Abstract
Engram cells storing episodic memories are allocated to separate neuronal ensembles. However, how these ensembles maintain their stability to drive precise memory expression, and whether their destabilization contributes to aging-related memory deficits, remain elusive. Here, we show that during contextual fear memory consolidation, neuronal pentraxin 1 (NPTX1) in Fos transcription-dependent ensemble (F-RAM) of the dentate gyrus (DG) promotes memory expression in the fear context. NPTX1 facilitates Kv7.2 channel-mediated inhibition of engram cell hyperexcitability, thereby restricting the response of these cells to excitatory inputs from medial entorhinal cortex. Meanwhile, NPTX2 enhances the perisomatic inhibition of Npas4 transcription-dependent ensemble (N-RAM) by parvalbumin+ (PV+) interneurons, thereby preventing fear memory overgeneralization. Pharmacological activation of Kv7.2 channels or chemogenetic activation of PV+ interneurons repaired memory deficits caused by engram-specific NPTX depletion. Contextual fear memory precision and NPTX expression in DG engram cells were decreased in aged mice. Overexpressing NPTX1 in F-RAM ensemble or the AMPAR-binding domain of NPTX2 in N-RAM ensemble rescued contextual fear memory deficits. These findings elucidate that the coordination of NPTX1 and NPTX2 prevents engram ensembles from becoming hyperactive and provide a causal link between engram network destabilization and aging-related contextual fear memory deficits.
Subject terms: Cell biology, Mechanisms of disease
Introduction
Specific episodic memories are believed to be encoded in sparse neuronal ensembles (engram cells) that are activated by learning experiences.1,2 Neurons recruited to engrams are more stably connected than those that are not.3 Memory accessibility and precision depend on the stabilization of engram network during memory consolidation.4,5 The specificity of these changes suggests a complex regulation of both excitatory and inhibitory inputs, as well as broader circuit changes. However, the molecular mechanisms underlying the maintenance of the dormant yet stable engram networks remain unknown. In addition, memory precision progressively declines with age, and is considered as one of the predominant hallmarks of aging-related cognitive dysfunctions.6,7 Aging-related maladaptive changes in the functional properties of neurons include both decreased and increased neuronal excitability, depending on the neuronal cell type and brain region,8 while the mechanistic link between the disturbed engram network and aging-related memory deficits remains to be elucidated.
Cell-adhesion molecules (CAMs) are a superfamily that play an irreplaceable role in synaptic plasticity through mediating the bidirectional organization of synaptic compartments.9 Neuronal pentraxins (NPTXs) are a subfamily of CAMs primarily expressed in excitatory neurons. NPTXs consist of NPTX1, NPTX2 and their receptor NPTXR.10 Pre-synaptically secreted NPTX1 and NPTX2 form disulfide-linked heteromultimers with post-synaptic NPTXR to promote the clustering of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) type glutamate receptors (AMPARs).11,12 Subsequent studies identified NPTX1 as both a pro-apoptotic protein13 and an inhibitor of excitatory synaptic transmission.14 In contrast, NPTX2 maintains excitatory homeostasis by adaptively enhancing circuit inhibition.15,16 These findings suggest that NPTX1 and NPTX2 may serve as potential substrates for stabilizing the excitatory and inhibitory inputs onto distinct neuronal ensembles. NPTXs are also considered as prognostic biomarkers for neurodegeneration, with a significant decrease in their expression levels in Alzheimer’s disease (AD), schizophrenia or even mild cognitive impairment patients.17–19 However, the causal link between NPTX reduction-induced engram network destabilization and aging-related memory deficits remain unclear.
The tracing of neuronal circuits involved in specific memory formation is enabled by utilizing the immediate early gene (IEG)-dependent robust activity marking (RAM) system.20 Dentate gyrus (DG) engram cells contain functionally divergent neuronal ensembles defined by Fos or Npas4 activation-dependent transcriptional outputs (F-RAM or N-RAM). These two ensembles drive precise memory expression by recruiting excitatory and inhibitory (E/I) circuits.21 NPTX1 and NPTX2 are both expressed in DG.22 In this study, we found that the maintenance of engram network stability requires NPTX1 to restrict the excitatory inputs from medial entorhinal cortex (MEC), and NPTX2 to promote the inhibitory inputs from parvalbumin+ (PV+) interneurons during the consolidation of contextual fear memory. Engram network hyperactivity caused by NPTX downregulation in DG engram ensembles contributes to aging-related memory deficits. Our findings elucidate the critical role of NPTXs in stabilizing the DG engram network during memory consolidation by controlling excitatory and inhibitory inputs, and establish a causal link between engram network destabilization and aging-related memory deficits.
Results
NPTX1 restricts MEC excitatory inputs onto DG engram ensembles
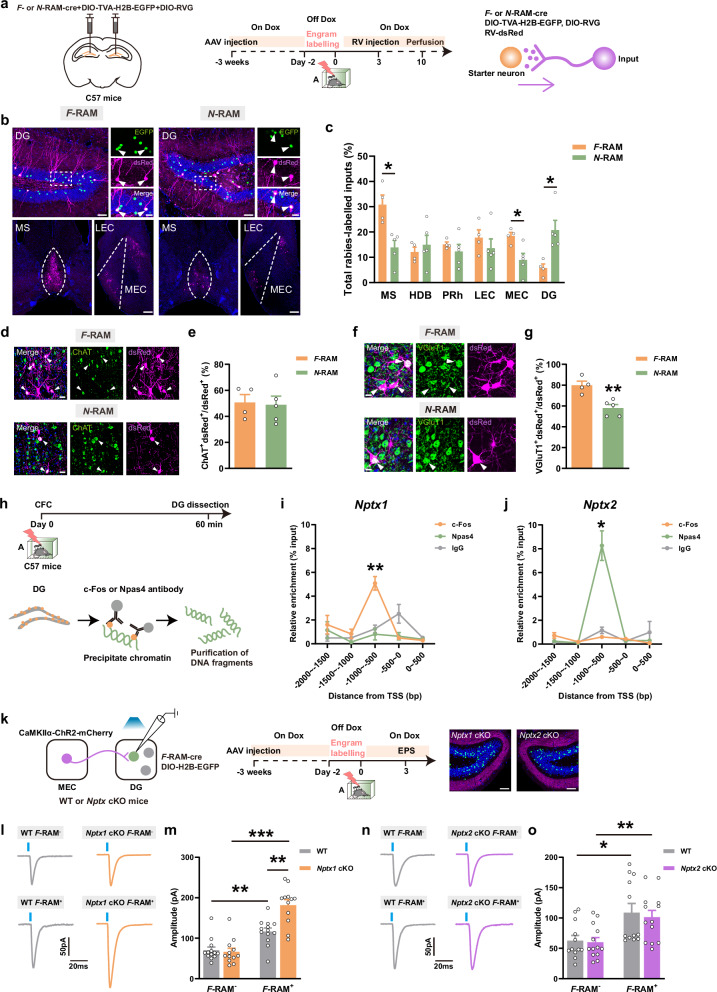
F-RAM and N-RAM systems were employed to label engram ensembles with the mKate2 reporter after doxycycline (Dox) removal.21 Npas4-CreERT2 mice in which the endogenous Npas4 promoter drives the expression of CreERT2 were generated to label Npas4+ ensemble with the EGFP reporter by 4-hydroxytamoxifen (4-OHT) intraperitoneal (i.p.) injection (Supplementary information, Fig. S1a–d). A combination of these two activity-dependent labeling strategies (Supplementary information, Fig. S1e) was used simultaneously to label engrams activated by contextual fear conditioning (CFC, 0.5 mA, 1 s, 3 trials). The EGFP+ neurons exhibited substantial overlap with mKate2+ N-RAM ensemble but minimal colocalization with mKate2+ F-RAM ensemble (Supplementary information, Fig. S1f, g). These results confirm that engram cells in DG labeled by F-RAM and N-RAM reporters are distinct populations.
AAV-F-RAM and AAV-N-RAM promoters driving expression of Cre recombinase (AAV-F-RAM-Cre and AAV-N-RAM-Cre) were generated. The number of tagged F- or N-RAM engram cells was significantly increased in the off Dox-CFC group (Supplementary information, Fig. S2). The upstream inputs of F- and N-RAM neuronal ensembles from medial septum (MS), horizontal limb of the diagonal band (HDB), perirhinal cortex (PRh), lateral entorhinal cortex (LEC), MEC and DG were detected by rabies virus (RV) tracing (Fig. 1a, b). F-RAM ensemble formed more connections with MS and MEC, while N-RAM ensemble received more inputs from DG local neurons (Fig. 1c). Nearly half of the dsRed+ inputs from MS to F-RAM and N-RAM neurons expressed the cholinergic neuron marker, choline acetyltransferase (ChAT) (Fig. 1d, e). However, F-RAM neurons received more excitatory inputs from MEC than N-RAM neurons (Fig. 1f, g).
Fig. 2. The effects of Nptx1 or Nptx2 depletion on engram excitability and membrane expression of Kv7.2.
a Diagram of AAV injection. b Experimental scheme to label F-RAM and N-RAM engram ensembles. EPS, electrophysiology; IHC, immunohistochemistry. c Representative images of F-RAM engram cells in DG of Nptx1 and Nptx2 cKO mice. Green: F-RAM engram cells of Nptx1 and Nptx2 cKO mice, EGFP; blue: DAPI. Scale bars, 20 μm. d, h Representative AP traces induced by depolarizing current injections (100 pA) recorded from WT and Nptx cKO mice. e Input-output curves of AP spikes vs injected currents of WT and Nptx1 cKO mice. WT, n = 22 neurons from 4 mice; Nptx1 cKO, n = 23 neurons from 4 mice. f RMP of WT and Nptx1 cKO mice. WT, n = 22 neurons from 4 mice; Nptx1 cKO, n = 23 neurons from 4 mice. g Rheobase of WT and Nptx1 cKO mice. WT, n = 22 neurons from 4 mice; Nptx1 cKO, n = 23 neurons from 4 mice. i Input-output curves of AP spikes vs injected currents of WT and Nptx2 cKO mice. WT, n = 15 neurons from 4 mice; Nptx2 cKO, n = 16 neurons from 3 mice. j RMP of WT and Nptx2 cKO mice. WT, n = 15 neurons from 4 mice; Nptx2 cKO, n = 16 neurons from 3 mice. k Rheobase of WT and Nptx2 cKO mice. WT, n = 15 neurons from 4 mice; Nptx2 cKO, n = 16 neurons from 3 mice. l Co-IP of NPTX1 with Kv7.2 in DG. m, o Representative confocal images of engram cells colocalizing with Kv7.2. Green: F-RAM engram cells, EGFP; purple: Kv7.2; blue: DAPI. Dashed white lines outline cell membrane. Scale bars, 5 μm. n Quantification of membrane Kv7.2 fluorescence intensity of WT and Nptx1 cKO neurons. WT, n = 53 neurons from 5 mice; Nptx1 cKO, n = 48 neurons from 6 mice. p Quantification of Kv7.2 fluorescence intensity of WT and Nptx2 cKO neurons. WT, n = 38 neurons from 5 mice; Nptx1 cKO, n = 33 neurons from 6 mice. q Representative traces of IM current recorded before and after application of XE991. r Normalized IM current recorded at –30 mV before and after application of XE991. n = 6 neurons from 3 mice. s Diagram of AAV injection. t Representative traces of IM current recorded from WT and Nptx1 cKO mice with or without Kcnq2 knockdown. u Average IM current amplitudes recorded at –30 mV from WT and Nptx1 cKO mice with or without Kcnq2 knockdown. WT-sgScramble, n = 14 neurons from 3 mice; Nptx1 cKO-sgScramble, n = 12 neurons from 3 mice; WT-sgKcnq2, n = 14 neurons from 3 mice; Nptx1 cKO-sgKcnq2, n = 15 neurons from 3 mice. Data are presented as mean ± SEM; **P < 0.01, ***P < 0.001.
