Abstract
Modulating macrophage function is an effective strategy for treating atherosclerosis. Our previous research shows that tilianin (Til) effectively regulates macrophage polarization. This immune modulation positions Til as a promising plant-derived therapeutic agent with potential for atherosclerosis treatment and management. Due to its biopharmaceutics classification system (BCS) IV drug properties, it is a challenge to delivering Til to macrophages in atherosclerotic plaques, especially via the oral route. Herein, we introduced a folate-modified oral Til nanocrystal liposome (FA-Lipo@Til NCs) that showed enhanced mucus permeability and transmembrane transport ability across the intestinal epithelium. It could subsequently target and accumulate in macrophages within aortic plaques. After three months of oral treatment with FA-Lipo@Til NCs in apolipoprotein E deficient (ApoE−/−) mice with atherosclerosis, significant therapeutic effects were observed. The treatment effectively stabilized atherosclerotic plaques. Additionally, FA-Lipo@Til NCs effectively inhibited reactive oxygen species (ROS) production in macrophages, thereby reducing oxidative stress. The treatment also promoted macrophage polarization towards the anti-inflammatory M2 phenotype, enhancing their ability to clear apoptotic cells (efferocytosis) and resolving local inflammation. Notably, throughout the treatment period, significant alterations in blood lipid levels were observed. In summary, FA-Lipo@Til NCs offer a targeted and effective approach to regulate macrophage polarization while also hindering the advancement of atherosclerosis. Besides, our study proposes a promising therapeutic approach for atherosclerosis by leveraging an innovative oral targeted delivery system for BCS IV drugs.
Keywords: Tilianin, Nanocrystal liposomes, Folate, Oral targeting, Atherosclerosis
Graphical abstract
Folate-modified tilianin nanocrystalline liposomes (FA-Lipo@Til NCs) are natural nanoparticles designed to oral target macrophages in atherosclerotic plaques, facilitating anti-atherosclerotic therapy by enhancing macrophage polarization (M2 phenotype) and efferocytosis.

1. Introduction
Atherosclerosis constitutes the fundamental pathological basis for a variety of cardiovascular diseases, including peripheral artery disease, coronary heart disease, stroke, and other potentially fatal conditions [1]. Due to its alarmingly high prevalence, atherosclerosis has attracted considerable attention as a major concern both in medical fields and in society, presenting a profound threat to global health and economic stability [[2], [3], [4]]. According to the American Heart Association (AHA) and the World Heart Federation, cardiovascular diseases account for nearly 33 % of all global deaths, with ischemic heart disease and stroke representing the majority of these fatalities [5]. The incidence of cardiovascular disease has significantly increased in recent years. Therefore, improving the capacity for early diagnosis and treatment of atherosclerosis has become a critical priority and a central focus of ongoing medical research [6,7].
Recent studies have highlighted that macrophages represent the predominant immune cells within atherosclerotic plaques. Their intrinsic properties, such as lipid accumulation, phenotype plasticity, and the secretion of inflammatory mediators, have a profound impact on the inflammatory state and stability of the plaques [[8], [9], [10]]. As atherosclerosis advances, circulating monocytes are recruited to sites of endothelial injury, where they undergo differentiation into macrophages [[11], [12], [13], [14]]. Upon infiltrating tissues, macrophages display remarkable plasticity, allowing them to dynamically transition between M1 and M2 phenotypes in response to local microenvironmental factors and stimuli [15]. M1 macrophages, which respond to pathogenic stimuli, compromise plaque integrity, thereby accelerating the formation of vulnerable plaques. In contrast, M2 macrophages have beneficial effects by enhancing plaque stability and mitigating the progression of vulnerable plaques [16,17]. The balance between these phenotypes plays a crucial role in modulating plaque progression, immune responses, and the overall stability of atherosclerotic lesions. Furthermore, as the inflammatory process advances, apoptotic cells transition into necrotic cells, ultimately contributing to the enlargement of necrotic core regions within plaques [18]. Efferocytosis by macrophages plays a pivotal role in mitigating atherosclerosis, a process significantly enhanced by polarization towards the M2 phenotype [17]. Macrophages have emerged as promising therapeutic targets for atherosclerosis treatment, as modulating their function offers potential to attenuate inflammation and create conditions that impede disease progression. Atherosclerotic plaques are known to harbor macrophages that express high levels of folic acid receptor β (FR-β), as demonstrated by Xuejun Wen et al. [19]. Recombinant immunotoxins and FA-conjugated therapeutics can selectively target these macrophages by binding to FR-β, presenting an effective strategy for treating atherosclerosis [[20], [21], [22]].
Tilianin (Til), a naturally occurring flavonoid glycoside, is abundantly sourced from a wide range of medicinal plants [23,24]. Its distinctive structure, comprising triterpenoids and polyphenolic hydroxyl groups linked to sugar moieties, imparts exceptional chemical properties and physiological activities [[25], [26], [27], [28], [29], [30]]. Consequently, Til acts as a key bioactive agent in alleviating tissue damage and dampening inflammation during pathological inflammatory responses [26,27]. In our previous studies, we demonstrated that Til promotes macrophage polarization toward the anti-inflammatory M2 phenotype. This phenotypic transition not only suppresses inflammatory responses but also underscores Til's therapeutic potential in modulating immune activity under chronic inflammatory conditions [31]. Additional studies have reported that Til exerts vascular protective effects, primarily through macrophage modulation, thereby supporting its potential in the treatment of atherosclerosis [26,32,33]. Notably, for atherosclerosis and other chronic inflammatory diseases, oral administration offers considerable advantages over parenteral or localized delivery routes [[34], [35], [36], [37]]. Its non-invasive profile markedly enhances patient compliance, making it particularly suitable for sustained therapeutic regimens and facilitating administration in home-based or outpatient care settings. Furthermore, oral delivery eliminates injection-associated risks, such as infection and tissue trauma, and, when integrated with advanced drug delivery systems, enables sustained or targeted release, thereby enhancing therapeutic efficacy while mitigating systemic adverse effects. Nonetheless, the oral delivery of Til remains significantly constrained by its exceptionally low aqueous solubility (approximately 0.00157 g/L at 37 °C) and limited permeability across the gastrointestinal epithelium. These physicochemical limitations categorize Til as a Biopharmaceutics Classification System (BCS) Class IV compound, defined by poor solubility and low membrane permeability. As a result, these intrinsic properties present formidable challenges for pharmaceutical development and substantially impede Til's clinical translation and therapeutic deployment [38,39]. To address the challenges of Til's poor solubility and limited oral bioavailability, various formulation strategies have been explored, such as lysophosphatidylcholine composite phospholipid liposomes and lysophosphatidylcholine-PLGA block copolymer nanoparticles [31,40]. Though these formulations improved its oral absorption to a certain extent, the circulating Til struggles to accumulate in plaques. In our previous study, we employed nanocrystals (NCs) to enhance the solubility of Til [31]. The findings revealed significant improvements in Til's solubility, cellular uptake, and anti-inflammatory efficacy. As a carrier-free drug delivery platform, NCs offer several distinct advantages that contribute to these enhancements: (i) Reducing particle size markedly increases the specific surface area, accelerating drug dissolution in biological fluids, consistent with the Noyes-Whitney equation [41]; (ii) As predicted by the Ostwald–Freundlich equation, nanoscale reduction elevates surface free energy, thereby enhancing the apparent solubility of drug molecules [42]; (iii) The gastrointestinal tract features a porous mucosal barrier, through which NCs, due to their nanoscale dimensions, can more readily diffuse and strongly adhere, thereby extending residence time and facilitating enhanced mucosal absorption [43]. Together, these physicochemical features synergistically promote improved dissolution kinetics and significantly enhance the oral bioavailability of poorly water-soluble drugs [43]. Consequently, drug NCs have emerged as a compelling formulation strategy, attracting increasing interest across the pharmaceutical landscape [44].
Although nanocrystal technology offers considerable advantages in improving drug solubility and bioavailability, several inherent limitations remain. One major challenge lies in the complete suppression of nanocrystal growth, especially in liquid dispersion systems, where heightened thermodynamic and molecular interactions promote particle aggregation, ultimately compromising both stability and therapeutic efficacy [45,46]. Moreover, unmodified nanocrystals inherently lack active targeting capabilities, limiting their accumulation at specific pathological sites such as atherosclerotic plaques [47,48].
To overcome these challenges, we engineered a FA-modified phospholipid bilayer capable of encapsulating Til NCs, resulting in the construction of a targeted nanodelivery system, denoted FA-Lipo@Til NCs (see Scheme 1). Compared to the traditional method of encapsulating Til in its free molecular form, pre-formulation into nanocrystals offers distinct advantages. Owing to their limited aqueous solubility, poorly soluble drugs in their free molecular form often suffer from inefficient encapsulation, rapid leakage, and diminished liposomal stability [[49], [50], [51]]. In contrast, nanocrystals exhibit a well-defined crystalline architecture and high drug payload, facilitating their stable integration into phospholipid bilayers in the solid state. This strategy significantly enhances both the drug-loading capacity and physicochemical stability of the liposomal formulation, effectively reducing premature drug release during storage and systemic circulation [52]. Consequently, the delivery system can selectively target macrophages at atherosclerotic lesion sites, promoting enhanced accumulation within the plaque. Consequently, the delivery system can selectively target macrophages at atherosclerotic lesion sites, promoting enhanced accumulation within the plaque. We subsequently investigated the oral absorption pathway of Til, its plaque-targeting efficacy, therapeutic impact on atherosclerosis, underlying molecular mechanisms, and conducted preliminary safety evaluations.
Schematic 1.
Schematic representation of FA-Lipo@Til NCs as orally administered biomimetic nanocarriers targeting macrophages within atherosclerotic plaques for anti-atherosclerotic therapy. (a) The synthesis process of Til NCs and FA-Lipo@Til NCs. (b) The translocation of FA-Lipo@Til NCs across mucus and epithelial barriers. (c) The multifaceted anti-atherosclerotic mechanisms of FA-Lipo@Til NCs, including selective targeting of plaque-resident macrophages and restoration of efferocytosis through modulation of macrophage polarization toward the anti-inflammatory M2 phenotype.
2. Experimental
2.1. Materials
Til and its standards (purity >98 %) were obtained from Chengdu Herbpurify Co., Ltd. (Chengdu, China). FA-PEG2000-Chol and mPEG2000-Chol were obtained from Xian Qiyue Biotechnology Co., LTD. (Xian, China). Tocopherol polyethylene glycol succinate (TPGS), Polyvinyl alcohol (PVA), soybean phospholipid and cholesterol (Chol) were obtained from Ouyi Biomaterial Co.,Ltd.(Chengdu, China). N-acetylcysteine (NAC), fixation buffer and mucin from porcine stomach were provided by SigmaAldrich Co., Ltd. (St. Louis, MO, USA). Coumarin 6 (Cou 6), red cell membrane fluorescent probe (DiD), agarose, cytochalasin D, chlorpromazine, amiloride hydrochloride, nystatin, folic acid (FA), bafilomycin A1, monensin, brefeldin A, intracellular staining permeabilization wash buffer, cell staining buffer and Cell Counting Kit-8 (CCK-8) were obtained from Vazyme Biotech (Nanjing, China).Allophycocyanin (APC)-anti-mouse CD86 antibody, phycoerythrin (PE)-anti-mouse CD206 antibody, fluorescein isothiocyanate (FITC)-anti-mouse F4/80 antibody and 4′,6-diamidino-2-phenylindole (DAPI) were provided by BioLegend, Inc. (Beijing, China). CD16/32 was provided by Becton, Dickinson and Company (Beijing, China). Formic acid, polyvinyl alcohol N,N-Dimethylformamide (DMF), methanol and acetone were sourced from Beijing Zhonglian United Technology Co., Ltd. (Beijing, China). Absolute ethanol and acetonitrile were obtained from Wenhanhanrui Co., Ltd. (Chengdu, China).
2.2. Cell lines and animals
Cell Lines: RAW264.7 and Caco-2 cell lines, obtained from the American Type Culture Collection (ATCC), were cultured at Sichuan University for experimental purposes. RAW264.7 cells were maintained in Dulbecco's Modified Eagle Medium (DMEM, Gibco, NY, USA) supplemented with fetal bovine serum (FBS), penicillin, and streptomycin, while Caco-2 cells were grown in Minimal Essential Medium (MEM, Gibco, NY, USA) with equivalent additives. Both cell types were incubated in a cell incubator (BB15, TS, USA).
Animals: In vivo studies utilized male Sprague-Dawley (SD) rats, C57BL/6J mice, and apolipoprotein E deficient (ApoE−/−) mice, which were sourced from Beijing HFK Bioscience Co., Ltd. All animal experiments were approved by the Institutional Animal Care and Use Committee of the State Key Laboratory of Biotherapy, Sichuan University (Approval No. 20221110006). For atherosclerosis modeling, ApoE−/− mice were placed on a high-fat diet (HFD, H10540, HUAFUKANG, China) for 12–14 weeks [53], with the presence of atherosclerotic plaques confirmed through Oil Red O staining. This model was employed in subsequent investigations of biodistribution, therapeutic effects on atherosclerosis, and safety profiling, with C57BL/6J mice serving as healthy controls.
