Abstract
Mycobacterium ulcerans is an emerging environmental pathogen which causes chronic skin ulcers (i.e., Buruli ulcer) in otherwise healthy humans living in tropical countries, particularly those in Africa. In spite of epidemiological and PCR data linking M. ulcerans to water, the mode of transmission of this organism remains elusive. To determine the role of aquatic insects in the transmission of M. ulcerans, we have set up an experimental model with aquariums that mimic aquatic microenvironments. We report that M. ulcerans may be transmitted to laboratory mice by the bite of aquatic bugs (Naucoridae) that are infected with this organism. In addition, M. ulcerans appears to be localized exclusively within salivary glands of these insects, where it can both survive and multiply without causing any observable damage in the insect tissues. Subsequently, we isolated M. ulcerans from wild aquatic insects collected from a zone in the Daloa region of Ivory Coast where Buruli ulcer is endemic. Taken together, these results point to aquatic insects as a possible vector of M. ulcerans.
Mycobacterium ulcerans, an environmental mycobacterial species, is the etiologic agent of Buruli ulcer, a disease that usually begins with a painless nodule or papule on the skin that, in the absence of effective treatment, slowly evolves into extensive skin ulceration in otherwise healthy children and adults. Given that the drug treatment for this disease is still disappointing (3), the only effective care is by surgical excision of necrotic and infected tissues. Such treatment requires long hospital stays and cannot prevent functional disabilities. The prevalence of Buruli ulcer has been increasing dramatically in West Africa for the last 10 years; it is now the third most common mycobacterial infection in humans, exceeded only by tuberculosis and leprosy (1).
Since the study by the Uganda Buruli Group (19), it is assumed that infection by M. ulcerans is related to swampy areas of tropical countries. Interestingly, M. ulcerans has been detected by direct PCR both in environmental waters and in water bugs (11, 12, 15). This finding suggests that people who live in swampy areas of tropical countries become infected through minor wounds or skin abrasions that come in contact with the mycobacterium-infected water. An attractive hypothesis for a possible mode of transmission to humans was recently proposed by Portaels et al. (10): water-filtering hosts (fish, mollusks) concentrate the M. ulcerans bacteria present in water or mud and discharge them again to this environment, where they are then ingested by aquatic predators such as beetles and water bugs; these insects, in turn, may transmit the disease to humans by biting. Despite several extensive investigations using PCR to identify M. ulcerans in aquatic environments, the role of water insects as an intermediate host has thus far remained elusive. In this report, we demonstrate that (i) infected aquatic insects were able to transmit M. ulcerans to laboratory mice by biting; (ii) after experimental infection of the insects, M. ulcerans was located in their salivary glands; and (iii) the salivary glands of some aquatic bugs collected from an area where Buruli ulcer is endemic naturally harbored M. ulcerans, as assessed both by PCR and by positive mycobacterial cultures.
MATERIALS AND METHODS
Bacterial strains.
M. ulcerans (strain 01G897) was originally isolated from a skin biopsy sample from a human patient from French Guiana (4) and was passaged in Löwenstein-Jensen medium at 30°C (Sanofi Diagnostics Pasteur, Marnes la Coquette, France). The mycobacterial strains Mycobacterium chelonae (6B0139), Mycobacterium fortuitum (10B0345), and Mycobacterium kansasii (11B0014) were isolated from water, while Mycobacterium marinum (8B0432) was isolated from a patient. All strains were cultured under the same conditions. Inocula were obtained from exponential-phase cultures and adjusted to a concentration of 3.3 × 107/ml in 0.15 M NaCl (14).
Aquatic insects.
A large number of creeping water bugs were captured on the right bank of the Lobo River downstream, from the bridge of the Daloa-Zoukougbeu road in March 2001 just before the rainy season. Eighty adult Naucoridae sharing the same characteristics as those collected in France were examined. They belonged to the Naucoris genus, but their species was not determined definitively prior to the removal of their salivary glands for culture and diagnostic PCR.
Experimental infection of aquatic bugs.
