Abstract
Cercosporin is a non-host-specific polyketide toxin produced by many species of plant pathogens belonging to the genus Cercospora. This red-pigmented, light-activated toxin is an important pathogenicity determinant for Cercospora species. In this study, we screened 244 bacterial isolates representing 12 different genera for the ability to degrade cercosporin. Cercosporin degradation was determined by screening for the presence of cleared zones surrounding colonies on cercosporin-containing culture medium and was confirmed by assaying the kinetics of degradation in liquid medium. Bacteria belonging to four different genera exhibited the cercosporin-degrading phenotype. The isolates with the greatest cercosporin-degrading activity belonged to Xanthomonas campestris pv. zinniae and X. campestris pv. pruni. Isolates of these pathovars removed over 90% of the cercosporin from culture medium within 48 h. Bacterial degradation of red cercosporin was accompanied by a shift in the color of the growth medium to brown and then green. The disappearance of cercosporin was accompanied by the appearance of a transient green product, designated xanosporic acid. Xanosporic acid and its more stable lactone derivative, xanosporolactone, are nontoxic to cercosporin-sensitive fungi and to plant tissue and are labile in the presence of light. Detailed spectroscopic analysis (to be reported in a separate publication) of xanosporolactone revealed that cercosporin loses one methoxyl group and gains one oxygen atom in the bacterial conversion. The resulting chromophore (4,9-dihydroxy-3-oxaperlylen-10H-10-one) has never been reported before but is biosynthetically plausible via oxygen insertion by a cytochrome P-450 enzyme.
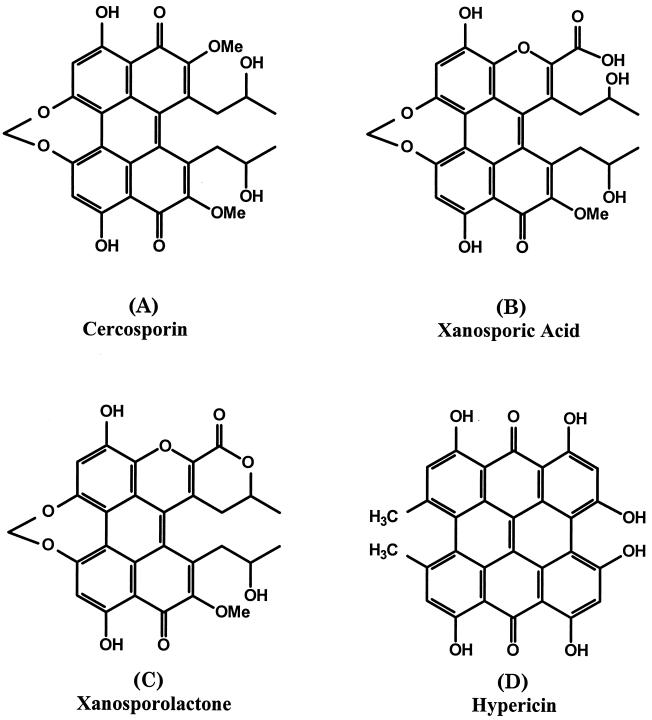
The fungal genus Cercospora contains many species capable of infecting a large number of diverse and economically important plants. The wide host range and worldwide distribution of these fungi, combined with the lack of robust resistance in host plants, have led to difficulties in designing adequate control strategies. The success of this group of fungi as pathogens is attributed to their production of a toxin, cercosporin, which is considered to be a primary pathogenicity factor (15). Cercosporin [1,12-bis(2-hydroxypropyl)-2,11-dimethoxy-6,7-methylenedioxy-4,9-dihydroxyperylene-3,10-quinone] (Fig. 1) is a photoactive, non-host-specific polyketide toxin (12).
FIG. 1.
Structures of cercosporin, xanosporic acid, xanosporolactone, and hypericin. Spectral analysis indicated that the green degradation product produced in culture is xanosporic acid, which is converted to its more stable lactone form during acidification for extraction from the medium.
Cercosporin, which was first isolated in 1957 by Kuyama and Tamura (31), belongs to a unique group of molecules known as photosensitizers (compounds that require light for cellular toxicity). Upon illumination, an effective photosensitizer absorbs light energy and enters the activated triplet state, which then interacts with oxygen, either via a reducing substrate to produce superoxide or through direct energy transfer to produce singlet oxygen (39). Light-induced production of both singlet oxygen and superoxide from cercosporin has been confirmed (16, 17, 32). Such active oxygen species cause oxidation of biomolecules, including lipids, vitamins, carbohydrates, proteins, and nucleic acids (24, 25, 47). Cercosporin localizes in the host plant's cellular membranes, where the activated oxygen induces lipid peroxidation and cross-linking, leading to membrane rigidity, electrolyte leakage, and ultimately cellular death (7, 14, 33). The nonspecific mode of action of cercosporin has made it difficult to breed plants for disease resistance, and only limited disease resistance has been reported for select agronomic crops, including corn, sugar beet, and soybean (9, 36, 38, 44, 46).
Several lines of evidence indicate that cercosporin plays a crucial role in the ability of Cercospora species to infect host plants. The toxin is produced by many members of the genus and can be extracted from infected tissue (18, 27, 31). Membrane damage in infected tissue is consistent with the mode of action of cercosporin (13, 22), and the requirement of light for symptom development in Cercospora diseases is well documented in several hosts (5, 6, 42). The most compelling evidence for the role of cercosporin in disease development is the finding that cercosporin-deficient mutants of Cercospora kikuchii are not pathogenic on soybean (43). These data suggest that strategies that interfere with cercosporin, such as toxin resistance or toxin degradation, may be useful for developing resistance to Cercospora diseases. Current control strategies include host resistance, cultural practices, and chemical applications, which may be used in conjunction with disease prediction models (8, 10, 36, 44, 46). With the exception of one report for rice (3), host resistance to Cercospora diseases has not been correlated with resistance to cercosporin or interference with cercosporin production or action. A long-term goal of our work is to identify the cercosporin degradation genes and to transfer them into plants in order to develop transgenic resistance to Cercospora diseases.