2.3. Preparation and characterization of FA-Lipo@Til NCs
2.3.1. Preparation of FA-Lipo@Til NCs
The anti-solvent precipitation ultrasonication method was first utilized to prepare Til NCs [31,54]. The Crude Til was dissolved in a 1:1 (v:v) mixture of DMF and ethanol. The organic phase was then combined with an aqueous solution containing PVA and TPGS stabilizers (oil-to-water ratio, 1:20). The mixture was sonicated in an ice bath at 130 W for 25 min, and the residual organic solvent was subsequently removed by dialysis.
The preparation of FA-Lipo@Til NCs was initiated by dissolving soybean phospholipid, cholesterol, mPEG2000-Chol, and FA-PEG2000-Chol in 2 mL of ethanol, maintaining a mass ratio of 6:1.5:0.25:0.25. The organic solvent was evaporated under reduced pressure, resulting in the formation of a uniform lipid film. Next, 2 mL of Til NCs was added to the lipid film and hydrated at 60 °C for 1 h. The mixture was then subjected to sonication at 120 W for 10 min, resulting in the formation of FA-Lipo@Til NCs. For comparison, the unmodified Lipo@Til NCs were prepared using the same procedure without the addition of FA-PEG2000-Chol.
To enhance the tracking and analytical capabilities, the formulations were labeled with Cou 6 or DiD. A precise quantity of Cou 6 or DiD was introduced into the organic phase containing dissolved Til. The subsequent steps followed the previously established method for Til NCs preparation. The obtained samples were centrifuged to remove free Cou 6 or DiD. After discarding the supernatant, the remaining pellet was sonicated and re-dispersed in deionized water. The centrifugation-redispersion process was repeated three times to ensure optimal uniformity and purity, yielding Til NCs conjugated with Cou 6 (Til NCs/Cou 6) or DiD (Til NCs/DiD). Furthermore, FA-Lipo@Til NCs labeled with either Cou 6 or DiD were prepared by incorporating Til NCs/Cou 6 or Til NCs/DiD, following the previously described methodology.
2.3.2. Transmission electron microscopy (TEM)
The morphology of Til NCs and FA-Lipo@Til NCs was then observed using TEM with a JEM-2000EXII instrument (JEOL, Tokyo, Japan).
2.3.3. Fourier transform infrared spectroscopy (FTIR) analysis
Lyophilized samples were analyzed using a Nicolet 6700 spectrometer (Thermo Scientific) with KBr pellet method. Spectra were collected from 4000 to 400 cm−1 at 0.09 cm−1 resolution (65 scans/sec). Samples were mounted between CaF2 windows (12 mm) and equilibrated for 10 min prior to measurement. Data processing (peak identification, baseline correction, normalization) was performed using Omnic 8.0 software.
2.4. Interaction between FA-Lipo@Til NCs and mucus
2.4.1. Turbidity measurements
The preparation of the mucus suspension followed a procedure consistent with previous studies [55]. Mucin powder (20 mg/mL) extracted from porcine stomach was dissolved in ultrapure water and incubated overnight at 37 °C. The suspension was then centrifuged at 1000 rpm for 30 min, and the supernatant was collected. Equal volumes of mucin (1 mg/mL) and nanoparticle suspension (1 mg/mL) were mixed and incubated at 37 °C with shaking for various time points. Absorbance was measured at 650 nm using a microplate reader (MK3, Thermo, USA).
2.4.2. Permeation assay using agarose gel
An agarose gel model [56] was utilized to assess the permeability of various nanoparticles through mucus. Agarose (0.3 % w/v) was dissolved in heated water, transferred into small vials, and allowed to cool and solidify. Once solidified, a mucin solution (20 mg/mL) was evenly spread over the gel surface, and Cou 6-labeled nanoparticles (250 μL, 200 ng/mL) were applied. The mixture was incubated at 37 °C for 6 h. After incubation, the mucus layer was gently removed, and the gel was washed three times with deionized water to eliminate any residual contaminants. Lastly, the gel was liquefied, and its optical characteristics were evaluated at 466 nm using a microplate reader.
2.4.3. Transmucosa transport study
2.4.3.1. Penetration test using transwell system
A 24-well Transwell plate model was used to evaluate nanoparticle permeability through mucus [57]. A mucin solution (20 mg/mL) was added to the apical chamber, and Hank's Balanced Salt Solution (HBSS) to the basolateral chamber. The system was incubated at 37 °C for 30 min. Next, 100 μL of Cou 6-labeled nanoparticle suspension (200 ng/mL) was introduced to the apical chamber. Basolateral samples were collected at intervals, and Cou 6 concentration was measured. The apparent permeability coefficient (Papp) was calculated for each formulation.
| (1) |
Among them, dQ/dt was the cumulative amount of preparation transported through the upper layer of the Transwell plate to the lower layer side, The initial concentration on the upper layer side was C0, and the membrane area was A (cm2).
2.4.3.2. Penetration test using in situ intestinal loop model
NAC is commonly employed as a mucolytic agent, acting by breaking both hydrogen and disulfide bonds in mucus, which reduces its viscosity and facilitates its clearance [58]. The in situ intestinal absorption study was performed according to the protocols outlined in the current study [59]. Briefly, rats were fasted for 12 h and then anesthetized with urethane (20 mg/kg). The jejunal segment was carefully excised along the abdominal midline and cleaned. In the control group, the intestinal loop was incubated with the formulations for 2 h without any additional treatment. In the post-treatment group, the intestinal loop was initially incubated with the formulations for 2 h, followed by the removal of the mucus layer using a 0.2 % NAC solution. In the pre-treatment group, the mucus layer was first removed with 0.2 % NAC, after which the loop was incubated with the formulations for 2 h. Following these procedures, the rats were euthanized, and the intestinal segments were processed for nuclear labeling, permeabilization, sectioning, fixation, and dehydration. Finally, the samples were mounted and analyzed using confocal laser scanning microscopy (CLSM; LSM980/STED, Carl Zeiss).
2.5. Cellular uptake
2.5.1. Cytotoxicity analysis
Caco-2 and RAW264.7 cells (1 × 105 cells/well) were seeded in 96-well plates and incubated overnight. The cells were treated with various concentrations of Til formulations for 24 or 48 h. After treatment, 10 μL of CCK-8 solution was added, and the plates were incubated for 2 h at 37 °C. Cell viability was measured by absorbance at 450 nm using a microplate reader.
2.5.2. Intracellular uptake in RAW264.7 and Caco-2
Caco-2 and RAW264.7 cells (1 × 105 cells/well) were seeded into 15-mm confocal dishes and incubated for 24 h. RAW264.7 cells were stimulated with LPS (100 ng/mL) and IFN-γ (20 ng/mL) for 12 h. After medium replacement, Cou 6-labeled Til formulations were added and incubated for 2 h. The cells were then washed with PBS, fixed with 4 % paraformaldehyde, and stained with DAPI (5 μg/mL) to visualize nuclei. Cellular uptake was assessed using CLSM. For flow cytometry (FCM), fluorescence from 10,000 cells was measured using a BD FCM Calibur (BD, USA) [60].
2.5.3. Mechanisms of cellular uptake and intracellular transport
Caco-2 cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 48 h. Then, 1 mL of solution containing cytochalasin D (1 μg/mL), chlorpromazine (20 μg/mL), amiloride hydrochloride (10 μg/mL), nystatin (10 μg/mL), and folic acid (20 μg/mL) was added, while the control group received PBS without inhibitors. After 30 min, Cou 6-labeled Til formulations were added. After 2 h, fluorescence intensity was measured by FCM to assess uptake and distribution [61,62].
Caco-2 cells were seeded in 24-well plates and incubated with 100 ng/mL of Cou 6-labeled Til formulations for 4 h. The cells were then treated with fresh medium containing selective inhibitors—brefeldin A (25 μg/mL) [63], monensin (32.5 μg/mL) [64], and bafilomycin A1 (62.28 μg/mL) [65]—for 2 h to assess their effects on nanoparticle uptake. FCM was used for quantitative analysis, with untreated cells as the control.
2.5.4. Intracellular transport through the monolayer
Caco-2 cells (1 × 104 cells/well) were seeded onto the apical surface of Transwell 3462 membranes (Corning Costar, NY, USA). Fresh medium was added to both the apical and basolateral chambers to sustain optimal culture conditions. After 21 days, the cells formed a confluent monolayer [66].
Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs were added to the apical compartments at Til concentrations of 25, 50, and 100 μg to evaluate transcellular transport. At specified intervals, 50 μL samples were withdrawn from the basolateral compartments and replaced with fresh medium. Til and nanocarrier concentrations in the samples were quantified by high-performance liquid chromatography (HPLC).
2.6. The targeting of FA-Lipo@Til NCs
2.6.1. Macrophage targeting after transcellular monolayers in vitro
A Caco-2/RAW264.7 co-culture model was established. Caco-2 cells were cultured to form a monolayer (Section 2.5.4). RAW264.7 cells (1 × 105 cells/well) were seeded into the basolateral chamber and incubated for 24 h. To induce a specific response, LPS (100 ng/mL) and IFN-γ (20 ng/mL) were added, finalizing the co-culture model for subsequent experiments.
To assess the interaction between nanoparticles and RAW264.7 cells in the Caco-2/RAW264.7 co-culture model, the apical chamber was treated with Cou 6-labeled Til formulations (50 μg/mL) for 4 h. RAW264.7 cell coverslips were fixed with 300 μL of fixative for 20 min, then stained with 1 % DAPI for 10 min. The coverslips were inverted onto slides pre-treated with anti-fade reagent and analyzed using CLSM.
Next, Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs were introduced into the apical chamber at concentrations of 25, 50, and 100 μg/mL and incubated for 4 h. After the incubation, the culture medium was removed, and the cells were rinsed with 300 μL of purified water before vortexing. The RAW264.7 cells were then harvested, lysed using an ultrasonic cell disruptor for 1 min, and analyzed by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS, Agilent, USA) to quantify Til uptake.
2.6.2. Ex vivo fluorescent imaging and biodistribution analysis
The ApoE−/− mice (n = 3 per group) were randomly assigned to receive either saline, Til NCs/DiD, Lipo@Til NCs/DiD, or FA-Lipo@Til NCs/DiD (with DiD at 20 μg/mL, 200 μL) via oral gavage. After 8 h, their aortae along with visceral organs were collected for imaging, which was performed using the IVIS Lumina Series III system (PerkinElmer, Waltham, USA) [53].
2.7. Pharmacodynamic study in vitro and vivo
2.7.1. Pharmacodynamic study in vitro
2.7.1.1. Study of macrophage polarization
RAW264.7 cells were plated in 24-well plates and stimulated. After 24 h of treatment with Til formulations (10 μg/mL), cells were incubated with fluorochrome-conjugated antibodies and anti-mouse CD16/32 at 4 °C for 10 min. The cells were then stained with the M1 marker, CD86, for 30 min at 4 °C, followed by fixation and permeabilization. For M2 marker staining, cells were incubated with CD206 for 30 min at 4 °C [67]. After two washes with staining buffer, the cells were analyzed by FCM.
2.7.1.2. Study of macrophage efferocytosis
RAW264.7 cells were seeded in 24-well plates and stimulated. After 24 h of incubation with Til formulations (10 μg/mL) at 37 °C, the cells were stained with 3 μM CellTracker Dark Red dye, then harvested and washed. To induce apoptosis, untreated RAW264.7 cells were treated with 2 μM cyclosporine for 24 h, washed with serum-free DMEM, stained with 3 μM CellTracker Green dye for 30–40 min, and collected. Apoptotic cells and macrophages were co-cultured at a 1:3 ratio in 12-well plates for 4–6 h, then harvested and washed. FCM was used to analyze apoptotic cells and macrophages [68].
2.7.1.3. Anti-ROS study
RAW264.7 cells were plated in 24-well plates and stimulated. After 24 h of incubation with Til formulations (10 μg/mL) at 37 °C, the cells were rinsed and incubated with 20 μM dichlorodihydrofluorescein diacetate (DCFH-DA) in serum-free medium for 30 min. The cells were washed twice with staining buffer and collected for FCM analysis [69].
2.7.2. Pharmacodynamic study in vivo
2.7.2.1. Treatment of atherosclerosis ApoE−/− mice
The development of the atherosclerosis model is described in Section 2.2. Mice (n = 6 per group) were administered saline (Model), Crude Til, Til NCs, Lipo@Til NCs, or FA-Lipo@Til NCs (4 mg/kg) for 12 weeks on a HFD. Body weight was monitored biweekly. After an overnight fast with access to water, the mice were perfused with cold saline through the right atrium until clear outflow was achieved. Major organs were then excised, and the aorta and its branches were dissected. Tissues were either fixed or stored at −80 °C. A separate cohort of male C57BL/6J mice was used as healthy controls [68].
