Abstract
Background and Objective
The COVID-19 pandemic has significantly influenced vaccination strategies and public health policies. Discrete choice experiments have emerged as a valuable tool for understanding preferences regarding vaccination. This study systematically reviews discrete choice experiments conducted on COVID-19 public vaccination preferences to identify key determinants influencing vaccine uptake and to assess methodological approaches used in these studies.
Methods
A systematic literature search was conducted across major databases, including PubMed, Scopus, and Web of Science, to identify discrete choice experiments focusing on COVID-19 vaccination preferences up to 31 December, 2024. Attribute categorization into five dimensions Outcome, Process, Cost, Trust, and Framing was performed and quality appraised according to the DIRECT checklist. Conditional relative importance as well as geographical differences were assessed.
Results
The review identified 58 studies employing discrete choice experiments that assessed public COVID-19 vaccine preferences. Among attribute categories, outcome-related factors were the most frequently used and had the highest relative importance. Other commonly evaluated attributes included cost, origin/brand, and required doses. A notable geographic disparity was observed, with studies being unevenly distributed across different regions. Methodological heterogeneity was observed in attribute selection and experimental design.
Conclusions
This review emphasizes the importance of considering individual preferences into vaccination strategies to enhance uptake, particularly in preparation for future pandemics. The findings reveal that vaccine effectiveness and safety are key concerns for individuals. Future research could focus on increasing representation of underexamined regions in preference studies to better inform local policymakers in developing effective vaccination programs for future health crises.
Clinical Trial Registration
This review was prospectively registered in PROSPERO (International Prospective Register of Systematic Reviews) with the ID CRD42025543234.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40271-025-00753-7.
Key Points for Decision Makers
| Discrete choice experiments on COVID-19 vaccination predominantly include attributes related to effectiveness and safety (outcome attributes), though notable regional differences exist. |
| Outcome attributes were the most important in most studies, while Trust, Cost, Process, and Framing attributes were rarely ranked as the highest priority. |
| Physicians and policymakers should consider these findings when communicating vaccination information for COVID-19 and future pandemics. |
Introduction
The COVID-19 pandemic has profoundly impacted global health, economic stability, and social structures, necessitating rapid public health responses and vaccination campaigns. By the end of 2024, the virus had infected over 776 million people worldwide, leading to more than 7 million deaths [1]. As governments and healthcare organizations scrambled to control the spread of SARS-CoV-2, vaccination emerged as a cornerstone of pandemic management. By the end of 2021, several vaccines had been authorized for emergency use, and mass vaccination campaigns were underway globally. These vaccines proved to be highly effective in reducing severe illness, hospitalizations, and deaths [2].
However, the success of vaccination campaigns depends not only on vaccine availability but also on public acceptance and adherence, which are influenced by a complex interplay of factors including information regarding perceived risks, benefits, and accessibility [3, 4]. Vaccination adherence can be a critical factor in determining the effectiveness of vaccination campaigns [5–7]. High levels of adherence are essential for achieving herd immunity and reducing the burden on healthcare systems. Despite the clear benefits of vaccination, global efforts were hampered by vaccine hesitancy, driven by a range of factors, including concerns about vaccine safety, misinformation, political polarization, and historical mistrust in healthcare systems [8]. Understanding the factors influencing both vaccine hesitancy and adherence is essential for ensuring adequate vaccination coverage.
Discrete choice experiments (DCEs) have become a prominent method for understanding preferences in health economics, including vaccination decisions and adherence [9–12]. By presenting respondents with hypothetical scenarios involving trade-offs between attributes, DCEs allow researchers to quantify the relative importance of factors influencing decision making. This methodology has proven particularly valuable in identifying trade-offs, barriers, and facilitators to vaccination adherence, such as vaccine efficacy, side effects, and cost [13].
Since the start of the COVID-19 pandemic, DCEs were widely used to investigate public preferences for COVID-19 vaccines. These studies provided critical insights into how individuals prioritize vaccine attributes as well as the role of demographic factors in shaping preferences [14, 15]. Such insights may inform policy decisions and communication strategies, potentially helping to address vaccine hesitancy and optimize vaccine rollouts. While the immediate utility of COVID-19 DCE studies is evident, their implications may extend beyond the current pandemic as they can reflect how the population may respond to different vaccination strategies and policies. Understanding the lessons learned from stated preference studies regarding the COVID-19 pandemic can guide future research and policy for managing future pandemics and designing appropriate vaccination campaigns.
While a systematic review by Huang et al. [16] did provide a comprehensive overview of DCEs in this field, our study expands on their findings in several ways. Their review included studies up to April 2023, while new studies have been published recently. Furthermore, Huang et al. acknowledged that the studies included in their review used different attributes, reflecting diverse research focuses. While they synthesized these attributes to inform future DCEs, they did not distinguish between vaccine characteristics and broader policy aspects. In contrast, our study focuses specifically on public preferences for vaccination characteristics to provide clearer insights into commonly used attributes and their importance for influencing vaccination uptake and adherence. Additionally, the authors noted that classifying attributes into outcome, cost, and process categories was insufficient. We address this by adding two new categories to better capture the full range of attributes. Additionally, while Huang et al. highlighted the limitations of the PREFS checklist for assessing DCE studies, we use the more comprehensive DIRECT checklist, which, to our knowledge, is newly applied in a systematic review of DCEs. Furthermore, as the World Health Organization revoked the status of COVID-19 being a public health emergency of international concern on 5 May, 2023, studies conducted after this date might show a relevant change in preferences regarding vaccination attributes, which is why this study aims at analyzing how preferences may have changed over time and included studies after the cutoff date by Huang et al. [16, 17].
Last, a secondary objective of our study was to analyze geographic differences in DCEs on COVID-19 vaccination preferences. Understanding regional variations in preferences is crucial for policymakers, as these differences may significantly influence vaccine adherence and the effectiveness of public health strategies. In summary, this systematic literature review synthesizes findings from DCEs, focusing on public preferences for the characteristics of COVID-19 vaccination. By examining the methodologies, results, and implications of these studies, we aim to derive actionable lessons for future pandemics and vaccination campaigns. Through this analysis, we contribute to the growing body of literature on health preference research and offer practical recommendations for policymakers and researchers to enhance the design and delivery of vaccination campaigns in future global health crises.
Methods
This systematic review follows the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) 2020 guidelines to ensure transparency and reproducibility, as well as the PRISMA-Search [18] and PRESS guidelines [19] for developing a rigorous and comprehensive literature search process. Accordingly, the completed PRISMA checklist is reported in Appendix A of the Electronic Supplementary Material (ESM). Furthermore, a protocol for the systematic review was registered in PROSPERO (International Prospective Register of Systematic Reviews) with the ID CRD42025543234.
Literature Search
A comprehensive search strategy was developed to identify studies that provide quantitative stated preference data through DCEs regarding COVID-19 vaccination. The development of final search strategies, presented in Appendix B of the ESM, was supported by experienced researchers (CB, MH, and NKS), informed by previous research, and included a combination of keywords and subject headings related to “COVID-19 vaccination,” “discrete choice experiments,” and “stated preferences.” Initial candidate search terms were expanded and validated using draft search strategies. For validation purposes, three known relevant studies meeting all inclusion criteria were identified a priori and successfully retrieved using the final strategies.
The electronic databases MEDLINE, Embase (both via Ovid), and Scopus were searched for relevant articles. In addition to electronic searches, experts in the field were contacted to identify additional studies. Furthermore, manual searches including bibliography screening of the included studies were conducted.
The search was limited to peer-reviewed articles published in English up to 31 December, 2024. This restriction was based on previous findings indicating limited utility of non-English studies in similar contexts [20, 21].
Study Records
The literature search results were processed using Covidence, a software platform specifically designed for managing systematic reviews (www.covidence.org). After importing all identified records into Covidence, duplicate entries were automatically removed by the software. The remaining titles and abstracts were then independently screened by two reviewers (ELH and JD or CB) against the inclusion criteria outlined in Table 1. Articles deemed potentially relevant were retrieved in a full-text format for a detailed assessment. Discrepancies between the reviewers were addressed through discussion, and a third reviewer was consulted when a consensus could not be achieved. The reasons for excluding articles at the full-text review stage were recorded to maintain transparency in the study selection process.
Table 1.
Inclusion criteria
| Population | People of any demographic (i.e., age, ethnicity, sex) |
|---|---|
| Intervention/comparator | Vaccination for COVID-19 |
| Timing | Studies published until 31 December, 2024 |
| Study design | Peer-reviewed discrete choice experiments |
| Language | English |
Data Extraction
The process of data extraction and analysis was structured and systematic. All included studies were summarized using a predefined data extraction form that was developed based on previous research and adapted for the purpose of this review. To ensure its reliability and relevance, the form was tested on three studies before data extraction began, and insights gained during this pretesting phase were used to refine it further.
The finalized extraction form captured the following categories:
General study information: title, authors, journal, year of publication, country, availability, time and duration of data collection.
Population characteristics: total number of participants, mean age, and response rate.
Methodological details: study design, data collection method (e.g., online, face-to-face), elicitation method for attributes and levels, the specific attributes and levels included in the questionnaire, whether a pilot study was conducted, number of choice sets/tasks per participant, number of attributes per choice set/task, maximum levels per attribute, and number of alternatives per choice set/task.
Results: key findings, including conditional relative importance values, and attribute rankings.
The extracted information was used to perform a narrative synthesis, and results were summarized in tabular formats to facilitate a comparison across studies.