Microorganisms are attractive sources of genes that code for proteins that modify complex organic molecules. In 1993 Robeson et al. filed a patent describing a method to identify bacteria with the capacity to degrade cercosporin (37). Bacteria identified by this method (Bacillus thuringiensis, Pseudomonas fluorescens biovar V, Mycobacterium sp., and Bacillus subtilis) were not resistant to cercosporin in the light, yet they were able to degrade it in the dark. Degradation was determined by the ability of the bacterial isolates to generate a cleared zone in cercosporin-containing medium. To further demonstrate degradation, 14C-labeled cercosporin was incorporated into solid culture medium and incubated with cercosporin-degrading bacteria. The amount of radioactive carbon in cleared zones was significantly less than the amount observed in medium retaining the red color of cercosporin.
This study was performed to document degradation of the complex polyketide toxin cercosporin with the expectation that the information obtained may lead to future novel control strategies for cercosporin-producing pathogens. The objectives of this study were to identify the diversity of bacteria with the ability to degrade cercosporin, to investigate the kinetics of toxin degradation, and to isolate the product of cercosporin degradation in an attempt to elucidate how the degrading reactions proceed. Here we present the results of our screening analysis and of our characterization of the degrading phenotype and an initial description of the nontoxic cercosporin breakdown product not observed by Robeson et al.
MATERIALS AND METHODS
Bacterial cultures.
The bacterial isolates tested were obtained from the sources indicated in Table 1. Unless otherwise noted, isolates were maintained on Luria-Bertani (LB) medium (per liter: 10 g of NaCl, 10 g of tryptone, 5 g of yeast extract; pH 7.2) and were grown at 28°C.
TABLE 1.
Degradation of cercosporin by bacterial isolatesa
| Organism | No. of isolates tested | No. of isolates able to degradeb | Degrading isolate(s) | % Loss of cercosporinc |
|---|---|---|---|---|
| Xanthomonas campestris pv. pruni | 23 | 5 | XCP-11 | 87 |
| XCP-14 | 91 | |||
| XCP-77 | 96 | |||
| XCP-78 | 86 | |||
| BL-9 | 87 | |||
| Xanthomonas campestris pv. campestris | 2 | 0 | ||
| Xanthomonas campestris pv. pelargoniae | 2 | 0 | ||
| Xanthomonas campestris pv. vesicatoria | 3 | 0 | ||
| Xanthomonas campestris pv. zinniae | 32 | 32 | XCZ-1 to XCZ-32 | 85-99 |
| Pseudomonas syringae pv. glycinea | 5 | 0 | ||
| Pseudomonas syringae pv. tomato | 3 | 0 | ||
| Pseudomonas syringae pv. pisi | 3 | 1 | PSP-1 | 78 |
| Pseudomonas syringae pv. coronafaciens | 2 | 0 | ||
| Pseudomonas syringae pv. lachrymans | 2 | 0 | ||
| Pseudomonas syringae pv. syringae | 8 | 0 | ||
| Pseudomonas syringae pv. phaseolicola | 5 | 0 | ||
| Pseudomonas syringae pv. tabaci | 10 | 0 | ||
| Pseudomonas syringae unknown pathovar | 3 | 0 | ||
| Pseudomonas viridiflava | 3 | 0 | ||
| Pseudomonas unknown fluorescing pathovar | 28 | 0 | ||
| Pseudomonas unknown nonfluorescing pathovar | 23 | 0 | ||
| Pseudomonas unknown pathovar | 50 | 0 | ||
| Ralstonia solanacearum | 15 | 0 | ||
| Escherichia coli | 2 | 0 | ||
| Agrobacterium tumefaciens | 2 | 0 | ||
| Rhodococcus equi | 1 | 0 | ||
| Rhodococcus rhodochrous | 1 | 0 | ||
| Mycobacterium smegmatis | 1 | 1 | Sp7EIBW | 98 |
| Mycobacterium convolutum | 1 | 0 | ||
| Mycobacterium vaccae | 1 | 0 | ||
| Zoogloea ramigera | 1 | 0 | ||
| Gordona terrae | 1 | 0 | ||
| Nocardia asteroides | 1 | 0 | ||
| Bacillus megaterium | 1 | 0 | ||
| Bacillus subtilis | 1 | 1 | BS-1 | 82 |
| Bacillus cereus | 1 | 1 | BC-1 | 83 |
| Propylene users | 2 | 0 | ||
| Propane users | 2 | 2 | Sp2, Sp3 | 91-98 |
| Erwinia carotovora pv. atroseptica | 1 | 0 | ||
| Erwinia carotovora pv. carotovora | 1 | 0 | ||
| Erwinia chrysanthemi | 1 | 0 |
Isolates were obtained from the following sources: Xanthomonas and Erwina isolates, David Ritchie, North Carolina State University Department of Plant Pathology; Pseudomonas and Ralstonia isolates, Peter Lindgren, North Carolina State University Department of Plant Pathology, and Daniel Kluepfel, Clemson University Department of Plant Pathology and Physiology; and Rhodococcus, Mycobacterium, Zoogloea ramigera, Gordona terrae, Nocardia asteroides, and Bacillus isolates and the propylene and propane users, Jerome Perry, North Carolina State University Department of Microbiology. The Agrobacterium tumefaciens and Escherichia coli isolates were strains in use in our laboratory.
Degradation was determined by the ability to generate a clear zone extending into cercosporin-containing LB medium within 12 days.
Loss of cercosporin was assayed by removing cleared medium surrounding colonies, extracting cercosporin into acetone, and calculating the absorbance spectrophotometrically.
Cercosporin stocks and chemicals.
Cercosporin was extracted and purified from mycelial tissue of C. kikuchii as previously described (12), except that cercosporin was crystallized directly from the chloroform extract without column purification. Dried crystals were stored in the dark at −20°C. For working stocks, crystals were dissolved in 0.1 N NaOH that was subsequently added to medium at the appropriate dilution with a corresponding equal volume of 0.1 N HCl. All chemicals were of the highest purity available and were used as received. Unless otherwise noted, all organic chemicals were purchased from Fisher Scientific (Pittsburgh, Pa.).
Screen for cercosporin-degrading bacteria.