Adult water bugs, 2.5 cm long and belonging to the family Naucoridae (Naucoris cimicoides), were collected from swamps in western France. They were housed in an aquarium filled with 28°C water from their natural environment and exposed to a photoperiod of 12 h of light and 12 h of dark, without being fed for 7 days, and then they were separated into two groups. The first aquarium harbored a group of 120 insects, each of which was fed only one 15- to 20-day-old grub of Phormia terrae novae (Verminière de l'Ouest, Tremblay, France) infected by inoculation with 106 M. ulcerans bacteria in a volume of 30 μl by using a 25-gauge needle. The grubs were used as food immediately after they were inoculated. Thirty insects of the control group were isolated in another aquarium and similarly fed with one noninfected grub. After 8 h, during which the grubs were available to be eaten, each group of insects was transferred to another clean water aquarium and left without any food for 2 weeks. The live insects then received noninfected food twice a week; every 2 weeks for the next 105 days, three insects were sacrificed for detection of M. ulcerans either by culture or by PCR. For histological studies, insects were sacrificed at 15, 30, 60, 90, and 105 days after being infected with M. ulcerans. Four groups of 10 insects infected with M. chelonae, M. fortuitum, M. kansasii, or M. marinum were monitored under the same conditions for histological studies.
Transmission of M. ulcerans infection to mice by bite.
Thirty days after experimental infection, 10 insects from each group were allowed to bite in turn the tails of 10 mice. Twenty female BALB/c mice (Iffa Credo, Saint-Germain sur l'Arbresle, France), 4 to 6 weeks old, were maintained under conventional conditions during experimentation. They were anesthetized by an intramuscular injection of ketamine (88 mg/kg of body weight), and their tails were immersed in an aquarium for 10 s to be bitten by one bug, either infected or not infected (control group). Every week the two groups of 10 mice were examined clinically. When specific inflammatory lesions appeared, the animals were sacrificed and infected tissues were withdrawn for detection of pathogens.
Production and purification of IgG.
Antibodies to M. ulcerans (strain 01G897) were raised in New Zealand White rabbits (Iffa Credo). After 4 weeks of culture on Löwenstein- Jensen medium at 30°C, M. ulcerans bacteria were harvested and washed three times by centrifugation (3,000 × g) for 30 min with phosphate-buffered saline (PBS) containing 4% formalin. After 2 h of incubation at 37°C, they were washed again three times and resuspended at 5 × 106/ml in PBS. For 3 weeks at weekly intervals, rabbits were subcutaneously inoculated with a 500-μl bacterial suspension mixed with 500 μl of incomplete Freund's adjuvant (Sigma-Aldrich, St. Louis, Mo.) into an emulsion. Two weeks later, the rabbit sera were harvested and high-titer fractions were pooled. The immunoglobulin (IgG) fraction was precipitated by 50% ammonium sulfate and purified with a DEAE column (Interchim, Montluçon, France) according to the manufacturer's instructions. The specificities of the antibodies were checked by indirect immunofluorescence with different mycobacterial strains: M. chelonae, M. fortuitum, M. kansasii, and M. marinum.
Isolation of mycobacteria by culture.
The tissue specimens were weighed, minced with disposable scalpels in a petri dish, and ground with a Potter-Elvehjem homogenizer (size 22; Kimble/Kontes, Vineland, N.J.) in 0.15 M NaCl to obtain a 10-fold dilution. Smears of suspensions were stained by the Ziehl-Neelsen procedure. The suspensions were decontaminated with an equal volume of N-acetyl-l-cysteine sodium hydroxide (2%), and cultures were developed by inoculating 0.2 ml into two Löwenstein-Jensen slants (Difco Laboratories, Detroit, Mich.).
To isolate M. ulcerans from the salivary glands of insects which were captured in Ivory Coast, the specimens were processed by immunomagnetic separation to concentrate and purify the pathogen as previously developed (12). The amount of antibody required for optimal coating of the immunomagnetic particles was determined to be 20 μg in 200 μl of PBS, pH 7.2, containing 0.1% bovine serum albumin (BSA). This mixture was incubated with 35 μl of 2.8-μm-diameter immunomagnetic particles (6 × 108 to 7 × 108 beads per ml) precoated with sheep anti-rabbit IgG (Dynald, Skoyen, Norway), with bidirectional mixing at 4°C for 12 h. Beads were washed six times for 3 min each with PBS-0.1% BSA.
Immunostaining.
Insect tissues were fixed in 10% neutral buffered formalin for 15 days for routine embedding in paraffin. Five-micrometer-thick sections of tissue were deparaffined with xylene for 30 min and rehydrated at 20°C in a graded ethanol series (95, 90, 70, and 50%). Tissue sections were stained for acid-fast bacilli (AFB) with Ziehl-Neelsen stain or overlaid with 200 μl of purified rabbit IgG in PBS-0.1% BSA for 10 h at 4°C. The detection was performed with alkaline phosphatase-conjugated sheep IgG anti-rabbit IgG (Interchim). The tissues were counterstained with light green dye.
