell encapsulation and interaction [82].
Collagen, a naturally derived scaffold, is an insoluble fibrous protein found in the extracellular matrix and serves as a key structural component of connective tissues in both humans and animals. It demonstrates excellent biocompatibility, mineralization properties, and is susceptible to enzymatic degradation [82,88]. Collagen-based hydrogels can be used for encapsulating dental pulp stem/progenitor cells, either alone or in combination with bioactive agents, to mimic the interaction between cells and extracellular matrices in vivo and support the regeneration of pulp-like tissues [88–90]. Therefore, these scaffolds can regulate cell growth, making them valuable in tissue engineering and suitable for use as drug delivery systems in dentistry. In a subcutaneous implantation model in mice, Zhang et al. demonstrated the formation of structures resembling normal pulp-dentin tissue within root slices filled with a collagen gel containing DPSCs and exosome-like vesicles derived from Hertwig’s epithelial root sheath (HERS) [90].
Although collagen degrades quickly and has modest overall strength, its excellent tensile properties still make it a valuable scaffold for regenerating pulp tissue [91]. Crosslinking can further enhance its mechanical and physical performance [91,92]; however, traditional agents such as glutaraldehyde may reduce cell viability and compromise biocompatibility. Consequently, researchers are exploring alternative crosslinkers, including flavonoids, that enhance cellular differentiation without compromising cell survival [91]. Thanks to their compatibility with a wide range of bioactive molecules, collagen-based scaffolds remain one of the most promising natural polymers for pulp tissue engineering applications [93,94].
Given the well-known drawbacks of collagen-based hydrogels, including low mechanical strength, rapid degradation, and contraction, thermo-responsive “smart” systems made from synthetic polymers have emerged as an attractive alternative. These materials gel in situ, crosslink quickly, and can adapt in real-time to shifts in the local microenvironment [82].
3.3. Growth factor-mediated regeneration
During dentinogenesis, growth factors become incorporated into the dentin matrix and are subsequently trapped within the mineralized tissue [20]. These molecules have distinct functions in tissue regeneration (Figure 3) and are released when dentin is demineralized, a process that occurs in dental caries, during vital pulp therapy, or in REPs after exposure to chelating agents. Once liberated, growth factors orchestrate cellular behavior by directing the migration, proliferation, and differentiation of cells essential for pulp-dentin regeneration, including odontoblasts, fibroblasts, vascular endothelial cells, and nerve fibers.
Figure 3.

Schematic diagram illustrating bioactive molecules that promote tissue ingrowth into the root canal and mineralization of the canal walls (elements created with BioRender.com).
Recent work in regenerative endodontics now focuses on both the selection of bioactive signals and the methods for delivering them to the root canal, with the goal of enhancing cell homing and promoting pulp-like tissue regeneration. By controlling when and where specific molecules are released inside the canal, researchers hope to make outcomes in REPs more predictable. Chemotactic growth factors and cytokines present in the canal lumen attract cementoblast and osteoblast progenitors, which migrate inward and lay down new cementum and bone. Building on this principle, investigators have created an injectable alkaline hydrogel embedded with gelatin microspheres that activate endogenous TGF-β1 under alkaline conditions [95]. This material offers sustained release of TGF-β1, encourages the migration of BMMSCs, and supports the in vivo formation of neurovascular and mineralized tissues without the need for cell transplantation or added growth factor supplements [95].
Concentrated growth factor (CGF), an autologous biomaterial derived from platelet concentrates, also shows strong regenerative potential [96]. It consists of a dense, three-dimensional fibrin network rich in PDGF-BB, insulin-like growth factor 1 (IGF-1), TGF-β1, basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF) [96]. This synergistic blend of scaffold and cytokines stimulates BMMSC proliferation in a dose-dependent manner, supporting hard-tissue formation [97]. In addition, the high levels of chemotactic factors released from CGF, particularly PDGF-BB and bFGF, appear crucial for enhancing the motility of SCAPs [96]. Outcomes can vary, however, because the scaffold’s performance depends on both the target cell type and the specific mix of growth factors and cytokines liberated from the platelets [96].
Although still underexplored in endodontics, gene therapy has been applied in regenerative medicine as a promising strategy for the controlled delivery of signaling molecules. By introducing genes encoding specific growth factors directly into target cells, gene therapy enables the sustained and localized production of bioactive signals [98]. This approach offers the potential for precise regulation of cellular behavior, particularly through the targeted delivery of growth factors to the pulp-dentin complex.
3.4. Advances in 3D bioprinting
One of the latest trends is the advancement of 3D printed scaffolds with biomimetic structures. This technology, already well-studied in regenerative medicine, offers a powerful way to build patient-specific scaffolds that closely replicate the native extracellular matrix of dental pulp [99,100]. This strategy has already proven effective for pulp-tissue regeneration [101]. Researchers have utilized a range of biomaterials, including natural and synthetic polymers, as well as decellularized ECM, to fabricate 3D-printed constructs for endodontic applications. To better replicate the native microenvironment, hybrid bioactive inks, also known as bioinks, are being developed.
Material selection should be based on essential criteria, including stem cell bioactivity, processability, mechanical compatibility with the target tissue, and favorable physical properties such as porosity, surface texture, wettability, and viscosity [100]. Innovative bioprinting techniques, including sequential multimodal bioprinting of hydrogels, continuous multimaterial bioprinting, and coaxial extrusion of cell-laden hydrogels, are gaining traction in the field due to their ability to generate relatively thick, well-vascularized, and biomimetic tissue constructs [99]. 3D bioprinting enables precise control over the spatial deposition of stem cells, biomaterials, and bioactive agents in a layer-by-layer manner within engineered tissue [102]. Beyond its capacity to create constructs with high fidelity and customized structures, this technology also offers superior production efficiency compared to conventional fabrication methods [100].