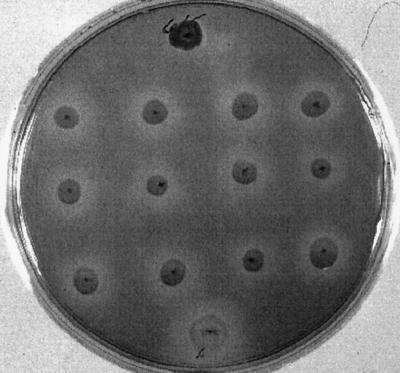
Bacterial isolates were grown on solid LB medium for 48 h or until colonies were visible. Each isolate was then inoculated onto NACE medium (nutrient agar [Difco] containing 50 μM cercosporin) and incubated in the dark for 12 days at 28°C. The solidified medium was red due to the addition of cercosporin, and toxin degradation was indicated by a clear zone surrounding a bacterial colony (Fig. 2). The concentration of cercosporin remaining in the medium was determined by removing the medium directly below a colony with a no. 3 cork borer and extracting the crushed medium for 12 h in 250 μl of acetone at room temperature in the dark. Remaining agar particles were removed by centrifugation, and the supernatant was assayed for cercosporin. Concentrations of cercosporin were calculated spectrophotometrically from the absorbance measured at cercosporin's absorbance maximum at 473 nm by using 26,600 as the molar extinction coefficient (32).
FIG. 2.
Plate assay for cercosporin degradation. Bacteria were inoculated onto plates containing NACE medium and incubated in the dark for 12 days at 28°C. The degrading isolates (all isolates except the top center isolate) retained the normal colony color and showed distinct clearing of the cercosporin in the medium surrounding the colonies. The nondegrading isolate (top center isolate) was purple and did not produce the distinct halo produced by the degrading isolates.
Time course of cercosporin degradation.
One-milliliter portions of log-phase liquid cultures of the cercosporin-degrading isolates Xanthomonas campestris pv. pruni XCP-77 and X. campestris pv. zinniae XCZ-3 and the nondegrading isolate X. campestris pv. pruni XCP-76 were inoculated into flasks containing 50 ml of LB broth with 60 μM cercosporin and were incubated in the dark at 28°C with constant agitation. Every 12 h for a 110-h period, 5 ml of each growing culture was removed and stored at −20oC until it was analyzed. Each sample was extracted with 2 ml of chloroform. To determine the amount of cercosporin in each sample, the chloroform phase was assayed spectrophotometrically, and the absorbance at 473 nm was determined.
Time course of breakdown product accumulation.
Log-phase cultures of isolate XCZ-3 in LB medium containing 50 μM cercosporin were incubated in the dark at 28°C for 108 h. A 5-ml sample of culture medium was removed every 12 h. Cercosporin and the green breakdown product were isolated from each sample as follows. The sample was briefly shaken in 2 ml of chloroform and then centrifuged at 9,715 × g for 2 min to separate the aqueous and organic phases. The organic phase containing any nondegraded cercosporin was removed. The green breakdown product was removed from the remaining aqueous phase, following adjustment of the pH of the sample to 2.0 with 1 N HCl, by an additional chloroform extraction. The chloroform was evaporated from the sample, and the residue was resuspended in acetone. The absorbance for cercosporin and the absorbance for the breakdown product were measured at 472 and 451 nm, respectively (the absorbance maxima of the two molecules).
Isolation of the green product.
Liquid medium cultures of isolate XCZ-3 containing 50 μM cercosporin were incubated in the dark at 28°C for 48 to 60 h, at which time the pH of the medium was 8.4 and the green degradation product (subsequently identified as xanosporic acid [Fig. 1]) was present as a water-soluble anion. Residual cercosporin was removed by adding 0.25 volume of chloroform, shaking, and allowing the phases to separate. The organic layer was removed, and the aqueous layer was acidified to pH 2 with 1 N HCl, which was subsequently shown to cause lactonization of xanosporic acid to form xanosporolactone (Fig. 1). A second chloroform extraction was performed, and the organic phase containing the green xanosporolactone product was collected and centrifuged at 9,715 × g to separate the cellular debris and the remaining aqueous phase. The chloroform was evaporated, and the resulting green solid was dissolved in acetone and stored in the dark at −20°C.
Further purification of the green xanosporolactone product was achieved by using thin-layer chromatography (TLC). The partially purified extract was loaded onto 500-μm Whatman silica gel plates and was resolved by using a solvent system consisting of ethyl acetate, hexane, methanol, and water (6:4:1.5:1). A green band at an Rf of 0.84 was scraped off each glass plate support, and the product was eluted from the silica gel matrix with ethyl acetate. After evaporation of the ethyl acetate, the residue was dissolved in 40% acetonitrile-water. Final purification was done by passing the extract over a C8 Accu-Bond solid-phase extraction resin (J & W Scientific, Folsom, Calif.). The extract was applied to the column, and the column was washed with 2 bed volumes of 40% acetonitrile-water and 4 bed volumes of hexane to elute any contaminants. The green product retained by the C8 matrix was eluted with 1 bed volume of acetone and stored at −20°C in the dark. The final purity of the green xanosporolactone product was assayed by using TLC plates containing a fluorescent indicator. No fluorescing quenching or phosphomolybdic acid-oxidizable impurities were detected. Due to the high degree of sensitivity of the green xanosporolactone product to light, extraction and purification were performed under conditions that minimized light exposure.
Toxicity assays.
Two assays were used to determine the toxicity of the green product. First, the toxicity of the unpurified xanosporic acid product was tested with a cercosporin-sensitive mutant of Cercospora nicotianae (CS-8) (26). Flasks containing malt medium (per liter: 15 g of malt extract, 3 g of peptone, 30 g of glucose) supplemented with 10 μM cercosporin were inoculated with XCZ-3 and incubated in the dark at 28°C with constant agitation. After 8 days of incubation, the bacteria were removed from the medium by centrifugation at 10,000 rpm for 10 min. Ground mycelium of C. nicotianae mutant CS-8 was then inoculated into the bacterium-free medium and grown for 7 days in the light (54 microeinsteins·m−2·s−1). The controls included cercosporin-containing medium preinoculated with XCP-76, malt medium alone (no cercosporin) preincubated with isolate XCZ-3, and malt medium alone with no preincubation. The growth of mutant CS-8 in all preparations was determined by harvesting the fungal mass by vacuum filtration, lyophilizing the fungal mass, and weighing the dried material.