DNA preparation.
DNA was extracted from cell pellets by the method of Boom et al. (2), with minor modifications. After centrifugation at 3,500 × g for 30 min, the pellets were washed in 100 μl of PBS and then resuspended in 900 μl of lysis buffer (5 M thiocyanate guanidine, 0.1 M Tris-HCl [pH 6.4], 0.2 M EDTA, 2.6% Triton X-100) in the presence of 20 μl of either silica particles or a diatom suspension (Organon Teknika, Boxtel, The Netherlands). After centrifugation at 5,000 × g for 3 min, the sediment was resuspended in 800 μl of washing buffer (5 M thiocyanate guanidine, 0.1 M Tris-HCl [pH 6.4]) and then washed with a mixture of 70% ethanol and acetone and dried for 10 min at 56°C. The DNA was eluted with twice-distilled water.
PCR.
A nested PCR of the repetitive insertion sequence IS2404 (13) was performed to detect M. ulcerans. Ten-microliter aliquots of purified DNA were amplified in a buffer supplied with Taq polymerase (Promega) in 100-μl reaction mixtures containing 1 U of Taq polymerase, 20 pM concentrations of the primers, 2 mM MgCl2, 10 mM Tris-HCl (pH 8.4), and a 200 μM concentration (each) of the deoxynucleoside triphosphates. The primers of the first PCR were MU1 (5′-GGCAGGCTGCAGATGGCATA-3′) and MU2 (5′-GGCAGTTACTTCACTGCACA-3′), which amplify a 549-bp sequence (13). The reactions were performed in a PTC-150 Minicycler (MJ Research, Waltham, Mass.). After an initial denaturation at 95°C for 10 min, the DNA was amplified by 30 cycles of 1-min steps at 94, 64, and 72°C. For the second PCR (7), 1 μl from the first-run product was amplified in a 20-μl reaction mixture with the primers PGP3 (5′-GGCGCAGATCAACTTCGCGGT-3′) and PGP4 (5′-CTGCGTGGTGCTTTACGCGC-3′). Cycling was as follows: denaturation at 95°C for 5 min; amplification for 40 cycles of 1-min steps at 94, 66, and 72°C; and a final extension step at 72°C for 10 min. The amplified DNA was subjected to electrophoresis through 2% agarose gel (Interchim) and detected by ethidium bromide staining and UV transillumination.
RESULTS
Transmission of M. ulcerans infection to mice.
Seven of the 10 BALB/c mice whose tails had been bitten by the infected insects developed a nonulcerative inflammatory lesion with edema at the point of the bite 57 to 89 days after being bitten (Fig. 1). Cultures and PCR performed on tail lesion specimens were positive for M. ulcerans (Fig. 2A). No lesions occurred in mice bitten by uninfected control bugs.
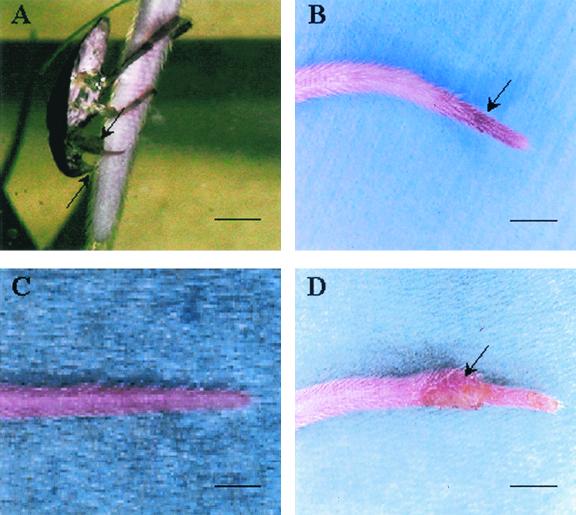
FIG. 1.