Quality Assessment
The quality of the included studies was evaluated by two independent reviewers (DN and ELH) using the DIRECT checklist from the Equator Network [22]. Although the DIRECT checklist is primarily a reporting checklist, it also assesses multiple dimensions of study quality that are crucial for evaluating the robustness of a study. This checklist provides a detailed framework for assessing critical aspects of stated preference studies, including the purpose and rationale of the study, attributes and levels used in the experimental design, construction of choice tasks and survey design, including randomization and data collection methods, econometric and statistical analysis approaches, and reporting of results and conclusions.
To ensure consistency and objectivity in scoring, an assessment guide was developed prior to initiating the quality assessment (see Appendix C of the ESM). Each of the 18 items in the checklist was assigned a rating scale that ranged from 0 points (not reported) to 2 points (fully reported), with criteria and examples provided to define when a score of 1 point or 2 points was appropriate. Therefore, studies could reach a minimum score of 0 points and a maximum score of 36 points. Discrepancies in quality assessments were resolved through discussion, and if a consensus was not achieved, a third reviewer was consulted. The results of the quality assessment were systematically documented and reported.
Data Analysis
Attributes were analyzed according to their frequency across all included studies, as well as regarding the conditional relative importance (CRI). If the CRI was not reported by the authors, the ISPOR Conjoint Analysis Good Research Practices Task Force range method was used to calculate CRI, if the necessary information was included [23]. This approach assesses the CRI of an attribute by computing the maximum range between two coefficient levels for that attribute. Attributes are then ranked based on their CRI, which is determined by dividing the range of the coefficient values for the specific attribute by the total range sum of all attributes. The maximum range for each attribute is calculated as the difference between its highest and lowest coefficient estimates. When an attribute exhibits a larger range compared with others, it suggests that the levels within that attribute are more sensitive to changes, resulting in a greater influence on respondents’ preferences and, therefore, a higher CRI for that attribute.
For studies that did not provide an overall CRI across all respondents, such as those employing latent class models or those that did not summarize cross-country results, the CRI for each attribute was determined based on the CRI for each subgroup. If a single subgroup encompassed more than 50% of respondents, the preference ranking of that subgroup was used to reflect the findings of the paper. If no subgroup reached the 50% threshold, subgroup findings were aggregated, and the attribute ranked highest (and second highest) across 50% of respondents cumulatively was selected as the most important (and second most important). If coding was not indicated, calculations were run both for dummy and effects coding. In the case of differences in the most or second most important attribute, the paper was excluded from the analysis regarding CRI. Otherwise, the paper was included in the analysis.
Attributes were subsequently classified into five categories, Outcome, Process, Cost, Framing, and Trust, to provide a comprehensive overview of the included studies. This categorization builds on the framework proposed by Bien et al. [24] and was expanded to incorporate additional contextual and perceptual factors that were consistently employed by the included studies. The expansion beyond the traditional categories was motivated not only by limitations identified in previous research [16], but also by the polarized discussions surrounding the role of trust, as well as the framing mechanisms employed in real-life vaccination policies, both of which were appropriately reflected in the attributes used by the included studies.
The Outcome category encompassed attributes related to vaccine efficacy, duration of protection, side effects, and the time that the vaccine needs to work. The Process category included attributes concerning the mode and frequency of administration, number of doses, mode and site of administration, availability, and appointment scheduling aspects such as office hours of waiting time. Cost attributes covered financial considerations such as direct vaccine costs.
The Framing category included attributes related to information on vaccination uptake or adherence within the respondent’s social group or general population, the perceived health risk of non-vaccination, incentives for vaccination, media coverage, obligations such as vaccination mandates, and consequences such as quarantine requirements for non-vaccination. The Trust category encompassed attributes concerning the vaccine’s origin, brand, development time and trial information, recommender, and supporting evidence. Within these categories, the frequency of attributes was assessed and compared across the included studies to identify common trends and key drivers of decision making. No meta-analysis or statistical tests were performed.
Results
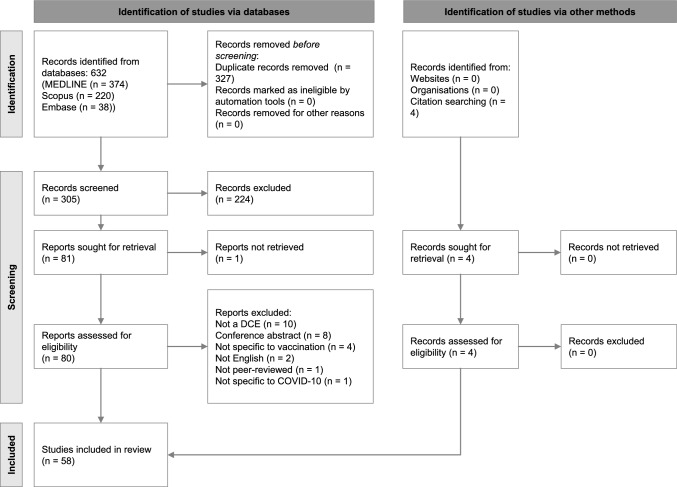
The database searches initially identified 632 records, of which 327 duplicates were removed. The remaining 305 records proceeded to title and abstract screening, where 224 studies were excluded for not meeting the inclusion criteria. This left 81 studies for a full-text review, though one study could not be retrieved. During the full-text assessment, 26 studies were excluded, with detailed reasons for exclusion provided in Appendix D of the ESM.
Additionally, four records were identified during manual searches of the included studies. Ultimately, 58 studies met the inclusion criteria and were included in the analysis. The study selection process is detailed in the PRISMA flow diagram presented in Fig. 1.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) 2020 flow chart for systematic reviews [25]. DCE discrete choice experiment
Study Characteristics
Table 2 provides an overview of the included studies, their key characteristics, and study design. Of the 58 included studies, ten were published prior to the availability of the first COVID-19 vaccine in the UK in December 2020 [14, 26–34]. Additionally, five studies conducted at least part of their data collection before this milestone [35–39]. In contrast, data collection for 41 studies took place after the vaccine’s availability was announced [39–79], while two studies did not specify their data collection period [80, 81]. Regarding publication timelines, the distribution of studies over time reflects the evolving research focus during the pandemic. Three studies were published in 2020 [14, 26, 29], followed by 13 in 2021 [27, 30, 31, 34, 35, 39, 49, 52, 55, 57, 59, 61, 81], 20 in 2022 [28, 32, 33, 36, 37, 41, 44, 48, 50, 51, 53, 56, 58, 63, 65, 70–72, 76, 79], 14 in 2023 [38, 40, 42, 43, 47, 54, 60, 64, 66–68, 73, 75, 78], and 8 in 2024 [45, 46, 62, 69, 74, 77, 78, 80].
Table 2.