Fig. 1. RV tracing of the upstream inputs to DG F- and N-RAM neuronal ensembles and the effects of Nptx depletion on the response to MEC excitatory inputs.
a Diagram of virus injection and experimental scheme to trace upstream inputs of DG F-RAM and N-RAM ensembles. b Representative confocal images of DG starter neurons in F-RAM and N-RAM ensembles and their respective upstream inputs. Green: F-RAM or N-RAM engram cells, EGFP; purple: dsRed; blue: DAPI. White arrows indicate the starter neurons. Scale bars: top left, 50 μm; top right, 10 μm; bottom, 100 μm. c Percentages of total inputs from each upstream site relative to total quantified inputs. F-RAM, n = 4 mice; N-RAM, n = 5 mice. d Representative confocal images of upstream MS dsRed+ cells immunostained with ChAT. Green: ChAT; purple: dsRed; blue: DAPI. White arrows indicate the colocalized cells. Scale bars, 10 μm. e Percentages of colocalized cells in MS total dsRed+ cells. F-RAM, n = 4 mice; N-RAM, n = 5 mice. f Representative confocal images of upstream MEC dsRed+ cells immunostained with vGluT1. Green: vGluT1; purple: dsRed; blue: DAPI. White arrows indicate the colocalized cells. Scale bars, 5 μm. g Percentages of colocalized cells in MEC total dsRed+ cells. F-RAM, n = 4 mice; N-RAM, n = 5 mice. h Experimental scheme of ChIP assay in DG. i ChIP-qPCR analysis showing the enrichment of c-Fos and Npas4 at genomic sequences located from –2000 bp upstream to 500 bp downstream of Nptx1 TSS. c-Fos, n = 4 from 12 mice; Npas4, n = 4 from 12 mice; IgG, n = 4 from 12 mice. j ChIP-qPCR analysis showing the enrichment of c-Fos and Npas4 at genomic sequences located from –2000 bp upstream to 500 bp downstream of Nptx2 TSS. c-Fos, n = 4 from 12 mice; Npas4, n = 3 from 9 mice; IgG, n = 4 from 12 mice. k Diagram of AAV injection, experimental scheme and representative expression of ChR2 in MEC projection neurons and DG engram cells. Green: F-RAM engram cells of Nptx1 and Nptx2 cKO mice, EGFP; purple: MEC projections, mCherry; blue: DAPI. Scale bars, 50 μm. l, n Representative traces of oEPSC recorded from WT and Nptx cKO mice. m Quantification of oEPSC amplitudes recorded from WT and Nptx1 cKO mice. WT-F-RAM–, n = 13 neurons from 4 mice; Nptx1 cKO-F-RAM–, n = 12 neurons from 4 mice; WT-F-RAM+, n = 13 neurons from 4 mice; Nptx1 cKO-F-RAM+, n = 12 neurons from 4 mice. o Quantification of oEPSC amplitudes recorded from WT and Nptx2 cKO mice. WT-F-RAM–, n = 12 neurons from 4 mice; Nptx2 cKO-F-RAM–, n = 13 neurons from 4 mice; WT-F-RAM+, n = 12 neurons from 4 mice; Nptx2 cKO-F-RAM+, n = 13 neurons from 4 mice. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
NPTXs were found to be predominantly involved in E/I synaptic homeostasis rather than cholinergic signaling.12,23 Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) on the DG of mice was performed 1 h after fear conditioning using anti-c-Fos and anti-Npas4 antibodies (Fig. 1h). C-Fos was preferentially enriched in the transcription starting site (TSS) of Nptx1 ranging from –1000 bp to –500 bp, whereas Npas4 was preferentially enriched in the TSS of Nptx2 ranging from –1000 bp to –500 bp (Fig. 1i, j), suggesting that NPTX1 and NPTX2 could be preferentially recruited by Fos and Npas4 to regulate the plasticity of the formed engram circuits, respectively.
Nptx1fl/fl, Nptx2fl/fl conditional knockout (Nptx1 cKO, Nptx2 cKO) mice were generated (Supplementary information, Fig. S3a, b). Ribosome-associated transcripts of F-RAM and N-RAM ensembles were enriched from Nptx cKO mice and their wild-type (WT) littermates 3 days after CFC (Supplementary information, Fig. S3c, d), and the knockout efficiency of Nptx1 and Nptx2 in F- or N-RAM ensembles were verified by qPCR. Nptx2 expression remained unaffected when Nptx1 was depleted, and vice versa (Supplementary information, Fig. S3e–l). The expression of NPTX1 and NPTX2 proteins in F-RAM and N-RAM ensembles were examined 1.5 h, 6 h, 24 h and 72 h after CFC. Compared with the WT groups, NPTX proteins in engram cells of the cKO groups were decreased at 24 h and 72 h (Supplementary information, Figs. S4, S5). The delayed effect of Cre-dependent knockout does not affect NPTX expression during fear conditioning; rather, it exerts an effect during memory consolidation, once the engram circuits have formed.
To test the role of NPTXs in the excitatory connection between MEC and DG F-RAM ensemble, AAV-CaMKIIα-ChR2-mCherry was injected into MEC of Nptx1 cKO, Nptx2 cKO mice and their WT littermates, and the virus mixture of AAV-F-RAM-Cre with Cre-dependent AAV-DIO-H2B-EGFP was injected into DG to knock out Nptxs in F-RAM ensemble. Opto-evoked excitatory post-synaptic currents (oEPSCs) from MEC were recorded on EGFP– (F-RAM–) and EGFP+ (F-RAM+) cells (Fig. 1k). The amplitude of oEPSCs recorded from EGFP+ cells was larger in the Nptx1 cKO group (Fig. 1l, m), whereas this effect was not found in the Nptx2 cKO mice (Fig. 1n, o). These data indicate that NPTX1 plays an important role in dampening the excitatory synaptic transmission between MEC and DG F-RAM ensemble.
NPTX1 facilitates M-current and membrane expression of Kv7.2 in DG F-RAM ensemble
Action potential (AP) recordings were performed to examine whether neuronal excitability of the engram ensembles was changed after Nptx knockout (Fig. 2a–c). F-RAM engram cells lacking Nptx1, but not Nptx2, exhibited upward shift of the neuronal spiking curve after step-increment current injections, lower resting membrane potential (RMP) and rheobase (Fig. 2d–k), indicating increased excitability of F-RAM engram cells. KCNQ2 (Kv7.2) pairs with the KCNQ3 (Kv7.3) subunit to form KCNQ2/3 heterotetramers, which are the primary molecular subunits of M-channel involved in regulating neuronal excitability.24,25 Co-immunoprecipitation (co-IP) of the DG lysate confirmed the interaction between NPTX1 and Kv7.2 (Fig. 2l). Immunostaining analysis further revealed that Nptx1 ablation decreased Kv7.2 membrane expression in F-RAM cells (Fig. 2m, n), which was not observed in Nptx2 cKO group (Fig. 2o, p). Application of the KCNQ2/3-selective blocker XE991 resulted in a reduction of M-current (IM) amplitude on DG F-RAM engram cells (Fig. 2q, r). Nptx1 cKO exerted the similar inhibitory effect to XE991 on IM amplitude, while this effect was blunted when Kcnq2 was simultaneously knocked down in Nptx1 cKO F-RAM ensemble by using CRISPR-Cas9-Kcnq2-sgRNA25 (Fig. 2s–u). Taken together, these results indicate that NPTX1 restrains the excitatory synaptic transmission between MEC and DG F-RAM ensemble by facilitating Kv7.2-mediated inhibition of neuronal hyperexcitability.
NPTX2 in N-RAM ensemble facilitates the perisomatic inhibition by PV+ interneurons in DG
The results of RV tracing (Fig. 1c) and ChIP-qPCR (Fig. 1j) led us to wonder whether NPTX2 was specifically involved in N-RAM ensemble-dependent inhibitory synaptic connection in DG. PV+ and somatostatin+ (SST+) interneurons are two major subtypes of GABAergic interneurons in DG26 that separately target perisomatic compartments and distal apical dendrites of granule cells.27 F-RAM and N-RAM ensembles received equal amount of SST+ inputs (Supplementary information, Fig. S6a). To test the involvement of NPTXs in the synaptic transmission between N-RAM ensemble and SST+ interneurons, the viral mixture of AAV-N-RAM-Cre, AAV-DIO-ChR2-EYFP, AAV-SST-Flp and Flp recombinase-dependent AAV-fDIO-mCherry was injected into DG of Nptx1 cKO, Nptx2 cKO mice and their WT littermates. The specificity of AAV-SST-Flp28,29 was confirmed by immunostaining with anti-SST antibody (Supplementary information, Fig. S6c, d). Opto-evoked paired-pulse ratio (PPR) and AMPAR/N-methyl-D-aspartate receptor (NMDAR) (A/N) ratio were recorded in SST+ interneurons according to mCherry expression, morphology and location (Supplementary information, Fig. S6e, f). Depletion of Nptxs in N-RAM ensemble induced increased PPR of SST+ interneurons (Supplementary information, Fig. S6g, h, k, l), consistent with previous studies that NPTX family promotes pre-synaptic glutamate release.23,30 However, Nptx knockout in N-RAM ensemble exerted no effect on A/N ratio of SST+ interneurons (Supplementary information, Fig. S6i, j, m, n).
N-RAM neurons received more PV+ inputs than F-RAM neurons in DG (Fig. 3a, b). To investigate the involvement of NPTX2 in the synaptic transmission between N-RAM ensemble and PV+ interneurons, the viral mixture of AAV-N-RAM-Cre, AAV-DIO-ChR2-EYFP, AAV-PV-Flp and AAV-fDIO-mCherry was injected into DG of Nptx1 cKO, Nptx2 cKO mice and their WT littermates. The specificity of AAV-PV-Flp28,31 was confirmed by immunostaining with anti-PV antibody (Supplementary information, Fig. S7a). PPR and A/N ratio were recorded in PV+ interneurons (Fig. 3c, d). The weakened pre-synaptic glutamate release was observed in both Nptx1 cKO and Nptx2 cKO mice (Supplementary information, Fig. S7b–e), while decreased AMPAR-mediated EPSC and A/N ratio in PV+ interneurons were only found in Nptx2 cKO mice (Fig. 3e–j). Immunostaining showed that Nptx2 depletion in N-RAM ensemble reduced the membrane expression of AMPAR subunit GluA4 in PV+ interneurons (Fig. 3k, l), which is consistent with previous findings that NPTX2 binds GluA4 in PV+ interneurons to regulate the network excitatory/inhibitory balance.15,32 Moreover, depletion of Nptxs in F-RAM ensemble exerted no effect on the A/N ratios of postsynaptic PV+ or SST+ interneurons (Supplementary information, Fig. S8). These data demonstrate that NPTX2 is specifically involved in mediating the plasticity of the circuits between PV+ interneurons and the N-RAM ensemble in DG. To examine whether NPTX2-dependent plasticity also applies to GABAergic CCK+ cells, the viral mixture of AAV-N-RAM-Cre, AAV-DIO-ChR2-EYFP with AAV-vGAT2-Flp33–35-dependent AAV-CCK-fDIO-mCherry was injected into DG of WT and Nptx2 cKO mice to label the GABAergic CCK+ cells (Supplementary information, Fig. S9a–c). Opto-evoked PPR and A/N ratio of mCherry+ CCK+ interneurons were unaffected by Nptx2 depletion in N-RAM ensemble (Supplementary information, Fig. S9d–g). These data further suggest that NPTX2-dependent interneuron plasticity is specific to PV+ interneurons.
