Abstract
Barth syndrome (BTHS) is an X-linked recessive disorder characterized by cardiomyopathy, skeletal muscle myopathy and fatigue, growth restriction, and neutropenia. Neutropenia increases the risk of life-threatening bacterial infections, a major cause of death in individuals with BTHS. Currently, there is no curative treatment for BTHS or associated neutropenia. The development of therapeutic strategies to correct BTHS-associated neutropenia has been hindered by a limited understanding of the underlying molecular mechanisms involved. BTHS is caused by a mutation in the Tafazzin gene encoding a transacylase required for the maturation of cardiolipin, an inner mitochondrial membrane phospholipid crucial for mitochondrial structure and function. We introduced a BTHS patient’s point mutation (TAZD75H) into the mouse Tafazzin enzyme’s critical acyltransferase site using CRISPR/Cas9-mediated genome editing, resulting in a patient-tailored point mutant knock-in BTHS model (TazD75H) that expresses a stable mutant TazD75H protein lacking transacylase activity. TazD75H mice were then used to investigate how loss of Tafazzin enzymatic activity impacts hematopoiesis. Male TazD75H mice exhibited impaired granulopoiesis and neutropenia secondary to impaired function of hematopoietic progenitors. Furthermore, they demonstrated age-dependent neutrophil maturation impairment reflecting the variable neutropenia observed in BTHS patients. Additionally, male TazD75H mice exhibit chronic lymphopenia that persists post TazD75H bone marrow transplantation. Mechanistically, the TAZD75H point mutation caused hematopoietic cell mitochondrial dysfunction in patient-derived immortalized TAZD75H lymphoblasts, increasing reactive oxygen species production and mitochondrial membrane depolarization. Likewise, Cyclosporine A treatment rescued these mitochondrial phenotypes in vitro, confirming TAZD75H mitochondrial dysfunction. Overall, our findings demonstrate that mitochondrial dysfunction secondary to TAFAZZIN loss of enzymatic function underlies BTHS-associated neutropenia and lymphopenia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12015-025-10945-1.
Keywords: Patient-tailored mouse model, Tafazzin, Barth syndrome, Neutropenia, Hematopoietic stem cells
Introduction
Barth syndrome (BTHS; OMIM# 302060) is a rare, life-threatening Xq28-linked mitochondrial disorder caused by mutations in the Tafazzin gene. BTHS primarily affects males and is characterized by cardiomyopathy, neutropenia, skeletal myopathy, and failure to thrive, typically presenting in infancy or early childhood [1–5]. BTHS is the most common X-linked mitochondrial disorder, and as with most mitochondriopathies, there is no cure [5, 6].
TAFAZZIN encodes an enzymatic transacylase required for the formation of mature cardiolipin (CL), a prominent inner mitochondrial membrane (IMM) phospholipid [3–7]. CL is vital for maintaining proper mitochondrial oxidative phosphorylation (OxPhos) in skeletal muscle, including electron transport chain activity and ATP production, as well as mitochondrial morphology and mitophagy [3–9]. BTHS patient tissues and cells exhibit reduced CL levels, altered mitochondrial structure, and decreased OxPhos functional capacity [4, 7, 9]. More than 200 different TAFAZZIN mutations cause BTHS, with variable outcomes; most result in mutant TAFAZZIN proteins [10–13]. Moreover, the TAFAZZIN protein performs nonenzymatic functions; it senses IMM curvature, is essential for glycerolipid acyltransferase catalysis, and binds various adenine nucleotide metabolism and protein complexes at its hydrophilic domain [14–17]. Consequently, the spectrum and severity of BTHS symptoms can vary, genotype‒phenotype correlations are incompletely understood, and the natural course of disease progression throughout the patient’s lifetime remains unclear [3, 5–18].
Among the most serious complications of BTHS is neutropenia, a pathological disease resulting from lower-than-normal circulating neutrophil levels, increasing infection risk [19–21]. Neutropenia can present before birth in BTHS patients [22] and is often the initial finding [23, 24]; furthermore, patients often succumb to bacterial infections secondary to repeated life-threatening infections [5, 20, 21]. However, the mechanisms driving neutropenia in BTHS are not well elucidated [21, 23], and its presentation is variable. Some patients do not exhibit neutropenia [5, 25], whereas others demonstrate intermittent and unpredictable, chronic and severe, or cyclical presentations [23, 24]; as well as compensatory monocytosis help to compensate for neutrophil deficiency [20, 23]. Thus, there is an urgent need to characterize neutropenia in patient-derived models to enable the development of targeted therapies for BTHS and other neutropenia conditions.
Neutrophils are the most prevalent circulatory immune cells involved in the innate immune response to infection and tissue remodeling [25, 26]. These cells are short-lived [27], necessitating continual replenishment from bone marrow hematopoietic stem cells (HSCs). BTHS-derived lymphoblasts exhibit reduced CL levels and abnormal mitochondrial structures [28]. Nevertheless, it remains unclear whether BTHS neutropenia arises from defective granulopoiesis, increased neutrophil apoptosis, accelerated clearance, or functional defects.
In this study, we aimed to address this knowledge gap in a patient-tailored mouse model and patient-derived lymphoblasts. We examined granulopoiesis in a mouse model expressing a novel patient-derived TAFAZZIN variant (D75H substitution within the H(X)4D acyltransferase motif; TazD75H [13]) generated by CRISPR/Cas9 genetic modification and in immortalized TAZD75H lymphoblasts from this BTHS patient. Our study provides a patient-relevant platform for elucidating the mechanisms of neutrophil dysregulation in BTHS and identifying potential therapeutic targets.
Methods
Animal Models
Tafazzin (MGI:109626) point mutant knock-in mice (herein termed TazD75H) with a pathogenic mutation (D75H: G > C substitution within Asp223 in coding exon 2) were generated via CRISPR/Cas9 gene editing, maintained on a C57BL/6J background and bred/genotyped as previously described [13]. BoyJ male mice used for transplantation were obtained from the onsite Indiana University Simon Comprehensive Cancer Center (IUSCCC) In Vivo Therapeutics Core. All the mice were housed in a specific pathogen-free facility in the Animal Resource Center at IU School of Medicine, and the Institutional Animal Care and Use Committee of IU School of Medicine approved all the experimental procedures.
Cytospin Preparation and Wright‒Giemsa Staining
Venous blood was drawn from the lateral tail veins of 5-day-old, 4-week-old, and 8- to 10-month-old wt male (♂) and TazD75H♂ littermates and collected in EDTA-coated tubes. Complete blood counts (CBCs) were obtained via a Hemavet 950FS (Drew Scientific), as described [13]. Primary bone marrow was flushed from 4-week-old wt♂ and TazD75H♂ littermate femurs and tibias and then processed to create a single-cell suspension suitable for cytospinning. Blood and cells were spun onto glass slides (Cytospin 4 Cytocentrifuge, Thermo Scientific), dried for 20 min, fixed in methanol, and stained with either Giemsa (Sigma) or Wright‒Giemsa (Siemens). Images were acquired with an Axioscope2 (Zeiss) instrument equipped with a 100× oil immersion objective, and the image brightness was adjusted with Photoshop (Adobe).
Histology
10 μm frozen wt♂ and TazD75H♂ adult spleens sections (by Leica CM3000 Cryostat) were collected on Superfrost plus slides (VWR) and air dried for 30–90 min (n = 3 spleens/genotype). Following brief 4% paraformaldehyde fixation and PBS washing, sections were probed with anti-neutrophil (Ly-6G and Ly-6 C) antibody (1:400, Abcam, clone NIMP-R14) and signal detected using the ABC kit and a rat-specific secondary antibody (Vectorstain). DAB/hydrogen peroxide was used to visualize immunosignals. Antibody diluent (Vectorstain), without primary antibodies, was used for negative control. For each assay, serial sections were examined, and a specific signal was only noted when present in at least three consecutive serial sections.
Flow Cytometry Analysis
Bone marrow-derived HSCs and progenitor (HPC) cell phenotypes were identified via flow cytometry in single-cell suspensions from four-week-old ♂ mice. The following phenotyping markers were used: APC, FITC or Pacific Blue™ mouse lineage cocktail (CD3, Gr-1, CD11b, CD45R, and Ter119; APC cocktail from BD Bioscience; FITC and Pacific Blue™ cocktail from BioLegend), PE-CF594-anti-Ly6A/E (a.k.a. Sca-1; clone D7; BD Biosciences), APC or APC-H7-anti-CD117 (a.k.a. c-Kit; clone 2B8; BD Biosciences), APC- or PE-anti-CD135 (a.k.a. Flt3; clone A2F10.1; BD Biosciences), PE- or BV421-anti-CD34 (clone RAM34; BD Biosciences), PerCP-Cy™5.5-anti-CD16/CD32 (a.k.a. FcγR; clone 2.4G2; BD Biosciences), and BV421-anti-CD127 (a.k.a. IL-7R; clone SB/199; BD Biosciences). The HSC and HPC populations were defined as LK- or myeloid progenitor-enriched cells: Lin-Sca-1-c-Kit+, LSK cells: Lin- Sca-+ c-Kit+, long-term (LT)-HSCs: LSK Flt3- CD34-, short-term (ST)-HSCs: LSK Flt3- CD34+, multipotent progenitors (MPPs): LSK Flt3+ CD34+, common myeloid progenitors (CMPs): LK FcγRlo CD34+, granulocyte- monocyte progenitors (GMPs): LK FcγRhi CD34+, megakaryocyte-erythrocyte progenitors (MEPs): LK FcγR- CD34-, and common lymphoid progenitors (CLPs): Lin- Sca-1lo c-Kitlo Flt3+ IL-7R+. Neutrophils were defined as Ly-6G+ (APC anti-Ly-6G; clone 1A8; BioLegend) and CD11b+ (FITC anti-CD11b; clone M1/70; BioLegend). Experiments were performed on a modified FACSLSR II cytometer (BD Biosciences) and analyzed with FlowJo version 7.6.3 software (TreeStar).
MitoTracker™ Green FM (Invitrogen) staining was performed according to the manufacturer’s instructions and as described previously [29–31]. JC-1 staining, utilizing a MitoProbe™ JC-1 Assay Kit (Invitrogen), was performed according to the manufacturer’s instructions and as described previously [29]. To detect mitochondrial superoxide, we incubated ♂ lymphoblasts in medium containing MitoSOX reagent (Invitrogen) at 37 °C for 20 min, washed twice, and then analyzed via flow cytometry. Positive control cells were incubated in medium containing 9 µM H2O2 for 50 min prior to the addition of MitoSOX.