General characteristics and design of discrete choice experiments of COVID-19 vaccinations
| References | Year of publication | Time of data collection | Country and region | Partici-pants | Adminis-tration | Selection of attributes and levels | Model | Choice sets | Attri-butes | Max no. of levels | Pilot study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [15] | 2024 | 07/2022–08/2023 | 22 countries/6 continents (Global) | 50,242 | Online | Literature review, expert discussion | Multinomial logit | 12 | 7 | 5 | Yes |
| [40] | 2023 | 02–04/2021 | Hong Kong (Asia) | 2032 | Online | Focus group discussion | Nested logit, latent class | 8 | 7 | 3 | Yes |
| [41] | 2022 | 05–06/2021 | India (Asia) | 1371 | Online | Literature review | Conditional logit, mixed logit | 6 | 7 | 4 | No |
| [42] | 2023 | 03–09/2021 | Hungary (Europe) | 1,011 | In person | Expert discussion, focus group discussion | Random parameter logit, hybrid random parameter logit | 8 | 4 | 5 | Yes |
| [43] | 2023 | 06/2021 | UK (Europe) | 149 | Online | Literature review, expert discussion, focus group discussion | Random parameter logit, latent class | 12 | 5 | 4 | Yes |
| [26] | 2020 | 03/2020 | Australia (Oceania) | 2136 | Online | Literature review | Latent class | 8 | 7 | 8 | Yes |
| [44] | 2022 | 01/2021 | Germany (Europe) | 1556 | Online | Not specified | Hierarchical multinomial logit | 10 | 6 | 5 | No |
| [45] | 2024 | 12/2021–03/2022 | New Zealand (Oceania) | 611 | Online | Literature review, expert discussion | Not specified | Varying | 13 | 5 | Yes |
| [46] | 2024 | 10–12/2021 | China (Asia) | 1509 | Online and offline | Literature review, expert discussion, focus group discussion | Conditional logit, latent class | 10 | 5 | 3 | Yes |
| [47] | 2023 | 01–03/2021 | China (Asia) | 293 | Offline, not specified | Literature review, expert discussion | Multinomial logit | 12 | 5 | 4 | Yes |
| [35] | 2021 | 09/2020–03/2021 | USA (America) | 1661 | Online | Not specified | OLS regression | 6 | 8 | 3 | No |
| [27] | 2021 | 11/2020 | USA (America) | 1153 | Online | Literature review, expert discussion | Latent class (opt-out inflated logit) | 8 | 5 | 4 | No |
| [48] | 2022 | 03–07/2021 | Iran (Asia) | 410 | Online and in person | Previous research, expert discussion, policy maker discussion | Conditional logit | 9 and 10 | 5 | 5 | No |
| [28] | 2022 | 10–11/2020 | USA (America) | 2723 | Online | Literature review, focus group discussion | Conditional logit, random parameters logit, latent class | 7 | 9 | 4 | Yes |
| [49] | 2021 | 10/2020–01/2021 | France (Europe) | 4346 | Online | Literature review | Random intercept logit | 8 | 5 | 4 | Yes |
| [29] | 2020 | 06–07/2020 | China (Asia) | 1236 | Online | Literature review | Mixed logit | 10 | 6 | 6 | Yes |
| [50] | 2022 | 01–02/2021 | India, UK, Germany, Italy, Spain (Global) | 2565 | Online | Not specified | Conditional logit | 11 | 6 | 5 | Yes |
| [51] | 2022 | 03–05/2021 | Czech Republic (Europe) | 445 | Online | Literature review | Hierarchical Bayes | Not specified | 6 | 4 | Yes |
| [52] | 2021 | 03/2021 | USA (America) | 2895 | Online | Literature review, expert discussion | Mixed logit, latent class | 10 | 7 | 5 | Yes |
| [36] | 2022 | 07–09/2021 | Hong Kong (Asia) | 3423 | Online | Previous research, expert discussion | Mixed logit | 8 | 7 | 4 | No |
| [53] | 2022 | 11–12/2021 | South Africa (Africa) | 1836 | Online | Previous research, expert discussion | Mixed effects logit | 8 | 7 | 4 | No |
| [80] | 2024 | Not specified | Ghana (Africa) | 150 | Offline, not specified | Not specified | Conditional probit | 8 | 4 | 2 | No |
| [54] | 2023 | 05–08/2021 | Canada (America) | 551 | Online | Previous research | Multinomial logit | 10 | 4 | 6 | Yes |
| [37] | 2022 | 07/2020–03/2021 | 18 countries/6 continents (Global) | 13,128 | Online and offline | Not specified | Ordered logit, nested logit, latent class | 6 | 9 | 6 | No |
| [30] | 2021 | 08/2020 | China (Asia) | 873 | Online | Expert discussion, focus group discussion | Multinominal mixed effects logit model | 6 | 7 | 4 | Yes |
| [55] | 2021 | 02/2022 | Hong Kong (Asia) | 1773 | Online | Previous research, focus group discussion | Nested logit, mixed logit | 8 | 7 | 3 | Yes |
| [56] | 2022 | 09–10/2021 | Hong Kong (Asia) | 298 | Online | Literature review, previous research | Mixed logit | 8 | 7 | 3 | Yes |
| [57] | 2021 | 02–03/2021 | Japan (Asia) | 15,000 | Online | Not specified | Augmented inverse propensity score weighting | 5 | 6 | 5 | No |
| [38] | 2023 | 10–12/2020 | Namibia (Africa) | 269 | Online and in person | Not specified | Latent class (multinomial logit) | 6 | 9 | 6 | No |
| [14] | 2020 | 07/ 2020 | USA (America) | 1971 | Online | Previous research | OLS regression | 5 | 7 | 4 | Yes |
| [58] | 2022 | 03/2021 | USA (America) | 1421 | Online | Literature review, focus group discussion | Mixed logit | 7 | 9 | 4 | No |
| [81] | 2021 | Not specified | China (Asia) | 1883 | Online | Literature review, expert discussion, focus group discussion | Conditional logit, latent class | 8 | 7 | 3 | Yes |
| [59] | 2021 | 01–02/2021 | Hong Kong (Asia) | 194 | Online | Not specified | Conditional logit | 6 | 6 | 6 | No |
| [60] | 2023 | 01–02/2021 | China, USA (Global) | 1604 | Online | Literature review, expert discussion | Conditional logit | 13 | 6 | 5 | No |
| [61] | 2021 | 01–02/2021 | China, USA (Global) | 2480 | Online | Literature review, expert discussion | Conditional logit | Not specified | 6 | 5 | No |
| [31] | 2021 | 08–09/2020 | UK (Europe) | 1501 | Online | Previous research | Conditional logit | 6 | 5 | 3 | No |
| [32] | 2022 | 10–11/2020 | Canada (America) | 1599 | Online | Literature review, expert discussion, focus group discussion | Mixed logit, latent class | 12 | 7 | 6 | Yes |
| [62] | 2024 | 04–06/2021 | Canada (America) | 1883 | Online | Literature review, expert discussion, focus group discussion | Mixed logit, latent class | 12 | 7 | 6 | Yes |
| [33] | 2022 | 11/2020 | Netherlands (Europe) | 895 | Online | Previous research, expert discussion, focus group discussion, policy maker discussion | Mixed logit | 8 | 4 | 8 | Yes |
| [63] | 2022 | 10–11/2021 | Philippines (Oceania) | 865 | Online | Based on real interventions | Not specified | Not specified | 7 | 5 | No |
| [64] | 2023 | 04–05/2022 | Indonesia, Vietnam (Asia) | 1000 | Online | Previous research, focus group discussion | Mixed logit | 3 | 3 | 4 | Yes |
| [65] | 2022 | 10–11/2021 | USA (America) | 1456 | Online | Literature review | Random parameter logit | 11 | 5 | 3 | No |
| [66] | 2023 | 04–07/2021 | Iran (Asia) | 678 | Online | Previous research, expert discussion | Conditional logit, mixed logit | 12 | 7 | 4 | No |
| [67] | 2023 | 02–03/2022 | USA (America) | 544 | Online | Literature review, expert discussion, focus group discussion | Mixed logit, latent class | 10 | 7 | 3 | Yes |
| [68] | 2023 | 05–06/2021 | USA (America) | 1040 | Online | Literature review | Bayesian logit regression, latent class | 6 | 6 | 4 | Yes |
| [69] | 2024 | 07–08/2023 | Canada, Germany, UK, USA (Global) | 2000 | Online | Literature review, social listening, expert discussion | Multinomial logit, mixed logit | 12 | 6 | 4 | No |
| [34] | 2021 | 06–07/2020 | France (Europe) | 1942 | Online | Literature review, expert discussion | Conditional logit | 8 | 4 | 4 | No |
| [70] | 2022 | 05–06/2021 | China (Asia) | 849 | In person | Literature review, expert discussion | Conditional logit, mixed logit, latent class | 8 | 6 | 3 | Yes |
| [71] | 2022 | 05/2021 | China (Asia) | 1138 | In person | Previous research | Conditional logit, mixed logit | 9 | 6 | 3 | No |
| [72] | 2022 | 03/2021 | Malaysia (Asia) | 2028 | Online | Literature review, expert discussion, policy maker discussion | Mixed logit, nested logit | 10 | 5 | 4 | Yes |
| [73] | 2023 | 04–08/2022 | Vietnam (Asia) | 871 | Online | Literature review | Hierarchical Bayes | 7 | 6 | 5 | Yes |
| [74] | 2024 | 04–08/2022 | Vietnam (Asia) | 891 | Online | Literature review | Hierarchical Bayes | 7 | 6 | 5 | No |
| [39] | 2021 | 11–12/2020 | France (Europe) | 4415 | Online | Not specified | Conditional logit | 8 | 5 | 5 | No |
| [75] | 2023 | 03–06/2023 | Australia (Oceania) | 1007 | Online | Literature review, focus group discussion | Mixed logit, latent class conditional logit | 10 | 6 | 5 | Yes |
| [76] | 2022 | 01/2021 | China (Asia) | 1576 | Online | Literature review, expert discussion | Random parameter logit, latent class | 4 | 4 | 6 | Yes |
| [77] | 2024 | 06–07/2021 | China (Asia) | 12,000 | Online | Literature review, focus group discussion | Mixed logit | 12 | 7 | 4 | Yes |
| [78] | 2023 | 01/2021 | Hong Kong (Asia) | 2733 | Online | Not specified | Average marginal component effects (linear regression) | 3 | 6 | 5 | No |
| [79] | 2022 | 07–08/2021 | China (Asia) | 1200 | Online | Literature review, expert discussion | Conditional logit | 11 | 6 | 5 | No |
Max maximum, OLS ordinary least squares
Geographically, the studies covered a diverse range of regions. The majority (24 studies, 41%) were conducted in Asian countries [29, 30, 36, 40, 41, 46–48, 55–57, 59, 64, 66, 70–74, 76–79, 81], followed by 12 studies in the Americas (21%) [14, 27, 28, 32, 35, 52, 54, 58, 62, 65, 67, 68], nine in Europe (16%) [31, 33, 34, 39, 42–44, 49, 51], four in Oceania (7%) [39, 45, 63, 75], and three in Africa (5%) [38, 75, 80]. Additionally, six studies (10%) included participants across multiple continents, providing a broader global perspective [15, 37, 50, 60, 61, 69].
Sample sizes varied significantly, with a range from 149 [43] to 50,242 participants [15], an average sample size of 2919 participants, and a median of 1479 respondents.
Quality Assessment
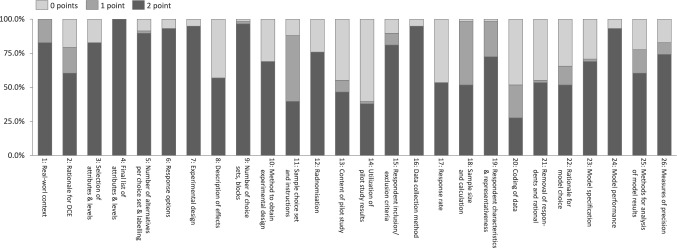
On average, the included studies scored 39 out of 52 possible points on the DIRECT checklist. Figure 2 provides insight into the overall study quality rating per DIRECT checklist item, a detailed list can be found in Appendix E of the ESM. The highest scoring item, and the only one where all studies received 2 points, was the reporting of the final list of attributes and levels (item 4). This was followed by the description of choice sets and blocks (item 9), data collection method (item 16), response options (item 6), and experimental design (item 7), each averaging 1.9 points. Likewise, reporting of model performance (item 24) also scored 1.9 points, though studies automatically received 2 points for this item if they reported a single model, as goodness-of-fit comparisons were not applicable.