2.7.2.2. Histological analysis
The effectiveness of FA-Lipo@Til NCs in reducing atherosclerotic plaques was evaluated through histological and serum biochemical analyses. Following treatment, aortas and serum samples were collected from all mice. The samples were then sectioned. For morphometric analysis, sections were stained with hematoxylin and eosin as well as Oil Red O, and the total lesion and necrotic core sizes were measured. Histomorphological analysis was performed under a microscope. Serum levels were subsequently measured using an automated biochemical analysis system (Qiyang Intelligent Technology, China).
2.7.2.3. In vivo evaluation of the macrophage polarization and anti-ROS ability
The remaining aortic roots were paraffin-embedded, sectioned, and stained for macrophage markers. Immunohistochemical staining was performed for the phenotypic markers CD206 (M2), CD86 (M1), and CD68 (macrophage) following incubation. In parallel, the aortic roots were cryosectioned. Dihydroethidium (DHE) staining was used for immunofluorescent detection of ROS, providing insights into the potential mechanism for atherosclerotic plaque reduction following treatment. Additionally, serum cytokine concentrations were quantified using ELISA kits, following the manufacturer's protocols.
2.8. Assessment of in vivo safety
Atherosclerotic ApoE−/− mice were treated for 12 weeks as described in Section 2.7.2.1, with body weight monitored bi-weekly. At the end of the treatment, after the final dose, the mice were euthanized, and blood and key organs were collected. Tissue samples were stained with hematoxylin and eosin (H&E), and routine blood tests and biochemical analyses were performed using a Roche Cobas C501 platform (Roche, Switzerland).
2.9. Analytical methods
Til quantification was conducted using a Waters e2695 HPLC system (Waters, Shanghai, China), with detection at 323 nm. Separation was performed on a Reliasil C18 column (4.6 mm × 250 mm, 5 μm), with a mobile phase consisting of 25 % acetonitrile and 75 % 0.5 % formic acid. The column temperature was maintained at 40 °C, and the flow rate was set at 1.0 mL/min, in accordance with established protocols [24].
Til quantification was performed using an AB Sciex QTRAP 5500 system, which integrates an Exion LC HPLC system with a triple quadrupole mass spectrometer (Sciex) [38,70,71]. The HPLC system was equipped with BD pumps, an autosampler, a degasser, a column oven, and a controller, while the mass spectrometer utilized an electrospray ionization source. Til separation was achieved on a Zorbax SB-AqC18 column (4.6 mm × 250 mm, 5 μm), with detection at 323 nm. The mobile phase consisted of 0.1 % formic acid aqueous solution (A) and acetonitrile (B), with a flow rate of 1.0 mL/min. The column was maintained at ambient temperature, and a 10 μL injection volume was used. The mass spectrometer settings and MRM transitions for Til quantification are detailed in Table S1 and Table S2. Data analysis was conducted using Analyst software.
2.10. Statistical analysis
Statistical analysis was performed using Origin Software (2023, Redwood, EA, USA). Data are presented as mean ± standard deviation (SD). Differences between two groups were assessed using a two-tailed Student's t-test, with statistical significance set at p < 0.05.
3. Results and discussion
3.1. Characterization and mucus layer permeability study of FA-Lipo@Til NCs
The morphology of Til NCs was needle-like as characterized previously [31] and freshly prepared (Fig. 1A). Since Til NCs had a positive surface charge of 10.41 ± 0.48 mV [31] after encapsulated with the lipid layer, it presented a more smooth and glossy surface, indicating a successful coating (Fig. 1B). In parallel, FTIR analysis was performed to investigate the functional group composition and structural evolution during the synthesis of FA-Lipo@Til NCs. As shown in Fig. S1, significant alterations in characteristic peaks were observed throughout the fabrication process. The distinctive absorption bands of Til NCs, including the phenolic O-H stretching (3422 cm−1), carbonyl C=O vibration (1660 cm−1), and aromatic C=C stretching (1606-1497 cm−1), underwent noticeable shifts upon incorporation of PVA/TPGS. The emergence of new peaks at 2907 cm−1 (aliphatic C-H stretching) and 1733 cm−1 (ester C=O stretching), along with the red-shift of hydroxyl peak to 3304 cm−1, confirmed successful blending and enhanced aqueous dispersibility through hydrogen bonding. Subsequent phospholipid modification was evidenced by intensified peaks at 2917/2849 cm−1 (phospholipid alkyl chains) and 1086 cm−1 (P-O stretching), accompanied by hydrogen bond reorganization (3364 cm−1) and strengthened hydrophobic interactions (1625/1466 cm−1). Although FA conjugation caused minimal spectral changes due to its low content, the enhanced intensity at 1733/1090 cm−1 (C=O/C-O) and disappearance of certain aromatic peaks demonstrated its successful incorporation via non-covalent interactions, ultimately yielding the stable FA-Lipo@Til NCs composite system.
Fig. 1.
Characterization and mucus layer permeability study of FA-Lipo@Til NCs. (A, B) TEM images of Til NCs and FA-Lipo@Til NCs, with a scale bar of 50 nm. (C) The turbidity changes of the mucin-nanoparticle mixture over time were examined. (D) A schematic diagram showing the method used to assess nanoparticle penetration through the agarose gel layer and the Transwell system. (E) The permeability of nanoparticles was evaluated using the agarose gel layer. (F-G) The cumulative release curves and Papp values of different nanoparticles were evaluated using the Transwell system. Data are presented as mean ± standard deviation (n = 3), and statistical analysis was performed using one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns indicates no significant difference.
Similarly, in our prior study, we successfully developed FA-Lipo@Til NCs. This nanocarrier system was designed to overcome Til's poor aqueous solubility and reduce gastrointestinal toxicity associated with enterohepatic circulation. The resulting FA-Lipo@Til NCs exhibited favorable physicochemical characteristics, including a uniform particle size (∼140 nm), neutral surface charge, and high encapsulation efficiency [72]. Notably, FA-Lipo@Til NCs demonstrated substantial controlled release properties. In comparison to Til NCs, Til release from FA-Lipo@Til NCs was markedly slower and more sustained, especially in Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF). This controlled release behavior is primarily attributed to the phospholipid bilayer, which serves as an effective barrier, modulating Til release and preventing premature diffusion of the drug. In more complex media, such as SGF and SIF, Til NCs demonstrated a significantly enhanced release profile, with the nanocrystals exhibiting improved solubility due to their reduced particle size and increased surface area. Furthermore, the FA modification had a negligible effect on the release profile, suggesting that the formulation remains stable and capable of delivering a sustained, controlled drug release throughout the gastrointestinal tract. These findings have been conclusively demonstrated in our previous research [72].
The interaction between mucin and a variety of nanoparticles was systematically assessed using the turbidimetric technique [73]. As illustrated in Fig. 1C, the turbidity of each formulation exhibited a rapid increase within the first 0.5 h, followed by a plateau, suggesting that equilibrium had been established between the nanoparticles and the mucus fibers. Notably, Til NCs demonstrated a turbidity value of ΔA = 0.104, indicative of a more pronounced interaction with the mucus fibers. These findings suggest that the electrostatic interaction between Til NCs and mucin plays a pivotal role in facilitating this enhanced interaction, resulting in the aggregation of nanoparticles within the mucus matrix. In contrast, the turbidities of Lipo@Til NCs and FA-Lipo@Til NCs were recorded at 0.073 and 0.052, respectively, both of which were notably lower than that of Til NCs. The observed lower turbidity values suggest a reduced interaction with the mucus fibers, potentially attributed to the protective roles of the lipid bilayer and PEG modification. It is proposed that the lipid bilayer and PEG modifications serve to attenuate the electrostatic interactions between mucin and Til NCs via steric hindrance, thereby facilitating the enhanced ability of nanoparticles to traverse the mucus layer [74,75]. These findings underscore the crucial role of surface modifications in regulating the interaction between nanoparticles and mucus, a factor critical to optimizing the efficiency of drug delivery.
Then, the permeability of FA-Lipo@Til NCs was investigated using agarose gel layers and mucus solution sourced from porcine stomach (Fig. 1D). As shown in Fig. 1E, in the agarose gel-layer model, the penetration rates of Lipo@Til NCs (38.73 ± 3.4 %) and FA-Lipo@Til NCs (38.62 ± 1.8 %) were significantly higher than that of Til NCs (26.16 ± 4.6 %) (p < 0.001). Furthermore, in mucus solution model, the accumulation of each formulation increased progressively over time (Fig. 1F). FA-Lipo@Til NCs and Lipo@Til NCs showed higher accumulation amounts. The Papp values of FA-Lipo@Til NCs and Lipo@Til NCs were (6.09 ± 0.8) × 10−6 cm/s and (5.52 ± 0.9) × 10−6 cm/s, which were 1.3- and 1.2- fold compared to Til NCs ((4.66 ± 0.8) × 10−6 cm/s) (Fig. 1G). These findings suggested that FA-Lipo@Til NCs exhibited superior permeability through the mucus layer.
3.2. Cellular uptake and mechanism study
The epithelial layer plays a pivotal role in oral drug administration, acting as the primary barrier to drug absorption [[76], [77], [78]]. In order to more accurately simulate and investigate this physiological process, Caco-2 cells were employed in the present study. These cells are capable of forming a monolayer that closely mimics the intestinal epithelium in vitro, and are extensively utilized in studies investigating oral drug absorption. As depicted in Fig. 2A, after incubation for 2 h, FA-Lipo@Til NCs/Cou 6 showed distinct green fluorescence, revealing the highest absorption. Moreover, FA-Lipo@Til NCs exhibited a significantly higher mean fluorescence intensity (MFI) than both Lipo@Til NCs and Til NCs (p < 0.05), as quantified by FCM (Fig. 2B and C). To evaluate the uptake of FA-Lipo@Til NCs/Cou 6 in RAW264.7 cells, and confocal laser scanning microscopy (CLSM) was employed to observe the internalization of the nanoparticles. From Fig. S2A, FA-Lipo@Til NCs demonstrated the most intense green fluorescence, indicating the highest uptake efficiency among the formulations tested. Conversely, Til NCs and Lipo@Til NCs displayed weaker fluorescence signals, suggesting reduced uptake efficiency. Subsequently, the quantitative analysis was conducted using flow cytometer (FCM). The results corroborated the CLSM findings (Fig. S1B–C), and FA-Lipo@Til NCs had a 1.28 and 1.54 higher cellular uptake than Til NCs and Lipo@Til NCs. This increased uptake can likely be attributed to the up-regulation of FRs on the surface of the macrophages membrane following lipopolysaccharide (LPS) and interferon-γ (IFN-γ) stimulation, enabling FA-Lipo@Til NCs to more efficiently enter cells via receptor-mediated endocytosis [20].
Fig. 2.
Cellular uptake and mechanism study. (A) Fluorescence microscopy images of Caco-2 cells following a 2-h incubation with various nanoparticles. Green: Cou 6-labeled formulations. Blue: Cell nuclei were stained with DAPI. Scale bars = 20 μm. (B, C) Representative FCM histograms and quantified MFI data for the cellular uptake in Caco-2 cells. (D-F) Relative uptake of the Cou 6-labeled formulations by Caco-2 cells following the addition of an endocytosis inhibitor. Data are shown as the mean ± S.D. (n = 3) and were analyzed by one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, a targeted inhibitory approach was employed to investigate the internalization mechanism of the formulations. Specifically, actin-mediated endocytosis was inhibited using cytochalasin D, while clathrin-mediated endocytosis was blocked with chlorpromazine. Nystatin was administered to inhibit caveolin-mediated endocytosis, amiloride hydrochloride was used to block macropinocytosis-induced phagocytosis, and folate was introduced to inhibit FR-mediated pathways [79]. As shown in Fig. 2D–F, the uptake of Til NCs, Lipo@Til NCs and FA-Lipo@Til NCs underwent an energy-dependent pathway with the implication of reduced internalization at 4 °C. Apart from this, the uptake of Til NCs (Fig. 2D) was significantly reduced (p < 0.001) following treatment with cytochalasin D, suggesting actin-mediated processes. Besides, both clathrin-mediated endocytosis and macropinocytosis (p < 0.05) were similarly linked to the uptake of Til NCs/Cou 6. As illustrated in Fig. 2E, treatment with cytochalasin D (p < 0.01) and nystatin (p < 0.001) significantly reduced the uptake of Lipo@Til NCs, suggesting the involvement of actin-mediated and caveolin-dependent endocytic pathways (Fig. 2E). Caveolin-mediated endocytosis predominantly governs the internalization of lipid-based materials [80], a process that circumvents the lysosomal degradation pathway [81]. Moreover, the enhanced compatibility between the lipid bilayer and the cell membrane contributes to a significantly higher uptake of Lipo@Til NCs relative to Til NCs. For FA-Lipo@Til NCs, except for cytochalasin D (p < 0.05) and nystatin (p < 0.001), folic acid significantly (p < 0.0001) inhibited the internalization of FA-Lipo@Til NCs, revealing actin-mediated, caveolin-dependent and FR-mediated absorption (Fig. 2F). These results indicated that the cellular uptake advantage of FA-Lipo@Til NCs is primarily due to the modification of the folate ligand and the encapsulation by the lipid bilayer.