Hematopoietic Progenitor Cell Colony and Tritiated Thymidine Kill Assays
Hematopoietic clonogenic progenitors from 4-week-old wt♂ and TazD75H♂ bone marrow cells flushed from femurs were grown in 1% methylcellulose culture medium with 0.1 mM hemin (Sigma-Aldrich), 30% FBS, 1 U/mL recombinant human erythropoietin (rhEPO; Amgen), 50 ng/mL recombinant mouse stem cell factor (rmSCF; R&D Systems), and 5% vol/vol pokeweed mitogen mouse spleen cell conditioned medium at 5 × 104 cells/mL per plate (3 plates per mouse). Colonies were scored after 6 days of incubation in 5% CO2 and low (5%) O2 in a humidified chamber. Granulocyte‒macrophage colony-forming units (CFU‒GM), erythrocyte burst-forming units (BFU‒E), and granulocyte, erythrocyte, macrophage, and megakaryocyte colony-forming units (CFU‒GEMM) were distinguished by examining colony morphology. The total number of colonies per femur was calculated as previously described [30, 31]. For high-specific activity tritiated thymidine kill assays, bone marrow cells were treated with 50 µCi high-specific activity [3H]Tdr (20 Ci/mmol; DuPont NEN) at room temperature (20–22 °C) for 40 min and then washed in PBS before plating for HPC colony assays (as previously described [31, 32]).
Bone Marrow Transplantation and ROS Assessment
Bone marrow were harvested from either 4-week-old or 6-month-old wt♂ and TazD75H♂ mice, and low-density mononuclear cells (LDMNCs) were isolated as previously described [29–33]. Recipient male BoyJ mice (6‒8 weeks old) from the onsite IUSCCC In Vivo Therapeutics Core were subjected to whole-body irradiation with 1,100 cGy (n = 10/genotype) and transplanted intravenously with 2 × 106 donor bone marrow LDMNCs after 24 h. For in vivo hematopoietic assessment, CBC analysis was performed 1 to 10 months post-transplantation. For reactive oxygen species (ROS) examination, 8-weeks post transplantation, marrow was harvested and ROS levels assessed using the CellROX Deep Red Reagent, as per the manufacturer’s instructions (Thermo Fisher Scientific). Briefly, single-cell suspensions were prepared from freshly isolated adult marrow. After red blood cell lysis and washing, cells were resuspended in pre-warmed Roswell Park Memorial Institute (RPMI) containing 10% FBS. CellROX Deep Red was added at a final concentration of 3 µM, and cells were incubated at 37 °C for 30 min in the dark. Following incubation, cells were washed twice with ice-cold HBSS/FBS buffer and surface immunostaining for hematopoietic markers was performed on ice using fluorophore-conjugated antibodies (Lineage cocktail BV421, Sca-1-BV711, KIT-BV785, BioLegend) and cells were washed with HBSS with + 2% FBS to remove unbound antibodies, and immediately analyzed by flow cytometry without fixation.
BTHS Patient Lymphoblast Line and BTHS Patient Blood Data
TAZD75H patient lymphocytes were isolated from fresh blood samples and subjected to Epstein–Barr virus transformation as previously described [34]. Control EBV-immortalized human lymphoblasts (NCI-BL1672) were obtained from the American Type Culture Collection. Both cell types were cultured in RPMI medium supplemented with 10% FBS, 1% sodium pyruvate, and 1% penicillin–streptomycin. Lymphocyte collection and transformation were approved by the Johns Hopkins University Institutional Review Board (NA_00098987: ‘Investigation into clinical, metabolic and molecular factors in Barth syndrome’; PI H.J. Vernon). For the collection of BTHS data, the study was longitudinal and observational in nature. This study was approved by the Johns Hopkins University IRB protocol (NA_0008316: ‘Clinical Studies in Barth Syndrome’; PI H.J. Vernon). All procedures were conducted per the ethical standards of the responsible committee on human experimentation (institutional and national) and the tenets of the Declaration of Helsinki of 1975, as revised in 2013. Informed written consent was obtained from the parents or legal guardians of all participants in the study.
Transmission Electron Microscopy
For electron microscopy (EM), patient TAZD75H lymphoblasts were placed on coverslips and fixed in 2% glutaraldehyde fixative in 0.1 M cacodylate buffer (pH 7.2) overnight, processed, and imaged on a Phillips400 microscope at the IU EM Core as previously described [13]. Examiners were blinded to the genotype, and the data were analyzed via ImageJ (version 1.54j).
Detection of Oxidative Phosphorylation Complexes
Mitochondria were isolated from human lymphoblasts using the Mitochondria Isolation Kit for Cultured Cells (Abcam, ab110171) as per manufacturer’s instructions. Mitochondria were lysed using M-PER buffer Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) containing protease and phosphatase inhibitors. The lysates were denatured for 15 min at 37 °C and proteins were resolved using a 10–20% gradient gel (Novex™, Thermo Fisher Scientific). Protein was transferred to PVDF using high pH CAPS transfer buffer, and blots were probed with Total OXPHOS Rodent WB Antibody Cocktail (1:250, Abcam) for intact electron transport complexes as per the manufacturer’s instructions. VDAC1 (1:2000, Abcam) was used as loading control.
Cyclosporine A Treatment
Cyclosporine A (Cayman Chemical) was dissolved in DMSO and added to the lymphoblast cultures at a final concentration of 10 µM. An equal volume of pure DMSO was added to control cells. The total exposure duration was 90 min at 37 °C.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software). One-way ANOVA with post hoc Tukey’s multiple comparison test or Student’s t-test were used to determine significant differences between groups. A p value below 0.05 was considered to indicate significance. The ROUT test (Robust regression and Outlier Removal Test) on GraphPad was used to determine any outliers. The data in the figures are presented as the means ± SEMs.
Results
Impaired Granulopoiesis and Neutropenia in TazD75H Male Mice
We identified a novel TAFAZZIN variant causing a D75H substitution in the critical acyltransferase site of the TAFAZZIN enzyme in a 1.5-year-old male BTHS patient exhibiting growth deficiency, hypotonia, gross motor delay, and noncompaction cardiomyopathy. Significantly, additional BTHS mutations are present around residue D75 (D74E, D75N, D75R, D76R, D76L) at the border of the H(X)4D acyltransferase domain, and ~ 47% of residues in proximity to D75 carry pathogenic mutations [10]. Importantly, this TAZD75H patient initially presented with recurrent fever, monocytosis and neutropenia and requires continual G-CSF therapy [35] to stimulate his bone marrow to induce granulopoiesis and neutrophil release to help prevent infections. Thus, we examined baseline hematopoiesis in a CRISPR/Cas9 knock-in mutant mouse model harboring this D75H point mutation (referred to hereafter as TazD75H) in the critical TAZ acyltransferase domain required to remodel immature monolysocardiolipin (MLCL) to mature (18:2)4 cardiolipin (CL) [13].
Male TazD75H mice exhibited BTHS phenotypes and an elevated adult blood MLCL: CL ratio (diagnostic feature of BTHS [7]). Furthermore, only ~ 50% of TazD75H♂ mice survived perinatally (the other ~ 50% die in utero or at birth likely due to cardiovascular defects [36, 37]), whilst the remaining TazD75H♂ all die prematurely by ~ 11 months of age [13]. Thus, we analyzed surviving neonatal, juvenile, and mature adult TazD75H♂ mice. Peripheral blood smear assessment revealed that both 5-day-old and 4-week-old TazD75H♂ mice exhibit significantly fewer neutrophils than their wt♂ littermates, whereas 8–10-month-old adult TazD75H♂ and wt♂ mice exhibit similar neutrophil levels (Supplemental Fig. 1 A, B). These data suggest that the mild-to-moderate neutropenia mediated by the point mutation in TazD75H♂ mice is apparent only in young mice and likely present from birth.
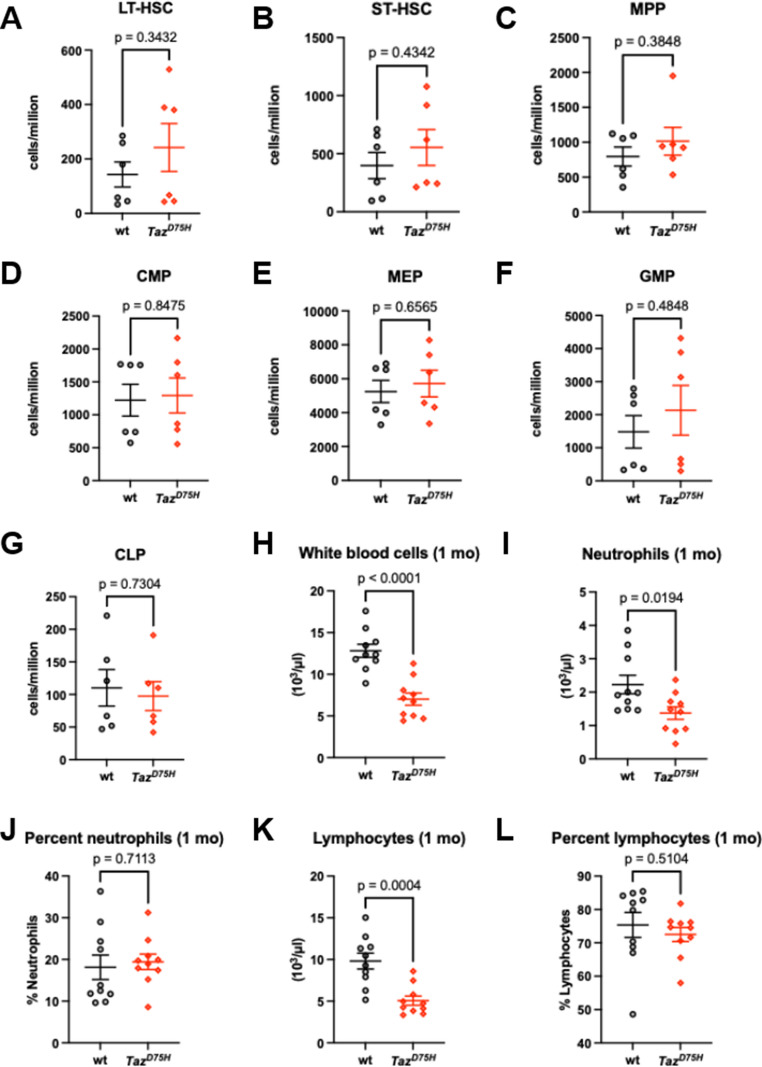
Consistent with prior published data [13], we observed significant variability in neutrophil number reduction in neonatal and juvenile TazD75H♂ mice, reflecting the variability observed in BTHS [2, 5, 19, 23]. Given these peripheral smear data, we examined cytopsin preparations from 4-week-old mice and revealed that the bone marrow of both TazD75H♂ and wt♂ mice exhibit a comparable spectrum of differentiating neutrophil precursors, including a significant number of immature band and ring neutrophils with some mature segmented neutrophils in both genotypes (Supplemental Fig. 1 C). This finding indicates that immature myeloblast precursors in TazD75H♂ mice can at least undergo preliminary differentiation. Additionally, we immunophenotypically quantified HSCs and HPCs in the bone marrow of 4-week-old TazD75H♂ and wt♂ mice via flow cytometry (Fig. 1). Compared to wt♂ littermate controls, long-term HSCs (LT-HSCs) and short-term HSCs (ST-HSCs) in TazD75H♂ mice were not significantly different (Fig. 1A, B). Similarly, there were no significant differences in the numbers of MPPs, CMPs, GMPs, MEPs, or CLPs in TazD75H♂ versus wt♂ mouse bone marrow samples (Fig. 1C–G). Interestingly, Complete blood counts (CBC) analyses revealed that 4-week-old TazD75H♂ mice exhibited a significant reduction in the absolute number of neutrophils (p = 0.0194 by t-test; n = 10 mice/genotype; Fig. 1I). Overall, TazD75H♂ mice presented decreased absolute white blood cell (WBC) and lymphocyte counts (Fig. 1H, K). Taken together, these data suggest that the TazD75H mutation results in neonatal/juvenile dysfunctional maturation at the final neutrophil differentiation step under homeostatic conditions.