Inkjet-based printing, one of the earliest techniques applied to tissue engineering, operates by ejecting droplets of bioink in a precise, layer-by-layer manner [103]. This approach is cost-effective and compatible with low-viscosity bioinks, offering high resolution and generating minimal shear stress on cells, thereby preserving cell viability [104]. However, to prevent nozzle clogging, inkjet bioprinting is confined to low-viscosity bioinks and may not effectively produce constructs with high cell densities or large volumes, which limits its application in fabricating complex, large-scale tissues. In contrast, extrusion-based bioprinting, one of the most widely used methods, encapsulates cells in a hydrogel, which is then extruded through a robotically controlled syringe. Although extrusion-based printing typically incurs higher operational costs than inkjet systems, it offers greater versatility by accommodating a wider range of bioink viscosities and supporting unrestricted cell densities [105,106]. This makes it well-suited for creating larger and more complex tissue constructs. However, extrusion-based bioprinting poses several challenges, including cell death due to shear stress and the need to optimize bioink viscosity to minimize nozzle clogging, which can limit the resolution of printed constructs [106]. Compared to conventional extrusion methods, using cellular aggregate bioinks with precultured hydrogels can help mesenchymal stem cells (MSCs) maintain proliferation and stemness, and may enhance their differentiation in lower elastic moduli hydrogels [107]. Moreover, a previous study proposed a multimaterial extrusion bioprinting platform designed to continuously deposit multiple coded bioinks, with rapid and smooth switching between reservoirs, allowing for the efficient fabrication of complex scaffolds [108].
The future of regenerative strategies is increasingly related to the promise of in situ printing [109]. Recent progress in in situ 3D printing enables the direct deposition of scaffolds or therapeutic agents at the site of a defect, allowing for customized, less invasive treatments while minimizing contamination during handling and transfer. This approach relies on the physiological environment of the patients to support cell proliferation and differentiation, enabling interaction with the surrounding tissues. A major challenge in in situ 3D printing is accurately fabricating soft tissues in the confined and complex regions of the root canal space. Minimizing surgical trauma requires small printing tools; however, the softness of these tissues can cause deformation and misalignment during printing [109]. Moreover, this technology demands proper training for personnel operating the printer and technological advancements, particularly to address workspace limitations in REPs.
Therefore, given the promising potential of combining 3D printing with the delivery of stem cells and bioactive molecules within the root canal to achieve predictable pulp-like tissue regeneration, several factors require careful consideration, as each technique and chosen scaffold material has its own strengths and limitations. Overall, the mechanical properties and crosslinking mechanisms of the bioinks must be thoroughly evaluated, as they significantly affect both cell viability and printability [110]. All these aspects need more rigorous investigation, particularly in regenerative endodontics, before progressing to clinical application. In this regard, laboratory research in regenerative medicine has provided the basis for the rapid growth of biotechnology and biomaterials development. Therefore, preliminary studies employing well-established semi-orthotopic and orthotopic regenerative models, accompanied by detailed histological and molecular analyses, are essential for generating robust evidence to support translational research and for establishing standardized clinical protocols that incorporate combined therapeutic approaches. In addition to selecting appropriate bioink characteristics, researchers have investigated strategies to promote vascularization within bioprinted constructs, such as co-culturing HUVECs with region-specific stem cells [111] and exposing constructs to short-term hypoxia during post-processing [112].
Among the various bioinks under investigation, GelMA has emerged as a versatile matrix for engineering dental pulp tissue. In addition to its favorable physicochemical properties, excellent biocompatibility, and ability to sustain the controlled release of multiple growth factors, GelMA undergoes photocrosslinking upon exposure to ultraviolet light in the presence of a photoinitiator. This allows the formation of stable gel-like structures, making it highly suitable for 3D printing applications, while also enabling the sustained and controlled release of various drugs [113,114]. Park et al. demonstrated that the conjugation of bone morphogenetic protein (BMP) into a GelMA-based bioink formulation allowed for the retention of this peptide in the bioprinted construct for more than 50% over three weeks, while significantly enhancing the expression of odontogenic-related genes in human DPSCs [113].
Chitosan and alginate, two versatile natural polymers, often serve as the backbone of composite bioinks because their physicochemical properties can be tuned, and they print reliably. Yet, chitosan-based hydrogels still suffer from modest mechanical strength and can be challenging to sterilize for bioprinting. To overcome these drawbacks, researchers are testing chemical modifications and co-crosslinking with complementary molecules to enhance both strength and stability [115]. At the same time, achieving consistent vasculogenesis remains a key hurdle in functional pulp regeneration. A recent study demonstrated that a collagen-rich, cell-laden bioink can vascularize the root canal space without shrinking, suggesting significant promise for building vascularized pulp-like tissue [111]. Another encouraging approach utilizes printed GelMA hydrogel microspheres seeded with stem cells; these constructs have already demonstrated the stimulation of angiogenic, neurogenic, and odontogenic differentiation in preclinical studies [116,117].