3.3. Transmembrane transport study
To effectively target atherosclerotic plaques, the nanoparticles must traverse intestinal barriers to reach the target site. Thus, the transmembrane transport capacity was studied using an in situ intestinal loop model [82]. N-acetylcysteine (NAC) was administered to relax the mucus of the intestinal loop samples as presented in Fig. 3A. After treatment, the slices of the samples were observed by CLSM. As illustrated in Fig. 3B and C, FA-Lipo@Til NCs/Cou 6 showed superior intestinal absorption with or without NAC treatment, indicating its excellent mucus penetration ability and cellular uptake capacity. What's more, for Til NCs/Cou 6, the absorption amount in pre-treatment group was 1.71- and 1.38-fold higher than in post-treatment group and untreated group. This phenomenon can primarily be attributed to the strong electrostatic interactions between the positively charged Til NCs and mucin, which hindered their ability to penetrate the mucus layer [[83], [84], [85]]. These results align with the findings from the previously discussed study on the mucus layer permeability of FA-Lipo@Til NCs. However, upon removal of the mucus pre-incubation, Til NCs/Cou 6 exhibited the highest internalization. This may be attributed to the positive charge of Til NCs/Cou 6 that promoted cellular uptake. The charge of Lipo@Til NCs and FA-Lipo@Til NCs is neutralized by the lipid bilayer encapsulation, thereby enhancing the affinity of these particles for mucus and intestinal absorption. Additionally, the incorporation of folate subsequently increased the transmembrane efficiency of FA-Lipo@Til NCs.
Fig. 3.
Transmembrane transport study. (A) A schematic of research on the interaction between Til NCs/Cou 6, Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs/Cou 6 and mucus layer by removing mucus in the rat in situ intestine ring model. (B) Fluorescence imaging of Til NCs/Cou 6, Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs/Cou 6 uptaken in intestinal sections after removing mucus in the rat in situ intestine ring model. Green: Cou 6-labeled different formulations, including Til NCs/Cou 6, Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs/Cou 6. Blue: cell nucei were stained with DAPI. Scale bars = 500 μm. (C) Quantitative fluorescence intensity data for CLSM. (D) Scheme for investigating the transepithelial transport behavior of the different nanoparticles across the Caco-2 cell monolayer. (E) Time-dependent transmembrane transport profiles of different formulations containing various Til concentrations on a Caco-2 monolayer. (F) Papp values of different formulations containing various Til concentrations on a Caco-2 monolayer. (G-I) Effects of three inhibitors on the exocytosis of Caco-2 cells on Til NCs/Cou 6, Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs/Cou 6. Data are shown as the mean ± S.D. (n = 3) and were analyzed by one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 and ns denotes no significance. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Further, the transcytosis of FA-Lipo@Til NCs was investigated using the Transwell system [86]. Caco-2 cell monolayers with transepithelial electrical resistance (TEER) values exceeding 500 Ω cm2 were used in the study. The transmembrane transport efficiency of Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs was evaluated over a 4-h period (Fig. 3D). The results (Fig. 3E) indicated that Til accumulation was concentration-dependent, progressively increasing with the dose ranging from 25 μg to 100 μg showing a consistent trend. Til NCs were transported to the basolateral side at a rate three times higher after 4 h compared to Crude Til at the dose of 100 μg. Besides, the cumulative amounts of Lipo@Til NCs and FA-Lipo@Til NCs were 1.62- and 1.84-fold higher than that of Crude Til. Then,the Papp was calculated to evaluate the rate at which they traverse the Caco-2 monolayer. When dosed at 100 μg, FA-Lipo@Til NCs exhibited superior transport capacity across the cell monolayer, with Papp values 1.84-fold and 5.54-fold greater than those of Til NCs and Crude Til respectively (Fig. 3F), indicating that the lipid bilayer coating and FA-modification facilitated not only improved cell internalization but also the transportation efficiency. Moreover, the TEER values were stable throughout the duration of the experiment (Fig. S3), effectively ruling out paracellular transport and reinforcing the notion that the transcellular route served as the primary mechanism for all formulations.
To investigate the exocytosis pathway of FA-Lipo@Til NCs, the specific inhibitors: bafilomycin A1 (62.28 μg/mL) [63], monensin (32.5 μg/mL) [64] and brefeldin A (25 μg/mL) [87] were employed to block lysosomal function, Golgi activity, and endoplasmic reticulum (ER)-to-Golgi transport pathway, respectively. As demonstrated in Fig. 3G, bafilomycin A1 significantly inhibited the exocytosis of Til NCs/Cou 6 (P < 0.001), highlighted its critical role in this process. Additionally, Fig. 3H and I reveal that Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs/Cou 6 displayed a more pronounced inhibition of exocytosis when treated with monensin and brefeldin A, compared to Til NCs/Cou 6. These findings suggest that Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs/Cou 6 are more frequently trafficked from the ER to the Golgi complex. This may be due to the outer lipid bilayer coating, which allowed for better fusion and transmission between the membrane-bound organelles [88]. Whereas Til NCs/Cou 6 tend to undergo more frequent vesicle reactivation and re-transport after cellular entry.
3.4. Plaque-targeting study
To assess the targeting efficiency of FA-Lipo@Til NCs toward macrophages within plaques after crossing the intestinal mucosal barrier, a Transwell system was established. A schematic diagram of the Caco-2/RAW264.7 co-culture model is shown in Fig. 4A. Fig. 4B and C illustrated the cellular internalization of RAW264.7 cells after the formulations were transport through the Caco-2 monolayer. From the results, after 4 h incubation, FA-Lipo@Til NCs/Cou 6 and Lipo@Til NCs/Cou 6 exhibited pronounced green fluorescence and higher cellular uptake compared to Til NCs/Cou 6, suggesting distinct transcytosis and macrophage uptake capacity. In addition, FA-Lipo@Til NCs/Cou 6 showed an enhanced intracellular level of Til compared to Lipo@Til NCs/Cou 6 across various dose gradients (25 μg, p < 0.001; 50 μg, p < 0.01; 100 μg, p < 0.01). In the inflammatory microenvironment of the plaque, macrophages are predominantly of the M1 phenotype, which express FR [[19], [20], [21], [22],89]. RAW264.7 cells are a commercially used in vitro macrophage model that differentiate into the M1 phenotype under the stimulation of LPS and IFN-γ, and also highly express FRs. Therefore, RAW264.7 cells are commonly used to evaluate the targeted uptake of FA-modified nanoparticles. Moreover, in this study, FA-Lipo@Til NCs/Cou 6 demonstrated excellent potential for enhancing transcytosis in the small intestine and targeting inflammatory macrophages.
Fig. 4.
Plaque-targeting study. (A) Schematic representation of uptake in RAW264.7 targeted across monolayer cell membranes by CLSM observation. (B) CLSM was used to qualitatively detect the uptake of different preparations across the monolayer cell membrane targeting RAW264.7. Green: Cou 6-labeled different formulations, including Til NCs/Cou 6, Lipo@Til NCs/Cou 6 and FA-Lipo@Til NCs. Blue: cell nucei were stained with DAPI. Scale bars = 20 μm. (C) The amount of Til (25, 50 and 100 μg) taken up by RAW264.7 after crossing the monolayer cell membrane within 4 h of different formulations of Til. (D) Representative ex vivo fluorescence images and (E) quantitative results illustrating the distribution of FA-Lipo@Til NCs/DiD in aortas harvested from plaque-bearing ApoE−/− mice 8 h after oral administration. (F) Ex vivo fluorescence images of hearts and aortas in atherosclerotic mice. (G) The fluorescence images of liver, spleen, lung and kidney organs from plaquebearing ApoE−/− mice. Data are shown as the mean ± S.D. (n = 3) and were analyzed by one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 and ns denotes no significance. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The capacity of FA-Lipo@Til NCs to target atherosclerotic plaques in apolipoprotein E deficient (ApoE−/−) mice following oral administration was also evaluated. Specifically, owing to its specific binding to macrophage-overexpressing FR, FA-Lipo@Til NCs/DiD exhibited greater accumulation in atherosclerotic plaques, including the aortic root, aortic arch, and abdominal aorta, than Lipo@Til NCs/DiD (Fig. 4D and E). In alignment with in vitro findings, these results suggested that FA-Lipo@Til NCs possess potent plaque-targeting capabilities in vivo after oral gavage. Additionally, the gastrointestinal absorption profiles of different formulations in mice were investigated (Fig. S4). At 8 h post-oral administration, FA-Lipo@Til NCs were almost completely cleared from the stomach and exhibited minimal distribution throughout the intestinal tract, indicating their superior trans-mucosal absorption efficiency. In contrast, the Til NCs group displayed significant residual fluorescence signals in the stomach, ileum, cecum, and colon, suggesting prolonged retention in various gastrointestinal segments. These observations further support that the positively charged surface of Til NCs leads to their entrapment within the gastric mucosa and intestinal mucus layer, thereby impeding efficient absorption. In other organs, all formulations exhibited distribution across various tissues, with the liver showing the highest accumulation (Fig. 4F and G). Notably, FA-Lipo@Til NCs/DiD significantly reduced the accumulation of Til NCs/DiD in the liver. This may be due to the encapsulation by the lipid bilayer, which reduced protein adsorption and enhanced the anti-phagocytic activity of the nanoparticles. At the same time, the modification with FA also increased the targeted accumulation of Til at the plaque site.
3.5. Effect on macrophage functions in vitro
3.5.1. Macrophage phenotypic transition inducing M1 to M2 repolarization
Macrophages play a pivotal role in the pathogenesis of atherosclerosis, orchestrating both the onset and progression of the disease [[90], [91], [92]]. Classically activated M1 macrophages primarily mediate antigen presentation and drive pro-inflammatory immune responses [93], while alternatively activated M2 macrophages facilitate the resolution of inflammation and promote tissue repair. An increasing body of evidence indicates that an elevated CD86+/CD206+ ratio is closely associated with the exacerbation of atherosclerotic lesions [[94], [95], [96]]. Accordingly, therapeutic strategies that modulate macrophage polarization hold substantial promise for restoring immune homeostasis within atherosclerotic plaques and mitigating chronic vascular inflammation [97,98].
To investigate this, we quantitatively analyzed the proportions of M1 and M2 macrophages, as well as the M1/M2 ratio, using FCM. The gating strategy is depicted in Fig. 5A, and the corresponding quantitative results are presented in Fig. 5(B–E). In the model group, CD86+ macrophages constituted 64.59 ± 12.05 % of the total population, whereas CD206+ macrophages accounted for only 7.14 ± 0.23 %, reflecting a pronounced skew toward a pro-inflammatory M1 phenotype (Fig. 5B). Notably, among the four treatment groups, only FA-Lipo@Til NCs induced a statistically significant reduction in CD86+ macrophages (p < 0.01), while the others showed no appreciable change (Fig. 5C). This selective downregulation is likely attributable to folate receptor (FR-β)-mediated endocytosis, given the high expression of FR-β on activated M1 macrophages. This receptor-targeted delivery likely enhanced intracellular Til accumulation, potentiating its local anti-inflammatory effects and consequently downregulating M1-associated markers. n contrast, all treatment groups promoted a significant upregulation of CD206+ macrophages, indicative of enhanced M2 polarization, with the FA-Lipo@Til NCs group showing the most pronounced effect (p < 0.001) (Fig. 5D). Consistent with these observations, the M1/M2 ratio declined across all treatment groups, reflecting a progressive shift towards an anti-inflammatory phenotype—most notably in the FA-Lipo@Til NCs group (Fig. 5E).
Fig. 5.
Pharmacodynamic study of FA-Lipo@Til NCs in vitro. (A) Gating strategy used for analysis of the M1-like and M2-like macrophages expression in RAW264.7 macrophages. (B) Representative FCM histograms and quantitative analysis of (C) CD86+, (D) CD206+ and (E) CD86+/CD206+ expression in the RAW264.7 macrophages treated with Crude Til, Til NCs, Lipo@Til NCs and FA-Lipo@Til NCs. (F) FCM results depicting the efferocytosis activity of macrophages after exposure to FA-Lipo@Til NCs. (G) Quantitative assessment of efferocytosis following FA-Lipo@Til NCs treatment. (H) Representative flow cytometry histograms and (I) quantitative analysis of intracellular ROS generation. (Data are shown as the mean ± S.D. (n = 3) and were analyzed by one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 and ns denotes no significance.