Fig. 1.
Hematopoiesis is impaired in TazD75H♂ male mice. (A-G) Quantitative flow cytometry immunophenotyping of TazD75H♂ and wt♂ mouse bone marrow reveals the relative abundance of long- and short-term HSCs (A, B) and lineage-specific myeloid progenitors (C–G). The graphs present pooled data (means ± SEMs) from two independent experiments (n = 3/genotype/experiment). P values were calculated by t-test. (H-L) CBCs in 3-5-week-old (1 mo) TazD75H♂ and wt♂ mice revealed significant differences in the absolute numbers of total white blood cells, neutrophils, and lymphocytes (H, I, K). Graphs represent means ± SEM (n = 10 mice/genotype), and p values were calculated by t-test
Ex Vivo Hematopoietic Progenitor Cell Colony Analysis
To evaluate the functionality of hematopoietic myeloid progenitors in TazD75H♂ mice, we performed ex vivo HPC colony assays, as previously described [32]. Given the observed chronic growth deficiency [13] of TazD75H♂ mice, the data are shown both as absolute cell number/femur and as adjusted to body weight (Fig. 2). These assays revealed impaired differentiation within all myeloid progenitors examined in the bone marrow of 4-week-old TazD75H♂ versus wt♂ mice. Compared with that in wt♂ mice, the number of myeloid progenitor cells capable of forming colonies consisting of 20 or more granulocytes and macrophages (GMPs/CFU-GM) was significantly reduced in the bone marrow of TazD75H♂ versus wt♂ mice (Fig. 2A, B). Similarly, the number of CMPs capable of forming colonies containing erythroid, megakaryocyte, granulocyte, and megakaryocyte (CFU-GEMM) cells was reduced in TazD75H♂ versus wt♂ mice (Fig. 2G, H), as were the number of cells capable of forming colonies consisting predominantly of erythroblasts (BFUs; Fig. 2D, E). The decrease in CFU-GM, BFU-E, and CFU-GEMM numbers in the TazD75H♂ mouse femurs in the colony formation assays was associated with a decrease in the number of myeloid progenitors in the S-phase at the time of plating, as defined by the high specific activity of the tritiated thymidine kill assay (Fig. 2C, F, I). In order to examine whether mutant bone marrow cellularity itself was perturbed by the TazD75H mutation, CBCs revealed 4-week-old TazD75H♂ bone marrow exhibited a trend toward reduced cellularity, suggesting a transient impairment during early hematopoietic development (Fig. 2J). In contrast, adult TazD75H♂ displayed normal bone marrow cellularity, indicating that the early deficit is resolved over time. Moreover, bone marrow cellularity in TazD75H♂ recipients at 8 weeks post-transplant was comparable to wt♂ recipients (Fig. 2J), further supporting the notion that any early defects are not sustained, and that the mutant marrow environment can recover or compensate functionally under steady-state and regenerative conditions.
Fig. 2.
Decreased function of hematopoietic myeloid progenitors in TazD75H♂ bone marrow. Colony-forming units (CFUs) for granulocyte-macrophage (A), burst-forming unit-erythrocyte (D), and granulocyte-erythrocyte-macrophage-megakaryocyte (G) numbers per femur of TazD75H♂ and wt♂ mice as determined by colony assay. (B, E, H) Body weight-adjusted numbers of bone marrow CFUs. (C, F, I) The percentage of HPCs cycling per femur was determined via a thymidine kill assay. For the CFU data, the graphs represent pooled data (means ± SEMs) from two independent experiments (n = 3/genotype/experiment). For the thymidine kill data, the graphs represent data from a single experiment (n = 3/genotype). (J) Total bone marrow cellularity (total number of bone marrow cells/mouse) of juvenile and adult TazD75H♂ and wt♂ bone marrow, as well as adult TazD75H♂ and wt♂ bone marrow 8 weeks post-transplantation. (n = 2–5 mice/genotype)
In parallel, we assessed the colony-forming ability of HPC populations from splenocytes isolated from TazD75H♂ or wt♂ littermates. Interestingly, we did not observe decreased colony-forming ability in splenocyte-derived HPCs from TazD75H mice (Supplemental Fig. 2). Notably, when adjusted for body weight, CFU-GM colony formation was significantly increased in TazD75H♂ versus wt♂ mice, as was the percentage of CFU-GM in S-phase at the time of plating (Supplemental Fig. 2B, C), suggesting a potential compensatory mechanism for the impaired bone marrow granulopoiesis and subsequent neutropenia observed in juvenile TazD75H♂ mice. However, the CBC results indicate that this extramedullary hematopoiesis in the spleen does not fully compensate for the loss of function of the myeloid progenitor cells producing neutrophils in the mutant bone marrow. Immunohistochemistry using an anti-neutrophil marker antibody revealed that cellularity and neutrophil numbers were comparable in TazD75H♂ and wt♂ adult spleens (Supplementary Fig. 2 J). Whether this is due to differences in adhesion or migration or perhaps excessive secretion of cytokines responsible for mobilizing HSPCs and/or neutrophils remains to be determined. Taken together, these data suggest an impaired mutant capacity to generate and differentiate bone marrow HPCs into mature neutrophils, which likely underlies the TazD75H♂ neonatal/juvenile neutropenia phenotype and diminished absolute WBC/lymphocyte counts.
In Vivo Hematopoiesis Analysis
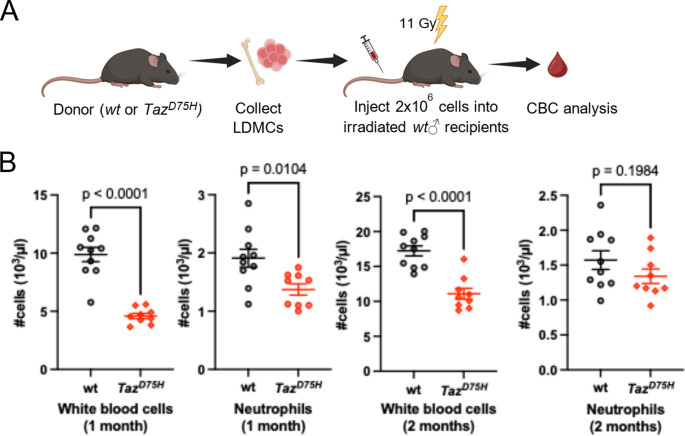
To directly assess the impact of the TazD75H mutation on hematopoiesis in vivo, we transplanted low-density mononuclear cells (LDMCs) isolated from the bone marrow of 4-week-old TazD75H♂ or wt♂ mice into lethally irradiated wt♂ recipient mice (6–8 weeks of age, Fig. 3A). One month after transplantation, CBC analysis of peripheral blood revealed significant reductions in total WBCs and neutrophils in TazD75H♂ bone marrow recipients (Fig. 3B). However, 2 months after transplantation, recipients of TazD75H♂ bone marrow no longer exhibited neutropenia, despite a sustained significant reduction in total WBC count (Fig. 3B and Supplemental Fig. 3). These data suggest that the neutropenia is temporary even through transplantation, confirming a correction in neutrophil production with time. Moreover, CBC analysis of TazD75H♂ and wt♂ mice revealed TazD75H♂ reduced RBCs and hemoglobin (Supplemental Fig. 4 A). Additionally, CBC analysis following 8 weeks post transplantation of 6-month-old TazD75H♂ or wt♂ bone marrow revealed persistent reduction of absolute lymphocyte numbers along with anemic features; including reduced RBCs, hemoglobin, and hematocrit in TazD75H♂ transplants compared to unaffected wt♂ bone marrow transplants (Supplemental Fig. 4B). As TazD75H♂ adult lymphocyte frequencies are low, there is enrichment of relative neutrophil frequency (% not absolute numbers), confirming that lymphopenia is the chief adult TazD75H♂ hematopoietic defect. Together, these findings confirm that the TazD75H mutation impairs both granulopoiesis and neutrophil maturation in vivo, with early transient disruption of neutrophil morphogenesis associated with mouse age, which is reflective of the phenotype observed in some BTHS patients [2, 5, 22, 23, 38].
Fig. 3.