Fig. 3. The effects of Nptx2 depletion on the functional connection between DG local PV+ interneurons and N-RAM ensemble.
a Representative confocal images of DG local dsRed+ cells immunostained with PV. Green: EGFP; purple: dsRed; yellow: PV; blue: DAPI. White arrows indicate the colocalized cells. Scale bars, 5 μm. b Percentages of colocalized cells in DG total PV+ cells. F-RAM, n = 4 mice; N-RAM, n = 5 mice. c Diagram of AAV injection and experimental scheme to label N-RAM engram ensembles. d Diagram of photostimulation and whole-cell patch clamp recordings (left) and representative images of engram cells and PV+ interneurons (right). Green: N-RAM engram cells of Nptx1 and Nptx2 cKO mice, EYFP; purple: PV+ interneurons, mCherry; blue: DAPI. Scale bars, 10 μm. e, g Representative traces of opto-evoked AMPA-EPSC and NMDA-EPSC recorded from WT and Nptx cKO mice. f Average A/N ratio recorded from WT and Nptx1 cKO mice. WT, n = 13 neurons from 4 mice; Nptx1 cKO, n = 13 neurons from 4 mice. h Average A/N ratio recorded from WT and Nptx2 cKO mice. WT, n = 16 neurons from 3 mice; Nptx2 cKO, n = 17 neurons from 5 mice. i, j Average AMPA-EPSC (i) and NMDA-EPSC (j) amplitudes recorded from WT and Nptx2 cKO mice. WT, n = 16 neurons from 3 mice; Nptx2 cKO, n = 17 neurons from 5 mice. k Representative confocal images of GluA4 colocalizing with PV+ interneurons. Green: N-RAM engram cells; purple: PV+ interneurons; yellow: GluA4. Scale bars, 10 μm. l Quantification of GluA4 membrane expression on PV+ interneurons in WT and Nptx2 cKO mice. WT, n = 32 neurons from 5 mice; Nptx2 cKO, n = 37 neurons from 5 mice. m Diagram of AAV injection and experimental scheme to label N-RAM engram ensembles. n Diagram of photostimulation and whole-cell patch clamp recordings (left) and representative images of N-RAM engram cells and PV+ interneurons (right). Green: N-RAM engram cells, EGFP; purple: PV+ interneurons, mCherry; blue: DAPI. Scale bar, 20 μm. o, q Representative traces of opto-evoked PPR (o) and oIPSC (q) recorded from neurons transfected with Scramble or Nptx2 shRNA. p, r Quantification of PPR (p) and oIPSC (r) amplitudes recorded from neurons transfected with Scramble or Nptx2 shRNA. For PPR recording, Scramble, n = 21 from 4 mice; Nptx2 shRNA, n = 22 from 4 mice. For oIPSC recording, Scramble, n = 25 from 4 mice; Nptx2 shRNA, n = 24 from 4 mice. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
The disturbed PV+ interneuron plasticity caused by Nptx2 depletion in N-RAM ensemble led us to speculate that the inhibitory inputs received by the N-RAM ensemble was affected. A viral mixture of AAV-N-RAM-Cre, AAV-Flex-EGFP-Nptx2 shRNA (Nptx2 sh)22 or Scramble shRNA (Scramble), and AAV-fDIO-ChR2-mCherry was injected into DG of PV-Flpe mice. Opto-evoked inhibitory post-synaptic currents (oIPSCs) and PPR were then recorded in N-RAM cells (Fig. 3m, n). Nptx2 knockdown in N-RAM ensemble increased PPR (Fig. 3o, p) and decreased IPSC amplitude evoked by optical activation of PV+ interneurons (Fig. 3q, r). These data indicate a reduction in pre-synaptic γ-aminobutyric acid (GABA) release and the weakened inhibitory inputs to N-RAM engram cells. Taken together, these data indicate that NPTX2 in N-RAM ensemble facilitates the inhibitory synaptic transmission between DG N-RAM engram cells and PV+ interneurons by the AMPAR-dependent mechanism.
NPTX1 in DG F-RAM ensemble and NPTX2 in DG N-RAM ensemble facilitate the precise expression of contextual fear memory
To investigate the role of NPTX-dependent maintenance of engram network stability in regulating specific behavioral outputs, the viral mixture of AAV-F-RAM-Cre or AAV-N-RAM-Cre combined with AAV-DIO-EYFP was injected into DG of young Nptx1 cKO, Nptx2 cKO mice and their respective WT littermates. Mice were tested on day 3 in fear context A and non-fear context C after CFC (Fig. 4a–c, h–j). The freezing levels during conditioning were not different between Nptx1 cKO, Nptx2 cKO mice and their WT littermates (Supplementary information, Fig. S10). Interestingly, Nptx1 depletion in F-RAM ensemble decreased freezing in context A (Fig. 4d, e; Supplementary information, Fig. S11a–f), whereas Nptx2 depletion in F-RAM ensemble caused no difference in freezing levels in either context (Fig. 4f, g). Nptx1 knockout in DG N-RAM ensemble did not affect the freezing levels in either context (Fig. 4k, l), whereas Nptx2 knockout in DG N-RAM ensemble increased the freezing levels in context C on day 3 (Fig. 4m, n; Supplementary information, Fig S11k–p), indicating overgeneralization of contextual fear memory. Other types of memories, such as novel object recognition (NOR) and novel place recognition (NPR) were preserved when Nptxs were knocked out in F-RAM and N-RAM neurons (Supplementary information, Fig. S11g–j, q–t).
Fig. 4. The effects of Nptx depletion in F-RAM and N-RAM ensembles on the expression of contextual fear memory.
a, h, o Diagram of AAV injection. b, i, p Experimental scheme of CFC and memory retrieval. c, j, q Representative images of F- and N-RAM engram cells in DG. Green: F-RAM or N-RAM ensemble, EYFP; blue: DAPI. Scale bar, 100 μm. d, e Freezing percentage (d) and discrimination index (e) of WT and Nptx1 cKO mice tested in context A and context C at day 3 (F-RAM). WT, n = 14 mice; Nptx1 cKO, n = 11 mice. f, g Freezing percentage (f) and discrimination index (g) of WT and Nptx2 cKO mice tested in context A and context C at day 3 (F-RAM). WT, n = 14 mice; Nptx2 cKO, n = 13 mice. k, l Freezing percentage (k) and discrimination index (l) of WT and Nptx1 cKO mice tested in context A and context C at day 3 (N-RAM). WT, n = 18 mice; Nptx1 cKO, n = 15 mice. m, n Freezing percentage (m) and discrimination index (n) of WT and Nptx2 cKO mice tested in context A and context C at day 3 (N-RAM). WT, n = 13 mice; Nptx2 cKO, n = 12 mice. r, s Freezing percentage (r) and discrimination index (s) of WT and Nptx1 cKO mice tested in context A and context C at day 3 (F-RAM). WT, n = 11 mice; Nptx1 cKO, n = 11 mice. t, u Freezing percentage (t) and discrimination index (u) of WT and Nptx2 cKO mice tested in context A and context C at day 3 (N-RAM). WT, n = 10 mice; Nptx2 cKO, n = 9 mice. Data are presented as mean ± SEM; *P < 0.05.
Furthermore, knocking down Nptx1 or Nptx2 in F- or N-RAM neurons (activated during exposure to a novel context N, distinct from context A or C) did not significantly alter the freezing levels in Nptx cKO mice compared to WT controls (Fig. 4o–u), indicating that NPTXs exert a fear context-specific regulatory effect on memory precision. In addition, Nptx depletion in both ensembles had no effect on mouse’s locomotion, anxiety or depression levels (Supplementary information, Figs. S12–S14).
Taken together, these data suggest that the precise expression of contextual fear memory, characterized by high freezing levels in the fear context and low levels in the non-fear context, requires the coordinated function of NPTX1 and NPTX2 in distinct ensembles.
Pharmacological activation of Kv7.2 channels or chemogenetic activation of PV+ interneurons ameliorates contextual fear memory deficits induced by NPTX depletion in DG engrams
The Kv7 activator retigabine (a clinically used anticonvulsant36), was injected intraperitoneally 30 min before mice were tested in the fear context A and non-fear context C (Fig. 5a–c). Retigabine had no effect on the freezing levels of WT mice in either context, whereas it restored the reduced freezing levels in context A when NPTX1 was depleted in F-RAM ensemble (Fig. 5d–g; Supplementary information, Fig. S15a, b).
Fig. 5. The effects of activating Kv7.2 or DG PV+ interneurons on memory deficits induced by Nptx depletion in DG engram ensembles.
a, h Diagram of AAV injection. b, i Experimental scheme of memory retrieval test. c Representative images of F- and N-RAM engram cells in DG. Green: F-RAM and N-RAM ensembles, EYFP; blue: DAPI. Scale bars, 100 μm. d, e Freezing percentage (d) and discrimination index (e) of WT-Vehicle, WT-Retigabine, Nptx1 cKO-Vehicle and Nptx1 cKO-Retigabine mice (F-RAM). WT-Vehicle, n = 12 mice; WT-Retigabine, n = 13 mice; Nptx1 cKO-Vehicle, n = 10 mice; Nptx1 cKO-Retigabine, n = 12 mice. f, g Freezing percentage (f) and discrimination index (g) of WT-Vehicle, WT-Retigabine, Nptx1 cKO-Vehicle and Nptx1 cKO-Retigabine mice (N-RAM). WT-Vehicle, n = 10 mice; WT-Retigabine, n = 11 mice; Nptx1 cKO-Vehicle, n = 11 mice; Nptx1 cKO-Retigabine, n = 10 mice. j Representative images of F- and N-RAM engram cells in DG. Green: F-RAM and N-RAM ensembles, EGFP; purple: PV+ interneurons, mCherry; blue: DAPI. Scale bars, 100 μm. k, l Freezing percentage (k) and discrimination index (l) of Scramble-mCherry, Scramble-hM3Dq, Nptx2 sh-mCherry and Nptx2 sh-hM3Dq groups (F-RAM). Scramble-mcherry, n = 10 mice; Scramble-hM3Dq, n = 11 mice; Nptx2 sh-mcherry, n = 10 mice; Nptx2 sh-hM3Dq, n = 12 mice. m, n Freezing percentage (m) and discrimination index (n) of Scramble-mCherry, Scramble-hM3Dq, Nptx2 sh-mCherry and Nptx2 sh-hM3Dq groups (N-RAM). Scramble-mcherry, n = 16 mice; Scramble-hM3Dq, n = 15 mice; Nptx2 sh-mcherry, n = 15 mice; Nptx2 sh-hM3Dq, n = 14 mice. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01.
To restore the activity of PV+ interneurons after Nptx2 depletion in N-RAM ensemble, the designer receptors exclusively activated by designer drugs (DREADDs) were used to activate PV+ interneurons. The viral mixture of AAV-N-RAM-Cre, AAV-Flex-EGFP-Nptx2 shRNA and AAV-fDIO-hM3Dq-mCherry was injected into the DG of PV-Flpe mice and clozapine N-oxide (CNO) was administered via i.p. injection 30 min before memory test (Fig. 5h–j). Chemogenetic activation of PV+ interneurons in DG did not affect either freezing levels or discrimination index in control groups expressing Scramble shRNA, but increased the discrimination index when Nptx2 was knocked down in N-RAM ensemble (Fig. 5k–n; Supplementary information, Fig. S15c, d).