Fig. 2.
Percentage of studies with 0, 1, or 2 points according to the DIRECT checklist item
Across broader ranking categories, studies performed best in description of attributes and levels and experimental design (both averaging 1.8 points). This was followed by purpose and rationale, sample and data collection, and reporting of results, each averaging 1.6 points.
The lowest scoring items included reporting of how information from pilot studies was used (item 14) and indication of data coding (item 20), both averaging 0.8 points. Other areas with relatively low scores were the description of pilot study subjects with 1.0 points (item 13) and reporting of respondent removal and reasons (item 21), response rates (item 17), and identified effects in study design (item 8), averaging 1.1 points respectively.
When analyzing regional differences, studies from Oceania tended to have the highest reporting quality, with an average score of 41 points. This was followed by studies from Europe and the Americas, which each scored an average of 40 points, and studies from Asia, which averaged 39 points. In contrast, global studies—those encompassing multiple continents—scored slightly lower at 35 points, while studies conducted in Africa had the lowest average score of 34 points. These differences could suggest some regional variations in adherence to reporting standards, potentially influenced by journal requirements, research funding, or institutional expectations.
Study Design and Choice Sets
Table 2 also provides a comprehensive overview of the relevant aspects of study and choice set design. The process for selecting attributes and levels was explicitly described in 48 of the 58 studies (83%). Among these, the majority (44 studies, 76%) based their selection on a literature review or previous research [14, 15, 26–29, 32–34, 36, 41, 43, 45–49, 51–56, 58, 60–62, 64–77, 79, 81], while expert discussions were incorporated in 26 studies (45%) [15, 27, 30, 32–34, 36, 42, 43, 45–48, 52, 53, 60–62, 66, 67, 69, 70, 72, 76, 79, 81], and focus group discussions were conducted in 16 studies (28%) [28, 30, 32, 33, 40, 42, 43, 46, 55, 58, 62, 64, 67, 75, 77, 81]. Only three studies (5%) mentioned discussions with policymakers [33, 48, 72]. Additionally, one study (2%) relied solely on real-life interventions [63], and another (2%) used a social listening approach [69]. A mixed-methods approach was adopted in 33 studies (57%), with the most frequent combination being literature review/previous research and expert discussions (24 studies, 41%), followed by literature review/previous research and focus group discussions (13 studies, 22%).
Regarding pilot testing, 32 studies (55%) tested their study instruments [14, 15, 26, 28–30, 32, 33, 40, 42, 43, 45–47, 49–52, 54–56, 62, 64, 67, 68, 70, 72, 73, 75–77, 81], while the remaining 26 studies (45%) did not report any pilot testing [27, 31, 34–39, 44, 48, 53, 57–61, 63, 65, 66, 69, 71, 74, 77–80]. The number of choice sets per participant varied between three [64, 78] and 13 [60], with eight choice sets being the most common (15 studies, 26%) [26, 27, 33, 34, 36, 39, 40, 42, 49, 53, 55, 56, 70, 80, 81]. However, three studies (6%) did not clearly report the number of choice sets used [51, 59, 63], and two studies used a varying number of choice sets (4%) [45, 48].
In terms of attributes, most studies included seven attributes (18 studies, 31%) [14, 15, 26, 30, 32, 36, 40, 41, 52, 53, 55, 56, 62, 63, 66, 67, 77, 81] or six attributes (17 studies, 29%) [29, 44, 50, 51, 57, 59–61, 68–71, 73–75, 78, 79], with the total number range from 3 to 13 and an average of 6.2 attributes per study. The maximum number of levels per attribute varied between two [80] and eight [26, 33], with most studies using four levels (19 studies, 33%) [14, 27, 28, 30, 34, 36, 41, 43, 47, 49, 51, 53, 58, 64, 66, 68, 69, 72, 77].
Main Insights Regarding Preferences
Attribute Classification
All 58 studies, encompassing a total of 362 attributes, were included in the attribute classification process. However, six attributes were excluded because of either representing a mix of two categories or lacking a sufficient description in the respective papers to enable accurate categorization. Figure 3 provides a visual overview of the attribute classification for the remaining 356 attributes included, while a detailed list can be found in Appendix F of the ESM.
Fig. 3.
Classification of attributes of included discrete choice experiments
The outcome category comprised attributes related to vaccine effectiveness and side effects. Attributes under effectiveness, the largest subgroup (n = 100), included prevention of infection, prevention of hospitalization, duration of protection, and the time required for the vaccine to take effect. Attributes addressing side effects (n = 71) covered the risks of mild or severe reactions, including death and flares in autoimmune conditions (Fig. 3).
Process attributes related to various logistical aspects of vaccination, such as appointment setting, time until vaccine availability, number and frequency of required doses, mode of administration, and vaccination site. The most common attributes in this category focused on the dose number and frequency (n = 23), followed by the vaccination site (n = 21), appointment setting (including office hours and waiting time, n = 7), mode of administration (n = 6), and vaccine availability (n = 6).
Trust attributes primarily addressed vaccine origin and branding (n = 25), followed by the recommending institution, such as a general practitioner or health organization (n = 11), and the vaccine type (e.g., messenger RNA or adenovirus, n = 8). Additional trust-related attributes included clinical evidence (n = 6) and information on development time and clinical trials (n = 2).
Framing attributes captured factors related to the presentation and perception of vaccination. These included obligations or incentives for vaccination (e.g., monetary rewards or restrictions for unvaccinated individuals, n = 17), social influences on vaccine uptake or adherence (e.g., vaccination rates among peers or the general population, n = 14), and perceived health risks of non-vaccination (e.g., risks of infection, hospitalization, severe illness, or death, n = 10). Less commonly, framing attributes covered priority group information (n = 2), and media coverage (n = 1).
Overall, the majority of attributes were classified under the Outcome category (171, 47%), followed by Process attributes (63, 17%), Trust attributes (52, 14%), Framing attributes (44, 12%), and Cost attributes (26, 7%).
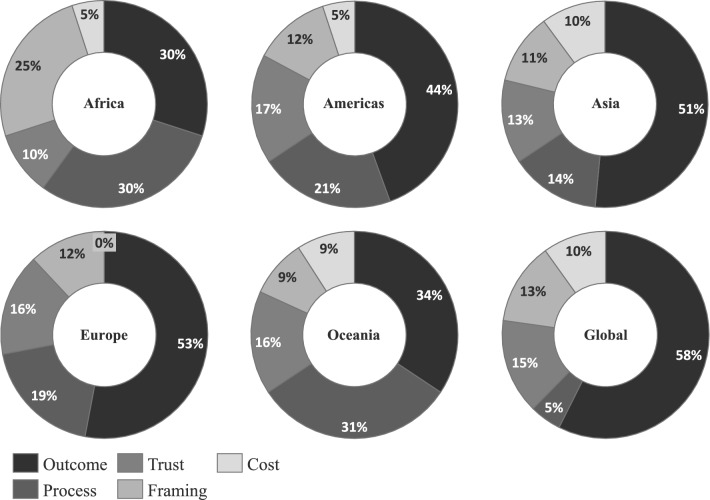
The findings reveal distinct regional differences, as illustrated in Fig. 4. Outcome attributes were most frequently used in global studies (58%), followed by those conducted in Europe (53%) and Asia (51%). In contrast, their use was notably lower in studies from Oceania (34%) and Africa (30%).
Fig. 4.
Geographic difference of attribute utilization in percent
Framing attributes showed a high regional variation, being particularly prominent in African studies (25%), and relatively underrepresented in studies conducted in Oceania (9%) compared with an overall average of 12% across other regions. Process attributes were also more common in Oceanian (31%) and African studies (30%) compared with the overall average of 18%, whereas global studies included only two Process attributes (5%).
Cost attributes (7% overall) were absent from European studies, less frequently used in studies from Africa and the Americas (5% each) and more frequently used in studies from Oceania (9%), Asian and Global studies (10% each), and European studies (12%). A similar pattern was observed for Trust attributes, which did not show strong regional clustering aside from African studies having fewer Trust attributes incorporated (10%) than the average of 15%. No relevant differences could be observed when differentiating regarding the timing of data collection.
CRI of DCEs Regarding Preferences for COVID-19 Vaccination
In this analysis, a total of 31 studies (53%) explicitly reported at least partial data on the CRI of attributes [14, 29, 43–47, 50, 51, 54, 55, 58–65, 67–76, 79, 81], while 19 studies (33%) provided enough information to allow for its calculation using the range method [26, 30–34, 36, 39–41, 48, 49, 52, 53, 57, 66, 77, 78, 80]. Among these 19 studies, one did not offer sufficient details on variable coding; however, as dummy and effects coding produced identical results for the first two attribute ranks, it was still included in the analysis [40]. Another study lacked aggregated results for its reported subgroups, but as the ranking remained consistent for the first two attributes, it was also retained [30].