The TazD75H mutation induces impaired granulopoiesis in vivo. (A) Schematic of the bone marrow transplantation experiments. LDMNCs collected from TazD75H♂ and wt♂ mice were transplanted into irradiated wt♂ recipients (n = 10 recipient mice/genotype). Peripheral blood was collected for CBC analysis at one- and two-months post transplantation. (B) Plots showing the quantification of the indicated cell types. P values were calculated via unpaired t-tests. Results from one TazD75H♂ recipient mouse were identified as outlier by the ROUT t-test and were thus omitted from the statistical analysis
Mitochondrial Dysfunction in Hematopoietic Cells from TazD75H♂ Mice
Next, we sought to determine the molecular phenotype underlying the impaired granulopoiesis observed in TazD75H♂ mice. As mitochondrial defects are a hallmark of BTHS [3, 5] and as TAZ itself impacts many aspects of mitochondrial structure and function [1, 39, 40], we examined the mitochondrial content and membrane potential (Δψm) across HSC and HPC populations in TazD75H♂ and wt♂ mice. Like TazD75H♂ heart, skeletal muscle and livers [13], the TazD75H protein is expressed at equivalent steady-state levels to wt♂ in adult bone marrows (Supplemental Fig. 5 A). Flow cytometry analysis of live MitoTracker Red-stained 4-week-old ♂ bone marrow hematopoietic cells revealed statistically comparable mitochondrial content in TazD75H♂ and wt♂ mouse lineage-negative (LSK and LK) and lineage-positive cells (Fig. 4A). However, JC-1 red/green fluorescence analysis of the mitochondrial Δψm revealed that TazD75H♂ versus wt♂ mouse bone marrow hematopoietic cells presented a significant decrease in all the mutant cell populations examined (Fig. 4B). As the mitochondrial Δψm reflects the overall health and function of mitochondria [41], our finding that mitochondrial content is unperturbed in TazD75H♂ mice but that mutant mitochondria are depolarized is indicative of impaired mutant mitochondrial function. Moreover, the finding that the mitochondrial Δψm of mutant lineage-negative cells is also reduced reinforces our hypothesis that the TazD75H defect likely occurs at the stem cell level.
Fig. 4.
Impaired mitochondrial function in TazD75H♂ bone marrow. (A, B) Flow cytometry assessment of the mitochondrial content (MitoTracker Red fluorescence intensity; A) and membrane potential (JC-1 red/green fluorescence intensity ratio; B) of hematopoietic cells derived from the bone marrow of 4-week-old TazD75H♂ and wt♂ mice. The graphs present pooled data (means ± SEMs) from two independent experiments (n = 3 mice of each genotype/experiment); p values were calculated via t-tests
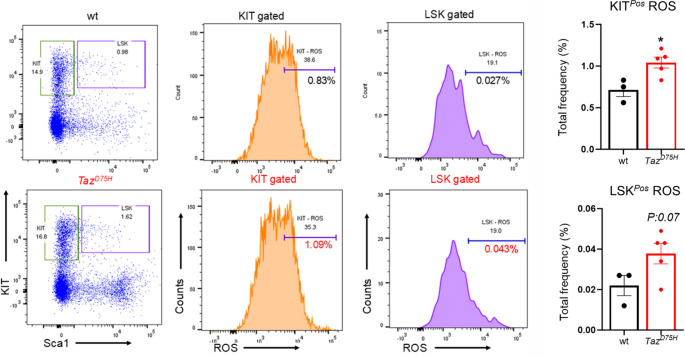
To directly assess myeloid progenitor function, we transplanted 2 million bone marrow cells from either TazD75H♂ or wt♂ donor mice into lethally irradiated C57BL/6 recipients and monitored the development of BTHS–associated hematologic features (Fig. 5). Peripheral blood analysis at 8 weeks post-transplant revealed an increase in circulating neutrophils and monocytes percentages along with a reduction in erythroid parameters including RBCs, hemoglobin and hematocrit in TazD75H♂ marrow compared to wt♂ marrow (Supplemental Fig. 4). We then assessed ROS levels within bone marrow progenitor populations and found that LK (lineage negative c-Kit positive) myeloid progenitors from TazD75H♂ recipients exhibited significantly elevated ROS levels (Fig. 5). Notably, TAZD75H patient CBCs confirm G-CSF-responsive neutropenia and a relative (% increase) monocytosis. Further, our TAZD75H patient has an intermittent mild microcytic anemia of unclear etiology but could be due to iron deficiency, chronic disease, or marrow dysfunction (Table 1). Combined these clinical data confirm granulopoietic dysregulation and are suggestive of anemia for those with the TAZD75H mutation.
Fig. 5.
Significant accumulation of ROS in TazD75H♂ myeloid progenitors. Flow cytometric analysis of ROS levels in hematopoietic cells at 8 weeks post-bone marrow transplantation of adult wt♂ and TazD75H♂ mice. Representative flow cytometry profiles illustrate ROS levels within bone marrow myeloid progenitor (LK or KITPos) and LSK (LinNeg Sca-1Pos c-KitPos) populations. Quantitative analysis is shown in the accompanying graphs, with data expressed as mean ± SEM. (n = 3–5 mice per group. *p ≤ 0.05)
Table 1.
Complete blood count and differential for patients with BTHS
| Reference range + units | Patient TAZD75H # | Patient A | Patient B | Patient C | |
|---|---|---|---|---|---|
| Age | Years | 14–16 ♂ | 39 ♂ | 42 ♂ | 43 ♂ |
| G-CSF therapy | Yes | No | No | Yes | |
| WBC | 4.5–11.00 k/cumm |
4.66 (3.6-6) |
4.03 | 3.25 | 5.1 |
| RBC | 4.5–5.9 106/cumm |
5.11 (4.76–5.80) |
4.45 | 5.77 | 5.75 |
| Hb | 13.9–16.3 g/dL |
12.82 (11.9–14.5) |
14.1 | 13.7 | 16.8 |
| HCT | 41–53% |
37.98 (35.7–42.3) |
43.6 | 45.7 | 51.8 |
| Platelets | 150–350 k/cumm |
257.5 (246–269) |
129 | 357 | 120 |
| Neutrophil % | 40–70% |
30.6 (11–50) |
14.6 | 7.8 | 52.6 |
| Monocyte % | 2–11% |
19.5 (14–29) |
42.7 | 23.4 | 8.2 |
| Lymphocyte % | 24–44% |
42.87 (37–52) |
35.2 | 53.8 | 30.2 |
| Eosinophil % | 1–4% |
4.62 (3–8) |
6 | 13.2 | 7.6 |
| Neutrophil absolute | 1.5–7.8 k/cumm |
1.51 (0.4–2.7) |
0.59 | 0.25 | 2.68 |
| Lymphocyte absolute | 1.1–4.80 k/cumm |
1.94 (1.7–2.7) |
1.42 | 1.75 | 1.54 |
| Monocyte absolute | 0.1–1.2 k/cumm |
0.8 (0.6–1.1) |
1.72 | 0.76 | 0.42 |
| Red Cell Distribution Width (RDW) | 11.5–14.5% |
16.25 (15.4–17.2) |
11.7 | 19.6 | 13.7 |
| Mean Corpuscular Volume (MCV) | 81–99 fL |
74.75 (73–76) |
98.0 | 79.2 | 90.1 |
| Mean Corpuscular Hemoglobin (MCH) | 27.0–34.0 pg |
25.25 (24.9–25.4) |
31.7 | 23.7 | 29.2 |
Each BTHS patient has been genetically confirmed to have pathogenic variants in TAFAZZIN and elevated plasma MLCL/CL ratios. Our TAZD75H patient (#) exhibits G-CSF-responsive neutropenia, a relative monocytosis and a mild intermittent microcytic anemia. Data represent the average (with TAZD75H ranges included) of eight CBC results over two years. Thrombocytopenia is present in patients A and C, and low-normal lymphocyte counts are present in patients A-C
Cyclosporine Rescues Mitochondrial Damage in Patient TAZD75H Lymphoblasts
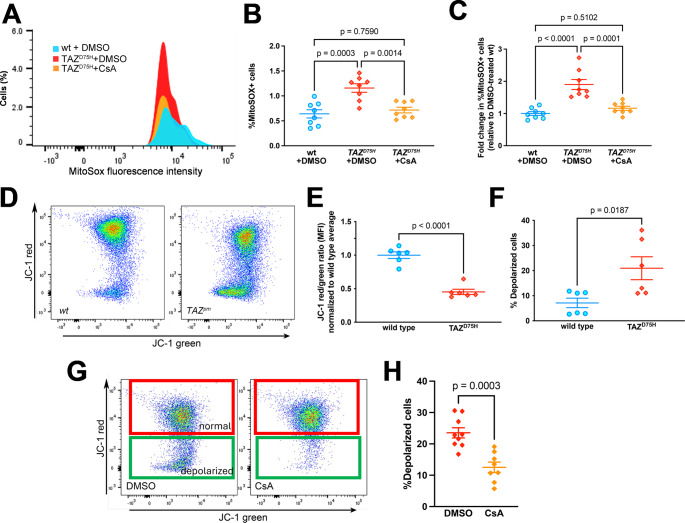
To confirm that mitochondrial dysfunction is secondary to the TAZD75H mutation in BTHS neutrophils and their progenitors, we utilized immortalized lymphoblasts derived from our BTHS patient (TAZD75H♂ cells). Significantly, the TAZD75H protein is expressed in TAZD75H♂ cells at comparable levels to wildtype TAFAZZIN in control EBV-immortalized human male lymphoblasts (Supplemental Fig. 5B). Importantly, TEM analysis of these cells revealed abnormally disorganized swollen and empty mitochondria (Supplemental Fig. 6), irregular perinuclear clustering, and mitochondrial clumping, indicating a stress response and/or mitochondrial dysfunction [42]. Consistent with these findings, TAZD75H♂ lymphoblasts exhibited a trend of reduced expression of select electron chain transport complexes (Supplemental Fig. 7). Furthermore, flow cytometry analysis revealed elevated mitochondrial superoxide and loss of mitochondrial Δψm in TAZD75H♂ versus control lymphoblasts (Fig. 6). Indeed, extensive studies across various murine models have demonstrated that even modest increases in mitochondrial ROS, often in the range of 1.2–1.5-fold above baseline, are sufficient to induce measurable mitochondrial dysfunction. This includes loss of Δψm, mtDNA damage, altered mitochondrial dynamics, and skewing of stem or progenitor cell fate [43, 44]. Thus, even small subsets of MitoSOX⁺ cells or subtle ROS elevations in our model are biologically meaningful, especially when corroborated by structural mitochondrial damage and molecular anomalies as observed in our TEM and Western analysis (see Supplementary Figs. 6 and 7).
Fig. 6.