Transmission of M. ulcerans infection to BALB/c mice by biting insects (Naucoridae). (A) Water bug (N. cimicoides). Note its rostrum (lower arrow) and raptorial front legs (upper arrow), the femurs of which are greatly thickened, flattened, and adapted for grasping prey. A few minutes after a bite of the tail of a mouse by a Naucoris bug, a slight local erythema is noted (arrow) (B) which disappeared within 3 days (C). (D) After 57 days, active inflammatory lesions develop at the same site (arrow). This observation was made with six other mice. Scale bars: 0.5 cm (A) and 1 cm (B to D).
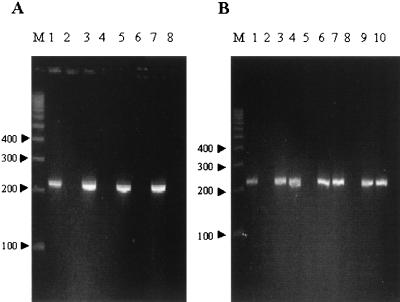
FIG. 2.
Identification of M. ulcerans based on detection of the IS2404 nested-PCR product. (A) Lanes contain a M. ulcerans DNA-positive control (lane 1); mock-specimen negative controls (lanes 2, 4, 6, and 8); and extractions from a water bug (Naucoridae) experimentally infected by M. ulcerans (strain 1G897) (lane 3), from skin biopsy material (day 57) from the tail of a BALB/c mouse bitten by an infected bug (lane 5), and from bacterial colonies isolated by culture from the salivary glands of a water bug (Naucoridae) (lane 7). (B) Nested-PCR amplification of DNAs extracted from M. ulcerans (lane 1) and salivary glands of Naucoridae captured in France that were experimentally infected by M. ulcerans (strain 1G897) (lane 3) and salivary glands of Naucoridae captured in Ivory Coast that were naturally infected by M. ulcerans (lane 4) or were not infected (lane 5). PCR was also performed on the two isolates (Nau.CI.001 and Nau.CI.002) cultured on Löwenstein-Jensen medium (lanes 6 and 7). Eight weeks after subcutaneous inoculation of BALB/c mouse tails with 104 bacilli (Nau.CI.001 and Nau.CI.002), PCR was performed on skin biopsy material from the mouse tails (lanes 9 and 10). Lanes 2 and 8 contain mock-specimen negative controls. Lane M contains molecular size markers (100-bp ladder; Gensura, San Diego, Calif.).
Localization of M. ulcerans in insect tissues.
Histological sections were made on bugs that had been fed grubs experimentally infected with M. ulcerans. AFB were observed in the accessory and main salivary glands (Fig. 3A to E) and in canals that drain these glands into the rostrum and onto the silks of the raptorial legs (Fig. 3F). The presence of M. ulcerans in insect salivary tissues was confirmed by PCR (Fig. 2A), culture, and immunochemistry (Fig. 3E). M. ulcerans was not detected in other insect tissues by PCR.
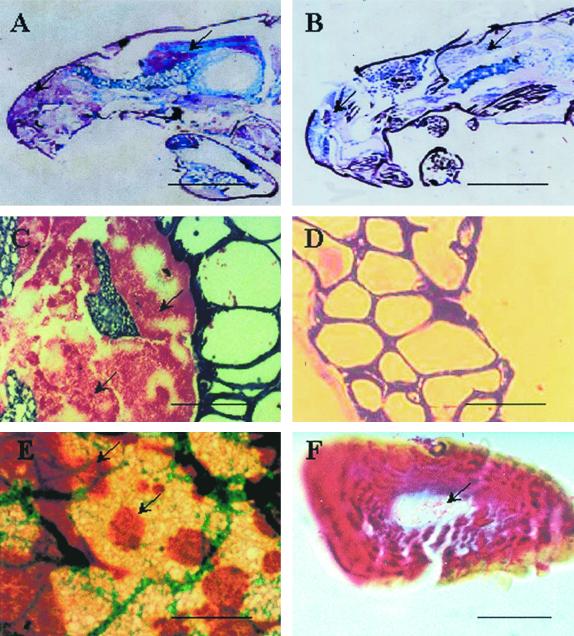
FIG. 3.
Localization of M. ulcerans in 5-μm-thick sections of tissues from infected water bugs. (A) Ziehl-Neelsen staining of sagittal sections of an experimentally infected bug of the order Naucoridae shows colonies of AFB restricted to the principal (left arrow) and accessory (right arrow) salivary glands. The latter are infiltrated by aggregates of AFB (C) and are immunostained with brown dye (arrows) and counterstained with light-green dye (E). No AFB were observed in tissues of noninfected insects (control group) (B) or in their salivary glands (D). The lumens of salivary canals in a silk (F) covering a raptorial leg show many AFB. Scale bars: 0.5 cm (A and B), 100 μm (C to E), and 25 μm (F).