The toxicity of the purified xanosporolactone product was measured by a plant infiltration assay. Eight-week-old leaf tissue of tobacco cultivar Burley-21 was treated with a solution of the chromatographically purified green product (ca. 1, 5, 10, and 50 μM) dissolved in 20% acetone. The concentrations were adjusted by using the molar extinction coefficient of reduced cercosporin (17,600), which also has a green chromophore, and were therefore approximate. Cercosporin controls were infiltrated at concentrations of 0.8 and 1.5 μM, along with a control consisting of 20% acetone alone. Each concentration of the green xanosporolactone product and controls was infiltrated at two sites per leaf on three different plants. The plants were allowed to grow in the greenhouse, and lesions were evaluated after 4 and 8 days.
Spectral analysis of the purified xanosporolactone product.
The absorbance of each sample examined in acetone was determined with a Beckman DU650 spectrophotometer. Electrospray ionization experiments were performed with a Micromass Quattro II triple quadrupole mass spectrometer equipped with an electrospray ionization source (Micromass, Inc., Manchester, United Kingdom); each sample was injected in acetonitrile-water (80:20), and the mass spectra were acquired at cone voltage settings of 30 and 60 eV. Low- and high-resolution mass spectra were measured with direct probe fast atom bombardment (FAB) by using a JOEL HX110HF spectrometer. Both electrospray ionization and FAB experiments were conducted at the Mass Spectrometry Laboratory for Biotechnology Research, Department of Chemistry, North Carolina State University.
RESULTS
Identification of cercosporin-degrading bacteria.
To identify cercosporin-degrading bacteria, we screened 244 isolates representing over 12 genera containing 23 different species in this study (Table 1). Isolates were initially screened visually for the production of a cleared zone surrounding colonies in cercosporin-containing solid medium, as well as for colony color (Fig. 2). An initial transient discoloration of the medium immediately around the colony was observed for most isolates, perhaps due to cercosporin diffusing into the bacterial cells. Isolates unable to degrade cercosporin produced a limited clear area restricted to the medium immediately under the colony and concurrently turned purple, suggesting that cercosporin uptake or adsorption occurred. A few isolates established a permanent and expanded clear zone in the medium, suggesting that degradation of the cercosporin occurred in that area. Cercosporin-degrading isolates were then rescreened, and degrading activity was quantified by extracting the medium below each colony and determining the concentration of cercosporin spectrophotometrically. Quantification of the cercosporin in the cleared zones revealed a loss of over 85% of the compound (Table 1), suggesting that the bacteria actively degraded the toxin. The isolates with the greatest ability to degrade cercosporin (as defined by removal of more than 85% of the cercosporin) were X. campestris pv. pruni isolates XCP-11, XCP-14, XCP-77, XCP-78, and BL-9, 32 strains of X. campestris pv. zinniae (isolates XCZ-1 through XCZ-32), Mycobacterium smegmatis strain Sp7EIBW, and two unidentified bacteria able to utilize propane as a carbon source. Two of these isolates (X. campestris pv. pruni XCP-77 and X. campestris pv. zinniae XCZ-3) were chosen for further investigation. An isolate unable to degrade cercosporin (X. campestris pv. pruni strain XCP-76) was used as a negative control.
Characterization of the degrading phenotype.
Cercosporin-degrading isolates XCZ-3 and XCP-77 were examined for the ability to use cercosporin as a sole carbon source when they were grown on a minimal salts medium amended with 30 μM cercosporin. These two isolates were unable to grow in the dark on this medium, indicating that they were not able to use cercosporin as a sole carbon source (data not shown). These data suggest that the degrading bacteria derive no carbon from the degradation of cercosporin.
The degrading isolate XCZ-3 and the nondegrading isolate XCP-76 were assayed for the ability to grow on cercosporin-containing medium in the light. These two isolates were spotted on LB medium containing 0.7, 1.0, 1.25, and 2 μM cercosporin and incubated in the presence of constant light (75 microeinsteins·m−2·s−1). XCZ-3 was able to grow on 0, 1.0, and 1.25 μM cercosporin, whereas XCP-76 was unable to grow on cercosporin at any of these concentrations. Thus, the ability to degrade cercosporin provides a small but measurable increase in cercosporin resistance.
Cercosporin-degrading isolate XCZ-3 was tested for the ability to degrade hypericin (Fig. 1), a phenanthroperylenequinone photosensitizer produced by the herb St. John's wort (Hypericum perforatum). XCZ-3 was grown in LB medium with 20 μg of hypericin per ml. After 60 h, the amount of hypericin in the culture medium was assayed spectrophotometrically. At this time, no loss of hypericin from the culture medium was detected (data not shown). Thus, XCZ-3 is not able to degrade the phenanthroperylenequinone, indicating that the degradation reaction is specific for structural constituents found in the cercosporin molecule but not in hypericin.
Time course for cercosporin degradation and accumulation of the breakdown product.
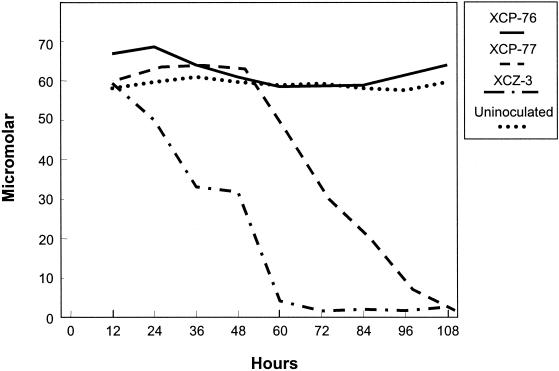
The kinetics of cercosporin degradation was determined by growing isolates XCP-76, XCP-77, and XCZ-3 in the dark in cercosporin-containing LB medium and quantifying the cercosporin concentration spectrophotometrically at 12-h intervals up to 108 h. The experiment was performed three times with comparable results; the results of one experiment are shown in Fig. 3. In the absence of light, cercosporin is stable in liquid medium, as shown by the lack of change in the cercosporin concentration in medium lacking bacteria. Nondegrading isolate XCP-76 did not cause any loss of cercosporin. The two cercosporin-degrading isolates (XCP-77 and XCZ-3) decreased the concentration of cercosporin in the medium to nondetectable levels. In all experiments, isolate XCZ-3 initiated cercosporin breakdown earlier than isolate XCP-77. Once breakdown was initiated, the rates and overall extents of degradation were similar for the two isolates.