Cyclosporine suppresses superoxide production and restores mitochondrial membrane potential in BTHS patient lymphoblasts. (A) Representative flow cytometry histograms and quantification (B) of MitoSOX-positive populations in patient TAZD75H and control lymphoblasts treated with DMSO versus TAZD75H lymphoblasts treated with CsA (10µM). (C) Quantification of fold-change in the percentage of MitoSOX-positive cells detected by flow cytometry. Data is from two independent experiments, where n = 8 per condition. P values were calculated by one-way ANOVA. (D) Representative flow cytometry dot plots and quantification (E) of red versus green emission in JC-1-stained control and TAZD75H lymphoblasts. (F) Quantification of percent depolarized (i.e. percentage of cells positive for green JC-1 fluorescence) lymphoblasts. (G) Representative flow cytometry dot plots of red versus green emission in JC-1-stained TAZD75H lymphoblasts treated with DMSO or CsA. (H) Quantification of percent depolarized (i.e. percentage of cells positive for green JC-1 fluorescence) TAZD75H lymphoblasts after treatment with DMSO or CsA (10µM). Data is from two independent experiments (n ≥ 8 technical replicates/condition). P values were calculated by t-test
Under oxidative stress, the mitochondrial matrix-resident cyclophilin D (CypD) translocates to the IMM, opening the mitochondrial permeability transition pore (mPTP). This phenomenon rapidly increases the permeability of the IMM, resulting in mitochondrial swelling and membrane depolarization, potentiating cell death [45–49]. The mPTP‒CypD signaling axis is activated by ROS in HSCs, and this pathway has been successfully targeted to increase life span in a mouse model of lethal mitochondrial myopathy via the clinically approved small molecule inhibitor Cyclosporine A (CsA) [50]. CsA binds and inhibits CypD, prohibiting mPTP induction [51, 52]. To evaluate whether CsA could normalize superoxide levels and the mitochondrial Δψm in BTHS, we treated TAZD75H♂ lymphoblasts with CsA (or DMSO as a control) and assayed the mitochondrial superoxide levels and Δψm through MitoSOX and JC-1 staining, respectively. Flow cytometry analysis revealed that exposure to CsA reduced TAZD75H♂ cell mitochondrial superoxide to control levels (Fig. 6A–C; Supplemental Fig. 8) and reduced the percentage of TAZD75H♂ cells with mitochondrial membrane depolarization (Fig. 6G, H). These results suggest that CsA, and potentially other pharmacological agents that target the mPTP-CypD axis, may be therapeutically beneficial for treating BTHS hematopoietic defects.
Discussion
In this study, we demonstrated that surviving patient-tailored knock-in mutant TazD75H♂ mice, which recapitulate the major BTHS-associated phenotypes [13, 53], exhibit impaired granulopoiesis and early-onset neutropenia. Using CBC analysis and ex vivo/in vivo HPC and hematopoietic cell analyses, we found that TazD75H♂ mice exhibit an age-dependent transient impairment of juvenile neutrophil maturation and persistent lymphopenia. Notably, we also demonstrated that myeloid progenitor cells from juvenile TazD75H♂ mice are functionally abnormal. Specifically, both mutant lineage-negative (LSK and LK myeloid progenitors) and lineage-positive bone marrow cells exhibit decreased mitochondrial membrane potentials, which likely impairs energy production and signaling pathways. Importantly, this confinement to the neonatal and juvenile stages is consistent with reports that BTHS neutropenia can be observed before birth [22]. Furthermore, the lack of neutropenia and absence of changes in TazD75H♂ neutrophil CD45.2+ percentages in 10-month-old wt♂ transplanted mice confirmed a juvenile-restricted effect and a similar adult mutant reconstitution potential. As neutrophils have a brief lifespan [25], our observations in the TazD75H♂-cell transplanted wt♂ model suggest that cell-autonomous changes within the Taz gene underlie the observed TazD75H♂ granulopoiesis defects.
The TazD75H model results indicate a unique granulopoiesis phenotype distinct from that associated with existing Tafazzin alleles. Notably, in contrast to all the other Tafazzin alleles, the TazD75H allele lacks acyltransferase activity but expresses a stable mutant protein, resulting in a buildup of immature MLCL relative to CL in the TazD75H♂ mouse circulation [13]. In previous studies using the doxycycline-inducible transgenic Tafazzin shRNA-mediated model [54, 55], in which wildtype Tafazzin levels are reduced via doxycycline feeding in the in utero and postnatal stages, 50% of the 2-month-old TazKD♂ mice demonstrated neutropenia, as did 20% of the wt controls (with doxycycline administered to both). Similarly, an in vitro analysis of bone marrow mesenchymal stem cells from 3–5-month-old TazKD♂ mice revealed that increased ROS generation and glycolysis can promote activated B lymphocyte reprogramming [56]. However, when Tafazzin shRNA-knockdown mice were fed doxycycline at 7.6–14.3 weeks of age, neutrophil numbers were unaffected in adult TazKD♂ mice despite an abnormal increase in the MLCL/CL ratio [57]. Given that doxycycline itself can impair mitochondrial function [55] and decrease the number of circulating neutrophils [58], the distinct TazKD♂ phenotype likely confirms that Tafazzin knockdown affects neutrophils only when administered in utero/neonatally [56]. These data support a Taz-specific requirement for juvenile neutrophil morphogenesis.
Conversely, null TazKO♂ mice that systemically lack Tafazzin [36] demonstrate reduced circulating neutrophil numbers at 6 months, whereas the neonatal/juvenile hematopoietic status remains unknown. Moreover, the use of the estrogen receptor-Hoxb8 fusion system to conditionally immortalize GMPs from TazKO♂ and wt mouse E14-E16 fetal livers [21] revealed that null myeloid progenitor development was largely unaffected; however, the transplantation of null fetal liver cells caused mild and consistent leukopenia. Likewise, the absence of Tafazzin in TazKO♂ fetal livers did not cause any significant differences in neutrophil development or function, including phagocytosis, cytokine production, or ROS [21]. Whether the various Tafazzin allele differences in neutrophil phenotypes are reflective of inherent BTHS variability, a subset of BTHS patients, or are due to lower wildtype Tafazzin levels, a complete absence of Tafazzin and/or the presence of a stable TazD75H mutant protein remains to be determined. Significantly, characterization of TAFAZZIN variants demonstrated BTHS-causing mutations frequently result in expression of stable mutant proteins with variable phenotypes [11, 12, 18]. Thus, BTHS is not solely driven by loss of TAFAZZIN protein. Additionally, TAFAZZIN can sense IMM curvature and its hydrophilic domain (which is intact in the TazD75H protein) is known to bind complexes containing ADP/ATP-carrier and -synthase, the Prohibitin/Dnajb6 complex, and drives glycerolipid catalysis [4, 14–17]. Suggestively, while TazD75H♂ hearts exhibit dysregulated p53/p53-target, autophagic AMPK/LC3B and senescent p16 signaling variations, none of these effectors are mis-expressed in TazKO♂ hearts [13]. Given TAFAZZIN has additional non-enzymatic functions and there are known signaling disparities between the alleles, these findings suggest the TAZD75H mutant protein retains partial function, conferring pathogenicity through a distinct mechanism-of-action.
Although the mechanism underlying the age-specific presentation of neutropenia remains uncertain, declining CL content in aged versus young HSCs [59] could indicate that CL is less essential during adult neutrophil replenishment. Aging itself has also been shown to alter neutrophil production and the ability of neutrophils to respond to infections [60]. Additionally, the spatiotemporal-restricted requirement of Tafazzin in HSCs, HPCs, lymphoblasts, and neutrophils remains unknown, allied to the finding that cardiomyocyte-restricted conditional knockouts of Tafazzin are surprisingly viable [36]. Further examination of the role of impaired granulopoiesis in Tafazzin allele-associated partial in utero/newborn mutant lethality [13, 36, 37] and in prenatal BTHS is warranted [5]. Likewise, as fetal HPCs can respond to prenatal inflammation in utero, and the fetal response can shape postnatal hematopoiesis and immune cell function [61, 62], it remains to be determined whether neonatal/juvenile impairment of mutant neutrophil maturation may drive subsequent WBC/lymphogenic alterations in TazD75H♂ adults. Although neutropenia is the primary BTHS hematologic disorder, both monocytosis and dysregulated bone marrow promyelocyte-myelocyte maturation are also observed in BTHS [20, 23, 24], and there is a report of BTHS-associated B cell lymphopenia and hypogammaglobulinemia [38]. Moreover, anemia has also been reported [63, 64] even though the number of published cases in BTHS are currently limited. Significantly, bone marrow transplantation data confirmed aberrant TazD75H♂ myeloid progenitor expansion leading to increased circulating neutrophils and monocytes percentages along with a reduction of RBCs, hemoglobin and hematocrit (see Fig. 5). Notably, the TAZD75H patient exhibits neutropenia, monocytosis and iron deficiency anemia (Table 1). Additionally, we identified three adult BTHS patients, two who were neutropenic and one receiving G-CSF treatment, whose lymphocyte counts are at the “low end of normal” and two were thrombocytopenic (Table 1). Therefore, we suggest that comprehensive immunology evaluation of BTHS patients is likely to reveal a constellation of hematological findings and may offer valuable phenotype-genotype information and insights.
To investigate the role of TAFAZZIN enzymatic activity in BTHS hematopoietic cells, we examined the mitochondrial structure and function of immortalized TAZD75H♂ lymphoblasts. Like the TazKO♂ mouse [13] and BTHS patient cells [65], TAZD75H♂ lymphoblasts exhibited ultrastructural cristae defects and abnormally swollen mitochondria. Furthermore, TAZD75H♂ lymphoblast mitochondria were functionally affected and displayed increased superoxide levels and reduced mitochondrial membrane potential, both indicators of mitochondrial stress. Indeed, an absence of CL from the IMM can drive electron leakage from the electron transfer system, defective NADPH oxidative stress signaling, and reduced mitochondrial Δψm in BTHS [65–67]. Importantly, BTHS bone marrow neutrophils demonstrate impaired differentiation and maturation, whereas circulating BTHS neutrophils demonstrate an immature phenotype and a dysregulated neutrophil inflammatory response [24]. Similarly, shRNA-mediated TAFAZZIN knockdown in several leukemic cell lines is sufficient to alter stemness and differentiation and induce cell cycle arrest but not apoptosis [57]. Further, BTHS lymphoblasts lack a mature CL and present increased superoxide anion levels and loss of mitochondrial Δψm [28, 65]. As we found that CsA (an immunosuppressant) can restore TAZD75H♂ lymphoblast mitochondrial potential and reduce superoxide levels, this suggests beneficial antioxidant and functional mitochondrial effects. Furthermore, CsA can prevent mitochondrial swelling and membrane depolarization by targeting mitochondrial CypD, a key component in mPTP opening, leading to mitochondrial dysfunction [45–49]. Although the hematopoietic status of CypD expression is uncertain, CypD can contribute to normal neutrophil activation [68] and is upregulated in TazKD♂ mice [69]; thus, these data suggest that therapeutic targeting of the mPTP‒CypD axis may be beneficial. Indeed, elamipretide (a mitochondrial-targeting peptide) is currently used to treat BTHS [6, 70], as it inhibits mPTP opening, prevents oxidative damage, and helps maintain cristae integrity. Future studies will be needed to directly test whether CsA reverses TAZD75H-mediated neutropenia in vivo or in an ex vivo neutrophil culture system. Taken together, these data support our hypothesis that a lack of TAFAZZIN acyltransferase activity is sufficient to phenocopy BTHS neutropenia and drive mitochondrial dysfunction, demonstrating that normal granulopoiesis requires CL phospholipids.