Furthermore, one study that only presented findings from a latent class model without mean attribute coefficients was included by applying the range method to the largest latent class, each representing more than 50% of respondents [26]. However, eight studies lacked the necessary data for such calculations and were therefore excluded [26–28, 35, 37, 38, 42, 56].
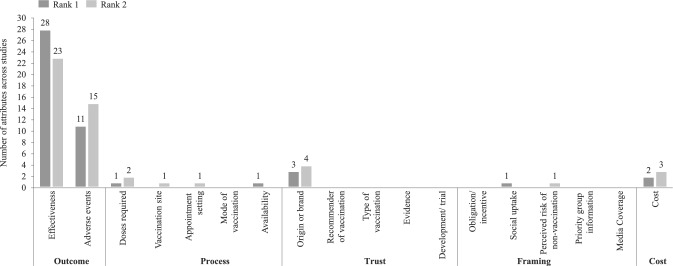
As a result, a total of 50 studies spanning 98,824 respondents were ultimately included in the analysis. Figure 5 illustrates the attribute ranking according to the CRI for the first two ranks, a comprehensive ranking breakdown across studies is provided in Appendix F of the ESM.
Fig. 5.
Attribute ranking according to the conditional relative importance
When examining the dataset, the majority of included studies (39, 78%) ranked Outcome attributes as the most important [14, 29–33, 36, 40, 43, 45–51, 53–55, 58–66, 68–71, 74–78, 80, 81], encompassing a total of 64,866 respondents (66% of respondents of studies included in this analysis). Trust was ranked most important by three studies (6% of studies, 22% or respondents) [34, 39, 57], while Process attributes (5% of respondents) [26, 52] and Cost attributes (2% of respondents) [67, 79] were ranked most important by two studies each (4% of studies). Framing (1% of respondents) was only the most important attribute in one study (2% of studies) [41]. Similar tendencies were observed for the second rank across studies and attribute categories.
Within the Outcome category ranked first, Effectiveness was the most chosen attribute (28 studies) [14, 29–32, 36, 40, 43, 48–51, 53–55, 58, 60–63, 66, 68–70, 76–78, 81], while Side effects was ranked first by 11 studies [33, 45–47, 59, 64, 65, 71, 74, 75, 80].
When examining attribute rankings across regions, minor differences emerged regarding the most important attribute. In all regions, Outcome attributes were consistently ranked as the most important. The only notable variation was in the second most important attribute: in Global studies that included participants from multiple continents, Cost was ranked the second most important attribute, whereas in the overall model, Outcome attributes held this position as well.
Similarly, no relevant differences were found when comparing studies based on their data collection as well as publication timing. Differentiating studies by whether they were conducted before or after vaccines became available, or differentiation by publication date showed no meaningful variation in attribute rankings.
Discussion
This systematic review aimed to synthesize evidence on public preferences related to COVID-19 vaccine attributes up until 31 December, 2024, focusing on the CRI of various attributes across a diverse set of studies. A total of 58 studies were included in the final analysis, providing insights from a wide range of geographic locations and participant groups. As the world continues to manage COVID-19 and future pandemics remain a looming possibility, understanding these preferences may become crucial for designing targeted vaccination campaigns that can boost public trust and vaccination uptake or adherence [82]. While this review provides valuable knowledge about COVID-19 vaccine preferences, the findings also reveal several key trends, methodological considerations, and areas for future research.
The studies included in this review covered a broad range of geographic regions, with the highest number conducted in Asia (24 studies) and the Americas (12 studies), followed by smaller contributions from Europe (9 studies), Oceania (4 studies), and Africa (3 studies). The inclusion of studies from multiple continents is a notable strength, as it offers a global perspective on vaccine preferences. However, regional differences in healthcare infrastructure, vaccination policies, and pandemic responses could influence respondents’ priorities [83–85]. These were reflected in the utilization of attributes, which varied across regions and may influence patient decision making. It could be expected that for instance, in regions with limited access to healthcare, cost-related attributes might weigh more heavily, whereas in countries with well-established vaccination programs, attributes related to efficacy or side effects may be prioritized [86, 87]. These tendencies were not indicated by our analysis; however, future studies could explore these regional variations more deeply to tailor public health strategies accordingly.
The overall reporting quality score of the included studies (39 out of 52 points on average) suggests that the studies employed fairly rigorous methodologies and is also reflected in the earlier systematic review by Huang et al. However, areas such as pilot testing, data removal, and response rates were less frequently reported. These gaps highlight opportunities for improvement in the transparency and robustness of future COVID-19 preference studies, which are critical for informing vaccine rollout strategies during both ongoing vaccination campaigns and potential future pandemics. It is worth noting that studies from Europe and Oceania generally scored higher on average compared with other regions. This could reflect more established research infrastructures in these regions, offering lessons for other areas on how to design and implement preference studies that better capture public opinion.
A significant finding from this review is the dominance of Outcome attributes, specifically vaccine effectiveness, side effects, and duration of protection, in determining the most important factors for vaccine preference. This finding is in line with the previous systematic review conducted by Huang et al. [16]. The prioritization of effectiveness, including attributes such as prevention of infection and hospitalization, is not surprising given the urgent global need to control COVID-19 and potential future pandemics. This further emphasizes the critical role that health outcomes, particularly efficacy, played in shaping public preferences for the COVID-19 vaccine and underscores the importance of communicating the tangible benefits of vaccination, such as a reduced risk of severe disease and death, in public health campaigns. As new variants of COVID-19 and future pandemic threats emerge, focusing on the effectiveness of vaccines in preventing these outcomes will likely remain a central message for public health authorities to emphasize.
An interesting finding from this review is the relatively low ranking of Framing attributes, such as social norms, vaccination mandates, and incentives, in determining vaccine preferences. Given the immense global restrictions and the widespread debates and protests surrounding these measures during the COVID-19 pandemic [88, 89], one might have expected framing factors to play a more prominent role in shaping public opinion. The pandemic was marked by strong political and social discourse around vaccination requirements, mandates, and incentives [90], yet these framing elements were not as influential as might have been anticipated. This result suggests that, while Framing attributes certainly contributed to shaping public attitudes, they were secondary to more direct health-related concerns, such as vaccine effectiveness and side effects.
The relatively minor role played by Cost and Trust attributes in the ranking of vaccine preferences for COVID-19 suggests that, for many respondents, these were secondary concerns compared to the immediate health implications of the vaccine. This finding aligns with earlier research on vaccines, particularly for influenza, where attributes related to clinical effectiveness and safety consistently emerged as the primary drivers of vaccine preference, while cost and trust-related factors played a more limited role. For instance, Xia et al. and Li et al. found that attributes such as vaccine efficacy, risk of side effects, and protection duration were the dominant influences on decision making, with cost ranked lower in importance. However, this pattern contrasts with findings from other contexts, particularly in low-income settings or among vaccine-hesitant groups, where affordability and trust have been shown to significantly influence uptake [91, 92]. Crawshaw et al. reported that migrants and underserved populations in Europe experienced structural barriers, including cost and institutional distrust, which shaped their vaccine-related decisions [93]. Dubé et al. emphasized that in specific settings, such as vaccination decisions of new mothers for their infants, trust in healthcare providers and government authorities may become a crucial determinant of vaccine acceptance [94]. As shown by Godoy-Ramirez et al., among undocumented migrants in Sweden, barriers such as fear of being reported to authorities, unclear entitlement to services, and a lack of trust in the healthcare system significantly affected the vaccine uptake, demonstrating that trust and systemic accessibility can be more decisive than clinical concerns in marginalized populations [95]. Furthermore, Fifer et al. showed that when the severity of the influenza season was perceived as unchanged compared to the previous year, out-of-pocket cost emerged as the most important attribute influencing vaccination decisions among Australian adults [96]. These mixed findings suggest that the salience of cost and trust is highly context dependent, shaped by factors such as healthcare access, economic conditions, social inclusion, and perceived institutional legitimacy. Therefore, while these attributes ranked lower overall in this review, they may assume a more prominent role in specific populations or under different circumstances—an area that future research should explore more explicitly.
To our knowledge, this is one of the first systematic reviews to utilize the DIRECT checklist for evaluating the quality of studies employing DCEs. The checklist offers valuable insights into whether key aspects of study design, data collection, and analysis are transparently and comprehensively reported. However, it is important to emphasize that the DIRECT checklist is a reporting tool, not a measure of methodological quality. It does not assess the scientific rigor, internal validity, or robustness of the study’s design or analytical approach. As such, studies may receive high scores on the checklist for thorough reporting but still have underlying methodological weaknesses, such as sample bias or inappropriate modeling techniques, which could affect the reliability of the findings. However, although the checklist was primarily developed as a reporting checklist, it has provided valuable insights into the methodological quality of the included studies and allowed for a comprehensive appraisal of study quality. Nevertheless, future research could benefit from additional guidance on how to effectively use the DIRECT checklist for quality appraisal. For some items, such as those related to model performance reporting and effects identification, providing more detailed guidance on applicability in various contexts could help ensure that studies are assessed in a uniform and comprehensive manner, ultimately improving the rigor and reliability of DCE research in public health.
Based on the findings of this review, several actionable lessons can be derived for future pandemics and vaccination campaigns: first, vaccine effectiveness should remain a central component of public health messaging, as it consistently emerged as the most important attribute across studies. Second, while framing elements such as mandates or social norms generated significant public discourse, they appeared to have limited influence on preference formation, suggesting that these should be carefully evaluated and targeted, rather than relied upon as primary motivators. Finally, trust in the recommending authority or vaccine origin—though ranked lower overall—remains a sensitive dimension, particularly in politicized environments, and should be proactively addressed through transparent communication.