These data suggest that the deficits in contextual fear memory induced by NPTX depletion in distinct DG engram ensembles can be ameliorated by activation of Kv7.2 channels or DG PV+ interneurons.
Downregulation of NPTX1 and NPTX2 in DG engram cells in aged mice
Maladaptive changes in expression of functional synaptic proteins, which destabilize neuronal network and impair cognition, represent central hallmarks of physiological brain aging.8 NPTXs are considered as biomarkers of synaptic dysfunction and cognitive impairment.10,19 To investigate whether Nptx expression in DG changes during physiological aging, young (3 months) and aged (18 months) mice were sacrificed 15 min, 30 min, 60 min and 120 min after CFC or under home cage (HC) conditions, single molecule fluorescence in situ hybridization (smFISH) was used to assess their mRNA expression (Fig. 6a–e).
Fig. 6. Nptx expression in DG engram cells in young and aged mice.
a Experimental scheme for smFISH. b, c Representative confocal images of Nptx1 colocalizing with Fos and Npas4 30 min after CFC in DG of young (b) and aged (c) mice. Green: Nptx1; purple: Fos; blue: DAPI. White arrows indicate the colocalized cells. Scale bars: top, 30 μm; bottom, 10 μm. d, e Representative confocal images of Nptx2 colocalizing with Fos and Npas4 30 min after CFC in DG of young (d) and aged (e) mice. Green: Nptx2; purple: Npas4; blue: DAPI. White arrows indicate the colocalized cells. Scale bars: top, 30 μm; bottom, 10 μm. f, g Fos+ (f) and Npas4+ (g) cell counts in DG. Young HC, n = 4 mice, Aged HC, n = 5 mice; Young 15 min, n = 5 mice, Aged 15 min, n = 5 mice; Young 30 min, n = 4 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 4 mice, Aged 60 min, n = 5 mice; Young 120 min, n = 4 mice, Aged 120 min, n = 5 mice. h Fluorescence intensity of overall Nptx1 mRNA in DG. Young HC, n = 4 mice, Aged HC, n = 4 mice; Young 15 min, n = 5 mice, Aged 15 min, n = 5 mice; Young 30 min, n = 5 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 5 mice, Aged 60 min, n = 5 mice; Young 120 min, n = 4 mice, Aged 120 min, n = 5 mice. i Fluorescence intensity of Nptx1 mRNA in Fos+ ensemble in DG. Young HC, n = 4 mice, Aged HC, n = 4 mice; Young 15 min, n = 5 mice, Aged 15 min, n = 5 mice; Young 30 min, n = 5 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 5 mice, Aged 60 min, n = 5 mice; Young 120 min, n = 4 mice, Aged 120 min, n = 5 mice. j Fluorescence intensity of Nptx1 mRNA in Npas4+ ensemble in DG. Young HC, n = 5 mice, Aged HC, n = 4 mice; Young 15 min, n = 5 mice, Aged 15 min, n = 4 mice; Young 30 min, n = 5 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 5 mice, Aged 60 min, n = 4 mice; Young 120 min, n = 3 mice, Aged 120 min, n = 5 mice. k Nptx2+ cell counts in DG. Young HC, n = 5 mice, Aged HC, n = 4 mice; Young 15 min, n = 4 mice, Aged 15 min, n = 5 mice; Young 30 min, n = 5 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 5 mice, Aged 60 min, n = 5 mice; Young 120 min, n = 5 mice, Aged 120 min, n = 4 mice. l Fluorescence intensity of Nptx2 mRNA in Fos+ ensemble in DG. Young HC, n = 4 mice, Aged HC, n = 4 mice; Young 15 min, n = 5 mice, Aged 15 min, n = 4 mice; Young 30 min, n = 6 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 5 mice, Aged 60 min, n = 4 mice; Young 120 min, n = 5 mice, Aged 120 min, n = 4 mice. m Fluorescence intensity of Nptx2 mRNA in Npas4+ ensemble in DG. Young HC, n = 4 mice, Aged HC, n = 4 mice; Young 15 min, n = 5 mice, Aged 15 min, n = 4 mice; Young 30 min, n = 5 mice, Aged 30 min, n = 4 mice; Young 60 min, n = 5 mice, Aged 60 min, n = 4 mice; Young 120 min, n = 4 mice, Aged 120 min, n = 4 mice. n Diagram of AAV injection and experimental scheme to label F-RAM and N-RAM cells. o Representative images of F- and N-RAM engram cells in DG of young and aged mice. p F-RAM and N-RAM cell counts in DG of young and aged mice. Young F-RAM, n = 6; Young N-RAM, n = 7; Aged F-RAM, n = 5; Aged N-RAM, n = 4. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
The numbers of Fos+ and Npas4+ cells after CFC were decreased in DG of aged mice, especially at 30 min and 60 min, indicating decreased activation of neurons during learning in aged mice (Fig. 6f, g). Nptx1 was expressed in all DG granule cells. Although the overall Nptx1 mRNA fluorescence intensity was not significantly decreased in aged mice (Fig. 6h), the specific expression of Nptx1 in Fos+ and Npas4+ engram cells was reduced (Fig. 6i, j). NPTX2 was also seen as a neuronal IEG protein,17 both the number of Nptx2+ cells and the average fluorescence intensity of Nptx2 mRNA in Fos+ or Npas4+ engram cells were decreased in aged mice (Fig. 6k–m). Consistent with the decreased number of Fos+ and Npas4+ cells in DG after CFC, the number of cells labeled by the F- and N-RAM systems were both reduced in DG of aged mice (Fig. 6n–p). These findings indicate the downregulation of Nptx1 and Nptx2 in Fos+ and Npas4+ engram cells in aged mice.
NPTX overexpression in DG engram cells rescued contextual fear memory deficits in aged mice
Ribosome-associated transcripts of F-RAM and N-RAM ensembles that were activated in HC or by CFC were analyzed (Supplementary information, Fig. S16a). Gene ontology (GO) analysis revealed clusters of differentially expressed genes (DEGs) related to synaptic plasticity and cell communication between F-RAM and N-RAM ensembles. The difference in these clusters became more significant after CFC (Supplementary information, Fig. S16b). In addition, a small number of DEGs were overlapped between F- and N-RAM ensembles during aging (Supplementary information, Fig. S16c, d). In F-RAM ensemble, DEGs from young and aged mice showed enrichment in pathways regulating neuronal excitability (e.g., membrane potential and potassium ion import across plasma membrane). In contrast, DEGs in N-RAM were enriched in the AMPAR complex and others (Supplementary information, Fig. S16e). This differential enrichment suggests that distinct pathways are involved in engram network plasticity related to aging in F- and N-RAM ensembles.
As aging is always accompanied with a decline in memory precision,37–39 the expression of contextual fear memory in young and aged mice was further assessed. When exposed to the mild fear conditioning (0.3 mA, 1 s, 1 trial), aged mice froze less in the fear context A (Fig. 7a, c, d), which suggested memory retrieval impairment. However, when exposed to the strong fear conditioning (0.5 mA, 1 s, 3 trials), aged mice froze more in the non-fear context C, indicating memory overgeneralization (Fig. 7b, e, f). These results demonstrate that the precise expression of contextual fear memory is impaired in aged mice.
Fig. 7. Overexpression of NPTX1 in F-RAM ensemble rescued contextual memory imprecision in aged mice.
a, b Experimental scheme of CFC to test memory expression. c, d Freezing percentage (c) and discrimination index (d) of young and aged mice. Young, n = 11 mice; Aged, n = 10 mice. e, f Freezing percentage (e) and discrimination index (f) of young and aged mice. Young, n = 12 mice; Aged, n = 11 mice. g Diagram of AAV injection. h Experimental scheme for IM recording. i Representative traces of IM currents in F-RAM cells of young and aged mice. j Average IM current amplitudes recorded at –30 mV from young and aged mice. Young, n = 14 neurons from 3 mice; Aged, n = 15 neurons from 3 mice. k Representative confocal images of c-Fos+ cells colocalizing with Kv7.2. Green: c-Fos+ engram cells; purple: Kv7.2; blue: DAPI. Dashed white lines outline cell membrane. Scale bars, 5 μm. l Average membrane Kv7.2 fluorescence intensity of c-Fos+ neurons in young and aged mice. Young, n = 88 from 6 mice; Aged, n = 64 from 6 mice. m Co-IP of NPTX1 with Kv7.2 in DG of young and aged mice. n Quantification of immunoprecipitated Kv7.2 in young and aged mice. Young, n = 4 mice; Aged, n = 5 mice. o Diagram of AAV injection. p Experimental scheme of memory retrieval test. q Representative expression of NPTX1 in F- or N-RAM engram cells in DG. Green: F-RAM or N-RAM ensemble, EYFP or EGFP; blue: DAPI. Scale bars, 100 μm. r, s Freezing percentage (r) and discrimination index (s) of EYFP- and Nptx1-overexpressing aged groups (F-RAM). EYFP, n = 11 mice; Nptx1, n = 12 mice. t, u Freezing percentage (t) and discrimination index (u) of EYFP- and Nptx1-overexpressing aged groups (N-RAM). EYFP, n = 14 mice; Nptx1, n = 9 mice. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Decreased IM current (Fig. 7g–j), Kv7.2 membrane expression (Fig. 7k, l) as well as NPTX1–Kv7.2 interaction in the DG (Fig. 7m, n; Supplementary information, S17a) were observed in aged mice. AAV-DIO-Nptx1-EYFP was constructed and delivered with AAV-F-RAM-Cre or AAV-N-RAM-Cre into DG to overexpress NPTX1 in F- or N-RAM ensemble (Fig. 7o; Supplementary information, Fig. S17b). Aged mice were subjected to the mild fear conditioning protocol (0.3 mA, 1 s, one shock). Overexpression of NPTX1 in F-RAM ensemble, but not in N-RAM ensemble, increased the freezing levels of aged mice in the fear context A (Fig. 7o–u; Supplementary information, Fig. S17c, d).
AAVs encoding N-RAM-Cre, DIO-ChR2-EYFP, PV-flp and fDIO-mCherry were delivered into DG of young and aged mice (Fig. 8a–c). Impaired AMPAR-mediated EPSC (Fig. 8d–g), reduced GluA4 membrane expression in PV+ interneurons (Fig. 8h, i) and decreased PV+ fluorescence intensity surrounding Npas4+ engram cells (Fig. 8j, k) were observed in aged mice. These results are consistent with the phenotype of Nptx2 depletion in N-RAM ensemble of young mice (Fig. 3). Overexpression of the AMPAR binding domain of NPTX2 (NPTX2-PTX)22 in N-RAM ensemble, but not in F-RAM ensemble, rescued the overgeneralization of fear memory in aged mice (Fig. 8l–r; Supplementary information, Fig. S17e, f). However, in young mice, overexpression of NPTX1 or NPTX2-PTX in F- and N-RAM ensembles failed to alter freezing levels in context A or C (Supplementary information, Fig. S18). These data confirm that the hyperactivity of DG engram network caused by NPTX downregulation underlies aging-associated deficits in contextual fear memory, and re-stabilizing the NPTX1-dependent MEC-DG excitatory circuit and NPTX2-dependent DG PV+ interneuron inhibitory circuit is able to repair contextual fear memory deficits in aged mice.