4. Future perspectives
Due to the functional versatility of hydrogels, microsphere fabrication has become a promising strategy for developing injectable drug delivery systems. Notably, this technique has already been successfully applied in other areas of regenerative medicine. Similarly, the core-shell scaffold design has acquired attention for its multifunctional characteristics, which are respected for both controlled delivery and tissue engineering applications. This configuration, consisting of a central core encapsulated by an outer shell, allows for the incorporation and targeted release of cells and bioactive molecules [118].
Building on the principles of 3D bioprinting, 4D printing was first introduced by the Massachusetts Institute of Technology in 2012 and has since become a promising advanced manufacturing method for regenerative medicine. In 4D printing, fabricated structures can undergo transformations in shape, properties, or function when exposed to specific stimuli after printing, such as light, heat, magnetic or electrical fields, pH changes, or combinations of these factors [119]. This technology creates scaffolds that encapsulate cells and growth factors while dynamically regulating the microenvironment. It offers a new, largely unexplored way to mimic natural tooth development and repair [100]. Therefore, combining the advantages of polymers as scaffolds, such as their ability to mimic the native ECM, favorable mechanical properties, thermal stability, improved durability, and cost-effectiveness, alongside the potential to integrate cells and bioactive agents through 3D bioprinting techniques, it becomes possible to manufacture standardized scaffolds on a large scale, thereby achieving more reliable and consistent results in regenerative endodontics.
Well-designed 3D models help to elucidate cellular behavior and interactions involved in disease progression and healing, being crucial for optimizing future strategies in REPs. Organoid technology, miniaturized, self-organizing 3D cultures derived from stem cells, can recapitulate key features of the injured dentin-pulp complex and now serves as a powerful platform for probing cell-cell crosstalk and evaluating novel biomaterials in regenerative endodontics [120]. An apical papilla organoid, for example, permits detailed analysis of interactions between SCAPs and macrophages during inflammation and healing [121]. Other studies have engineered dentin-pulp-like organoids from mesenchymal stem cells to evaluate the performance of biomaterials [122] and have co-cultured DPSCs with periodontal ligament stem/progenitor cells (PDLSCs) to construct a root organoid that mimics cell-cell interactions and in vivo dental tissue repair [123].
Three-dimensional printed scaffolds combined with gene-editing technologies could enable truly personalized regenerative endodontic therapies. Gene therapy provides a means to correct pathogenic mutations and modulate gene expression with precision [124,125]. Applied to REPs, it could up-regulate genes that drive mineralization and angiogenesis [126], enabling regeneration of a fully functional pulp-dentin complex rather than relying solely on biomaterial performance [127]. Yet major hurdles remain, such as ethical and safety considerations, the demand for exact gene-editing control, high costs (especially in low and middle-income regions), and the technical difficulty of delivering therapy within the intricate rootcanal anatomy [127,128]. Ongoing research aimed at refining delivery and optimizing scaffold architecture is essential before these approaches can be rigorously evaluated and translated into clinical practice.
5. Conclusions
Following Ostby’s demonstration of the effectiveness of evoked bleeding in promoting continued root development in necrotic teeth, there has been growing interest in elucidating the signaling pathways and physiological processes underlying tissue repair. Despite significant advancements in recent decades, true dental pulp regeneration remains the ultimate goal for researchers and continues to face substantial challenges, particularly in terms of morphological characterization and functional properties of newly formed tissue. In addition to the critical need to eliminate residual bacteria within root canal spaces, the application of novel tissue engineering strategies and biomaterials has garnered considerable attention.
In scaffolding systems, there is growing recognition that balancing cell-homing-based regeneration with cell-based therapies is essential to overcome the limitations of each approach and achieve predictable clinical outcomes. Diverse polymers are indispensable to these systems: they provide structural support for cells, enable drug delivery, maintain scaffold stability, and ensure biocompatibility, among other key functions. Consequently, the choice of polymer remains dependent on the specific requirements of the application and the research objectives.
Integrating controlled growth-factor delivery, undifferentiated stem cells, gene editing, and 3D-printed scaffolds could shift REPs from repair to true regeneration. At the same time, organoid models clarify mechanisms and accelerate the translation of research from the bench to the bedside. Nevertheless, despite extensive preclinical studies, translating functional dental pulp regeneration from animal models to clinical practice remains challenging in regenerative endodontics. In translational research, animal testing is essential prior to human clinical trials. Although the dental anatomy of larger animal species more closely resembles the human dentoalveolar structure [129], rat molars are frequently employed as an experimental model for REPs. Rat molars provide a cost-effective option and exhibit anatomical, biological, and physiological characteristics similar to those of human molars [130,131]. Additionally, they exhibit similar proportions of mineralized tissues and share comparable structural characteristics of the pulp chamber and pulp tissue with human teeth [131]. Although these animal models remain the most reliable sources of histological information on the performance of novel therapeutic approaches and materials in REPs, limitations include the regenerative potential and immunogenicity of DPSCs among species, variations in blood supply, and root canal morphology compared to humans [132]. Moreover, the difficulty of replicating the complex microenvironment of human dental pulp and the lack of long-term efficacy data from clinical trials remain major challenges within basic research. To facilitate clinical translation, clinical trials must address ethical and regulatory issues and focus on strategies to reduce the time to regeneration while ensuring that new methods are both safe and effective.