FIG. 3.
Kinetics of cercosporin degradation by bacteria: concentrations of cercosporin present in cercosporin-containing medium alone (Uninoculated) or following inoculation with a nondegrading isolate (X. campestris pv. pruni XCP-76) or two degrading isolates (X. campestris pv. pruni XCP-77 and X. campestris pv. zinniae XCZ-3) at various times after inoculation. The cercosporin concentration was determined by extracting cercosporin from the medium and measuring the absorbance of the organic extract at 473 nm. The cercosporin concentrations in the uninoculated control and in the preparation inoculated with XCP-76 did not decrease over time. The kinetics of cercosporin degradation by XCP-77 and XCZ-3 were similar except for a 48-h lag in the initiation of degradation by XCP-77.
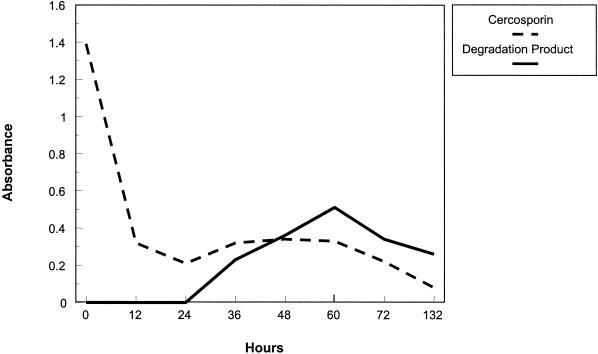
As cercosporin was lost from the culture medium, a green compound accumulated. A time course study was conducted to look at the loss of cercosporin and the accumulation of the green product relative to each other. The results are shown in Fig. 4. The cercosporin concentration decreased rapidly during the first 24 h. The product was first detected at 24 h, and the concentration increased until 60 h, after which it gradually decreased. These data support visual observations that the green pigment is lost from culture medium with time. The difference between the time that cercosporin disappeared and the time that the green product was detected in the culture medium can be attributed to sequestration of cercosporin in the bacterial pellet at early time points in a form in which the cercosporin is not extractable with CHCl3 (unpublished results). TLC of culture extracts taken after 10 and 120 h of incubation confirmed that cercosporin was converted to a green product with an altered Rf.
FIG. 4.
Kinetics of loss of cercosporin and accumulation of the green product in cercosporin-containing medium inoculated with the cercosporin-degrading bacterium X. campestris pv. zinniae isolate XCZ-3. Compounds were extracted and quantified as described in Materials and Methods. Following a decrease in the cercosporin concentration during the first 12 h after inoculation, the breakdown product was first detected at 24 h and the concentration increased until 60 h. After 60 h, there was a dramatic decrease in the amount of the breakdown product, suggesting that further metabolism occurred. By 132 h, all cercosporin was removed from the culture medium. This experiment was repeated once, and the two experiments yielded comparable results.
Identification of the cercosporin breakdown product.
The green breakdown product was extracted from acidified culture medium with chloroform and was separated by TLC. TLC resolved a unique green compound with an Rf of 0.84, compared to an Rf of 0.77 for cercosporin, when a solvent system consisting of ethyl acetate, hexane, methanol, and water (6:4:1.5:1) was used. The silica matrix containing the green compound was scraped from the glass support, and the sample was analyzed by spectrophotometry and FAB. FAB showed a single peak. Over time, the green breakdown product was converted to a colorless molecule(s) that was not identifiable on TLC plates with or without a fluorescence indicator or when plates were treated with phosphomolybdic acid (data not shown).
The TLC-purified green product was characterized further. Spectral analysis of this product identified it as xanosporolactone (Fig. 1). Subsequent analysis (T. K. Mitchell, F. Alejos-Gonzalez, H. Gracz, D. A. Danehower, M. E. Daub, and W. S. Chilton, unpublished data) indicated that the form produced by XCZ-3 in culture is xanosporic acid (Fig. 1), which upon acid extraction by the method used in this study is converted to the lactone form. The acid form is highly labile, so the product was isolated as the lactone, which we completely characterized. The absorbance and fluorescence maxima for xanosporolactone are 451 and 580 nm (dual absorbance peaks) and 557 nm, respectively, compared to maxima of 473 and 606 nm for cercosporin. Positive and negative FAB experiments revealed that the mass of xanosporolactone is 518 mass units, 16 mass units less than the mass of cercosporin (534 mass units). High-resolution mass spectra obtained by using positive FAB ionization showed that the exact mass of xanosporolactone is 518.1232 mass units and that the most probable elemental composition is C28H22O10 (518.1212 mass units). When xanosporolactone is compared to cercosporin (C29H26O10), the compositional difference between the two molecules is one carbon and four hydrogen atoms. The hydrogen magnetic resonance spectroscopy elemental formula of xanosporolactone (C28H22O10) indicates that there is a formal loss of CH4 from cercosporin (C29H26O10). However, nuclear magnetic resonance data indicate that there is a loss of one methoxyl group and therefore a gain of one oxygen atom (—CH3OH + O = —CH4), with the creation of one carboxylic acid equivalent. Since the nuclear magnetic resonance data for substituents on three of the rings of xanosporolactone are almost identical to the data for cercosporin, all of the reactions must occur in a single ring. A plausible biodegradation pathway would be initiated by cytochrome P-450 monooxygenase-mediated insertion of oxygen into one of the quinonoid rings of cercosporin, expanding the ring to a seven-member cyclic ester (lactone). Subsequent, and possibly spontaneous, hydrolytic opening of the lactone ring could be followed by spontaneous recyclization to a six-member oxygen-containing ring accompanied by loss of the methoxy group to form the previously unknown 4,9-dihydroxy-3-oxaperylene-10H-10-one chromophore of xanosporolactone. A detailed spectroscopic and structural analysis will be described in a separate publication (Mitchell et al., submitted for publication).
Bioassay of xanosporic acid and xanosporolactone toxicities.