Fig. 8. Overexpression of NPTX2 in N-RAM ensemble rescued contextual memory overgeneralization.
a Diagram of AAV injection. b Experimental scheme to label N-RAM engram ensembles. c Diagram of photostimulation and whole-cell patch clamp recordings. d Representative traces of opto-evoked AMPA-EPSC and NMDA-EPSC recorded from young and aged mice. e Average A/N ratio recorded from young and aged mice. f, g Average AMPA-EPSC (f) and NMDA-EPSC (g) amplitudes recorded from young and aged mice. Young, n = 9 neurons from 3 mice; Aged, n = 9 neurons from 3 mice. h Representative confocal images of GluA4 colocalizing with PV+ interneurons. Green: PV+ interneurons; purple: GluA4. Scale bars, 10 μm. i Quantification of GluA4 membrane expression on PV+ interneurons in young and aged mice. Young, n = 28 neurons from 4 mice; Aged, n = 23 neurons from 5 mice. j Representative confocal images of PV neurites around Npas4+ engram cells. Green: Npas4+ engram cells; purple: PV; blue: DAPI. Dashed white lines outline PV neurites. Scale bars, 5 μm. k Average fluorescence intensity of PV neurites around Npas4+ neurons in young and aged mice. Young, n = 160 neurons from 6 mice; Aged, n = 94 neurons from 6 mice. l Diagram of AAV injection. m Experimental scheme of memory retrieval test. n Representative expression of NPTX2-PTX in F- or N-RAM engram cells in DG. Green: F-RAM or N-RAM ensemble, EYFP or EGFP; blue: DAPI. Scale bars, 100 μm. o, p Freezing percentage (o) and discrimination index (p) of EGFP- and Nptx2-PTX-overexpressing aged groups (F-RAM). EGFP, n = 11 mice; Nptx2-PTX, n = 11 mice. q, r Freezing percentage (q) and discrimination index (r) of EGFP- and Nptx2-PTX-overexpressing aged groups (N-RAM). EGFP, n = 13 mice; Nptx2-PTX, n = 10 mice. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
During learning, neuronal ensembles that recruit excitatory and inhibitory circuits are activated by changes in synaptic connectivity. However, it is unclear how the recruited engram network maintains its stability. The consolidation of newly acquired memories requires gene transcription and translation. The transcription of IEGs (beyond their mRNA expression) activates downstream targets that maintain memory stability. Our study identified NPTXs as the molecular substrates that prevent DG engram network hyperactivity during memory consolidation and maintain the precision of contextual fear memory (Fig. 9). Although NPTX upregulation following CFC occurred in cells expressing endogenous Fos and Npas4 (Fig. 6), specific behavioral outputs were observed when Nptxs were knocked out in F- or N-RAM ensemble with a delay (24–72 h after CFC). These ensembles are tagged by activated Fos and Npas4 proteins, which drive meaningful transcriptional outputs. ChIP-qPCR results (Fig. 1h–j) showed that c-Fos was preferentially enriched in the TSS region of Nptx1, whereas Npas4 was significantly enriched in the TSS region of Nptx2 following CFC. Previous study21 and our data (Supplementary information, Fig. S1) demonstrated anatomical segregation between F- and N-RAM ensembles. The functional specificity of NPTXs should be placed into specific engram circuits to be manifested.
Fig. 9. Working model illustrating main findings.
During contextual fear memory learning (encoding), Fos transcription- and Npas4 transcription-dependent engram ensembles (F-RAM and N-RAM) are activated to preferentially recruit excitatory and inhibitory inputs. During memory consolidation, NPTX1 functions in F-RAM ensemble to restrict the response of this ensemble to MEC excitatory inputs, and NPTX2 functions in N-RAM ensemble to facilitate the recruitment of inhibitory inputs from PV+ interneurons. NPTX1 and NPTX2 cooperate to suppress engram network hyperactivity, thereby ensuring precise memory expression during retrieval. Downregulation of NPTX1 and NPTX2 in engram cells of aged mice contributes to destabilization of the engram network, resulting in contextual fear memory deficits.
NPTX1 plays a critical role in stabilizing the excitatory synaptic connection between MEC and DG F-RAM cells via facilitating Kv7.2 membrane expression-dependent inhibition of neuronal hyperexcitability to promote contextual fear memory retrieval. Our data demonstrated that NPTX1 interacts with the potassium channel Kv7.2, although this interaction may not be direct and requires other auxiliary proteins such as syntaxin.40 This finding provides a potential mechanism by which NPTX1 regulates neuronal excitability independently of its classical role in glutamate signaling. As a secretory protein, NPTX1 could form complexes with Kv7.2 and facilitate Kv7.2 membrane trafficking. The hyperexcitability observed in Nptx1 cKO mice may result from the impaired trafficking and membrane enrichment of Kv7.2. The contextual fear memory impairment caused by Nptx1 depletion in F-RAM ensemble contrasted with Sun et al.’s21 finding that optogenetic inhibition of MEC-DG circuit suppressed fear memory generalization. This is probably because optogenetic manipulation was transient, while Nptx1 depletion mimics the sustained neuronal activation during memory consolidation. This persistent hyperexcitability in engram cells decreased the signal-to-noise ratio of excitatory transmission from MEC to F-RAM ensemble during memory retrieval. Furthermore, accumulating evidence indicates that MEC inputs to DG regulate memory retrieval.41,42
NPTX2 stabilizes the inhibitory synaptic connection between DG local PV+ interneurons and N-RAM ensemble to suppress overgeneralization of contextual fear memory. This result was consistent with Sun et al.’s report that N-RAM ensemble contributed to memory discrimination.21 Their finding confirmed the critical role of CCK+ interneurons, while our finding illustrated the importance of NPTX2-dependent PV+ interneuron plasticity. We hypothesize that N-RAM ensemble-dependent fear memory discrimination requires the inhibitory inputs from both CCK+ and PV+ interneurons, as they were found to exert complementary roles in recruiting perisomatic inhibition.43,44 Notably, NPTX2 is specifically involved in regulating the functional connection between PV+ interneurons and N-RAM engram cells. NPTX2 was identified as a downstream effector of Npas4.45,46 This accounted for the coincidence that both Nptx2 knockout and Npas4 depletion47 impaired perisomatic inhibition. As a neuronal IEG, Npas4 upregulates perisomatic inhibitory synapses when activated46,48 to scale down the level of network activity, but the specific mechanism remains to be elucidated. Our study provides the downstream molecular mechanism for Npas4 transcription-dependent mediation of contextual fear memory precision. The functional specificity of NPTX2 in N-RAM ensemble (but not in F-RAM ensemble) for contextual fear memory discrimination was further supported by Ee-Lynn et al.’s study that knockdown of Nptx2 in Fos+ ensemble had no effect on PV+ interneuron-mediated perisomatic inhibition.49
Memory precision changes dynamically across the lifespan. Young individuals initially form only gist-like memories, which gradually develop into precise episodic memories as the brain matures. Subsequently, the ability to retrieve specific information declines with age. Understanding the maladaptive changes in engram network stability during aging may provide insights into combating aging-induced cognitive deficits. NPTXs are classic synaptic proteins that are also prognostic biomarkers in cognitive and mental disorders, such as AD and schizophrenia.17,18 Nptx2 was also seen as a neuronal IEG. Our smFISH results showed that the total number of Nptx2+ cells and the expression of Nptx2 in engram cells were both downregulated in DG during aging, whereas the overall downregulation of Nptx1 was not observed in aged mice, indicating that NPTX2 is more sensitive to aging. That is to say, during aging, Nptx2 downregulation-induced disruption of local perisomatic inhibition in DG engram precedes Nptx1 downregulation-induced disruption of long-range MEC-DG excitatory projection; this probably explains why generalization always emerges before amnesia in aged individuals. The finding of Ramsaran et al. showed that extracellular perineuronal net-dependent functional maturation of PV+ interneurons promotes sparse engram formation and memory precision during brain development.50 Our study elucidated that engram network hyperactivity, caused by NPTX downregulation, contributed to aging-related contextual fear memory deficits and provided potential synaptic and molecular strategies to mitigate different phenotypes of aging-related contextual fear memory imprecision, such as amnesia and overgeneralization.
Another interesting finding in our study is that the number of Fos+ and Npas4+ cells, as well as the number of F- and N-RAM tagged cells, was significantly reduced in aged brain (Fig. 6), which was in accordance with previous studies.51–53 Decreased activation and expression of IEGs in aged animals may accelerate cell senescence and increase the susceptibility to neurodegeneration, thereby impairing cognition and decreasing lifespan.47,54 In addition, memory impairments were found in c-Fos or Npas4 KO mice,55–57 indicating that Fos- and Npas4-dependent transcriptional activation is essential for neuronal network plasticity and cognitive maintenance. The disruption of this process may also contribute to aging-related memory deficits.
However, there are some limitations in our study. (1) Npas4 can regulate the transcription of Fos or itself,48 indicating the complex regulatory network between these IEGs. Whether Fos and Npas4 competitively bind to downstream genes, or whether Npas4+ and Fos+ neurons can mutually transform, remains to be investigated. (2) Unlike Nptx2, Nptx1 is consistently expressed in the brain, it is unknown whether there exists a causal relationship between NPTX1-dependent inhibition of neuronal hyperexcitability and engram formation. (3) It cannot be denied that memory encoding and consolidation involve multiple interacting molecular and circuit-level processes beyond NPTX regulation. The downregulation of NPTX1 and NPTX2 in specific DG circuits that encode the contextual fear memory could represent a key mechanism underlying contextual fear memory deficits during aging. According to our RNA-seq data (Supplementary information, Fig. S16), other molecules that contribute to Fos- and Npas4-dependent circuit plasticity could be further investigated. (4) The present study exclusively relying on the CFC paradigm as a measure of memory precision and generalization is limiting, as it does not fully capture the complexity of memory processes.
Materials and Methods
Animals
All animals used in this study were listed as follows: adult C57BL/6J male mice (3 months) from the SLAC Laboratory Animal Company (Shanghai, China), aged C57BL/6J male mice (16–18 months) from the Aniphe Biolaboratory Inc. (Nanjing, China) and PV-Flpe (021191) mice from the Jackson Laboratory (CA, USA). CRISPR-Cas9-mediated construction of Nptx1fl/fl, Nptx2fl/fl conditional knockout (Nptx1 cKO, Nptx2 cKO) mice targeting exon 3 and exon 2, respectively, were generated by the Biocytogen Co. (Beijing, China) and Npas4-CreERT2 mice were generated by Shanghai Model Organisms Center (Shanghai, China). Npas4-CreERT2 and PV-Flpe mice were bred to C57BL/6J mice for more than six generations, Nptx1fl/fl, Nptx2fl/fl mice and their respective WT littermates were obtained from self-crossing of Nptx1fl/+, Nptx2fl/+ (heterozygous) mice. Genotypes were determined by PCR of mouse toe DNA samples. The primers for genotyping PCR were provided in Supplementary information, Table S1. Male offsprings at 8–12 weeks of age were used in the following experiments, which were randomly assigned to groups. All mice were housed on a 12 h light/dark cycle (light on from 8 a.m. to 8 p.m.) with access to food and water ad libitum. All experiment procedures were strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by Animal Care and Use Committee of the animal facility at Fudan University.