Interestingly, the apparent reduction in myeloid progenitor colony formation in vitro (see Fig. 2), despite preserved in vivo output (see Fig. 1), points to a context-dependent functional defect that is likely influenced by cytokine signaling or the microenvironment, rather than intrinsic depletion or only dysfunction of progenitor populations. This suggests that the myeloid progenitors in TazD75H♂ marrow remain functionally competent in vivo due to the presence of essential regulatory cues derived from stromal signals, cell-cell interactions, and finely tuned cytokine gradients, which may not adequately replicate in vitro colony assays. Alternatively, the in vitro myeloid progenitor assay may recapitulate aspects of stress hematopoiesis which are required for the deficiency in progenitors and not present in the transplant model. A particularly relevant factor in this context is IL-6, a pleiotropic cytokine, which is shown to be consistently elevated in the serum of BTHS patients [71]. IL-6 primarily induces differentiation and suppresses proliferation. Sustained IL-6 exposure can induce promote ROS generation, mitochondrial dysfunction, HSPC exhaustion, and drive pathological remodeling of the niche [72, 73]. In vivo, the hematopoietic niche may regulate the effects of elevated IL-6 through compensatory anti-inflammatory pathways or niche-protective mechanisms, thereby preserving baseline myelopoiesis [74–76]. However, under in vitro conditions, where cells are removed from their native regulatory microenvironment and exposed to cytokine cocktails this buffering is absent, reflecting a more stressed scenario and indicate that the defect in GMPs may require specific stressors to elicit the phenotype. As a result, the suppressive influence of IL-6 may become unmasked, leading to impaired colony-forming capacity, as higher IL-6 in vivo could also help the mice overcome the myeloid progenitor deficiency since it drives myeloid differentiation. We speculate that excessive IL-6 signaling might be a key contributor to the observed discrepancies between in vitro functional effect on CFU and in vivo phenotypic expression of progenitors seen TazD75H♂ bone marrow cells. Conversely, the high levels of IL-6 in vivo may promote myelopoiesis and increase progenitors thereby circumventing the deficiency in myeloid progenitors observed in vitro.
Although several groups have investigated the mechanisms by which TAFAZZIN mutations result in BTHS-related cardiac and skeletal muscle defects [36, 37, 55, 77], the reason BTHS patients have fewer neutrophils remains unclear. Our data demonstrate a novel stage-restricted defect within mouse and patient D75H-mutant neutrophil maturation associated with deficient hematopoietic progenitor differentiation, abnormal depolarization of mutant hematopoietic cell mitochondrial membranes, and ROS buildup. One limitation of our study is the lack of serial CBC measurements from individual mice over time. A longitudinal study of absolute cell counts from individual mice will be necessary to detect periodic neutropenia. Future studies are also needed to assess whether TAZD75H HSCs can function appropriately via in vivo competition assays and transplantation into secondary hosts to confirm whether the initial defect is at the level of stem cells. Additionally, the functions of both reduced neonatal/juvenile and unchanged adult TAZD75H neutrophils need to be examined for their bacterial killing capacity and NET formation ability [78]. Also, as some BTHS patients and TazD75H♂ exhibit 3-methylglutaconic aciduria [5, 7, 13], the effect of elevated 3-methylglutaconic upon granulopoiesis warrants further investigation. In summary, our work demonstrated that impaired hematopoiesis in BTHS patients can be recapitulated in vivo in mice by editing a patient-specific TAFAZZIN point mutation into the murine genome. This patient-tailored TazD75H♂ mouse model recapitulates the clinical hallmarks of BTHS neutropenia and demonstrates other hallmark BTHS phenotypes in nonhematopoietic tissues [13, 53], accompanied by structural and functional mitochondrial defects. This work enables future in vivo studies to enhance our mechanistic understanding of hematopoietic defects in BTHS and develop treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This paper is dedicated to the memory of Grzegorz Nalepa and Hal Broxmeyer, both extraordinary scientists, mentors, and human beings. We are also grateful to Tiffini Allen for her continued support and to Drs. Kathleen Overholt and Linda Resar for their observations. We thank Dr. Zejin Sun for his assistance. We thank the Flow Cytometry Core, In vivo Therapeutic Core and the Center for Electron Microscopy at Indiana University for their technical assistance. These studies were supported, in part, by the Indiana University Simon Comprehensive Cancer Center, Riley Children’s Foundation and National Institutes of Health grants R01 HL159436 and U54 DK106846.
Author Contributions
Elizabeth A. Sierra Potchanant: Methodology, validation, formal analysis, investigation, writing, conceptualization. Maegan L. Capitano: Methodology, validation, formal analysis, investigation, writing, conceptualization, funding. Baskar Ramdas: Data generation, methodology, analysis. Donna M. Edwards: Data generation, methodology, analysis. Scott Cooper: Data generation, methodology, analysis. James Ropa: Data generation, methodology, analysis. S. Louise Pay: Editing of manuscript. Aditya Sheth: Data generation, methodology, analysis. Paige L. Snider: Data generation, methodology, analysis. Hilary J. Vernon: Generated cell line and patient data. Ngoc-Tung Tran: Writing/revision. Reuben Kapur: Writing/revision. Simon J. Conway: Methodology, validation, formal analysis, investigation, writing/revision, conceptualization, funding.
Funding
These studies were supported, in part, by the Indiana University Simon Comprehensive Cancer Center, Riley Children’s Foundation and National Institutes of Health grants R01 HL159436 and U54 DK106846.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval
All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (Revised 2020) and were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee (Indianapolis, IN, USA). For cell line generation and collection of patient data, all procedures were conducted per the ethical standards of the responsible committee on human experimentation (institutional and national) and the tenets of the Declaration of Helsinki of 1975, as revised in 2013. Informed written consent was obtained from the patients and parents or legal guardians of all participants in the study.
Consent for Publication
All authors consent to publication.
Consent to Participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: The original published version of this article unfortunately contained a mistake. In this article, Simon J. Conway should have been denoted as a corresponding author. The error occurred during the typesetting and proofing process.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elizabeth A. Sierra Potchanant and Maegan L. Capitano contributed equally to this work.
Change history
9/11/2025
The original online version of this article was revised: The original published version of this article unfortunately contained a mistake. In this article, Simon J. Conway should have been denoted as a corresponding author. The error occurred during the typesetting and proofing process.
Change history
9/30/2025
A Correction to this paper has been published: 10.1007/s12015-025-10983-9
Contributor Information
Maegan L. Capitano, Email: malcapit@iu.edu
Simon J. Conway, Email: siconway@iu.edu
References
- 1.Barth, P. G., Scholte, H. R., Berden, J. A., Van der Klei-Van Moorsel, J. M., Luyt-Houwen, I. E., Van ‘t Veer-Korthof, E. T., Van der Harten, J. J., & Sobotka-Plojhar, M. A. (1983). An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. Journal of the Neurological Sciences, 62(1–3), 327–355. [DOI] [PubMed] [Google Scholar]
- 2.Bione, S., D’Adamo, P., Maestrini, E., Gedeon, A. K., Bolhuis, P. A., & Toniolo, D. (1996). A novel X-linked gene, G4.5. Is responsible for Barth syndrome. Nature Genetics, 12(4), 385–389. [DOI] [PubMed] [Google Scholar]
- 3.Chin, M. T., & Conway, S. J. (2020). Role of Tafazzin in mitochondrial function, development and disease. Journal of Developmental Biology, 8(2), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garlid, A. O., Schaffer, C. T., Kim, J., Bhatt, H., Guevara-Gonzalez, V., & Ping, P. (2020). TAZ encodes tafazzin, a transacylase essential for Cardiolipin formation and central to the etiology of Barth syndrome. Gene, 726, 144148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke, S. L., Bowron, A., Gonzalez, I. L., Groves, S. J., Newbury-Ecob, R., Clayton, N., Martin, R. P., Tsai-Goodman, B., Garratt, V., Ashworth, M., Bowen, V. M., McCurdy, K. R., Damin, M. K., Spencer, C. T., Toth, M. J., Kelley, R. I., & Steward, C. G. (2013). Barth syndrome. Orphanet Journal of Rare Diseases, 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson, R., Jefferies, J., Wang, S., Pu, W. T., Takemoto, C., Hornby, B., Heyman, A., Chin, M. T., & Vernon, H. J. (2022). Current and future treatment approaches for Barth syndrome. Journal of Inherited Metabolic Disease, 45(1), 17–28. [DOI] [PubMed] [Google Scholar]
- 7.Vernon, H. J., Sandlers, Y., McClellan, R., & Kelley, R. I. (2014). Clinical laboratory studies in Barth syndrome. Molecular Genetics and Metabolism, 112(2), 143–147. [DOI] [PubMed] [Google Scholar]
- 8.Schlame, M., & Greenberg, M. L. (2017). Biosynthesis, remodeling and turnover of mitochondrial Cardiolipin. Biochim Biophys Acta Mol Cell Biol Lipids, 1862(1), 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie, M., Lazarou, M., Thorburn, D. R., & Ryan, M. T. (2006). J mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. Molecular Biology, 361(3), 462–469. [Google Scholar]
- 10.Gonzalez, I. L., & Human TAFAZZIN variants database (Version 2023 July). Retrieved from Barth Syndrome Foundation.