3.5.2. Macrophage efferocytosis study
Apoptotic cells (ACs) are known to preferentially accumulate within the necrotic core of atherosclerotic plaques, where they contribute significantly to the destabilization of the plaque and increase the likelihood of rupture. The presence of a high number of ACs in this region can exacerbate inflammatory responses, leading to further plaque vulnerability. Consequently, the efficient clearance of ACs by macrophages becomes a critical process in maintaining the structural integrity and homeostasis of atherosclerotic plaques. This clearance is not only essential for limiting the inflammatory burden but also for preventing the progression of plaque instability. Macrophage polarization plays a central role in modulating this process, as the functional state of macrophages, influenced by their polarization towards either pro-inflammatory M1 or anti-inflammatory M2 phenotypes, directly impacts their capacity to phagocytize ACs and regulate plaque health [99]. To investigate this process, we examined macrophage efferocytosis following treatment with different formulations. LPS- and IFN-γ-activated RAW264.7 cells were utilized as the model for phagocytic macrophages, while cyclosporin A-treated RAW264.7 cells were employed to simulate ACs. These two cell populations were co-cultured and subjected to treatment with all test formulations, enabling us to assess the effects on efferocytosis. As shown in Fig. 5F and G, the phagocytic macrophages exhibited a limited capacity for AC engulfment in the absence of Til treatment, accounting for only (13.75 ± 0.21)% of their total efferocytosis activity. An enhancement of phagocytic capacity was observed when treated with Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs, with FA-Lipo@Til NCs demonstrating the highest phagocytic activity and efferocytosis effect (p < 0.001).
3.5.3. ROS scavenging capability
The generation of ROS is a key factor in driving the inflammatory processes associated with atherosclerosis. Studies have demonstrated that FA-Lipo@Til NCs can efficiently scavenge ROS upon stimulation with LPS and IFN-γ. To assess intracellular ROS levels, the fluorescent probe dichlorodihydrofluorescein diacetate (DCFH-DA) was employed. Upon cellular uptake, DCFH-DA is hydrolyzed by intracellular esterases to form DCFH. The presence of ROS within the cell then induces oxidation of DCFH to dichlorodihydrofluorescein (DCF), which generates a green fluorescence signal. In contrast, untreated (unstimulated) cells exhibit minimal fluorescence, reflecting the lack of substantial ROS production [100].
As shown in Fig. 5H and I, the ROS levels in the model group were approximately 8 times higher than those in the control group. After treatment, the ROS scavenging activity followed the order: FA-Lipo@Til NCs > Lipo@Til NCs > Til NCs > Crude Til (Fig. 5I). Among these, FA-Lipo@Til NCs demonstrated the most significant reduction in ROS levels, indicating its potential to effectively inhibit excessive ROS production (p < 0.001). In summary, FA-Lipo@Til NCs, by modulating macrophage function, show significant potential as a promising therapeutic candidate for atherosclerosis.
3.6. In vivo anti-atherosclerosis activity
To evaluate the therapeutic potential of FA-Lipo@Til NCs, ApoE−/− mice were subjected to a high-fat diet (HFD) for a duration of 12 weeks, promoting the formation of atherosclerotic plaques [53]. Upon plaque development, the mice were randomly allocated into five distinct treatment groups. Each group received daily oral doses of either saline, Crude Til, Til NCs, Lipo@Til NCs, or FA-Lipo@Til NCs (4 mg/kg). The modeling and dosing regimen is shown in Fig. 6A. Fig. 6B and D displayed oil red O (ORO) staining pictures and quantitative analysis results of the entire aortas from each group and the distinct atherosclerotic plaques manifested as red patches. In the model group, the average plaque area accounted for 51.53 %, indicating the atherosclerotic disease was successfully induced. Following intragastric administration of Crude Til, a significant reduction in plaque area was observed (p < 0.05). In comparison, the average aortic plaque area was reduced by approximately 28 % with Til NCs, accounting for 37.19 % of the total area compared to model group. The plaque areas were further diminished to 30.90 % and 25.46 % after administration of Lipo@Til NCs and FA-Lipo@Til NCs, respectively, demonstrating a more pronounced therapeutic effect. Specifically, FA-Lipo@Til NCs exhibited a superior effect relative to Til NCs, resulting in a 2-fold decrease compared to model group. These findings suggest that FA-Lipo@Til NCs possess a noteworthy efficacy in the treatment of atherosclerosis.
Fig. 6.
Antiatherosclerotic activity of FA-Lipo@Til NCs in ApoE−/− mice. (A) Schematic of the treatment protocol for the study. (B) ORO-stained aorta images from C57BL/6J control mice and ApoE−/− mice subjected to different treatments. Scale bar = 3 mm. (C) Representative micrographs of aortic roots stained with ORO, H&E, and Masson's trichrome from C57BL/6J (healthy) and ApoE−/− mice following administration of the specified formulations. Scale bar = 200 μm. (D) Quantification of ORO-positive regions across the entire aortic tissue. (E-G) Measurement of ORO-positive areas, necrotic core size, and collagen content relative to plaque area in aortic root cross-sections from ApoE−/− mice treated with different formulations. Data are shown as the mean ± S.D. (n = 6) and were analyzed by one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 and ns denotes no significance.
To comprehensively assess the extent of the lesions, histological analysis was conducted on the cross-sections of the aortic roots (Fig. 6C). Fig. 6E–G and Fig. S5 represent the percentages and absolute areas of the detected samples. A quantitative assessment of ORO staining in the aortic root was presented in Fig. 6E and S3A. The average plaque area in the aortic root of the model was approximately 45.83 %. After treatment, the average plaque area in the aortic root was reduced to 36.50 % (p < 0.05), 31.00 % (p < 0.0001), and 26.83 % (p < 0.0001) for the Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs groups, respectively. The results indicated that FA-Lipo@Til NCs had the strongest effect in inhibiting the enlargement of atherosclerotic plaques (p < 0.0001).
Unstable plaques are prone to dislodgment, potentially accumulating in the arterial lumen, where they may precipitate thrombosis and pose significant health risks. Consequently, to evaluate the stability of the atherosclerotic plaques, H&E and Masson staining techniques were employed to characterize the necrotic core and collagen. As shown in Fig. 6F and Fig. S5B, the model group showed a significant number of clear areas devoid of H&E staining, identified as the necrotic regions (marked with red arrows). These necrotic areas made up about 26.50 % of the total area, showing a large necrotic zone with many cholesterol crystals. After administration of Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs, the areas of the necrotic core (indicated by red arrows) decreased to 23.66 % (p < 0.05), 23.17 % (p < 0.05), 19.50 % (p < 0.001), and 16.33 % (p < 0.001) for each respective group. FA-Lipo@Til NCs had the strongest effect on stabilizing the necrotic core.
Masson staining was also employed to further assess the collagen content (depicted in blue) within the plaque area (Fig. 6G and Fig. S5C). From the results, the collagen content in the model group (12.00 %) was significantly lower than the control group (15.83 %). The Crude Til group and the Til NCs group showed little changes compared to control group. This lack of increase may be attributed to the rapid metabolism of the Til NCs in the gastrointestinal tract, as well as the limited solubility of Crude Til [70,71,101]. The collagen content in the Lipo@Til NCs group and the FA-Lipo@Til NCs group were significantly increased (p < 0.001, p < 0.0001). Concurrently, the FA-Lipo@Til NCs demonstrated a markedly higher collagen concentration (p < 0.05) than Lipo@Til NCs. These findings indicated that FA-Lipo@Til NCs possessed the notable capability to stabilize atherosclerotic plaques and effectively inhibit the progression of atherosclerosis.
3.7. Evaluation of macrophage polarization and anti-ROS ability in vivo
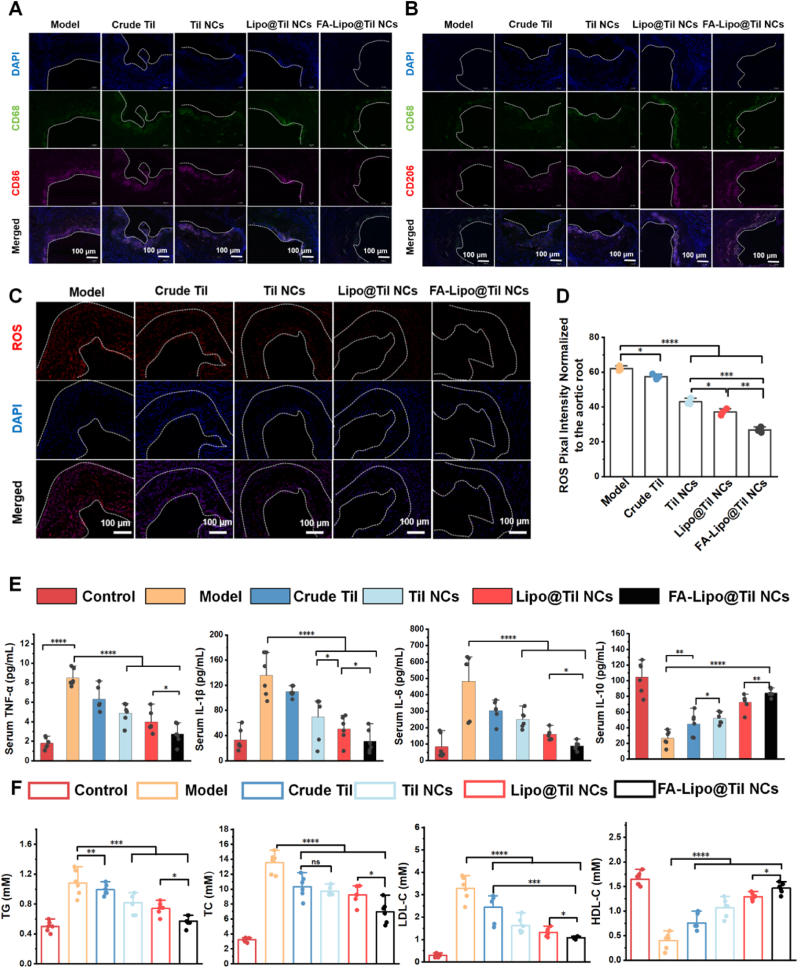
Immunofluorescent staining was employed to examine macrophage polarization-related biomarkers in aortic root sections derived from ApoE−/− mice following various therapeutic interventions [102]. As shown in Fig. 7A and B, different treatments resulted in a significant decrease in CD86 expression (Fig. 7A), with CD86 being a marker associated with macrophage activation and pro-inflammatory responses. The reduction in CD86 fluorescence intensity suggests that these formulations may have the potential to modulate immune responses in the aortic root, thereby playing a role in alleviating inflammation in atherosclerosis. This finding indicates that these treatments could have therapeutic implications for regulating macrophage-mediated inflammation and potentially mitigating the progression of atherosclerotic lesions. In contrast, a progressive increase in CD206 fluorescence intensity was observed correspondingly (Fig. 7B), with the FA-Lipo@Til NCs exhibiting the most pronounced effect. The results corroborated the previously obtained in vitro findings (as depicted in Fig. 5A–E).
Fig. 7.
The ability of FA-Lipo@Til NCs to regulate macrophage polarization in atherosclerotic plaques. Representative immunofluorescence images displaying the macrophage marker CD68 (green), (A) the M1 marker CD86 (red), (B) the M2 marker CD206 (red) and DAPI (blue) for nuclear staining. Scale bar = 100 μm. (C, D) Representative sections stained with DHE (red) and DAPI (blue), accompanied by quantitative analysis of aortic roots from ApoE−/− mice with atherosclerosis, treated with various formulations (saline, Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs). Scale bar = 200 μm. (E) Serum levels in ApoE−/− mice post-treatment with different formulations. (F) Serum concentrations in ApoE−/− mice after various treatments. Data are shown as the mean ± S.D. (n = 6) and were analyzed by one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 and ns denotes no significance. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
DHE staining was used to assess the ROS levels in aortic tissue, and the results indicated a significant increase in oxidative stress in the model group, as shown in Fig. 7C and D. In contrast, in the treatment groups (Crude Til, Til NCs, Lipo@Til NCs, and FA-Lipo@Til NCs), a marked reduction in red fluorescence was observed in the aortic root plaques compared to the model group (FA-Lipo@Til NCs > Lipo@Til NCs > Til NCs > Crude Til), indicating a decrease in ROS levels. This trend may be attributed to the FA modification, which enhanced the accumulation of Til in the aortic root plaques and promoted macrophage polarization toward the M2 phenotype, thereby effectively inhibiting ROS production.