Viral vectors
AAV-Fos-RAM-d2tTA-TRE-mKate2 and AAV-Npas4-RAM-d2tTA-TRE-mKate2 plasmids were kind gifts from Prof. Yingxi Lin (The University of Texas Southwestern Medical Center, TX, USA). To generate AAV-Fos-RAM-d2tTA-TRE-Cre and AAV-Npas4-RAM-d2tTA-TRE-Cre plasmids, mKate2 in AAV-Fos-RAM-d2tTA-TRE-mKate2 and AAV-Npas4-RAM-d2tTA-TRE-mKate2 plasmids was replaced with the Cre sequence obtained by PCR from pAAV-Cre-GFP (Addgene: 68544). pAAV-CMV-bGlobin-Flex-EGFP-MIR30-Scramble-shRNA, pAAV-CMV-bGlobin-Flex-EGFP-MIR30-Nptx2-shRNA and pAAV-CMV-DIO-Nptx2-PTX-P2A-EGFP were used in our previous paper.22 To generate pAAV-Ef1α-DIO-Nptx1-T2A-EYFP, the coding sequence for mouse Nptx1 with T2A was generated by Azenta US, Inc. (Suzhou, China) and subcloned into pAAV-Ef1α-DIO-EYFP (Addgene: 27056). Adeno-associated viruses (AAVs) described above were packaged by OBiO Technology Co., Ltd. (Shanghai, China) into serotype 9. AAV9-Ef1α-Flex-NBL10 (69971) and AAV9-Ef1α-DIO-ChR2-EYFP (S0199-9-H50) were purchased from Taitool Bioscience Co., Ltd. (Shanghai, China). AAV9-Ef1α-DIO-H2B-EGFP (PT-0258), AAV9-Ef1α-DIO-EYFP (PT-0012), AAV9-CaMKIIα-ChR2-mCherry (PT-0297), AAV9-VGAT2-Flp (PT-2501, vector backbone from BrainVTA), AAV9-CCK-fDIO-mCherry (PT-8508, vector backbone from Addgene: 114471), AAV8-Ef1α-DIO-TVA-H2B-EGFP (PT-0021), AAV8-Ef1α-DIO-RVG (PT-0023) and RV-ENVA-ΔG-dsRed (R01002) were purchased from BrainVTA Co., Ltd. (Wuhan, China). AAV9-PV-Flp (BC-0430, vector backbone from Addgene: 22914), AAV9-SST-Flp (BC-0429, vector backbone from Addgene: 22913), AAV9-Ef1α-fDIO-ChR2-mCherry (BC-0113), AAV9-Ef1α-fDIO-mCherry (BC-0193) and AAV9-Ef1α-fDIO-hM3Dq-mCherry (BC-0495) were purchased from Brain Case Co., Ltd. (Shenzhen, China).
Stereotaxic surgery
Mice were anesthetized with 2% isoflurane and placed in a stereotaxic instrument (RWD Life Science, Shenzhen, China). Microinjections were performed using 33-gauge needles connected to a 10 μL microsyringe (Hamilton, Bonaduz, Switzerland), which were under the control of a UMP3 ultra micropump (World Precision Instruments, FL, USA). The coordinates relative to bregma were listed as follows: anterior-posterior (AP) – 1.9 mm; medial-lateral (ML) ± 1.1 mm; dorsal-ventral (DV) – 2.1 mm for DG and AP – 4.7 mm; ML ± 3.2 mm; DV – 4.5 mm for MEC. The needle was slowly lowered to the target site and remained for at least 3 min after injection. The injection volume per site was 0.3 μL in DG and 0.4 μL in MEC. The final titer of all the AAVs used for infections were at least 1 × 1012 V.G./mL except for AAV-SST-Flp, AAV-PV-Flp, AAV-VGAT2-Flp, AAV-Fos-RAM-d2tTA-TRE-Cre and AAV-Npas4-RAM-d2tTA-TRE-Cre, which were 1:1000 diluted. For RV infections, the final titer used was 1 × 108 IFU/mL. After surgery, all mice were given at least 3 weeks to recover before behavioral experiments or electrophysiological recordings, and the efficiency of virus infection was verified by immunostaining. Only the mice with virus infection in correct places were chosen for further analysis.
Engram labeling
To label engram cells, Fos-RAM-d2tTA-TRE (F-RAM) and Npas4-RAM-d2tTA-TRE (N-RAM) systems, whose gene expression was under the control of the tetracycline responsive element (TRE), were used through AAV infusion. All mice were kept on Dox (Huamaike Bio, 360304) containing food (40 mg/kg) one day before virus injection. 48 h (day –2) before engram labeling, mice were fed with regular food instead of Dox diet. Then CFC or novel context exposure (context N) was performed on day 0 to label F-RAM or N-RAM engram cells in DG, after labeling, mice were put on Dox-containing food again immediately. Mice were fed at least 3 days to allow sufficient protein expression before subsequent experiments.
RV tracing of inputs
To study the respective monosynaptic inputs of F-RAM or N-RAM ensembles in DG, RV trans-synaptic tracing experiment was performed. Mice were infected with AAV9-Fos-RAM-d2tTA-TRE-Cre or AAV9-Npas4-RAM-d2tTA-TRE-Cre, AAV helper (AAV8-Ef1α-DIO-TVA-H2B-EGFP and AAV8-Ef1α-DIO-RVG). On day 3 after engram labeling, RV (RV-ENVA-ΔG-dsRed) was injected into DG at the same coordinates, and then mice were housed in the BSL2 facility for 1 week to allow rabies spread and dsRed expression before perfusion (day 10). For RV tracing analysis, consecutive brain slices from AP + 2.8 mm to AP – 5.0 mm (50 μm thickness) selected from every fifth slice were collected. DsRed+ cells were manually counted by an experimenter who was blind to the experimental condition. The percentage of rabies-labeled inputs was calculated as follows: dsRed+ cells in each brain region/total dsRed+ cells per mouse.
CFC
Before CFC was performed, mice were handled daily in a holding room for 3 days. For experiments with Dox-dependent ensemble labeling, on the third handling day, the Dox diet was replaced with regular food (off Dox) and CFC assay was typically carried out 48 h after the last handling session. CFC was performed in the conditioning chamber (Med-Associates, St. Albans, VT, USA) and the procedure was composed of conditioning and test sessions.
On the conditioning day (day 0), mice were firstly transported into the holding room and allowed to habituate for at least 30 min, then transported into the behavioral room and placed into context A, a square plexiglass observational chamber with stainless steel bars connected to a shock generator on the floor for conditioning. Two individual conditioning protocols were used in our study, for mice that conditioned to the 360 s protocol, three foot shocks (0.5 mA, 1 s each) at 180 s, 240 s and 300 s were given and mice were taken out 60 s after termination of the third foot shock, for mice that conditioned to the 180 s protocol, one single foot shock (0.3 mA, 1 s) at 120 s was given and mice were taken out 60 s after termination of the foot shock.
The test session was carried out on day 3. Mice were put back into fear context A for 180 s, and were placed into a non-fear context (context C, a triangular chamber with white, smooth plastic floor and black cover) for 180 s 6 h later. For immunohistochemistry, mice were tested for memory expression in only one context, either context A or C, and sacrificed 1 h later.
The freezing percentage was automatically analyzed by software (Med-Associates) with freezing defined as absence of movement for at least 1 s. The discrimination index was calculated as follows: (the freezing percentage in context A – the freezing percentage in context C)/(the freezing percentage in context A + the freezing percentage in context C).
NOR
Mice were allowed to explore two identical objects (object A) presented in two different places for 10 min, and after 1 h delay, mice were put back to the chamber for 5 min with one object replaced by a novel one (object B).
NPR
Mice were allowed to explore two identical objects (object C) presented in two different places for 10 min, and after 1 h delay, mice were put back to the chamber for 5 min with one object moved to another place. The time spent exploring each object in NOR and NPR memory tasks were analyzed by an experimenter who was blind to the project and the discrimination ratio was calculated as follows: (time exploring the novel/displaced objects – time exploring the familiar/stationary objects)/time exploring both objects.
Open field test
Spontaneous locomotor activity was carried out as we previously reported.58 In short, mice were placed in the center of an open arena (40 cm × 40 cm) at the beginning of the test, and were allowed to freely explore the arena for 20 min. Distance traveled in the arena and time in center zone were quantified using a TopScan automated detection system (CleverSys, Reston, VA, USA).
O-maze test
The O-maze was 70 cm in diameter and 70 cm high off the ground, and consisted of two open arms (7 cm wide) without walls, two closed arms that were enclosed by vertical walls. Mice were gently placed into the open arms, and their behaviors were recorded for 6 min via a TopScan automated detection system (CleverSys, Reston, VA, USA) located above the maze.
Tail suspension test (TST)
Mice were suspended 20 cm above a solid surface by the use of adhesive tape applied to the tail, and their behaviors were recorded for 6 min. Immobility time was defined as absence of struggling for at least 1 s and latency to immobility was defined as the time from the beginning of TST to mouse’s first absence of struggling for at least 1 s, which were manually analyzed by an experimenter who was blind to the experimental condition.
Drug injections
CNO (Sigma, C0832) was dissolved in saline, and i.p. injected at a concentration of 1 mg/kg 30 min before memory retrieval. The KCNQ2/3 activator, retigabine (Supelco, 90221) was diluted in 5% DMSO (Sigma, 276855) and administered i.p. at 1 mg/kg 30 min before memory retrieval with 5% DMSO as control. 4-OHT (Sigma, H6278) was dissolved in ethanol at 20 mg/mL and stored at –20 °C. The ethanol was evaporated at 95 °C and corn oil (Thermo Fisher Scientific, C40543) was added to give a final concentration of 10 mg/mL 4-OHT, which was i.p. injected 2 h before memory conditioning with corn oil as control.
Whole-cell patch-clamp recording in brain slices
Mice were subjected to electrophysiological recordings on day 3 following CFC. Preparation of living acute brain slices (300 μm) for analyzing engram synaptic connections and engram intrinsic excitability was performed as previously described59 only with minor modifications. Briefly, mice’s brains were cut on a vibratome (Thermo Fisher Scientific, MA, USA) in carbogenated (95% O2, 5% CO2) ice-cold cutting solution containing: 93 mM NMDG, 2.5 mM KCl, 1.25 mM NaH2PO4, 30 mM NaHCO3, 20 mM HEPES, 25 mM glucose, 2 mM thiourea, 5 mM Na-ascorbate, 3 mM Na-pyruvate, 0.5 mM CaCl2 and 10 mM MgCl2, 300–310 mOsm, pH adjusted to 7.3 with HCl. After initial recovery at 32 °C for 10 min, slices were transferred to carbogenated HEPES holding ACSF (92 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 30 mM NaHCO3, 20 mM HEPES, 25 mM glucose, 2 mM thiourea, 5 mM Na-ascorbate, 3 mM Na-pyruvate, 2 mM CaCl2 and 2 mM MgCl2, 300–310 mOsm, pH 7.4) and incubated for over 45 min before recording. Whole-cell patch-clamp recordings were performed in carbogenated recording ACSF (119 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 24 mM NaHCO3, 12.5 mM glucose, 2 mM CaCl2 and 2 mM MgCl2 (300–310 mOsm, pH 7.3–7.4) at a rate of 1.5 mL/min (30–32 °C) with an EPC-10 amplifier and Pulse v8.78 software (HEKA Elektronik, Lambrecht/Pfalz, Germany). Recorded neurons were identified visually by location, morphology, size and fluorescence, and recordings were performed using borosilicate glass pipettes (5–7 mΩ tip resistance).