The toxicities of xanosporic acid and xanosporolactone were tested by using a cercosporin-sensitive mutant of C. nicotianae and tobacco leaf tissue, respectively. The results of the fungal assay for xansoporic acid toxicity are shown in Table 2. When the cercosporin-sensitive mutant CS-8 was grown in cercosporin-containing medium preinoculated with nondegrading isolate XCP-76, cercosporin in the medium inhibited the growth of the fungus. When cercosporin was converted to xanosporic acid by preinoculation with the degrading isolate XCZ-3, CS-8 grew to levels equivalent to those in nontreated malt medium or in non-cercosporin-containing medium preinoculated with XCZ-3.
TABLE 2.
Assay of toxicity of xanosporic acid to a cercosporin-sensitive mutant (CS-8) of C. nicotianaea
| Treatment | Fungal growth (g [dry wt])
|
||
|---|---|---|---|
| Cercosporin | Preincubated bacteria | Expt 1 | Expt 2 |
| + | XCZ-3b | 2.6 | 3.4 |
| + | XCP-76c | 0.3 | 0.5 |
| − | XCZ-3 | 3.8 | 4.8 |
| − | None | 4.1 | 3.4 |
CS-8 was grown in cercosporin-containing medium following preincubation with the cercosporin-degrading organism X. campestris pv. zinniae isolate XCZ-3 to produce xanosporic acid. Fungal growth was compared to fungal growth in cercosporin-containing medium preincubated with the nondegrading organism X. campestris pv. pruni isolate XCP-76 (no loss of cercosporin) or in medium lacking cercosporin, both alone and following preincubation with XCZ-3.
Cercosporin was converted to xanosporic acid.
Cercosporin was not converted to xanosporic acid.
The toxicity of purified xanosporolactone was tested by infiltration into Burley-21 tobacco leaf tissue. When cercosporin was infiltrated into leaves, necrotic lesions formed after 4 days of incubation under normal greenhouse conditions when concentrations greater than 0.9 μM were used (data not shown). Xanosporolactone was infiltrated into leaves at concentrations of 0, 1, 6, 10, and 50 μM (estimated as described in Materials and Methods). After 8 days, no visible necrotic lesions had formed with any of the concentrations infiltrated, indicating that there was no toxicity.
DISCUSSION
Many fungi in the genus Cercospora have developed the ability to produce a molecule (cercosporin) that is highly toxic to most organisms, including plants, animals, and microorganisms (15). As cercosporin's role in the pathogenesis of this group of fungi is well documented (12, 43), research efforts directed towards alleviating the impact of the toxin hold promise for control of these ubiquitous diseases. Currently, resistance, chemicals, and cultural practices are used to control Cercospora diseases (8, 9, 19, 28). Studies related to the toxin have focused mostly on the mechanisms of resistance in the producing fungi and on understanding cercosporin biosynthesis. Neither of these approaches has provided practical disease control yet.
The long-term goal of our studies is to develop a strategy for the control of diseases caused by Cercospora species by transforming hosts with genes encoding proteins that can degrade cercosporin to nontoxic products. There are a number of precedents for this approach. For example, resistance to a trichothecene mycotoxin has been obtained by mobilizing into sensitive yeast a gene encoding an enzyme that acetylates the toxin (30). While yeasts are not natural hosts for fungi that produce trichothecenes, this study showed that genes involved in detoxification may be transferred to and protect toxin-sensitive organisms. A gene that codes for tabtoxin-specific degradation has provided resistance in transformed tobacco plants to the tabtoxin-producing bacterial pathogen Pseudomonas syringae pv. tabaci (2, 48). A gene (VR-ERE) isolated from Eutypa lata-tolerant grapevines, which encodes a protein that reduces the toxin eutypine to its corresponding alcohol, conferred resistance to eutypine in grapevine cells, although it has yet to be shown that whole-plant resistance can be achieved (11, 21).
We report identification of bacterial isolates that have the capacity to detoxify and degrade the fungal toxin cercosporin. Of 244 bacterial isolates screened, 40 were able to degrade 85% or more of the cercosporin in the growth medium. The best degrading isolates were X. campestris pv. zinniae isolate XCZ-3, X. campestris pv. pruni isolate XCP-77, and two unidentified propane users. Cercosporin-degrading bacteria previously identified by Robeson et al. (37) were originally isolated from plant tissue infected with Cercospora; thus, the tissue contained cercosporin. The degrading isolates which we found were isolated from plants with no known infestation of cercosporin-producing fungi. There is no intuitive reason why these bacteria have this capability. No naturally occurring perylenequinones have been identified yet from Xanthomonas species or their host plants (4). Furthermore, Cercospora species are not known to be significant members of the microflora of the leaf surfaces of these hosts. However, Xanthomonas species have been identified that are capable of degrading diverse organic compounds that they may not commonly encounter in nature (1, 23, 34, 35, 41, 45). Selected examples of aromatic molecules degraded by Xanthomonas spp. include protocatechuate, phenylalanine, lignins, toluene, fuel oil, and styrene (1, 20, 29, 40, 45).
It is interesting that of the 43 isolates able to degrade cercosporin, 32 were X. campestris pv. zinniae isolates. Every isolate tested belonging to this pathovar was able to degrade cercosporin, even though the 32 isolates were isolated at different times (1985 through 1990) and came from diverse locations, including Raleigh, N.C., Lansing, Mich., and Winchester, Va. It is curious that for the other bacteria tested, not all of the isolates belonging to the same pathovar are capable of degrading cercosporin. These data suggest that there is heterogeneity within the pathovars studied.
Time course studies showed that degradation of cercosporin in liquid medium by XCZ-3 is rapid. Degradation is initiated within the first 12 h of culture growth and is completed by 60 h, giving an estimated rate of degradation of 0.83 μM·h−1. During the process of degradation, the toxin is transiently converted into a green nontoxic compound, which we isolated, purified, and identified as xanosporic acid via its lactone derivative. Time course experiments also showed that as cercosporin is degraded, the concentration of xanosporic acid increases; however, there is a distinct lag between the time that cercosporin is lost from the medium and the time that xanosporic acid accumulates. At this time, we are not able to explain the temporal lag between cercosporin degradation and detectable xanosporic acid accumulation. However, experiments whose results are not presented here have suggested that rapid sequestration of cercosporin in the capsular polysaccharide rather than internal degradation by the bacteria is the cause of this lag. Cell extracts of cultures previously containing XCZ-3 were tested for the ability to degrade cercosporin. In no experiment, and under no conditions, did the level of cercosporin decrease in response to these extracts, suggesting that cercosporin is not degraded outside the cell (data not shown).