Acknowledgments
Figures 2(A–D) were reprinted/adapted from Figures 2, 4(B,D,E) from the following source: Journal of Endodontics, 50(12), Narayanam R, Cardoso LM, Dos Reis-Prado AH, de Carvalho ABG, Anselmi C, Mahmoud AH, Fenno JC, Dal-Fabbro R, Bottino MC. Antimicrobial Silk Fibroin Methacrylated Scaffolds for Regenerative Endodontics, 1752–1760, doi: 10.1016/j.joen.2024.08.004, © (2024), with permission from Elsevier.
Funding Statement
This study was funded in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES - Brasil (n. 88887.649870/2021–00), and by the National Institutes of Health (NIH – National Institute of Dental and Craniofacial Research/NIDCR, grant R01DE031476).
Article highlights
Evoked bleeding (fibrin clot) is the most common scaffold in regenerative endodontics; however, challenges such as cell loss and difficulty with bleeding drive the search for alternative scaffolding matrices.
Innovations in polymeric scaffold designs, including injectable hydrogels and 3D bioprinting, have gained clinical attention by allowing minimally invasive and personalized scaffolds in REPs.
Regenerative endodontics faces challenges from infection control and complex root canal anatomy; injectable hydrogels offer controlled release of antibacterial cargo while maintaining a cell-friendly environment.
Combining strategies like 3D-printed constructs with stem cells and bioactive agents can shift outcomes from repair to true regeneration.
Revolutionary technologies, such as organoids and gene therapy, can clarify cellular interactions, personalize treatments, and bridge the gap between laboratory research and clinical translation.
Most histological information on novel strategies for REPs comes from animal studies; clinical translation and long-term efficacy evaluation are encouraged.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Author contributions
Alexandre Henrique dos Reis-Prado: conceptualization; data curation; formal analysis; investigation; visualization; methodology; writing – original draft. Francine Benetti: investigation; methodology; resources; supervision; writing – review & editing. Marco Cicero Bottino: conceptualization; investigation; methodology; project administration; resources; supervision; writing – review & editing. Renan Dal-Fabbro: conceptualization; formal analysis; investigation; methodology; writing – review & editing; project administration.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hargreaves KM, Diogenes A, Teixeira FB.. Treatment options: biological basis of regenerative endodontic procedures. J Endod. 2013;39(3 Suppl):S30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabeti M, Ghobrial D, Zanjir M, et al. Treatment outcomes of regenerative endodontic therapy in immature permanent teeth with pulpal necrosis: a systematic review and network meta-analysis. Int Endod J. 2024;57(3):238–255. [DOI] [PubMed] [Google Scholar]
- 3.Huang GT, Sonoyama W, Liu Y, et al. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tewari N, Devi P, Sampath S, et al. Comparative effectiveness of regenerative endodontic treatment versus apexification for necrotic immature permanent teeth with or without apical periodontitis: an umbrella review. Dent Traumatol. 2025;41(3):263–282. [DOI] [PubMed] [Google Scholar]
- 5.Kahler SL, Shetty S, Andreasen FM, et al. The effect of long-term dressing with calcium hydroxide on the fracture susceptibility of Teeth. J Endod. 2018;44(3):464–469. [DOI] [PubMed] [Google Scholar]
- 6.Law AS. Considerations for regeneration procedures. J Endod. 2013;39(3):S44–S56. [DOI] [PubMed] [Google Scholar]
- 7.Wei X, Yang M, Yue L, et al. Expert consensus on regenerative endodontic procedures. Int J Oral Sci. 2022;14(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostby BN. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol Scand. 1961;19:324–353. [PubMed] [Google Scholar]
- 9.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17(4):185–187. [DOI] [PubMed] [Google Scholar]; •• First paper demonstrating successful revascularization in humans.
- 10.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196–200. [DOI] [PubMed] [Google Scholar]; •• This paper introduces the modern clinical protocol for regenerative/revascularization therapy and proves that a necrotic immature tooth could continue root development.
- 11.Palma PJ, Martins J, Diogo P, et al. Does apical papilla survive and develop in apical periodontitis presence after regenerative endodontic procedures? Appl Sci. 2019. [cited. DOI: 10.3390/app9193942 [DOI] [Google Scholar]
- 12.Ishizaka R, Iohara K, Murakami M, et al. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33(7):2109–2118. [DOI] [PubMed] [Google Scholar]
- 13.Chen YJ, Zhao YH, Zhao YJ, et al. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res. 2015;361(2):439–455. [DOI] [PubMed] [Google Scholar]
- 14.Cai S, Zhang W, Chen W. PDGFRβ(+)/c-kit(+) pulp cells are odontoblastic progenitors capable of producing dentin-like structure in vitro and in vivo. BMC Oral Health. 2016;16(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Thibodeau B, Trope M, et al. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36(1):56–63. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi N, Yamauchi S, Nagaoka H, et al. Tissue engineering strategies for immature teeth with apical periodontitis. J Endod. 2011;37(3):390–397. [DOI] [PubMed] [Google Scholar]
- 17.Torabinejad M, Faras H, Corr R, et al. Histologic examinations of teeth treated with 2 scaffolds: a pilot animal investigation. J Endod. 2014;40(4):515–520. [DOI] [PubMed] [Google Scholar]
- 18.Meschi N, Hilkens P, Lambrichts I, et al. Regenerative endodontic procedure of an infected immature permanent human tooth: an immunohistological study. Clin Oral Investig. 2016;20(4):807–814. [DOI] [PubMed] [Google Scholar]
- 19.Arslan H, Şahin Y, Topçuoğlu HS, et al. Histologic evaluation of regenerated tissues in the pulp spaces of teeth with mature roots at the Time of the regenerative endodontic procedures. J Endod. 2019;45(11):1384–1389. [DOI] [PubMed] [Google Scholar]