To record A/N ratios, neurons were voltage-clamped at –70 mV to record AMPAR-mediated EPSCs and at +40 mV to record dual-component EPSCs containing NMDA receptor (NMDAR) EPSCs. To calculate the A/N ratios, the peak current of the AMPA EPSC at –70 mV was compared with the value of the NMDA EPSC after stimulation start time 50 ms at +40 mV. Intracellular solution used was: 127.5 mM cesium methanesulfonate, 7.5 mM CsCl, 10 mM HEPES, 2.5 mM MgCl2, 4 mM Mg-ATP, 0.4 mM Na3-GTP, 10 mM sodium phosphocreatine, 0.6 mM EGTA (290–300 mOsm, pH 7.2).
To record light-evoked EPSCs (oEPSC), a TTL-driven light-emitting diode (Lumen Dynamics) was used to generate photostimulation consisting of a single wide-field blue flash (470 nm, 1 ms duration) for photostimulation of ChR2-expressing terminals. The laser intensity was measured at the focal plane of the slice when delivered through the 40× water-immersion objective lens (Nikon, Japan). Slices containing MEC ChR2-expressing terminals were chosen for oEPSC recording in the presence of 1 μM Tetrodotoxin (TTX, Tocris, 1078), 100 μM 4-aminopyridine (4-AP, Sigma, 275875) and 100 μM picrotoxin (PTX, Tocris, 1128), neurons were voltage-clamped at –70 mV and the stimulus intensity was 0.1 mW at 470 nm. To record PPR and A/N ratio, DG slices containing ChR2-expressing neurons were chosen to perform patch-clamp recordings in the presence of 100 μM PTX and the stimulus intensity was 0.1 mW at 470 nm. The AMPAR-mediated EPSCs were evoked by paired photostimulation of 50-ms interval for 10 consecutive traces, and PPR was determined as the peak amplitude ratio of the second to the first EPSC. To record light-evoked IPSCs (oIPSC) and PPR, neurons were clamped at +10 mV in the presence of 1 μM TTX, 100 μM 4-AP, 20 μM CNQX (Sigma, C127) and 50 μM D-AP5 (Tocris, 0106) and the stimulus duration and intensity were 1 ms, 0.2 mW at 470 nm. The recording protocols and intracellular solution used were the same as above.
To record AP, current-clamp was used and membrane potentials were measured in response to intracellular injection of step currents (1000 ms duration, magnitudes ranging from –150 pA to 250 pA in steps of 10 pA) with the addition of 20 μM CNQX, 50 μM D-AP5 and 100 μM PTX into ACSF. Intracellular solution used was: 135 mM potassium-gluconate, 4 mM KCl, 2 mM NaCl, 10 mM HEPES, 4 mM EGTA, 4 mM Mg-ATP, 0.3 mM Na3-GTP, 10 mM sodium phosphocreatine (280–290 mOsm, pH 7.3).
To record and isolate IM, intracellular solution used was the same as above. 0.2 mM CdCl2 (Sigma, 202908), 1 μM TTX, 10 μM ZD7288 (Tocris, 1000), and 4 mM 4-AP to block voltage-dependent Cav, Nav, HCN and Kv1 channels, and 20 μM CNQX, 50 μM D-AP5 and 100 μM PTX to block synaptic activity were added into ACSF. To isolate IM, the following protocol as previously reported60 with minor modifications was applied to the recording cells: (1) a 1 s step from the holding potential (–70 mV) to –10 mV was applied to activate IM while inactivating most other voltage-gated currents, (2) a 1 s step from –60 mV to –10 mV with 10 mV increments to elicit IM tail current, and (3) a 1 s step to –10 mV, before returning to –70 mV.
The signals were acquired at 10 kHz and filtered at 2 kHz. The series resistance was < 30 mΩ. Data were analyzed with Mini Analysis Program (Synaptosoft, Fort Lee, NJ, USA) or pCLAMP10.7 (Molecular Devices, San Jose, CA, USA) by an experimenter who was blind to the experimental condition.
Ribosome-associated mRNA purification/RiboTag
DG tissues from the mice injected with AAV9-F/N-RAM-Cre and AAV9-Flex-NBL10 (Cre-dependent expression of N-terminus of ribosomal subunit protein Rpl10a (NBL10)61,62) following fear conditioning (day 3) were quickly isolated and used for enrichment of ribosome-associated transcripts as described previously.22 The brain tissue (DG) was homogenized in supplemented hybridization buffer (HB-S) containing dithiothreitol (Sigma, D9760), cycloheximide (Cayman, 14126), heparin (Tocris, 2812), protease inhibitors (Roche, 04693116001), and RNase inhibitor (Vazyme, R301). The supernatant was incubated with anti-hemagglutinin (HA) antibody (Sigma, H6908) and Dynabeads Protein G (Invitrogen, 10003D) for 12 h. Purified mRNA was eluted from the Dynabeads using TRIzolTM LS (Invitrogen, 10296010). An Agilent RNA 6000 Pico Kit (Agilent, 5067-1513) and an Agilent 2100 bioanalyzer were used to evaluate the quality of purified mRNA. Purified mRNA samples with RNA integrity number < 7 were discarded.
Sequence processing and data analysis
The library was prepared with VAHTSTM mRNA-seq V3 Library Prep Kit for Illumina (Vazyme, NR611) and sequenced on a HiSeq 4000 (Illumina) by Novogene Technology Co., Ltd. (Beijing, China). Raw reads were cleaned with FASTX-toolkit to remove adapter contamination and low-quality reads (quality score < 28). The clipped reads were aligned to mouse reference sequence (GRCm38/mm10) using HISAT2.63 Cuffdiff-generated FPKM count matrix was used for subsequent analysis. Significance was drawn with analysis of variance (ANOVA) and clustered using Shannon entropy-based method.64 Genes with > 1.5-fold expression changes and significant difference (P < 0.05) were selected for further analysis.
smFISH
Fixed-frozen brain tissues were sliced into 10-μm coronal sections and baked at 60 °C for 30 min. Then slices were incubated with H2O2 for 10 min at room temperature (RT), and target retrieval and protease III incubation were performed using RNAscope® 2.5 Universal Pretreatment Reagents (Advanced Cell Diagnostics, 322000, 322381). smFISH probes for all genes examined: Nptx1 (505421), Nptx2 (316901), Fos (316921) and Npas4 (423431) were hybridized for 2 h. After hybridization, RNAscope® Multiplex Fluorescent Detection Kit v2 (Advanced Cell Diagnostics, 323110) was used to amplify signals. Images were acquired by using the Nikon A1 confocal microscope (Tokyo, Japan). Regions of interest were circled and the intensity within the regions was analyzed by Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA). For cell counting analysis, consecutive brain slices from AP – 1.2 mm to AP – 2.0 mm (10 μm thickness) were selected from every tenth slice, 8 slices per mouse were collected, and the numbers of mice were included in Supplementary information, Table S2.
Immunohistochemistry
Mice were perfused transcardially with ice-cold saline followed by 4% paraformaldehyde (PFA, dissolved in 0.1 M PBS). The brains were removed and fixed in 4% PFA overnight. Then the brains were dehydrated in 30% sucrose solutions at 4 °C for 72 h before being sliced into 30-μm or 40-μm coronal sections (selected from every fifth slice). Slices were incubated with primary antibodies in blocking solution containing 0.3% Triton X-100 overnight at 4 °C. Slices were washed with 0.1 M PBS, and then incubated with secondary antibody at RT for 1.5 h. After being washed in PBS, slices were mounted in anti-quenching mounting medium (Southern Biotech, 0100). Primary antibodies used were: anti-NPTX1 (1:400, Alomone Labs, ANR-191), anti-NPTX2 (1:500, Proteintech, 10889-1-AP), anti-Fos (1:500, Synaptic Systems, 226017), anti-parvalbumin (1:100, Thermo Fisher Scientific, PA1-933), anti-PV (1:500, Oasis, OB-PGP005-01), anti-GluA4 (1:500, Merck, AB1508), anti-Somatostatin (SST, 1:200, Oasis, OB-PRB111-01), anti-ChAT (1:100, Merck, AB143), anti-VGluT1 (1:500, Synaptic Systems, 135302), anti-GAD67 (1:500, Merck, MAB5406), anti-cholecystokinin (CCK, 1:1000, Merck, C2581) and anti-KCNQ2 (1:100, Alomone Labs, APC-050). Secondary antibodies used were: goat anti-rat 488 (1:1000, Jackson Immuno Research, 112-545-167), goat anti-rabbit 488 (1:1000, Jackson Immuno Research, 111-545-144), donkey anti-guinea pig 488 (1:1000, Jackson Immuno Research, 706-545-148), goat anti-rabbit Cy3 (1:1000, Jackson Immuno Research, 111-165-144), donkey anti-rat Cy3 (1:1000, Jackson Immuno Research, 712-165-153) and donkey anti-rat 647 (1:500, Jackson Immuno Research, 712-605-150). Kv7.2 and GluA4 membrane expression analysis were adapted from one of the latest researches from our lab.65 Images were acquired using a Nikon-A1 confocal microscope (Tokyo, Japan) with a 20× objective lens or a 60× objective oil lens. Data were analyzed blindly to the group using Image-Pro Plus 6.0 and ImageJ (Fiji). For cell counting analysis, consecutive brain slices from AP – 1.2 mm to AP – 2.0 mm (40 μm thickness) selected from every fifth slice, 4 slices per mouse were collected, and the numbers of mice were included in Supplementary information, Table S2.
ChIP
Adult C57BL/6J male mice were subjected to CFC (0.5 mA, 1 s, 3 times), and 1 h later, DG tissues were quickly isolated. ChIP was performed using the ChIP Assay Kit (Beyotime, P2078) according to the manufacturer’s instructions with modifications. Briefly, the samples were minced and crosslinked with 1.5% formaldehyde at RT for 12 min, followed by quenching with glycine for 5 min. The samples were then homogenized and chromatin was sheared by incubating with nuclei lysis buffer, followed by sonication (100 W, 30 s on/off cycles; 10 cycles) to disrupt the nuclear membrane. For immunoprecipitation, the diluted chromatin was incubated overnight at 4 °C with 4 μg anti-IgG (Cell Signaling Technology, 2729S), anti-Fos (Cell Signaling Technology, 2250) or anti-Npas4 antibody (Activity Signaling, AS-AB18A-100) with continuous rotation, followed by an additional 1 h incubation with 30 μL Protein A + G Agarose. DNA was then eluted and purified and subjected to qPCR. ChIP-qPCR results were calculated as a percentage of input DNA.
Reverse transcription-quantitative PCR
Reverse transcription was completed using HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, R212). The cDNA was subjected to qPCR using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q711) and Eppendorf Mastercycler PCR System. The mRNA expression of Nptx1 and Nptx2 was normalized to that of the internal control Gapdh. All primers used for PCR amplification were listed in Supplementary information, Table S1.