Elucidation of the structure of xanosporic acid may allow identification of the mechanism(s) involved in the early steps of cercosporin degradation. The enzymatic reaction may be as simple as a cytochrome P-450-mediated oxygen insertion. Insertion of an oxygen atom into one of the quinone rings of cercosporin is an example of the well-known Bayer-Villager chemical oxidation, which is also observed in several biological oxidations, including oxidation of aflatoxin B1 to aflatoxin G1. In this case, the responsible enzyme has properties of a cytochrome P-450 monooxygenase (microsomal localization and inhibition by P-450 inhibitors).
Acknowledgments
This research was supported by a grant from Pioneer HyBrid International.
We thank Gary Payne, Pete Lindgren, and Marilyn Ehrenshaft for their suggestions and consultations.
REFERENCES
- 1.Arnold, M., A. Reittu, A. von Wright, P. J. Martikainen, and M.-L. Suihko. 1997. Bacterial degradation of styrene in waste gasses using a peat filter. Appl. Microbiol. Biotechnol. 48:738-744. [DOI] [PubMed] [Google Scholar]
- 2.Batchvarova, R., V. Nikolaeva, S. Slavov, S. Bossolova, V. Valkov, S. Atanassova, S. Guelemerov, A. Atanassov, and H. Anzai. 1998. Transgenic tobacco cultivars resistant to Pseudomonas syringae pv. tabaci. Theor. Appl. Genet. 97:986-989. [Google Scholar]
- 3.Batchvarova, R. B., V. S. Reddy, and J. Bennett. 1992. Cellular resistance in rice to cercosporin, a toxin of Cercospora. Phytopathology 82:642-646. [Google Scholar]
- 4.Buckingham, J. 1994. Dictionary of natural products, 1st ed., vol. 7. Chapman and Hall, London, United Kingdom.
- 5.Calpouzos, L. 1966. Action of oil in the control of plant disease. Annu. Rev. Phytopathol. 4:369-390. [Google Scholar]
- 6.Calpouzos, L., and G. F. Stalknecht. 1967. Symptoms of Cercospora leaf spot of sugar beets influenced by light intensity. Phytopathology 57:799-800. [Google Scholar]
- 7.Cavallini, L., A. Binoldi, F. Macri, and A. Vianello. 1979. Lipid peroxidation induced by cercosporin as a possible determinant of its toxicity. Chem. Biol. Interact. 28:139-146. [DOI] [PubMed] [Google Scholar]
- 8.Coates, S. T., and D. G. White. 1998. Inheritance of resistance to gray leaf spot in crosses involving selected resistant inbred lines of corn. Phytopathology 88:972-982. [DOI] [PubMed] [Google Scholar]
- 9.Coates, S. T., and D. G. White. 1994. Sources of resistance to gray leaf spot of corn. Plant Dis. 78:1153-1155. [Google Scholar]
- 10.Cold, J. M. J., M. D. Laing, and D. C. Nowell. 1997. Chemical control of maize grey leaf spot. Crop Prot. 16:265-271. [Google Scholar]
- 11.Colrat, S., C. Deswarte, A. Latche, A. Klaebe, M. Bouzayen, J. Fallot, and J. P. Roustan. 1999. Enzymatic detoxification of eutypine, a toxin from Eutypa lata, by Vitis vinifera cells: partial purification of an NADPH-dependent aldehyde reductase. Planta 207:544-550. [Google Scholar]
- 12.Daub, M. E. 1982. Cercosporin, a photosensitizing toxin from Cercospora species. Phytopathology 72:370-374. [Google Scholar]
- 13.Daub, M. E. 1987. The fungal photosensitizer cercosporin and its role in plant disease, p. 271-280. In J. R. Heitz and K. R. Downum (ed.), Light-activated pesticides. American Chemical Society, Washington, D.C.
- 14.Daub, M. E. 1982. Peroxidation of tobacco membrane lipids by the photosensitizing toxin, cercosporin. Plant Physiol. 69:1361-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daub, M. E., and M. Ehrenshaft. 2000. The photoactive cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 38:461-490. [DOI] [PubMed] [Google Scholar]
- 16.Daub, M. E., and R. P. Hangarter. 1983. Production of singlet oxygen and superoxide by the fungal toxin, cercosporin. Plant Physiol. 73:855-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrowolski, D. C., and C. S. Foote. 1983. Chemistry of singlet oxygen 46. Quantum yield of cercosporin-sensitized singlet oxygen formation. Angew. Chem. Int. Ed. Engl. 95:729-730. [Google Scholar]
- 18.Fajola, A. O. 1978. Cercosporin, a phytotoxin from Cercospora species. Physiol. Plant Pathol. 13:157-164. [Google Scholar]
- 19.Fajola, A. O., and S. O. Alasoadura. 1973. Chemical control of the frog-eye disease (Cercospora nicotianae) of tobacco (Nicotiana tabacum) in Nigeria. Ann. Appl. Biol. 74:219-224. [Google Scholar]
- 20.Grishchenkov, V. G., R. R. Gayazov, V. G. Tokarev, V. V. Kochetkov, A. E. Filonov, and A. M. Boronin. 1997. Bacterial strains degrading fuel oil: microbial degradation under laboratory conditions. Appl. Biochem. Microbiol. 33:378-381. [Google Scholar]
- 21.Guillen, P., M. Guis, G. Martinez-Reina, S. Colrat, S. Dalmayrac, C. Deswarte, M. Bouzayen, J. P. Roustan, J. Fallot, J. C. Pech, and A. Latche. 1998. A novel NADPH-dependent aldehyde reductase gene from Vigna radiata confers resistance to the grapevine fungal toxin eutypine. Plant J. 16:335-343. [DOI] [PubMed] [Google Scholar]
- 22.Gwinn, K. D., D. A. Stelzig, and J. L. Brooks. 1987. Effects of corn plant-age and cultivar on resistance to Cercospora zeae-maydis and sensitivity to cercosporin. Plant Dis. 71:603-606. [Google Scholar]