- 20.Reis-Prado AHD, Oliveira SC, Goto J, et al. Influence of ethylenediaminetetraacetic acid irrigation on the regenerative endodontic procedure in an immature rat molar model. Int Endod J. 2023;56(1):69–79. [DOI] [PubMed] [Google Scholar]
- 21.Reis-Prado AHD, Rahimnejad M, Dal-Fabbro R, et al. Injectable thermosensitive antibiotic-laden chitosan hydrogel for regenerative endodontics. Bioact Mater. 2025;46:406–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis-Prado AHD, Maia CA, Nunes GP, et al. Top 100 most-cited scientific articles in regenerative endodontics 2019–2023: a bibliometric analysis. Int Endod J. 2024;57(10):1434–1452. [DOI] [PubMed] [Google Scholar]
- 23.Verma P, Nosrat A, Kim JR, et al. Effect of residual bacteria on the outcome of pulp regeneration in vivo. J Dent Res. 2017;96(1):100–106. [DOI] [PubMed] [Google Scholar]
- 24.Yanpiset K, Trope M. Pulp revascularization of replanted immature dog teeth after different treatment methods. Endod Dent Traumatol. 2000;16(5):211–217. [DOI] [PubMed] [Google Scholar]
- 25.Ruparel NB, Teixeira FB, Ferraz CC, et al. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38(10):1372–1375. [DOI] [PubMed] [Google Scholar]
- 26.Kamocki K, Nor JE, Bottino MC. Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability-in vitro studies. Arch Oral Biol. 2015;60(8):1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro JS, Munchow EA, Ferreira Bordini EA, et al. Antimicrobial therapeutics in regenerative endodontics: a scoping review. J Endod. 2020;46(9S):S115–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanam R, Cardoso LM, Dos Reis-Prado AH, et al. Antimicrobial silk fibroin methacrylated scaffolds for regenerative endodontics. J Endod. 2024;50(12):1752–1760 e2. [DOI] [PubMed] [Google Scholar]
- 29.Aksel H, Mahjour F, Bosaid F, et al. Antimicrobial activity and biocompatibility of antibiotic-loaded chitosan hydrogels as a potential scaffold in regenerative endodontic treatment. J Endod. 2020;46(12):1867–1875. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro JS, Sanz CK, Munchow EA, et al. Photocrosslinkable methacrylated gelatin hydrogel as a cell-friendly injectable delivery system for chlorhexidine in regenerative endodontics. Dent Mater. 2022;38(9):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubey N, Ribeiro JS, Zhang Z, et al. Gelatin methacryloyl hydrogel as an injectable scaffold with multi-therapeutic effects to promote antimicrobial disinfection and angiogenesis for regenerative endodontics. J Mater Chem B. 2023;11(17):3823–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alghofaily M, Almana A, Alrayes J, et al. Chitosan-gelatin scaffolds loaded with different antibiotic formulations for regenerative endodontic procedures promote biocompatibility and antibacterial activity. J Funct Biomater. 2024;15(7):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dal-Fabbro R, Huang YC, Toledo PTA, et al. Injectable methacrylated gelatin hydrogel for safe sodium hypochlorite delivery in endodontics. Gels. 2023;9(11):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang L, Cui W. Biomedical application of photo-crosslinked gelatin hydrogels. J Leather Sci Eng. 2021;3(1):3. [Google Scholar]
- 35.Namazi SS, Mahmoud AH, Dal-Fabbro R, et al. Multifunctional and biodegradable methacrylated gelatin/Aloe vera nanofibers for endodontic disinfection and immunomodulation. Biomater Adv. 2023;150:213427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashouri F, Beyranvand F, Beigi Boroujeni N. Macrophage polarization in wound healing: role of aloe vera/chitosan nanohydrogel. Drug Deliv Transl Res. 2019;9(6):1027–1042. [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro JS, Daghrery A, Dubey N, et al. Hybrid antimicrobial hydrogel as injectable therapeutics for Oral infection ablation. Biomacromolecules. 2020;21(9):3945–3956. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Y, Tian J, Kong S, et al. SrCuSi(4) O(10)/GelMA composite hydrogel-mediated vital pulp therapy: integrating antibacterial property and enhanced pulp regeneration activity. Adv Healthc Mater. 2023;12(24):e2300546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palasuk J, Kamocki K, Hippenmeyer L, et al. Bimix antimicrobial scaffolds for regenerative endodontics. J Endod. 2014;40(11):1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D, Kim SG. Cell homing strategies in regenerative endodontic therapy. Cells. 2025;14(3):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nygaard-Ostby B, Hjortdal O. Tissue formation in the root canal following pulp removal. Scand J Dent Res. 1971;79(5):333–349. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Lu J, Jiang Q, et al. Biomaterial scaffolds for clinical procedures in endodontic regeneration. Bioact Mater. 2022;12:257–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13(7):1231–1256. [DOI] [PubMed] [Google Scholar]
- 44.Bi J, Liu Y, Liu XM, et al. Platelet-rich fibrin improves the osteo-/Odontogenic differentiation of stem cells from apical papilla via the extracellular signal-regulated protein kinase signaling pathway. J Endod. 2020;46(5):648–654. [DOI] [PubMed] [Google Scholar]
- 45.Pan J, Luo L, Jiang Z, et al. The effect of injectable platelet-rich fibrin and platelet-rich fibrin in regenerative endodontics: a comparative in vitro study. J Appl Oral Sci. 2024;32:e20230449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rios-Osorio N, Caviedes-Bucheli J, Jimenez-Pena O, et al. Comparative outcomes of platelet concentrates and blood clot scaffolds for regenerative endodontic procedures: a systematic review of randomized controlled clinical trials. J Clin Exp Dent. 2023;15(3):e239–e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miron RJ, Fujioka-Kobayashi M, Hernandez M, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig. 2017;21(8):2619–2627. [DOI] [PubMed] [Google Scholar]
- 48.Kubesch A, Barbeck M, Al-Maawi S, et al. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets. 2019;30(3):329–340. [DOI] [PubMed] [Google Scholar]
- 49.Yan H, De Deus G, Kristoffersen IM, et al. Regenerative endodontics by cell homing: a review of recent clinical trials. J Endod. 2023;49(1):4–17. [DOI] [PubMed] [Google Scholar]