- 11.Xu, Y., Zhang, S., Malhotra, A., Edelman-Novemsky, I., Ma, J., Kruppa, A., Cernicica, C., Blais, S., Neubert, T. A., Ren, M., & Schlame, M. (2009). Characterization of Tafazzin splice variants from humans and fruit flies. Journal of Biological Chemistry, 284(42), 29230–29239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu, Y. W., Galbraith, L., Herndon, J. D., Lu, Y. L., Pras-Raves, M., Vervaart, M., Van Kampen, A., Luyf, A., Koehler, C. M., McCaffery, J. M., Gottlieb, E., Vaz, F. M., & Claypool, S. M. (2016). Defining functional classes of Barth syndrome mutation in humans. Human Molecular Genetics, 25(9), 1754–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snider, P. L., Sierra Potchanant, E. A., Sun, Z., Edwards, D. M., Chan, K. K., Matias, C., Awata, J., Sheth, A., Pride, P. M., Payne, R. M., Rubart, M., Brault, J. J., Chin, M. T., Nalepa, G., & Conway, S. J. (2024). A Barth syndrome Patient-Derived D75H point mutation in TAFAZZIN drives progressive cardiomyopathy in mice. International Journal of Molecular Sciences, 25(15), 8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath, R. J., & Rock, C. O. (1998). A conserved histidine is essential for glycerolipid acyltransferase catalysis. Journal of Bacteriology, 180, 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claypool, S. M., McCaffery, J. M., & Koehler, C. M. (2006). Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. Journal of Cell Biology, 174(3), 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claypool, S. M., Oktay, Y., Boontheung, P., Loo, J. A., & Koehler, C. M. (2008). Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. Journal of Cell Biology, 182(5), 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter-Dennerlein, R., Korwitz, A., Haag, M., Tatsuta, T., Dargazanli, S., Baker, M., Decker, T., Lamkemeyer, T., Rugarli, E. I., & Langer, T. (2014). DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with prohibitins to regulate Cardiolipin remodeling. Cell Metabolism, 20(1), 158–171. [DOI] [PubMed] [Google Scholar]
- 18.Johnston, J., Kelley, R. I., Feigenbaum, A., Cox, G. F., Iyer, G. S., Funanage, V. L., & Proujansky, R. (1997). Mutation characterization and genotype-phenotype correlation in Barth syndrome. American Journal of Human Genetics, 61(5), 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley, R. I., Cheatham, J. P., Clark, B. J., Nigro, M. A., Powell, B. R., Sherwood, G. W., Sladky, J. T., & Swisher, W. P. (1991). X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. Journal of Pediatrics, 119(5), 738–747. [DOI] [PubMed] [Google Scholar]
- 20.Dale, D. C., Bolyard, A. A., Marrero, T. M., Bonilla, M. A., Phan, L., & Steward, C. (2013). Barth syndrome and neutropenia. Blood, 122(21), 3465. [Google Scholar]
- 21.Sohn, J., Milosevic, J., Brouse, T., Aziz, N., Elkhoury, J., Wang, S., Hauschild, A., van Gastel, N., Cetinbas, M., Tufa, S. F., Keene, D. R., Sadreyev, R. I., Pu, W. T., & Sykes, D. B. (2022). A new murine model of Barth syndrome neutropenia links TAFAZZIN deficiency to increased ER stress-induced apoptosis. Blood Advances, 6(8), 2557–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferies, J. L. (2013). Barth syndrome. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 163C(3), 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steward, C. G., Groves, S. J., Taylor, C. T., Maisenbacher, M. K., Versluys, B., Newbury-Ecob, R. A., Ozsahin, H., Damin, M. K., Bowen, V. M., McCurdy, K. R., Mackey, M. C., Bolyard, A. A., & Dale, D. C. (2019). Neutropenia in Barth syndrome: Characteristics, risks, and management. Current Opinion in Hematology, 26(1), 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zakrzewski, P., Rice, C. M., Fleming, K., Cela, D., Groves, S. J., Ponce-Garcia, F. M., Gibbs, W., Roberts, K., Pike, T., Strathdee, D., Anderson, E., Nobbs, A. H., Toye, A. M., Steward, C., & Amulic, B. (2025). Tafazzin regulates neutrophil maturation and inflammatory response. Embo Reports, 26(6), 1590–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolaczkowska, E., & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology, 13(3), 159–175. [DOI] [PubMed] [Google Scholar]
- 26.Evrard, M., Kwok, I. W. H., Chong, S. Z., Teng, K. W. W., Becht, E., Chen, J., Sieow, J. L., Penny, H. L., Ching, G. C., Devi, S., Adrover, J. M., Li, J. L. Y., Liong, K. H., Tan, L., Poon, Z., Foo, S., Chua, J. W., Su, I. H., Balabanian, K., Bachelerie, F., Biswas, S. K., Larbi, A., Hwang, W. Y. K., Madan, V., Koeffler, H. P., Wong, S. C., Newell, E. W., Hidalgo, A., Ginhoux, F., & Ng, L. G. (2018). Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity, 48(2), 364–379e8. [DOI] [PubMed] [Google Scholar]
- 27.Pillay, J., den Braber, I., Vrisekoop, N., Kwast, L. M., de Boer, R. J., Borghans, J. A., Tesselaar, K., & Koenderman, L. (2010). In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood, 116(4), 625–627. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalvez, F., D’Aurelio, M., Boutant, M., Moustapha, A., Puech, J. P., Landes, T., Arnauné-Pelloquin, L., Vial, G., Taleux, N., Slomianny, C., Wanders, R. J., Houtkooper, R. H., Bellenguer, P., Møller, I. M., Gottlieb, E., Vaz, F. M., Manfredi, G., & Petit, P. X. (2013). Barth syndrome: Cellular compensation of mitochondrial dysfunction and apoptosis Inhibition due to changes in Cardiolipin remodeling linked to Tafazzin (TAZ) gene mutation. Biochimica Et Biophysica Acta, 1832(8), 1194–1206. [DOI] [PubMed] [Google Scholar]
- 29.Mantel, C. R., O’Leary, H. A., Chitteti, B. R., Huang, X., Cooper, S., Hangoc, G., Brustovetsky, N., Srour, E. F., Lee, M. R., Messina-Graham, S., Haas, D. M., Falah, N., Kapur, R., Pelus, L. M., Bardeesy, N., Fitamant, J., Ivan, M., Kim, K. S., & Broxmeyer, H. E. (2015). Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell, 161(7), 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capitano, M. L., Mor-Vaknin, N., Saha, A. K., Cooper, S., Legendre, M., Guo, H., Contreras-Galindo, R., Kappes, F., Sartor, M. A., Lee, C. T., Huang, X., Markovitz, D. M., & Broxmeyer, H. E. (2019). Secreted nuclear protein DEK regulates hematopoiesis through CXCR2 signaling. Journal of Clinical Investigation, 129(6), 2555–2570.
- 31.Capitano, M. L., Mohamad, S. F., Cooper, S., Guo, B., Huang, X., Gunawan, A. M., Sampson, C., Ropa, J., Srour, E. F., Orschell, C. M., & Broxmeyer, H. E. (2021). Mitigating oxygen stress enhances aged mouse hematopoietic stem cell numbers and function. Journal of Clinical Investigation, 131(1), e140177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broxmeyer, H. E., Hoggatt, J., O’Leary, H. A., Mantel, C., Chitteti, B. R., Cooper, S., Messina-Graham, S., Hangoc, G., Farag, S., Rohrabaugh, S. L., Ou, X., Speth, J., Pelus, L. M., Srour, E. F., & Campbell, T. B. (2012). Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nature Medicine, 18(12), 1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerabona, D., Sun, Z., & Nalepa, G. (2016). Leukemia and chromosomal instability in aged Fancc-/- mice. Experimental Hematology, 44(5), 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tosato, G., & Cohen, J. I. (2007). Generation of Epstein-Barr virus (EBV)-immortalized B cell lines. Current Protocols In Immunology / Edited By John E. Coligan. [Et Al.].;Chap. 7:7.22.1–7.22.4.