While this review provides a comprehensive overview of COVID-19 vaccine preferences, there are several limitations that warrant consideration. First, the temporal variability of the studies included, with some conducted before the availability of vaccines, could introduce bias in how vaccine attributes were perceived. The evolution of public understanding and attitudes toward vaccines as the pandemic unfolded may have shifted the relative importance of different attributes. Although our study has not found relevant differences in this regard, future research could focus on pre-pandemic data to better understand how policies and incentives could support upfront adherence with vaccination protocols.
Furthermore, this systematic review exclusively included preference studies that employed DCEs as opposed to methods such as best-worst ranking or other forms of conjoint analysis. While synthesizing findings across methodologies can be difficult and reduce generalizability, a broader inclusion of studies could have provided additional insight and enriched the analysis as seen both within COVID-19 as well as in other contexts [97, 98]. Similarly, this study focused on the CRI, while information on predicted probabilities of vaccine uptake were not extracted from the included studies but could provide important insights that future research might address. A further limitation in the studies reviewed is the inconsistency in how side effects were defined and reported. In some studies, side effects encompassed severe outcomes such as anaphylactic shock or death, while in others, only mild side effects, such as fever or headache, were considered. This variation in the scope of side effects could have influenced participants’ perceptions of risk differently across studies. For example, including severe side effects may have amplified concerns about vaccine safety, whereas focusing solely on mild side effects might have downplayed the perceived risks. This inconsistency complicates comparisons between studies and may limit the ability to draw generalizable conclusions regarding public preferences for vaccine safety.
Last, we did not examine the interaction of demographic factors, such as age, sex, health status, and vaccine hesitancy, with vaccine preferences or provide insight into pairwise comparisons of attributes. Given the varying concerns different demographic groups may have regarding COVID-19 vaccination and the impact of demographic interaction on vaccine preferences, it could be valuable for future studies to incorporate such detailed subgroup analyses as well as analyses regarding other reasons for heterogeneity. For example, Raskin et al. estimated the potential impact of respondents’ sociodemographic characteristics on preferences for prioritization strategies for COVID-19 vaccination [99]. They found that different subgroups showed different preferences according to their sociodemographic characteristics. Schwarzinger et al. identified associations between outright refusal and specific demographic characteristics (e.g., sex, age, education level, and chronic conditions), but not with information on collective benefits such as herd immunity or general practitioner recommendations. Similarly, Hess et al. reported that only 6.5% of respondents rejected vaccination regardless of the presented attributes, including those related to framing. Konstantinus et al. explored anti-vaccination behavior and found that rejection was primarily driven by the perception that none of the attribute-defined options was sufficiently desirable, though the study did not specify which attributes influenced that judgment.
Understanding how specific populations weigh different vaccine attributes could be crucial for designing targeted interventions that address the unique concerns of diverse groups and could therefore be a valuable aspect in future research. Furthermore, we did not analyze preference heterogeneity, although some studies reported differences among respondents, for example, through latent class analyses, which showed a varied willingness to be vaccinated [43].
Conclusions
This systematic review highlights the central role of outcome attributes, particularly vaccine effectiveness and safety, in shaping public preferences for COVID-19 vaccination. These findings are important not only for guiding the design of current vaccination campaigns but also for informing strategies for future pandemics. As the world moves forward, public health authorities should prioritize communicating the tangible benefits of vaccination, particularly in terms of efficacy and safety, while addressing cost and trust-related concerns, particularly in regions where access to vaccines may be more limited. In addition to refining preference assessment methodologies specifically within the COVID-19 context, future research could leverage lessons learned from studies conducted in the context of this pandemic such as this systematic review to guide the design of stated preference studies for future pandemics. This includes evaluating which attributes are most consistently prioritized across different studies, how CRI rankings vary by region or population group, and how these insights can enhance the development of effective vaccination strategies in future global health crises. The lessons learned from the COVID-19 vaccination campaigns may become valuable in informing vaccination campaigns in light of future pandemics and health crises.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No funding was received for the conduct of this study.
Conflicts of interest/competing interests
Eva-Lotta Hinzpeter, Nadja Kairies-Schwarz, Charlotte Beaudart, Jonathan Douxfils, Dweeti Nayak, and Mickaël Hiligsmann have no conflicts of interests that are directly relevant to the content of this article. Mickaël Hiligsmann is an Editorial Board Member of The Patient. He was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data analyzed during this study are included in this article and its supplementary information files.
Code availability
Not applicable.
Authors’ contributions
The search strategies were prepared by CB and ELH, and reviewed by MH and NKS. The literature review was conducted by JD, CB, and ELH and supported by NKS and MH. Data extraction was performed by ELH and quality appraisal was conducted by DN and ELH, the data analysis was carried out by MH and ELH. The first draft of the manuscript was written by ELH, and all authors provided intensive feedback throughout the development and read and approved the final paper. All authors sufficiently contributed to this research according to ICMJE criteria to qualify as a listed author.
References
- 1.World Health Organization. COVID-19 epidemiological update, 24 December 2024. 2024. https://www.who.int/publications/m/item/covid-19-epidemiological-update---24-december-2024. Accessed 18 June 2025.
- 2.U.S. Centers For Disease Control And Prevention. Vaccine effectiveness studies. 2024. https://www.cdc.gov/covid/php/surveillance/vaccine-effectiveness-studies.html. Accessed 30 Mar 2025.
- 3.Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337–48. 10.1038/s41562-021-01056-1. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Lu X, Lai X, Lyu Y, Zhang H, Fenghuang Y, et al. The changing acceptance of COVID-19 vaccination in different epidemic phases in China: a longitudinal study. Vaccines (Basel). 2021;9(3):191. 10.3390/vaccines9030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartsch SM, O’Shea KJ, Ferguson MC, Bottazzi ME, Wedlock PT, Strych U, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59:493–503. 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandmann F, Jit M, Andrews N, Buckley HL, Campbell H, Ribeiro S, et al. Evaluating the impact of a continued maternal pertussis immunisation programme in England: a modelling study and cost-effectiveness analysis. Vaccine. 2021;39:4500–9. 10.1016/j.vaccine.2021.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinzpeter E-L, Nagendra L, Kairies-Schwarz N, Beaudart C, Hiligsmann M. Stated preferences of at-risk populations for the treatment of osteoporosis: a systematic review. Patient. 2024;17:619–34. 10.1007/s40271-024-00714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridman A, Gershon R, Gneezy A. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS ONE. 2021;16: e0250123. 10.1371/journal.pone.0250123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–72. 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 10.Determann D, Korfage IJ, Lambooij MS, Bliemer M, Richardus JH, Steyerberg EW, et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS ONE. 2014;9: e102505. 10.1371/journal.pone.0102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diks ME, Hiligsmann M, van der Putten IM. Vaccine preferences driving vaccine-decision making of different target groups: a systematic review of choice-based experiments. BMC Infect Dis. 2021;21:879. 10.1186/s12879-021-06398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willems D, Hinzpeter E-L, van der Zee HH, Sayed CJ, Ingram JR, Beaudart C, et al. Patient preferences in the management of hidradenitis suppurativa: results of a multinational discrete choice experiment in Europe. Patient. 2023;16:153–64. 10.1007/s40271-022-00614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lack A, Hiligsmann M, Bloem P, Tünneßen M, Hutubessy R. Parent, provider and vaccinee preferences for HPV vaccination: a systematic review of discrete choice experiments. Vaccine. 2020;38:7226–38. 10.1016/j.vaccine.2020.08.078. [DOI] [PubMed] [Google Scholar]
- 14.Kreps S, Prasad S, Brownstein JS, Hswen Y, Garibaldi BT, Zhang B, et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3: e2025594. 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonini M, Genie MG, Attema AE, Attwell K, Balogh ZJ, Behmane D, et al. Public preferences for vaccination campaigns in the COVID-19 endemic phase: Insights from the VaxPref database. Health Policy Technol. 2024;13: 100849. 10.1016/j.hlpt.2024.100849. [Google Scholar]
- 16.Huang Y, Feng S, Zhao Y, Wang H, Jiang H. Preferences for COVID-19 vaccines: systematic literature review of discrete choice experiments. JMIR Public Health Surveill. 2024;10: e56546. 10.3390/vaccines11050974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. 2023. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic?adgroupsurvey=%7Badgroupsurvey%7D&gclid=EAIaIQobChMI4Ojtsdbe_gIVjQRyCh07igt4EAAYASACEgJ9pfD_BwE&fbclid=IwAR2M8EAyiSrAodhK9p-X582nHkP2AigpSX8pYIsLsPwqYh4SG26RGokGe7E. Accessed 18 Junw 2025.