- 50.Mooney DJ, Powell C, Piana J, et al. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12(6):865–868. [DOI] [PubMed] [Google Scholar]
- 51.Dissanayaka WL, Hargreaves KM, Jin L, et al. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A. 2015;21(3–4):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iohara K, Fujita M, Ariji Y, et al. Assessment of pulp regeneration induced by stem cell therapy by magnetic resonance imaging. J Endod. 2016;42(3):397–401. [DOI] [PubMed] [Google Scholar]
- 53.El Ashiry EA, Alamoudi NM, El Ashiry MK, et al. Tissue engineering of necrotic dental pulp of immature teeth with apical periodontitis in dogs: radiographic and histological evaluation. J Clin Pediatr Dent. 2018;42(5):373–382. [DOI] [PubMed] [Google Scholar]
- 54.Brizuela C, Meza G, Urrejola D, et al. Cell-based regenerative endodontics for treatment of periapical lesions: a randomized, controlled phase I/II clinical trial. J Dent Res. 2020;99(5):523–529. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Sosa JF, Diaz-Solano D, Wittig O, et al. Dental pulp regeneration induced by allogenic mesenchymal stromal cell transplantation in a mature tooth: a case report. J Endod. 2022;48(6):736–740. [DOI] [PubMed] [Google Scholar]
- 56.Yoshihashi N. Feasibility and outcomes of cell-based regenerative endodontic therapy in postautogenous transplantation of a mature tooth: a case report. J Endod. 2025;51(1):85–93. [DOI] [PubMed] [Google Scholar]
- 57.Wen R, Wang X, Lu Y, et al. The combined application of rat bone marrow mesenchymal stem cells and bioceramic materials in the regeneration of dental pulp-like tissues. Int J Clin Exp Pathol. 2020;13(7):1492–1499. [PMC free article] [PubMed] [Google Scholar]
- 58.Sequeira DB, Oliveira AR, Seabra CM, et al. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: in vivo interaction with two bioactive materials. Clin Oral Investig. 2021;25(9):5317–5329. [DOI] [PubMed] [Google Scholar]
- 59.Syed-Picard FN, Hl R, Kumta PN, et al. Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J Dent Res. 2014;93(3):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dissanayaka WL, Zhu L, Hargreaves KM, et al. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J Dent Res. 2014;93(12):1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itoh Y, Sasaki JI, Hashimoto M, et al. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J Dent Res. 2018;97(10):1137–1143. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed GM, Abouauf EA, AbuBakr N, et al. Cell-based transplantation versus cell homing approaches for pulp-dentin complex regeneration. Stem Cells Int. 2021;2021:8483668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Socci MC, Rodriguez G, Oliva E, et al. Polymeric materials, advances and applications in tissue engineering: a review. Bioengineering (basel). 2023;10(2):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamid Akash MS, Kanwal R, Chen S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym Rev. 2015;55(3):371–406. [Google Scholar]
- 65.Yu H, Zhang X, Song W, et al. Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells. J Endod. 2019;45(6):706–715. [DOI] [PubMed] [Google Scholar]
- 66.Leite ML, de Oliveira Ribeiro RA, Soares DG, et al. Poly(caprolactone)-aligned nanofibers associated with fibronectin-loaded collagen hydrogel as a potent bioactive scaffold for cell-free regenerative endodontics. Int Endod J. 2022;55(12):1359–1371. [DOI] [PubMed] [Google Scholar]
- 67.Castro JI, Astudillo S, Mina Hernandez JH, et al. Synthesis, characterization, and optimization studies of Polycaprolactone/Polylactic acid/Titanium dioxide Nanoparticle/Orange essential oil membranes for biomedical applications. Polym (basel). 2022;15(1):2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prescott RS, Alsanea R, Fayad MI, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34(4):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iohara K, Imabayashi K, Ishizaka R, et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17(15–16):1911–1920. [DOI] [PubMed] [Google Scholar]
- 70.Liao F, Chen Y, Li Z, et al. A novel bioactive three-dimensional beta-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J Mater Sci Mater Med. 2010;21(2):489–496. [DOI] [PubMed] [Google Scholar]
- 71.Sancilio S, Gallorini M, Di Nisio C, et al. Alginate/hydroxyapatite-based nanocomposite scaffolds for bone tissue engineering improve dental pulp biomineralization and differentiation. Stem Cells Int. 2018;2018:9643721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu N, Chatzistavrou X, Ge L, et al. Biological properties of modified bioactive glass on dental pulp cells. J Dent. 2019;83:18–26. [DOI] [PubMed] [Google Scholar]
- 73.Sakai VT, Zhang Z, Dong Z, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89(8):791–796. [DOI] [PubMed] [Google Scholar]
- 74.Zou H, Wang G, Song F, et al. Investigation of human dental pulp cells on a potential injectable Poly(lactic-co-glycolic acid) microsphere scaffold. J Endod. 2017;43(5):745–750. [DOI] [PubMed] [Google Scholar]
- 75.Gangolli RA, Devlin SM, Gerstenhaber JA, et al. A Bilayered poly (lactic-co-glycolic acid) scaffold provides differential cues for the differentiation of dental pulp stem cells. Tissue Eng Part A. 2019;25(3–4):224–233. [DOI] [PubMed] [Google Scholar]
- 76.Cho YI, Choi JS, Jeong SY, et al. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010;6(12):4725–4733. [DOI] [PubMed] [Google Scholar]
- 77.Eap S, Becavin T, Keller L, et al. Nanofibers implant functionalized by neural growth factor as a strategy to innervate a bioengineered tooth. Adv Healthc Mater. 2014;3(3):386–391. [DOI] [PubMed] [Google Scholar]
- 78.Bottino MC, Kamocki K, Yassen GH, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92(11):963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang GT, Yamaza T, Shea LD, et al. Stem/Progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16(2):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identified stem cells from the apical papilla (SCAP) and their role in root development highlight the importance of stem cell biology in regenerative endodontics (REPs).