3.8. Inflammatory cytokines and blood lipid study
Atherosclerosis is closely linked to vascular inflammation and dyslipidemia, both crucial factors in its progression [103]. To evaluate the effects of treatment on inflammatory cytokine levels, we measured key cytokines. As shown in Fig. 7E, the model group exhibited a significant increase in tumor necrosis factor (TNF)-α (8.17 pg/mL), IL-1β (150.48 pg/mL), and interleukin (IL)-6 (585.89 pg/mL), indicating a pronounced inflammatory response. In contrast, IL-10 levels were markedly reduced to 34.12 pg/mL, suggesting a decrease in anti-inflammatory cytokines. These findings confirm the successful establishment of the atherosclerosis model with a robust inflammatory response. Following treatment, the FA-Lipo@Til NCs group demonstrated significantly lower levels of IL-6, TNF-α, and IL-1β, while IL-10 concentrations were notably higher, signaling a reduction in inflammation. This indicates that FA-Lipo@Til NCs effectively modulate immune responses, reduce pro-inflammatory cytokine production, and boost anti-inflammatory cytokine levels. As shown in Fig. 7F, the model group exhibited a significant rise in blood total cholesterol (TG), triglycerides (TC), and low density lipoprotein cholesterol (LDL-C) levels, compared to the control group (p < 0.0001), along with a marked decrease in HDL-C. After treatment, the FA-Lipo@Til NCs group showed the most substantial reduction in TG, TC, and LDL-C levels among all treatment groups (Crude Til, Til NCs, and Lipo@Til NCs), while high density lipoprotein cholesterol (HDL-C) levels were significantly increased. These findings highlight the superior lipid-lowering effect of FA-Lipo@Til NCs, suggesting that Til encapsulated in FA-modified liposomes enhances its lipid-regulating therapeutic efficacy. Collectively, these findings indicate that FA-Lipo@Til NCs can effectively regulate macrophage polarization towards the anti-inflammatory phenotype, promote an anti-inflammatory environment within the aorta, and subsequently reduce blood cholesterol levels in ApoE−/− mice predisposed to atherosclerosis.
3.9. In vitro and In vivo safety evalution of FA-Lipo@Til NCs
Cytotoxicity assay was employed to evaluate the toxicity of FA-Lipo@Til NCs in vitro. As shown in Fig. S6, FA-Lipo@Til NCs exhibited no significant cytotoxic effects on RAW264.7 and Caco-2 cell lines.
The long-term safety of FA-Lipo@Til NCs in treating atherosclerosis was also evaluated. Throughout a 12-week gavage period, the body weight of the mice exhibited a consistent trend, with no significant variations observed among the treatment groups (Fig. 8A). Furthermore, no notable differences were detected among the groups regarding key organ indices, including those of the liver, heart, spleen, lungs, and kidneys (Fig. 8B). Histopathological examination using H&E staining further confirmed the absence of significant organ damage in animals treated with FA-Lipo@Til NCs (Fig. 8C). Following treatment with FA-Lipo@Til NCs, serum markers indicative of liver and kidney function—such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and and creatinine (CRE) were consistent with those observed in the saline-treated control group and remained well within the normal physiological range (Fig. 8D). In addition, comprehensive blood analyses were conducted, and as depicted in Fig. 8E, the levels of Hemoglobin (HGB), red blood cell (RBC), white blood cell (WBC) and platelet (PLT) in all groups fell within the normal limits, with no significant fluctuations. These initial findings suggested that the hematological system of the mice was not adversely affected by the treatments with the various formulations. As previously noted, this study demonstrated that FA-Lipo@Til NCs exhibited favorable safety in long-term, normal-dose treated animal models, while also confirming its low cytotoxicity.
Fig. 8.
In vivo biosafety evaluation of FA-Lipo@Til NCs treatment for 12 weeks. (A) Time-course body weight of ApoE−/− mice during various treatments. (B) Organ index of ApoE−/− mice during various treatments. (C) H&E staining images of major organs collected from ApoE−/− mice after receiving various treatments. Scale bars = 100 μm. (D) Clinical serum biochemistry analysis of ALT, AST, BUN, and CRE. (E) Clinical routine serum analysis of HGBs, WBCs, RBCs and PLTs. Data are shown as the mean ± S.D. (n = 6). No obvious abnormalities were found in any of the groups.
4. Conclusion
In summary, we have developed a novel targeted oral nanomedicine of Til (FA-Lipo@Til NCs), specifically engineered for the treatment of atherosclerosis. Following a comprehensive evaluation, several noteworthy characteristics of FA-Lipo@Til NCs have been identified: First, the modification of FA and the lipid bilayer significantly enhanced mucus permeability and transmembrane transport efficiency through the intestinal epithelial cells, thereby bypassing the intestinal mucosal barrier. Second, the FA-targeting strategy facilitated the extensive penetration of Til into atherosclerotic plaques in ApoE−/− mice. Furthermore, after three months of oral administration, FA-Lipo@Til NCs significantly reduced the lesion area, plaque area, and necrotic core areas within the aortic root. Concurrently, Til modulated macrophage polarization towards the M2 phenotype, promoting efferocytosis and reducing ROS generation, thereby attenuating inflammatory responses. FA-Lipo@Til NCs also effectively lower blood lipid levels, offering a comprehensive strategy for the treatment of atherosclerosis. Importantly, this study introduces Til as a targeting oral nanomedicine in the form of FA-Lipo@Til NCs, which successfully bypasses the intestinal barrier and target atherosclerotic plaques in the aortas. The results highlight the benefits of custom oral delivery systems in treating atherosclerosis.
CRediT authorship contribution statement
Min Sun: Writing – original draft, Methodology, Data curation, Conceptualization. Mengran Guo: Writing – review & editing, Supervision, Conceptualization. Zhongshan He: Writing – review & editing, Supervision, Conceptualization. Yaoyao Luo: Supervision, Methodology. Huiling Yang: Visualization. Yupei Zhang: Visualization. Yuntao Gao: Visualization, Investigation. Xuli Ruan: Visualization. Ruyue Liu: Visualization, Software. Jingrun Li: Visualization. Wenjiang Cao: Visualization. Chuansheng Huang: Visualization. Liping Wang: Visualization. Yong Yuan: Visualization. Xiangrong Song: Writing – review & editing, Supervision, Resources, Conceptualization. Xinchun Wang: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
All animal care and experimental protocols comply with the relevant
Laws, regulations and standards concerning animal welfare ethics. All animal experiments were approved by the Institutional Animal Care and Use Committee of the State Key Laboratory of Biotherapy, Sichuan University (Approval No. 20221110006).
Funding
This research was supported by the Science and Technology Project of Xinjiang Production and Construction Corps, China (No. 2022AB020, 2023AB045, 2024AB071); the Tianshan Elite Leading Talent Program in the Pharmaceutical Field, China (No. TSYC202401A016); the Youth Fund of the National Natural Science Foundation of China (No. 81960766, 81860747, 82104081); the Sichuan Province Science and Technology Support Program, China (No. 2023NSFSC1682); and the NMPA Key Laboratory for Technical Research on Drug Products In Vitro and In Vivo Correlation Open Program, China (No. 2024-KFKT-001).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the group members of Drug delivery system (DDS) group, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, for the help during experiments. The graphical abstract was created with writer, presentation and spreadsheets (WPS) office.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2025.102204.
Contributor Information
Xiangrong Song, Email: songxr@scu.edu.cn.
Xinchun Wang, Email: cwjwxc@163.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Chen W., Li Z., Zhao Y., Chen Y., Huang R. Global and national burden of atherosclerosis from 1990 to 2019: trend analysis based on the global burden of disease study 2019. Chin. Med. J. 2023;136(20):2442–2450. doi: 10.1097/cm9.0000000000002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoshino T., Sissani L., Labreuche J., Ducrocq G., Lavallee P.C., Meseguer E., Guidoux C., Cabrejo L., Hobeanu C., Gongora-Rivera F., Touboul P.-J., Steg P.G., Amarenco P., Investigators A. Prevalence of systemic atherosclerosis burdens and overlapping stroke etiologies and their associations with long-term vascular prognosis in stroke with intracranial atherosclerotic disease. JAMA Neurol. 2018;75(2):203–211. doi: 10.1001/jamaneurol.2017.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J., Chen Y., Jiang N., Li Z., Xu S. Burden of peripheral artery disease and its attributable risk factors in 204 countries and territories from 1990 to 2019. Front. Cardiovasc. Med. 2022;9(1) doi: 10.3389/fcvm.2022.868370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan C., Wu S., Xu W., Zhang J. Global, regional, and national burden of ischaemic heart disease and its trends, 1990-2019. Public Health. 2023;223(4):57–66. doi: 10.1016/j.puhe.2023.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Momtazmanesh S., Moghaddam S.S., Ghamari S.-H., Rad E.M., Rezaei N., Shobeiri P. Global burden of chronic respiratory diseases and risk factors, 1990-2019: an update from the global burden of disease study 2019. eClinicalMedicine. 2023;59(3) doi: 10.1016/j.eclinm.2023.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demir Y. The behaviour of some antihypertension drugs on human serum paraoxonase-1: an important protector enzyme against atherosclerosis. J. Pharm. Pharmacol. 2019;71(10):1576–1583. doi: 10.1111/jphp.13144. [DOI] [PubMed] [Google Scholar]
- 7.Demir Y. Naphthoquinones, benzoquinones, and anthraquinones: molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular diseases. Drug Dev. Res. 2020;81(5):628–636. doi: 10.1002/ddr.21667. [DOI] [PubMed] [Google Scholar]
- 8.Barrett T.J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 2020;40(1):20–33. doi: 10.1161/atvbaha.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Back M., Yurdagul A., Jr., Tabas I., Oorni K., Kovanen P.T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019;16(7):389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C., Wang H., Guo L., Cui Y., Zou C., Hu J., Zhang H., Yang G., Zhou W. Multifunctional nanomedicine for targeted atherosclerosis therapy: activating plaque clearance Cascade and suppressing inflammation. ACS Nano. 2025;19(3):3339–3361. doi: 10.1021/acsnano.4c12131. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe R., Hashimoto M. Pathogenic role of monocytes/macrophages in large vessel vasculitis. Front. Immunol. 2022;13(2) doi: 10.3389/fimmu.2022.859502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 13.Lin J.-D., Nishi H., Poles J., Niu X., McCauley C., Rahman K., Brown E.J., Yeung S.T., Vozhilla N., Weinstock A., Ramsey S.A., Fisher E.A., Loke P n. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang C., Wang H., Guo L., Zou C., Hu J., Zhang H., Zhou W., Yang G. CpG-Conjugated silver nanoparticles as a multifunctional nanomedicine to promote macrophage efferocytosis and repolarization for atherosclerosis therapy. ACS Appl. Mater. Interfaces. 2023;15(45):52162–52179. doi: 10.1021/acsami.3c11227. [DOI] [PubMed] [Google Scholar]
- 15.de Meyer G.R.Y., Zurek M., Puylaert P., Martinet W. Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat. Rev. Cardiol. 2024;21(7):312–325. doi: 10.1038/s41569-023-00957-0. [DOI] [PubMed] [Google Scholar]
- 16.Jinnouchi H., Guo L., Sakamoto A., Torii S., Sato Y., Cornelissen A., Kuntz S., Paek K.H., Fernandez R., Fuller D., Gadhoke N., Surve D., Romero M., Kolodgie F.D., Virmani R., Finn A.V. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell. Mol. Life Sci. 2020;77(10):1919–1932. doi: 10.1007/s00018-019-03371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potor L., Hendrik Z., Patsalos A., Katona E., Mehes G., Poliska S., Csosz E., Kallo G., Komaromi I., Combi Z., Posta N., Sikura K.E., Petho D., Oros M., Vereb G., Toth C., Gergely P., Nagy L., Balla G., Balla J. Oxidation of hemoglobin drives a proatherogenic polarization of macrophages in human atherosclerosis. Antioxidants Redox Signal. 2021;35(12):917–950. doi: 10.1089/ars.2020.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su W., Liang L., Zhou L., Cao Y., Zhou X., Liu S., Wang Q., Zhang H. Macrophage paired immunoglobulin-like receptor B deficiency promotes peripheral atherosclerosis in apolipoprotein E-Deficient mice. Front. Cell Dev. Biol. 2022;9(2) doi: 10.3389/fcell.2021.783954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen X., Zeng X., Liu J., Zeng X., Zhang Y., Cheng X., Huang J., Li Y., Zhuang R., Zhang X., Guo Z. In vivo comparative study of radioiodinated folate receptor targeting albumin probes for atherosclerosis plaque imaging. Bioconjug. Chem. 2023;34(12):2387–2397. doi: 10.1021/acs.bioconjchem.3c00486. [DOI] [PubMed] [Google Scholar]