- 35.Edwards, D., Potchanant, E. S., Huang, X., Sun, Z., Capitano, M., Miller, C., He, Y., Broxmeyer, H. E., & Nalepa, G. (2017). Patient-Tailored mouse genome editing recapitulates hematopoietic and systemic manifestations of Barth syndrome. Blood, 130(Supplement 1), 775. [Google Scholar]
- 36.Wang, S., Li, Y., Xu, Y., Ma, Q., Lin, Z., Schlame, M., Bezzerides, V. J., Strathdee, D., & Pu, W. T. (2020). AAV gene therapy prevents and reverses heart failure in a murine knockout model of Barth syndrome. Circulation Research, 126(8), 1024–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phoon, C. K., Acehan, D., Schlame, M., Stokes, D. L., Edelman-Novemsky, I., Yu, D., Xu, Y., Viswanathan, N., & Ren, M. (2012). Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. Journal of the American Heart Association, 1(2), jah3–e000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudlaty, E., Agnihotri, N., & Khojah, A. (2022). Hypogammaglobulinaemia and B cell lymphopaenia in Barth syndrome. BMJ Case Reports CP, 15, e249254. [Google Scholar]
- 39.Houtkooper, R. H., Turkenburg, M., Poll-The, B. T., Karall, D., Pérez-Cerdá, C., Morrone, A., Malvagia, S., Wanders, R. J., Kulik, W., & Vaz, F. M. (2009). The enigmatic role of Tafazzin in Cardiolipin metabolism. Biochimica Et Biophysica Acta, 1788(10), 2003–2014. [DOI] [PubMed] [Google Scholar]
- 40.Duncan, A. L. (2020). Monolysocardiolipin (MLCL) interactions with mitochondrial membrane proteins. Biochemical Society Transactions, 48(3), 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zorova, L. D., Popkov, V. A., Plotnikov, E. Y., Silachev, D. N., Pevzner, I. B., Jankauskas, S. S., Babenko, V. A., Zorov, S. D., Balakireva, A. V., Juhaszova, M., Sollott, S. J., & Zorov, D. B. (2018). Mitochondrial membrane potential. Analytical Biochemistry, 552, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka, Y., Kanai, Y., Okada, Y., Nonaka, S., Takeda, S., Harada, A., & Hirokawa, N. (1998). Targeted disruption of mouse conventional Kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell, 93(7), 1147–1158. [DOI] [PubMed] [Google Scholar]
- 43.Jang, Y. Y., & Sharkis, S. J. (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that May reside in the low-oxygenic niche. Blood, 110(8), 3056–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porto, M. L., Rodrigues, B. P., Menezes, T. N., Ceschim, S. L., Casarini, D. E., Gava, A. L., Pereira, T. M., Vasquez, E. C., Campagnaro, B. P., & Meyrelles, S. S. (2015). Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. Journal of Biomedical Science, 22, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connern, C. P., & Halestrap, A. P. (1994). Recruitment of mitochondrial Cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. The Biochemical Journal, 302(Pt 2), 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan, P. G., Rabchevsky, A. G., Waldmeier, P. C., & Springer, J. E. (2005). Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? Journal of Neuroscience Research, 79(1–2), 231–239. [DOI] [PubMed] [Google Scholar]
- 47.Tsujimoto, Y., & Shimizu, S. (2007). Role of the mitochondrial membrane permeability transition in cell death. Apoptosis, 12(5), 835–840. [DOI] [PubMed] [Google Scholar]
- 48.Rao, V. K., Carlson, E. A., & Yan, S. S. (2014). Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochimica Et Biophysica Acta, 1842(8), 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briston, T., Roberts, M., Lewis, S., Powney, B., Szabadkai, J. M. S., & Duchen, G. (2017). Mitochondrial permeability transition pore: Sensitivity to opening and mechanistic dependence on substrate availability. Scientific Reports, 7(1), 10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gineste, C., Hernandez, A., Ivarsson, N., Cheng, A. J., Naess, K., Wibom, R., Lesko, N., Bruhn, H., Wedell, A., Freyer, C., Zhang, S. J., Carlstrom, M., Lanner, J. T., Andersson, D. C., Bruton, J. D., Wredenberg, A., & Westerblad, H. (2015). Cyclophilin D, a target for counteracting skeletal muscle dysfunction in mitochondrial myopathy. Human Molecular Genetics, 24(23), 6580–6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halestrap, A. P., & Davidson, A. M. (1990). Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. The Biochemical Journal, 268(1), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGuinness, O., Yafei, N., Costi, A., & Crompton, M. (1990). The presence of two classes of high-affinity cyclosporin A binding sites in mitochondria. Evidence that the minor component is involved in the opening of an inner-membrane Ca(2+)-dependent pore. European Journal of Biochemistry, 194(2), 671–679. [DOI] [PubMed] [Google Scholar]
- 53.Snider, P. L., Sierra Potchanant, E. A., Matias, C., Edwards, D. M., Brault, J. J., & Conway, S. J. (2024). The loss of Tafazzin transacetylase activity is sufficient to drive testicular infertility. Journal of Developmental Biology, 12(4), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soustek, M. S., Falk, D. J., Mah, C. S., Toth, M. J., Schlame, M., Lewin, A. S., & Byrne, B. J. (2011). Characterization of a Transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Human Gene Therapy, 22(7), 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren, M., Miller, P. C., Schlame, M., & Phoon, C. K. L. (2019). A critical appraisal of the Tafazzin knockdown mouse model of Barth syndrome: What have we learned about pathogenesis and potential treatments? American Journal of Physiology Heart and Circulatory Physiology, 317(6), H1183–H1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zegallai, H. M., Abu-El-Rub, E., Olayinka-Adefemi, F., Cole, L. K., Sparagna, G. C., Marshall, A. J., & Hatch, G. M. (2022). Tafazzin deficiency in mouse mesenchymal stem cells promote reprogramming of activated B lymphocytes toward immunosuppressive phenotypes. The Faseb Journal, 36(8), e22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seneviratne, A. K., Xu, M., Henao, J. J. A., Fajardo, V. A., Hao, Z., Voisin, V., Xu, G. W., Hurren, R., Kim, S., MacLean, N., Wang, X., Gronda, M., Jeyaraju, D., Jitkova, Y., Ketela, T., Mullokandov, M., Sharon, D., Thomas, G., Chouinard-Watkins, R., Hawley, J. R., Schafer, C., Yau, H. L., Khuchua, Z., Aman, A., Al-Awar, R., Gross, A., Claypool, S. M., Bazinet, R. P., Lupien, M., Chan, S., De Carvalho, D. D., Minden, M. D., Bader, G. D., Stark, K. D., LeBlanc, P., & Schimmer, A. D. (2019). The mitochondrial transacylase, tafazzin, regulates for AML stemness by modulating intracellular levels of phospholipids. Cell Stem Cell, 24(4), 621–636e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdul-Hussien, H., Hanemaaijer, R., Verheijen, J. H., van Bockel, J. H., Geelkerken, R. H., & Lindeman, J. H. (2009). Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. Journal of Vascular Surgery, 49(3), 741–749. [DOI] [PubMed] [Google Scholar]
- 59.Devyani Sharma, D., Xu, J., & Filippi, M. D. (2023). Decline in Cardiolipin in hematopoietic stem cell during aging alters their regenerative potential. Blood, 142(Supplement 1), 2674. [Google Scholar]
- 60.Hornigold, K., Chu, J. Y., Chetwynd, S. A., Machin, P. A., Crossland, L., Pantarelli, C., Anderson, K. E., Hawkins, P. T., Segonds-Pichon, A., Oxley, D., & Welch, H. C. E. (2022). Age-related decline in the resistance of mice to bacterial infection and in LPS/TLR4 pathway-dependent neutrophil responses. Frontiers in Immunology, 13, 888415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López, D. A., Apostol, A. C., Lebish, E. J., Valencia, C. H., Romero-Mulero, M. C., Pavlovich, P. V., Hernandez, G. E., Forsberg, E. C., Cabezas-Wallscheid, N., & Beaudin, A. E. (2022). Prenatal inflammation perturbs murine fetal hematopoietic development and causes persistent changes to postnatal immunity. Cell Reports, 41(8), 111677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bee, G. C. W., Lokken-Toyli, K. L., Yeung, S. T., Rodriguez, L., Zangari, T., Anderson, E. E., Ghosh, S., Rothlin, C. V., Brodin, P., Khanna, K. M., & Weiser, J. N. (2023). Age-dependent differences in efferocytosis determine the outcome of opsonophagocytic protection from invasive pathogens. Immunity, 56(6), 1255–1268e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferri, L., Donati, M. A., Funghini, S., Cavicchi, C., Pensato, V., Gellera, C., Natacci, F., Spaccini, L., Gasperini, S., Vaz, F. M., Cooper, D. N., Guerrini, R., & Morrone, A. (2015). Intra-individual plasticity of the TAZ gene leading to different heritable mutations in siblings with Barth syndrome. European Journal of Human Genetics, 23(12), 1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clayton, N. (2018). Understanding Feeding in Barth Syndrome. Barth Syndrome Foundation website. https://www.barthsyndrome.org/file_download/inline/d4951f0f-7937-49c5-8c86-37aed9f57ad3
- 65.Xu, Y., Sutachan, J. J., Plesken, H., Kelley, R. I., & Schlame, M. (2005). Characterization of lymphoblast mitochondria from patients with Barth syndrome. Laboratory Investigation, 85(6), 823–830. [DOI] [PubMed] [Google Scholar]
- 66.Dudek, J. (2017). Role of Cardiolipin in mitochondrial signaling pathways. Frontiers in Cell and Developmental Biology, 5, 90.
- 67.Choudhuri, S., Chowdhury, I. H., & Garg, N. J. (2020). Mitochondrial regulation of macrophage response against pathogens. Frontiers in Immunology, 11, 622602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Downey, J., Randolph, H. E., Pernet, E., Tran, K. A., Khader, S. A., King, I. L., Barreiro, L. B., & Divangahi, M. (2022). Mitochondrial Cyclophilin D promotes disease tolerance by licensing NK cell development and IL-22 production against influenza virus. Cell Reports, 39(12), 110974. [DOI] [PubMed] [Google Scholar]
- 69.Jang, S., Lewis, T. S., Powers, C., Khuchua, Z., Baines, C. P., Wipf, P., & Javadov, S. (2017). Elucidating mitochondrial electron transport chain supercomplexes in the heart during Ischemia-Reperfusion. Antioxidants & Redox Signaling, 27(1), 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson, W. R., Manuel, R., Abbruscato, A., Carr, J., Campbell, J., Hornby, B., Vaz, F. M., & Vernon, H. J. (2024). Long-term efficacy and safety of Elamipretide in patients with Barth syndrome: 168-week open-label extension results of TAZPOWER. Genetics in Medicine, 26(7), 101138. [DOI] [PubMed] [Google Scholar]
- 71.Wilson, L. D., Al-Majid, S., Rakovski, C. S., & Schwindt, C. D. (2012). Higher IL-6 and IL6:IGF ratio in patients with Barth syndrome. Journal of Inflammation (London), 9(1), 25. [Google Scholar]
- 72.Didion, S. P. (2017). Cellular and oxidative mechanisms associated with Interleukin-6 signaling in the vasculature. International Journal of Molecular Sciences, 18(12), 2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh, S., Jakubison, B., & Keller, J. R. (2020). Protection of hematopoietic stem cells from stress-induced exhaustion and aging. Current Opinion in Hematology, 27(4), 225–231. [DOI] [PubMed] [Google Scholar]
- 74.Reynaud, D., Pietras, E., Barry-Holson, K., Mir, A., Binnewies, M., Jeanne, M., Sala-Torra, O., Radich, J. P., & Passegué, E. (2011). IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell, 20(5), 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crane, G. M., Jeffery, E., & Morrison, S. J. (2017). Adult Haematopoietic stem cell niches. Nature Reviews Immunology, 17(9), 573–590. [DOI] [PubMed] [Google Scholar]
- 76.Méndez-Ferrer, S., Bonnet, D., Steensma, D. P., Hasserjian, R. P., Ghobrial, I. M., Gribben, J. G., Andreeff, M., & Krause, D. S. (2020). Bone marrow niches in haematological malignancies. Nature Reviews Cancer, 20(5), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki-Hatano, S., Saha, M., Rizzo, S. A., Witko, R. L., Gosiker, B. J., Ramanathan, M., Soustek, M. S., Jones, M. D., Kang, P. B., Byrne, B. J., Cade, W. T., & Pacak, C. A. (2019). AAV-Mediated TAZ gene replacement restores mitochondrial and cardioskeletal function in Barth syndrome. Human Gene Therapy, 30(2), 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran, N. T., Graf, R., Wulf-Goldenberg, A., Stecklum, M., Strauß, G., Kühn, R., Kocks, C., Rajewsky, K., & Chu, V. T. (2020). CRISPR-Cas9-Mediated ELANE mutation correction in hematopoietic stem and progenitor cells to treat severe congenital neutropenia. Molecular Therapy, 28(12), 2621–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.