- 80.Jazayeri HE, Lee SM, Kuhn L, et al. Polymeric scaffolds for dental pulp tissue engineering: a review. Dent Mater. 2020;36(2):e47–e58. [DOI] [PubMed] [Google Scholar]
- 81.Munim SA, Raza ZA. Poly(lactic acid) based hydrogels: formation, characteristics and biomedical applications. J Porous Mater. 2019;26(3):881–901. [Google Scholar]
- 82.Dissanayaka WL, Zhang C. Scaffold-based and scaffold-free strategies in dental pulp regeneration. J Endod. 2020;46(9s):S81–s89. [DOI] [PubMed] [Google Scholar]
- 83.Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34(8):962–969. [DOI] [PubMed] [Google Scholar]
- 84.Kuang R, Zhang Z, Jin X, et al. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv Healthc Mater. 2015;4(13):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bekhouche M, Bolon M, Charriaud F, et al. Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering. J Mater Chem B. 2020;8(36):8422–8432. [DOI] [PubMed] [Google Scholar]
- 86.Galler KM, Cavender AC, Koeklue U, et al. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen Med. 2011;6(2):191–200. [DOI] [PubMed] [Google Scholar]
- 87.Sugiaman VK, Jeffrey NS. Polymeric scaffolds used in dental pulp regeneration by tissue engineering approach. Polym (Basel). 2023;15(5):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Q, Yuan C, Liu L, et al. Effect of 3-dimensional collagen fibrous scaffolds with different pore sizes on pulp regeneration. J Endod. 2022;48(12):1493–1501. [DOI] [PubMed] [Google Scholar]
- 89.Iohara K, Murakami M, Takeuchi N, et al. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med. 2013;2(10):818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang S, Yang Y, Jia S, et al. Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics. 2020;10(13):5914–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim ES, Lim MJ, Min KS, et al. Effects of epicatechin, a crosslinking agent, on human dental pulp cells cultured in collagen scaffolds. J Appl Oral Sci. 2016;24(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhand C, Ong ST, Dwivedi N. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials. 2016;104:323–338. [DOI] [PubMed] [Google Scholar]
- 93.Nakao K, Itoh M, Tomita Y, et al. FGF-2 potently induces both proliferation and DSP expression in collagen type I gel cultures of adult incisor immature pulp cells. Biochem Biophys Res Commun. 2004;325(3):1052–1059. [DOI] [PubMed] [Google Scholar]
- 94.Kim JY, Xin X, Moioli EK, et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 2010;16(10):3023–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S, Niu Y, Jia P, et al. Alkaline activation of endogenous latent TGFbeta1 by an injectable hydrogel directs cell homing for in situ complex tissue regeneration. Bioact Mater. 2022;15:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong S, Li L, Cai W, et al. The potential application of concentrated growth factor in regenerative endodontics. Int Endod J. 2019;52(5):646–655. [DOI] [PubMed] [Google Scholar]
- 97.Yu B, Wang Z. Effect of concentrated growth factors on beagle periodontal ligament stem cells in vitro. Mol Med Rep. 2014;9(1):235–242. [DOI] [PubMed] [Google Scholar]
- 98.Hargreaves KM, Giesler T, Henry M, et al. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34(7 Suppl):S51–6. [DOI] [PubMed] [Google Scholar]
- 99.Groll J, Boland T, Blunk T. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016;8(1):013001. [DOI] [PubMed] [Google Scholar]
- 100.Zhao F, Zhang Z, Guo W. The 3-dimensional printing for dental tissue regeneration: the state of the art and future challenges. Front Bioeng Biotechnol. 2024;12:1356580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bottino MC, Pankajakshan D, Nör JE. Advanced scaffolds for dental pulp and periodontal regeneration. Dent Clin North Am. 2017;61(4):689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. [DOI] [PubMed] [Google Scholar]
- 103.Ma Y, Xie L, Yang B, et al. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol Bioeng. 2019;116(2):452–468. [DOI] [PubMed] [Google Scholar]
- 104.Salar Amoli M, EzEldeen M, Jacobs R, et al. Materials for Dentoalveolar Bioprinting: current state of the art. Biomedicines. 2021;10(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramesh S, Harrysson OLA, Rao PK, et al. Extrusion bioprinting: recent progress, challenges, and future opportunities. Bioprinting. 2021;21:e00116. [Google Scholar]
- 106.Budharaju H, Sundaramurthi D, Sethuraman S. Embedded 3D bioprinting - an emerging strategy to fabricate biomimetic & large vascularized tissue constructs. Bioact Mater. 2024;32:356–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang L, Li Z, Yao B, et al. Extrusion bioprinting of cellular aggregates improves mesenchymal stem cell proliferation and differentiation. Biomater Adv. 2023;149:213369. [DOI] [PubMed] [Google Scholar]