- 18.Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10:39. 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–44. 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 21.Dobrescu AI, Nussbaumer-Streit B, Klerings I, Wagner G, Persad E, Sommer I, et al. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: a systematic review. J Clin Epidemiol. 2021;137:209–17. 10.1016/j.jclinepi.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Ride J, Goranitis I, Meng Y, LaBond C, Lancsar E. A reporting checklist for discrete choice experiments in health: the DIRECT Checklist. Pharmacoeconomics. 2024;42:1161–75. 10.1007/s40273-024-01431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health: a checklist. A report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–13. 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsmann M. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient. 2017;10:553–65. 10.1007/s40271-017-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borriello A, Master D, Pellegrini A, Rose JM. Preferences for a COVID-19 vaccine in Australia. Vaccine. 2021;39:473–9. 10.1016/j.vaccine.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig BM. United States COVID-19 vaccination preferences (CVP): 2020 hindsight. Patient. 2021;14:309–18. 10.1007/s40271-021-00508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daziano RA. A choice experiment assessment of stated early response to COVID-19 vaccines in the USA. Health Econ Rev. 2022;12:23. 10.1186/s13561-022-00368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong D, Xu RH, Wong EL-Y, Hung C-T, Feng D, Feng Z, et al. Public preference for COVID-19 vaccines in China: a discrete choice experiment. Health Expect. 2020;23:1543–78. 10.1111/hex.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Wagner AL, Chen Y, Jaime E, Hu X, Wu S, et al. Would COVID-19 vaccination willingness increase if mobile technologies prohibit unvaccinated individuals from public spaces? A nationwide discrete choice experiment from China. Vaccine. 2022;40:7466–75. 10.1016/j.vaccine.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPhedran R, Toombs B. Efficacy or delivery? An online discrete choice experiment to explore preferences for COVID-19 vaccines in the UK. Econ Lett. 2021;200: 109747. 10.1016/j.econlet.2021.109747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morillon GF, Poder TG. Public preferences for a COVID-19 vaccination program in Quebec: a discrete choice experiment. Pharmacoeconomics. 2022;40:341–54. 10.1007/s40273-021-01124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouter N, de Ruijter A, Ardine de Wit G, Lambooij MS, van Wijhe M, van Exel J, et al. “Please, you go first!” preferences for a COVID-19 vaccine among adults in the Netherlands. Soc Sci Med. 2022;292:114626. 10.1016/j.socscimed.2021.114626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6:e210–21. 10.1016/S2468-2667(21)00012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu H, Liu S. Light at the end of the tunnel: influence of vaccine availability and vaccination intention on people’s consideration of the COVID-19 vaccine. Soc Sci Med. 2021;286: 114315. 10.1016/j.socscimed.2021.114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung LWY, Zhao J, Yan VKC, Blais JE, Chan JCH, Li STH, et al. COVID-19 vaccination preferences of university students and staff in Hong Kong. JAMA Netw Open. 2022;5: e2212681. 10.1001/jamanetworkopen.2022.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess S, Lancsar E, Mariel P, Meyerhoff J, Song F, van den Broek-Altenburg E, et al. The path towards herd immunity: predicting COVID-19 vaccination uptake through results from a stated choice study across six continents. Soc Sci Med. 2022;298: 114800. 10.1016/j.socscimed.2022.114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konstantinus A, Konstantinus I. Choice preference and willingness to pay for COVID-19 vaccination in Namibia. Vaccine X. 2023;14: 100324. 10.1016/j.jvacx.2023.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velardo F, Watson V, Arwidson P, Alla F, Luchini S, Schwarzinger M, et al. Regional differences in COVID-19 vaccine hesitancy in December 2020: a natural experiment in the French Working-Age Population. Vaccines (Basel). 2021;9:1364. 10.3390/vaccines9111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asim S, Wang K, Nichini E, Yip FF, Zhu L, Fung HCE, et al. COVID-19 vaccination preferences among non-Chinese migrants in Hong Kong: discrete choice experiment. JMIR Public Health Surveill. 2023;9: e40587. 10.2196/40587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bansal P, Raj A, Mani Shukla D, Sunder N. COVID-19 vaccine preferences in India. Vaccine. 2022;40:2242–6. 10.1016/j.vaccine.2022.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaga Z, Czine P, Takacs B, Szilagyi A, Szekeres R, Wachal Z, et al. Examination of preferences for COVID-19 vaccines in Hungary based on their properties: examining the ompact of pandemic awareness with a hybrid choice approach. Int J Environ Res Public Health. 2023;20:1270. 10.3390/ijerph20021270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böger S, van Bergen I, Beaudart C, Cheung KL, Hiligsmann M. Preference of young adults for COVID-19 vaccination in the United Kingdom: a discrete choice experiment. Expert Rev Pharmacoecon Outcomes Res. 2023;23:921–31. 10.1080/14737167.2023.2223983. [DOI] [PubMed] [Google Scholar]
- 44.Bughin J, Cincera M, Kiepfer E, Reykowska D, Philippi F, Żyszkiewicz M, et al. Vaccination or NPI? A conjoint analysis of German citizens’ preferences in the context of the COVID-19 pandemic. Eur J Health Econ. 2023;24:39–52. 10.1007/s10198-022-01450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan AHY, Tao M, Marsh S, Petousis-Harris H. Vaccine decision making in New Zealand: a discrete choice experiment. BMC Public Health. 2024;24:447. 10.1186/s12889-024-17865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang S, Xu B, Xi H, Shao Y. Investigating the influencing factors of vaccination decisions for newly developed and established vaccines: a comparative study based on latent class logit models in China. Front Public Health. 2024;12:1455718. 10.3389/fpubh.2024.1455718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Wang J, Yi M, Xu H, Liang H. The COVID-19 vaccination decision-making preferences of elderly people: a discrete choice experiment. Sci Rep. 2023;13:5242. 10.1038/s41598-023-32471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darrudi A, Daroudi R, Yunesian M, Akbari SA. Public preferences and willingness to pay for a COVID-19 vaccine in Iran: a discrete choice experiment. Pharmacoecon Open. 2022;6:669–79. 10.1007/s41669-022-00359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Díaz Luévano C, Sicsic J, Pellissier G, Chyderiotis S, Arwidson P, Olivier C, et al. Quantifying healthcare and welfare sector workers’ preferences around COVID-19 vaccination: a cross-sectional, single-profile discrete-choice experiment in France. BMJ Open. 2021;11: e055148. 10.1136/bmjopen-2021-055148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong Y, He Z, Liu T, Huang J, Zhang CJP, Akinwunmi B, Ming W-K. Acceptance of and preference for COVID-19 vaccination in India, the United Kingdom, Germany, Italy, and Spain: an international cross-sectional study. Vaccines (Basel). 2022;10(6):832. 10.3390/vaccines10060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donin G, Erfányuková A, Ivlev I. Factors affecting young adults’ decision making to undergo COVID-19 vaccination: a patient preference study. Vaccines (Basel). 2022;10(2):265. 10.3390/vaccines10020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eshun-Wilson I, Mody A, Tram KH, Bradley C, Sheve A, Fox B, et al. Preferences for COVID-19 vaccine distribution strategies in the US: a discrete choice survey. PLoS ONE. 2021;16: e0256394. 10.1371/journal.pone.0256394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George G, Strauss M, Lansdell E, Nadesan-Reddy N, Moroe N, Reddy T, et al. South African university staff and students’ perspectives, preferences, and drivers of hesitancy regarding COVID-19 vaccines: a multi-methods study. Vaccines (Basel). 2022;10:1250. 10.3390/vaccines10081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hazlewood GS, Colmegna I, Hitchon C, Fortin PR, Bernatsky S, Clarke AE, et al. Preferences for COVID-19 vaccination in people with chronic immune-mediated inflammatory diseases. J Rheumatol. 2023;50:949–57. 10.3899/jrheum.220697. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Wong EL-Y, Cheung AW-L, Yau PS-Y, Chung VC-H, Wong CH-L, et al. Influence of vaccination characteristics on COVID-19 vaccine acceptance among working-age people in Hong Kong, China: a discrete choice experiment. Front Public Health. 2021;9:793533. 10.3389/fpubh.2021.793533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K, Wong EL-Y, Cheung AW-L, Chung VC-H, Wong CH-L, Dong D, et al. Impact of information framing and vaccination characteristics on parental COVID-19 vaccine acceptance for children: a discrete choice experiment. Eur J Pediatr. 2022;181:3839–49. 10.1007/s00431-022-04586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawata K, Nakabayashi M. Determinants of COVID-19 vaccine preference: a survey study in Japan. SSM Popul Health. 2021;15: 100902. 10.1016/j.ssmph.2021.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krueger R, Daziano RA. Stated choice analysis of preferences for COVID-19 vaccines using the Choquet integral. J Choice Model. 2022;45: 100385. 10.1016/j.jocm.2022.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Chong MY, Chan CY, Chan VWS, Tong X. COVID-19 vaccine preferences among university students in Hong Kong: a discrete choice experiment. BMC Res Notes. 2021;14:421. 10.1186/s13104-021-05841-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Yang L, Tian G, Feng B, Jia X, He Z, et al. Understanding influencing attributes of COVID-19 vaccine preference and willingness-to-pay among Chinese and American middle-aged and elderly adults: a discrete choice experiment and propensity score matching study. Front Public Health. 2023;11:1067218. 10.3389/fpubh.2023.1067218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu T, He Z, Huang J, Yan N, Chen Q, Huang F, et al. A comparison of vaccine hesitancy of COVID-19 vaccination in China and the United States. Vaccines (Basel). 