When aquatic bugs were fed grubs experimentally infected with other mycobacteria known to be present in an aquatic environment, i.e., M. kansasii, a slowly growing species, and M. chelonae, M. fortuitum, and M. marinum, three rapidly growing species, no AFB were observed in their salivary glands or in other tissues. Surprisingly, the presence of M. ulcerans in salivary glands was not temporary but permanent. Up through the 105th day after the N. cimicoides insects were fed infected grubs, sections of the insects' salivary glands stained either with Ziehl-Neelsen stain or with specific antibodies exhibited an extremely large number of M. ulcerans bacteria. Moreover, not only did M. ulcerans survive in the salivary glands of the bugs, it also multiplied locally, as suggested by the presence of mycobacterial clusters (Fig. 3A and C). In three N. cimicoides insects fed grubs, each containing 106 M. ulcerans AFB, we harvested 105 days later approximately 108 AFB from the accessory salivary glands of each bug with the method developed by Shepard and McRae to enumerate M. leprae bacteria in the footpads of mice (14). Very few insects (<5%) died during the 105-day incubation period, with no significant difference in results between the infected group and the control group.
PCR analysis of water bugs from the Daloa region.
The Daloa region of Ivory Coast is known to be heavily affected by Buruli ulcer disease (9). Many villages in the rural area are close to the main river Lobo and are situated among a large number of swamps. This prompted us to capture various aquatic insects, including eighty Naucoridae, in an area between Daloa and Zoukougbeu where Buruli ulcer disease is endemic. Since our former findings demonstrated that M. ulcerans was restricted to insects' salivary glands, PCR and cultures were performed on these tissues. M. ulcerans was detected by PCR in 5 of 80 Naucoridae (Fig. 2B). Specimens from two of the insects produced a positive culture on Löwenstein-Jensen medium and grew only at 30°C. PCR performed on the two isolates demonstrated specific sequences of M. ulcerans (Fig. 2B). These strains were named Nau.CI.001 and Nau.CI.002. About 104 bacilli of each isolate (Nau.CI.001, Nau.CI.002, and 01G897) were inoculated subcutaneously into the tails of five mice, and 8 weeks later, the same signs of infection (inflammatory lesions with edema) were observed in all five mice (Fig. 4); PCR performed on infected tissues with isolates Nau.CI.001 and Nau.CI.002 were positive for M. ulcerans (Fig. 2B).
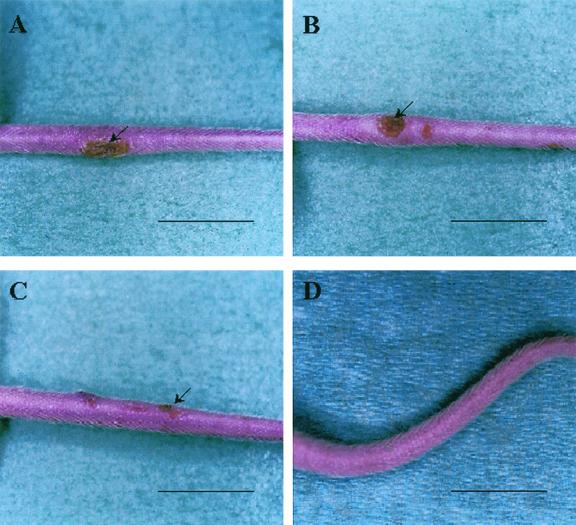
FIG. 4.
Cutaneous lesions of a mouse tail due to M. ulcerans isolated from the salivary glands of a Naucoris bug captured in Ivory Coast. (A) BALB/c mouse tail showing an inflammatory ulceration (arrow) 8 weeks after subcutaneous inoculation of 104 bacilli (strain 01G897). (B and C) The same lesions (arrow) were observed in mouse tails inoculated with Nau.CI.001 (B) and Nau.CI.002 (C), which were isolated by culture from the salivary glands of water bugs captured in Ivory Coast. (D) Control mouse tail. Scale bars: 1.5 cm.