Co-IP and western blotting
The DG tissues of adult C57BL/6J male mice were rapidly extracted and homogenized in radio-immunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors for 30 min. The protein lysate concentration was determined using bicinchoninic acid kit. Protein samples were incubated with 3 µg anti-NPTX1 antibody (Santa Cruz Biotechnology, sc-374199) or anti-IgG antibody (Cell Signaling Technology, 5415S) for 4 h at 4 °C. Pierce Protein A/G magnetic beads (Thermo Fisher Scientific, 88803) were added to each sample and the mixture was rotated at 4 °C overnight. The attached protein was then eluted and used for further assay. Before western blotting assay, 50 µg protein samples were boiled for 6 min at 85 °C, and were separated by SDS-PAGE under 120 V for 1.5 h. Proteins were then transferred to the polyvinylidene fluoride (PVDF) membrane at 100 mV for 100 min. The membrane was firstly washed with Tris-buffered saline with Tween 20 (TBST), followed by blocking in 5% skimmed milk for 2 h. The membrane was then incubated with the following primary antibodies anti-NPTX1 (1:100, Santa Cruz Biotechnology, sc-374199) and anti-KCNQ2 (1:500, Abcam, ab22897) at 4 °C overnight. On the next day, the membrane was washed with TBST and incubated with IRDye 700DX- or 800DX-conjugated anti-rabbit or anti-mouse IgG (1:50,000, Rockland Immunochemicals Inc.) for 2 h at RT. Protein bands were visualized using Odyssey (LI-COR Biosciences). The immunoblots were analyzed with Image J.
Statistics
All data were presented as mean ± SEM. Sample sizes were based on our previous research.22,58,66 Statistical analyses were performed by SPSS 20.0 software (IBM, Armonk, NY, USA). The normality test of the data sets was performed by the Shapiro-Wilk test or the Kolmogorov-Smirnov test if n > 50, and homogeneity of variance test of the data sets was performed by the Levene’s test. Two-tailed unpaired t-test was used for comparing two independent groups. Multiple group comparisons were assessed using one-way ANOVA, two-way ANOVA or two-way repeated measures ANOVA, followed by the Bonferroni’s post-hoc test when significant main effects or interactions were detected. Mann–Whitney U test or Kruskal-Wallis H test was used when normality was violated. Full statistical analyses corresponding to each figure are provided in Supplementary information, Table S2. *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary information
Acknowledgements
This work was supported by grants from the STI2030-Major Projects (2021ZD0203500 to F.W. and L.M., 2021ZD0202100 to X.L.), the National Natural Science Foundation of China (32222033 to F.W., 32330041 and 82021002 to L.M., 32450102 and 32171041 to X.L., 32270660 to Q.L.), the CAMS Innovation Fund for Medical Sciences (2021-I2M-5-009 to L.M. and X.L.). We thank Prof. Yingxi Lin (The University of Texas Southwestern Medical Center, TX, USA) for providing the AAV-Fos-RAM-d2tTA-TRE-mKate2 and AAV-Npas4-RAM-d2tTA-TRE-mKate2 plasmids.
Author contributions
F.W., L.M., and T.J. designed the research. T.J. and Y.Y. conducted the AAV vector construction, virus injection, immunostaining, co-IP, smFISH, Ribo Tag purification and the behavioral experiments. T.J. carried out the electrophysiological recordings. N.H., Y.G., Y.Z., and Q.L. contributed to the bioinformatics analysis. T.J., Y.Y., J.Y., and X.L. analyzed the data. T.J. carried out the statistical analysis and drafted the manuscript. L.M. and F.W. supervised the project and revised the manuscript.
Data availability
The RNA-seq data of F- and N-RAM engram cells have been submitted to NCBI Gene Expression Omnibus (GEO) under accession number PRJNA1067898.
Code availability
Custom codes will be provided upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tao Jin, Yang Yang, Yu Guo.
Supplementary information
The online version contains supplementary material available at 10.1038/s41422-025-01157-w.
References
- 1.Josselyn, S. A. & Tonegawa, S. Memory engrams: recalling the past and imagining the future. Science367, eaaw4325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonegawa, S., Morrissey, M. D. & Kitamura, T. The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci.19, 485–498 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Castello-Waldow, T. P. et al. Hippocampal neurons with stable excitatory connectivity become part of neuronal representations. PLoS Biol.18, e3000928 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesuis, S. L. et al. Glucocorticoids promote fear generalization by increasing the size of a dentate gyrus engram cell population. Biol. Psychiatry90, 494–504 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Josselyn, S. A., Kohler, S. & Frankland, P. W. Finding the engram. Nat. Rev. Neurosci.16, 521–534 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Luo, L. & Craik, F. I. Aging and memory: a cognitive approach. Can. J. Psychiatry53, 346–353 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Fan, X., Wheatley, E. G. & Villeda, S. A. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu. Rev. Neurosci.40, 251–272 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bieri, G., Schroer, A. B. & Villeda, S. A. Blood-to-brain communication in aging and rejuvenation. Nat. Neurosci.26, 379–393 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Sudhof, T. C. Towards an understanding of synapse formation. Neuron100, 276–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez de San Jose, N. et al. Neuronal pentraxins as biomarkers of synaptic activity: from physiological functions to pathological changes in neurodegeneration. J. Neural Transm.129, 207–230 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho, R. W. et al. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron57, 858–871 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu, D. et al. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron39, 513–528 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Abad, M. A., Enguita, M., DeGregorio-Rocasolano, N., Ferrer, I. & Trullas, R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-beta and is overexpressed in dystrophic neurites in Alzheimer’s brain. J. Neurosci.26, 12735–12747 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueiro-Silva, J. et al. Neuronal pentraxin 1 negatively regulates excitatory synapse density and synaptic plasticity. J. Neurosci.35, 5504–5521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, M. C. et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci.13, 1090–1097 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, Y. et al. Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron79, 335–346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao, M. F. et al. A biomarker-authenticated model of schizophrenia implicating NPTX2 loss of function. Sci. Adv.7, eabf6935 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mravinacová, S. et al. CSF protein ratios with enhanced potential to reflect Alzheimer’s disease pathology and neurodegeneration. Mol. Neurodegener.19, 15 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soldan, A. et al. NPTX2 in cerebrospinal fluid predicts the progression from normal cognition to mild cognitive impairment. Ann. Neurol.94, 620–631 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen, A. T. et al. A robust activity marking system for exploring active neuronal ensembles. Elife5, e13918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun, X. et al. Functionally distinct neuronal ensembles within the memory engram. Cell181, 410–423.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, Z. et al. Retrieval-driven hippocampal NPTX2 plasticity facilitates the extinction of cocaine-associated context memory. Biol. Psychiatry87, 979–991 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Mariga, A. et al. Definition of a bidirectional activity-dependent pathway involving BDNF and Narp. Cell Rep.13, 1747–1756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delmas, P. & Brown, D. A. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci.6, 850–862 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Li, S. B. et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science375, eabh3021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topolnik, L. & Tamboli, S. The role of inhibitory circuits in hippocampal memory processing. Nat. Rev. Neurosci.23, 476–492 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci.15, 769–775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Y. et al. Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol. Psychiatry87, 843–856 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Huang, Z. et al. A disinhibitory microcircuit of the orbitofrontal cortex mediates cocaine preference in mice. Mol. Psychiatry29, 3160–3169 (2024). [DOI] [PubMed] [Google Scholar]
- 30.Cummings, D. M. et al. Neuronal and peripheral pentraxins modify glutamate release and may interact in blood-brain barrier failure. Cereb. Cortex27, 3437–3448 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Fang, S. et al. Sexually dimorphic control of affective state processing and empathic behaviors. Neuron112, 1498–1517.e8 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Pelkey, K. A. et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron85, 1257–1272 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, L. et al. Cocaine induces locomotor sensitization through a dopamine-dependent VTA-mPFC-FrA cortico-cortical pathway in male mice. Nat. Commun.14, 1568 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou, C. et al. YTHDF1 in periaqueductal gray inhibitory neurons contributes to morphine withdrawal responses in mice. BMC Med.22, 406 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Q. et al. An iterative neural processing sequence orchestrates feeding. Neuron111, 1651–1665.e5 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Blackburn-Munro, G., Dalby-Brown, W., Mirza, N. R., Mikkelsen, J. D. & Blackburn-Munro, R. E. Retigabine: chemical synthesis to clinical application. CNS Drug Rev.11, 1–20 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo, N. et al. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med.24, 438–449 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen, P. T. et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell182, 388–403.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAvoy, K. M. et al. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron91, 1356–1373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regev, N. et al. Selective interaction of syntaxin 1A with KCNQ2: possible implications for specific modulation of presynaptic activity. PLoS One 4, e6586 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luna, V. M. et al. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science364, 578–583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin, H. et al. A visual-cue-dependent memory circuit for place navigation. Neuron99, 47–55.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartos, M. & Elgueta, C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J. Physiol.590, 669–681 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klausberger, T. et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci.25, 9782–9793 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, X. & Lin, Y. Npas4: linking neuronal activity to memory. Trends Neurosci.39, 264–275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiegel, I. et al. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell157, 1216–1229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollina, E. A. et al. A NPAS4-NuA4 complex couples synaptic activity to DNA repair. Nature614, 732–741 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloodgood, B. L., Sharma, N., Browne, H. A., Trepman, A. Z. & Greenberg, M. E. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature503, 121–125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yap, E. L. et al. Bidirectional perisomatic inhibitory plasticity of a Fos neuronal network. Nature590, 115–121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsaran, A. I. et al. A shift in the mechanisms controlling hippocampal engram formation during brain maturation. Science380, 543–551 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Rogue, P. J., Ritz, M. F. & Malviya, A. N. Impaired gene transcription and nuclear protein kinase C activation in the brain and liver of aged rats. FEBS Lett.334, 351–354 (1993). [DOI] [PubMed] [Google Scholar]
- 52.Sutin, E. L., Dement, W. C., Heller, H. C. & Kilduff, T. S. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol. Aging14, 441–446 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Qiu, J. et al. Decreased Npas4 and Arc mRNA levels in the hippocampus of aged memory-impaired wild-type but not memory preserved 11beta-HSD1 deficient mice. J. Neuroendocrinol.28, n/a (2016). [DOI] [PMC free article] [PubMed]
- 54.Singh, A. K. et al. Anti-aging biomaterial sturgeon chondroitin sulfate upregulating anti-oxidant and SIRT-1/c-fos gene expression to reprogram stem cell senescence and prolong longevity. Biomater. Sci.11, 4522–4536 (2023). [DOI] [PubMed] [Google Scholar]
- 55.Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med.20, 659–663 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weng, F. J. et al. Npas4 is a critical regulator of learning-induced plasticity at mossy fiber-CA3 synapses during contextual memory formation. Neuron97, 1137–1152.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramamoorthi, K. et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science334, 1669–1675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin, T. et al. Dorsal hippocampus- and ACC-projecting medial septum neurons differentially contribute to the recollection of episodic-like memory. FASEB J.34, 11741–11753 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Ting, J. T., Daigle, T. L., Chen, Q. & Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol.1183, 221–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez, J. P. et al. Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron110, 2283–2298.e9 (2022). [DOI] [PubMed] [Google Scholar]
- 61.Ekstrand, M. I. et al. Molecular profiling of neurons based on connectivity. Cell157, 1230–1242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, F. et al. Morphine- and foot shock-responsive neuronal ensembles in the VTA possess different connectivity and biased GPCR signaling pathway. Theranostics14, 1126–1146 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol.37, 907–915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh, S. & Chan, C. K. Analysis of RNA-seq data using TopHat and Cufflinks. Methods Mol. Biol.1374, 339–361 (2016). [DOI] [PubMed] [Google Scholar]
- 65.He, G. et al. Persistent increase of accumbens cocaine ensemble excitability induced by IRK downregulation after withdrawal mediates the incubation of cocaine craving. Mol. Psychiatry28, 448–462 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou, Y. et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat. Neurosci.22, 1986–1999 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data of F- and N-RAM engram cells have been submitted to NCBI Gene Expression Omnibus (GEO) under accession number PRJNA1067898.
Custom codes will be provided upon request.