2021;9(6):649. 10.3390/vaccines9060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morillon GF, Poder TG. Which factors drive the choice of the French-speaking Quebec population towards a COVID-19 vaccination programme: a discrete-choice experiment. Health Expect. 2024;27: e13963. 10.1111/hex.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ong AKS, Prasetyo YT, Lagura FC, Ramos RN, Salazar JML, Sigua KM, et al. Young adult preference analysis on the attributes of COVID-19 vaccine in the Philippines: a conjoint analysis approach. Public Health Pract (Oxf). 2022;4: 100300. 10.1016/j.puhip.2022.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozdemir S, Ng S, Huynh VA, Mühlbacher A, Tan HK, Finkelstein EA. Trade-offs between vaccine effectiveness and vaccine safety: personal versus policy decisions. Pharmacoecon Open. 2023;7:915–26. 10.1007/s41669-023-00442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panchalingam T, Shi Y. Parental refusal and hesitancy of vaccinating children against COVID-19: findings from a nationally representative sample of parents in the US. Prev Med. 2022;164:107288. 10.1016/j.ypmed.2022.107288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parvizi S, Mehrara M, Taiebnia A. Investigating preferences of the covid-19 vaccine among individuals in Iran: discrete choice experiment analysis. Health Sci Rep. 2023;6: e1332. 10.1002/hsr2.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poudel N, Ngorsuraches S. A Preference-based value assessment of the fear of COVID-19 contagion. Patient Prefer Adherence. 2023;17:3435–48. 10.2147/PPA.S431148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prosser LA, Wagner AL, Wittenberg E, Zikmund-Fisher BJ, Rose AM, Pike J. A discrete choice analysis comparing COVID-19 vaccination decisions for children and adults. JAMA Netw Open. 2023;6: e2253582. 10.1001/jamanetworkopen.2022.53582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salisbury D, Lazarus JV, Waite N, Lehmann C, Sri Bhashyam S, de La Cruz M, et al. COVID-19 vaccine preferences in general populations in Canada, Germany, the United Kingdom, and the United States: discrete choice experiment. JMIR Public Health Surveill. 2024;10: e57242. 10.2196/57242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Nicholas S, Maitland E, Leng A. Individual preferences for COVID-19 vaccination under the China’s 2021 national vaccination policy: a discrete choice experiment study. Vaccines (Basel). 2022;10:543. 10.3390/vaccines10040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S, Maitland E, Wang T, Nicholas S, Leng A. Student COVID-19 vaccination preferences in China: a discrete choice experiment. Front Public Health. 2022;10: 997900. 10.3389/fpubh.2022.997900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teh HS, Woon YL, Leong CT, Hing NYL, Mien TYS, Roope LSJ, et al. Malaysian public preferences and decision making for COVID-19 vaccination: a discrete choice experiment. Lancet Reg Health West Pac. 2022;27: 100534. 10.1016/j.lanwpc.2022.100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran BX, Do AL, Boyer L, Auquier P, Le HT, Le Vu MN, et al. Preference and willingness to pay for the regular COVID-19 booster shot in the Vietnamese population: theory-driven discrete choice experiment. JMIR Public Health Surveill. 2023;9: e43055. 10.2196/43055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tran BX, Dang DS, Dang THT, Le TT, Hoang TP, Boyer L, et al. Assessing willingness to pay for children’s COVID-19 vaccination among healthcare providers and users using a theory-based discrete choice experiment. Sci Rep. 2024;14:22352. 10.1038/s41598-024-71857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang B, McDonough J, Chen G, Ong JJ, Marshall H. Sociodemographic factors and attitudes associated with Australian parental acceptance of paediatric COVID-19 vaccination. Vaccine. 2023;41:7608–17. 10.1016/j.vaccine.2023.11.024. [DOI] [PubMed] [Google Scholar]
- 76.Xiao J, Wang F, Wang M, Ma Z. Attribute nonattendance in COVID-19 vaccine choice: a discrete choice experiment based on Chinese public preference. Health Expect. 2022;25:959–70. 10.1111/hex.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu F, Jiao L, Chen Q, Wang Q, de Allegri M, Cao Z, et al. Preferences regarding COVID-19 vaccination among 12,000 adults in China: a cross-sectional discrete choice experiment. PLoS Glob Public Health. 2024;4: e0003387. 10.1371/journal.pgph.0003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue PHR, Lau HPB, Ng SM, Chan LWC, Yuen S. When to be vaccinated? What to consider? Modelling decision-making and time preference for COVID-19 vaccine through a conjoint experiment. Vaccine. 2023;41:6300–8. 10.1016/j.vaccine.2023.08.068. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Ge P, Li X, Yin M, Wang Y, Ming W, et al. Personality effects on Chinese public preference for the COVID-19 vaccination: discrete choice experiment and latent profile analysis study. Int J Environ Res Public Health. 2022;19(8):4842. 10.3390/ijerph19084842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gyasi SF, Kumi W, Kwofie C. Factors influencing individual vaccine preferences for COVID-19 in the Sunyani Municipality, Ghana: an observational study using discrete choice experiment analysis. Health Sci Rep. 2024;7: e2263. 10.1002/hsr2.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leng A, Maitland E, Wang S, Nicholas S, Liu R, Wang J. Individual preferences for COVID-19 vaccination in China. Vaccine. 2021;39:247–54. 10.1016/j.vaccine.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harvard T.H. Chan School of Public Health. The next pandemic: not if, but when. 2024.: https://hsph.harvard.edu/news/next-pandemic-not-if-but-when/#:~:text=September%2012%2C%202024%20%E2%80%93%20As%20new,for%2C%20and%20managing%20future%20outbreaks. Accessed 18 June 2025.
- 83.Arsenault C, Lewis TP, Kapoor NR, Okiro EA, Leslie HH, Armeni P, et al. Health system quality and COVID-19 vaccination: a cross-sectional analysis in 14 countries. Lancet Glob Health. 2024;12:e156–65. 10.1016/S2214-109X(23)00490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santangelo OE, Provenzano S, Di Martino G, Ferrara P. COVID-19 vaccination and public health: addressing global, regional, and within-country inequalities. Vaccines (Basel). 2024;12(8):885. 10.3389/fpubh.2021.673542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lacy A, Khan MM, Deb Nath N, Das P, Igoe M, Lenhart S, et al. Geographic disparities and predictors of COVID-19 vaccination in Missouri: a retrospective ecological study. Front Public Health. 2024;12:1329382. 10.3389/fpubh.2024.1329382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ali HA, Hartner A-M, Echeverria-Londono S, Roth J, Li X, Abbas K, et al. Vaccine equity in low and middle income countries: a systematic review and meta-analysis. Int J Equity Health. 2022;21:82. 10.1016/S0140-6736(21)00617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolobova I, Nyaku MK, Karakusevic A, Bridge D, Fotheringham I, O’Brien M. Vaccine uptake and barriers to vaccination among at-risk adult populations in the US. Hum Vaccin Immunother. 2022;18:2055422. 10.1177/003335491312800203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Zwet K, Barros AI, van Engers TM, Sloot PMA. Emergence of protests during the COVID-19 pandemic: quantitative models to explore the contributions of societal conditions. Humanit Soc Sci Commun. 2022;9:287. 10.1057/s41599-022-01082-y. [Google Scholar]
- 89.Rohlinger DA, Meyer DS. Protest during a pandemic: how Covid-19 affected social movements in the United States. Am Behav Sci. 2024;68:810–28. 10.1177/00027642221132179. [Google Scholar]
- 90.Martin S, Vanderslott S. “Any idea how fast ‘It’s just a mask!’ can turn into ‘It’s just a vaccine!’”: from mask mandates to vaccine mandates during the COVID-19 pandemic. Vaccine. 2022;40:7488–99. 10.1371/journal.pone.0246317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S, Gong T, Chen G, Liu P, Lai X, Rong H, et al. Parental preference for influenza vaccine for children in China: a discrete choice experiment. BMJ Open. 2022;12: e055725. 10.1542/peds.2010-1722P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xia Y, Wang M, Hu M, Wang Y, Yuan B, Zhu D, et al. Preferences and willingness to pay for herpes zoster vaccination among Chinese adults: discrete choice experiment. JMIR Public Health Surveill. 2024;10: e51242. 10.1080/21645515.2023.2167439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crawshaw AF, Farah Y, Deal A, Rustage K, Hayward SE, Carter J, et al. Defining the determinants of vaccine uptake and undervaccination in migrant populations in Europe to improve routine and COVID-19 vaccine uptake: a systematic review. Lancet Infect Dis. 2022;22:e254–66. 10.1016/S1473-3099(22)00066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9:1763–73. 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Godoy-Ramirez K, Byström E, Lindstrand A, Butler R, Ascher H, Kulane A. Exploring childhood immunization among undocumented migrants in Sweden: following qualitative study and the World Health Organizations Guide to Tailoring Immunization Programmes (TIP). Public Health. 2019;171:97–105. 10.1016/j.puhe.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 96.Fifer S, Toh L, Yu D, Young K, Menche J. Australian preferences for influenza vaccine attributes and cost: a discrete choice experiment. Hum Vaccin Immunother. 2025;21:2440164. 10.1080/21645515.2024.2440164. [DOI] [PubMed] [Google Scholar]
- 97.Beckham SW, Crossnohere NL, Gross M, Bridges JFP. Eliciting preferences for HIV prevention technologies: a systematic review. Patient. 2021;14:151–74. 10.1007/s40271-020-00486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang E, Jia Y, Zhu X, Wang L, Yan Y, Liu K, et al. COVID-19 vaccine preferences in China: a comparison of discrete choice experiment and profile case best-worst scaling. Pharmacoecon Open. 2025;9:399–413. 10.1007/s41669-025-00559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raskin N, Hiligsmann M, Luyten J, Tubeuf S, Grigoriev A, Kessels R. Socio-demographic differences in citizen’ preferences for distributing a scarce, lifesaving resource: a case study using COVID-19 vaccine distribution in Belgium. Vaccine. 2025;55: 126997. 10.1016/j.vaccine.2025.126997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.