DISCUSSION
The identification of the environmental source of M. ulcerans has long been hampered by the slow growth of the bacterium in culture. This problem was recently circumvented by the development of a PCR assay that uses specific primers for M. ulcerans (7, 13, 16, 17). This assay has enabled the accumulation of significant evidence to suggest an environmental reservoir associated with slow-flowing or stagnant waters (11, 12, 15). After considering the various suspected agents, Portaels et al. proposed the hypothesis (10) that human beings as well as domestic and wild animals could be contaminated or infected by biting insects such as water bugs. Aquatic bugs are cosmopolite insects found throughout temperate and tropical regions especially rich in freshwater. They represent about 10% of all species of Hemiptera associated with water and belong to two series of the suborder Heteroptera: the Nepomorpha, which includes four superfamilies whose members spend most of their time under water, and the Naucoroidea, which includes a single family, the Naucoridae, whose members are commonly termed creeping water bugs. We have chosen members of the Naucoridae for the validation of our experimental model of the transmission of M. ulcerans by aquatic bugs. Whether found in temperate countries like France or tropical ones like Ivory Coast, aquatic bugs exhibit the same way of life, preying, according to their size, on mollusks, snails, young fishes, and the adults and larvae of other insects that they capture with their raptorial front legs and bite with their rostrum (Fig. 1A). These insects can inflict painful bites on humans as well. In Ivory Coast, where Buruli ulcer is endemic, the water bugs are present in swamps and rivers, where human activities such as farming, fishing, and bathing take place. Our present findings describing the transmission of M. ulcerans from water bugs to mice are in good agreement with the possibility of this mode of transmission to humans by bites. Also in strong support of this hypothesis was the localization of M. ulcerans within the salivary glands of Naucoridae. Local physiological conditions of this niche appear to fit the survival and the replication needs of M. ulcerans but not those of other mycobacteria. Surprisingly, infiltration of the salivary glands of Naucoridae by M. ulcerans does not seem to be accompanied by any tissue damage similar to the ulcerative skin lesions developed by bitten individuals and mediated by the cytotoxic activity of the mycolactone (6) and other toxins produced by M. ulcerans (5). The inactivation of the latter toxins could be the result of salivary enzymatic activities which remain to be determined. The removal of salivary glands has enabled us to isolate for the first time two strains phenotypically related to M. ulcerans from two of five water bugs of the family Naucoridae. All these data are in line with the low estimate reported elsewhere (5 insects out of 95 tested positive by PCR for M. ulcerans) (11) and the rather low frequency of Buruli ulcer.
Many other devastating human diseases are transmitted by insects and tick vectors. The saliva of bloodsucking arthropods is an important factor in the transmission of arboviruses by ticks and mosquitoes (8) and in the transmission of Leishmania protozoa by sand flies (18). Compared to the number of insect-borne human-disease-causing viruses, the number of insect-borne bacteria that are known to cause illness in humans is very small. The oriental rat flea is the primary vector of Yersinia pestis in the transmission of the black plague. Unlike most of these vectors, the aquatic insects of the family Naucoroidea which we have studied have the distinctive feature of being neither hematophagous nor phytophagous. Interestingly, among the Heteroptera, other members of the family Cimicidae with a terrestrial habitat live exclusively as ectoparasites by feeding on the blood of mammals. Chagas’ disease, caused by a flagellate protozoan parasite, Trypanosoma cruzi, is transmitted to humans by triatomine insects.
In conclusion, the mouse model for the transmission of Buruli ulcer described in this report provides the first strong evidence implicating insects in the transmission of a mycobacterial disease recognized as an important public health problem in West Africa. The spread of the disease might be explained by environmental changes caused by the development of agriculture, such as the construction of artificial lakes for irrigation and the extension of swamps for rice growing and fish breeding. The identities and the distribution of various infected aquatic insects, particularly among the Nepomorpha, as well as various putative strains of M. ulcerans require further investigation to understand the epidemiology of M. ulcerans infections and the environmental ecology of this organism. Under certain environmental conditions of starvation or drought, water bugs may fly to find water in which to live, so they may colonize new swampy areas and create new foci for the spread of Buruli ulcer. However, this possibility does not exclude other ways of transmission or even other environmental reservoirs.
Acknowledgments
We thank A. Rochetaing, F. Lagarce, S. Sourice, and J. P. Gislard for technical assistance; R. Luzé and P. Bobard for capturing water bugs; F. Portaels for helpful discussions; and J. Grosset, M. Bonneville, G. Kubica, and T. Stinear for critically reading the manuscript.