- 108.Liu W, Zhang YS, Heinrich MA, et al. Rapid continuous multimaterial extrusion bioprinting. Adv Mater. 2017;29(3). doi: 10.1002/adma.201604630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang J, Zhou G, Jiang Q, et al. In situ 3D bioprinting: The future of regenerative medicine. Fundamental Res. 2025;18:43. [Google Scholar]
- 110.Chen XB, Fazel anvari-Yazdi A, Duan X, et al. Biomaterials/Bioinks and extrusion bioprinting. Bioact Mater. 2023;28:511–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duarte Campos DF, Zhang S, Kreimendahl F, et al. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect Tissue Res. 2020;61(2):205–215. [DOI] [PubMed] [Google Scholar]
- 112.Kuss MA, Harms R, Wu S, et al. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017;7(47):29312–29320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park JH, Gillispie GJ, Copus JS, et al. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication. 2020;12(3):035029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li P, Zhang M, Chen Z, et al. Tissue-engineered injectable gelatin-methacryloyl hydrogel-based adjunctive therapy for intervertebral disc degeneration. ACS Omega. 2023;8(15):13509–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salar Amoli M, Anand R, EzEldeen M. The development of a 3D printable chitosan-based copolymer with tunable properties for dentoalveolar regeneration. Carbohydr Polym. 2022;289:119441. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q, Yang T, Zhang R, et al. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021;136:441–455. [DOI] [PubMed] [Google Scholar]
- 117.Qian Y, Gong J, Lu K, et al. DLP printed hDPSC-loaded GelMA microsphere regenerates dental pulp and repairs spinal cord. Biomaterials. 2023;299:122137. [DOI] [PubMed] [Google Scholar]
- 118.Perez RA, Kim HW. Core-shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 2015;21:2–19. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Cui H, Esworthy T, et al. Emerging 4D printing strategies for next-generation tissue regeneration and medical devices. Adv Mater. 2022;34(20):e2109198. [DOI] [PubMed] [Google Scholar]
- 120.Li FC, Kishen A. 3D organoids for regenerative endodontics. Biomolecules. 2023;13(6):900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li FC, Hussein H, Magalhaes M, et al. Deciphering stem cell from apical papilla-macrophage choreography using a novel 3-dimensional organoid system. J Endod. 2022;48(8):1063–1072 e7. [DOI] [PubMed] [Google Scholar]
- 122.Jeong SY, Lee S, Choi WH, et al. Fabrication of dentin-pulp-like organoids using dental-pulp stem cells. Cells. 2020;9(3):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calabrese TC, Rothermund K, Gabe CM, et al. Self-assembly of tooth root organoid from postnatal human dental stem cells. Tissue Eng Part A. 2024;30(9–10):404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fu B, Shen J, Chen Y, et al. Narrative review of gene modification: applications in three-dimensional (3D) bioprinting. Ann Transl Med. 2021;9(19):1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hosseinkhani H, Domb AJ, Sharifzadeh G, et al. Gene therapy for regenerative medicine. Pharmaceutics. 2023;15(3):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alothman FA, Hakami LS, Alnasser A, et al. Recent advances in regenerative endodontics: a review of current techniques and future directions. Cureus. 2024;16(11):e74121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farjaminejad R, Farjaminejad S, Garcia-Godoy F. Regenerative endodontic therapies: harnessing stem cells, scaffolds, and growth factors. Polym (basel). 2025;17(11):1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Umapathy VR, Natarajan PM, Swamikannu B. Regenerative strategies in dentistry: harnessing stem cells, biomaterials and bioactive materials for tissue repair. Biomolecules. 2025;15(4):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kantarci A, Hasturk H, Van Dyke TE. Animal models for periodontal regeneration and peri-implant responses. Periodontol 2000. 2015;68(1):66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sasaki T, Kawamata-Kido H. Providing an environment for reparative dentine induction in amputated rat molar pulp by high molecular-weight hyaluronic acid. Arch Oral Biol. 1995;40(3):209–219. [DOI] [PubMed] [Google Scholar]
- 131.Dammaschke T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim. 2010;44(1):1–6. [DOI] [PubMed] [Google Scholar]
- 132.Xie Z, Shen Z, Zhan P, et al. Functional dental pulp regeneration: basic research and clinical translation. Int J Mol Sci. 2021;22(16):8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bottino MC, Albuquerque MTP, Azabi A, et al. A novel patient-specific three-dimensional drug delivery construct for regenerative endodontics. J Biomed Mater Res B Appl Biomater. 2019;107(5):1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]