- 23.Hamann, C., J. Hegemann, and A. Hildebrandt. 1999. Detection of polycyclic aromatic hydrocarbon degradation genes in different soil bacteria by polymerase chain reaction and DNA hybridization. FEMS Microbiol. Lett. 173:255-263. [DOI] [PubMed] [Google Scholar]
- 24.Ito, T. 1981. Dye binding and photodynamic action. Photochem. Photobiol. 33:947-955. [Google Scholar]
- 25.Ito, T. 1982. Photodynamic agents as tools for cell biology. Photochem. Photobiol. Rev. 7:141-186. [Google Scholar]
- 26.Jenns, A. E., and M. E. Daub. 1995. Characterization of mutants of Cercospora nicotianae sensitive to the toxin cercosporin. Phytopathology 85:906-912. [Google Scholar]
- 27.Jenns, A. E., M. E. Daub, and R. G. Upchurch. 1989. Regulation of cercosporin accumulation in culture by medium and temperature manipulation. Phytopathology 79:213-219. [Google Scholar]
- 28.Johnson, C. S., and M. K. Beute. 1986. The role of partial resistance in the management of cercospora leaf spot of peanut in North Carolina. Phytopathology 76:468-472. [Google Scholar]
- 29.Kern, H. W., and T. K. Kirk. 1987. Influence of molecular size and ligninase pretreatment on degradation of lignins by Xanthomonas sp. strain 99. Appl. Environ. Microbiol. 53:2242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura, M., I. Keneko, M. Komiyama, A. Takatsuke, H. Koshino, K. Yoneyama, and I. Yamagushi. 1998. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins-cloning and characterization of Tri101. J. Biol. Chem. 273:1654-1661. [DOI] [PubMed] [Google Scholar]
- 31.Kuyama, S., and T. Tamura. 1957. Cercosporin. A pigment of Cercospora kikuchii Matsumoto et Tomoyasu. I. Cultivation of fungus, isolation and purification of pigment. J. Am. Chem. Soc. 79:5725-5726. [Google Scholar]
- 32.Leisman, G. B., and M. E. Daub. 1992. Singlet oxygen yields, optical properties, and phototoxicity of reduced derivatives of the photosensitizer cercosporin. Photochem. Photobiol. 55:373-379. [Google Scholar]
- 33.Macri, F., and A. Vianello. 1979. Photodynamic activity of cercosporin on plant tissue. Plant Cell Environ. 2:262-271. [Google Scholar]
- 34.Masaphy, S., T. Fahima, D. Levanon, Y. Henis, and U. Mingelgrin. 1996. Parathion degradation by Xanthomonas sp. and its crude enzyme extract in clay suspensions. J. Environ. Qual. 25:1248-1255. [Google Scholar]
- 35.McGhee, I., and R. G. Burns. 1995. Biodegradation of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2-mentyl-4-chlorophenoxyacetic acid (MCPA) in contaminated soils. Appl. Soil Ecol. 2:143-154. [Google Scholar]
- 36.Orth, C. E., and W. Schuh. 1994. Resistance of 17 soybean cultivars to foliar, latent, and seed infection by Cercospora kikuchii. Plant Dis. 78:661-664. [Google Scholar]
- 37.Robeson, J. R., M. A. F. Jalal, and R. B. Simpsom. November 1993. Methods for identifying cercosporin-degrading microorganisms. U.S. patent 5,262,306.
- 38.Rossi, V. 1995. Effect of host resistance in decreasing infection rate of Cercospora leaf spot epidemics on sugarbeet. Phytopathol. Mediterr. 34:149-156. [Google Scholar]
- 39.Spikes, J. D. 1989. Photosensitization, p. 79-110. In K. C. Smith (ed.), The science of photobiology, 2nd ed. Plenum Press, New York, N.Y.
- 40.Su, J. J., and D. Kafkewitz. 1996. Toluene and xylene degradation by a denitrifying strain of Xanthomonas maltophilia with limited or no oxygen. Chemosphere 32:1843-1850. [Google Scholar]
- 41.Tchelet, R., D. Levanon, U. Mingelgrin, and Y. Henis. 1993. Parathion degradation by a Pseudomonas sp. and a Xanthomonas sp. and by their crude enzyme extracts as affected by some cations. Soil Biol. Biochem. 25:1665-1671. [Google Scholar]
- 42.Thorold, C. A. 1940. Cultivation of bananas under shade for the control of leaf spot disease. Trop. Agric. Trin. 17:213-214. [Google Scholar]
- 43.Upchurch, R. G., D. C. Walker, J. A. Rollins, M. Ehrenshaft, and M. E. Daub. 1991. Mutants of Cercospora kikuchii altered in cercosporin synthesis and pathogenicity. Appl. Environ. Microbiol. 57:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward, J. M. J., E. L. Stromberg, D. C. Nowell, and F. W. Nutter. 1999. Grey leaf spot, a disease of global importance in maize production. Plant Dis. 83:884-895. [DOI] [PubMed] [Google Scholar]
- 45.William, F., and A. Mahadevan. 1980. Degradation of aromatic compounds by Xanthomonas species. J. Plant Dis. Prot. 87:738-744. [Google Scholar]
- 46.Windels, C. E., H. A. Lamey, D. Hilde, J. Widner, and T. Knudsen. 1998. A cercospora leaf spot model for sugar beet. Plant Dis. 82:716-726. [DOI] [PubMed] [Google Scholar]
- 47.Yasaei, P. M., G. C. Yang, C. R. Warner, D. H. Daniels, and Y. Ku. 1996. Singlet oxygen oxidation of lipids resulting from photochemical sensitizers in the presence of antioxidants. J. Am. Oil Chem. Soc. 73:1177-1181. [Google Scholar]
- 48.Yoneyama, K., and H. Anzai. 1993. Transgenic plants resistant to diseases by the detoxification of toxins, p. 115-137. In I. Chet (ed.), Bio/technology in plant disease control. Wiley-Liss, Inc., New York, N.Y.