We are also grateful for the predoctoral fellowship from l'Association Française Raoul Follereau (L.M.). This work was supported by grants from the Raoul Follereau Association.
REFERENCES
- 1.Asiedu, K., R. Sherpbier, and M. C. Raviglione. 2000. Buruli ulcer Mycobacterium ulcerans infection. WHO Global Buruli Ulcer initiative. Report 2000. World Health Organization, Geneva, Switzerland.
- 2.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darie, H., T. Le Guyadec, and J. E. Touze. 1993. Epidemiological and clinical aspects of Buruli ulcer in Ivory Coast: 124 recent cases. Bull. Soc. Pathol. Exot. 86:272-276. (In French.) [PubMed] [Google Scholar]
- 4.De Gentile, P. L., C. Mahaza, F. Rolland, B. Carbonnelle, J. L. Verret, and D. Chabasse. 1992. Cutaneous ulcer from Mycobacterium ulcerans. Apropos of 1 case in French Guyana. Bull. Soc. Pathol. Exot. 85:212-214. (In French.) [PubMed] [Google Scholar]
- 5.Dobos, K. M., P. L. Small, M. Deslauriers, F. D. Quinn, and C. H. King. 2001. Mycobacterium ulcerans cytotoxicity in an adipose cell model. Infect. Immun. 69:7182-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George, K. M., L. Pascopella, D. M. Welty, and P. L. C. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guimaraes-Peres, A., F. Portaels, P. de Rijk, K. Fissette, S. R. Pattyn, J.-P. van Vooren, and P.-A. Fonteyne. 1999. Comparison of two PCRs for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 37:206-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, L. D., W. R. Kaufman, and P. A. Nuttall. 1992. Modification of the skin feeding site by tick saliva mediates virus transmission. Experientia 48:779-782. [DOI] [PubMed] [Google Scholar]
- 9.Marston, B. J., M. O. Diallo, C. R. Horsburgh, Jr., I. Diomande, M. Z. Saki, J. M. Kanga, G. Patrice, H. B. Lipman, S. M. Ostroff, and R. C. Good. 1995. Emergence of Buruli ulcer disease in the Daloa region of Côte d'Ivoire. Am. J. Trop. Med. Hyg. 52:219-224. [DOI] [PubMed] [Google Scholar]
- 10.Portaels, F., K. Chemlal, P. Elsen, P. D. Johnson, J. A. Hayman, J. Hibble, R. Kirkwood, and W. M. Meyers. 2001. Mycobacterium ulcerans in wild animals. Rev. Sci. Tech. Off. Int. Epizoot. 20:252-264. [DOI] [PubMed] [Google Scholar]
- 11.Portaels, F., P.-A. Fonteyne, and W. M. Meyers. 1999. Insects in transmission of Mycobacterium ulcerans infection. Lancet 353:986.. [DOI] [PubMed] [Google Scholar]
- 12.Roberts, B., and R. Hirst. 1997. Immunomagnetic separation and PCR for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 35:2709-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and P. D. R. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria Int. J. Lepr. 36:78-82. [PubMed] [Google Scholar]
- 15.Stinear, T., J. K. Davies, G. A. Jenkin, J. A. Hayman, F. Oppedisano, and P. D. R. Johnson. 2000. Identification of Mycobacterium ulcerans in the environment from regions in southeast Australia in which it is endemic with sequence capture-PCR. Appl. Environ. Microbiol. 66:3206-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stinear, T., J. K. Davies, G. A. Jenkin, F. Portaels, B. C. Ross, F. Oppedisano, M. Purcell, J. A. Hayman, and P. D. R. Johnson. 2000. A simple PCR method for rapid genotype analysis of Mycobacterium ulcerans. J. Clin. Microbiol. 38:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stinear, T., B. C. Ross, J. K. Davies, L. Marino, R. M. Robins-Browne, F. Oppedisano, A. Sievers, and P. D. R. Johnson. 1999. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J. Clin. Microbiol. 37:1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titus, R. G., and J. M. Ribeiro. 1988. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 239:1306-1308. [DOI] [PubMed] [Google Scholar]
- 19.Uganda Buruli Group. 1971. Epidemiology of Mycobacterium ulcerans infection at Kinyara, Uganda. Trans. R. Soc. Trop. Med. Hyg. 65:763-775. [DOI] [PubMed] [Google Scholar]