- 20.Guo M., He Z., Jin Z., Huang L., Yuan J., Qin S., Wang X., Cao L., Song X. Oral nanoparticles containing naringenin suppress atherosclerotic progression by targeting delivery to plaque macrophages. Nano Res. 2023;16(1):925–937. doi: 10.1007/s12274-022-4808-2. [DOI] [Google Scholar]
- 21.Xiang Y., Liang B., Zhang X., Qiu X., Deng Q., Yu L., Yu H., Lu Z., Zheng F. Atheroprotective mechanism by which folic acid regulates monocyte subsets and function through DNA methylation. Clin. Epigenet. 2022;14(1):32. doi: 10.1186/s13148-022-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan S., Liu H., Gao F., Luo H., Lin H., Meng L., Jiang C., Guo Y., Chi J., Guo H. Folic acid delays development of atherosclerosis in low-density lipoprotein receptor-deficient mice. J. Cell Mol. Med. 2018;22(6):3183–3191. doi: 10.1111/jcmm.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Abreu O., Torres-Piedra M., Garcia-Jimenez S., Ibarra-Barajas M., Villalobos-Molina R., Montes S, Rembao D., Estrada-Soto S. Dose-dependent antihypertensive determination and toxicological studies of tilianin isolated from Agastache mexicana. J. Ethnopharmacol. 2013;146(1):187–191. doi: 10.1016/j.jep.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Wei J., Cao P., Wang J., Kang W. Analysis of tilianin and acacetin in Agastache rugosa by high-performance liquid chromatography with ionic liquids-ultrasound based extraction. Chem. Cent. J. 2016;10(3):76. doi: 10.1186/s13065-016-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberto Garcia-Diaz J., Navarrete-Vazquez G., Garcia-Jimenez S., Hidalgo-Figueroa S., Almanza-Perez J.C., Javier Alarcon-Aguilar F., Gomez-Zamudio J., Cruz M., Ibarra-Barajas M., Estrada-Soto S. Antidiabetic, antihyperlipidemic and anti-inflammatory effects of tilianin in streptozotocin-nicotinamide diabetic rats. Biomed. Pharmacother. 2016;83(1):667–675. doi: 10.1016/j.biopha.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Nam K.-H., Choi J.-H., Seo Y.-J., Lee Y.-M., Won Y.-S., Lee M.-R., Lee M.-N., Lee M.-N., Park J.-G., Kim Y.-M., Kim H.-C., Lee C.-H., Lee H.-K., Oh S.-R., Oh G.T. Inhibitory effects of tilianin on the expression of inducible nitric oxide synthase in low density lipoprotein receptor deficiency mice. Exp. Mol. Med. 2006;38(4):445–452. doi: 10.1038/emm.2006.52. [DOI] [PubMed] [Google Scholar]
- 27.Oh H.M., Kang Y.J., Lee Y.S., Park M.K., Kim S.H., Kim H.J., Seo H.G., Lee J.H., Chang K.C. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264.7 cells from hydrogen peroxide-induced injury. J. Ethnopharmacol. 2006;103(2):229–235. doi: 10.1016/j.jep.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Eva Gonzalez-Trujano M., Ponce-Munoz H., Hidalgo-Figueroa S., Navanete-Vazquez G., Estrada-Soto S. Depressant effects of Agastache mexicana methanol extract and one of major metabolites tilianin. Asian Pac. J. Tropical Med. 2015;8(3):185–190. doi: 10.1016/s1995-7645(14)60312-6. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H., Fang J., Xing J., Wang L., Wang Q., Wang Y., Li Z., Liu R. Tilianin mediates neuroprotection against ischemic injury by attenuating CaMKII-dependent mitochondrion-mediated apoptosis and MAPK/NF-κB signaling. Life Sci. 2019;216(1):233–245. doi: 10.1016/j.lfs.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Zeng C., Jiang W., Zheng R., He C., Li J., Xing J. Cardioprotection of tilianin ameliorates myocardial ischemia-reperfusion injury: role of the apoptotic signaling pathway. PLoS One. 2018;13(3):38–45. doi: 10.1371/journal.pone.0193845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M., Guo M., He Z., Luo Y., He X., Huang C., Yuan Y., Zhao Y., Song X., Wang X. Enhanced anti-inflammatory activity of tilianin based on the novel amorphous nanocrystals. Pharmaceuticals. 2024;17(5):654. doi: 10.3390/ph17050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam K.W., Kim J., Hong J.J., Choi J.H., Mar W., Cho M.H., Kim Y.M., Oh S.R., Lee H.K., Nam K.H., Oh G.T. Inhibition of cytokine-induced IκB kinase activation as a mechanism contributing to the anti-atherogenic activity of tilianin in hyperlipidemic mice. Atherosclerosis. 2005;180(1):27–35. doi: 10.1016/j.atherosclerosis.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Shen W., Anwaier G., Cao Y., Lian G., Chen C., Liu S., Tuerdi N., Qi R. Atheroprotective mechanisms of tilianin by inhibiting inflammation through down-regulating NF-κB pathway and foam cells formation. Front. Physiol. 2019;10(1):825. doi: 10.3389/fphys.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Wang J., Xu J., Zhang J., Xu S., Zhang Q., Huang J., Peng J., Xu H., Du Q., Gong Z. Fabrication of a polysaccharide-Protein/Protein complex stabilized oral nanoemulsion to facilitate the therapeutic effects of 1,8-Cineole on atherosclerosis. ACS Nano. 2023;17(10):9090–9109. doi: 10.1021/acsnano.2c12230. [DOI] [PubMed] [Google Scholar]
- 35.Guo M., He Z., Jin Z., Huang L., Yuan J., Qin S., Wang X., Cao L., Song X. Oral nanoparticles containing naringenin suppress atherosclerotic progression by targeting delivery to plaque macrophages. Nano Res. 2023;16(1):925–937. doi: 10.1007/s12274-022-4808-2. [DOI] [Google Scholar]
- 36.Fang H., Huang L., Lv F., Hu B., Liu H., Huang Z., Sun Y., Zhou W., Wang X. Dual-responsive targeted atherosclerosis therapy through a multi-effective nanoplatform with anti-inflammatory, lipid-regulating and autophagy. Chem. Eng. J. 2023;454 doi: 10.1016/j.cej.2022.140067. [DOI] [Google Scholar]
- 37.Yuan J., Guo M., Zhao S., Li J., Wang X., Yang J., Jin Z., Song X. Core-shell lipid-polymeric nanoparticles for enhanced oral bioavailability and antihypertensive efficacy of KY5 peptide. Chin. Chem. Lett. 2023;34(4) doi: 10.1016/j.cclet.2022.107943. [DOI] [Google Scholar]
- 38.Hernandez-Abreu O., Estrada-Soto S., Rivera-Leyva J.C., Peregrina-Lucano A., Avila-Villareal G., Villalobos-Molina R. Pharmacokinetic study of tilianin after oral administration in wistar rats by HPLC. Rev. Bras. Farmacogn. 2024;34(5):1001–1011. doi: 10.1007/s43450-024-00541-8. [DOI] [Google Scholar]
- 39.Vijayarani K.R., Govindarajulu M., Ramesh S., Alturki M., Majrashi M., Fujihashi A., Almaghrabi M., Kirubakaran N., Ren J., Babu R.J., Smith F., Moore T., Dhanasekaran M. Enhanced bioavailability of boswellic acid by piper Longum: a computational and pharmacokinetic study. Front. Pharmacol. 2020;11(1) doi: 10.3389/fphar.2020.551911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng C., Jiang W., Tan M., Yang X., He C., Huang W., Xing J. Optimization of the process variables of tilianin-loaded composite phospholipid liposomes based on response surface-central composite design and pharmacokinetic study. Eur. J. Pharmaceut. Sci. 2016;85(1):123–131. doi: 10.1016/j.ejps.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Kesisoglou F., Panmai S., Wu Y. Nanosizing - oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007;59(7):631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Yu Q., Wu X., Zhu Q., Wu W., Chen Z., Li Y., Lu Y. Enhanced transdermal delivery of meloxicam by nanocrystals: preparation, In Vitro and In Vivo evaluation. Asian J. Pharm. Sci. 2018;13(6):518–526. doi: 10.1016/j.ajps.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao L., Liu G., Ma J., Wang X., Zhou L., Li X., Wang F. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm. Res. 2013;30(2):307–324. doi: 10.1007/s11095-012-0889-z. [DOI] [PubMed] [Google Scholar]
- 44.Fontana F., Figueiredo P., Zhang P., Hirvonen J.T., Liu D., Santos H.A. Production of pure drug nanocrystals and nano co-crystals by confinement methods. Adv. Drug Deliv. Rev. 2018;131:3–21. doi: 10.1016/j.addr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Yang H., Kim H., Jung S., Seo H., Nida S.K., Yoo S.-Y., Lee J. Pharmaceutical strategies for stabilizing drug nanocrystals. Curr. Pharm. Des. 2018;24(21):2362–2374. doi: 10.2174/1381612824666180515125247. [DOI] [PubMed] [Google Scholar]
- 46.Malamatari M., Taylor K.M.G., Malamataris S., Douroumis D., Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov. Today. 2018;23(3):534–547. doi: 10.1016/j.drudis.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y., Tan X., Fan X., Zhao L., Wang S., He H., Yin T., Zhang Y., Tang X., Jian L., Jin J., Gou J. Current strategies for oral delivery of BCS IV drug nanocrystals: challenges, solutions and future trends. Expet Opin. Drug Deliv. 2021;18(9):1211–1228. doi: 10.1080/17425247.2021.1903428. [DOI] [PubMed] [Google Scholar]
- 48.Lhaglham P., Jiramonai L., Jia Y., Huang B., Huang Y., Gao X., Zhang J., Liang X.-J., Zhu M. Drug nanocrystals: surface engineering and its applications in targeted delivery. iScience. 2024;27(11) doi: 10.1016/j.isci.2024.111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu P., Chen G., Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4) doi: 10.3390/molecules27041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamam H., Park J., Gadalla H.H., Masters A.R., Abdel-Aleem J.A., Abdelrahman S.I., Abdelrahman A.A., Lyle L.T., Yeo Y. Development of liposomal gemcitabine with high drug loading capacity. Mol. Pharm. 2019;16(7):2858–2871. doi: 10.1021/acs.molpharmaceut.8b01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeitler R., Glader C., Koenig G., Kaplan J., Tetyczka C., Remmelgas J., Mussbacher M., Froehlich E., Roblegg E. On the structure, stability, and cell uptake of nanostructured lipid carriers for drug delivery. Mol. Pharm. 2024;21(7):3674–3683. doi: 10.1021/acs.molpharmaceut.4c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Z., Chen W., Hu K., Luo Y., Zeng W., He X., Li T., Ouyang J., Li Y., Xie L., Zhang Y., Xu Q., Yang S., Guo M., Zou W., Li Y., Huang L., Chen L., Zhang X., Saiding Q., Wang R., Zhang M.-R., Kong N., Xie T., Song X., Tao W. Resolvin D1 delivery to lesional macrophages using antioxidative Black phosphorus nanosheets for atherosclerosis treatment. Nat. Nanotechnol. 2024;19(9):1386–1398. doi: 10.1038/s41565-024-01687-1. [DOI] [PubMed] [Google Scholar]
- 54.Guan W., Liu Y., Ding S., Wang Y. Ciprofloxacin nanocrystals and N-acetylcysteine co-solidified powders for pulmonary drug delivery: development and in vitro and in vivo characterization. J. Nanoparticle Res. 2022;24(2):41. doi: 10.1007/s11051-022-05427-1. [DOI] [Google Scholar]
- 55.Poinard B., Kamaluddin S., Tan A.Q.Q., Neoh K.G., Kah J.C.Y. Polydopamine coating enhances mucopenetration and cell uptake of nanoparticles. ACS Appl. Mater. Interfaces. 2019;11(5):4777–4789. doi: 10.1021/acsami.8b18107. [DOI] [PubMed] [Google Scholar]
- 56.Hu S., Pei X., Duan L., Zhu Z., Liu Y., Chen J., Chen T., Ji P., Wan Q., Wang J. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 2021;12(1):1689. doi: 10.1038/s41467-021-21989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leal J., Peng X., Liu X., Arasappan D., Wylie D.C., Schwartz S.H., Fullmer J.J., McWilliams B.C., Smyth H.D.C., Ghosh D. Peptides as surface coatings of nanoparticles that penetrate human cystic fibrosis sputum and uniformly distribute in vivo following pulmonary delivery. J. Contr. Release. 2020;322:457–469. doi: 10.1016/j.jconrel.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marlinda A.R., An'amt M.N., Yusoff N., Sagadevan S., Wahab Y.A., Johan M.R. Recent progress in nitrates and nitrites sensor with graphene-based nanocomposites as electrocatalysts. Trends Environ. Anal. Chem. 2022;34(2) doi: 10.1016/j.teac.2022.e00162. [DOI] [Google Scholar]
- 59.Guo S., Liang Y., Liu L., Yin M., Wang A., Sun K., Li Y., Shi Y. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: size, surface charge and pro-hydrophobics. J. Nanobiotechnol. 2021;19(1):32. doi: 10.1186/s12951-021-00770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang Y., Tang Z., Li P., Tang K., Ma Z., Wang Y., Wang X., Li C. Precise delivery of nanomedicines to M2 macrophages by combining "Eat Me/Don't Eat Me" Ssgnals and Iis Aaticancer Aaplication. ACS Nano. 2021;15(11):18100–18112. doi: 10.1021/acsnano.1c06707. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y., Deng P., Tian Y., Feng J., Xiao J., Li J., Liu J., Li G., He Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020;18(1):112. doi: 10.1186/s12951-020-00672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahlawat J., Barroso G.G., Asil S.M., Alvarado M., Armendariz I., Bernal J., Carabaza X., Chavez S., Cruz P., Escalante V., Estorga S., Fernandez D., Lozano C., Marrufo M., Ahmad N., Negrete S., Olvera K., Parada X., Portillo B., Ramirez A., Ramos R., Rodriguez V., Rojas P., Romero J., Suarez D., Urueta G., Viel S., Narayan M. Nanocarriers as potential drug delivery candidates for overcoming the blood-brain barrier: challenges and possibilities. ACS Omega. 2020;5(22):12583–12595. doi: 10.1021/acsomega.0c01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sipos A., Kim K.-J., Chow R.H., Flodby P., Borok Z., Crandall E.D. Alveolar epithelial cell processing of nanoparticles activates autophagy and lysosomal exocytosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;315(2):L286–L300. doi: 10.1152/ajplung.00108.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J., Li M., Wang M., Xu H., Wang Z., Li Y., Ding B., Gao J. Effects of the surface charge of polyamidoamine dendrimers on cellular exocytosis and the exocytosis mechanism in multidrug-resistant breast cancer cells. J. Nanobiotechnol. 2021;19(1):135. doi: 10.1186/s12951-021-00881-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Cao S., Alam M.N.A., Raabe M., Michel-Souzy S., Wang Z., Wagner M., Ermakova A., Cornelissen J.J.L.M., Weil T. Fluorescent nanodiamonds encapsulated by cowpea chlorotic mottle virus (CCMV) proteins for intracellular 3D-trajectory analysis. J. Mater. Chem. B. 2021;9(28):5621–5627. doi: 10.1039/d1tb00890k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X., Bakker W., Sang Y., Rietjens I.M.C.M. Absorption and intracellular accumulation of food-borne dicarbonyl precursors of advanced glycation end-product in a Caco-2 human cell transwell model. Food Chem. 2024;452(2) doi: 10.1016/j.foodchem.2024.139532. [DOI] [PubMed] [Google Scholar]
- 67.Liang M., Wang Q., Zhang S., Lan Q., Wang R., Tan E., Zhou L., Wang C., Wang H., Cheng Y. Polypyridiniums with inherent autophagy-inducing activity for atherosclerosis treatment by intracellularly Co-Delivering two antioxidant enzymes. Adv. Mater. 2024;36(46) doi: 10.1002/adma.202409015. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J., Wang Z., Liao Y., Tong J., Gao R., Zeng Z., Bai Y., Wei Y., Guo X. Black phosphorus nanoplatform coated with platelet membrane improves inhibition of atherosclerosis progression through macrophage targeting and efferocytosis. Acta Biomater. 2024;192(15):377–393. doi: 10.1016/j.actbio.2024.11.041. [DOI] [PubMed] [Google Scholar]
- 69.Feng M., Liu L., Qu Z., Zhang B., Wang Y., Yan L., Kong L. CRISPR/Cas9 knockout of MTA1 enhanced RANKL-induced osteoclastogenesis in RAW264.7 cells partly via increasing ROS activities. J. Cell Mol. Med. 2023;27(5):701–713. doi: 10.1111/jcmm.17692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai P., Zhu L., Luo F., Lu L., Li Q., Wang L., Wang Y., Wang X., Hu M., Liu Z. Triple recycling processes impact systemic and local bioavailability of orally administered flavonoids. AAPS J. 2015;17(3):723–736. doi: 10.1208/s12248-015-9732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai P., Luo F., Wang Y., Jiang H., Wang L., Zhang G., Zhu L., Hu M., Wang X., Lu L., Liu Z. Species- and gender-dependent differences in the glucuronidation of a flavonoid glucoside and its aglycone determined using expressed UGT enzymes and microsomes. Biopharm Drug Dispos. 2015;36(9):622–635. doi: 10.1002/bdd.1989. [DOI] [PubMed] [Google Scholar]
- 72.Sun M., Guo M., He Z., Luo Y., Yang H., Zhang Y., Li J., Cao W., Huang C., Wang L., Song X., Wang X. Enhancing oral bioavailability and reducing gastrointestinal toxicity of Tilianin via folic acid-modified nanocrystal liposomes. J. Drug Deliv. Sci. Technol. 2025;108 doi: 10.1016/j.jddst.2025.106914. [DOI] [Google Scholar]
- 73.Alp G., Aydogan N. Lipid-based mucus penetrating nanoparticles and their biophysical interactions with pulmonary mucus layer. Eur. J. Pharm. Biopharm. 2020;149(4):45–57. doi: 10.1016/j.ejpb.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 74.Champion J.A., Pho T. Surface engineering of protein nanoparticles modulates transport, adsorption, and uptake in mucus. ACS Appl. Mater. Interfaces. 2022;14(46):4670. doi: 10.1021/acsami.2c14670. [DOI] [PubMed] [Google Scholar]
- 75.Gao Y., He Y., Zhang H., Zhang Y., Gao T., Wang J.-H., Wang S. Zwitterion-functionalized mesoporous silica nanoparticles for enhancing oral delivery of protein drugs by overcoming multiple gastrointestinal barriers. J. Colloid Interface Sci. 2021;582(A):364–375. doi: 10.1016/j.jcis.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019;18(1):19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 77.Macedo A.S., Castro P.M., Roque L., Thome N.G., Reis C.P., Pintado M.E., Fonte P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Contr. Release. 2020;320(1):125–141. doi: 10.1016/j.jconrel.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Ribeiro I.R.S., da Silva R.F., Rabelo R.S., Marin T.M., Bettini J., Cardoso M.B. Flowing through gastrointestinal barriers with model nanoparticles: from complex fluids to model human intestinal epithelium permeation. ACS Appl. Mater. Interfaces. 2023;15(30):36025–36035. doi: 10.1021/acsami.3c07048. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z., Su C. Effects of folic acid deficiency on genetic damage in colorectal cancer cells. Am. J. Transl. Res. 2023;15(5):3162–3171. [PMC free article] [PubMed] [Google Scholar]
- 80.Vykoukal J.V., Fahrmann J.F., Thompson T.C. Caveolin and lipid domains-close companions in managing cellular pathways. Cancer Metastasis Rev. 2020;39(2):341–342. doi: 10.1007/s10555-020-09891-w. [DOI] [PubMed] [Google Scholar]
- 81.Ding L., Yao C., Yin X., Li C., Huang Y., Wu M., Wang B., Guo X., Wang Y., Wu M. Size, shape, and protein Corona determine cellular uptake and removal mechanisms of gold nanoparticles. Small. 2018;14(42):1451. doi: 10.1002/smll.201801451. [DOI] [PubMed] [Google Scholar]
- 82.Valibeknejad M., Abdoli S.M., Alizadeh R., Mihaila S.M., Raoof A. Insights into transport in mucus barrier: exploring particle penetration through the intestinal mucus layer. J. Drug Deliv. Sci. Technol. 2023;86(1) doi: 10.1016/j.jddst.2023.104752. [DOI] [Google Scholar]
- 83.Lai S.K., Wang Y.-Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61(2):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byrne J., Huang H.-W., McRae J.C., Babaee S., Soltani A., Becker S.L., Traverso G. Devices for drug delivery in the gastrointestinal tract: a review of systems physically interacting with the mucosa for enhanced delivery. Adv. Drug Deliv. Rev. 2021;177 doi: 10.1016/j.addr.2021.113926. [DOI] [PubMed] [Google Scholar]
- 85.Hua X., Zhu Q., Liu Y., Zhou S., Huang P., Li Q., Liu S. A double tangential flow filtration-based microfluidic device for highly efficient separation and enrichment of exosomes. Anal. Chim. Acta. 2023;1258(2) doi: 10.1016/j.aca.2023.341160. [DOI] [PubMed] [Google Scholar]
- 86.Hu M., Li Y., Huang J., Wang X., Han J. Electrospun scaffold for biomimic culture of Caco-2 cell monolayer as an in vitro intestinal model. ACS Appl. Bio Mater. 2021;4(2):1340–1349. doi: 10.1021/acsabm.0c01230. [DOI] [PubMed] [Google Scholar]
- 87.Shin K., Song S., Song Y.H., Hahn S., Kim J.-H., Jeong I.-C., Sung J., Lee K.T. Intracellular transport dynamics of upconverting nanoparticles in living cells. Biophys. J. 2020;118(3):574A. 574A. [Google Scholar]
- 88.Cohen S., Valm A.M., Lippincott-Schwartz J. Interacting organelles. Curr. Opin. Cell Biol. 2018;53(5):84–91. doi: 10.1016/j.ceb.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yi Y.-S. Folate receptor-targeted diagnostics and therapeutics for inflammatory diseases. Immune Netw. 2016;16(6):337–343. doi: 10.4110/in.2016.16.6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canfran-Duque A., Rotllan N., Zhang X., Andres-Blasco I., Thompson B.M., Sun J., Price N.L., Fernandez-Fuertes M., Fowler J.W., Gomez-Coronado D., Sessa W.C., Giannarelli C., Schneider R.J., Tellides G., McDonald J.G., Fernandez-Hernando C., Suarez Y. Macrophage-derived 25-Hydroxycholesterol promotes vascular inflammation, atherogenesis, and lesion remodeling. Circulation. 2023;147(5):388–408. doi: 10.1161/circulationaha.122.059062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu X., Guo J.-W., Lin X.-C., Tuo Y.-H., Peng W.-L., He S.-Y., Li Z.-Q., Ye Y.-C., Yu J., Zhang F.-R., Ma M.-M., Shang J.-Y., Lv X.-F., Zhou A.-D., Ouyang Y., Wang C., Pang R.-P., Sun J.-X., Ou J.-S., Zhou J.-G., Liang S.-J. Macrophage NFAT c3 prevents foam cell formation and atherosclerosis: evidence and mechanisms. Eur. Heart J. 2021;42(47):4847–4861. doi: 10.1093/eurheartj/ehab660. [DOI] [PubMed] [Google Scholar]
- 92.Ding Y., Wang Y., Hu Q. Recent advances in overcoming barriers to cell-based delivery systems for cancer immunotherapy. Explorations. 2022;2(3):106. doi: 10.1002/exp.20210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chazaud B. Inflammation and skeletal muscle regeneration: leave it to the macrophages. Trends Immunol. 2020;41(6):481–492. doi: 10.1016/j.it.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Wang J., Li J., Yang Z., Chen Y., Shen H., Chen L., Chen Y., Shen Z. The characteristic of resident macrophages and their therapeutic potential for myocardial infarction. Curr. Probl. Cardiol. 2023;48(5):1–12. doi: 10.1016/j.cpcardiol.2022.101570. [DOI] [PubMed] [Google Scholar]
- 95.Kim S.Y., Nair M.G. Macrophages in wound healing: activation and plasticity. Immunol. Cell Biol. 2019;97(3):258–267. doi: 10.1111/imcb.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun K., Li Y-y, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct. Targeted Ther. 2021;6(1):79. doi: 10.1038/s41392-020-00455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arabpour M., Saghazadeh A., Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021;97(6) doi: 10.1016/j.intimp.2021.107823. [DOI] [PubMed] [Google Scholar]
- 98.Chen S., Yang J., Wei Y., Wei X. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell. Mol. Immunol. 2020;17(1):36–49. doi: 10.1038/s41423-019-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sukka S.R., Ampomah P.B., Darville L.N.F., Ngai D., Wang X., Kuriakose G., Xiao Y., Shi J., Koomen J.M., McCusker R.H., Tabas I. Efferocytosis drives a tryptophan metabolism pathway in macrophages to promote tissue resolution (SEP, 10.1038/s42255-024-01115-7, 2024) Nat. Metab. 2024;6(11):2204. doi: 10.1038/s42255-024-01142-4. 2204. [DOI] [PubMed] [Google Scholar]
- 100.Ding L., Chen Y., Zhang B., Hu R., Dai C., Dong C., Huang H. Living macrophage-delivered tetrapod PdH nanoenzyme for targeted atherosclerosis management by ROS scavenging, hydrogen anti-inflammation, and autophagy activation. ACS Nano. 2022;16(10):15959–15976. doi: 10.1021/acsnano.2c03422. [DOI] [PubMed] [Google Scholar]
- 101.Khattulanuar F.S., Sekar M., Fuloria S., Gan S.H., Rani N.N.I.M., Ravi S., Chidambaram K., Begum M.Y., Azad A.K., Jeyabalan S., Dhiravidamani A., Thangavelu L., Lum P.T., Subramaniyan V., Wu Y.S., Sathasivam K.V., Fuloria N.K. Tilianin: a potential natural lead molecule for new drug design and development for the treatment of cardiovascular disorders. Molecules. 2022;27(3):673. doi: 10.3390/molecules27030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu H., Sheng J., Wang Z., Zu Z., Xiang K., Qi J., Wang Z., Lu G., Zhang L. Tannic acid-poloxamer self-assembled nanoparticles for advanced atherosclerosis therapy by regulation of macrophage polarization. J. Mater. Chem. B. 2024;12(19):4708–4716. doi: 10.1039/d3tb01157g. [DOI] [PubMed] [Google Scholar]
- 103.Xiao Y., Huang X., Xia Y., Ding M., Li A., Yang B., She Q. Role of dysregulated macrophage subpopulation ratios and functional changes in the development of coronary atherosclerosis. J. Gene Med. 2024;26(1):3626. doi: 10.1002/jgm.3626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.